Abstract
Curcumin is a natural product widely consumed by humans. It has many biological properties. In this study, we investigated the radiosensitive effect of curcumin on thyroid cancer cells against cellular toxicity induced by 131-I. Human thyroid cancer and human non-malignant fibroblast cells (HFFF2) were treated with 131-I and/or curcumin at different concentrations (5, 10 and 25 µg/ml) for 48 h. The cell proliferation was measured by determination of the surviving cells by using MTT assay. Our results showed that curcumin increased the killing effect of 131-I on thyroid cancer cells, while it exerted no toxicity on HFFF2 cells. This result shows a promising effect of curcumin on the enhancement of therapeutic effects of 131-I in patients.
Keywords: 131-I, curcumin, anti-proliferation, MTT, thyroid cancer cell
Introduction
Radioiodine-131 (131I) has been used as the first line of treatment for hyperthyroidism, Graves’ disease and differentiated thyroid cancer. It has a physical half-life of 8.02 days and emits gamma rays and beta particles (Sawin et al., 1997, Zanzonico, 1997, Robbins et al., 2005). It concentrates in thyroid cells and kills tumor cells, yet it has several side effects such as sialadenitis, gastrointestinal symptoms, xerostomia, temporary bone-marrow suppression and neoplasia (Bushnell et al., 1992, Noaparast et al., 2013). 131I may also induce genetic damage and chromosomal instability in normal cells that may result in secondary malignancies (Baugnet-Mahieu et al., 1994, Watanabe et al., 2004, Hosseinimehr et al., 2013). The cytotoxic effect of 131I is mainly related to beta particles. Ionizing radiation causes cellular injury mainly by producing reactive oxygen species (ROS). ROS can induce lipid peroxidation and damage to cellular membranes and critical macromolecules such as DNA (Little, 2000, Noaparas et al., 2013). Curcumin is a major component of turmeric, produced from the rhizome of the plant Curcuma longa (Chendil et al., 2004). Many studies have indicated that curcumin has strong pharmacological activities such as anti-oxidant, anti-cancer (Kuttan et al., 1985), anti-microbial effects (Negi et al., 1999). Curcumin can scavenge free radicals and protect the cellular macromolecules against oxidative stress (Kalpana et al., 2004, Polasa et al., 2004, Singh et al., 2012). Recently we showed that curcumin protected human lymphocytes against genotoxicity induced by 131I and it significantly reduced the DNA damage induced by 131I in vitro (Shafaghati et al., 2014). Although curcumin exhibited protective effects on chromosome damage induced by 131I in normal cells, its effect on thyroid cancer cells during 131I treatment is not clear.
The aim of this study was to determine the therapeutic effect of curcumin on cell death induced by 131I in thyroid human cancer cells and human non-malignant fibroblast cells in vitro.
Materials and methods
Cell lines
Human non-malignant skin fibroblasts (HFFF2) and human thyroid cancer (Thr.C1-PI 33) cell line were obtained from the Iranian Pasteur Institute (Tehran). The cells were grown at 37°C and 5% CO2 in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS), penicillin 100 IU/mL, and streptomycin 100 µg/ml, all of which were obtained from Gibco (Invitrogen, USA).
MTT assay
Thyroid cancer and HFFF2 cells were subjected to cell proliferation assay by using MTT. The MTT colorimetric assay is used for evaluation of cell toxicity. The MTT test is based on the strength of mitochondrial enzymes to decrease MTT (pale yellow) to formazan crystals (dark blue). Owing to their impenetrability through the cell membrane, formazan crystals collect in cells (Ashrafi et al., 2012). Cells (20,000) were seeded in 96-well plates. After 24 h incubation, the cells were treated with various concentrations of curcumin (CM) (5, 10 and 25 µg/ml) and were incubated at 37 °C and 5% CO2. After 48 h incubation, 20 µL of MTT (5 mg/mL in phosphate buffer saline) was added to each well, and the cells were incubated for 4 hours. After removal of the medium, dimethyl sulfoxide (DMSO) was used to solubilize the formazan compounds and the cell plates were shaken for 10 minutes. The absorbance of every culture well was read on an ELISA Reader (Bioteck, USA). Cells without any treatment were used as control for comparison of absorbance and cell survival.
Irradiation protocol
Cells were seeded in 96-well plates. After 24 h incubation, the cells were treated with various concentrations of CM (5, 10 and 25 µg/ml) and incubated at 37°C and 5% CO2. After 2h incubation, the diluted solution of 131I was added at the dose of 10 µCi (100 µl) to each well and incubated for 48 h. MTT assay was performed according to the above protocol.
Statistical analysis
Data were presented as mean ± standard deviation (SD) of four experiments. Data were compared and the differences were considered significant if the p-value<0.05.
Results
Effect of curcumin on cell proliferation in thyroid cancer and HFFF2 cells
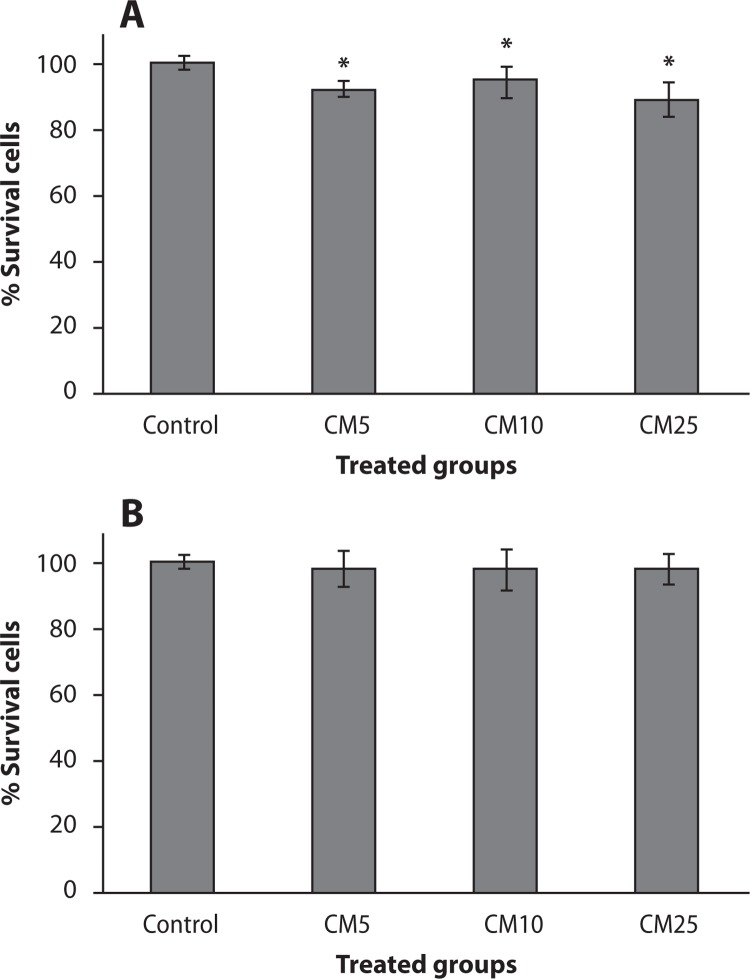
The effect of curcumin on cell proliferation in thyroid cancer and HFFF2 cells is shown in Figure 1. In thyroid cancer cells, a statistically significantly reduced cell proliferation was observed in curcumin treatments at concentrations of 5, 10 and 25 µg/ml (p<0.02). The percentage of survival in thyroid cancer cells was 92.5±2.4, 95±4.9 and 89.4±5.3 at concentrations of 5, 10 and 25 µg/ml, respectively. A statistically significant difference was observed between the doses of 5, 10 and 25 µg/ml of curcumin with control for cellular anti-proliferation (Figure 1A). No significant toxicity was observed in HFFF2 cells treated by any of the doses of curcumin (Figure 1B).
Figure 1.
Effect of curcumin (CM) at different concentrations (5, 10 and 25 µg/ml) on thyroid cancer cells (A) and non-malignant fibroblast cells (HFFF2) (B). Cell proliferation was assayed with MTT test. *p<0.05, comparison CM5, CM10 and CM25 with control
Effect of curcumin and 131I combination on cell proliferation in thyroid cancer and HFFF2 cells
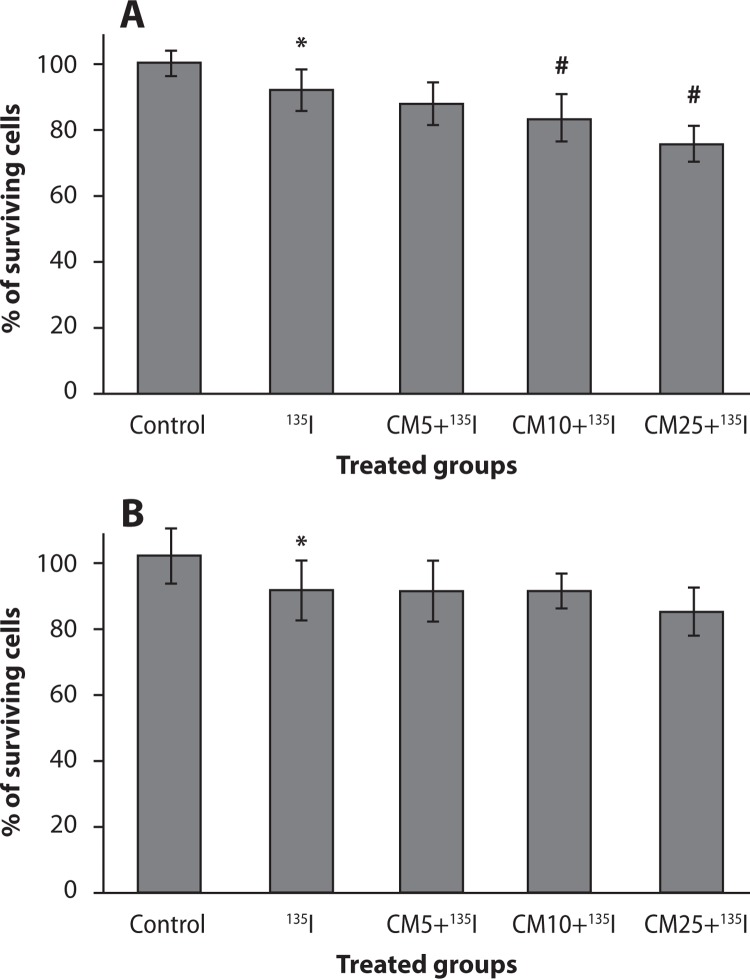
The combination effects of curcumin and 131I on the percentage of cell proliferation in control, curcumin-pretreated, and/or 131I treated thyroid cancer and HFFF2 cells are shown in Figure 2. 131I significantly reduced the survival rate in thyroid cancer cells by 91%. Thyroid cancer cell proliferation was reduced in pre-treated curcumin groups. Curcumin reduced the percentage of cell survival to 87±6%, 83±7% and 75±5% at concentrations 5, 10 and 25 µg/ml, respectively. Curcumin significantly increased cell death in the dose of 10 and 25 µg/ml in combination with 131I as compared to 131I alone (p<0.05). These results show that curcumin has a synergistic effect with 131I on cell growth inhibition in thyroid cancer cells; it is related to the radiosensitive effect of curcumin on thyroid cancer cells treated with 131I. Interestingly, curcumin at all doses of 5, 10 and 25 µg/ml did not show any enhancement of toxicity on HFFF2 cells in combination with 131I.
Figure 2.
Effect of curcumin (CM) at different concentrations (5, 10 and 25 µg/ml) in combination with 131I on thyroid cancer cells (A) and non-malignant fibroblast cells (HFFF2) (B). Cell proliferation was assayed with MTT test. *p<0.05, comparison control group with 131I group; #p<0.05, comparison CM10 and CM25 groups with 131I group
Discussion
In this study, we observed that curcumin exerted a radiosensitive effect on thyroid cancer cells; it reduced significantly cell growth in combination with 131I. Curcumin did not exhibit any cellular toxicity in non-malignant fibroblast cells (HFFF2) treated at the same doses with 131I. Iodine-131 is widely used for the treatment of thyroid-related diseases. High-dose radioiodine treatment is associated with dose-limited side effects. 131I emits gamma and beta rays; the latter ones have a short range board with higher destroying effects on cells as compared to gamma rays. Induction of oxidative stress is one of the main mechanisms for therapeutic and /or side effects of 131I. Oxidative stress may cause DNA damage. Several studies showed that curcumin exerted radioprotective effects on normal cells such as human lymphocytes and fibrosis in the rat lung. Protective effects of curcumin are related to free radical scavenging and enhancement of enzymatic and non-enzymatic antioxidants like GSH in cells treated with curcumin (Srinivasan et al., 2006, Cho et al., 2013).
Recently we showed that curcumin significantly protected human lymphocytes from genotoxicity induced by 131I. Curcumin reduced micronuclei frequency in lymphocytes in combination with 131I (Shafaghati et al., 2014). In this study we tried to evaluate the effect of curcumin on thyroid cancer cell, because it was hypothesized that curcumin could enhance cellular toxicity induced by 131I in thyroid cancer cells. Our results showed that curcumin increased radiation toxicity in thyroid cancer cells and it was showed no toxicity on non-malignant human cells induced by 131I. These results are promising for using this natural product in combination with 131I therapy in patients. Curcumin has been shown to affect mediated several cell signaling pathways such as apoptosis (activation of caspases and down regulation of anti-apoptotic gene products) (Agrawal et al., 2010). Also, curcumin sensitized human cancer cells on exposure to external gamma radiation, which is a dual benefit t of curcumin in patients with cancer therapy (Kunnumakkara et al., 2008, Goel et al., 2010, Lopez-Jornet et al., 2011).
Our findings indicate that curcumin is a promising natural product for patients on radioiodine therapy by radiosensitizing thyroid cancer cells in combination with 131I.
Acknowledgments
This study was supported by a grant from Mazandaran University of Medical Sciences, Sari, Iran. This research was the subject of a Pharm.D thesis of A.H. Hosseini as a student of Mazandaran University of Medical Sciences.
Conflict of interest statement
The authors declared no potential conflict of interest with respect to the authorship, and/or publication of this study.
REFERENCES
- Agrawal DK, Mishra PK. Curcumin and its analogues: potential anticancer agents. Med Res Rev. 2010;30:818–860. doi: 10.1002/med.20188. [DOI] [PubMed] [Google Scholar]
- Ashrafi SA, Hosseinimehr SJ, Varmira K, Abedi SM. Radioimmunotherapy with (131)I-bevacizumab as a specific molecule for cells with overexpression of the vascular endothelial growth factor. Cancer Biother Radiopharm. 2012;27:420–425. doi: 10.1089/cbr.2012.1224. [DOI] [PubMed] [Google Scholar]
- Baugnet-Mahieu L, Lemaire M, Leonard ED, Leonard A, Gerber GB. Chromosome aberrations after treatment with radioactive iodine for thyroid cancer. Radiat Res. 1994;140:429–431. [PubMed] [Google Scholar]
- Bushnell Dl, Boles MA, Kaufman GE, Wadas MA, Barnes WE. Complications, sequela and dosimetry of Iodine-131 therapy for thyroid carcinoma. J Nucl Med. 1992;33:2214–2221. [PubMed] [Google Scholar]
- Chendil D, Ranga RS, Meigooni D, Sathishkumar S, Ahmed MA. Curcumin confers radiosensitizing effect in prostate cancer cell line Pc-3. Oncogene. 2004;23(8):1599–607. doi: 10.1038/sj.onc.1207284. [DOI] [PubMed] [Google Scholar]
- Cho YJ, Yi CO, Jeon BT, Jeong YY, Kang GM, Lee JE, et al. Curcumin attenuates radiation-induced inflammation and fibrosis in rat lungs. Korean J Physiol Pharmacol. 2013;17:267–274. doi: 10.4196/kjpp.2013.17.4.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel A, Aggarwal BB. Curcumin, the golden spice from indian saffron, is a chemosensitizer and radiosensitizer for tumors and chemoprotector and radioprotector for normal organs. Nutr Cancer. 2010;62:919–930. doi: 10.1080/01635581.2010.509835. [DOI] [PubMed] [Google Scholar]
- Hosseinimehr SJ, Shafaghati N, Hedayati M. Genotoxicity induced by iodine-131 in human cultured lymphocytes. Interdiscip Toxicol. 2013;6:74–76. doi: 10.2478/intox-2013-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalpana C, Menon VP. Curcumin ameliorates oxidative stress during nicotine-induced lung toxicity in Wistar rats. Ital J Biochem. 2004;53:82–86. [PubMed] [Google Scholar]
- Kunnumakkara AB, Diagaradjane P, Guha S, Deorukhkar A, Shentu S, Aggarwal BB, et al. Curcumin sensitizes human colorectal cancer xenografts in nude mice to gamma-radiation by targeting nuclear factor-kappab-regulated gene products. Clin Cancer Res. 2008;14:2128–2136. doi: 10.1158/1078-0432.CCR-07-4722. [DOI] [PubMed] [Google Scholar]
- Kuttan R, Bhanumathy P, Nirmala K, George MC. Potential anticancer activity of turmeric (Curcuma longa) Cancer Lett. 1985;29:197–202. doi: 10.1016/0304-3835(85)90159-4. [DOI] [PubMed] [Google Scholar]
- Little JB. Radiation Carcinogenesis. Carcinogenesis. 2000;21:397–404. doi: 10.1093/carcin/21.3.397. [DOI] [PubMed] [Google Scholar]
- Lopez-Jornet P, Camacho-Alonso F, Gomez-Garcia F. Effect of curcumin and irradiation in Pe/Ca-Pj15 oral squamous cell carcinoma. Acta Odontol Scand. 2011;69:269–273. doi: 10.3109/00016357.2011.554864. [DOI] [PubMed] [Google Scholar]
- Negi PS, Jayaprakasha GK, Jagan Mohan Rao L, Sakariah KK. Antibacterial activity of turmeric oil: A byproduct from curcumin manufacture. J Agric Food Chem. 1999;47:4297–4300. doi: 10.1021/jf990308d. [DOI] [PubMed] [Google Scholar]
- Noaparast Z, Hosseinimehr SJ. Radioprotective agents for the prevention of side effects induced by radioiodine-131 therapy. Future Oncology. 2013;9:1145–1159. doi: 10.2217/fon.13.79. [DOI] [PubMed] [Google Scholar]
- Polasa K, Naidu AN, Ravindranath I, Krishnaswamy K. Inhibition of B(a)P induced strand breaks in presence of curcumin. Mutat Res-Gen Tox En. 2004;557:203–213. doi: 10.1016/j.mrgentox.2003.10.016. [DOI] [PubMed] [Google Scholar]
- Robbins RJ, Schlumberger MJ. The evolving role of 131I for the treatment of differentiated thyroid carcinoma. J Nucl Med. 2005;46:28S–37S. [PubMed] [Google Scholar]
- Sawin Ct, Becker DV. Radioiodine and the treatment of hyperthyroidism: The early history. Thyroid. 1997;7:163–176. doi: 10.1089/thy.1997.7.163. [DOI] [PubMed] [Google Scholar]
- Shafaghati N, Hedayati N, Hosseinimehr SJ. Protective effects of curcumin against genotoxicity induced by 131-iodine in human cultured lymphocyte cells. Pharmacognosy Magazine. 2014;10:106–110. doi: 10.4103/0973-1296.131020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh G, Sharma S, Choudhary N, Yadav S, Chauhan R, Dwivedi J. Evaluation of radioprotective properties of curcuma longa rhizome extract: a cytogenetic analysis in cancer. Pharmacognosy Communications. 2012;2:44–49. [Google Scholar]
- Srinivasan M, Rajendra Prasad N, Menon VP. Protective effect of curcumin on gamma-radiation induced dna damage and lipid peroxidation in cultured human lymphocytes. Mutat Res. 2006;611:96–103. doi: 10.1016/j.mrgentox.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Watanabe N, Kanegane H, Kinuya S, Shuke N, Yokoyama K, Kato H, et al. The radiotoxicity of 131I therapy of thyroid cancer: assessment by micronucleus assay of b lymphocytes. J Nucl Med. 2004;45:608–611. [PubMed] [Google Scholar]
- Zanzonico PB. Radiation dose to patients and relatives incident to 131I therapy. Thyroid. 1997;7:199–204. doi: 10.1089/thy.1997.7.199. [DOI] [PubMed] [Google Scholar]