Abstract
AIM: To determine the prognostic significance of preoperative serum neutrophil-lymphocyte ratio (NLR) in esophageal squamous cell carcinoma (ESCC).
METHODS: Data from 371 eligible patients with ESCC who had undergone surgery with curative intent at our institution between October 2000 and May 2007 were retrospectively recruited for analysis. The cutoff value of NLR was 3.0 as determined by the receiver operating characteristic curve, which discriminated between survival and death; the area under the curve was 0.709, and the sensitivity and specificity were 66.1% and 69.1%, respectively, at the cutoff point. The correlation between the NLR and clinicopathological characteristics was analyzed using a χ2 test. The prognostic influence of the NLR and other clinicopathological factors on cancer-specific survival (CSS) and recurrence-free survival (RFS) was studied using the Kaplan-Meier method. To evaluate the independent prognostic value of NLR, multivariate Cox regression models were applied.
RESULTS: The median age of the patients was 57.0 years, and 276/371 (74.4%) patients were male. The NLR was ≤ 3.0 in 80.1% (297/371) of the patients, and the remaining 19.9% (74/371) had an NLR > 3.0. Median postoperative follow-up was 66.0 mo [interquartile range (IQR): 49.0-76.0 mo], with a follow-up rate of 94%. Follow-up was not significantly different between patients with an NLR ≤ and > 3.0 (63.13 ± 1.64 vs 61.52 ± 3.66, P = 0.711). However, higher preoperative serum NLR was associated with significantly increased risks of higher pathological tumor status (P = 0.007). A significant, independent association between high preoperative serum NLR and poor clinical outcome was identified in a multivariate analysis for CSS (HR = 1.591; P = 0.007) and RFS (HR = 1.525; P = 0.013). Moreover, when patients were stratified by pathological tumor-node-metastasis (TNM) staging, the adverse effects of preoperative serum NLR on CSS (HR = 2.294; P = 0.008) and RFS (HR = 2.273; P = 0.008) were greatest in those patients with stage IIIA disease.
CONCLUSION: Preoperative serum NLR is a useful prognostic marker to complement TNM staging for operable ESCC patients, particularly in patients with stage IIIA disease.
Keywords: Esophageal squamous cell carcinoma, Neutrophil-lymphocyte ratio, Prognosis, Radical esophagectomy
Core tip: From a relatively large cohort of esophageal squamous cell carcinoma patients who underwent esophagectomy without neoadjuvant treatment and extended follow-up, we determined that an elevated preoperative serum neutrophil-lymphocyte ratio (NLR) is a negative prognostic factor. When stratified by pathological tumor-node-metastasis staging, the adverse effects of NLR were greatest in patients with stage IIIA disease.
INTRODUCTION
Recent estimations rank esophageal cancer as the fifth deadliest cancer in men and eighth in women worldwide, and esophageal cancer was responsible for 406800 deaths in 2008[1]. The major pathological type of this cancer is esophageal squamous cell carcinoma (ESCC), which is most frequently found among individuals in the region ranging from Northern Iran to North-Central China[1]. Most predictive models for prognosis utilize tumor-node-metastasis (TNM) staging[2], albeit with discrepant results within the same stage. Therefore, there is an urgent demand for new parameters in risk stratification to complement TNM staging to improve individualized treatment.
Over the past two decades, the interaction between cancer and the inflammatory system has been increasingly recognized[3-5]. The direct and indirect interactions between neoplastic and inflammatory cells can cause DNA damage, promote angiogenesis, inhibit apoptosis, and increase propensity for metastasis[3-7]. The role of the systemic inflammatory response as an indicator of prognosis has also been recognized in various primary operable malignancies[8]. The prognostic value of the serum neutrophil-lymphocyte ratio (NLR), which represents an inexpensive, routine, and repeatable marker of systemic inflammation, has been well studied in lung, gastric, bladder, pancreatic, and biliary tract cancers[9-13]. However, the value of the NLR in ESCC patients is ill defined, because relevant studies have inconsistent results due to short-term follow-up[14-16], small sample sizes[15,16], receipt of neoadjuvant therapy[15,16], differences in histological types[15,16], and omission of pathologic (p)TNM staging in survival analyses[14]. Thus, the aim of the present study was to investigate the association of preoperative serum NLR with clinicopathological features in patients with ESCC, as well as to evaluate the risks of tumor recurrence and mortality for patients treated with radical esophagectomy.
MATERIALS AND METHODS
Patient characteristics
Between October 2000 and May 2007, 424 consecutive patients with histologically confirmed primary ESCC and who were treated with curative-intent esophagectomy at the Cancer Center of Sun Yat-Sen University were retrospectively reviewed. The exclusion criteria were as follows: (1) patients who received previous anti-inflammatory drugs within 1 wk; (2) patients who had received neoadjuvant treatment; (3) patients who had previous or other concomitant cancer; (4) patients with noncurative resection; (5) patients who had perioperative mortality; (6) patients with stage 0, stage IV, or pathological tumor (pT)4 disease; (7) patients for whom the records of preoperative complete blood cell counts with differential were unavailable; (8) patients who died from non-tumor causes; and (9) patients for whom follow-up data were not detailed or complete.
Data on NLR were retrospectively retrieved from the medical records. All of the patient blood samples were collected within 1 wk before surgery. When multiple values existed for a patient, those closest to the date of esophagectomy were used. Clinicopathological data including patient age, sex, tumor location, tumor differentiation, pT and pathological node (pN) status, and pathological (p)TNM staging were also collected. A single pathologist re-reviewed all esophagectomy pathologic specimens. Tumor differentiation was determined based on the criteria proposed by the World Health Organization classification of Tumors of the Digestive System (2010 version). pTNM staging was based on the American Joint Committee on Cancer staging manual, 7th edition[2]. This study was approved by the Medical Ethics Committee of the Cancer Center at Sun Yat-Sen University.
Surgical strategy
All patients included in this study were treated with curative resection by total or subtotal thoracic esophagectomy and thoracoabdominal lymphadenectomy, which included the subcarinal, paraesophageal, pulmonary ligament, diaphragmatic, paracardial, and left gastric artery lymph nodes. Esophageal reconstruction was performed using the stomach, colon, or jejunum.
Patient follow-up
At our institution, follow-up after esophagectomy is generally recommended quarterly for the first year, semiannually for the following 2 years, and annually thereafter for patients without evidence of recurrent disease. Recording of medical history, physical examination, and contrast-enhanced computed tomography scans of the neck, thorax, and upper abdomen, including the liver and adrenals, were routinely performed. The last follow-up was in June 2014, which included verification of the clinical attendance records and direct telecommunication with the patient or their family.
NLR cutoff determination and primary outcomes
The cutoff value of NLR was defined as 3.0 based on the receiver operating characteristic curve, which discriminated between survival and death; the area under the curve was 0.709, and the sensitivity and specificity were 66.1% and 69.1%, respectively, at the cutoff point. The primary outcomes were cancer-specific survival (CSS), defined as the time between the operation and death due to cancer, and recurrence-free survival (RFS), defined as the time between the operation and local and/or distant soft tissue recurrence.
Statistical analysis
All analyses were carried out using SPSS version 20.0 (IBM Corp., Armonk, NY, United States). The correlations between NLR and clinicopathological characteristics were analyzed using the χ2 test. CSS and RFS were estimated using the Kaplan-Meier method, and the differences were assessed by the log-rank test. All significant parameters identified by univariate analysis were evaluated by multivariate analysis using the Cox proportional hazards model. The strength of the association between predictors and survival was assessed by HRs with 95%CIs. Two-sided P < 0.05 was considered statistically significant. The statistical methods of this study were reviewed by Qing Liu at the Sun Yat-Sen University Cancer Center.
RESULTS
Data from a total of 371 eligible patients were included for analysis. Among these, 297 (80.1%) patients had an NLR ≤ 3.0 and 74 (19.9%) had an NLR > 3.0. The median age of the patients was 57.0 years, and 276 (74.4%) patients were male. The median duration of postoperative follow-up was 66.0 mo [interquartile range (IQR): 49.0-76.0 mo], during which 206/371 (55.5%) patients experienced disease recurrence at a median of 14.0 mo (IQR: 7.0-26.0 mo) after esophagectomy and 192/371 (51.8%) died of ESCC (median time to death: 18.0 mo, IQR: 11.0-27.0 mo). The follow-up rate was 94%. Follow-up duration was not significantly different between patients with an NLR ≤ and > 3.0 (63.13 ± 1.64 mo vs 61.52 ± 3.66 mo, P = 0.711).
Table 1 shows the distribution of clinicopathological characteristics according to NLR. Of the examined characteristics, only pT status was significantly associated with NLR (P = 0.007), with a preoperative serum NLR > 3.0 associated with a higher pT status.
Table 1.
Clinicopathological features according to neutrophil-lymphocyte ratio n (%)
| Variable |
NLR |
|||
| Cases | ≤ 3.0 | > 3.0 | P value1 | |
| Age (yr) | 0.112 | |||
| ≤ 57.02 | 201 (54.2) | 167 (83.1) | 34 (16.9) | |
| > 57.0 | 170 (45.8) | 130 (76.5) | 40 (23.5) | |
| Sex | 0.562 | |||
| Female | 95 (25.6) | 78 (82.1) | 17 (17.9) | |
| Male | 276 (74.4) | 219 (79.3) | 57 (20.7) | |
| Location | 0.372 | |||
| Upper | 23 (6.2) | 19 (82.6) | 4 (17.4) | |
| Middle | 256 (69.0) | 200 (78.1) | 56 (21.9) | |
| Lower | 92 (24.8) | 78 (84.8) | 14 (15.2) | |
| Differentiation | 0.487 | |||
| Well | 58 (15.6) | 46 (79.3) | 12 (20.7) | |
| Moderate | 245 (66.0) | 193 (78.8) | 52 (21.2) | |
| Poor | 68 (18.3) | 58 (85.3) | 10 (14.7) | |
| pT status | 0.007 | |||
| T1 | 17 (4.6) | 15 (88.2) | 2 (11.8) | |
| T2 | 88 (23.7) | 80 (90.9) | 8 (9.1) | |
| T3 | 266 (71.7) | 202 (75.9) | 64 (24.1) | |
| pN status | 0.592 | |||
| N0 | 199 (53.6) | 163 (81.9) | 36 (18.1) | |
| N1 | 102 (27.5) | 82 (80.4) | 20 (19.6) | |
| N2 | 55 (14.8) | 41 (74.5) | 14 (25.5) | |
| N3 | 15 (4.0) | 11 (73.3) | 4 (26.7) | |
| Stage | 0.566 | |||
| IB | 20 (5.4) | 17 (85.0) | 3 (15.0) | |
| IIA | 58 (15.6) | 50 (86.2) | 8 (13.8) | |
| IIB | 148 (39.9) | 119 (80.4) | 29 (19.6) | |
| IIIA | 83 (22.4) | 66 (79.5) | 17 (20.5) | |
| IIIB | 47 (12.7) | 34 (72.3) | 13 (27.7) | |
| IIIC | 15 (4.0) | 11 (73.3) | 4 (26.7) | |
| Total | 371 (100) | 297 (80.1) | 74 (19.9) | |
χ2 test;
Median age. NLR: Neutrophil-lymphocyte ratio; pN: Pathologic node; pT: Pathologic tumor.
The results of the univariate analysis revealed that tumor differentiation, pT and pN status, pTNM staging, and NLR were negatively associated with CSS and RFS (all P < 0.05) (Table 2). In addition, CSS was significantly associated with tumor location (P < 0.05). CSS and RFS were significantly shorter in patients with an NLR > 3.0 than those with an NLR ≤ 3.0 (all P ≤ 0.001) (Figure 1).
Table 2.
Univariate analysis of factors associated with recurrence-free survival and cancer-specific survival
| Variable | Cases (n) |
CSS (mo) |
RFS (mo) |
||||
| Mean | Median | P value | Mean | Median | P value | ||
| Age (yr) | 0.259 | 0.230 | |||||
| ≤ 57.01 | 201 | 61.9 | 42 | 54.7 | 32 | ||
| > 57.0 | 170 | 59.1 | 70 | 53.9 | 66 | ||
| Sex | 0.070 | 0.113 | |||||
| Female | 95 | 72.2 | NR | 62.3 | 74 | ||
| Male | 276 | 59.5 | 45 | 52.8 | 34 | ||
| Location | 0.033 | 0.127 | |||||
| Upper | 23 | 70.4 | NR | 52.2 | 58 | ||
| Middle | 256 | 52.7 | 41 | 48.8 | 35 | ||
| Lower | 92 | 74.8 | NR | 67.5 | 74 | ||
| Differentiation | 0.014 | 0.005 | |||||
| Well | 58 | 55.0 | 55 | 52.1 | 58 | ||
| Moderate | 245 | 67.9 | 70 | 59.0 | 65 | ||
| Poor | 68 | 44.0 | 25 | 38.1 | 22 | ||
| pT status | 0.003 | 0.008 | |||||
| T1 | 17 | 60.5 | NR | 55.5 | NR | ||
| T2 | 88 | 71.6 | 74 | 65.9 | 77 | ||
| T3 | 266 | 59.4 | 39 | 51.1 | 29 | ||
| pN status | < 0.001 | < 0.001 | |||||
| N0 | 199 | 80.9 | NR | 71.7 | 87 | ||
| N1 | 102 | 44.1 | 41 | 40.1 | 29 | ||
| N2 | 55 | 25.0 | 15 | 21.2 | 10 | ||
| N3 | 15 | 20.7 | 11 | 12.0 | 6 | ||
| Stage | < 0.001 | < 0.001 | |||||
| I | 20 | 64.1 | NR | 62.6 | NR | ||
| II | 206 | 77.4 | NR | 68.3 | 85 | ||
| III | 145 | 32.7 | 21 | 29.0 | 15 | ||
| NLR | < 0.001 | 0.001 | |||||
| ≤ 3.0 | 297 | 68.3 | 70 | 61.0 | 58 | ||
| > 3.0 | 74 | 41.2 | 24 | 38.3 | 17 | ||
Median age. CSS: Cancer-specific survival: NLR: Neutrophil-lymphocyte ratio; NR: Not reached; pN: Pathologic node; pT: Pathologic tumor; RFS: Recurrence-free survival.
Figure 1.
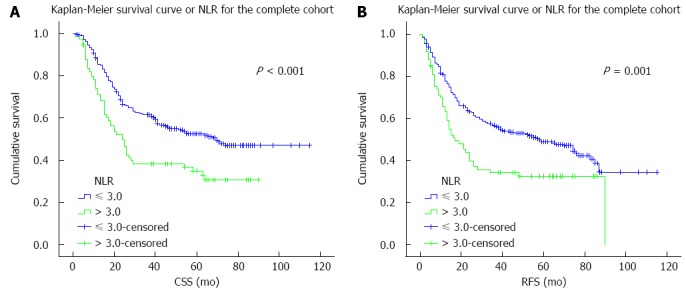
Survival curves for the complete cohort. A: Cancer-specific survival (CSS); B: Recurrence-free survival (RFS) curves for patients with esophageal squamous cell carcinoma according to preoperative serum neutrophil-lymphocyte ratio (NLR).
Multivariate analysis was performed using significant prognostic factors as determined by univariate analysis. Preoperative NLR remained independently associated with significantly increased risks for tumor recurrence and ESCC-related death (all P < 0.05) (Table 3). In addition, higher pN status was an adverse independent prognostic factor for CSS and RFS (all P < 0.001).
Table 3.
Results of multivariable cox regression analysis
| Prognostic factor | HR | 95%CI | P value |
| CSS | |||
| Location (upper vs middle vs lower) | 0.888 | 0.677-1.164 | 0.389 |
| Differentiation (well vs moderate vs poor) | 1.156 | 0.911-1.468 | 0.233 |
| pT status ( T1 vs T2 vs T3) | 1.101 | 0.778-1.559 | 0.586 |
| pN status (N0 vs N1 vs N2 vs N3) | 1.615 | 1.266-2.061 | < 0.001 |
| Stage (I vs II vs III) | 1.460 | 0.907-2.351 | 0.120 |
| NLR (≤ 3.0 vs > 3.0) | 1.591 | 1.132-2.235 | 0.007 |
| RFS | |||
| Differentiation (well vs moderate vs poor) | 1.177 | 0.934-1.482 | 0.167 |
| pT status (T1 vs T2 vs T3) | 1.088 | 0.782-1.515 | 0.615 |
| pN status (N0 vs N1 vs N2 vs N3) | 1.703 | 1.337-2.169 | < 0.001 |
| Stage (I vs II vs III) | 1.336 | 0.846-2.110 | 0.215 |
| NLR (≤ 3.0 vs > 3.0) | 1.525 | 1.094-2.126 | 0.013 |
NLR: Neutrophil-lymphocyte ratio; pN: Pathologic node; pT: Pathologic tumor; CSS: Cancer-specific survival; RFS: Recurrence-free survival.
The predictive effect of NLR on CSS and RFS was further assessed after stratification by pTNM staging. NLR was associated with disease outcome only in patients with stage IIIA disease (Table 4). Stage IIIA patients with an NLR > 3.0 had significantly worse CSS (HR = 2.294; P = 0.008) and RFS (HR = 2.273; P = 0.008) (Figure 2). Although stage IIB patients with NLR > 3.0 also showed worse survivals, the differences were not statistically significant. No differences were observed in patients with other pTNM stages.
Table 4.
Univariate analysis of neutrophil-lymphocyte ratio stratified by tumor staging n (%)
| TNM stage | Cases |
P value |
|
| CSS | RFS | ||
| IB | 20 (5.4) | 0.590 | 0.590 |
| IIA | 58 (15.6) | 0.987 | 0.977 |
| IIB | 148 (39.9) | 0.082 | 0.082 |
| IIIA | 83 (22.4) | 0.008 | 0.008 |
| IIIB | 47 (12.7) | 0.356 | 0.596 |
| IIIC | 15 (4.0) | 0.440 | 0.700 |
CSS: Cancer-specific survival; NLR: Neutrophil-lymphocyte ratio; RFS: Recurrence-free survival; TNM: Tumor-node-metastasis.
Figure 2.
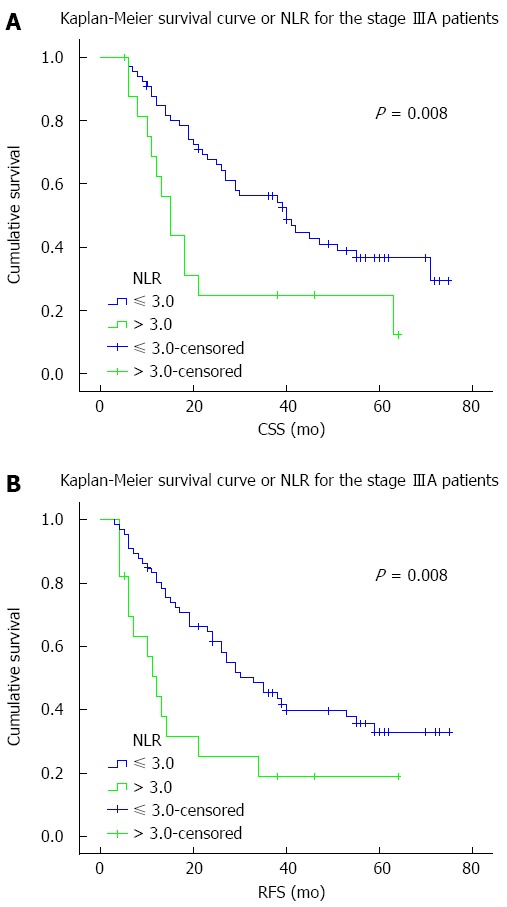
Survival curves for stage IIIA patients. A: Cancer-specific survival (CSS); B: Recurrence-free survival (RFS) curves according to preoperative serum neutrophil-lymphocyte ratio (NLR) for patients with pathological tumor-node-metastasis stage IIIA esophageal squamous cell carcinoma
DISCUSSION
This study shows that elevated preoperative serum NLR independently and adversely associates with ESCC prognosis, consistent with other studies[9-13] and meta-analyses[17-19]. For example, Li et al[17] reported significantly worse CSS (HR = 1.813) and RFS (HR = 2.102) in colorectal cancer patients with elevated pretreatment NLRs in a meta-analysis of 16 studies comprising 19 cohorts. Viers et al[13] reviewed 899 patients treated with radical cystectomy for urothelial carcinoma of the bladder and also found greater risks of recurrence (HR = 1.04) and cancer-related death (HR = 1.04). In addition, our findings indicate that elevated NLR correlates with pT status, suggesting that increased NLR favors ESCC tumor invasion. Similarly, previous studies have reported an association between preoperative serum NLR and pT status in gastric cancer and urothelial carcinoma of the bladder[11,13].
Although the mechanism underlying these findings is complex and has yet to be elucidated, the association between inflammation and cancer provides us with some clues. In cancer cells, oncogenes activate certain inflammatory transcriptional programs to produce various inflammatory mediators, including granulocyte and granulocyte-macrophage colony-stimulating factors[20], which can trigger the proliferation and differentiation of neutrophils in bone marrow[21]. Moreover, previous studies suggest that neutrophils from cancer patients are functionally different from those from healthy counterparts[22,23], and can facilitate tumor progression and affect patient outcome[24,25]. These neutrophils may inhibit T cells[26-28], which have anti-tumor activities and favorable prognostic significance[29,30]. These neutrophils may also secrete agents that promote tumor cellular proliferation, angiogenesis, invasion, and metastasis[31]. Consistent with these observations, studies have shown reduced tumor progression with inhibition of neutrophil infiltration[32,33].
The results of this study further demonstrate that the adverse effects of NLR on CSS and RFS are greatest in patients with stage IIIA disease. This result suggests that NLR is more sensitive as a prognostic factor in intermediate stage ESCC than in the other subgroups. However, studies with larger sample sizes are warranted to confirm these findings. The fact that the majority of the patients in our study with NLR > 3.0 had shorter CSS and RFS indicates that surgical resection alone was not sufficient. Preoperative serum NLR may facilitate the selection of patients with a high risk of disease recurrence and cancer-specific mortality to receive neoadjuvant or adjuvant therapy. However, further investigations evaluating the effect of systemic therapies with regard to NLR are necessary.
Although the results reveal the prognostic value of preoperative serum NLR, we acknowledge that there are some limitations of this study. Patients with stage 0, IA, an IV and pT4 disease were not included in the analyses. Future studies are warranted to confirm these preliminary results in all pTNM stages and to explore the optimal cutoff point for NLR, as well as the mechanism behind these phenomena, which would aid in the development of more effective disease management.
In conclusion, this study shows that elevated preoperative serum NLR is associated with tumor progression and represents a negative prognostic factor in patients who undergo esophagectomy for ESCC without neoadjuvant treatment, particularly those with stage IIIA disease. NLR measurement is routine and repeatable, and may be a useful adjunct to conventional TNM staging for future clinical trial enrollment and individualized treatments.
COMMENTS
Background
The interaction between cancer and the host inflammatory system has been increasingly recognized. The serum neutrophil-lymphocyte ratio (NLR) is a routine marker of systemic inflammation, and its prognostic value has been well studied in several cancer types. In this study, the prognostic value of the NLR was evaluated with long-term follow-up in a large cohort of patients with esophageal squamous cell carcinoma (ESCC), the primary pathological type of esophageal cancer.
Research frontiers
The use of new prognostic parameters can help improve risk stratification of cancer patients in order to instruct individualized treatment, which can improve the overall outcome in cancer patients.
Innovations and breakthroughs
With a relatively large cohort of ESCC patients who underwent esophagectomy without neoadjuvant treatment, we determined that elevated preoperative serum NLR is a negative prognostic factor. Moreover, we further examined the predictive effect of NLR with regard to pathological tumor-node-metastasis (TNM) staging, and found that the adverse effects of NLR were greatest in patients with stage IIIA ESCC.
Applications
As the measurement of serum NLR is inexpensive, routine, and repeatable, it may be a useful adjunct to conventional TNM staging that will instruct clinical trial enrollment and individualized treatment in the future.
Peer-review
This is a good study with a well contemplated statistical analysis. Giver the fact that literature data considering the preoperative serum neutrophil to lymphocyte ratio in SCC is scarce the present manuscript merits publication after a minor revision.
Footnotes
Supported by Research funding from the Science and Technology Planning Project of Guangdong Province, China, No. 2012B031800462 (to Zhang X); and the Sun Yat-Sen University Clinical Research 5010 Program (to Lin P).
Ethics approval: The study was reviewed and approved by the Sun Yat-Sen University Cancer Center Institutional Review Board.
Informed consent: All study participants, or their legal guardian, provided informed written consent prior to inclusion in the study.
Conflict-of-interest: None.
Data sharing: No additional data are available.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: November 19, 2014
First decision: December 26, 2014
Article in press: February 11, 2015
P- Reviewer: Sgourakis G S- Editor: Ma YJ L- Editor: Kerr C E- Editor: Ma S
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Rice TW, Blackstone EH, Rusch VW. 7th edition of the AJCC Cancer Staging Manual: esophagus and esophagogastric junction. Ann Surg Oncol. 2010;17:1721–1724. doi: 10.1245/s10434-010-1024-1. [DOI] [PubMed] [Google Scholar]
- 3.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 4.Baniyash M, Sade-Feldman M, Kanterman J. Chronic inflammation and cancer: suppressing the suppressors. Cancer Immunol Immunother. 2014;63:11–20. doi: 10.1007/s00262-013-1468-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jackson JR, Seed MP, Kircher CH, Willoughby DA, Winkler JD. The codependence of angiogenesis and chronic inflammation. FASEB J. 1997;11:457–465. [PubMed] [Google Scholar]
- 7.Jaiswal M, LaRusso NF, Burgart LJ, Gores GJ. Inflammatory cytokines induce DNA damage and inhibit DNA repair in cholangiocarcinoma cells by a nitric oxide-dependent mechanism. Cancer Res. 2000;60:184–190. [PubMed] [Google Scholar]
- 8.Roxburgh CS, McMillan DC. Role of systemic inflammatory response in predicting survival in patients with primary operable cancer. Future Oncol. 2010;6:149–163. doi: 10.2217/fon.09.136. [DOI] [PubMed] [Google Scholar]
- 9.McNamara MG, Templeton AJ, Maganti M, Walter T, Horgan AM, McKeever L, Min T, Amir E, Knox JJ. Neutrophil/lymphocyte ratio as a prognostic factor in biliary tract cancer. Eur J Cancer. 2014;50:1581–1589. doi: 10.1016/j.ejca.2014.02.015. [DOI] [PubMed] [Google Scholar]
- 10.Sarraf KM, Belcher E, Raevsky E, Nicholson AG, Goldstraw P, Lim E. Neutrophil/lymphocyte ratio and its association with survival after complete resection in non-small cell lung cancer. J Thorac Cardiovasc Surg. 2009;137:425–428. doi: 10.1016/j.jtcvs.2008.05.046. [DOI] [PubMed] [Google Scholar]
- 11.Shimada H, Takiguchi N, Kainuma O, Soda H, Ikeda A, Cho A, Miyazaki A, Gunji H, Yamamoto H, Nagata M. High preoperative neutrophil-lymphocyte ratio predicts poor survival in patients with gastric cancer. Gastric Cancer. 2010;13:170–176. doi: 10.1007/s10120-010-0554-3. [DOI] [PubMed] [Google Scholar]
- 12.Stotz M, Gerger A, Eisner F, Szkandera J, Loibner H, Ress AL, Kornprat P, AlZoughbi W, Seggewies FS, Lackner C, et al. Increased neutrophil-lymphocyte ratio is a poor prognostic factor in patients with primary operable and inoperable pancreatic cancer. Br J Cancer. 2013;109:416–421. doi: 10.1038/bjc.2013.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Viers BR, Boorjian SA, Frank I, Tarrell RF, Thapa P, Karnes RJ, Thompson RH, Tollefson MK. Pretreatment neutrophil-to-lymphocyte ratio is associated with advanced pathologic tumor stage and increased cancer-specific mortality among patients with urothelial carcinoma of the bladder undergoing radical cystectomy. Eur Urol. 2014;66:1157–1164. doi: 10.1016/j.eururo.2014.02.042. [DOI] [PubMed] [Google Scholar]
- 14.Feng JF, Huang Y, Liu JS. Combination of neutrophil lymphocyte ratio and platelet lymphocyte ratio is a useful predictor of postoperative survival in patients with esophageal squamous cell carcinoma. Onco Targets Ther. 2013;6:1605–1612. doi: 10.2147/OTT.S52501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rashid F, Waraich N, Bhatti I, Saha S, Khan RN, Ahmed J, Leeder PC, Larvin M, Iftikhar SY. A pre-operative elevated neutrophil: lymphocyte ratio does not predict survival from oesophageal cancer resection. World J Surg Oncol. 2010;8:1. doi: 10.1186/1477-7819-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharaiha RZ, Halazun KJ, Mirza F, Port JL, Lee PC, Neugut AI, Altorki NK, Abrams JA. Elevated preoperative neutrophil: lymphocyte ratio as a predictor of postoperative disease recurrence in esophageal cancer. Ann Surg Oncol. 2011;18:3362–3369. doi: 10.1245/s10434-011-1754-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li MX, Liu XM, Zhang XF, Zhang JF, Wang WL, Zhu Y, Dong J, Cheng JW, Liu ZW, Ma L, et al. Prognostic role of neutrophil-to-lymphocyte ratio in colorectal cancer: a systematic review and meta-analysis. Int J Cancer. 2014;134:2403–2413. doi: 10.1002/ijc.28536. [DOI] [PubMed] [Google Scholar]
- 18.Wei Y, Jiang YZ, Qian WH. Prognostic role of NLR in urinary cancers: a meta-analysis. PLoS One. 2014;9:e92079. doi: 10.1371/journal.pone.0092079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiao WK, Chen D, Li SQ, Fu SJ, Peng BG, Liang LJ. Prognostic significance of neutrophil-lymphocyte ratio in hepatocellular carcinoma: a meta-analysis. BMC Cancer. 2014;14:117. doi: 10.1186/1471-2407-14-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 21.Lord BI, Bronchud MH, Owens S, Chang J, Howell A, Souza L, Dexter TM. The kinetics of human granulopoiesis following treatment with granulocyte colony-stimulating factor in vivo. Proc Natl Acad Sci USA. 1989;86:9499–9503. doi: 10.1073/pnas.86.23.9499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dumitru CA, Moses K, Trellakis S, Lang S, Brandau S. Neutrophils and granulocytic myeloid-derived suppressor cells: immunophenotyping, cell biology and clinical relevance in human oncology. Cancer Immunol Immunother. 2012;61:1155–1167. doi: 10.1007/s00262-012-1294-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trellakis S, Farjah H, Bruderek K, Dumitru CA, Hoffmann TK, Lang S, Brandau S. Peripheral blood neutrophil granulocytes from patients with head and neck squamous cell carcinoma functionally differ from their counterparts in healthy donors. Int J Immunopathol Pharmacol. 2011;24:683–693. doi: 10.1177/039463201102400314. [DOI] [PubMed] [Google Scholar]
- 24.Donskov F. Immunomonitoring and prognostic relevance of neutrophils in clinical trials. Semin Cancer Biol. 2013;23:200–207. doi: 10.1016/j.semcancer.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 25.Dumitru CA, Lang S, Brandau S. Modulation of neutrophil granulocytes in the tumor microenvironment: mechanisms and consequences for tumor progression. Semin Cancer Biol. 2013;23:141–148. doi: 10.1016/j.semcancer.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 26.Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, Worthen GS, Albelda SM. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell. 2009;16:183–194. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hao S, Andersen M, Yu H. Detection of immune suppressive neutrophils in peripheral blood samples of cancer patients. Am J Blood Res. 2013;3:239–245. [PMC free article] [PubMed] [Google Scholar]
- 28.Schmielau J, Finn OJ. Activated granulocytes and granulocyte-derived hydrogen peroxide are the underlying mechanism of suppression of t-cell function in advanced cancer patients. Cancer Res. 2001;61:4756–4760. [PubMed] [Google Scholar]
- 29.Morgan RA, Dudley ME, Wunderlich JR, Hughes MS, Yang JC, Sherry RM, Royal RE, Topalian SL, Kammula US, Restifo NP, Zheng Z, Nahvi A, de Vries CR, Rogers-Freezer LJ, Mavroukakis SA, Rosenberg SA. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ogino S, Nosho K, Irahara N, Meyerhardt JA, Baba Y, Shima K, Glickman JN, Ferrone CR, Mino-Kenudson M, Tanaka N, et al. Lymphocytic reaction to colorectal cancer is associated with longer survival, independent of lymph node count, microsatellite instability, and CpG island methylator phenotype. Clin Cancer Res. 2009;15:6412–6420. doi: 10.1158/1078-0432.CCR-09-1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brandau S, Moses K, Lang S. The kinship of neutrophils and granulocytic myeloid-derived suppressor cells in cancer: cousins, siblings or twins? Semin Cancer Biol. 2013;23:171–182. doi: 10.1016/j.semcancer.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 32.Kazemfar K, Chen R, Nicholson K, Coppola D, Zhou JM, Chen X, Wei S, Blanck G. Combined IL-8 and TGF-beta blockade efficiently prevents neutrophil infiltrates into an A549-cell tumor. Immunol Lett. 2009;122:26–29. doi: 10.1016/j.imlet.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 33.Tazzyman S, Barry ST, Ashton S, Wood P, Blakey D, Lewis CE, Murdoch C. Inhibition of neutrophil infiltration into A549 lung tumors in vitro and in vivo using a CXCR2-specific antagonist is associated with reduced tumor growth. Int J Cancer. 2011;129:847–858. doi: 10.1002/ijc.25987. [DOI] [PubMed] [Google Scholar]


