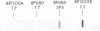Abstract
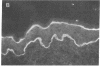
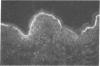
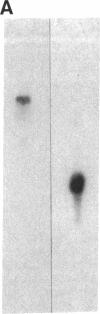
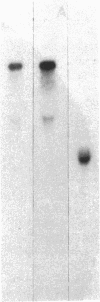
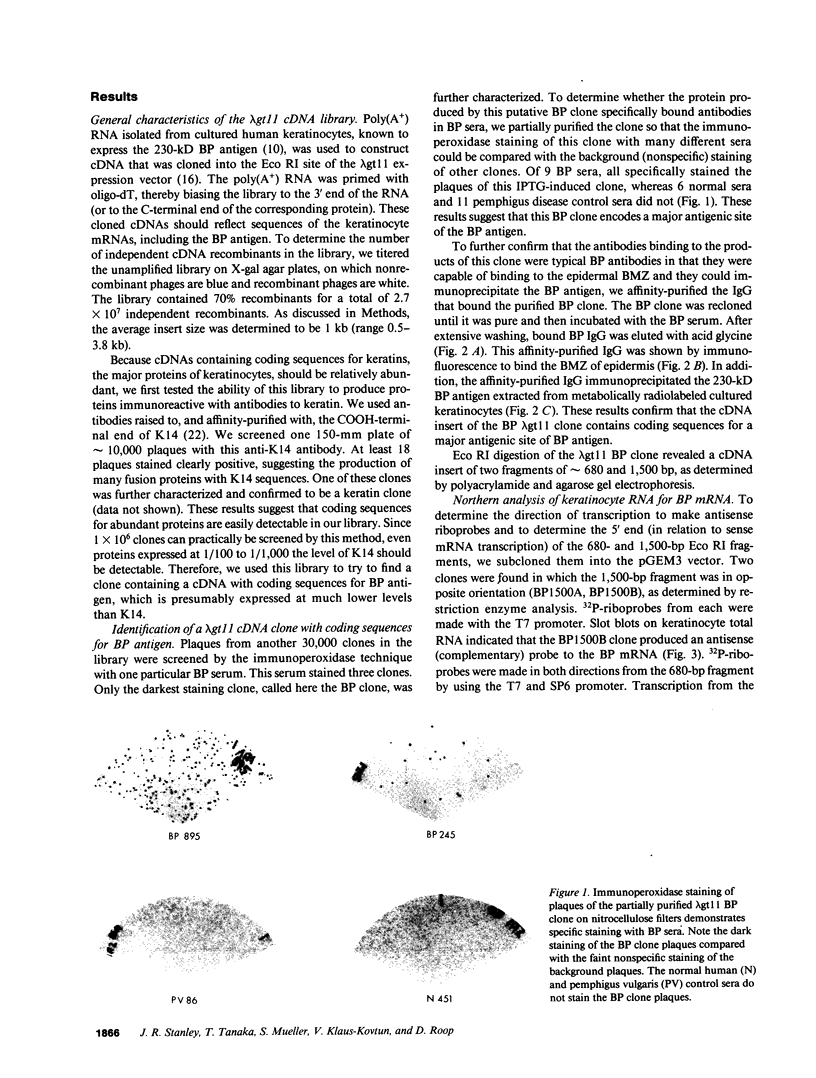
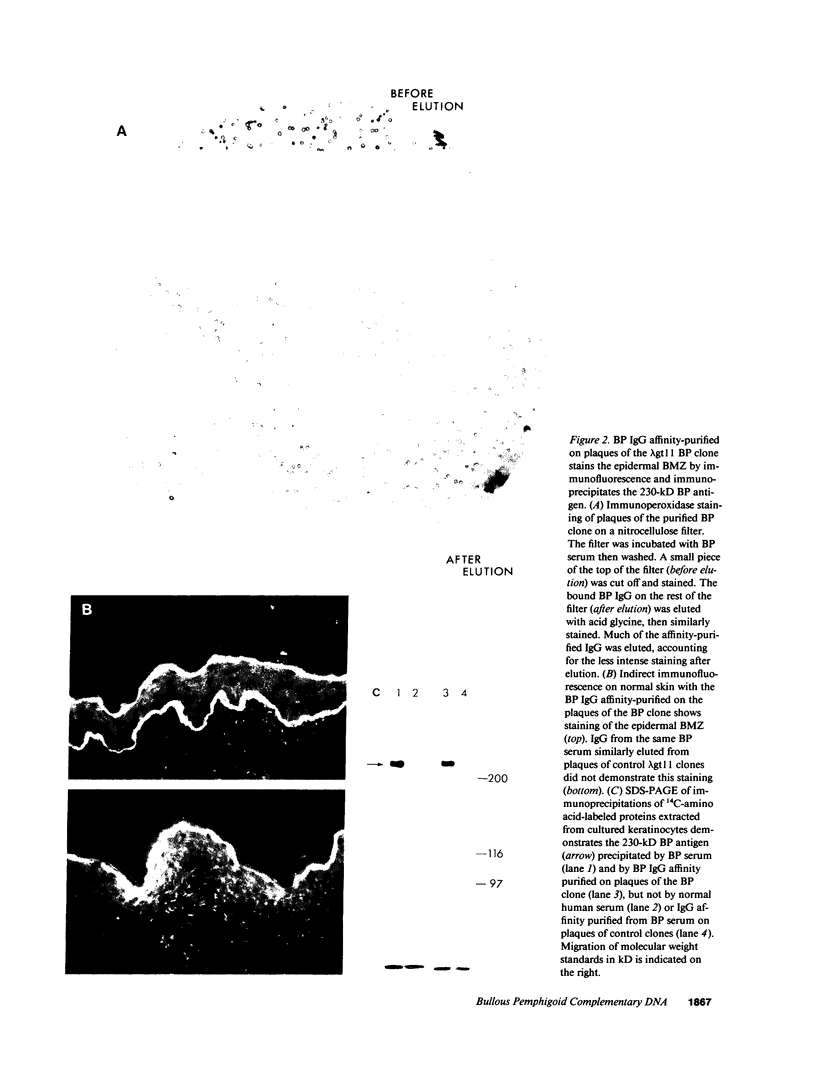
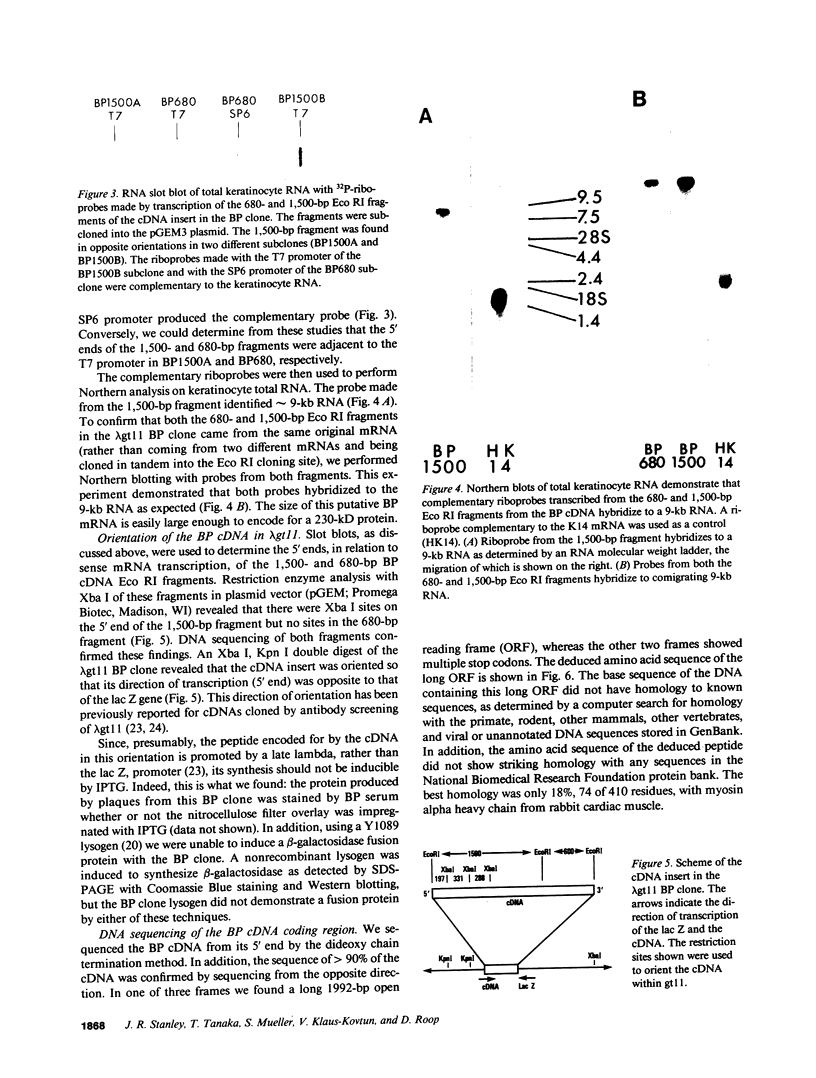
Autoantibodies from bullous pemphigoid (BP) patients define a 230-kD protein found in the basement membrane of stratified squamous epithelia. The purpose of this study was to isolate and characterize a cDNA clone with coding sequences for BP antigen. Poly(A+) RNA derived from total RNA of cultured keratinocytes was used, with oligo-dT priming, to construct a cDNA library in the lambda gt11 expression vector, which was screened by the immunoperoxidase method with one BP serum. One darkly stained clone, called here the BP clone, was further characterized. 9 of 9 BP sera, but none of 6 normal and 11 pemphigus sera, bound the plaques of this BP clone. Furthermore, BP IgG affinity purified on plaques of this clone, but not unrelated clones, bound the epidermal basement membrane by immunofluorescence and immunoprecipitated the 230-kD BP antigen from extracts of cultured keratinocytes. Eco RI digestion of the BP clone's cDNA insert demonstrated a 680- and 1,500-bp fragment. Northern blots of total keratinocyte RNA showed that complementary riboprobes transcribed from both fragments hybridized to a 9-kb RNA. Dideoxy DNA sequencing from the 5' end of the BP cDNA demonstrated a 1,992-bp open reading frame, encoding a peptide of 76 kD. This BP cDNA clone will be valuable for understanding the protein structure, expression, and gene organization of BP antigen.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beutner E. H., Jordon R. E., Chorzelski T. P. The immunopathology of pemphigus and bullous pemphigoid. J Invest Dermatol. 1968 Aug;51(2):63–80. [PubMed] [Google Scholar]
- Chambers J. C., Keene J. D. Isolation and analysis of cDNA clones expressing human lupus La antigen. Proc Natl Acad Sci U S A. 1985 Apr;82(7):2115–2119. doi: 10.1073/pnas.82.7.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dame J. B., Williams J. L., McCutchan T. F., Weber J. L., Wirtz R. A., Hockmeyer W. T., Maloy W. L., Haynes J. D., Schneider I., Roberts D. Structure of the gene encoding the immunodominant surface antigen on the sporozoite of the human malaria parasite Plasmodium falciparum. Science. 1984 Aug 10;225(4662):593–599. doi: 10.1126/science.6204383. [DOI] [PubMed] [Google Scholar]
- Diaz L. A., Weiss H. J., Calvanico N. J. Phylogenetic studies with pemphigus and pemphigoid antibodies. Acta Derm Venereol. 1978;58(6):537–540. [PubMed] [Google Scholar]
- Dropcho E. J., Chen Y. T., Posner J. B., Old L. J. Cloning of a brain protein identified by autoantibodies from a patient with paraneoplastic cerebellar degeneration. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4552–4556. doi: 10.1073/pnas.84.13.4552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto T., Wang J. C. Yeast DNA topoisomerase II is encoded by a single-copy, essential gene. Cell. 1984 Apr;36(4):1073–1080. doi: 10.1016/0092-8674(84)90057-6. [DOI] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Habets W. J., Sillekens P. T., Hoet M. H., Schalken J. A., Roebroek A. J., Leunissen J. A., van de Ven W. J., van Venrooij W. J. Analysis of a cDNA clone expressing a human autoimmune antigen: full-length sequence of the U2 small nuclear RNA-associated B" antigen. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2421–2425. doi: 10.1073/pnas.84.8.2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hintner H., Steinert P. M., Lawley T. J. Human upper epidermal cytoplasmic antibodies are directed against keratin intermediate filament proteins. J Clin Invest. 1983 Oct;72(4):1344–1351. doi: 10.1172/JCI111090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirayu H., Seto P., Magnusson R. P., Filetti S., Rapoport B. Molecular cloning and partial characterization of a new autoimmune thyroid disease-related antigen. J Clin Endocrinol Metab. 1987 Mar;64(3):578–584. doi: 10.1210/jcem-64-3-578. [DOI] [PubMed] [Google Scholar]
- Labib R. S., Anhalt G. J., Patel H. P., Mutasim D. F., Diaz L. A. Molecular heterogeneity of the bullous pemphigoid antigens as detected by immunoblotting. J Immunol. 1986 Feb 15;136(4):1231–1235. [PubMed] [Google Scholar]
- Mutasim D. F., Takahashi Y., Labib R. S., Anhalt G. J., Patel H. P., Diaz L. A. A pool of bullous pemphigoid antigen(s) is intracellular and associated with the basal cell cytoskeleton-hemidesmosome complex. J Invest Dermatol. 1985 Jan;84(1):47–53. doi: 10.1111/1523-1747.ep12274684. [DOI] [PubMed] [Google Scholar]
- Query C. C., Keene J. D. A human autoimmune protein associated with U1 RNA contains a region of homology that is cross-reactive with retroviral p30gag antigen. Cell. 1987 Oct 23;51(2):211–220. doi: 10.1016/0092-8674(87)90148-6. [DOI] [PubMed] [Google Scholar]
- Regnier M., Vaigot P., Michel S., Prunieras M. Localization of bullous pemphigoid antigen (BPA) in isolated human keratinocytes. J Invest Dermatol. 1985 Sep;85(3):187–190. doi: 10.1111/1523-1747.ep12276656. [DOI] [PubMed] [Google Scholar]
- Roop D. R., Cheng C. K., Toftgard R., Stanley J. R., Steinert P. M., Yuspa S. H. The use of cDNA clones and monospecific antibodies as probes to monitor keratin gene expression. Ann N Y Acad Sci. 1985;455:426–435. doi: 10.1111/j.1749-6632.1985.tb50426.x. [DOI] [PubMed] [Google Scholar]
- Sams W. M., Jr, Gammon W. R. Mechanism of lesion production in pemphigus and pemphigoid. J Am Acad Dermatol. 1982 Apr;6(4 Pt 1):431–452. doi: 10.1016/s0190-9622(82)70036-2. [DOI] [PubMed] [Google Scholar]
- Stanley J. R. A specific antigen-antibody interaction triggers the cellular pathophysiology of bullous pemphigoid. Br J Dermatol. 1985 Jul;113 (Suppl 28):67–73. doi: 10.1111/j.1365-2133.1985.tb15628.x. [DOI] [PubMed] [Google Scholar]
- Stanley J. R., Foidart J. M., Murray J. C., Martin G. R., Katz S. I. The epidermal cell which selectively adheres to a collagen substrate is the basal cell. J Invest Dermatol. 1980 Jan;74(1):54–58. doi: 10.1111/1523-1747.ep12514618. [DOI] [PubMed] [Google Scholar]
- Stanley J. R., Woodley D. T., Katz S. I. Identification and partial characterization of pemphigoid antigen extracted from normal human skin. J Invest Dermatol. 1984 Jan;82(1):108–111. doi: 10.1111/1523-1747.ep12259224. [DOI] [PubMed] [Google Scholar]
- Stanley J. R., Yuspa S. H. Specific epidermal protein markers are modulated during calcium-induced terminal differentiation. J Cell Biol. 1983 Jun;96(6):1809–1814. doi: 10.1083/jcb.96.6.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thivolet C. H., Hintner H. H., Stanley J. R. The effect of retinoic acid on the expression of pemphigus and pemphigoid antigens in cultured human keratinocytes. J Invest Dermatol. 1984 Apr;82(4):329–334. doi: 10.1111/1523-1747.ep12260634. [DOI] [PubMed] [Google Scholar]
- Tidman M. J., Eady R. A. Hemidesmosome heterogeneity in junctional epidermolysis bullosa revealed by morphometric analysis. J Invest Dermatol. 1986 Jan;86(1):51–56. doi: 10.1111/1523-1747.ep12283807. [DOI] [PubMed] [Google Scholar]
- Westgate G. E., Weaver A. C., Couchman J. R. Bullous pemphigoid antigen localization suggests an intracellular association with hemidesmosomes. J Invest Dermatol. 1985 Mar;84(3):218–224. doi: 10.1111/1523-1747.ep12265229. [DOI] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]