Abstract
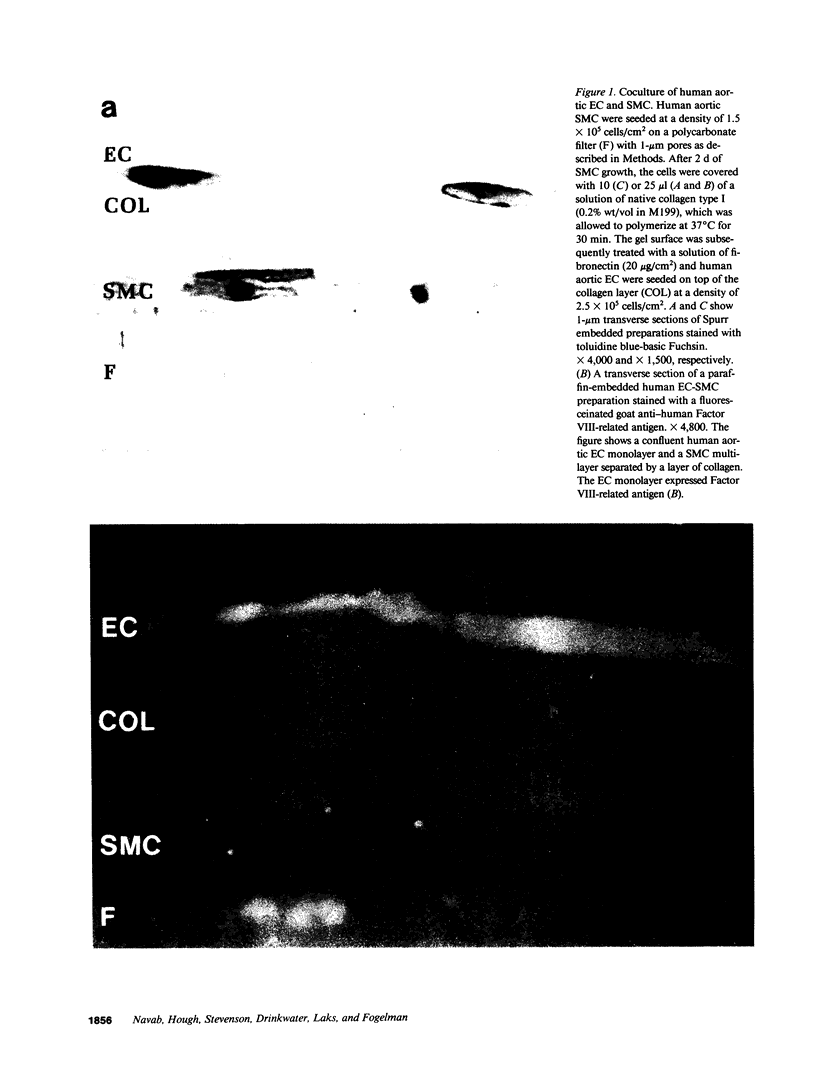
Human aortic endothelial cells (EC) and smooth muscle cells (SMC) were isolated and used to form a multilayer of EC-SMC separated by a layer of collagen. SMC and/or collagen layers exerted minimal effects on Na+ transport but impeded the transport of LDL. The presence of an endothelial monolayer markedly reduced the transport of Na+ and LDL. When monocytes were presented to the complete coculture, in the absence of added chemoattractant, one monocyte entered the subendothelial space for every one to three EC present. In contrast, neither collagen nor SMC plus collagen nor EC plus collagen induced comparable monocyte migration. Despite massive migration of monocytes into the coculture, no significant alteration in Na+ transport was observed. LDL transport into the preparation during massive monocyte migration increased modestly, but this was far less than the amount of LDL transported in the absence of an endothelial monolayer. We conclude that (a) the endothelial monolayer was the principal permeability barrier, (b) a substantial migration of monocytes occurred in the absence of added chemoattractant when both EC and SMC were present in the coculture, (c) endothelial barrier function was largely maintained after monocyte migration; and (d) these experiments indicate the need to study all three cell types (monocytes, EC, and SMC) together to understand the complex interactions that occur between these cells.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berliner J. A., Territo M., Almada L., Carter A., Shafonsky E., Fogelman A. M. Monocyte chemotactic factor produced by large vessel endothelial cells in vitro. Arteriosclerosis. 1986 May-Jun;6(3):254–258. doi: 10.1161/01.atv.6.3.254. [DOI] [PubMed] [Google Scholar]
- Bevilacqua M. P., Pober J. S., Wheeler M. E., Cotran R. S., Gimbrone M. A., Jr Interleukin-1 activation of vascular endothelium. Effects on procoagulant activity and leukocyte adhesion. Am J Pathol. 1985 Dec;121(3):394–403. [PMC free article] [PubMed] [Google Scholar]
- Bilheimer D. W., Eisenberg S., Levy R. I. The metabolism of very low density lipoprotein proteins. I. Preliminary in vitro and in vivo observations. Biochim Biophys Acta. 1972 Feb 21;260(2):212–221. doi: 10.1016/0005-2760(72)90034-3. [DOI] [PubMed] [Google Scholar]
- Caro C. G., Fitz-Gerald J. M., Schroter R. C. Atheroma and arterial wall shear. Observation, correlation and proposal of a shear dependent mass transfer mechanism for atherogenesis. Proc R Soc Lond B Biol Sci. 1971 Feb 16;177(1046):109–159. doi: 10.1098/rspb.1971.0019. [DOI] [PubMed] [Google Scholar]
- Chobanian A. V., Menzoian J. O., Shipman J., Heath K., Haudenschild C. C. Effects of endothelial denudation and cholesterol feeding on in vivo transport of albumin, glucose, and water across rabbit carotid artery. Circ Res. 1983 Dec;53(6):805–814. doi: 10.1161/01.res.53.6.805. [DOI] [PubMed] [Google Scholar]
- Colucci W. S., Brock T. A., Gimbrone M. A., Jr, Alexander R. W. Nonlinear relationship between alpha 1-adrenergic receptor occupancy and norepinephrine-stimulated calcium flux in cultured vascular smooth muscle cells. Mol Pharmacol. 1985 May;27(5):517–524. [PubMed] [Google Scholar]
- Cornhill J. F., Barrett W. A., Herderick E. E., Mahley R. W., Fry D. L. Topographic study of sudanophilic lesions in cholesterol-fed minipigs by image analysis. Arteriosclerosis. 1985 Sep-Oct;5(5):415–426. doi: 10.1161/01.atv.5.5.415. [DOI] [PubMed] [Google Scholar]
- DUFF G. L., McMILLAN G. C., RITCHIE A. C. The morphology of early atherosclerotic lesions of the aorta demonstrated by the surface technique in rabbits fed cholesterol; together with a description of the anatomy of the intima of the rabbit's aorta and the spontaneous lesions which occur in it. Am J Pathol. 1957 Sep-Oct;33(5):845–873. [PMC free article] [PubMed] [Google Scholar]
- Davies P. F., Truskey G. A., Warren H. B., O'Connor S. E., Eisenhaure B. H. Metabolic cooperation between vascular endothelial cells and smooth muscle cells in co-culture: changes in low density lipoprotein metabolism. J Cell Biol. 1985 Sep;101(3):871–879. doi: 10.1083/jcb.101.3.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogelman A. M., Elahi F., Sykes K., Van Lenten B. J., Territo M. C., Berliner J. A. Modification of the Recalde method for the isolation of human monocytes. J Lipid Res. 1988 Sep;29(9):1243–1247. [PubMed] [Google Scholar]
- Fogelman A. M., Haberland M. E., Seager J., Hokom M., Edwards P. A. Factors regulating the activities of the low density lipoprotein receptor and the scavenger receptor on human monocyte-macrophages. J Lipid Res. 1981 Sep;22(7):1131–1141. [PubMed] [Google Scholar]
- Freudenberg N., Riese K. H. Characterization of cells of the normal aortic endothelium of adult rats and changes due to endotoxin shock. I. Communication: light microscopy, autoradiography, DNA cytophotometry, and enzyme histochemistry. Beitr Pathol. 1976 Nov;159(2):125–142. doi: 10.1016/s0005-8165(76)80001-7. [DOI] [PubMed] [Google Scholar]
- Friedman M. H., Hutchins G. M., Bargeron C. B., Deters O. J., Mark F. F. Correlation between intimal thickness and fluid shear in human arteries. Atherosclerosis. 1981 Jun;39(3):425–436. doi: 10.1016/0021-9150(81)90027-7. [DOI] [PubMed] [Google Scholar]
- Furie M. B., Naprstek B. L., Silverstein S. C. Migration of neutrophils across monolayers of cultured microvascular endothelial cells. An in vitro model of leucocyte extravasation. J Cell Sci. 1987 Sep;88(Pt 2):161–175. doi: 10.1242/jcs.88.2.161. [DOI] [PubMed] [Google Scholar]
- Geer J. C. Fine structure of human aortic intimal thickening and fatty streaks. Lab Invest. 1965 Oct;14(10):1764–1783. [PubMed] [Google Scholar]
- Gerrity R. G., Naito H. K., Richardson M., Schwartz C. J. Dietary induced atherogenesis in swine. Morphology of the intima in prelesion stages. Am J Pathol. 1979 Jun;95(3):775–792. [PMC free article] [PubMed] [Google Scholar]
- Gerrity R. G., Schwartz C. J. Structural correlates of arterial endothelial permeability in the Evans blue model. Prog Biochem Pharmacol. 1977;13:134–137. [PubMed] [Google Scholar]
- Gown A. M., Tsukada T., Ross R. Human atherosclerosis. II. Immunocytochemical analysis of the cellular composition of human atherosclerotic lesions. Am J Pathol. 1986 Oct;125(1):191–207. [PMC free article] [PubMed] [Google Scholar]
- HAVEL R. J., EDER H. A., BRAGDON J. H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955 Sep;34(9):1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajjar D. P., Falcone D. J., Amberson J. B., Hefton J. M. Interaction of arterial cells. I. Endothelial cells alter cholesterol metabolism in co-cultured smooth muscle cells. J Lipid Res. 1985 Oct;26(10):1212–1223. [PubMed] [Google Scholar]
- Hajjar D. P., Marcus A. J., Hajjar K. A. Interactions of arterial cells. Studies on the mechanisms of endothelial cell modulation of cholesterol metabolism in co-cultured smooth muscle cells. J Biol Chem. 1987 May 25;262(15):6976–6981. [PubMed] [Google Scholar]
- Jones P. A. Construction of an artificial blood vessel wall from cultured endothelial and smooth muscle cells. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1882–1886. doi: 10.1073/pnas.76.4.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joris I., Stetz E., Majno G. Lymphocytes and monocytes in the aortic intima--An electron-microscopic study in the rat. Atherosclerosis. 1979 Nov;34(3):221–231. doi: 10.1016/s0021-9150(79)80003-9. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mazzone T., Jensen M., Chait A. Human arterial wall cells secrete factors that are chemotactic for monocytes. Proc Natl Acad Sci U S A. 1983 Aug;80(16):5094–5097. doi: 10.1073/pnas.80.16.5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrilees M. J., Scott L. Interaction of aortic endothelial and smooth muscle cells in culture. Effect on glycosaminoglycan levels. Atherosclerosis. 1981 May;39(2):147–161. doi: 10.1016/0021-9150(81)90064-2. [DOI] [PubMed] [Google Scholar]
- Migliorisi G., Folkes E., Pawlowski N., Cramer E. B. In vitro studies of human monocyte migration across endothelium in response to leukotriene B4 and f-Met-Leu-Phe. Am J Pathol. 1987 Apr;127(1):157–167. [PMC free article] [PubMed] [Google Scholar]
- Nash S., Stafford J., Madara J. L. Effects of polymorphonuclear leukocyte transmigration on the barrier function of cultured intestinal epithelial monolayers. J Clin Invest. 1987 Oct;80(4):1104–1113. doi: 10.1172/JCI113167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navab M., Hough G. P., Berliner J. A., Frank J. A., Fogelman A. M., Haberland M. E., Edwards P. A. Rabbit beta-migrating very low density lipoprotein increases endothelial macromolecular transport without altering electrical resistance. J Clin Invest. 1986 Aug;78(2):389–397. doi: 10.1172/JCI112589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navab M., Hough G. P., Van Lenten B. J., Berliner J. A., Fogelman A. M. Low density lipoproteins transfer bacterial lipopolysaccharides across endothelial monolayers in a biologically active form. J Clin Invest. 1988 Feb;81(2):601–605. doi: 10.1172/JCI113359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POOLE J. C., FLOREY H. W. Changes in the endothelium of the aorta and the behaviour of macrophages in experimental atheroma of rabbits. J Pathol Bacteriol. 1958 Apr;75(2):245–251. doi: 10.1002/path.1700750202. [DOI] [PubMed] [Google Scholar]
- Pawlowski N. A., Abraham E. L., Pontier S., Scott W. A., Cohn Z. A. Human monocyte-endothelial cell interaction in vitro. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8208–8212. doi: 10.1073/pnas.82.23.8208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitas R. E., Innerarity T. L., Weinstein J. N., Mahley R. W. Acetoacetylated lipoproteins used to distinguish fibroblasts from macrophages in vitro by fluorescence microscopy. Arteriosclerosis. 1981 May-Jun;1(3):177–185. doi: 10.1161/01.atv.1.3.177. [DOI] [PubMed] [Google Scholar]
- Ramirez C. A., Colton C. K., Smith K. A., Stemerman M. B., Lees R. S. Transport of 125I-albumin across normal and deendothelialized rabbit thoracic aorta in vivo. Arteriosclerosis. 1984 May-Jun;4(3):283–291. doi: 10.1161/01.atv.4.3.283. [DOI] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis--an update. N Engl J Med. 1986 Feb 20;314(8):488–500. doi: 10.1056/NEJM198602203140806. [DOI] [PubMed] [Google Scholar]
- STILL W. J., MARRIOTT P. R. COMPARATIVE MORPHOLOGY OF THE EARLY ATHEROSCLEROTIC LESION IN MAN AND CHOLESTEROL-ATHEROSCLEROSIS IN THE RABBIT AN ELECTRONMICROSCOPIC STUDY. J Atheroscler Res. 1964 Sep-Oct;4:373–386. doi: 10.1016/s0368-1319(64)80023-5. [DOI] [PubMed] [Google Scholar]
- Shasby D. M., Lind S. E., Shasby S. S., Goldsmith J. C., Hunninghake G. W. Reversible oxidant-induced increases in albumin transfer across cultured endothelium: alterations in cell shape and calcium homeostasis. Blood. 1985 Mar;65(3):605–614. [PubMed] [Google Scholar]
- Smith E. B., Staples E. M. Distribution of plasma proteins across the human aortic wall--barrier functions of endothelium and internal elastic lamina. Atherosclerosis. 1980 Dec;37(4):579–590. doi: 10.1016/0021-9150(80)90065-9. [DOI] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Stoll L. L., Spector A. A. Lipid transfer between endothelial and smooth muscle cells in coculture. J Cell Physiol. 1987 Oct;133(1):103–110. doi: 10.1002/jcp.1041330113. [DOI] [PubMed] [Google Scholar]
- Taylor R. F., Price T. H., Schwartz S. M., Dale D. C. Neutrophil-endothelial cell interactions on endothelial monolayers grown on micropore filters. J Clin Invest. 1981 Feb;67(2):584–587. doi: 10.1172/JCI110071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Territo M., Berliner J. A., Fogelman A. M. Effect of monocyte migration on low density lipoprotein transport across aortic endothelial cell monolayers. J Clin Invest. 1984 Dec;74(6):2279–2284. doi: 10.1172/JCI111655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton S. C., Mueller S. N., Levine E. M. Human endothelial cells: use of heparin in cloning and long-term serial cultivation. Science. 1983 Nov 11;222(4624):623–625. doi: 10.1126/science.6635659. [DOI] [PubMed] [Google Scholar]
- Weinberg C. B., Bell E. A blood vessel model constructed from collagen and cultured vascular cells. Science. 1986 Jan 24;231(4736):397–400. doi: 10.1126/science.2934816. [DOI] [PubMed] [Google Scholar]
- Zarins C. K., Giddens D. P., Bharadvaj B. K., Sottiurai V. S., Mabon R. F., Glagov S. Carotid bifurcation atherosclerosis. Quantitative correlation of plaque localization with flow velocity profiles and wall shear stress. Circ Res. 1983 Oct;53(4):502–514. doi: 10.1161/01.res.53.4.502. [DOI] [PubMed] [Google Scholar]
- van Buul-Wortelboer M. F., Brinkman H. J., Dingemans K. P., de Groot P. G., van Aken W. G., van Mourik J. A. Reconstitution of the vascular wall in vitro. A novel model to study interactions between endothelial and smooth muscle cells. Exp Cell Res. 1986 Jan;162(1):151–158. doi: 10.1016/0014-4827(86)90433-7. [DOI] [PubMed] [Google Scholar]