Abstract
The aim of the study was to evaluate the antioxidant action of SMe1EC2, the structural analogue of the hexahydropyridoindole antioxidant stobadine. The antiradical activity of SMe1EC2 was found to be higher when compared to stobadine, as determined both in cell-free model systems of AAPH-induced oxidation of dihydrorhodamine 123 and 2′,7′-dichloro-dihydrofluorescein diacetate, and in the cellular system of stimulated macrophages RAW264.7. Analysis of proliferation of HUVEC and HUVEC-ST cells revealed absence of cytotoxic effect of SMe1EC2 at concentrations below 100 µM. The antioxidant activity of SMe1EC2, superior to the parent drug stobadine, is accounted for by both the higher intrinsic free radical scavenging action and by the better bioavailability of the low-basicity SMe1EC2 relative to the high-basicity stobadine.
Keywords: antioxidant, hexahydropyridoindole, SMe1EC2, stobadine, oxidative stress
Introduction
Considering the antioxidant stobadine as a lead (Horakova and Stolc 1998), a number of structurally related hexahydropyridoindole congeners have been designed, synthesized and characterized (Juranek et al., 2010; Rackova et al., 2006, Stolc et al., 2008). Modification of the stobadine molecule by aromatic electron donating substitution was reported to enhance the intrinsic free radical scavenging activity, while variations of the N2 substituent provided a synthetically accessible way to modulate the biological availability by affecting both lipophilicity and basicity of the molecule, without changing significantly the free radical scavenging activity (Rackova et al., 2002; Rackova et al., 2006)
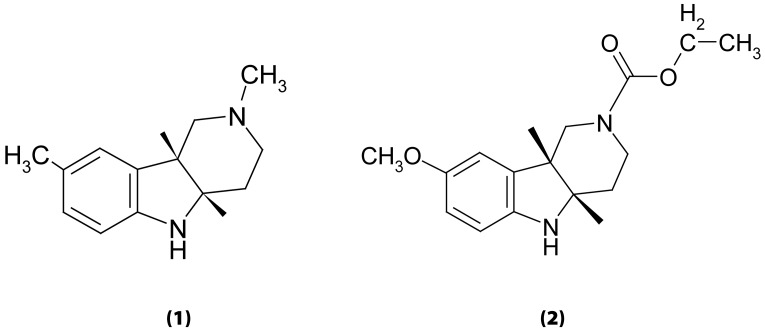
The subject of the present study was SMe1EC2, the 8-methoxy analogue of stobadine with an acyl substituent at the position N2 (Figure 1). In extensive preclinical studies, SMe1EC2 revealed significant neuroprotection in the murine model of acute head trauma (Stolc et al., 2006, 2008) and in in vitro rat hippocampal slices exposed to transient hypoxia/reoxygenation (Gasparova et al., 2009, 2010, 2011, 2014). Under conditions of experimental diabetes of rats, SMe1EC2 attenuated endothelial injury and restored the reduced endothelium-mediated relaxation in diabetic animals (Sotnikova et al., 2011). SMe1EC2 improved the viability of HT22 neuronal cells in culture exposed to high glucose and attenuated indices of oxidative stress (Rackova et al., 2009). The compound protected efficiently rat pancreatic INS-1E β cell cultures against cytotoxic effects of hydrogen peroxide (Rackova et al., 2011). Preclinical toxicology tests revealed a remarkably low acute toxicity of SMe1EC2 in mice, regardless the way of administration (Stolc et al., 2010). Contrary to stobadine, SMe1EC2 did no possess any α-adrenolytic action (Stolc et al., 2010). In a prenatal developmental toxicity study in rats, SMe1EC2 exerted neither embryotoxic nor teratogenic effects on rat fetuses and their postnatal development, nor were any signs of maternal toxicity found (Ujhazy et al., 2008, 2011).
Figure 1.
Chemical structure of stobadine, [(–)-cis-2,8-dimethyl-2,3,4,4a,5,9b-hexahydro-1H-pyrido[4,3b]indole, (1)] and SMe1EC2 [(±)-cis-8-methoxy-1,3,4,4a,5,9b-hexahydro-pyrido[4,3-b]indole-2-carboxylic acid ethyl ester, (2)].
In our previous study (Stefek et al., 2013), on applying a DPPH test, we reported efficient free radical scavenging activity of SMe1EC2, comparable with that of the standard trolox. In the cellular system of isolated erythrocytes, SMe1EC2 protected red blood cells against free-radical-initiated hemolysis. The overall antioxidant efficacy of SMe1EC2, relative to the reference antioxidant stobadine, was strongly affected by the lipophilicity of the initiating free radical species. In the system of t-BuOOH/isolated erythrocytes, a model cellular system of endogenously generated peroxyl radicals, SMe1EC2 significantly exceeded the parent stobadine in its antioxidant action.
The first part of the present study reports the evaluation of the intrinsic antiradical activity of SMe1EC2 in comparison with stobadine in the cell-free model system of 2,2‘-azobis(2-amidinopropane) hydrochloride (AAPH)-induced oxidation of dihydrorhodamine 123 (H2R123) and 2′,7′-dichloro-dihydrofluorescein diacetate (H2DCF DA). Further the overall antioxidant action of the compound was studied in the cellular system of mouse macrophages RAW 264.7. Finally, cytotoxicity of SMe1EC2 was examined in primary human umbilical vein endothelial cells (HUVEC) and immortalized human umbilical vein endothelial cells (HUVEC-ST).
Materials and methods
Chemicals
SMe1EC2 and stobadine (Figure 1) were synthesized at the Institute of Experimental Pharmacology and Toxicology, Slovak Academy of Sciences, and were available as hydrochlorides. AAPH was obtained from FLUKA Chemie GmbH (Steinheim, Germany). Cell culture reagents: fetal bovine serum, medium M199, Dulbecco's modified Eagle's medium (DMEM), collagenase II and antibiotics were purchased from Invitrogen (Paisley, UK). Epidermal growth factor (EGF) was supplied by Becton Dickinson (San Jose, CA, USA). Fluorescent probes, H2R123 and H2DCF DA, were obtained from Molecular Probes, Invitrogen Corporation (Leiden, The Netherlands). Other chemicals were purchased from local commercial sources and were of analytical grade quality.
Cell culture
HUVEC were isolated from veins of freshly collected umbilical cords, by collagenase type II digestion (Jaffe et al., 1973) and used for the experiments at passage 2–4. They were cultured in medium 199 containing 20% heat-inactivated fetal bovine serum, 10 U/ml penicillin, 50 µg/ml streptomycin, 5 µg/ml sodium heparin and 10 ng/ml epidermal growth factor (EGF). The cells were grown at 37 °C and 5% CO2 on plastic flasks coated with 1% gelatin. The cultured cells showed the typical cobblestone-like appearance, and were identified as endothelial cells by fluorescence activated cell sorting (FACS) analysis for von Willebrand factor with ECA-4 antibodies (kindly donated by Dr. Monica Spadofora-Ferreira, University of Sao Paulo, Brazil; Spadafora-Ferreira et al., 2000). SW620 colorectal adenocarcinoma cells were used as a negative control.
HUVEC-ST were cultured in OptiMEM medium supplemented with 3.5% heat-inactivated fetal bovine serum and antibiotics (10 U/ml penicillin, 50 µg/ml streptomycin). The HUVEC-ST cell line was obtained from Prof. C. Kieda (Orleans, France).
Mouse RAW 264.7 macrophages, kindly donated by Dr. Pawel Lipinski (Institute of Genetics and Animal Breeding, Jastrzebiec, Poland), were cultured in DMEM containing 10% fetal bovine serum heat-inactivated and 50 µg/ml gentamycin.
MTT assay
Proliferation of HUVEC/HUVEC-ST was assessed by measuring the ability of live cells to metabolize MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, to formazan. Cells were seeded onto gelatin-coated 96-well plates at a density of 3×103 cells per well (HUVEC) or 1×103 cells per well (HUVEC-ST). After overnight culture, the cells were treated for 72 h by stobadine and its derivatives in a concentration range of 5–200 µM. At the end of treatment, the cell monolayers were rinsed with HBSS and fresh medium containing MTT (final MTT concentration of 333 mg/ml) was added. After 3 h the medium was removed and formazan crystals were dissolved in DMSO. Absorbance was read at 590 nm.
Migration/Wound-healing Assay
Migration of cells was tracked using the Olympus automated phase-contrast microscope image analysis system. Scratch on confluent HUVEC monolayer was performed using the migration inserts (Ibidi®, Germany). Cells were washed twice with EBM-2 serum-free medium to remove detached and damaged cells and fresh complete growth medium was added. To exclude the influence of proliferation on wound closure, the medium was supplemented with an inhibitor of cell proliferation, mitomycin C (10 µg/ml) (Sigma). Wound size was measured a) immediately, b) 6h, and c) 12h after removing the insert. Estimation of ECs migration was performed using Metamorph software.
Migration of cells (% of recovery) was quantified by using the equation:
% R = [1 – (wound area at Tt/wound area at T0] × 100%
where:
Tt - wound area at indicated time after the injury
T0 - wound area immediately after the injury (0 h)
Results
Cell-free model
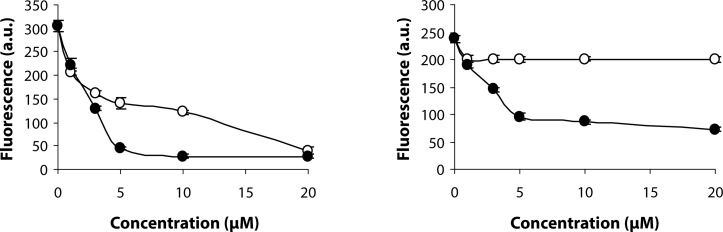
As shown in Figure 2, SMe1EC2 was found more efficient than stobadine in protecting H2R123 and H2DCF DA from AAPH-induced oxidation in a cell-free system.
Figure 2.
SMe1EC2 (-●-) and stobadine (-○-) protect H2R123 (a) and H2DCF DA (b) from AAPH induced oxidation in a cell-free system. Results are presented as means ± SD from at least three measurements.
Cellular systems
Studies in stimulated macrophages RAW 264.7
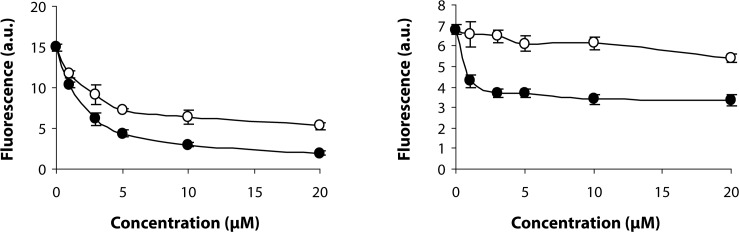
Determination of reactive oxygen/nitrogen species production by using fluorogenic probes confirmed the antioxidant properties of the compounds analyzed. Macrophages RAW 264.7 were used as a model. Nitric oxide production was stimulated by 16-h treatment of the cells with 100 ng/ml of lipopolysaccharide (LPS). Analysis of reactive oxygen species production was performed in cells stimulated with LPS (100 ng/ml, 16 h) and phorbol ester (PMA; 100 nM, 30 min), by using H2R123. Both compounds tested inhibited ROS/RNS production. SMe1EC2 revealed stronger antioxidant properties than stobadine (Figure 3). Based on H2DCF DA oxidation (Ischiropoulos et al., 1999), stobadine in the range of concentrations of 1–20 µM decreased the nitric oxide production by about 15%. The effect of SMe1EC2 was significantly stronger (inhibition was up to 50%).
Figure 3.
Effect of SMe1EC2 (-●-) and stobadine (-○-) on oxidation of H2R123 (a) or H2DCF DA (b) by RAW 264.7 macrophages. RAW 264.7 macrophages were stimulated with 100 µg/ml of LPS for 16 h and with 100 nM PMA for 30 min in a complete medium.
Proliferation of HUVEC and HUVEC-ST
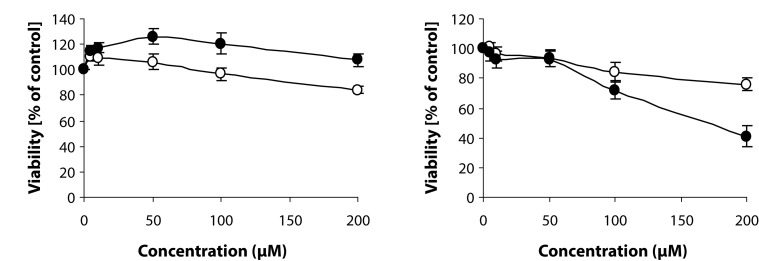
Analysis of proliferation under 72-h treatment of cells with stobadine and its derivative showed (Figure 4) that, in the range of concentrations 5–200 µM, SMe1EC2 slightly stimulated proliferation of HUVEC (Figure 4a, up to 120% of control), but only of primary and not of immortalized endothelial cells (HUVEC-ST), while stobadine had no significant effect on cell growth. In the case of HUVEC-ST, the cytotoxic effect of SMe1EC2 and stobadine was recorded at concentrations ≥ 100 µM (Figure 4b).
Figure 4.
Effect of SMe1EC2 (-●-) and stobadine (-○-) on proliferation of HUVEC (a) and HUVEC-ST (b). MTT assay after 72-h incubation of cells with the compounds.
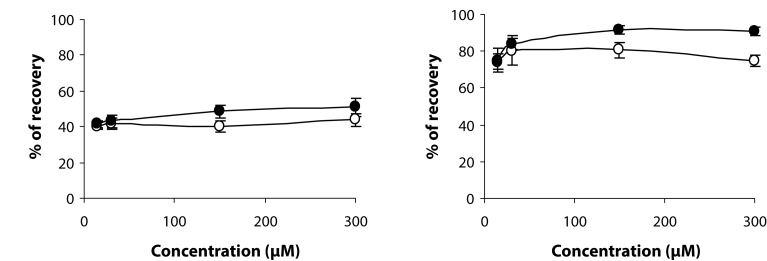
Migration of HUVECs by using wound-healing assay
Migration of HUVECs, by using wound-healing technique, was analyzed after 12 h and 24 h. The percentage of recovery was slightly increased in the case of SMe1EC2-treated cells. Stobadine did not exert any statistically significant effect (Figure 5).
Figure 5.
Effect of SMe1EC2 (-●-) and stobadine (-○-) on the migration of HUVEC after 12 (a) or 24 (b) hours.
Discussion
The pyridoindole stobadine has been postulated as a chain-breaking antioxidant characterized by the ability to scavenge chain-propagating peroxyl radicals (Steenken et al., 1992; Stefek et al., 1992; Kagan et al., 1993; Stefek and Trnkova 1996). The center of the antioxidant activity of stobadine and related substituted pyridoindoles was identified to reside at the indolic nitrogen (Rackova et al., 2002). Structural alterations in the close proximity of the indolic nitrogen, especially aromatic substitution in positions o and p, were found to influence the antioxidant efficacy (Rackova et al., 2002, Rackova et al., 2006). Moreover, alteration in the synthetically accessible position N2 provides the opportunity to vary basicity and lipophilicity of the compounds, thus optimizing bioavailability without affecting the intrinsic antiradical activity (Rackova et al., 2006).
The subject of the present study was SMe1EC2, the methoxy analogue of stobadine, whose acyl substituent at the position N2 was expected to decrease the basicity of this site without changing significantly the lipohilicity of the molecule. At the same time, the electron donating methoxy group at the aromatic position 8 was supposed to contribute to the elevation of the free radical scavenging activity compared to the parent stobadine.
In the first series of experiments, the intrinsic antioxidant activity of SMe1EC2 in comparison to stobadine was tested in a cell-free system comprising oxidant-sensitive fluorescent probes, H2R123 and H2DCF (Crow 1997; Kalyanaraman et al., 2012), exposed to AAPH-derived peroxyl radicals. Under the experimental conditions used, SMe1EC2 protected more efficiently H2R123 and H2DCF from their oxidation than did stobadine.
In homogeneous cell-free systems, antioxidant activity stems from an intrinsic chemical reactivity towards radicals. In membranes, however, the relative reactivities may be different since they are determined also by additional factors such as location of the antioxidant and radicals, ruled predominantly by their actual distribution ratios between water and lipid compartments. As we reported earlier (Stefek et al., 2013), SMe1EC2 and stobadine have similar lipophilicities, characterized by the corresponding log P values of 1.95 and 1.79, respectively. Yet their actual distribution ratios at pH 7.4 were shown to differ profoundly (calculated log DSMe1EC2=1.78 vs. calculated log Dstobadine=–0.05) as a result of basicity variance of SMe1EC2 vs. stobadine, characterized by respective pKa values, –3.7 vs. 8.5.
SMe1EC2 was found more effective than stobadine in scavenging reactive oxygen/nitrogen species in stimulated macrophage RAW 264.7 cell cultures by using H2R123 and H2DCF DA as fluorogenic probes.
Studies in HUVEC cell line revealed that both SMe1EC2 and stobadine, in concentrations up to 200 µM, did not decrease significantly the viability of the cells. On the other hand, SMe1EC2 slightly stimulated HUVEC proliferation at 50 µM concentration. Yet, in relation to HUVEC-ST, some cytotoxic effect of both compounds studied was recorded at concentrations ≥100 µM. Moreover, SMe1EC2 slightly stimulated the migration of HUVEC while stobadine failed to exert any effect.
To conclude, the present outcomes, in the context of preceding findings, indicate that modification of the hexahydropyridoindole skeleton of stobadine may yield congeners with increased antiradical efficacy and bioavailability, and that at reduced side effects.
Acknowledgements
Financial support by the grants VEGA 2/0067/11 and COST CM1001 action is gratefully acknowledged.
Declaration of interest
The authors report no conflict of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Crow JP. Dichlorodihydrofluorescein and dihydrorhodamine 123 are sensitive indicators of peroxynitrite in vitro: implications for intracellular measurement of reactive nitrogen and oxygen species. Nitric Oxide. 1997;1(2):145–157. doi: 10.1006/niox.1996.0113. [DOI] [PubMed] [Google Scholar]
- Ischiropoulos H, Gow A, Thom SR, Kooy NW, Royall JA, Crow JP. Detection of reactive nitrogen species using 2,7-dichlorodihydrofluorescein and dihydrorhodamine 123. Methods Enzymol. 1999;301:367–373. doi: 10.1016/s0076-6879(99)01100-3. [DOI] [PubMed] [Google Scholar]
- Gasparova Z, Janega P, Babal P, Snirc V, Stolc S, Mach M, Ujhazy E. Effect of the new pyridoindole antioxidant SMe1EC2 on functional deficits and oedema formation in rat hippocampus exposed to ischaemia in vitro. Neuro Endocrinol Lett. 2009;30:574–581. [PubMed] [Google Scholar]
- Gasparova Z, Ondrejickova O, Gajdosikova A, Gajdosik A, Snirc V, Stolc S. Oxidative stress induced by the Fe2+/ascorbic acid system or model ischemia in vitro: Effect of carvedilol and pyridoindole antioxidant SMe1EC2 in young and adult rat brain tissue. Interdiscip Toxicol. 2010;3:122–126. doi: 10.2478/v10102-010-0051-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparova Z, Snirc V, Stolc S. The new pyridoindole antioxidant SMe1EC2 and its intervention in hypoxia/hypoglycemia-induced impairment of longterm potentiation in rat hippocampus. Interdiscip Toxicol. 2011;4:56–61. doi: 10.2478/v10102-011-0011-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparova Z, Stara V, Stolc S. Effect of antioxidants on functional recovery after in vitro-induced ischemia and long-term potentiation recorded in pyramidal layer of the CA1 area of rat hippocampus. Gen Physiol Biophys. 2014;33(1):43–52. doi: 10.4149/gpb_2013062. [DOI] [PubMed] [Google Scholar]
- Horakova L, Stolc S. Antioxidant and pharmacodynamic effects of pyridoindole stobadine. Gen Pharmacol. 1998;30:627–638. doi: 10.1016/s0306-3623(97)00300-5. [DOI] [PubMed] [Google Scholar]
- Jaffe EA, Nachman RL, Becker CG, Minick CR. Culture of human endothelial cells derived from umbilical veins. J Clin Invest. 1973;52:2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juranek I, Horakova L, Rackova L, Stefek M. Antioxidants in treating pathologies involving oxidative damage: an update on medicinal chemistry and biological activity of stobadine and related pyridoindoles. Curr Med Chem. 2010;17:552–570. doi: 10.2174/092986710790416317. [DOI] [PubMed] [Google Scholar]
- Kagan VE, Tsuchiya M, Serbinova E, Packer L, Sies H. Interaction of the pyridoindole stobadine with peroxyl, superoxide and chromanoxyl radicals. Biochem Pharmacol. 1993;45:393–400. doi: 10.1016/0006-2952(93)90075-8. [DOI] [PubMed] [Google Scholar]
- Kalyanaraman B, Darley-Usmar V, Davies KJ, Dennery PA, Forman HJ, Grisham MB, Mann GE, Moore K, Roberts LJ, 2nd, Ischiropoulos H. Measuring reactive oxygen and nitrogen species with fluorescent probes: challenges and limitations. Free Radic Biol Med. 2012;52(1):1–6. doi: 10.1016/j.freeradbiomed.2011.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rackova L, Cumaoglu A, Bagriacik EU, Stefek M, Maechler P, Karasu C. Novel hexahydropyridoindole derivative as prospective agent against oxidative damage in pancreatic β cells. Med Chem. 2011;7:711–717. doi: 10.2174/157340611797928370. [DOI] [PubMed] [Google Scholar]
- Rackova L, Snirc V, Jung T, Stefek M, Karasu C, Grune T. Metabolism-induced oxidative stress is a mediator of glucose toxicity in HT22 neuronal cells. Free Radic Res. 2009;43:876–886. doi: 10.1080/10715760903104374. [DOI] [PubMed] [Google Scholar]
- Rackova L, Snirc V, Majekova M, Majek P, Stefek M. Free radical scavenging and antioxidant activities of substituted hexahydropyridoindoles. Quantitative structure-activity relationships. J Med Chem. 2006;49:2543–2548. doi: 10.1021/jm060041r. [DOI] [PubMed] [Google Scholar]
- Rackova L, Stefek M, Majekova M. Structural aspects of antioxidant activity of substituted pyridoindoles. Redox Rep. 2002;7(4):207–214. doi: 10.1179/135100002125000578. [DOI] [PubMed] [Google Scholar]
- Sotnikova R, Nedelcevova J, Navarova J, Nosalova V, Drabikova K, Szocs K, Krenek P, Kyselova Z, Bezek S, Knezl V, Drimal J, Broskova Z, Kristova V, Okruhlicova L, Bernatova I, Bauer V. Protection of the vascular endothelium in experimental situations. Interdiscip Toxicol. 2011;4:20–26. doi: 10.2478/v10102-011-0005-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spadafora-Ferreira M, Lopes AA, Coelho V, Guilherme L, Kalil J. Two novel anti-von Willebrand factor monoclonal antibodies. Thromb Res. 2000;97:3–13. doi: 10.1016/s0049-3848(99)00122-x. [DOI] [PubMed] [Google Scholar]
- Steenken S, Sunquist AR, Jovanovic SV, Crockett R, Sies H. Antioxidant activity of the pyridoindole stobadine. Pulse radiolytic characterization of one-electron-oxidized stobadine and quenching of singlet molecular oxygen. Chem Res Toxicol. 1992;5:355–360. doi: 10.1021/tx00027a006. [DOI] [PubMed] [Google Scholar]
- Stefek M, Masarykova M, Benes L. Inhibition of cumene hydroperoxide-induced lipid peroxidation by a novel pyridoindole antioxidant in rat liver microsomes. Pharmacol Toxicology. 1992;70:407–411. doi: 10.1111/j.1600-0773.1992.tb00498.x. [DOI] [PubMed] [Google Scholar]
- Stefek M, Milackova I, Juskova-Karasova M, Snirc V. Antioxidant action of the hexahydropyridoindole SMe1EC2 in the cellular system of isolated red blood cells in vitro. Redox Rep. 2013;18(2):71–75. doi: 10.1179/1351000213Y.0000000043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefek M, Trnkova Z. The pyridoindole antioxidant stobadine prevents alloxan-induced lipid peroxidation by inhibiting its propagation. Pharmacology and Toxicology. 1996;78:77–81. doi: 10.1111/j.1600-0773.1996.tb00183.x. [DOI] [PubMed] [Google Scholar]
- Stolc S, Povazanec F, Bauer V, Majekova M, Wilcox AL, Snirc V, Rackova L, Sotnikova R, Stefek M, Gasparova-Kvaltinova Z, Gajdosikova A, Mihalova D. Pyridoindole derivatives with antioxidant properties: synthesis, therapy and pharmaceutical remedies. Slovak Patent Agency. 2010:287506. [Google Scholar]
- Stolc S, Snirc V, Gajdosikova A, Gajdosik A, Gasparova Z, Ondrejickova O, Sotnikova R, Viola A, Rapta P, Jariabka P, Synekova I, Vajdova M, Zacharova S, Nemcek V, Krchnarova V. New pyridoindoles with antioxidant and neuroprotective actions. In: Bauer V, editor. Trends in Pharmacological Research. Bratislava: Institute of Experimental Pharmacology; 2008. pp. 118–136. [Google Scholar]
- Stolc S, Snirc V, Majekova M, Gasparova Z, Gajdosikova A, Stvrtina S. Development of the new group of indole-derived neuroprotective drugs affecting oxidative stress. Cell Mol Neurobiol. 2006;26(7–8):1495–1504. doi: 10.1007/s10571-006-9037-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ujhazy E, Dubovicky M, Ponechalova V, Navarova J, Brucknerova I, Snirc V, Mach M. Prenatal developmental toxicity study of the pyridoindole antioxidant SMe1EC2 in rats. Neuro Endocrinol Lett. 2008;29(5):639–643. [PubMed] [Google Scholar]
- Ujházy E, Mach M, Navarová J, Brucknerová I, Dubovický M. Safety assessment of the pyridoindole derivative SMe1EC2: developmental neurotoxicity study in rats. Interdiscip Toxicol. 2011;4(1):47–51. doi: 10.2478/v10102-011-0009-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurova-Nedelcevova J, Navarova J, Drabikova K, Jancinova V, Petrikova M, Bernatova I, Kristova V, Snirc V, Nosalova V, Sotnikova R. Participation of reactive oxygen species in diabetes-induced endothelial dysfunction. Neuro Endocrinol Lett. 2006;27(Suppl.2):168–171. [PubMed] [Google Scholar]