Abstract
A number of seaweed species are used as traditional foods and medicine in different parts of the world, including Asian countries. However, very few data on the anti-melanogenic effect of seaweed have been published. Undaria pinnatifida (Dolmiyeok), a brown alga, is a traditional food in Jeju Island, the southern regions of the Korea peninsula. In this study, ethylacetate extracts of U. pinnatifida (UPE) were examined for their anti-melanogenic potentials. Our results supports the finding that UPE down-regulated melanin content in a dose-dependent pattern. To clarify the target of UPE action in melanogenesis, we performed Western blotting for tyrosinase and microphthalmia-associated transcription factor (MITF), which are key melanogenic enzymes. UPE inhibited tyrosinase and MITF expressions in a dose-dependent manner. These results indicate that treatment with UPE significantly inhibits the melanogenesis in B16 cells, and may be effective in the whitening agent for the skin.
Keywords: Undaria pinnatifida, melanin, melanogenesis, tyrosinase, MITF
Introduction
There are many traditional foodstuffs in Korea. Undaria pinnatifida, called “Dolmiyeok or Miyeok” is one of them and is considered to be a healthy foodstuff in Korea. It is a very large brown alga, golden-brown in colour, related to the Laminaria species and other kelps. Adult specimens can grow to an overall length of between 1.5 and 3 m in less than a year – a rate of growth of up to one centimetre per day. Undaria pinnatifida is widely distributed along the coasts of Korea, Japan, China and Russia. Considering the traditional concept, several studies have focused on the beneficial effects of U. pinnatifida on anti-inflammatory (Khan et al., 2009; Yoo et al., 2012), anti-oxidant (Han et al., 2004; Hu et al., 2010; Moreira et al., 2010), antihypertensive (Suetsuna et al., 2004), antiviral (Thompson & Dragar, 2004; Hayashi et al., 2013), anticoagulating (Kim et al., 2010), and antiobesity (Okada et al., 2011) activities. However, the anti-melanogenic effect of U. pinnatifida extract has not been reported until now. Therefore, we conducted a detailed study to investigate the anti-melanogenic effects of U. pinnatifida extract in mouse B16 melanoma cells.
Melanin, produced by melanocytes in the basal layer of the epidermis, is principally responsible for skin colour and plays an important role in preventing skin damage caused by ultraviolet (UV) radiation. Melanin synthesis begins the conversion of L-tyrosine to 3,4-dihydroxyphenylalanine (L-DOPA) and then the oxidation of L-DOPA yields dopaquinone by tyrosinase enzyme, catalyzing the rate-limiting step for the melanin biosynthesis. This tyrosinase enzyme is involved in abnormal accumulation of melanin pigments, called hyperpigmentation (Wu et al., 2012; Chai et al., 2013). Therefore, tyrosinase inhibitors have been established as important constituents of whitening and depigmenting agents (Khan, 2012; Liang et al., 2013). However, side effects have been caused by the chemical inhibitors of tyrosinase. For example, arbutin caused a possible genotoxic effect and kojic acid, due to pigmented contact caused dermatitis. Thus the search for a safe and effective skin whitening agent is still a target of many studies in cosmetic industry (Yoon et al., 2010c; Kim et al., 2013).
A variety of seaweeds provide an interesting, largely unexplored source for the development of potential new drugs and skin-care cosmetics (Yang et al., 2010a;b;d; Yoon et al., 2010a; d). They have existed from antiquity to the present and have played significant roles in skin health and drug discovery, especially anti-inflammatory agents against skin diseases (Moon et al., 2011, Yang et al., 2010c; Yoon et al., 2010b). The seaweed extracts are also known to be inhibitors of melanin production, sometimes more potent than the classical inhibitors hydroquinone/arbutin or kojic acid, and not associated with side effects. Therefore, the present study focused on whether the ethyl acetate fraction from U. pinnatifida (UPE) inhibited melanin production and melanogenic protein expression in mouse B16 melanoma cells.
Materials and methods
Materials and solvent extraction
U. pinnatifida specimens were collected in April 2010 from Gapa Island, Korea. The specimen voucher (no. CSC-201) is deposited with Cosmetic Science Center, Department of Chemistry, Jeju National University, frozen and stored at –20°C until use. For extraction, the material was first ground into a fine powder and freeze-dried using a vacuum freeze-dryer. The dried powder (90g) was extracted with 80% ethanol (EtOH; 2 L) at room temperature for 24 h and then evaporated under vacuum. The evaporated EtOH extract (5g) was suspended in water (1L) and fractionated with ethyl acetate (EtOAc; 500mL). The yield and recovery of EtOAc fractions were 0.6535g and 13.1%, respectively.
Cell cultures
B16 murine melanoma cells were obtained from the Korean Cell Line Bank (Seoul). Cells were maintained in Dulbecco's modified Eagle's medium (DMEM), supplemented with 10% foetal bovine serum (FBS, Hyclone, Logan, UT, U.S.A.) and 1% penicillin-streptomycin (10,000 U/ml and 10,000 g/ml, respectively) in 5% CO2 at 37°C.
Cell viability assay
Cell viability assay was measured as described previously, with slight modification (Yoon et al., 2010c). Cell viability was determined by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Cells were seeded on 96-well plates, and drug treatment began 18 h after seeding, B16F10 murine melanoma cells were incubated with various concentrations of UPE for 72h at 37°C in a humidified 95% air and 5% CO2 atmosphere. MTT (1mg/mL in phosphate-buffer saline, PBS) was added to each well in a 1/10 volume of medium. Cells were incubated at 37°C for 4h. Finally, the supernatant was removed and the formazan crystals were dissolved in DMSO. Absorbance was measured at 570nm. Percent of cells showing cytotoxicity was determined relative to the control group.
Measurement of melanin contents
Extracellular melanin release was measured as described previously, with slight modification (Yoon et al., 2010c). Cells of the murine melanoma cell line, B16F10, plated at 1.0×105 cells/mL, were stimulated with α-MSH (50nM) and then incubated with aliquots of UPE (3.125, 6.25 and 12.5µg/mL) at 37°C for 72h; the cells were then washed in ice-cold phosphate-buffered saline. Briefly, the samples were incubated at 80°C for 1h in 1mL of 1N NaOH/10% DMSO and then vortexed to solubilize the melanin; the absorbance at 405nm was then measured. Further, the melanin content was determined based on the absorbance/µg of protein in the extract from each cell. The protein concentration of the cells was determined by using a protein assay kit (Pierce, Rockford, IL, USA).
Determination of cellular tyrosinase activity
Cellular tyrosinase activity was measured by the method of Yen et al. with some modification (Yen et al., 2012). Briefly, the culture method for determining cellular tyrosinase assay was similar to that for determining melanin content. After treatment with different concentrations of UPE for 72 h, the cells were collected after treatment with trypsin-EDTA and centrifuged at 15,000 rpm for 15 min to obtain cell pellets. The pellet solutions were frozen and thawed twice and then centrifuged at 15,000 rpm for 15 min. We added 80µL of the supernatant in a 96-well plate and mixed it with 20µL of 0.2% L-DOPA. After incubation for 1h, the optical densities were measured at 475nm using a microplate spectrophotometer. The inhibitory activity of the UPE treated cells was presented as percentage against that of the untreated cells.
Measurement of tyrosinase and MITF in melanoma B16/F10 cells by Western blot
To determine the amount of tyrosinase and MITF protein, Western blotting analysis was performed. B16 melanoma cells that had been stimulated by α-MSH (50nM) were treated with UPE (3.125, 6.25 and 12.5 µg/mL) for 3 days. After treatment, the cells were collected and lysed with cell lysis buffer [50mM Tris–HCl (pH 6.8), 2% SDS, 6% mercaptoethanol, 1% glycerol]. Whole cell lysates (5×104 cells equivalents per lane) were separated by 7.5% SDS-polyacrylamide gel electrophoresis as described previously and transferred to a polyvinylidene fluoride (PVDF) membrane. The membrane was blocked with 5% skimmed milk in phosphate-buffered saline containing 0.05% Tween 20. Tyrosinase and MITF bands were detected with rabbit polyclonal anti-tyrosinase antibody (dilution 1:1000) and rabbit polyclonal anti-MITF antibody (dilution 1:500), respectively, purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA), and then further incubated with horseradish peroxidase-conjugated anti-rabbit IgG antibody at a 1:5000 dilution. Bound antibodies were detected using an enhanced chemiluminescence kit (Amersham Biosciences, Buckinghamshire, UK), following the manufacturer's instructions. Loading control was assessed using anti-β-actin antibody. Positive bands were analysed using a gel image analysis instrument.
Statistical analysis
All data were obtained in triplicate and are represented as means ± standard error (SE). Significant differences between treatments were determined by Student's t test in one-way analysis of variance (ANOVA).
Results and discussion
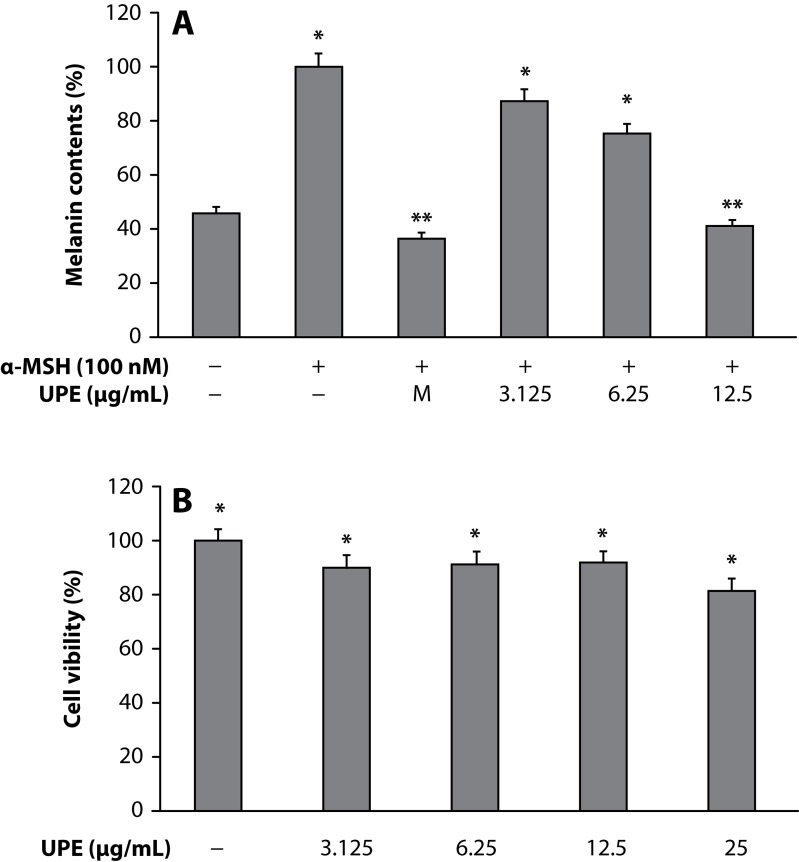
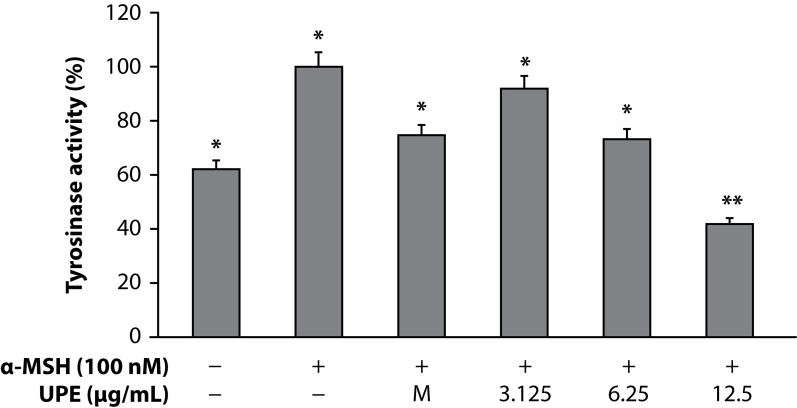
Melanin plays a crucial role in protecting the skin against harmful ultraviolet light, but overproduction and accumulation of melanin could create serious skin problems such as freckles, age pigment, and melasma. Thus, the inhibition of melanogenesis has been the focus on medicinal and cosmetic treatments for skin depigmenting and lightening. Therefore, this study focused on whether the ethyl acetate fraction from UPE inhibited melanin production and melanogenic protein expression in mouse B16 melanoma cells. In the present study, the changes in the melanin contents in the cells treated with UPE were evaluated for anti-melanogenesis activity. The melanin contents of cells were significantly attenuated by UPE in a dose-dependent manner (Figure 1A). In spite of a number of studies reporting on anti-melanogenic agents, such as hydroquinone, kojic acid, and arbutin, sometimes side effects such as irritation of the skin and exhibition of cell toxicity were observed. Therefore it is necessary to find potent natural products that act as anti-melanogenic agents without side effects. To investigate the cytotoxicity of UPE on cell proliferation, B16 murine melanoma cells were treated with various concentrations (3.125–25µg/mL) of UPE for 72h. As shown in Figure 1B, there was no significant difference in cell proliferation between control and UPE-treated cells until 12.5 µg/mL, suggesting that the inhibitory effects of UPE on melanin biosynthesis were not attributable to its cytotoxicity. Since cellular tyrosinase activity is also the major factor that stimulates melanin synthesis and ultimately induces melanogenesis, we determined to assess cellular tyrosinase activity for investigating the antimelanogenesis activity of UPE on B16 murine melanoma cells. B16 murine melanoma cells were pretreated with UPE at doses of 3.125–12.5µg/mL. UPE treatment significantly reduced the cellular tyrosinase activity in a dose-dependent manner compared to the control (Figure 2).
Figure 1.
Inhibitory effect of UPE on melanin content (A) and cell viability (B) of B16F10 cells. B16F10 cells (2.0×104 µg/mL) were pre-incubated for 18 h and the melanin content was assayed after incubation of the B16F10 cells treated with α-MSH (100 nM), melasolv (40 µM), and UPE (3.125, 6.25 and 12.5 µg/mL) for 72 h at 37°C in a 5% CO2 atmosphere. The absorbance was measured at 405 nm by ELISA. MTT assay was performed after incubation of the B16F10 cells treated with varying concentrations of UPE (3.125, 6.25 and 12.5 µg/mL) for 24 h at 37°C in a 5% CO2 atmosphere. The absorbance was measured at 570 nm with a spectrophotometer (Power Wave; Bio-tek, Winooski, VT). Values are the mean ± SEM of triplicate experiments. *p<0.05; **p<0.01.
Figure 2.
Inhibitory effect on tyrosinase activity of UPE in B16F10 cells. B16F10 cells (2.0×104 µg/mL) were pre-incubated for 18 h and tyrosinase activity was assessed after incubation of B16F10 cells treated with α-MSH (100 nM), melasolv (40 uM) and UPE (3.125, 6.25 and 12.5 µg/mL) for 72 h at 37°C in a 5% CO2 atmosphere. Absorbance was measured at 405 nm with a ELISA. Values are the mean ± SEM of triplicate experiments.*p<0.05; **p<0.01.
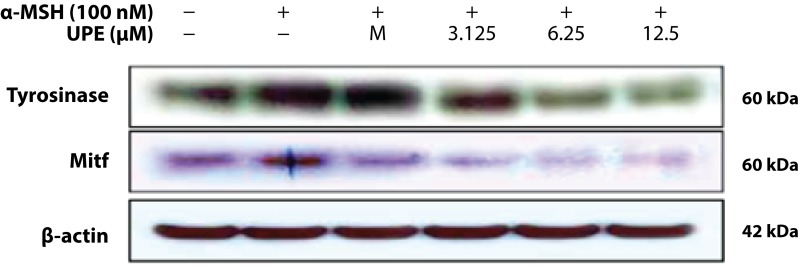
Human melanocytes are known to express tyrosinase and MITF is a factor that effectively transactivates tyrosinase. The expression of these proteins was evaluated using Western blot analysis. As compared with the untreated control values, UPE-treated cells showed dose-dependent decreases in tyrosinase and MITF expression (Figure 3).
Figure 3.
Inhibitory effect of the UPE on the protein level related to melanogenic factors in B16F10 cells. B16F10 cells (1.0×105cells/mL) were pre-incubated for 18 h and were stimulated with α-MSH (100nM) in the presence of melasolv (40 µM) and UPE (3.125, 6.25 and 12.5 µg/mL) for 24 h. The protein level was determined by immunoblotting.
In summary, we investigated the anti-melanogenic effects of UPE and related melanogenic activity. The present results suggest that MITF protein levels are reduced by UPE. The hypopigmentation effect of UPE may be the result of down-regulation of MITF gene expression, which would then repress the protein and gene expressions of tyrosinase. Therefore we suggest that UPE can be a useful inhibitor of melanogenesis and has beneficial effects in the treatment of hyperpigmentation disorders such as ephelis and oedema. However, the inhibitory mechanism of melanin production in B16 murine melanoma cells by UPE remains unclear. The investigation of the exact mechanisms and further in vivo experiments are needed to evaluate the possible use of UPE as a natural skin-whitening agent.
Acknowledgments
This research was financially supported by the Basic Science Research program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science of Technology (2011-0007254). We are grateful to Jeju technopark for providing research circumstances for this study.
REFERENCES
- Chai WM, Liu X, Hu YH, Feng HL, Jia YL, Guo YJ, Zhou HT, Chen QX. Antityrosinase and antimicrobial activities of furfuryl alcohol, furfural andfuroic acid. Int J Biol Macromol. 2013;57C:151–155. doi: 10.1016/j.ijbiomac.2013.02.019. [DOI] [PubMed] [Google Scholar]
- Han J, Kang S, Choue R, Kim H, Leem K, Chung S, Kim C, Chung J. Free radical scavenging effect of Diospyros kaki, Laminaria japonica and Undaria pinnatifida . Fitoterapia. 2002;73:710–712. doi: 10.1016/s0367-326x(02)00236-8. [DOI] [PubMed] [Google Scholar]
- Hayashi K, Lee JB, Nakano T, Hayashi T. Anti-influenza A virus characteristics of a fucoidan from sporophyll of Undaria pinnatifida in mice with normal and compromised immunity. Microbes Infect. 2013;15:302–309. doi: 10.1016/j.micinf.2012.12.004. [DOI] [PubMed] [Google Scholar]
- Hu T, Liu D, Chen Y, Wu J, Wang S. Antioxidant activity of sulfated polysaccharide fractions extracted from Undaria pinnitafida in vitro. Int J Biol Macromol. 2010;46:193–198. doi: 10.1016/j.ijbiomac.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Khan MN, Yoon SJ, Choi JS, Park NG, Lee HH, Cho JY, Hong YK. Anti-edema effects of brown seaweed (Undaria pinnatifida) extract on phorbol 12-myristate 13-acetate-induced mouse ear inflammation. Am J Chin Med. 2009;37:373–381. doi: 10.1142/S0192415X09006837. [DOI] [PubMed] [Google Scholar]
- Khan MT. Novel tyrosinase inhibitors from natural resources – their computational studies. Curr Med Chem. 2012;19:2262–2272. doi: 10.2174/092986712800229041. [DOI] [PubMed] [Google Scholar]
- Kim SS, Hyun CG, Choi YH, Lee NH. Tyrosinase inhibitory activities of the compounds isolated from Neolitsea aciculata (Blume) Koidz. J Enz Inhibit Med Chem. 2013;28(4):685–689. doi: 10.3109/14756366.2012.670806. [DOI] [PubMed] [Google Scholar]
- Kim WJ, Koo YK, Jung MK, Moon HR, Kim SM, Synytsya A, Yun-Choi HS, Kim YS, Park JK, Park YI. Anticoagulating activities of low-molecular weight fuco-oligosaccharides prepared by enzymatic digestion of fucoidan from the sporophyll of Korean Undaria pinnatifida . Arch Pharm Res. 2010;33:125–131. doi: 10.1007/s12272-010-2234-6. [DOI] [PubMed] [Google Scholar]
- Liang C, Lim JH, Kim SH, Kim DS. Dioscin: a synergistic tyrosinase inhibitor from the roots of Smilax china. Food Chem. 2012;134:1146–1148. doi: 10.1016/j.foodchem.2012.03.003. [DOI] [PubMed] [Google Scholar]
- Moon JY, Yang EJ, Kim SS, Kang JY, Kim GO, Lee NH, Hyun CG. Sasa quelpaertensis phenylpropanoid derivative suppresses lipopolysaccharide-induced nitric oxide synthase and cyclo-oxygenase-2 expressions in RAW 264.7 cells. Yakugaku Zasshi. 2011;131:961–967. doi: 10.1248/yakushi.131.961. [DOI] [PubMed] [Google Scholar]
- Moreira AS, González-Torres L, Olivero-David R, Bastida S, Benedi J, Sánchez-Muniz FJ. Wakame and Nori in restructured meats included in cholesterol-enriched diets affect the antioxidant enzyme gene expressions and activities in Wistar rats. Plant Foods Hum Nutr. 2010;65:290–298. doi: 10.1007/s11130-010-0179-z. [DOI] [PubMed] [Google Scholar]
- Okada T, Mizuno Y, Sibayama S, Hosokawa M, Miyashita K. Antiobesity effects of Undaria lipid capsules prepared with scallop phospholipids. J Food Sci. 2011;76:H2–6. doi: 10.1111/j.1750-3841.2010.01878.x. [DOI] [PubMed] [Google Scholar]
- Suetsuna K, Maekawa K, Chen JR. Antihypertensive effects of Undaria pinnatifida (wakame) peptide on blood pressure in spontaneously hypertensive rats. J Nutr Biochem. 2004;15:267–272. doi: 10.1016/j.jnutbio.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Thompson KD, Dragar C. Antiviral activity of Undaria pinnatifida against herpes simplex virus. Phytother Res. 2004;18:551–555. doi: 10.1002/ptr.1487. [DOI] [PubMed] [Google Scholar]
- Wu X, Yin S, Zhong J, Ding W, Wan J, Xie Z. Mushroom tyrosinase inhibitors from Aloe barbadensis Miller. Fitoterapia. 2012;83:1706–1711. doi: 10.1016/j.fitote.2012.09.028. [DOI] [PubMed] [Google Scholar]
- Yang EJ, Moon JY, Kim MJ, Kim DS, Kim CS, Lee WJ, Lee NH, Hyun CG. Inhibitory effect of Jeju endemic seaweeds on the production of pro-inflammatory mediators in mouse macrophage cell line RAW 264.7. J Zhejiang Univ Sci B. 2010;11:315–322. doi: 10.1631/jzus.B0900364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang EJ, Moon JY, Kim MJ, Kim DS, Lee WJ, Lee NH, Hyun CG. Anti-inflammatory effect of Petalonia binghamiae in LPS-induced macrophages is mediated by suppression of iNOS and COX-2. Int J Agri Biol. 2010;12:754–758. [Google Scholar]
- Yang EJ, Kim SS, Moon JY, Oh TH, Baik JS, Lee NH, Hyun CG. Inhibitory effects of Fortunella japonica var. margarita and Citrus sunki essential oils on nitric oxide production and skin pathogens. Acta Microbiol Immunol Hung. 2010;57:15–27. doi: 10.1556/AMicr.57.2010.1.2. [DOI] [PubMed] [Google Scholar]
- Yang EJ, Ham YM, Kim DS, Kim JY, Hong JP, Kim MJ, Moon JY, Lee WJ, Lee NH, Hyun CG. Ecklonia stolonifera inhibits lipopolysaccharide-induced production of nitric oxide, prostaglandin E2, and proinflammatory cytokines in RAW264.7 macrophages. Biologia. 2010;65:362–371. [Google Scholar]
- Yen FL, Wang MC, Liang CJ, Lee CW. Melanogenesis Inhibitor(s) from Phyla nodiflora Extract. Evid Complement Altern Med. 2012;2012:867494. doi: 10.1155/2012/867494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo MS, Shin JS, Choi HE, Cho YW, Bang MH, Baek NI, Lee KT. Fucosterol isolated from Undaria pinnatifida inhibits lipopolysaccharide-induced production of nitric oxide and pro-inflammatory cytokines via the inactivation of nuclear factor-(B and p38 mitogen-activated protein kinase in RAW264.7 macrophages. Food Chem. 2012;135:967–975. doi: 10.1016/j.foodchem.2012.05.039. [DOI] [PubMed] [Google Scholar]
- Yoon WJ, Kim DS, Yang EJ, Moon JY, Kim MJ, Lee WJ, Lee NH, Hyun CG. Inhibitory Effects of Enteromorpha prolifera on the production of nitric oxide, prostaglandin E2, and pro-inflammatory cytokines in RAW 264.7 cells. Lat Am J Pharm. 2010;29:165–170. [Google Scholar]
- Yoon WJ, Moon JY, Song G, Lee YK, Han MS, Lee JS, Ihm BS, Lee WJ, Lee NH, Hyun CG. Artemisia fukudo essential oil attenuates LPS-induced inflammation by suppressing NF-kappaB and MAPK activation in RAW 264.7 macrophages. Food Chem Toxicol. 2010;48:1222–1229. doi: 10.1016/j.fct.2010.02.014. [DOI] [PubMed] [Google Scholar]
- Yoon WJ, Kim MJ, Moon JY, Kang HJ, Kim GO, Lee NH, Hyun CG. Effect of palmitoleic acid on melanogenic protein expression in murine b16 melanoma. J Oleo Sci. 2010;59:315–319. doi: 10.5650/jos.59.315. [DOI] [PubMed] [Google Scholar]
- Yoon WJ, Ham YM, Lee WJ, Lee NH, Hyun CG. Brown alga Sargassum muticum inhibits proinflammatory cytokines, iNOS, and COX-2 expression in macrophage RAW 264.7 cells. Turk J Biol. 2010;34:25–34. [Google Scholar]