Abstract
AIM: To evaluate the 5-year survival after laparoscopic surgery vs open surgery for stages II and III rectal cancer.
METHODS: This study enrolled 406 consecutive patients who underwent curative resection for stages II and III rectal cancer between January 2000 and December 2009 [laparoscopic rectal resection (LRR), n = 152; open rectal resection (ORR), n = 254]. Clinical characteristics, operative outcomes, pathological outcomes, postoperative recovery, and 5-year survival outcomes were compared between the two groups.
RESULTS: Most of the clinical characteristics were similar except age (59 years vs 55 years, P = 0.033) between the LRR group and ORR group. The proportion of anterior resection was higher in the LRR group than that in the ORR group (81.6% vs 66.1%, P = 0.001). The LRR group had less estimated blood loss (50 mL vs 200 mL, P < 0.001) and a lower rate of blood transfusion (4.6% vs 11.8%, P = 0.019) compared to the ORR group. The pathological outcomes of the two groups were comparable. The LRR group was associated with faster recovery of bowel function (2.8 d vs 3.7 d, P < 0.001) and shorter postoperative hospital stay (11.7 d vs 13.7 d, P < 0.001). The median follow-up time was 63 mo in the LRR group and 65 mo in the ORR group. As for the survival outcomes, the 5-year local recurrence rate (16.0% vs 16.4%, P = 0.753), 5-year disease-free survival (DFS) rate (63.0% vs 63.1%, P = 0.589), and 5-year overall survival (OS) rate (68.1% vs 63.5%, P = 0.682) were comparable between the LRR group and the ORR group. Stage by stage, there were also no statistical differences between the LRR group and the ORR group in terms of the 5-year local recurrence rate (stage II: 6.3% vs 8.7%, P = 0.623; stage III: 26.4% vs 23.2%, P = 0.747), 5-year DFS rate (stage II: 77.5% vs 77.6%, P = 0.462; stage III: 46.5% vs 50.9%, P = 0.738), and 5-year OS rate (stage II: 81.4% vs 74.3%, P = 0.242; stage III: 53.9% vs 54.1%, P = 0.459).
CONCLUSION: LRR for stages II and III rectal cancer can yield comparable long-term survival while achieving short-term benefits compared to open surgery.
Keywords: Laparoscopic surgery, Locally advanced rectal cancer, Oncologic outcomes
Core tip: This retrospective study specially evaluates the oncologic outcomes after laparoscopic resection for locally advanced rectal cancer. Results suggest that laparoscopic rectal resection for stage II or III rectal cancer is a safe procedure, yielding comparable 5-year oncologic outcomes to open surgery.
INTRODUCTION
Laparoscopic rectal resection (LRR) is regarded as a technically demanding approach and many colorectal surgeons attempt LRR for early-stage rectal cancer or tumors in small size in their initial stages[1,2]. With the improvement in surgical technique and instruments, experienced surgeons have attempted to apply LRR for locally advanced rectal cancer. However, long-term oncologic outcomes of laparoscopic surgery for locally advanced rectal cancer remains controversial. Among the published trials comparing the long-term oncologic outcomes of rectal cancer between LRR and open rectal resection (ORR) groups[3-11], there were rare trials involving subgroup comparison of stage II or III rectal cancer[9]. The 5-year oncologic outcomes of the trial for stage II or III rectal cancer is not persuasive enough for the limited cases[9]. There were retrospective studies providing different long-term oncologic outcomes between laparoscopic and open surgery for stages II and III rectal cancer[12-18]. LRR for rectal cancer has been widely performed by Chinese colorectal surgeons. However, few studies evaluated long-term oncologic outcomes of LRR for stage II or III rectal cancer[12]. The present study was conducted to evaluate the long-term oncologic outcomes of LRR for stages II and III rectal cancer.
MATERIALS AND METHODS
Four hundred and sixty-two consecutive stages II and III rectal cancer patients who underwent LRR or ORR from January 2003 to December 2009 were extracted from the clinical colorectal database of Department of General Surgery of Nanfang Hospital, Southern Medical University, which is one of the members of the Southern Chinese Laparoscopic Colorectal Surgery Study (SCLASS) group[19]. After excluding 44 patients who underwent emergency resection and 12 patients with recurrent disease, 406 cases were finally included in the present study, with 152 undergoing LRR and 254 undergoing ORR.
The tumors were subdivided into three types according to the distances between their distal borders and the anal verge (upper rectal cancer, 10-15 cm; middle rectal cancer, 5-10 cm; and lower rectal cancer, < 5 cm). Patients chose the surgical approach of LRR or ORR based on an understanding of the risks and benefits inherent to laparoscopic and open resection after having received an extensive explanation without any pressure from the surgeon. LRR and ORR were performed by three colorectal surgeons who were experts in both laparoscopic and open procedures.
Before operation, all patients underwent colonoscopy plus biopsy, pelvic magnetic resonance imaging (MRI) or abdominal computed tomography (CT). A liquid diet was started after the first flatus had been passed. Patients were discharged if they were analgesia-free, afebrile and can tolerate food for 24 h, without major complications. Patients with high risk factors were recommended to receive postoperative adjuvant chemotherapy with 5-fluorouracil-based regimens. The last follow-up was December 2013.
Surgical technique
For laparoscopic surgery, the patients were placed in the Lloyd-Davis position with forced Trendelenburg (30°). The monitor was placed at the patient’s feet on the left side. The surgeon stood on the patient’s right side, the first assistant stood on the patient’s left side, and the second assistant holding the laparoscope stood at the patient’s cranial side next to the surgeon. Pneumoperitoneum was generated with a pressure of 12-15 mmHg. The five trocars were inserted: supraumbilical (10 mm), right iliospinale anterius medial 3 cm (10 mm), right rectus abdominis outer at the umbilical level (5 mm), midpoint of the line from left iliospinale anterius to umbilical (10 mm), and upper margin of the pubic bone 3 cm (5 mm). The medial-to-lateral approach was used, with the roots of the inferior mesenteric vascular pedicles being dissected with lymphadenectomy, and the mesentery and relevant bowel being mobilized in sequence. With preservation of the autonomic nerves, partial mesorectal excision was carried out for upper rectal cancer with a mesorectal margin of ≥ 5 cm distally to the cancer, and total mesorectal excision (TME) was performed for the middle and lower rectal cancer.
The patients who underwent laparoscopic anterior resection (AR) with intraabdominal anastomosis received a 4-6 cm incision in the left-lower quadrant of the abdomen for the removal of specimen and placement of the staple gun head, except for the patients undergoing protective ileostomy with their specimens removed through the stoma in the right-lower quadrant of the abdomen. No abdominal incision was made when patients underwent abdominal perineal resection (APR). Conversion to open procedure was decided when the surgeon was unable to complete the laparoscopic surgery.
Measured outcomes and definitions
This study compared the following variables: characteristics, surgical outcomes, pathologic results, postoperative recovery, and 5-year survival outcomes. Morbidity was defined as a complication that required additional treatment or prolonged hospital stay within 30 d after operation. Operative mortality was defined as death during or within 30 d after operation. Local recurrence was defined as the presence of radiologically confirmed or histologically proven tumor restricted to the anastomosis or in the pelvis within the region of the primary surgery. Patients that were transferred to open procedure were included in the LRR group.
Statistical analysis
SPSS 18.0 for windows (SPSS, Chicago, IL, United States) was used for all statistical analyses. A χ2 analysis or Student’s t-test, as appropriate, was used to assess for differences in patient characteristics, according to the surgical approach. Non-parametric equivalents were applied when normality and homogeneity assumptions were violated. Survival probability was estimated by the Kaplan-Meier method and compared by log-rank testing. The independent prognostic effect of surgical approaches on local recurrence, disease-free survival (DFS), and overall survival (OS) in rectal cancer were estimated using Cox proportional hazard regression models. All P-values were two-sided and P < 0.05 was considered statistically significant.
RESULTS
Clinical characteristics
There were no significant differences between the LRR and ORR groups in terms of sex, body mass index (BMI), ASA (American Society of Anesthesiologists) status, tumor location, neoadjuvant chemoradiotherapy, or postoperative chemotherapy. The median age of patients in the LRR group was older than that in the ORR group [59 (24-90) years vs 55 (19-86) years, P = 0.033]. The median follow-up time was 63 (28-112) mo in the LRR group and 65 (32-118) mo in the ORR group (P = 0.211) (Table 1).
Table 1.
Characteristics of the patients n (%)
| LRR (n = 152) | ORR (n = 254) | P value | |
| Age1 (yr) | 59 (24-90) | 55 (19-86) | 0.033 |
| Sex | 0.600 | ||
| Male | 95 (62.5) | 151 (59.4) | |
| Female | 57 (37.5) | 103 (40.6) | |
| BMI (kg/m2) | 22.7 (11.1) | 23.1 (9.5) | 0.369 |
| ASA | 0.542 | ||
| 1 | 36 | 50 | |
| 2 | 98 | 177 | |
| 3 | 18 | 27 | |
| Tumor location | 0.837 | ||
| Upper rectum (10.1-15.0 cm) | 39 (25.7) | 59 (23.2) | |
| Middle (5.1-10.0 cm) | 60 (39.5) | 101 (39.8) | |
| Lower rectum (0-5 cm) | 53 (34.9) | 94 (37.0) | |
| Tumor stage | 0.259 | ||
| II | 78 (51.3) | 115 (45.3) | |
| III | 74 (48.7) | 139 (54.7) | |
| Cell differentiation | 0.192 | ||
| Low | 15 (9.9) | 41 (16.1) | |
| Middle | 101 (66.4) | 161 (63.4) | |
| High | 36 (23.7) | 52 (20.5) | |
| Neoadjuvant chemoradiotherapy | 13 (8.6) | 16 (6.3) | 0.429 |
| Postoperative chemotherapy | 0.745 | ||
| Total cycle | 30 (19.7) | 51 (20.1) | |
| Partial cycle | 18 (11.8) | 24 (9.4) | |
| Abdominal surgery history | 21 (13.8) | 28 (11.0) | 0.433 |
| Follow-up1 (mo) | 63 (28-112) | 65 (32-118) | 0.211 |
Values are median (range).
Operative and pathological outcomes
The anterior resection rate was significantly higher in the LRR group than in the ORR group (81.6% vs 66.1%, P = 0.001). The operative times were comparable in the two groups. There was less estimated blood loss (50 mL vs 200 mL, P < 0.001) and a lower rate of blood transfusion (4.6% vs 11.8%, P = 0.019) in the LRR group than those in the ORR group. Conversion to open procedure was required in 7 (4.6%) patients in the LRR group, for difficulty in pelvic exposure in 3 cases, severe adhesion in 2, and uncontrolled bleeding in 2. The protective ileostomy rates were comparable between the two groups. There were no significant differences between the two groups in terms of the R0 resection rate, distances of proximal resection margin and distal resection margin, number of lymph nodes harvested, number of metastatic lymph nodes or tumor size (Table 2).
Table 2.
Operative and pathological outcomes
| LRR (n = 152) | ORR (n = 254) | P value | |
| Surgical procedure | 0.001 | ||
| Anterior resection | 124 (81.6) | 168 (66.1) | |
| Abdominoperineal resection | 28 (18.4) | 86 (33.9) | |
| Protective ileostomy | 32 (21.1) | 49 (19.3) | 0.701 |
| Operating time1, min | 188.9 (67.7) | 188.7 (65.4) | 0.982 |
| Estimated blood loss2, mL | 50 (20-1500) | 200 (60-3000) | < 0.001 |
| Blood transfusion | 7 (4.6) | 30 (11.8) | 0.019 |
| Conversion, n (%) | 7 (4.6) | ||
| Resection margin involvement | 0.809 | ||
| R0 | 144 (94.7) | 243 (95.7) | |
| R1 | 8 (5.3) | 11 (4.3) | |
| Proximal resection margin1, cm | 11.2 (3.4) | 11.7 (3.6) | 0.148 |
| Distal resection margin1, cm | 3.1 (1.7) | 3.0 (1.5) | 0.182 |
| No. of lymph nodes harvested1 | 12.9 (6.1) | 12.2 (6.2) | 0.240 |
| No. of metastatic lymph nodes | 1.7 (2.9) | 1.8 (3.3) | 0.760 |
| Tumor size, cm | 3.9 (1.2) | 4.0 (1.1) | 0.425 |
Values are mean ± SD;
Values are median (range).
Postoperative recovery
The time to first flatus (2.8 d vs 3.7 d, P < 0.001), to the start of a liquid diet (3.7 d vs 4.5 d, P < 0.001), and the time of postoperative hospital stay (11.7 d vs 13.7 d, P < 0.001) were significantly shorter in the LRR group than in the ORR group. Postoperative morbidity rate in the LRR group was similar to that in the ORR group (25.0% vs 22.4%, P = 0.907). The mortality rate after surgery between the two groups was comparable (2.6% vs 2.0%, P = 0.733) (Table 3).
Table 3.
Postoperative recovery outcomes n (%)
| Laparoscopic | Open | P value | |
| (n = 152) | (n = 254) | ||
| Time to first flatus, d | 2.8 (1.0) | 3.7 (1.1) | < 0.001 |
| Time to liquid diet, d | 3.7 (1.1) | 4.5 (1.3) | < 0.001 |
| Postoperative hospital stay, d | 11.7 (3.7) | 13.7 (4.3) | < 0.001 |
| Postoperative morbidity | 38 (25.0) | 66 (22.4) | 0.907 |
| Wound infection | 6 (3.9) | 11 (4.3) | 0.851 |
| Ileus | 4 (2.6) | 5 (2.0) | 0.733 |
| Anastomotic leak | 6 (3.9) | 5 (2.0) | 0.343 |
| Anastomotic stenosis | 1 (0.7) | 0 (0.0) | 0.377 |
| Hemorrhage | 4 (2.6) | 17 (6.7) | 0.103 |
| Abdominal infection | 5 (3.3) | 2 (0.8) | 0.108 |
| Respiratory infection | 6 (3.9) | 12 (4.7) | 0.807 |
| Cardiac disease | 1 (0.7) | 6 (2.4) | 0.264 |
| Renal failure | 0 (0.0) | 3 (1.2) | 0.296 |
| Urinary tract infection | 5 (3.3) | 3 (1.2) | 0.157 |
| Cerebrovascular diseases | 0 (0.0) | 2 (0.8) | 0.530 |
| Postoperative mortality | 4 (2.6) | 5 (2.0) | 0.733 |
Survival outcomes
There were no statistical differences in 5-year local recurrence rate between the LRR group and the ORR group (16.0% vs 16.4%, P = 0.753) (Figure 1A). Stage by stage, there were also no statistical differences between the two groups (stage II: 6.3% vs 8.7%, P = 0.623; stage III: 26.4% vs 23.2%, P = 0.747) (Figure 1B).
Figure 1.
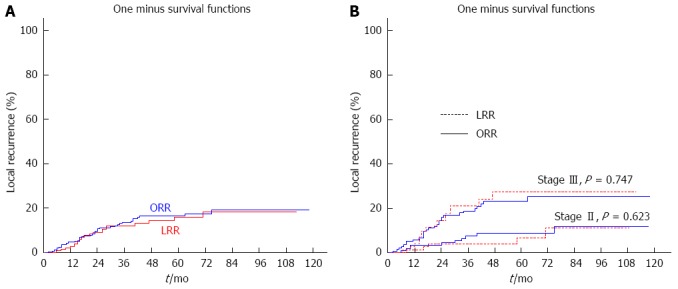
Cumulative local recurrence between the LRR and ORR groups. A: 5-year: 16.0% vs 16.4%, P = 0.753; B: 5-year: stage II 6.3% vs 8.7%, P = 0.623; stage III 26.4% vs 23.2%, P = 0.747.
The 5-year DFS were comparable between the LRR group and the ORR group (63.0% vs 63.1%, P = 0.589) (Figure 2A), and there were also no statistical differences in the 5-year DFS rates between the two groups when stratifying by tumor stage (stage II: 77.5% vs 77.6%, P = 0.462; stage III: 46.5% vs 50.9%, P = 0.738) (Figure 2B).
Figure 2.
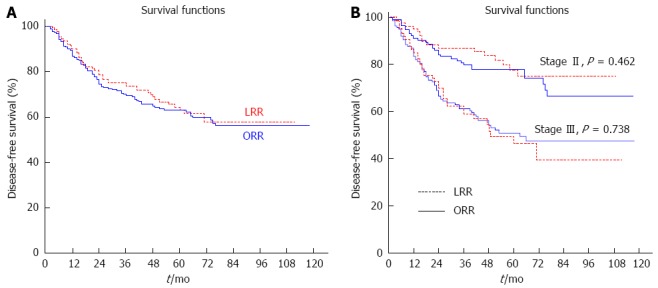
Cumulative disease-free survival between the LRR and ORR groups. A: 5-year: 63.0% vs 63.1%, P = 0.589; B: 5-year: stage II 77.5% vs 77.6%, P = 0.462; stage III 46.5% vs 50.9%, P = 0.738.
The 5-year OS rate was similar in the LRR group to that in the ORR group (68.1% vs 63.5%, P = 0.682) (Figure 3A). By subgroup analysis, the similar results were respectively conducted (stage II: 81.4% vs 74.3%, P = 0.242; stage III: 53.9% vs 54.1%, P = 0.459) (Figure 3B).
Figure 3.
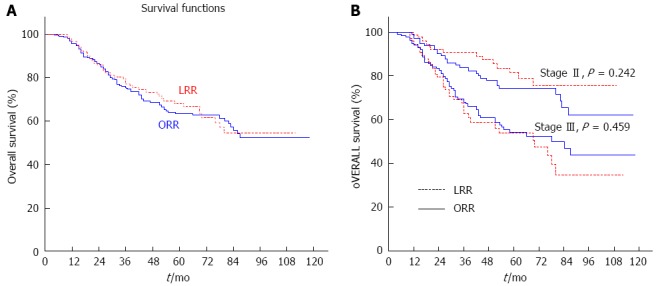
Cumulative overall survival between the LRR and ORR groups. A: 5-year: 68.1% vs 63.5%, P = 0.682; B: 5-year: stage II 81.4% vs 74.3%, P = 0.242; stage III 53.9% vs 54.1%, P = 0.459.
The multivariate Cox regression analysis showed that the laparoscopic approach was not associated with inferior local recurrence (HR = 0.796, 95%CI: 0.463-1.367, P = 0.408), DFS (HR = 0.847, 95%CI: 0.599-1.198, P = 0.348), or OS (HR = 1.044, 95%CI: 0.726-1.501, P = 0.817), after adjusting for covariates, such as age, gender, number of harvested lymph nodes, and tumor stage.
DISCUSSION
LRR was reported a technically demanding procedure for the difficulties in pelvic exposure and TME with the autonomic nerves preserved[12,20-22]. The application of LRR in early rectal cancer was usually accepted by laparoscopic surgeons[2,13]. Although more and more locally advanced rectal cancer cases have undergone LRR by experienced surgeons, there was still skepticism on the application of LRR for locally advanced rectal cancer for the limited data on the long-term oncologic outcomes. To date, the multi-center randomized controlled trial (RCT) of CLASSICC has provided the highest level of evidence on equivalent 3-year and 5-year local recurrence, DFS and OS rates of LRR compared to ORR[3,10]. However, there was no subgroup analysis of locally advanced rectal cancer in this trial and the maturity of the technique of laparoscopy had been questioned for a relatively high conversion rate and morbidity[23-25].
This study showed that, compared to ORR, LRR for locally advanced rectal cancer had less estimated blood loss, faster bowel function recovery, and, most importantly, similar 5-year local recurrence, DFS, and OS rates. The Cox regression indicated that surgical approach was not the factor that significantly impacted on 5-year local recurrence, DFS, and OS rates.
Studies demonstrated that circumferential resection margin (CRM) status was highly associated with long-term survival[26-28]. Pathological involvement of the CRM after rectal cancer surgery is a well-established prognostic indicator[29]. Clear CRMs are of great importance because the risk of local recurrence increases three to four times when these margins are invaded by tumor cells[30]. Changes in awareness of the requirements for pathologic evaluation of rectal cancer specimens began in 2007. However, the SCLASS database collected patient clinical data prior to 2009. Consequently, data on CRMs were not recorded in the current SCLASS database for the sake of consistency, which is one limitation of our study. Nevertheless, our results showed that laparoscopic approach did not have an adverse effect on local recurrence or DFS, which may be considered putative proxies for CRM. Wang et al[31] reported CRM involvement in patients with rectal cancer of approximately 1.9% at a leading single institution in China. This may indirectly reflect the status of CRM involvement in China. Additionally, during the last 20 years, the treatment of rectal cancer has changed dramatically. The reinforcement of TME by our understanding of the mesorectum and CRM has led to fewer positive margins and consequently fewer local recurrences[32].
The use of neoadjuvant therapy has only been highly recommended by NCCN guidelines in recent years, while the clinical data of the present study began from January 2003. This discrepancy contributed to the relatively low rate of patients who received neoadjuvant therapy in the present study. Only < 10% patients in our study had received treatment for rectal cancer with neoadjuvant therapy in both groups. Though a low rate of neoadjuvant therapy and adjuvant therapy, the proportions of them between the LRR group and ORR group were comparable, which helped result in similar long-term oncological outcomes between the two groups.
Although one RCT and two non-randomized studies have demonstrated that laparoscopic approach was an independent predictor of improved survival after colorectal surgery[14,33,34], laparoscopic approach was not associated with differences in survival or recurrence in our study. One of the reasons for the difference in our findings may be that > 50% of patients in the abovementioned studies were colon cancer patients. Therefore, their findings may not be applicable to rectal cancer. In our study, patient survival following LRR for rectal cancer was similar to survival following ORR, with acceptable complications and earlier recovery. The present study showed less estimated blood loss, a lower rate of blood transfusion, and quicker postoperative recovery. These non-inferior parameters relevant to surgical stress response and postoperative immune function of the LRR group may help result in non-inferior long-term oncological outcomes of the LRR group in the present study[35,36]. Together, the data suggest that it is not necessary to prove a survival benefit of LRR over ORR to justify its use in the treatment of rectal cancer.
The retrospective design was one main limitation of the present study and selective biases were unavoidable in the present study, such as the average age of the patients and the proportion of surgical procedure of AR and APR, though they had not significantly different impact on the long-term oncologic outcomes by Cox regression mode multivariate analysis. Furthermore, the rates of noeadjuvant and adjuvant treatment were low and the specimen lacked CRM status. Despite these limitations, this cohort study was specially created to compare local recurrence, DFS and OS rates between LRR and ORR for locally advanced rectal cancer in a Chinese population.
COMMENTS
Background
Long-term outcomes of randomized controlled trials are still needed to provide solid evidence on the efficacy of laparoscopic resection in the treatment of rectal cancer now. The published long-term oncologic outcomes of laparoscopic surgery for rectal cancer remain controversial. There are few studies comparing long-term oncologic outcomes between laparoscopic and open surgery focusing locally advanced rectal cancer.
Research frontiers
There were rare studies involving subgroup comparison of stage II or III rectal cancer among the published reports comparing the long-term oncologic outcomes of rectal cancer between laparoscopic surgery and open surgery. The 5-year oncologic outcomes of the laparoscopic surgery for stage II or III rectal cancer is not persuasive enough for the limited cases. Laparoscopic surgery for rectal cancer has been widely performed by Chinese colorectal surgeons. However, few studies evaluated long-term oncologic outcomes of laparoscopic rectal resection (LRR) for stage II or III rectal cancer.
Innovations and breakthroughs
Few studies evaluated long-term oncologic outcomes of laparoscopic surgery for stage II or III rectal cancer, especially for the Chinese population. This cohort study was specially created to compare local recurrence, disease-free survival and overall survival rates between LRR and open rectal resection for local advanced rectal cancer in a Chinese population.
Applications
The study results suggest that LRR for stages II and III rectal cancer can yield comparable 5-year survival rates while achieving short-term benefits compared to open surgery.
Terminology
Laparoscopic surgery is a minimally invasive approach for colorectal cancer. Since 2000, several multicenter studies on laparoscopic surgery for colon cancer suggest that it is associated with improved short-term results, but similar long-term survival rates. On the other hand, laparoscopic resection for rectal cancer remains controversial.
Peer-review
In this paper, the authors conducted a retrospective cohort study to evaluate long-term oncologic outcomes of LRR for stages II and III rectal cancer in a Chinese population. The paper was written basically in accordance with the recommendations of the Strengthening the Reporting of Observational Studies in Epidemiology Statement.
Footnotes
Supported by National Key Clinical Specialty Construction Project of China, the National High Technology Research and Development Program of China No. 2012AA021103; and the Research Fund of Public Welfare in Health Industry, National Health and Family Planning Commission of China, No. 201502039.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: November 6, 2014
First decision: November 26, 2014
Article in press: February 13, 2015
P- Reviewer: Jiang WJ S- Editor: Qi Y L- Editor: Wang TQ E- Editor: Ma S
References
- 1.Monson JR, Weiser MR, Buie WD, Chang GJ, Rafferty JF, Buie WD, Rafferty J. Practice parameters for the management of rectal cancer (revised) Dis Colon Rectum. 2013;56:535–550. doi: 10.1097/DCR.0b013e31828cb66c. [DOI] [PubMed] [Google Scholar]
- 2.Lee SD, Park SC, Park JW, Kim DY, Choi HS, Oh JH. Laparoscopic versus open surgery for stage I rectal cancer: long-term oncologic outcomes. World J Surg. 2013;37:646–651. doi: 10.1007/s00268-012-1846-z. [DOI] [PubMed] [Google Scholar]
- 3.Jayne DG, Thorpe HC, Copeland J, Quirke P, Brown JM, Guillou PJ. Five-year follow-up of the Medical Research Council CLASICC trial of laparoscopically assisted versus open surgery for colorectal cancer. Br J Surg. 2010;97:1638–1645. doi: 10.1002/bjs.7160. [DOI] [PubMed] [Google Scholar]
- 4.Leung KL, Kwok SP, Lam SC, Lee JF, Yiu RY, Ng SS, Lai PB, Lau WY. Laparoscopic resection of rectosigmoid carcinoma: prospective randomised trial. Lancet. 2004;363:1187–1192. doi: 10.1016/S0140-6736(04)15947-3. [DOI] [PubMed] [Google Scholar]
- 5.Lujan J, Valero G, Hernandez Q, Sanchez A, Frutos MD, Parrilla P. Randomized clinical trial comparing laparoscopic and open surgery in patients with rectal cancer. Br J Surg. 2009;96:982–989. doi: 10.1002/bjs.6662. [DOI] [PubMed] [Google Scholar]
- 6.Ng SS, Leung KL, Lee JF, Yiu RY, Li JC, Teoh AY, Leung WW. Laparoscopic-assisted versus open abdominoperineal resection for low rectal cancer: a prospective randomized trial. Ann Surg Oncol. 2008;15:2418–2425. doi: 10.1245/s10434-008-9895-0. [DOI] [PubMed] [Google Scholar]
- 7.Ng SS, Lee JF, Yiu RY, Li JC, Hon SS, Mak TW, Leung WW, Leung KL. Long-term oncologic outcomes of laparoscopic versus open surgery for rectal cancer: a pooled analysis of 3 randomized controlled trials. Ann Surg. 2014;259:139–147. doi: 10.1097/SLA.0b013e31828fe119. [DOI] [PubMed] [Google Scholar]
- 8.Ng SSM, Lee JFY, Yiu RYC, Li JCM, Hon SSF, Mak TWC, Ngo DKY, Leung WW, Leung KL. Laparoscopic-assisted versus open total mesorectal excision with anal sphincter preservation for mid and low rectal cancer: a prospective, randomized trial. Surg Endosc. 2013;28:297–306. doi: 10.1007/s00464-013-3187-x. [DOI] [PubMed] [Google Scholar]
- 9.Braga M, Frasson M, Vignali A, Zuliani W, Capretti G, Di Carlo V. Laparoscopic resection in rectal cancer patients: outcome and cost-benefit analysis. Dis Colon Rectum. 2007;50:464–471. doi: 10.1007/s10350-006-0798-5. [DOI] [PubMed] [Google Scholar]
- 10.Jayne DG, Guillou PJ, Thorpe H, Quirke P, Copeland J, Smith AM, Heath RM, Brown JM. Randomized trial of laparoscopic-assisted resection of colorectal carcinoma: 3-year results of the UK MRC CLASICC Trial Group. J Clin Oncol. 2007;25:3061–3068. doi: 10.1200/JCO.2006.09.7758. [DOI] [PubMed] [Google Scholar]
- 11.Liang X, Hou S, Liu H, Li Y, Jiang B, Bai W, Li G, Wang W, Feng Y, Guo J. Effectiveness and safety of laparoscopic resection versus open surgery in patients with rectal cancer: a randomized, controlled trial from China. J Laparoendosc Adv Surg Tech A. 2011;21:381–385. doi: 10.1089/lap.2010.0059. [DOI] [PubMed] [Google Scholar]
- 12.Li S, Chi P, Lin H, Lu X, Huang Y. Long-term outcomes of laparoscopic surgery versus open resection for middle and lower rectal cancer: an NTCLES study. Surg Endosc. 2011;25:3175–3182. doi: 10.1007/s00464-011-1683-4. [DOI] [PubMed] [Google Scholar]
- 13.Law WL, Poon JT, Fan JK, Lo SH. Comparison of outcome of open and laparoscopic resection for stage II and stage III rectal cancer. Ann Surg Oncol. 2009;16:1488–1493. doi: 10.1245/s10434-009-0418-4. [DOI] [PubMed] [Google Scholar]
- 14.Law WL, Poon JT, Fan JK, Lo OS. Survival following laparoscopic versus open resection for colorectal cancer. Int J Colorectal Dis. 2012;27:1077–1085. doi: 10.1007/s00384-012-1424-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morino M, Allaix ME, Giraudo G, Corno F, Garrone C. Laparoscopic versus open surgery for extraperitoneal rectal cancer: a prospective comparative study. Surg Endosc. 2005;19:1460–1467. doi: 10.1007/s00464-004-2001-1. [DOI] [PubMed] [Google Scholar]
- 16.Laurent C, Leblanc F, Wütrich P, Scheffler M, Rullier E. Laparoscopic versus open surgery for rectal cancer: long-term oncologic results. Ann Surg. 2009;250:54–61. doi: 10.1097/SLA.0b013e3181ad6511. [DOI] [PubMed] [Google Scholar]
- 17.Park JS, Choi GS, Jun SH, Park SY, Kim HJ. Long-term outcomes after laparoscopic surgery versus open surgery for rectal cancer: a propensity score analysis. Ann Surg Oncol. 2013;20:2633–2640. doi: 10.1245/s10434-013-2981-y. [DOI] [PubMed] [Google Scholar]
- 18.Patankar SK. 2002. Prospective Comparison ofLaparoscopic vs. Open Resections for Colorectal Adenocarcinoma over a Ten-Year Period. [DOI] [PubMed] [Google Scholar]
- 19.Liang YZ, Yu J, Zhang C, Wang YN, Cheng X, Huang F, Li GX. [Construction and application of evaluation system of laparoscopic colorectal surgery based on clinical data mining] Zhonghua Wei Chang Wai Ke Zazhi. 2010;13:741–744. [PubMed] [Google Scholar]
- 20.Morino M, Parini U, Giraudo G, Salval M, Brachet Contul R, Garrone C. Laparoscopic total mesorectal excision: a consecutive series of 100 patients. Ann Surg. 2003;237:335–342. doi: 10.1097/01.SLA.0000055270.48242.D2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Staudacher C, Di Palo S, Tamburini A, Vignali A, Orsenigo E. Total mesorectal excision (TME) with laparoscopic approach: 226 consecutive cases. Surg Oncol. 2007;16 Suppl 1:S113–S116. doi: 10.1016/j.suronc.2007.10.035. [DOI] [PubMed] [Google Scholar]
- 22.Bianchi PP, Rosati R, Bona S, Rottoli M, Elmore U, Ceriani C, Malesci A, Montorsi M. Laparoscopic surgery in rectal cancer: a prospective analysis of patient survival and outcomes. Dis Colon Rectum. 2007;50:2047–2053. doi: 10.1007/s10350-007-9055-9. [DOI] [PubMed] [Google Scholar]
- 23.Guillou PJ, Quirke P, Thorpe H, Walker J, Jayne DG, Smith AMH, Heath RM, Brown JM. Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial. Lancet. 2005;365:1718–1726. doi: 10.1016/S0140-6736(05)66545-2. [DOI] [PubMed] [Google Scholar]
- 24.Tan KY, Konishi F. Long-term results of laparoscopic colorectal cancer resection: current knowledge and what remains unclear. Surg Today. 2010;40:97–101. doi: 10.1007/s00595-009-4133-3. [DOI] [PubMed] [Google Scholar]
- 25.van der Pas MH, te Velde EA, Cuesta MA, Bonjer HJ. Favorable outcomes with laparoscopic surgery for rectal cancer. Surg Endosc. 2011;25:2060–2061. doi: 10.1007/s00464-010-1457-4. [DOI] [PubMed] [Google Scholar]
- 26.Bown EJ, Lloyd GM, Boyle KM, Miller AS. Rectal cancer: prognostic indicators of long-term outcome in patients considered for surgery. Int J Colorectal Dis. 2014;29:147–155. doi: 10.1007/s00384-013-1772-z. [DOI] [PubMed] [Google Scholar]
- 27.Trakarnsanga A, Gonen M, Shia J, Goodman KA, Nash GM, Temple LK, Guillem JG, Paty PB, Garcia-Aguilar J, Weiser MR. What is the significance of the circumferential margin in locally advanced rectal cancer after neoadjuvant chemoradiotherapy? Ann Surg Oncol. 2013;20:1179–1184. doi: 10.1245/s10434-012-2722-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bernstein TE, Endreseth BH, Romundstad P, Wibe A. Circumferential resection margin as a prognostic factor in rectal cancer. Br J Surg. 2009;96:1348–1357. doi: 10.1002/bjs.6739. [DOI] [PubMed] [Google Scholar]
- 29.Adam IJ, Mohamdee MO, Martin IG, Scott N, Finan PJ, Johnston D, Dixon MF, Quirke P. Role of circumferential margin involvement in the local recurrence of rectal cancer. Lancet. 1994;344:707–711. doi: 10.1016/s0140-6736(94)92206-3. [DOI] [PubMed] [Google Scholar]
- 30.Birbeck KF, Macklin CP, Tiffin NJ, Parsons W, Dixon MF, Mapstone NP, Abbott CR, Scott N, Finan PJ, Johnston D, et al. Rates of Circumferential Resection Margin Involvement Vary Between Surgeons and Predict Outcomes in Rectal Cancer Surgery. Ann Surg. 2002;35:49–57. doi: 10.1097/00000658-200204000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang C, Zhou ZG, Yu YY, Shu Y, Li Y, Yang L, Li L. Occurrence and prognostic value of circumferential resection margin involvement for patients with rectal cancer. Int J Colorectal Dis. 2009;24:385–390. doi: 10.1007/s00384-008-0624-8. [DOI] [PubMed] [Google Scholar]
- 32.Nagtegaal ID, Quirke P. What is the role for the circumferential margin in the modern treatment of rectal cancer? J Clin Oncol. 2008;26:303–312. doi: 10.1200/JCO.2007.12.7027. [DOI] [PubMed] [Google Scholar]
- 33.Lacy AM, Delgado S, Castells A, Prins HA, Arroyo V, Ibarzabal A, Pique JM. The long-term results of a randomized clinical trial of laparoscopy-assisted versus open surgery for colon cancer. Ann Surg. 2008;248:1–7. doi: 10.1097/SLA.0b013e31816a9d65. [DOI] [PubMed] [Google Scholar]
- 34.Capussotti L, Massucco P, Muratore A, Amisano M, Bima C, Zorzi D. Laparoscopy as a prognostic factor in curative resection for node positive colorectal cancer: results for a single-institution nonrandomized prospective trial. Surg Endosc. 2004;18:1130–1135. doi: 10.1007/s00464-003-9152-3. [DOI] [PubMed] [Google Scholar]
- 35.Edna TH, Bjerkeset T. Perioperative blood transfusions reduce long-term survival following surgery for colorectal cancer. Dis Colon Rectum. 1998;41:451–459. doi: 10.1007/BF02235758. [DOI] [PubMed] [Google Scholar]
- 36.Veenhof AA, Sietses C, von Blomberg BM, van Hoogstraten IM, vd Pas MH, Meijerink WJ, vd Peet DL, vd Tol MP, Bonjer HJ, Cuesta MA. The surgical stress response and postoperative immune function after laparoscopic or conventional total mesorectal excision in rectal cancer: a randomized trial. Int J Colorectal Dis. 2011;26:53–59. doi: 10.1007/s00384-010-1056-9. [DOI] [PMC free article] [PubMed] [Google Scholar]


