Abstract
AIM: To explore the effects of platelet count (PLT) and 11 platelet-based indices on postoperative recurrence of hepatocellular carcinoma (HCC).
METHODS: We retrospectively analyzed 172 HCC patients who were treated by partial hepatectomy. Preoperative data, including laboratory biochemical results, were used to calculate the 11 indices included in the analysis. We performed receiver operating characteristic curve analysis to determine the optimal cut-off values for predicting recurrence. Cumulative rates of HCC recurrence were calculated using Kaplan-Meier survival curves and differences were analyzed by log-rank tests. Multivariate analyses were performed to identify independent predictors of recurrence, early recurrence (within one year after surgery), and late recurrence in HCC. To obtain better prognostic models, PLT-based indices were analyzed separately after being expressed as binary and continuous variables. Two platelet-unrelated, validated HCC prognostic models were included in the analyses as reference indices. Additional analyses were performed after patients were stratified based on hepatitis B virus infection status, cirrhosis, and tumor size to investigate the significance of platelets in different subgroups.
RESULTS: In the study cohort, 44.2% (76/172) of patients experienced HCC recurrence, and 50.6% (87/172) died during a median follow-up time of 46 mo. PLT and five of the 11 platelet-related models were significant predisposing factors for recurrence (P < 0.05). Multivariate analysis indicated that, among the clinical parameters, presence of ascites, PLT ≥ 148 × 109/L, alkaline phosphatase ≥ 116 U/L, and tumor size ≥ 5 cm were independently associated with a higher risk of HCC recurrence (P < 0.05). Independent and significant models included the aspartate aminotransferase/PLT index, fibrosis index based on the four factors, fibro-quotient, aspartate aminotransferase/PLT/γ-glutamyl transpeptidase/alpha-fetoprotein index, and the PLT/age/alkaline phosphatase/alpha-fetoprotein/aspartate aminotransferase index. There were different risk factors between early and late recurrences, and PLT and these indices were more inclined to influence late recurrence. PLT was only predictive of recurrence in non-cirrhotic HCC patients, and was not influenced by tumor size, which was a critical confounder in our study.
CONCLUSION: PLT and PLT-based noninvasive models are effective tools for predicting postoperative recurrence, especially late recurrence. Larger cohorts are needed to validate our findings.
Keywords: Alkaline phosphatase, Alpha-fetoprotein, Aspartate aminotransferase, Blood platelets, Hepatocellular carcinoma, Recurrence
Core tip: The high risk of postoperative recurrence is one of the greatest problems plaguing potential curative treatment for hepatocellular carcinoma (HCC). Although several prognostic models have been proposed for HCC, these indices mainly focus on non-modifiable tumor characteristics. In contrast, platelet count is an improvable variable, and there are numerous platelet-based models associated with cirrhosis and HCC formation. We found that platelet count and nearly half of the established platelet-related models were independently associated with postoperative recurrence. We also demonstrated different risk factors between early and late recurrences, with platelets more likely to influence late recurrence.
INTRODUCTION
Hepatocellular carcinoma (HCC) is the sixth most prevalent neoplasm and the third most frequent cause of cancer mortality[1]. Major risk factors include excessive alcohol intake and infection with hepatitis C virus (HCV) and/or hepatitis B virus (HBV)[2], which represents the most prevalent etiology for HCC and cirrhosis. Thus, the majority of HCC patients are from areas where there is a high prevalence of HBV infection, such as Asia and Africa[2,3]. Although the diagnosis and treatment of HCC have dramatically improved, the prognosis remains unsatisfactory with an overall 5-year survival rate of 5%-6%[4]. One reason for this involves the high risk for postoperative recurrence. Although microvascular invasion has been implicated as a major factor[5,6], the majority of postoperative tumor recurrences are due to de novo cancers from the cirrhotic liver[5]. The identification of related predisposing risk factors will help to reduce recurrence rates.
Survival in HCC has been associated with platelet count (PLT)[7-9], which is also an independent predictor of hepatocarcinogenesis[10-12]. Platelets are involved in thrombosis, inflammatory responses, liver regeneration[13-15], and the regulation of angiogenesis[15,16]. PLTs are significantly decreased in cirrhotic patients[17], and can be used, along with several platelet-based noninvasive models, to detect hepatic cirrhosis in patients with HBV/HCV infection with high accuracy[18-21]. Thus, we hypothesized that platelets might play a crucial role in HCC relapse. However, few studies have reported the association between PLT/platelet-based indices and postoperative recurrence in HCC. Herein, we evaluated PLT and 11 platelet-related indices for predicting HCC recurrence. For these analyses, the Cancer of the Liver Italian Program (CLIP)[22], aspartate aminotransferase/alanine aminotransferase ratio (AAR)[23], and two platelet-unrelated prognostic models of HCC were used as references.
MATERIALS AND METHODS
Study population
A total of 172 histologically proven HCC patients over 18 years of age who were treated by hepatic resection at our hospital between December 2002 and July 2012 were enrolled in this study. Complete clinical, laboratory and follow-up information was available for all patients. Patients were excluded from the study due to: (1) coexistent hematologic diseases; (2) previous treatment for HCC; (3) intrahepatic cholangiocarcinoma; and (4) extrahepatic spread. This study was in compliance with the provisions of the 2013 version of the Declaration of Helsinki[24], and the protocol was approved by the Ethics Committee of our hospital.
Data collection
Electronic medical records were used to collect information concerning patient age, sex, etiology (HBV, HCV), cirrhosis status, ascites, preoperative laboratory data [levels of alanine aminotransferase, aspartate aminotransferase (AST), alkaline phosphatase (ALP), γ-glutamyl transpeptidase (GGT), PLT, prothrombin time (international normalized ratio), and alpha-fetoprotein (AFP)], operation notes, tumor characteristics (number, diameter of the largest lesion, vascular invasion), and pathologic reports. The primary outcome measure for the study was HCC recurrence. The secondary outcomes were early (within one year) and late recurrences.
Noninvasive platelet scoring models
The following scoring models were used in this study: AAR; CLIP; AAR-PLT score[25]; Pohl et al[26] index; age/PLT index[27]; cirrhosis discriminant score[28]; AST/PLT ratio index[29]; fibrosis index based on the four factors (FIB-4)[30]; fibro-quotient (FibroQ)[31]; Lok et al[32] index; Goteburg university cirrhosis index[33]; AST/PLT/GGT/AFP index (APGA)[34]; and the PLT/age/ALP/AFP/AST index (PAPAS)[35]. Index scores were calculated based on the formulas presented in Table 1.
Table 1.
Scoring of noninvasive models
| Index | Formula |
| CLIP | Sum of: |
| Child-Pugh: A = 0, B = 1, C = 2 | |
| Tumor morphology: Uninodular and extension ≤ 50% = 0, multinodular and extension ≤ 50% = 1, massive or extension ≥ 50% = 2 | |
| AFP: < 400 = 0, ≥ 400 = 1 | |
| Portal vein thrombosis: no = 0, yes = 1 | |
| Pohl | Positive: AAR > 1 and PLT < 150 |
| AARP | Positive: AAR > 1 or PLT < 150 |
| AAR | AST/ALT |
| API | Sum of: |
| Age (yr): < 30 = 0, 30-39 = 1, 40-49 = 2, 50-59 = 3, 60-69 = 4, ≥ 70 = 5 | |
| PLT: ≥ 225 = 0, 200-224 = 1, 175-199 = 2, 150-174 = 3, 125-149 = 4, < 125 = 5 | |
| CDS | Sum of: |
| PLT: > 340 = 0, 280–339 = 1, 220-279 = 2, 160-219 = 3, 100-159 = 4, 40-99 = 5, < 40 = 6 | |
| ALT/AST: > 1.7 = 0, 1.2-1.7 = 1, 0.6-1.1 = 2, < 0.6 = 3 | |
| PT: < 1.1 = 0, 1.1-1.4 = 1, > 1.4 = 2 | |
| APRI | AST × 100/PLT |
| FIB-4 | Age × AST/PLT × ALT1/2 |
| FibroQ | 10 × Age × AST × PT/ALT × PLT |
| Lok | Lok index = exp (log odds)/[1 - exp (log odds)] |
| Log odds = -5.56-0089 × PLT + 1.26 × AAR + 5.27 × PT | |
| GUCI | Normalized AST × PT × 100/PLT |
| APGA | Log (index) = 1.44 + 0.1490 × log (GGT) + 0.3308 × log (AST) - 0.5846 log × (PLT) + 0.1148 × log (AFP + 1) |
| PAPAS | Log (index + 1) = 0.025 + 0.0031 × age + 0.1483 × log (ALP) + 0.004 × log (AST) + 0.0908 × log (AFP + 1) - 0.028 × log (PLT) |
AAR: Aspartate aminotransferase/alanine aminotransferase ratio; AARP: AAR-platelet count score; AFP: Alpha-fetoprotein; ALP: Alkaline phosphatase; ALT: Alanine aminotransferase; APGA: Aspartate aminotransferase/platelet count/γ-glutamyl transpeptidase/alpha-fetoprotein index; API: Age/platelet count index; APRI: Aspartate aminotransferase/platelet count ratio index; AST: Aspartate aminotransferase; CDS: Cirrhosis discriminant score; CLIP: Cancer of the Liver Italian Program; FIB-4: Fibrosis index based on the four factors; FibroQ: Fibro-quotient; GGT: γ-glutamyl transpeptidase; GUCI: Goteburg University Cirrhosis Index; PAPAS: Platelet count/age/ALP/AFP/AST index; PLT: Platelet count; PT: Prothrombin time. AFP (ng/mL); Age (year); ALT (U/L); AST (U/L); GGT (U/L); PLT (109/L); PT (international normalized ratio).
HCC diagnosis, treatment and follow-up
HCC was initially diagnosed by computed tomography, magnetic resonance imaging and/or ultrasound findings. All patients underwent hepatectomy by either anatomic or non-anatomic resection on the basis of preoperative hepatic function and tumor characteristics. HCC diagnosis was confirmed by pathology of resected tumor tissues.
After discharge, all patients were regularly followed at outpatient clinics, by either computed tomography or magnetic resonance imaging, physical examination, abdominal ultrasound, chest X-ray, and serologic tests (including liver function and AFP level). The same evaluations were performed as follow-ups every 3 mo for the first year, every 4 mo for the second year, and every 6 mo thereafter. Recurrence was determined based on the emergence of clinical, radiologic, and/or pathologic evidence of HCC. Patients who had recurrence received salvage treatments, including further hepatic surgery, percutaneous ablation, or transcatheter arterial chemoembolization, as appropriate.
Statistical analysis
All statistical analyses were performed using PASW Statistics for Windows (version 18.0 software; SPSS Inc., Chicago, IL, United States). Student’s t and χ2 tests were used to compare continuous and categorical variables, respectively. The receiver operating characteristic curve was calculated to determine the optimal cut-off point (with the highest cumulative value of the sum of specificity and sensitivity) of each variable for detecting recurrence. Cumulative recurrence rates were estimated by the Kaplan-Meier method and differences were analyzed by log-rank tests. All variables found to be significant (P < 0.05) were then entered into a multivariate analysis with Cox proportional hazard regression models. Normally distributed (P > 0.05 from a Kolmogorov-Smirnov test) continuous variables are expressed as mean ± standard deviation (SD), otherwise they are presented as median (range).
RESULTS
Patient characteristics
The study population consisted of 139 men and 33 women with an overall mean age of 53.5 ± 10.5 years. Of these, 121 patients were infected with HBV and 59 were cirrhotic. The median survival time after surgical resection was 52 mo, with 1-, 3- and 5-year overall survival rates of 74.1%, 54.4% and 46.6%, respectively. During a median follow-up of 46 mo, 50.6% (87/172) of the patients died and 44.2% (76/172) developed recurrence. Demographic data, serologic tests, tumor characteristics and index scores of patients stratified by recurrence are summarized in Table 2. Of all the variables, only tumor size and CLIP score were significantly different between recurrent and non-recurrent patients.
Table 2.
Characteristics of patients stratified according to recurrence status
| Parameter | Recurrence | No recurrence | P value |
| (n = 76) | (n = 96) | ||
| Sex, male/female | 63/13 | 76/20 | 0.537 |
| Age, yr | 54.6 ± 10.3 | 52.7 ± 10.6 | 0.231 |
| Etiology, yes/no | |||
| HBV | 51/25 | 70/26 | 0.407 |
| Cirrhosis | 23/53 | 36/60 | 0.321 |
| Ascites | 11/65 | 6/90 | 0.073 |
| Laboratory results in U/L | |||
| ALT | 49 (11-1315) | 39 (7-1436) | 0.291 |
| AST | 50 (16-1075) | 40 (11-1493) | 0.308 |
| ALP | 111 (30-732) | 89 (36-867) | 0.149 |
| GGT | 80 (12-623) | 62 (14-914) | 0.084 |
| PLT as 109/L | 136 (41-370) | 117 (3-486) | 0.055 |
| PT (INR) | 1.06 (0.82-1.63) | 1.06 (0.73-1.62) | 0.697 |
| AFP, ≥ 200/< 200 ng/mL | 38/32 | 36/50 | 0.122 |
| Tumor | |||
| Size, ≥ 5/< 5 cm | 57/19 | 37/59 | < 0.001 |
| Type, multiple/single | 18/58 | 14/82 | 0.128 |
| Vascular invasion, yes/no | 8/68 | 6/90 | 0.308 |
| Noninvasive model score | |||
| CLIP | 1 (0-4) | 1 (0-4) | 0.027 |
| Pohl, negative/positive | 47/39 | 60/36 | 0.930 |
| AARP, negative/positive | 14/62 | 11/85 | 0.198 |
| AAR | 1.08 (0.13-3.29) | 1.02 (0.09-4.85) | 0.826 |
| API | 7 (1-10) | 7 (2-10) | 0.319 |
| CDS | 6 (2-9) | 6 (3-9) | 0.286 |
| APRI | 0.90 (0.16-19.71) | 0.90 (0.17-55.71) | 0.658 |
| FIB-4 | 2.90 (0.51-17.98) | 2.94 (0.40-81.57) | 0.384 |
| FibroQ | 4.68 (0.48-22.82) | 4.49 (0.14-108.25) | 0.224 |
| Lok index | 0.56 ± 0.23 | 0.58 ± 0.22 | 0.600 |
| GUCI | 1.04 (0.14-19.32) | 0.95 (0.17-83.56) | 0.624 |
| APGA | 22.95 (6.03-89.35) | 19.03 (5.81-92.94) | 0.136 |
| PAPAS | 3.67 (1.52-9.40) | 3.02 (1.67-5.75) | 0.136 |
| Survival in mo | 23 (5-120) | 87 (1-117) | 0.003 |
| Death, yes/no | 69/7 | 18/78 | < 0.001 |
AAR: Aspartate aminotransferase/alanine aminotransferase ratio; AARP: AAR-platelet count score; AFP: Alpha-fetoprotein; ALP: Alkaline phosphatase; ALT: Alanine aminotransferase; APGA: Aspartate aminotransferase/platelet count/γ-glutamyl transpeptidase/AFP index; API: Age/platelet count index; APRI: Aspartate aminotransferase/platelet count ratio index; AST: Aspartate aminotransferase; CDS: Cirrhosis discriminant score; CLIP: Cancer of the Liver Italian Program; FIB-4: Fibrosis index based on the four factors; FibroQ: Fibro-quotient; GGT: γ-glutamyl transpeptidase; GUCI: Goteburg University Cirrhosis Index; HBV: Hepatitis B virus; INR: International normalized ratio; PAPAS: Platelet count/age/ALP/AFP/AST index; PLT: Platelet count; PT: Prothrombin time. Values are expressed as mean ± SD, median (range), or n.
Determination of cut-off values for continuous variables
The receiver operating characteristic curve of PLT indicated that 148 × 109/L was a cut-off value, as it corresponded to the maximal sum of sensitivity plus specificity. Among the indices, only ALP [area under the curve (AUC) = 0.618, 95%CI: 0.528-0.708], CLIP (AUC = 0.636, 95%CI: 0.535-0.712), and PAPAS (AUC = 0.636, 95%CI: 0.548-0.724) were significant indicators for determining recurrence (P < 0.05) (Figure 1). A PAPAS cutoff of 2.41 presented a sensitivity of 85.5% and a specificity of 38.4%.
Figure 1.
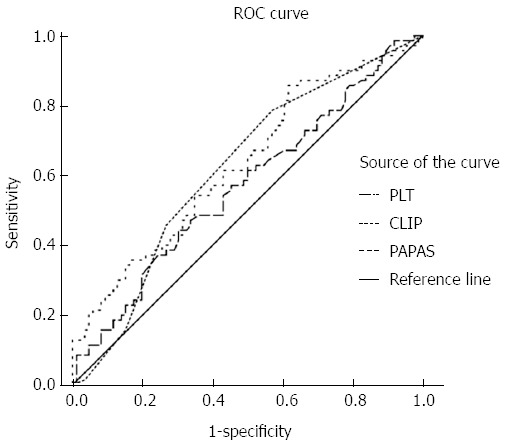
Predictive potentials for detecting recurrence. Receiver operating characteristic (ROC) curves for Cancer of the Liver Italian Program (CLIP) and platelet count/age/alkaline phosphatase/alpha-fetoprotein/aspartate aminotransferase (PAPAS) indices and platelet count (PLT).
Predictors of tumor recurrence
Figure 2A and B show the Kaplan-Meier cumulative recurrence and survival curves of the entire patient cohort. A log-rank analysis demonstrated that patients with ascites, AST ≥ 55 U/L, ALP ≥ 116 U/L, GGT ≥ 144 U/L, PLT ≥ 148 × 109/L, AFP ≥ 96 ng/mL, and tumor size ≥ 5 cm had an elevated probability of postoperative recurrence (Table 3). Scatter plots reveal the relationship between PLT and recurrence and overall survival. In addition, the boxplots further reflect the relations between platelets and postoperative recurrence (Figure 2C) as well as survival (Figure 2D). Among the 13 noninvasive indices, CLIP ≥ 1, AAR ≥ 1.07, APRI ≥ 1.94, FIB-4 ≥ 4.30, FibroQ ≥ 6.36, APGA ≥ 26.9, and PAPAS ≥ 2.41 were significantly associated with a high risk of relapse. Figure 3 shows the Kaplan-Meier cumulative recurrence curves stratified according to PLT and five of these platelet-based indices.
Figure 2.
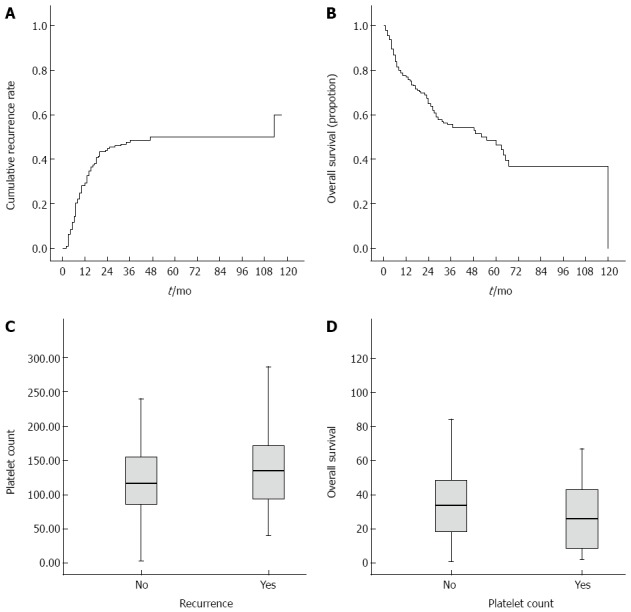
Survival and recurrence. Kaplan-Meier curves for cumulative recurrence (A) and overall survival (B). Boxplots comparing platelet count with recurrence (C) and overall survival (D).
Table 3.
Predictors of recurrence stratified according to recurrence time by Log-rank test
| Variable |
Recurrence, P value |
||
| Overall | Early | Late | |
| Men | 0.726 | 0.768 | 0.848 |
| Age ≥ 62 yr | 0.080 | 0.006 | 0.530 |
| HBV | 0.143 | 0.046 | 0.875 |
| Cirrhosis | 0.259 | 0.134 | 0.957 |
| Ascites | 0.022 | 0.219 | 0.028 |
| ALT ≥ 44 U/L | 0.086 | 0.118 | 0.429 |
| AST ≥ 55 U/L | < 0.001 | 0.005 | 0.001 |
| ALP ≥ 116 U/L | < 0.001 | 0.011 | 0.001 |
| GGT ≥ 144 U/L | < 0.001 | 0.004 | 0.007 |
| PLT ≥ 148 × 109/L | 0.021 | 0.376 | 0.009 |
| PT (INR) ≥ 1.17 | 0.398 | 0.390 | 0.792 |
| AFP ≥ 96 ng/mL | 0.003 | 0.005 | 0.246 |
| AFP ≥ 200 ng/mL | 0.008 | 0.011 | 0.316 |
| Tumor size ≥ 5 cm | < 0.001 | < 0.001 | 0.001 |
| Multiple tumors | 0.125 | 0.169 | 0.471 |
| Vascular invasion | 0.063 | 0.205 | 0.150 |
| CLIP ≥ 1 | < 0.001 | 0.001 | 0.116 |
| CLIP ≥ 2 | < 0.001 | < 0.001 | 0.386 |
| Positive Pohl | 0.748 | 0.683 | 0.993 |
| Positive AARP | 0.181 | 0.120 | 0.861 |
| AAR ≥ 1.07 | 0.048 | 0.491 | 0.021 |
| API ≥ 6 | 0.771 | 0.402 | 0.575 |
| CDS ≥ 8 | 0.678 | 0.972 | 0.466 |
| APRI ≥ 1.94 | 0.022 | 0.053 | 0.213 |
| APRI ≥ 0.56 | 0.080 | 0.054 | 0.665 |
| FIB-4 ≥ 4.3 | 0.044 | 0.130 | 0.152 |
| FibroQ ≥ 6.36 | 0.025 | 0.131 | 0.084 |
| Lok ≥ 0.33 | 0.836 | 0.698 | 0.870 |
| Lok ≥ 0.70 | 0.078 | 0.592 | 0.026 |
| GUCI ≥ 2.24 | 0.050 | 0.318 | 0.050 |
| GUCI ≥ 0.65 | 0.125 | 0.088 | 0.757 |
| APGA ≥ 26.9 | < 0.001 | < 0.001 | 0.333 |
| PAPAS ≥ 2.41 | < 0.001 | 0.002 | 0.034 |
| PAPAS ≥ 3.62 | < 0.001 | 0.001 | 0.024 |
AAR: Aspartate aminotransferase/alanine aminotransferase ratio; AARP: AAR-platelet count score; AFP: Alpha-fetoprotein; ALP: Alkaline phosphatase; ALT: Alanine aminotransferase; APGA: Aspartate aminotransferase/platelet count/γ-glutamyl transpeptidase/AFP index; API: Age/platelet count index; APRI: Aspartate aminotransferase/platelet count ratio index; AST: Aspartate aminotransferase; CDS: Cirrhosis discriminant score; CLIP: Cancer of the Liver Italian Program; FIB-4: Fibrosis index based on the four factors; FibroQ: Fibro-quotient; GGT: γ-glutamyl transpeptidase; GUCI: Goteburg University Cirrhosis Index; HBV: Hepatitis B virus; INR: International normalized ratio; PAPAS: Platelet count/age/ALP/AFP/AST index; PLT: Platelet count; PT: Prothrombin time.
Figure 3.
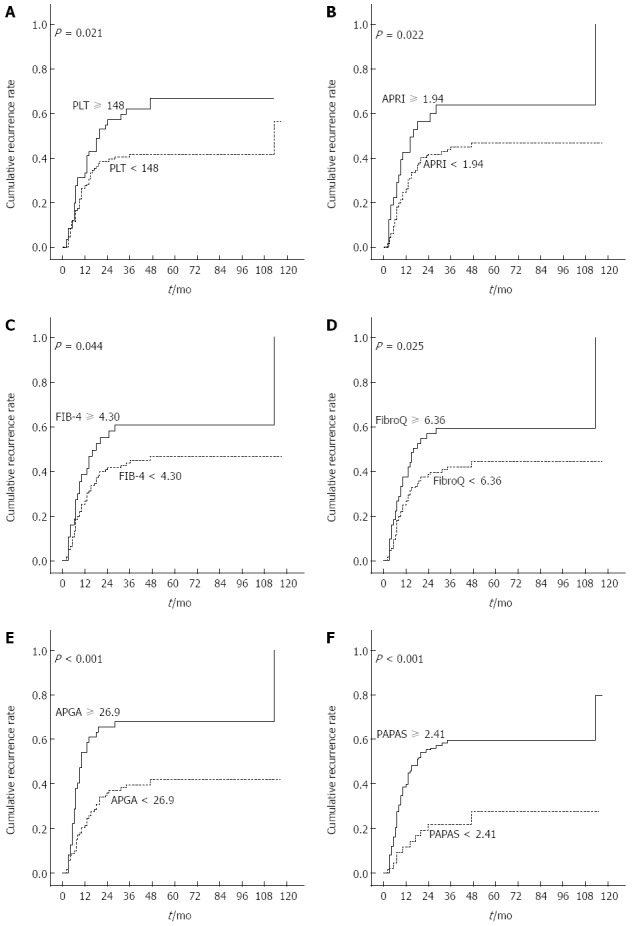
Cumulative recurrence. Kaplan-Meier curves of patients stratified according to A: Platelet count (PLT); B: Aspartate aminotransferase/platelet count ratio (APRI) index; C: Fibrosis index based on the four factors (FIB-4); D: Fibro-quotient (FibroQ); E: Aspartate aminotransferase/platelet count/γ-glutamyl transpeptidase/alpha-fetoprotein (APGA) index; F: Platelet count/age/alkaline phosphatase/alpha-fetoprotein/aspartate aminotransferase (PAPAS) index.
Multivariate analysis of biochemical variables expressed as binary variables revealed that ascites, ALP, PLT, and tumor size were independent risk factors for recurrence (Figure 4A). Among the noninvasive indices, multivariate analysis performed after adjusting for the seven predictive factors showed that APRI, FIB-4, FibroQ, APGA, and PAPAS were independent predictors for recurrence (Figure 4B). The two platelet-unrelated indices, CLIP and AAR, were not independently associated with recurrence. Further assessment of the noninvasive indices expressed as continuous variables (with original scores that were not converted into binary data) revealed that only APGA and PAPAS were independently related to recurrence (Figure 5).
Figure 4.
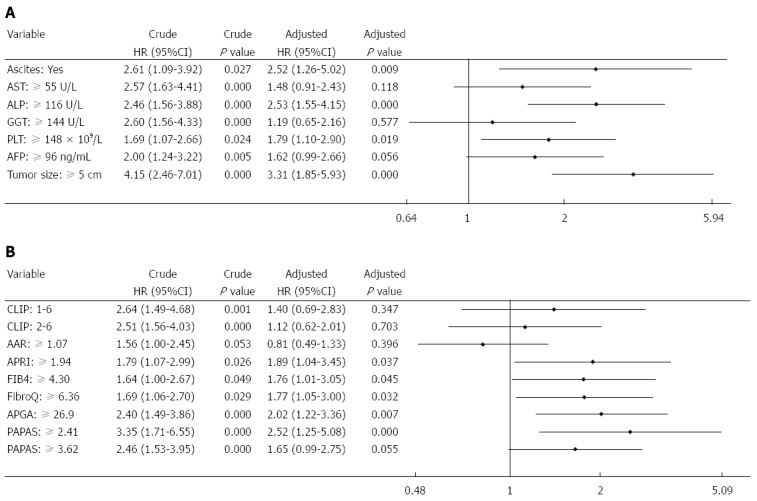
Multivariate analysis of prognostic indicators expressed as binary variables. A: Clinical and biochemical parameters; B: Noninvasive indices. AAR: Aspartate aminotransferase/alanine aminotransferase ratio; AFP: Alpha-fetoprotein; ALP: Alkaline phosphatase; APGA: Aspartate aminotransferase/platelet count/γ-glutamyl transpeptidase/alpha-fetoprotein index; APRI: Aspartate aminotransferase/platelet count ratio index; AST: Aspartate aminotransferase; CLIP: Cancer of the Liver Italian Program; FIB-4: Fibrosis index based on the four factors; FibroQ: Fibro-quotient; GGT: γ-glutamyl transpeptidase; HR: Hazard ratio; PAPAS: Platelet count/age/alkaline phosphatase/alpha- fetoprotein/aspartate aminotransferase index; PLT: Platelet count.
Figure 5.
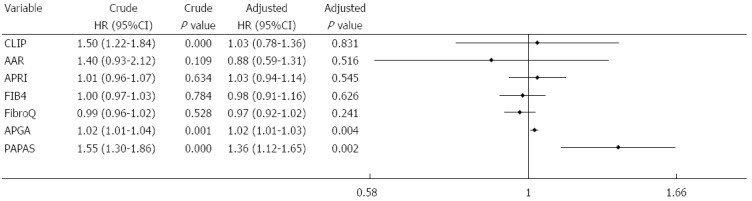
Multivariate analysis of noninvasive indices expressed as continuous variables. AAR: Aspartate aminotransferase/alanine aminotransferase ratio; APGA: Aspartate aminotransferase/platelet count/γ-glutamyl transpeptidase/alpha- fetoprotein index; APRI: Aspartate aminotransferase/platelet count ratio index; CLIP: Cancer of the Liver Italian Program; FIB-4: Fibrosis index based on the four factors; FibroQ: Fibro-quotient; HR: Hazard ratio; PAPAS: Platelet count/age/alkaline phosphatase/alpha-fetoprotein/aspartate aminotransferase index.
Predictors of early or late recurrence
Forty-six of the cases of recurrence occurred within 1 year of surgery. Factors significantly contributing to this early recurrence included elder age, HBV negativity, tumor size ≥ 5 cm, high AST, ALP, GGT and AFP levels, and elevated CLIP, APGA and PAPAS scores. Multivariate analysis showed that age, HBV infection, AFP, tumor size, and APGA were independent, valuable tools for predicting early recurrence (Figure 6). There were 30 late recurrences, and a log-rank test identified ascites, AST, ALP, GGT, PLT, tumor size, AAR, Lok index, and PAPAS score as associated factors. Among these, ALP, PLT, tumor size, Lok index, and PAPAS were significant independent predictors of late recurrence as determined by multivariate analysis.
Figure 6.
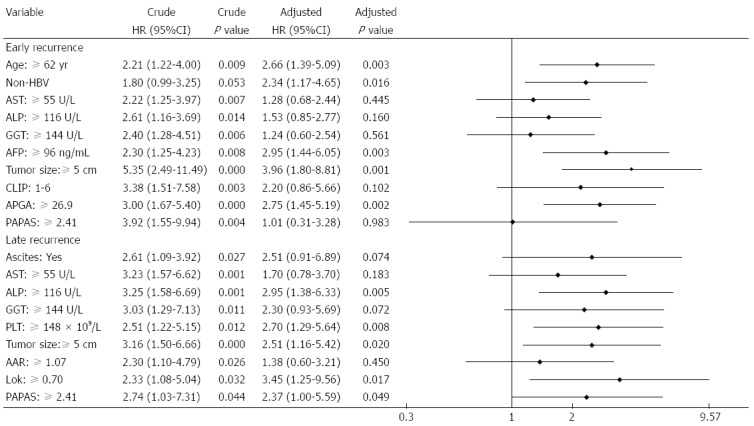
Predictors of early and late recurrences by multivariate analysis. AAR: Aspartate aminotransferase/alanine aminotransferase ratio; AFP: Alpha-fetoprotein; ALP: Alkaline phosphatase; APGA: Aspartate aminotransferase/platelet count/γ-glutamyl transpeptidase/AFP index; AST: Aspartate aminotransferase; CLIP: Cancer of the Liver Italian Program; GGT: γ-glutamyl transpeptidase; HBV: Hepatitis B virus; HR: Hazard ratio; PAPAS: Platelet count/age/alkaline phosphatase/alpha-fetoprotein/aspartate aminotransferase index; PLT: Platelet count.
Relationship between clinicopathologic features and PLT, APGA, and PAPAS status
As APGA and PAPAS were better tools to predict recurrence, we assessed their associations with several clinicopathologic features (Table 4). Patients with APGA ≥ 26.9 or PAPAS ≥ 2.41 had a significantly higher recurrence rate and larger tumors in comparison with patients with lower scores (P < 0.05).
Table 4.
Comparison of clinicopathologic features
| Variable |
PLT |
APGA |
PAPAS |
||||||
| < 148 × 109/L | ≥ 148 × 109/L | P value | < 26.9 | ≥ 26.9 | P value | < 2.41 | ≥ 2.41 | P value | |
| (n = 111) | (n = 61) | (n = 105) | (n = 51) | (n = 43) | (n = 113) | ||||
| HBV, yes/no | 82/29 | 39/22 | 0.172 | 75/30 | 38/13 | 0.686 | 32/11 | 81/32 | 0.732 |
| Sex, male/female | 90/21 | 49/12 | 0.904 | 85/20 | 42/9 | 0.833 | 36/7 | 91/22 | 0.647 |
| Median age in year | 52.6 | 55.2 | 0.115 | 54 | 52.1 | 0.263 | NA | NA | NA |
| Ascites, yes/no | 8/103 | 9/52 | 0.113 | 12/93 | 4/47 | 0.489 | 5/38 | 11/102 | 0.958 |
| Cirrhosis, yes/no | 43/68 | 16/45 | 0.098 | 33/72 | 21/30 | 0.230 | 15/28 | 39/74 | 0.965 |
| Tumor size, ≥ 5/< 5 cm | 55/56 | 39/22 | 0.070 | 51/54 | 38/13 | 0.002 | 14/29 | 75/38 | < 0.001 |
| Tumor type, multiple/single | 19/92 | 13/48 | 0.499 | 19/84 | 11/40 | 0.606 | 4/39 | 26/87 | 0.052 |
| Vascular invasion, yes/no | 11/100 | 3/58 | 0.252 | 7/98 | 6/45 | 0.440 | 2/41 | 11/102 | 0.482 |
| Recurrence, yes/no | 43/68 | 33/28 | 0.052 | 39/66 | 31/20 | 0.005 | 10/33 | 60/53 | 0.001 |
| Median survival in mo | 37.5 | 29.5 | 0.050 | 37.3 | 27.9 | 0.026 | 43.4 | 30.8 | 0.006 |
APGA: Aspartate aminotransferase/platelet count/γ-glutamyl transpeptidase/alpha-fetoprotein index; HBV: Hepatitis B virus; NA: Not applicable; PAPAS: Platelet count/age/alkaline phosphatase/alpha-fetoprotein/aspartate aminotransferase index; PLT: Platelet count.
Subgroup analysis according to presence of cirrhosis and tumor size
Previous studies showed that PLT and platelet-based indices were significantly associated with probable cirrhosis in patients with HBV/HCV. To clarify the subgroups of patients negatively influenced by preoperative PLT, APGA and PAPAS scores, patients were classified according to presence of cirrhosis irrespective of HBV infection. Interestingly, recurrence rates were significantly higher in non-cirrhotic patients with PLT ≥ 148 × 109/L, APGA ≥ 26.9 and PAPAS ≥ 2.41 (Ps < 0.05), but not in those with cirrhosis (Figure 7). In patients with cirrhosis, PLT values were not indicative of recurrence rates.
Figure 7.
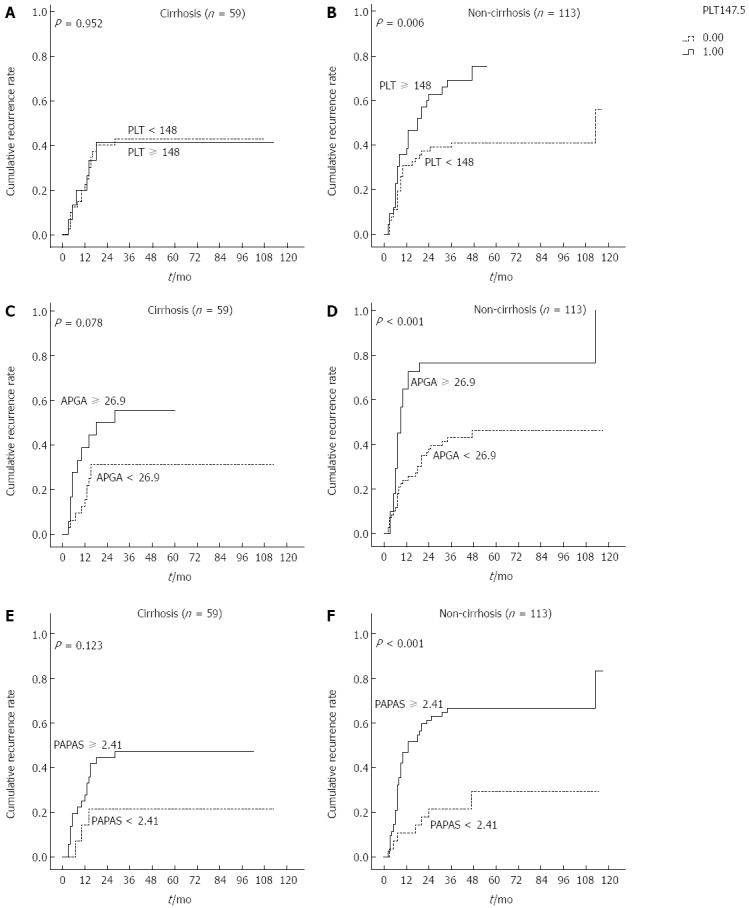
Cumulative recurrence curves of cirrhotic and non-cirrhotic patients. Kaplan-Meier curves of patients stratified according to A, B: Platelet count (PLT); C, D: Aspartate aminotransferase/PLT/γ-glutamyl transpeptidase/alpha-fetoprotein (APGA) index; E, F: PLT/age/alkaline phosphatase/alpha-fetoprotein/aspartate aminotransferase (PAPAS) index.
Tumor size was identified in our analyses as a possible predictor of tumor recurrence. Therefore, we investigated whether this factor influenced PLT and platelet-based models. Results show that PLT, PAGA, and PAPAS were all useful indictors for patients with large and small tumors (P < 0.05) (Figure 8). Further analyses revealed that cirrhosis and tumor size were not associated, and non-cirrhotic patients had a higher rate of elevated PLT in comparison with cirrhotic patients (39.8% vs 27.1%).
Figure 8.
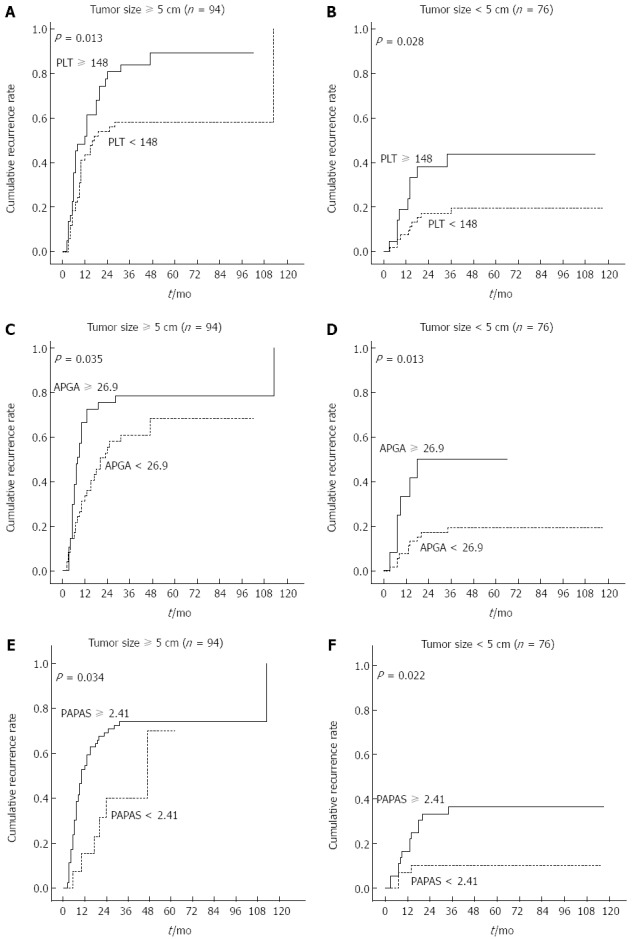
Cumulative recurrence curves of patients with large (≥ 5 cm) and small (< 5 cm) tumors. Kaplan-Meier curves of patients stratified according to A, B: Platelet count (PLT); C, D: Aspartate aminotransferase/PLT/γ-glutamyl transpeptidase/alpha-fetoprotein (APGA) index; E, F: PLT/age/alkaline phosphatase/alpha- fetoprotein/aspartate aminotransferase (PAPAS) index.
DISCUSSION
The high risk of recurrence is viewed as one of the greatest concerns plaguing HCC treatment[5]. The recurrence probability after curative therapy was 44.2% in our current study, within the reported range of 50%-100%[36]. Thus, it is crucial to identify the predisposing factors for recurrence and improve them before treatment. The roles of PLT and platelet-relative models for evaluating such factors were highlighted in this retrospective evaluation of 172 patients. The results confirmed that tumor size, ascites, PLT and ALP were independent prognostic factors of overall recurrence. We identified APRI, FIB-4, FibroQ, APGA, and PAPAS as independent predictors of recurrence.
Due to portal hypertension, a decrease in thrombopoietin production[17,37], and capture by the liver[13], PLT significantly decreases in cirrhotic patients[17]. Numerous observational studies have shown that thrombocytopenia is a major risk factor for the development of cirrhosis[18,20,38]. Moreover, PLT is associated with HCC[39], and several platelet-based indices have been identified as predictors of HCC formation. Tamaki et al[40] reported that an FIB-4 > 3.25 independently increased the risk of developing HCC by a factor of 1.7. However, it is unclear whether platelets accelerate or impede HCC occurrence, as Sitia et al[41] suggested that management of immune-mediated chronic HBV in an animal model with antiplatelet drugs, such as aspirin and clopidogrel, could prevent hepatocarcinogenesis.
The association between platelets and survival in HCC remains inconsistent and disputable as well. Buergy et al[42] demonstrated that an elevated PLT level at the time of diagnosis was associated with a shorter survival in many solid tumors, including lung cancer, pancreatic adenocarcinoma, gastric cancer, and HCC. Nouso et al[43] recruited 157 HCC patients with Child-Pugh class C cirrhosis and found that a PLT level > 80 × 109/L was an independent predictor of poor overall survival. In contrast, additional studies highlighted that thrombocytopenia/low PLT level adversely affected survival in HCC patients who received liver resection[44-47]. However, few studies have focused on the influence of PLT on postoperative recurrence. Although Amano et al[45] showed that decreased PLT was associated with a higher risk of recurrence in HCC, our study indicated that patients with a PLT level ≥ 148 × 109/L had a higher recurrence probability.
As prognosis of HCC remains far from satisfactory owing to the high incidence of tumor recurrence, several HCC prognostic models (such as CLIP[48-51]) have been proposed. However, these models mainly focus on non-modifiable tumor characteristics. Furthermore, tumor size and vascular invasion are not significant predictors of survival in HCC[52]. Thus, these models have certain limitations and there is an urgent need for effective, interventional models to evaluate the prognosis of HCC. In contrast, PLT is a correctable, inexpensive index and there are several established platelet-based models. However, the effects of these indices in predicting recurrence have not been extensively evaluated. This study demonstrates that nearly half of these indices were helpful predictors of recurrence, including APRI, FIB-4, FibroQ, APGA and PAPAS, which were more powerful prognostic tools than CLIP.
APRI is a valuable index for predicting survival, especially in patients with HBV infection or cirrhosis[7]. In our study, we found that an APRI ≥ 1.94 was independently associated with a high relapse risk. Although a prospective analysis by Seo et al[53] showed that API was an independent predictor of intrahepatic distance recurrence, our results found no such association. Our study also showed that an FIB-4 ≥ 4.3 was an indictor of recurrence, consistent with reports of HCC risk for patients with HIV infection[54] and for prognosis in nonalcoholic fatty liver disease[55]. Moreover, our multivariate analyses identified APGA and PAPAS as superior indicators, which have been seldom used in other studies. However, our study had a limited number of patients, and more studies are needed to determine their prognostic value.
A meta-analysis of 11 studies by Choi et al[56] confirmed that portal hypertension increases mortality, morbidity, complications and liver failure in HCC patients, and several studies demonstrated that thrombocytopenia, which reflects the degree of hypertension, induces similar outcomes[57-59]. Kubo et al[60] suggested that a low PLT in HCC patients with HCV was the only independent predictor of multicentric HCC. In addition, a decreased PLT level was significantly associated with elevated AFP levels[11,61,62], both of which are associated with HCC recurrence[63,64]. In contrast, serum PLT levels positively correlate with tumor size in HCC[39,65], and platelets can stimulate the growth and invasion of several HCC cell lines in vitro[66]. High PLT, such as in thrombocytosis, could also result in many adverse side effects, including deep vein thrombosis[67]. Thus, although thrombocytopenia and thrombocytosis are not contraindications for resection in HCC[44], it is still recommended to normalize serum PLT levels by prophylactic platelet transfusions or taking relevant agents before treatment.
There were some limitations in the current study. First and most important, although there was an adverse relationship between PLT and platelet-based indices, high levels of all predicted a high HCC recurrence. This dichotomy may due to the fact that although thrombocytopenia/low PLT has been identified as a crucial risk factor for HCC formation[10,11] and prognosis[44-47], it was identified as a favorable factor for HCC prognosis in our study and several others[42,43]. In addition, our limited study population included numerous variables that may have contributed to the discrepancy. Second, performances of the noninvasive indices for determining recurrence were all poor in our study. This could be due to the relatively small number of patients and a short follow-up period. Third, approximately three-quarters of the HCC cases in our cohort were caused by HBV infection, thus it is also necessary to validate our results in relation to HCV infection. Many of the formulas used in these indices were deduced in cohorts with[26-29,32,33] or partly with[23,31] HCV-infected individuals, or with HCV/HIV coinfection[30]. On the other hand, detection of cirrhosis with these indices has been validated in patients with chronic HCV in numerous studies[68-71]. These findings suggest that these noninvasive models may demonstrate better prognostic value in patients with HCV-positive HCC. Fourth, PLT, APGA, and PAPAS were significant predictors in patients without cirrhosis, but not in patients with cirrhosis. The limited number of individuals within each subgroup may explain this result, and thus further study is need for verification.
This study was the first to explore the performances of 11 platelet-based indices for detecting postoperative recurrence in HCC. Furthermore, these indices were compared with CLIP and AAR, two models that are not associated with platelets. We demonstrated that several PLT-based models were more valuable than the two PLT-unrelated indices. Additionally, stratification of patients demonstrated that PLT was not affected by tumor size, which was a major confounder in our study population. Taken together, the data show that in patients with HCC, PLT and several platelet-based indices might be effective tools to assess postoperative relapse, especially late relapse. As these significant prognostic models were noninvasive, inexpensive, and easy to calculate, our findings will be helpful for surgeons assessing postoperative recurrence probability in HCC.
ACKNOWLEDGMENTS
The authors gratefully acknowledge Ming-Hui Tai from the University of Kansas, United States, for polishing the manuscript.
COMMENTS
Background
The high risk of postoperative recurrence is one of the greatest problems plaguing potential curative treatment and hastening death for patients with hepatocellular carcinoma (HCC). It is essential to identify predisposing risk factors and endeavor to improve them before surgery. However, there are few variables currently available to accurately predict postoperative recurrence. Platelet count (PLT) is a simple parameter that has been closely associated with cirrhosis and HCC.
Research frontiers
PLT and platelet-based noninvasive indices have a high accuracy in detecting hepatic cirrhosis and HCC. The current study demonstrates that PLT and these indices are valuable tools to predict postoperative recurrence.
Innovations and breakthroughs
Several prognostic models for HCC have been established, mainly based on non-modifiable tumor characteristics. In contrast, PLT is improvable, and the authors highlight that PLT and platelet-based models are independently associated with recurrence, especially late recurrence (more than one year after liver resection). This study included PLT and 11 platelet-based models for assessing recurrence risk, which may be more useful in clinical application. Patients with a high PLT have a worse HCC prognosis, especially for those without cirrhosis.
Applications
By testing PLT and calculating several platelet-related indices before surgery, authors could estimate HCC recurrence probability after liver resection. These results may be used to reduce postoperative recurrence rate for patients by improving preoperative PLT.
Terminology
Platelets are involved in thrombosis, inflammatory responses, and liver regeneration via releasing several inflammatory mediators. PLT is a reflection of platelet function, with a normal range of 100 × 109/L-300 × 109/L.
Peer-review
The authors explored the complicated issue of predicting HCC recurrence and identified platelet-based indices as a possible tool. The results indicate that high platelet levels may predict recurrence, especially in non-cirrhotic patients.
Footnotes
Supported by National Natural Science Foundation of China, No. 30872482 and No. 81072051.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: July 30, 2014
First decision: August 15, 2014
Article in press: October 15, 2014
P- Reviewer: Chawla S, Chen YJ, Lin MS, Tanoglu A S- Editor: Gou SX L- Editor: Wang TQ E- Editor: Liu XM
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245–1255. doi: 10.1016/S0140-6736(11)61347-0. [DOI] [PubMed] [Google Scholar]
- 3.Kew MC. Epidemiology of chronic hepatitis B virus infection, hepatocellular carcinoma, and hepatitis B virus-induced hepatocellular carcinoma. Pathol Biol (Paris) 2010;58:273–277. doi: 10.1016/j.patbio.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 4.Buonaguro L, Petrizzo A, Tagliamonte M, Tornesello ML, Buonaguro FM. Challenges in cancer vaccine development for hepatocellular carcinoma. J Hepatol. 2013;59:897–903. doi: 10.1016/j.jhep.2013.05.031. [DOI] [PubMed] [Google Scholar]
- 5.Bruix J, Gores GJ, Mazzaferro V. Hepatocellular carcinoma: clinical frontiers and perspectives. Gut. 2014;63:844–855. doi: 10.1136/gutjnl-2013-306627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lim KC, Chow PK, Allen JC, Chia GS, Lim M, Cheow PC, Chung AY, Ooi LL, Tan SB. Microvascular invasion is a better predictor of tumor recurrence and overall survival following surgical resection for hepatocellular carcinoma compared to the Milan criteria. Ann Surg. 2011;254:108–113. doi: 10.1097/SLA.0b013e31821ad884. [DOI] [PubMed] [Google Scholar]
- 7.Shen SL, Fu SJ, Chen B, Kuang M, Li SQ, Hua YP, Liang LJ, Guo P, Hao Y, Peng BG. Preoperative aspartate aminotransferase to platelet ratio is an independent prognostic factor for hepatitis B-induced hepatocellular carcinoma after hepatic resection. Ann Surg Oncol. 2014;21:3802–3809. doi: 10.1245/s10434-014-3771-x. [DOI] [PubMed] [Google Scholar]
- 8.Xie H, Wang H, An W, Ma W, Qi R, Yang B, Liu C, Gao Y, Xu B, Wang W. The efficacy of radiofrequency ablation combined with transcatheter arterial chemoembolization for primary hepatocellular carcinoma in a cohort of 487 patients. PLoS One. 2014;9:e89081. doi: 10.1371/journal.pone.0089081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao WC, Zhang HB, Yang N, Fu Y, Qian W, Chen BD, Fan LF, Yang GS. Preoperative predictors of short-term survival after hepatectomy for multinodular hepatocellular carcinoma. World J Gastroenterol. 2012;18:3272–3281. doi: 10.3748/wjg.v18.i25.3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang JH, Chang KC, Kee KM, Chen PF, Yen YH, Tseng PL, Kuo YH, Tsai MC, Hung CH, Chen CH, et al. Hepatocellular carcinoma surveillance at 4- vs. 12-month intervals for patients with chronic viral hepatitis: a randomized study in community. Am J Gastroenterol. 2013;108:416–424. doi: 10.1038/ajg.2012.445. [DOI] [PubMed] [Google Scholar]
- 11.Akuta N, Suzuki F, Seko Y, Kawamura Y, Sezaki H, Suzuki Y, Hosaka T, Kobayashi M, Hara T, Kobayashi M, et al. Complicated relationships of amino acid substitution in hepatitis C virus core region and IL28B genotype influencing hepatocarcinogenesis. Hepatology. 2012;56:2134–2141. doi: 10.1002/hep.25949. [DOI] [PubMed] [Google Scholar]
- 12.Jun CH, Hong HJ, Chung MW, Park SY, Cho SB, Park CH, Joo YE, Kim HS, Choi SK, Rew JS. Risk factors for hepatocellular carcinoma in patients with drug-resistant chronic hepatitis B. World J Gastroenterol. 2013;19:6834–6841. doi: 10.3748/wjg.v19.i40.6834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kondo R, Yano H, Nakashima O, Tanikawa K, Nomura Y, Kage M. Accumulation of platelets in the liver may be an important contributory factor to thrombocytopenia and liver fibrosis in chronic hepatitis C. J Gastroenterol. 2013;48:526–534. doi: 10.1007/s00535-012-0656-2. [DOI] [PubMed] [Google Scholar]
- 14.Starlinger P, Assinger A, Haegele S, Wanek D, Zikeli S, Schauer D, Birner P, Fleischmann E, Gruenberger B, Brostjan C, et al. Evidence for serotonin as a relevant inducer of liver regeneration after liver resection in humans. Hepatology. 2014;60:257–266. doi: 10.1002/hep.26950. [DOI] [PubMed] [Google Scholar]
- 15.Walsh TG, Metharom P, Berndt MC. The functional role of platelets in the regulation of angiogenesis. Platelets. 2015;26:199–211. doi: 10.3109/09537104.2014.909022. [DOI] [PubMed] [Google Scholar]
- 16.Dineen SP, Roland CL, Toombs JE, Kelher M, Silliman CC, Brekken RA, Barnett CC. The acellular fraction of stored platelets promotes tumor cell invasion. J Surg Res. 2009;153:132–137. doi: 10.1016/j.jss.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 17.Hayashi H, Beppu T, Shirabe K, Maehara Y, Baba H. Management of thrombocytopenia due to liver cirrhosis: a review. World J Gastroenterol. 2014;20:2595–2605. doi: 10.3748/wjg.v20.i10.2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Udell JA, Wang CS, Tinmouth J, FitzGerald JM, Ayas NT, Simel DL, Schulzer M, Mak E, Yoshida EM. Does this patient with liver disease have cirrhosis? JAMA. 2012;307:832–842. doi: 10.1001/jama.2012.186. [DOI] [PubMed] [Google Scholar]
- 19.Shaheen AA, Myers RP. Diagnostic accuracy of the aspartate aminotransferase-to-platelet ratio index for the prediction of hepatitis C-related fibrosis: a systematic review. Hepatology. 2007;46:912–921. doi: 10.1002/hep.21835. [DOI] [PubMed] [Google Scholar]
- 20.Karasu Z, Tekin F, Ersoz G, Gunsar F, Batur Y, Ilter T, Akarca US. Liver fibrosis is associated with decreased peripheral platelet count in patients with chronic hepatitis B and C. Dig Dis Sci. 2007;52:1535–1539. doi: 10.1007/s10620-006-9144-y. [DOI] [PubMed] [Google Scholar]
- 21.Lee S, Kim do Y. Non-invasive diagnosis of hepatitis B virus-related cirrhosis. World J Gastroenterol. 2014;20:445–459. doi: 10.3748/wjg.v20.i2.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Llovet JM, Bruix J. Prospective validation of the Cancer of the Liver Italian Program (CLIP) score: a new prognostic system for patients with cirrhosis and hepatocellular carcinoma. Hepatology. 2000;32:679–680. doi: 10.1053/jhep.2000.16475. [DOI] [PubMed] [Google Scholar]
- 23.Giannini E, Botta F, Fasoli A, Ceppa P, Risso D, Lantieri PB, Celle G, Testa R. Progressive liver functional impairment is associated with an increase in AST/ALT ratio. Dig Dis Sci. 1999;44:1249–1253. doi: 10.1023/a:1026609231094. [DOI] [PubMed] [Google Scholar]
- 24.World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310:2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 25.Lee IC, Chan CC, Huang YH, Huo TI, Chu CJ, Lai CR, Lee PC, Su CW, Hung HH, Wu JC, et al. Comparative analysis of noninvasive models to predict early liver fibrosis in hepatitis B e Antigen-negative Chronic Hepatitis B. J Clin Gastroenterol. 2011;45:278–285. doi: 10.1097/MCG.0b013e3181dd5357. [DOI] [PubMed] [Google Scholar]
- 26.Pohl A, Behling C, Oliver D, Kilani M, Monson P, Hassanein T. Serum aminotransferase levels and platelet counts as predictors of degree of fibrosis in chronic hepatitis C virus infection. Am J Gastroenterol. 2001;96:3142–3146. doi: 10.1111/j.1572-0241.2001.05268.x. [DOI] [PubMed] [Google Scholar]
- 27.Poynard T, Bedossa P. Age and platelet count: a simple index for predicting the presence of histological lesions in patients with antibodies to hepatitis C virus. METAVIR and CLINIVIR Cooperative Study Groups. J Viral Hepat. 1997;4:199–208. doi: 10.1046/j.1365-2893.1997.00141.x. [DOI] [PubMed] [Google Scholar]
- 28.Bonacini M, Hadi G, Govindarajan S, Lindsay KL. Utility of a discriminant score for diagnosing advanced fibrosis or cirrhosis in patients with chronic hepatitis C virus infection. Am J Gastroenterol. 1997;92:1302–1304. [PubMed] [Google Scholar]
- 29.Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, Lok AS. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518–526. doi: 10.1053/jhep.2003.50346. [DOI] [PubMed] [Google Scholar]
- 30.Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, S Sulkowski M, Torriani FJ, Dieterich DT, Thomas DL, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317–1325. doi: 10.1002/hep.21178. [DOI] [PubMed] [Google Scholar]
- 31.Hsieh YY, Tung SY, Lee IL, Lee K, Shen CH, Wei KL, Chang TS, Chuang CS, Wu CS, Lin YH. FibroQ: an easy and useful noninvasive test for predicting liver fibrosis in patients with chronic viral hepatitis. Chang Gung Med J. 2009;32:614–622. [PubMed] [Google Scholar]
- 32.Lok AS, Ghany MG, Goodman ZD, Wright EC, Everson GT, Sterling RK, Everhart JE, Lindsay KL, Bonkovsky HL, Di Bisceglie AM, et al. Predicting cirrhosis in patients with hepatitis C based on standard laboratory tests: results of the HALT-C cohort. Hepatology. 2005;42:282–292. doi: 10.1002/hep.20772. [DOI] [PubMed] [Google Scholar]
- 33.Islam S, Antonsson L, Westin J, Lagging M. Cirrhosis in hepatitis C virus-infected patients can be excluded using an index of standard biochemical serum markers. Scand J Gastroenterol. 2005;40:867–872. doi: 10.1080/00365520510015674. [DOI] [PubMed] [Google Scholar]
- 34.Fung J, Lai CL, Fong DY, Yuen JC, Wong DK, Yuen MF. Correlation of liver biochemistry with liver stiffness in chronic hepatitis B and development of a predictive model for liver fibrosis. Liver Int. 2008;28:1408–1416. doi: 10.1111/j.1478-3231.2008.01784.x. [DOI] [PubMed] [Google Scholar]
- 35.Seto WK, Lee CF, Lai CL, Ip PP, Fong DY, Fung J, Wong DK, Yuen MF. A new model using routinely available clinical parameters to predict significant liver fibrosis in chronic hepatitis B. PLoS One. 2011;6:e23077. doi: 10.1371/journal.pone.0023077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaibori M, Kubo S, Nagano H, Hayashi M, Haji S, Nakai T, Ishizaki M, Matsui K, Uenishi T, Takemura S, et al. Clinicopathological features of recurrence in patients after 10-year disease-free survival following curative hepatic resection of hepatocellular carcinoma. World J Surg. 2013;37:820–828. doi: 10.1007/s00268-013-1902-3. [DOI] [PubMed] [Google Scholar]
- 37.Akuta N, Suzuki F, Kobayashi M, Hara T, Sezaki H, Suzuki Y, Hosaka T, Kobayashi M, Saitoh S, Ikeda K, et al. Correlation between hepatitis B virus surface antigen level and alpha-fetoprotein in patients free of hepatocellular carcinoma or severe hepatitis. J Med Virol. 2014;86:131–138. doi: 10.1002/jmv.23790. [DOI] [PubMed] [Google Scholar]
- 38.Carr BI, Guerra V. Hepatocellular carcinoma size: platelets, γ-glutamyl transpeptidase, and alkaline phosphatase. Oncology. 2013;85:153–159. doi: 10.1159/000354416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carr BI, Guerra V. Thrombocytosis and hepatocellular carcinoma. Dig Dis Sci. 2013;58:1790–1796. doi: 10.1007/s10620-012-2527-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tamaki N, Kurosaki M, Matsuda S, Muraoka M, Yasui Y, Suzuki S, Hosokawa T, Ueda K, Tsuchiya K, Nakanishi H, et al. Non-invasive prediction of hepatocellular carcinoma development using serum fibrosis marker in chronic hepatitis C patients. J Gastroenterol. 2014;49:1495–1503. doi: 10.1007/s00535-013-0914-y. [DOI] [PubMed] [Google Scholar]
- 41.Sitia G, Iannacone M, Guidotti LG. Anti-platelet therapy in the prevention of hepatitis B virus-associated hepatocellular carcinoma. J Hepatol. 2013;59:1135–1138. doi: 10.1016/j.jhep.2013.05.040. [DOI] [PubMed] [Google Scholar]
- 42.Buergy D, Wenz F, Groden C, Brockmann MA. Tumor-platelet interaction in solid tumors. Int J Cancer. 2012;130:2747–2760. doi: 10.1002/ijc.27441. [DOI] [PubMed] [Google Scholar]
- 43.Nouso K, Ito Y, Kuwaki K, Kobayashi Y, Nakamura S, Ohashi Y, Yamamoto K. Prognostic factors and treatment effects for hepatocellular carcinoma in Child C cirrhosis. Br J Cancer. 2008;98:1161–1165. doi: 10.1038/sj.bjc.6604282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roayaie S, Obeidat K, Sposito C, Mariani L, Bhoori S, Pellegrinelli A, Labow D, Llovet JM, Schwartz M, Mazzaferro V. Resection of hepatocellular cancer ≤2 cm: results from two Western centers. Hepatology. 2013;57:1426–1435. doi: 10.1002/hep.25832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Amano H, Tashiro H, Oshita A, Kobayashi T, Tanimoto Y, Kuroda S, Tazawa H, Itamoto T, Asahara T, Ohdan H. Significance of platelet count in the outcomes of hepatectomized patients with hepatocellular carcinoma exceeding the Milan criteria. J Gastrointest Surg. 2011;15:1173–1181. doi: 10.1007/s11605-011-1538-2. [DOI] [PubMed] [Google Scholar]
- 46.Wu WC, Chiou YY, Hung HH, Kao WY, Chou YH, Su CW, Wu JC, Huo TI, Huang YH, Lee KC, et al. Prognostic significance of computed tomography scan-derived splenic volume in hepatocellular carcinoma treated with radiofrequency ablation. J Clin Gastroenterol. 2012;46:789–795. doi: 10.1097/MCG.0b013e31825ceeb5. [DOI] [PubMed] [Google Scholar]
- 47.Kao WY, Chiou YY, Hung HH, Su CW, Chou YH, Huo TI, Huang YH, Wu WC, Lin HC, Lee SD, et al. Younger hepatocellular carcinoma patients have better prognosis after percutaneous radiofrequency ablation therapy. J Clin Gastroenterol. 2012;46:62–70. doi: 10.1097/MCG.0b013e31822b36cc. [DOI] [PubMed] [Google Scholar]
- 48.Memon K, Kulik LM, Lewandowski RJ, Wang E, Wang J, Ryu RK, Hickey R, Vouche M, Baker T, Ganger D, et al. Comparative study of staging systems for hepatocellular carcinoma in 428 patients treated with radioembolization. J Vasc Interv Radiol. 2014;25:1056–1066. doi: 10.1016/j.jvir.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gomaa AI, Hashim MS, Waked I. Comparing staging systems for predicting prognosis and survival in patients with hepatocellular carcinoma in Egypt. PLoS One. 2014;9:e90929. doi: 10.1371/journal.pone.0090929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grieco A, Pompili M, Caminiti G, Miele L, Covino M, Alfei B, Rapaccini GL, Gasbarrini G. Prognostic factors for survival in patients with early-intermediate hepatocellular carcinoma undergoing non-surgical therapy: comparison of Okuda, CLIP, and BCLC staging systems in a single Italian centre. Gut. 2005;54:411–418. doi: 10.1136/gut.2004.048124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim JH, Choi MS, Lee H, Kim do Y, Lee JH, Koh KC, Yoo BC, Paik SW, Rhee JC. Clinical features and prognosis of hepatocellular carcinoma in young patients from a hepatitis B-endemic area. J Gastroenterol Hepatol. 2006;21:588–594. doi: 10.1111/j.1440-1746.2005.04127.x. [DOI] [PubMed] [Google Scholar]
- 52.Kobayashi T, Itamoto T, Tashiro H, Amano H, Oshita A, Tanimoto Y, Kuroda S, Tazawa H, Ohdan H. Tumor-related factors do not influence the prognosis of solitary hepatocellular carcinoma after partial hepatectomy. J Hepatobiliary Pancreat Sci. 2011;18:689–699. doi: 10.1007/s00534-011-0379-4. [DOI] [PubMed] [Google Scholar]
- 53.Seo JY, Kim W, Kwon JH, Jin EH, Yu SJ, Kim HY, Jung YJ, Kim D, Kim YJ, Yoon JH, et al. Noninvasive fibrosis indices predict intrahepatic distant recurrence of hepatitis B-related hepatocellular carcinoma following radiofrequency ablation. Liver Int. 2013;33:884–893. doi: 10.1111/liv.12132. [DOI] [PubMed] [Google Scholar]
- 54.Park LS, Tate JP, Justice AC, Lo Re V, Lim JK, Bräu N, Brown ST, Butt AA, Gibert C, Goetz MB, et al. FIB-4 index is associated with hepatocellular carcinoma risk in HIV-infected patients. Cancer Epidemiol Biomarkers Prev. 2011;20:2512–2517. doi: 10.1158/1055-9965.EPI-11-0582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Angulo P, Bugianesi E, Bjornsson ES, Charatcharoenwitthaya P, Mills PR, Barrera F, Haflidadottir S, Day CP, George J. Simple noninvasive systems predict long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology. 2013;145:782–9.e4. doi: 10.1053/j.gastro.2013.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Choi SB, Kim HJ, Song TJ, Ahn HS, Choi SY. Influence of clinically significant portal hypertension on surgical outcomes and survival following hepatectomy for hepatocellular carcinoma: a systematic review and meta-analysis. J Hepatobiliary Pancreat Sci. 2014;21:639–647. doi: 10.1002/jhbp.124. [DOI] [PubMed] [Google Scholar]
- 57.Taketomi A, Kitagawa D, Itoh S, Harimoto N, Yamashita Y, Gion T, Shirabe K, Shimada M, Maehara Y. Trends in morbidity and mortality after hepatic resection for hepatocellular carcinoma: an institute’s experience with 625 patients. J Am Coll Surg. 2007;204:580–587. doi: 10.1016/j.jamcollsurg.2007.01.035. [DOI] [PubMed] [Google Scholar]
- 58.Maithel SK, Kneuertz PJ, Kooby DA, Scoggins CR, Weber SM, Martin RC, McMasters KM, Cho CS, Winslow ER, Wood WC, et al. Importance of low preoperative platelet count in selecting patients for resection of hepatocellular carcinoma: a multi-institutional analysis. J Am Coll Surg. 2011;212:638–648; discussion 648-650. doi: 10.1016/j.jamcollsurg.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee HS, Park SY, Kim SK, Kweon YO, Tak WY, Cho CM, Jeon SW, Jung MK, Park HG, Lee DW, et al. Thrombocytopenia represents a risk for deterioration of liver function after radiofrequency ablation in patients with hepatocellular carcinoma. Clin Mol Hepatol. 2012;18:302–308. doi: 10.3350/cmh.2012.18.3.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kubo S, Tanaka H, Shuto T, Takemura S, Yamamoto T, Uenishi T, Tanaka S, Ogawa M, Sakabe K, Yamazaki K, et al. Correlation between low platelet count and multicentricity of hepatocellular carcinoma in patients with chronic hepatitis C. Hepatol Res. 2004;30:221–225. doi: 10.1016/j.hepres.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 61.Chen TM, Huang PT, Tsai MH, Lin LF, Liu CC, Ho KS, Siauw CP, Chao PL, Tung JN. Predictors of alpha-fetoprotein elevation in patients with chronic hepatitis C, but not hepatocellular carcinoma, and its normalization after pegylated interferon alfa 2a-ribavirin combination therapy. J Gastroenterol Hepatol. 2007;22:669–675. doi: 10.1111/j.1440-1746.2007.04898.x. [DOI] [PubMed] [Google Scholar]
- 62.Kobeisy MA, Morsy KH, Galal M, Sayed SK, Ashmawy MM, Mohammad FM. Clinical significance of elevated alpha-foetoprotein (AFP) in patients with chronic hepatitis C without hepatocellular carcinoma in upper EGYPT. Arab J Gastroenterol. 2012;13:49–53. doi: 10.1016/j.ajg.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 63.Tateishi R, Shiina S, Yoshida H, Teratani T, Obi S, Yamashiki N, Yoshida H, Akamatsu M, Kawabe T, Omata M. Prediction of recurrence of hepatocellular carcinoma after curative ablation using three tumor markers. Hepatology. 2006;44:1518–1527. doi: 10.1002/hep.21408. [DOI] [PubMed] [Google Scholar]
- 64.Nouso K, Matsumoto E, Kobayashi Y, Nakamura S, Tanaka H, Osawa T, Ikeda H, Araki Y, Sakaguchi K, Shiratori Y. Risk factors for local and distant recurrence of hepatocellular carcinomas after local ablation therapies. J Gastroenterol Hepatol. 2008;23:453–458. doi: 10.1111/j.1440-1746.2007.05120.x. [DOI] [PubMed] [Google Scholar]
- 65.Sarpel U, Ayo D, Lobach I, Xu R, Newman E. Inverse relationship between cirrhosis and massive tumours in hepatocellular carcinoma. HPB (Oxford) 2012;14:741–745. doi: 10.1111/j.1477-2574.2012.00507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Carr BI, Cavallini A, D’Alessandro R, Refolo MG, Lippolis C, Mazzocca A, Messa C. Platelet extracts induce growth, migration and invasion in human hepatocellular carcinoma in vitro. BMC Cancer. 2014;14:43. doi: 10.1186/1471-2407-14-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pabinger I, Thaler J, Ay C. Biomarkers for prediction of venous thromboembolism in cancer. Blood. 2013;122:2011–2018. doi: 10.1182/blood-2013-04-460147. [DOI] [PubMed] [Google Scholar]
- 68.Lackner C, Struber G, Liegl B, Leibl S, Ofner P, Bankuti C, Bauer B, Stauber RE. Comparison and validation of simple noninvasive tests for prediction of fibrosis in chronic hepatitis C. Hepatology. 2005;41:1376–1382. doi: 10.1002/hep.20717. [DOI] [PubMed] [Google Scholar]
- 69.Giannini EG, Zaman A, Ceppa P, Mastracci L, Risso D, Testa R. A simple approach to noninvasively identifying significant fibrosis in chronic hepatitis C patients in clinical practice. J Clin Gastroenterol. 2006;40:521–527. doi: 10.1097/00004836-200607000-00011. [DOI] [PubMed] [Google Scholar]
- 70.Calès P, de Ledinghen V, Halfon P, Bacq Y, Leroy V, Boursier J, Foucher J, Bourlière M, de Muret A, Sturm N, et al. Evaluating the accuracy and increasing the reliable diagnosis rate of blood tests for liver fibrosis in chronic hepatitis C. Liver Int. 2008;28:1352–1362. doi: 10.1111/j.1478-3231.2008.01789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kayadibi H, Yasar B, Ozkara S, Serdar MA, Kurdas OO, Gonen C. The diagnostic accuracy of the Forns index, platelet count and AST to Platelet Ratio Index derived fibrosis index for the prediction of Hepatitis C virus-related significant liver fibrosis and cirrhosis. Scand J Clin Lab Invest. 2014;74:240–247. doi: 10.3109/00365513.2013.879392. [DOI] [PubMed] [Google Scholar]


