Abstract
AIM: To investigate the accuracy of endoscopic or biopsy diagnoses of superficial nonampullary duodenal epithelial tumors (NADETs).
METHODS: Clinicopathological data were reviewed for 84 superficial NADETs from 74 patients who underwent surgery or endoscopic resection between September 2002 and August 2014 at a single prefectural cancer center. Superficial NADETs were defined as lesions confined to the mucosa or submucosa. Demographic and clinicopathological data were retrieved from charts, endoscopic and pathologic reports. Endoscopic reports included endoscopic diagnosis, location, gross type, diameter, color, and presence or absence of biopsy. Endoscopic diagnoses were made by an endoscopist in charge of the examination before biopsy specimens were obtained. Endoscopic images were obtained using routine, front-view, high-resolution video endoscopy, and chromoendoscopy with indigocarmine was performed for all lesions. Endoscopic images were reviewed by at least two endoscopists to assess endoscopic findings indicative of carcinoma. Preoperative diagnoses based on endoscopy and biopsy findings were compared with histological diagnoses of resected specimens. Sensitivity, specificity, and accuracy were assessed for endoscopic diagnosis and biopsy diagnosis.
RESULTS: The majority (81%) of the lesions were located in the second portion of the duodenum. The median lesion diameter was 14.5 mm according to final histology. Surgery was performed for 49 lesions from 39 patients, and 35 lesions from 35 patients were endoscopically resected. Final histology confirmed 65 carcinomas, 15 adenomas, and 3 hyperplasias. A final diagnosis of duodenal carcinoma was made for 91% (52/57) of the lesions diagnosed as carcinoma by endoscopy and 93% (42/45) of the lesions diagnosed as carcinoma by biopsy. The sensitivity, specificity, and accuracy of endoscopic diagnoses were 80%, 72%, and 78%, respectively, whereas those of biopsy diagnoses were 72%, 80%, and 74%, respectively. Preoperative diagnoses of carcinomas were made in 88% (57/65) of the carcinoma lesions via endoscopy or biopsy. Endoscopic findings associated with carcinoma were red color, depression, and mixed-type morphology.
CONCLUSION: Preoperative endoscopy and biopsy showed similar accuracies in the diagnosis of carcinoma in patients with superficial NADETs.
Keywords: Biopsy, Endoscopic diagnosis, Duodenal adenoma, Duodenal carcinoma, Duodenal neoplasms
Core tip: An analysis of 84 resected lesions of superficial nonampullary duodenal epithelial tumors revealed that preoperative endoscopy and biopsy showed similar accuracies in the diagnosis of carcinoma or adenoma. Endoscopic findings associated with superficial duodenal carcinoma were red color, depression, and mixed-type morphology.
INTRODUCTION
Primary nonampullary duodenal epithelial tumors (NADETs) are extremely rare[1,2]. Patients with familial adenomatous polyposis (FAP) are known to have a high prevalence of duodenal adenomas (DAs), and prospective follow-up studies have demonstrated that such adenomas can progress slowly to carcinoma[3]. Sporadic nonampullary duodenal carcinoma (NADC) occurs de novo or because of the adenoma-carcinoma sequence, as observed in FAP patients[4]. Because the prognosis of advanced duodenal carcinomas is poor[5,6], early detection and treatment are essential.
Recently, a number of case studies have demonstrated an increase in endoscopic treatments for sporadic superficial NADETs, such as DAs or carcinomas[7-16], possibly because of the widespread use of esophagogastroduodenoscopy. Previous studies have shown that DAs and mucosal carcinomas are curable by local resection, with almost no risk of metastasis[17-19]. Endoscopic resection (ER) is a minimally invasive local treatment compared with surgical resection. However, ER of superficial NADETs is associated with a high risk of complications, such as bleeding and perforation[10,13,19]. Therefore, a preoperative diagnosis of superficial NADETs is required to distinguish between lesions that should be followed-up and those that require treatment[16,20,21]. However, because the incidence of sporadic NADC is extremely rare, endoscopic findings suggestive of early NADC have not yet been established. Although biopsies allow for the determination of histology and management strategies, their accuracy in diagnosing superficial NADETs remains unclear. This study aimed to identify the predictive endoscopic characteristics of carcinomas and compare the accuracy of endoscopy and biopsy in the diagnosis of superficial NADETs.
MATERIALS AND METHODS
This study included consecutive patients with superficial NADETs who were treated with endoscopy or surgical resection at the Shizuoka Cancer Center between September 2002 and August 2014. Superficial NADETs were defined as lesions confined to the mucosa or submucosa. The indications for resection for superficial NADETs at our institution include lesions diagnosed as carcinoma endoscopically or lesions diagnosed as high-grade adenoma or carcinoma via biopsy. Demographic and clinicopathological data were retrieved from charts, endoscopic and pathologic reports. Endoscopic reports included endoscopic diagnosis, location, gross type, diameter, color, and presence or absence of biopsy. Endoscopic images were obtained using routine, front-view, high-resolution video endoscopy (H260 or H260Z, Olympus, Tokyo, Japan). A duodenoscope or an enteroscope was not used in this study because all lesions were accessible using gastroscopes. Chromoendoscopy with indigocarmine was performed for all lesions. Images were reviewed by at least two endoscopists, and endoscopic findings, such as the presence of depressed areas, whitish villous color[22] and the presence of granules within the lesion, were recorded. Biopsy samples and resected specimens were pathologically assessed by one experienced pathologist and were graded according to the Vienna classification system[23].
Preoperative diagnoses based on endoscopy and biopsy findings were compared with histological diagnoses of resected specimens. Sensitivity, specificity, and accuracy were assessed for each modality, and the endoscopic findings indicative of carcinoma were assessed.
Gross type of NADETs
The gross types of superficial NADETs were classified using the criteria for colorectal tumors[24]. According to endoscopic features, the gross types included protruded pedunculated (Ip), protruded sessile (Is), semipedunculated (Isp), superficial elevated (IIa), and superficial shallow or depressed types (IIc). Mixed patterns, including IIa + Is or IIa + IIc, were diagnosed when more than one component was observed.
Endoscopic diagnosis of duodenal carcinomas
Because no criteria have been established for the endoscopic diagnosis of duodenal carcinomas, carcinoma or adenoma are currently diagnosed with reference to the procedures used for early gastric and colorectal carcinomas[25-29]. Whitish elevated lesions with lobulation or homogenous granules were considered adenoma (Figure 1), and lesions with depression, reddish component or heterogeneous granules were considered carcinoma (Figure 2). Endoscopic diagnoses were made by an endoscopist in charge of the examination before biopsy specimens were obtained.
Figure 1.
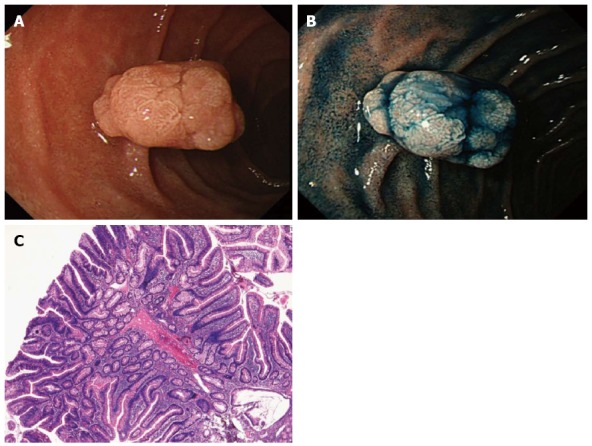
Case of duodenal adenoma. A: A whitish polypoid lesion is observed in the second portion of the duodenum; B: Chromoendoscopy clarifies the presence of lobulation. The lesion was diagnosed as a 0-Isp type adenoma; C: Histology of the biopsy specimen confirms a non-carcinomatous lesion, and the final diagnosis is a low-grade adenoma.
Figure 2.
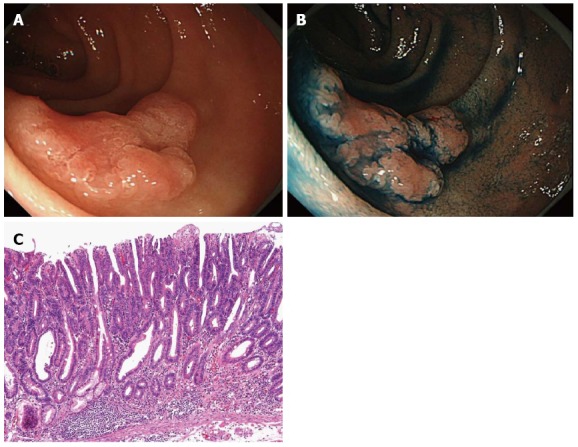
A case of duodenal carcinoma. A: A slightly elevated lesion with a reddish depression is observed in the second portion of the duodenum; B: Chromoendoscopy clarifies the presence of depressions. The lesion was diagnosed as a 0-IIa+IIc type intramucosal carcinoma; C: The final diagnosis is well-differentiated intramucosal adenocarcinoma.
Statistical analysis
Statistical analyses were performed using the chi-squared test followed by multivariate logistic regression analysis. Differences were considered significant when P was < 0.05. All analyses were performed using Excel statistics 2012 (Social Survey Research Information, Tokyo, Japan). The statistical methods of this study were reviewed by K. Mori from the Shizuoka Cancer Center. This retrospective study was approved by the institutional review board of our hospital (25-J155-25-1-3).
RESULTS
A total of 84 lesions from 74 patients were included in this study. The clinicopathological characteristics of the patients and lesions are shown in Table 1. The median age of the patients was 64 years, and 77% of the patients were male. The majority (81%) of the lesions were located in the second portion of the duodenum. The median lesion diameter was 14.5 mm according to final histology. The lesions were mostly elevated, although 4 lesions were of the depressed type (5%).
Table 1.
Clinicopathological characteristics of the 74 patients (84 lesions) n (%)
| Sex (male/female) | 54/21 |
| Age, yr, median (range) | 64 (33-84) |
| Location | |
| 1st portion | 12 (14) |
| 2nd portion | 68 (81) |
| 3rd portion | 4 (5) |
| Final lesion diameter, mm, median (range) | 14.5 (2-80) |
| Gross type | |
| Is, Isp, Ip | 17 (20) |
| IIa | 25 (30) |
| IIa + Is, IIa + IIc, Is + IIc | 38 (45) |
| IIc | 4 (5) |
| Biopsy histology | |
| Non-neoplastic | 2 (2) |
| LGA | 19 (23) |
| HGA | 7 (8) |
| Carcinoma | 46 (55) |
| NA | 10 (12) |
| Treatment | |
| Endoscopic resection | 35 (42) |
| Surgical resection | 49 (58) |
| Final histology | |
| Hyperplasia | 3 (4) |
| LGA | 10 (12) |
| HGA | 5 (6) |
| Carcinoma | 65 (77) |
| NE | 1 (1) |
LGA: Low-grade adenoma; HGA; High-grade adenoma; NA; Not assessed; NE: Not evaluable.
Biopsies were obtained from 74 lesions (88%). The median number of biopsies performed for each lesion was 1 (range 1-5). Among ten lesions in which biopsy was not performed, two were diagnosed as carcinoma endoscopically, and biopsy was not performed with the intent to avoid fibrosis related to biopsy. The other eight lesions were observed in two FAP patients (four in each patients), and all of them were diagnosed as adenoma endoscopically. These lesions were not biopsied because other larger lesions in the same patient were biopsied instead. Subsequently, 49 lesions from 39 patients were surgically resected, and 35 lesions from 35 patients were endoscopically resected. All resected lesions were sent for pathological assessment; however, one lesion was not available for pathologic assessment because of the presence of necrotic tissue. This lesion was diagnosed as carcinoma via biopsy and was endoscopically resected. The specimen was accidentally lost into the intestine immediately after resection and was collected from the stool the following day. Therefore, this lesion was excluded. Eventually, endoscopic and biopsy diagnoses were compared with final histological diagnoses in 83 and 73 lesions, respectively.
Comparisons of endoscopic diagnoses, biopsy diagnoses, and final histological diagnoses
Among the 57 lesions with endoscopic diagnoses of carcinoma, 52 (91%) were diagnosed as carcinomas, 3 were diagnosed as adenomas (2 high-grade, 1 low-grade), and 2 were diagnosed as hyperplasia via final histology. In contrast, among the 45 lesions diagnosed as carcinoma via biopsy, 42 (93%) were diagnosed as carcinomas and 3 were diagnosed as high-grade adenomas via final histology (Tables 2 and 3).
Table 2.
Diagnoses of endoscopy, biopsy, and final histology after treatment n (%)
|
Final histology |
|||||
| Non-neoplastic | LGA | HGA | Carcinoma | ||
| Endoscopic Dx (n = 83) | Non-neoplastic | 0 | 1 (50) | 0 | 1 (50) |
| (n = 2) | |||||
| Adenoma (n = 24) | 1 (4) | 8 (33) | 3 (13) | 12 (50) | |
| Carcinoma (n = 57) | 2 (3.5) | 1 (2) | 2 (3.5) | 52 (91) | |
| Biopsy Dx(n = 73) | Non-neoplastic | 0 | 2 (100) | 0 | 0 |
| (n = 2) | |||||
| LGA (n = 19) | 2 (11) | 5 (26) | 1 (5) | 11 (58) | |
| HGA (n = 7) | 1 (14) | 0 | 1 (14) | 5 (71) | |
| Carcinoma (n = 45) | 0 | 0 | 3 (7) | 42 (93) | |
Dx: Diagnosis; LGA: Low-grade adenoma; HGA: High-grade adenoma.
Table 3.
Comparison of endoscopic and biopsy diagnoses with final histology
|
Final histology |
|||
| Non-carcinoma | Carcinoma | ||
| Endoscopic Dx (n = 83) | Non-carcinoma (n = 26) | 12 | 14 |
| Carcinoma (n = 57) | 5 | 52 | |
| Sensitivity, 80%; Specificity, 72%; Accuracy, 78% | |||
| Biopsy Dx (n = 73) | Non-carcinoma (n = 28) | 12 | 16 |
| Carcinoma (n = 45) | 3 | 42 | |
| Sensitivity, 72%; Specificity, 80%; Accuracy, 74% | |||
Dx: Diagnosis.
Among the 24 lesions with endoscopic diagnoses of adenoma, 12 (50%) were finally diagnosed as carcinomas. Similarly, among the 26 lesions with biopsy diagnoses of adenoma (low- or high-grade), 16 (62%) were finally diagnosed as carcinomas.
Two lesions were diagnosed as non-neoplastic via endoscopy. Both of these appeared to be submucosal tumors in the first and second portions of the duodenum, respectively. One of these was diagnosed using biopsy as a carcinoma, which was consistent with the final histological diagnoses. The other lesion was diagnosed to be non-neoplastic via biopsy, and final histology indicated a low-grade adenoma.
The sensitivity, specificity, and accuracy of the endoscopic diagnoses were 80%, 72%, and 78%, respectively, whereas those of the biopsy diagnoses were 72%, 80%, and 74%, respectively.
Preoperative diagnoses of carcinomas were made in 88% (57/65) of the NADC cases via endoscopy or biopsy. All 8 lesions that were not preoperatively diagnosed as carcinoma were intramucosal cancers, and biopsies were not performed for 5 of these before resection.
Endoscopic findings associated with carcinoma
Preoperative factors associated with the final histology of adenomas or carcinomas are shown in Table 4. Univariate analyses showed that red color, presence of a depressed area, and gross types were associated with carcinomas. Preoperative sizes of 20 mm or larger were more often diagnosed as carcinoma, but the difference was not significant. Multivariate logistic regression analysis of preoperative endoscopic factors showed only red color as a factor indicative of carcinoma (Table 5).
Table 4.
Preoperative endoscopic factors associated with the final histology of adenoma or carcinoma
| Factor | Adenoma (n = 15) | Carcinoma (n = 65) | P value1 | |
| Location, n | 1st portion (n = 11) | 4 | 7 | NS |
| 2nd portion (n = 65) | 11 | 54 | ||
| 3rd portion (n = 4) | 0 | 4 | ||
| Preoperative diameter, mm, n | < 20 (n = 53) | 13 | 40 | 0.060 |
| ≥ 20 (n = 27) | 2 | 25 | ||
| Color, n | White or isocolor (n = 34) | 12 | 22 | 0.001 |
| Red (n = 46) | 3 | 43 | ||
| Depressed area, n | Present (n = 40) | 3 | 37 | 0.009 |
| Absent (n = 40) | 12 | 28 | ||
| Lobulation, n | Present (n = 42) | 9 | 33 | 0.500 |
| Absent (n = 38) | 6 | 32 | ||
| Marginal whitening, n | Present (n = 52) | 9 | 43 | NS |
| Absent (n = 28) | 6 | 22 | ||
| Gross type, n | Elevated type | 13 | 26 | 0.002 |
| (Is, Isp, Ip, IIa) (n = 39) | ||||
| Depressed type (IIc) (n = 4) | 0 | 4 | ||
| Mixed type (IIa + Is, IIa + IIc, Is + IIc) (n = 37) | 2 | 35 | ||
χ2 test. NS: Not significant.
Table 5.
Multivariate logistic regression analysis of endoscopic factors indicative of duodenal carcinoma
| Factor | OR | P value | 95%CI |
| Color | 2.360 | 0.038 | 1.04-5.32 |
| Lobulation | 0.553 | 0.389 | 0.14-2.13 |
| Marginal whitening | 0.546 | 0.359 | 0.15-1.98 |
| Gross type | 2.258 | 0.064 | 0.95-5.35 |
OR: Odds ratio.
DISCUSSION
In the present study, the diagnostic accuracy of endoscopy and biopsy was assessed in a large number of patients with superficial NADETs. Diagnostic accuracy was similar between endoscopy (78%) and biopsy (74%). However, the biopsy diagnoses (80%) had greater specificity than the endoscopic diagnoses (72%). Similar results were reported in a retrospective study by Goda et al[19] in which endoscopy diagnosis showed higher sensitivity (77%) and similar accuracy (75%) while showing lower specificity (72%) than those of biopsy diagnosis.
The necessity of obtaining biopsy specimens from superficial NADETs before treatment remains controversial. Because the duodenal wall is thin, the biopsy procedure itself may induce unintended fibrosis associated with the lesion, which may hamper subsequent ER. Moreover, histological proof of malignancy may be preferable prior to treatment with ER or surgery, which is associated with substantial risks of complication. Lesions that are endoscopically diagnosed as carcinoma, which can be treated by further ER, may not require biopsy because the sensitivity of endoscopy for the diagnoses of carcinoma is superior to that of biopsy (80% vs 72%). However, for lesions requiring surgical resection, the addition of biopsy to endoscopic diagnoses may provide improved sensitivity (88%) for the diagnosis of carcinoma.
Endoscopic observations of red color, depressed areas, and mixed gross type lesions were significant indicators of duodenal carcinomas. A previous case study reported that larger-sized lesions, those with components of depression (IIa+IIc type or IIc type) and those with red and dull surfaces tended to have a carcinomatous component[20]. Another study reported that erythematous color and nodular/rough surfaces were characteristics of high-grade adenoma, which were more likely to progress to carcinoma[21]. A multicenter study reported that a size of > 5 mm and red color were indicative factors of high-grade adenoma or carcinoma[19]. Our results were in accordance with these reports, and these factors are considered essential for the diagnosis of carcinoma in the duodenum, as well as for gastric lesions[26,30]. The major gross type of superficial NADETs is the elevated type. Therefore, observations of red color or depression within elevated lesions may reflect vascularization and proliferation of glands due to the presence of carcinomatous components. In this study, size was not a significant predictor of carcinoma. A few large carpet-like IIa lesions have been reported to increase their size as DA without any carcinomatous components[20]. This study included only two lesions of this type, and there were many other small-sized carcinomas. Developmental mechanisms for these large DA and small carcinoma lesions may differ, possibly reflecting differences between the adenoma-carcinoma sequence and the de novo sequence of carcinoma.
Discrepancies between biopsy diagnoses and final histological diagnoses have been reported for gastric epithelial lesions[30] and duodenal lesions[9,10,12,18,21]. In the present study, 19 lesions (26%, 19/73) were upgraded at final diagnosis. Among these, 11 were upgraded from low-grade adenoma to carcinoma, and another 5 were upgraded from high-grade adenoma to carcinoma. This discrepancy is likely to reflect the heterogeneity of tumors because many NADCs are considered to follow the adenoma-carcinoma sequence. Therefore, targeted biopsies from reddish depressed areas observed during endoscopy may help minimize the underestimation of biopsy diagnoses. Moreover, lesions with a biopsy diagnosis of high-grade adenoma should be considered for resection.
In this study, 2 lesions appeared to be submucosal tumors and were diagnosed as non-neoplastic via endoscopy, one lesion was diagnosed as carcinoma via biopsy. Submucosal tumors in the duodenum are often benign cysts or Brunner hyperplasia. In contrast, submucosal invasive carcinomas may appear to be submucosal tumors. Therefore, biopsies may be recommended for lesions that appear to be submucosal tumors to ensure the accurate and timely detection of carcinomas.
To overcome the diagnostic limitations of conventional white-light endoscopy with chomoendoscopy, magnifying endoscopy with or without narrow-band imaging may provide distinctions between potentially malignant lesions[22,31,32]. Heterogeneous patterns of irregular or invisible mucosal structures with irregular vascular patterns have been correlated with high-grade dysplasia (HGD) or intramucosal carcinomas[22,31,32]. However, the additional advantages of magnifying endoscopy remain unclear, and further studies are required to clarify these.
This study is limited by its retrospective design. Our center is specialized for the treatment of cancer patients; therefore, the high diagnostic performances of endoscopic diagnosis may have become a bias. Moreover, patients who were preoperatively diagnosed with adenoma endoscopically with a biopsy diagnosis of low-grade adenoma were followed-up without resection, leading to a potential selection bias. We tried to minimize the selection bias by including all patients who underwent resection and had a confirmed diagnosis based on the resected specimen. Therefore, the patient cohort in this study is likely to represent patients of therapeutic objectives. Most of the carcinomas included in this study were intramucosal, with only six lesions containing submucosal invasive carcinomas. Previous studies have shown that lesions with depressions (IIc, IIa+IIc) have a possibility of submucosal invasion, regardless of size[17,18]. In the present study, all four IIc lesions were intramucosal carcinomas. However, the small number of submucosal invasive carcinomas precluded the analyses of lesion depths. Further studies on the diagnosis of superficial NADETs according to tumor depth and magnifying endoscopic findings are required.
In conclusion, preoperative endoscopy and biopsy showed similar accuracy in the diagnosis of carcinoma in patients with superficial NADETs.
ACKNOWLEDGMENTS
The authors thank the biostatistician of Shizuoka Cancer Center, Mr. K. Mori for reviewing the statistical analysis.
COMMENTS
Background
Primary nonampullary duodenal epithelial tumors (NADETs) are extremely rare; however, recent studies have demonstrated an increase in endoscopic treatments for sporadic superficial NADETs such as duodenal adenoma or carcinoma. Endoscopic resection of superficial NADETs is associated with a high risk of complications; therefore, preoperative diagnosis to distinguish adenoma and carcinoma is essential. However, because the incidence of sporadic nonampullary duodenal carcinoma (NADC) is extremely rare, endoscopic findings suggestive of early NADC have not yet been established.
Research frontiers
The current research hotspot is to identify the predictive endoscopic characteristics of carcinomas and compare the accuracy of endoscopy and biopsy in the diagnosis of superficial NADETs.
Innovations and breakthroughs
Diagnostic accuracy was similar between endoscopy (78%) and biopsy (74%). However, biopsy diagnoses (80%) had greater specificity than endoscopic diagnoses (72%). The results were in accordance with the only study reported in the literature, which was based on a multicenter questionnaire survey.
Applications
Endoscopic observations of red color, depressed areas, and mixed gross type lesions were significant indicators of duodenal carcinomas. Biopsy may be omitted to prevent fibrosis associated with lesions scheduled for endoscopic treatment, because of the similar accuracy of endoscopy and biopsy in the diagnosis of carcinoma.
Terminology
Superficial nonampullary duodenal epithelial tumors were defined as duodenal adenomas or carcinomas confined to the mucosa or submucosa.
Peer-review
This is a well-written study with a significant number of patients with duodenal carcinomas. The results showed high diagnostic performances of endoscopic diagnosis in an expert center.
Footnotes
Ethics approval: This study was reviewed and approved by the Shizuoka Cancer Center institutional review board.
Informed consent: All patients gave written informed consent prior to their endoscopic examination. Informed consent for study inclusion was omitted due to the retrospective use of data extracted from past charts and reports.
Conflict-of-interest: The authors report no conflict-of-interest.
Data sharing: No additional data are available.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: November 6, 2014
First decision: November 26, 2014
Article in press: January 8, 2015
P- Reviewer: Cellier C S- Editor: Qi Y L- Editor: A E- Editor: Liu XM
References
- 1.Alwmark A, Andersson A, Lasson A. Primary carcinoma of the duodenum. Ann Surg. 1980;191:13–18. doi: 10.1097/00000658-198001000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yokoyama T, Saito D, Kondo H, Kido M, Hosokawa K, Shirao K, Yokota T, Yamaguchi H, Oguro Y, Ishikawa T, et al. Endoscopic diagnosis of malignant lesions of the duodenum. Stomach Int. 1993;28:641–649. [Google Scholar]
- 3.Vasen HF, Möslein G, Alonso A, Aretz S, Bernstein I, Bertario L, Blanco I, Bülow S, Burn J, Capella G, et al. Guidelines for the clinical management of familial adenomatous polyposis (FAP) Gut. 2008;57:704–713. doi: 10.1136/gut.2007.136127. [DOI] [PubMed] [Google Scholar]
- 4.Perzin KH, Bridge MF. Adenomas of the small intestine: a clinicopathologic review of 51 cases and a study of their relationship to carcinoma. Cancer. 1981;48:799–819. doi: 10.1002/1097-0142(19810801)48:3<799::aid-cncr2820480324>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 5.Barnes G, Romero L, Hess KR, Curley SA. Primary adenocarcinoma of the duodenum: management and survival in 67 patients. Ann Surg Oncol. 1994;1:73–78. doi: 10.1007/BF02303544. [DOI] [PubMed] [Google Scholar]
- 6.Santoro E, Sacchi M, Scutari F, Carboni F, Graziano F. Primary adenocarcinoma of the duodenum: treatment and survival in 89 patients. Hepatogastroenterology. 1997;44:1157–1163. [PubMed] [Google Scholar]
- 7.Hirasawa R, Iishi H, Tatsuta M, Ishiguro S. Clinicopathologic features and endoscopic resection of duodenal adenocarcinomas and adenomas with the submucosal saline injection technique. Gastrointest Endosc. 1997;46:507–513. doi: 10.1016/s0016-5107(97)70005-1. [DOI] [PubMed] [Google Scholar]
- 8.Oka S, Tanaka S, Nagata S, Hiyama T, Ito M, Kitadai Y, Yoshihara M, Haruma K, Chayama K. Clinicopathologic features and endoscopic resection of early primary nonampullary duodenal carcinoma. J Clin Gastroenterol. 2003;37:381–386. doi: 10.1097/00004836-200311000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Takahashi T, Ando T, Kabeshima Y, Kawakubo H, Shito M, Sugiura H, Omori T. Borderline cases between benignancy and malignancy of the duodenum diagnosed successfully by endoscopic submucosal dissection. Scand J Gastroenterol. 2009;44:1377–1383. doi: 10.3109/00365520903287551. [DOI] [PubMed] [Google Scholar]
- 10.Lépilliez V, Chemaly M, Ponchon T, Napoleon B, Saurin JC. Endoscopic resection of sporadic duodenal adenomas: an efficient technique with a substantial risk of delayed bleeding. Endoscopy. 2008;40:806–810. doi: 10.1055/s-2008-1077619. [DOI] [PubMed] [Google Scholar]
- 11.Alexander S, Bourke MJ, Williams SJ, Bailey A, Co J. EMR of large, sessile, sporadic nonampullary duodenal adenomas: technical aspects and long-term outcome (with videos) Gastrointest Endosc. 2009;69:66–73. doi: 10.1016/j.gie.2008.04.061. [DOI] [PubMed] [Google Scholar]
- 12.Endo M, Abiko Y, Oana S, Kudara N, Chiba T, Suzuki K, Koizuka H, Uesugi N, Sugai T. Usefulness of endoscopic treatment for duodenal adenoma. Dig Endosc. 2010;22:360–365. doi: 10.1111/j.1443-1661.2010.01014.x. [DOI] [PubMed] [Google Scholar]
- 13.Ono H, Nonaka S, Uedo N, Kaise M, Oyama T, Doyama H, Kokawa A, Kaneko K, Kodashima S, Tanabe S, et al. Clinical issues of duodenal EMR/ESD. Stomach Int. 2011;46:1669–1677. [Google Scholar]
- 14.Jung JH, Choi KD, Ahn JY, Lee JH, Jung HY, Choi KS, Lee GH, Song HJ, Kim DH, Kim MY, et al. Endoscopic submucosal dissection for sessile, nonampullary duodenal adenomas. Endoscopy. 2013;45:133–135. doi: 10.1055/s-0032-1326178. [DOI] [PubMed] [Google Scholar]
- 15.Matsumoto S, Miyatani H, Yoshida Y. Endoscopic submucosal dissection for duodenal tumors: a single-center experience. Endoscopy. 2013;45:136–137. doi: 10.1055/s-0032-1310123. [DOI] [PubMed] [Google Scholar]
- 16.Kakushima N, Ono H, Takao T, Kanemoto H, Sasaki K. Method and timing of resection of superficial nonampullary duodenal epithelial tumors. Dig Endosc. 2014;26:35–40. doi: 10.1111/den.12259. [DOI] [PubMed] [Google Scholar]
- 17.Nagatani K, Takekoshi T, Baba Y, Kaku S, Koizumi K, Fujii A, Ogata E, Ohta H, Nishi M, Kato Y, et al. Indications for endoscopic treatment of early duodenal cancer based on cases reported in the literature. Endosc Digest. 1993;5:969–976. [Google Scholar]
- 18.Fujisawa T, Tomofuji Y, Kuroda N, Hagino H, Sakamoto N, Sakashita M, Maeda M, Kouno T, Matsuno Y. A case of early duodenal cancer with tubulo-villous adenoma: report of a case and clinicopathological review of Japanese literature. Gastroenterol Endosc. 1995;37:2768–2775. [Google Scholar]
- 19.Goda K, Kikuchi D, Yamamoto Y, Takimoto K, Kakushima N, Morita Y, Doyama H, Gotoda T, Maehata Y, Abe N. Endoscopic diagnosis of superficial non-ampullary duodenal epithelial tumors in Japan: Multicenter case series. Digest Endosc. 2014;26:23–29. doi: 10.1111/den.12277. [DOI] [PubMed] [Google Scholar]
- 20.Yamanaka T, Yamamichi N, Konishi F. Clinico-pathological study of duodenal adenoma. Gastroenterol Endosc. 1987;29:3070–3079. [Google Scholar]
- 21.Okada K, Fujisaki J, Kasuga A, Omae M, Kubota M, Hirasawa T, Ishiyama A, Inamori M, Chino A, Yamamoto Y, et al. Sporadic nonampullary duodenal adenoma in the natural history of duodenal cancer: a study of follow-up surveillance. Am J Gastroenterol. 2011;106:357–364. doi: 10.1038/ajg.2010.422. [DOI] [PubMed] [Google Scholar]
- 22.Yoshimura N, Goda K, Tajiri H, Ikegami M, Nakayoshi T, Kaise M. Endoscopic features of nonampullary duodenal tumors with narrow-band imaging. Hepatogastroenterology. 2010;57:462–467. [PubMed] [Google Scholar]
- 23.Dixon MF. Gastrointestinal epithelial neoplasia: Vienna revisited. Gut. 2002;51:130–131. doi: 10.1136/gut.51.1.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Japanese Society for Cancer of the Colon and Rectum. Japanese classification of colorectal carcinoma. 8th ed. Tokyo: Kanehara Shuppan; 2013. pp. 9–10. [Google Scholar]
- 25.Shimamoto K, Misaki F, Murakami K, Ida K, Kawai K. Endoscopic diagnosis of Type I early gastric cancer. Stomach Int. 1971;6:48–53. [Google Scholar]
- 26.Fukutomi H, Takezawa H, Tani N, Kobayashi M, Sakita T. Endoscopic diagnosis of IIa type early gastric carcinoma. Stomach Int. 1971;6:55–61. [Google Scholar]
- 27.Saigenji K, Ohida M, Koizumi W, Tanabe S, Imaizumi H, Mitsuhashi T, Uesugi H. Endoscopic diagnosis of early gastric cancer: diagnosis of IIc type early gastric cancer by conventional and dye endoscopy. Stomach Int. 2000;35:25–35. [Google Scholar]
- 28.Maruyama M, Sasaki T, Yokoyama Y, Gondo M, Baba Y, Ninomiya K, Tajiri H, Ohashi M, Sugiyama N, Takekoshi T, et al. Further study on the diagnosis of early cancer of the large bowel, with special reference to reevaluation of diagnostic criteria and some problems on endoscopic polypectomy. Stomach Int. 1980;15:375–391. [Google Scholar]
- 29.Kudo S, Hayashi S, Miura K, Takano M, Saito S, Kashima Y. The clinicopathological features of flat and depressed type of early colorectal cancer. Stomach Int. 1989;24:317–329. [Google Scholar]
- 30.Takao M, Kakushima N, Takizawa K, Tanaka M, Yamaguchi Y, Matsubayashi H, Kusafuka K, Ono H. Discrepancies in histologic diagnoses of early gastric cancer between biopsy and endoscopic mucosal resection specimens. Gastric Cancer. 2012;15:91–96. doi: 10.1007/s10120-011-0075-8. [DOI] [PubMed] [Google Scholar]
- 31.Tanaka K, Toyoda H, Inoue H, Hamada Y, Aoki M, Kosaka R, Takamura M, Imoto I. Depressed-type early duodenal carcinoma (carcinoma in situ) observed by enhanced magnification endoscopy. Endoscopy. 2007;39 Suppl 1:E125–E126. doi: 10.1055/s-2007-966171. [DOI] [PubMed] [Google Scholar]
- 32.Onozato Y, Kakizaki S, Ishihara H, Sohara N, Iizuka H, Okamura S, Mori M, Ogawa T, Itoh H. Magnifying endoscopic findings of early duodenal adenocarcinoma in relation to the pathological findings. Endoscopy. 2008;40 Suppl 2:E92–E93. doi: 10.1055/s-2007-995573. [DOI] [PubMed] [Google Scholar]


