Abstract
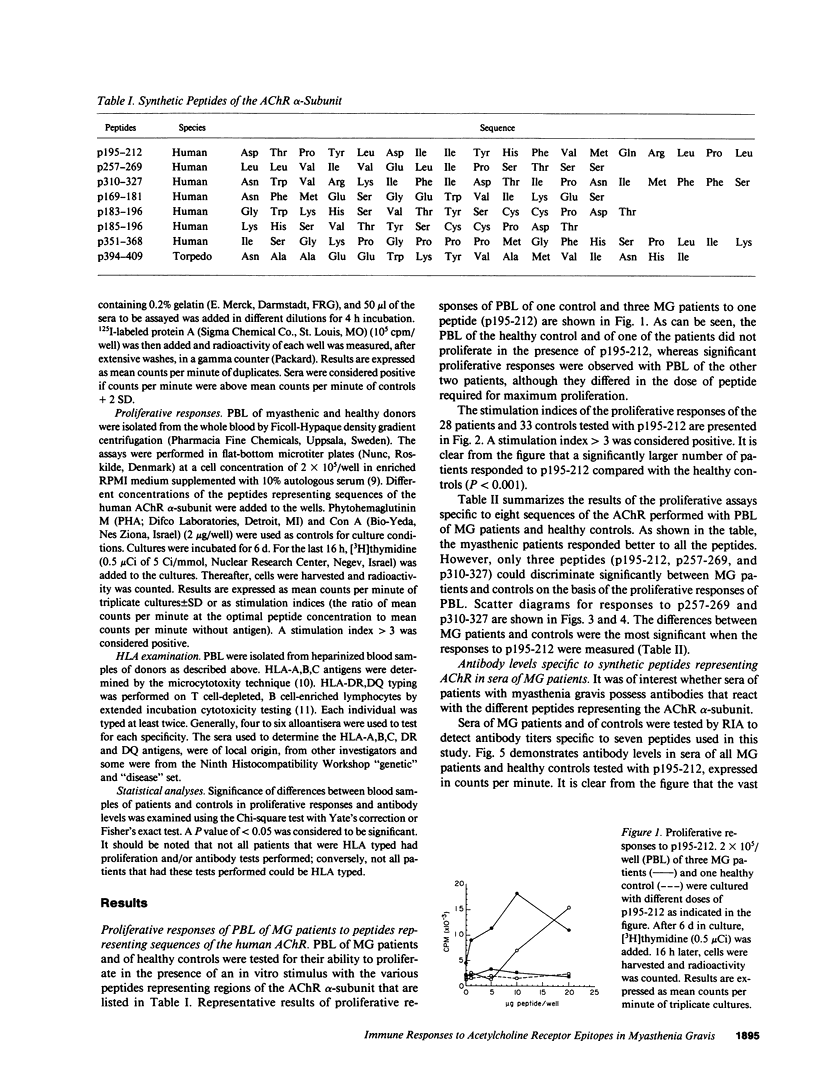
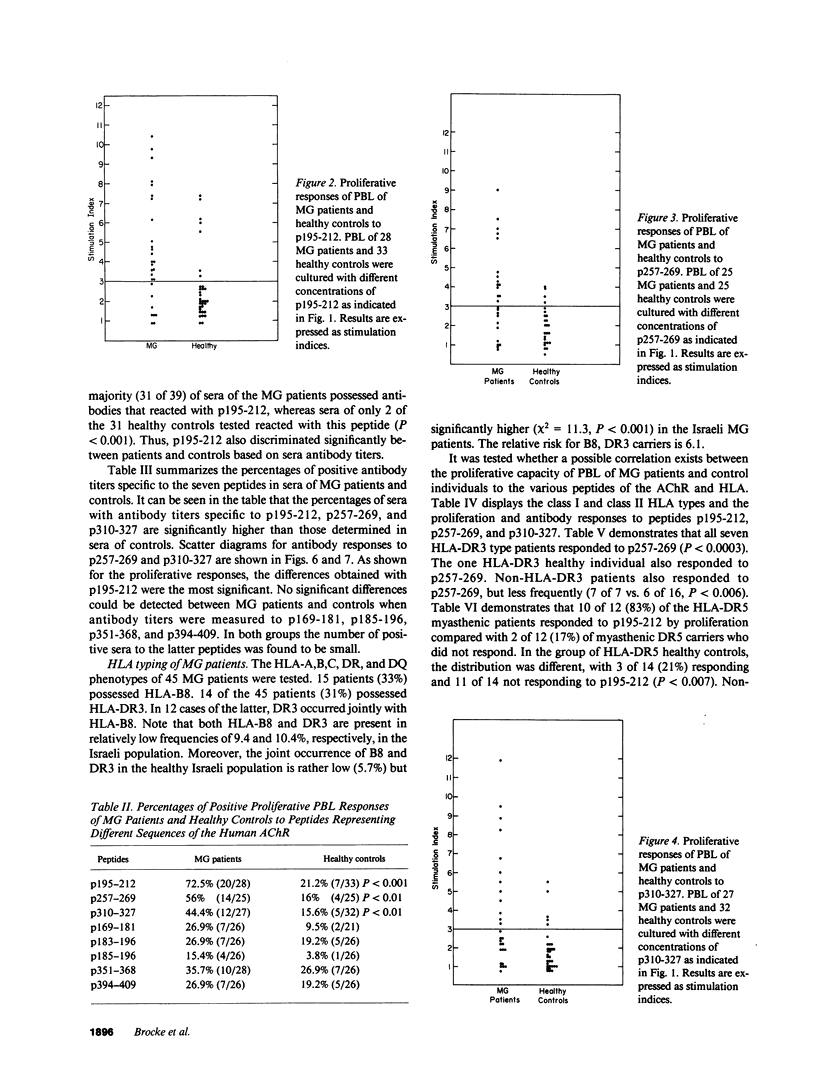
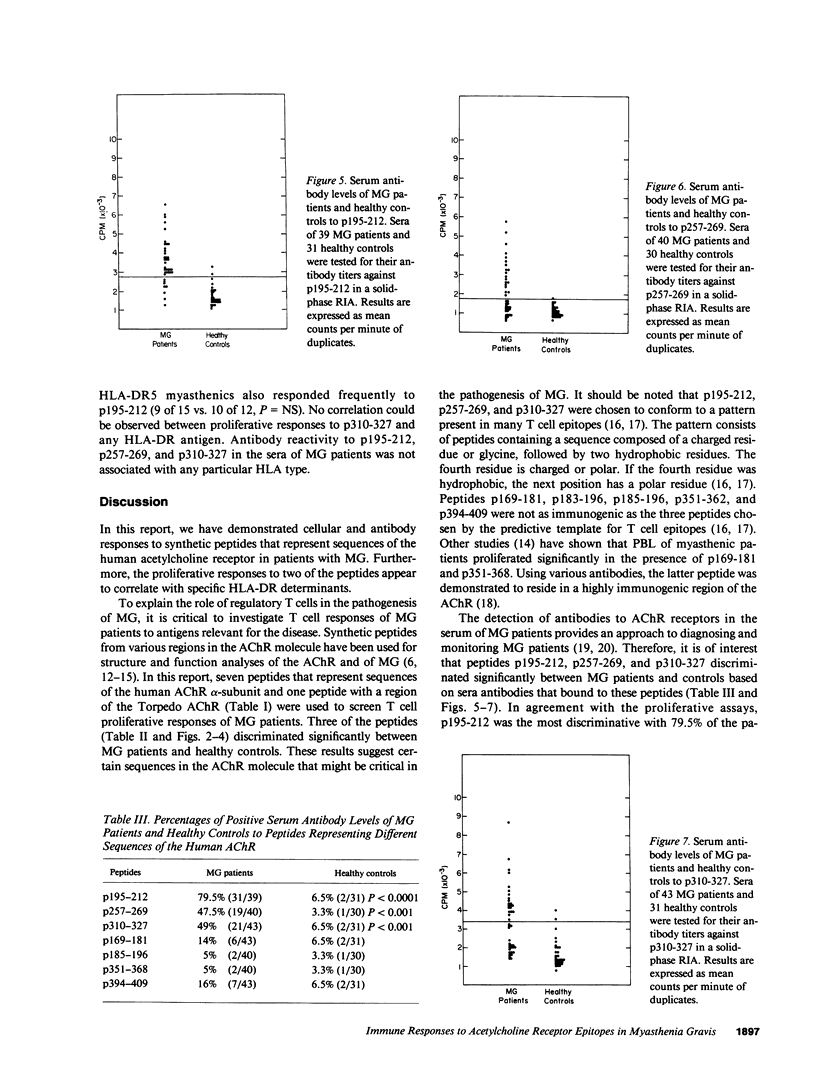
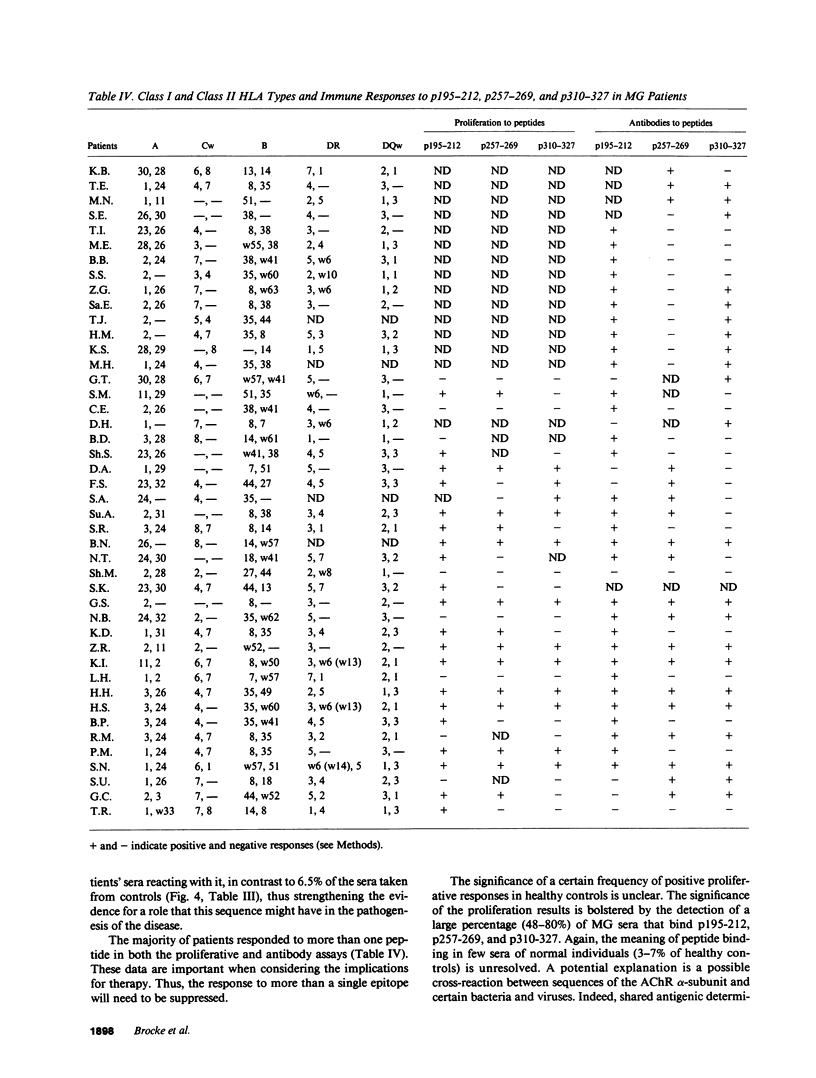
To investigate which parts of the acetylcholine receptor are involved in the initiation and development of myasthenia gravis (MG), peptides representing different sequences of the human acetylcholine receptor alpha-subunit were synthesized. These peptides were tested for their ability to stimulate T cells of myasthenic patients and healthy control patients in proliferation assays and to bind to sera antibodies. Three of eight peptides discriminated significantly between the two groups in the proliferation assay, as well as in their ability to bind to serum antibodies. HLA-DR3 and DR5 were associated with proliferative responses to specific AChR peptides in the group of myasthenics. Acetylcholine receptor epitopes that might play a specific role in myasthenia gravis thus were demonstrated.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramsky O., Brenner T., Lisak R. P., Zeidman A., Beyth Y. Significance in neonatal myasthenia gravis of inhibitory effect of amniotic fluid on binding of antibodies to acetylcholine receptor. Lancet. 1979 Dec 22;2(8156-8157):1333–1335. doi: 10.1016/s0140-6736(79)92815-0. [DOI] [PubMed] [Google Scholar]
- Atassi M. Z., Mulac-Jericevic B., Yokoi T., Manshouri T. Localization of the functional sites on the alpha chain of acetylcholine receptor. Fed Proc. 1987 Jun;46(8):2538–2547. [PubMed] [Google Scholar]
- Axelrod O., Mozes E. Analysis of the biological functions and fine specificity of (T,G)-A--L specific T cell clones. Immunobiology. 1986 Aug;172(1-2):99–109. doi: 10.1016/S0171-2985(86)80056-0. [DOI] [PubMed] [Google Scholar]
- Babbitt B. P., Allen P. M., Matsueda G., Haber E., Unanue E. R. Binding of immunogenic peptides to Ia histocompatibility molecules. 1985 Sep 26-Oct 2Nature. 317(6035):359–361. doi: 10.1038/317359a0. [DOI] [PubMed] [Google Scholar]
- Bell J., Rassenti L., Smoot S., Smith K., Newby C., Hohlfeld R., Toyka K., McDevitt H., Steinman L. HLA-DQ beta-chain polymorphism linked to myasthenia gravis. Lancet. 1986 May 10;1(8489):1058–1060. doi: 10.1016/s0140-6736(86)91330-9. [DOI] [PubMed] [Google Scholar]
- Brautbar C., Amar A., Cohen N., Oksenberg J., Cohen I., Kahana E., Bloch D., Alter M., Grosse-Wilde H. HLA-D typing in multiple sclerosis: Israelis tested with European homozygous typing cells. Tissue Antigens. 1982 Mar;19(3):189–197. doi: 10.1111/j.1399-0039.1982.tb01439.x. [DOI] [PubMed] [Google Scholar]
- Christadoss P., Lindstrom J. M., Melvold R. W., Talal N. Mutation at I-A beta chain prevents experimental autoimmune myasthenia gravis. Immunogenetics. 1985;21(1):33–38. doi: 10.1007/BF00372239. [DOI] [PubMed] [Google Scholar]
- Compston D. A., Vincent A., Newsom-Davis J., Batchelor J. R. Clinical, pathological, HLA antigen and immunological evidence for disease heterogeneity in myasthenia gravis. Brain. 1980 Sep;103(3):579–601. doi: 10.1093/brain/103.3.579. [DOI] [PubMed] [Google Scholar]
- Hohlfeld R., Toyka K. V., Heininger K., Grosse-Wilde H., Kalies I. Autoimmune human T lymphocytes specific for acetylcholine receptor. Nature. 1984 Jul 19;310(5974):244–246. doi: 10.1038/310244a0. [DOI] [PubMed] [Google Scholar]
- Hohlfeld R., Toyka K. V., Miner L. L., Walgrave S. L., Conti-Tronconi B. M. Amphipathic segment of the nicotinic receptor alpha subunit contains epitopes recognized by T lymphocytes in myasthenia gravis. J Clin Invest. 1988 Mar;81(3):657–660. doi: 10.1172/JCI113369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz D., Bentwich Z., Eshhar N., Löwy I., Mozes E. Immune response potential to poly(Tyr,Glu)-poly(DLAla)--poly(Lys) of human T cells of different donors. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4505–4509. doi: 10.1073/pnas.78.7.4505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limburg P. C., The T. H., Hummel-Tappel E., Oosterhuis H. J. Anti-acetylcholine receptor antibodies in myasthenia gravis. Part 1. Relation to clinical parameters in 250 patients. J Neurol Sci. 1983 Mar;58(3):357–370. doi: 10.1016/0022-510x(83)90095-3. [DOI] [PubMed] [Google Scholar]
- Lindstrom J. M., Seybold M. E., Lennon V. A., Whittingham S., Duane D. D. Antibody to acetylcholine receptor in myasthenia gravis. Prevalence, clinical correlates, and diagnostic value. Neurology. 1976 Nov;26(11):1054–1059. doi: 10.1212/wnl.26.11.1054. [DOI] [PubMed] [Google Scholar]
- Lindstrom J. Immunobiology of myasthenia gravis, experimental autoimmune myasthenia gravis, and Lambert-Eaton syndrome. Annu Rev Immunol. 1985;3:109–131. doi: 10.1146/annurev.iy.03.040185.000545. [DOI] [PubMed] [Google Scholar]
- McCormick D. J., Griesmann G. E., Huang Z. X., Lambert E. H., Lennon V. A. Myasthenogenicity of human acetylcholine receptor synthetic alpha-subunit peptide 125-147 does not require intramolecular disulfide cyclization. J Immunol. 1987 Oct 15;139(8):2615–2619. [PubMed] [Google Scholar]
- McQuillen D. P., Koethe S. M., McQuillen M. P. Cellular response to human acetylcholine receptor in patients with myasthenia gravis. J Neuroimmunol. 1983 Aug;5(1):59–65. doi: 10.1016/0165-5728(83)90026-7. [DOI] [PubMed] [Google Scholar]
- Mittal K. K., Mickey M. R., Singal D. P., Terasaki P. I. Serotyping for homotransplantation. 18. Refinement of microdroplet lymphocyte cytotoxicity test. Transplantation. 1968 Nov;6(8):913–927. doi: 10.1097/00007890-196811000-00006. [DOI] [PubMed] [Google Scholar]
- Neumann D., Barchan D., Safran A., Gershoni J. M., Fuchs S. Mapping of the alpha-bungarotoxin binding site within the alpha subunit of the acetylcholine receptor. Proc Natl Acad Sci U S A. 1986 May;83(9):3008–3011. doi: 10.1073/pnas.83.9.3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann D., Gershoni J. M., Fridkin M., Fuchs S. Antibodies to synthetic peptides as probes for the binding site on the alpha subunit of the acetylcholine receptor. Proc Natl Acad Sci U S A. 1985 May;82(10):3490–3493. doi: 10.1073/pnas.82.10.3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothbard J. B., Taylor W. R. A sequence pattern common to T cell epitopes. EMBO J. 1988 Jan;7(1):93–100. doi: 10.1002/j.1460-2075.1988.tb02787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev Y., Bentwich Z., Katz D., Brautbar C., Mozes E. (T,G)-A-L specific immune response potential and HLA typing of Israeli patients with systemic lupus erythematosus. Clin Exp Immunol. 1985 May;60(2):355–362. [PMC free article] [PubMed] [Google Scholar]
- Souroujon M. C., Neumann D., Pizzighella S., Safran A., Fuchs S. Localization of a highly immunogenic region on the acetylcholine receptor alpha-subunit. Biochem Biophys Res Commun. 1986 Feb 26;135(1):82–89. doi: 10.1016/0006-291x(86)90945-9. [DOI] [PubMed] [Google Scholar]
- Stefansson K., Dieperink M. E., Richman D. P., Gomez C. M., Marton L. S. Sharing of antigenic determinants between the nicotinic acetylcholine receptor and proteins in Escherichia coli, Proteus vulgaris, and Klebsiella pneumoniae. Possible role in the pathogenesis of myasthenia gravis. N Engl J Med. 1985 Jan 24;312(4):221–225. doi: 10.1056/NEJM198501243120407. [DOI] [PubMed] [Google Scholar]
- Terasaki P. I., Bernoco D., Park M. S., Ozturk G., Iwaki Y. Microdroplet testing for HLA-A, -B, -C, and -D antigens. The Phillip Levine Award Lecture. Am J Clin Pathol. 1978 Feb;69(2):103–120. doi: 10.1093/ajcp/69.2.103. [DOI] [PubMed] [Google Scholar]
- Tishler M., Brautbar C., Battat S., Dayan M., Mozes E. Analysis of the antigen specific helper T cell function and HLA-DR of Israeli patients with rheumatoid arthritis (RA). Tissue Antigens. 1987 Nov;30(5):229–234. doi: 10.1111/j.1399-0039.1987.tb01626.x. [DOI] [PubMed] [Google Scholar]
- Todd J. A., Acha-Orbea H., Bell J. I., Chao N., Fronek Z., Jacob C. O., McDermott M., Sinha A. A., Timmerman L., Steinman L. A molecular basis for MHC class II--associated autoimmunity. Science. 1988 May 20;240(4855):1003–1009. doi: 10.1126/science.3368786. [DOI] [PubMed] [Google Scholar]
- Waldor M. K., Sriram S., McDevitt H. O., Steinman L. In vivo therapy with monoclonal anti-I-A antibody suppresses immune responses to acetylcholine receptor. Proc Natl Acad Sci U S A. 1983 May;80(9):2713–2717. doi: 10.1073/pnas.80.9.2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson P. T., Lentz T. L., Hawrot E. Determination of the primary amino acid sequence specifying the alpha-bungarotoxin binding site on the alpha subunit of the acetylcholine receptor from Torpedo californica. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8790–8794. doi: 10.1073/pnas.82.24.8790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamvil S. S., Mitchell D. J., Moore A. C., Kitamura K., Steinman L., Rothbard J. B. T-cell epitope of the autoantigen myelin basic protein that induces encephalomyelitis. Nature. 1986 Nov 20;324(6094):258–260. doi: 10.1038/324258a0. [DOI] [PubMed] [Google Scholar]


