Abstract
Consumption of fluoride leads to several physiological disturbances in carbohydrate, lipid and antioxidant metabolisms. Averrhoa carambola L. fruit (Star fruit) is a commonly consumed fruit in tropical countries and is an ingredient in folklore medicines. As the fruits have high polyphenolic and antioxidant contents, the present study was undertaken to investigate the potential of star fruit as a dietary supplement in attenuating the fluoride induced hyperglycemia, hypercholesterolemia and oxidative stress in laboratory rats. A four-week exposure to fluoride caused sustained hyperglycemia, hyperlipidemia and oxidative stress and, when the diet was supplemented with star fruit powder, carbohydrate, lipid and antioxidant profiles were restored significantly. It is surmised that the antihyperglycemic, antihypercholesterolemic and antioxidant activities of star fruit in fluoride exposed rats could be due to the presence of polyphenols, flavonoids, saponins, phytosterols, ascorbic acid and fibers in the fruit, which are all well known regulators of carbohydrate, lipid and antioxidant metabolisms. These findings suggest that star fruit can be used as a dietary supplement in fluoride endemic regions to contain fluoride induced hyperglycemia, hyperlipidemia and oxidative stress.
Keywords: fluoride, star fruit, carbohydrate metabolism, lipid metabolism, oxidative stress
Introduction
Fluoride in excess amount can cause several ailments, viz. metabolic disturbances, endocrine dysfunctions and physiological alterations in the body (Jagtap et al., 2012; Susheela, 2007). Defluoridation of water is the only available option to reduce the fluoride content from water but the techniques are unaffordable and beyond the reach of underprivileged communities in fluoride endemic areas across the globe. Several natural adsorbents such as red soil, charcoal, brick, fly-ash, serpentine, and alum have been used to reduce the fluoride content in water (Chidambaram et al., 2003). Novel defluoridation techniques include the use of leaves of Azadirachta indica, Ficus religiosa and Acacia catechu and tamarind seeds (Jamode et al., 2004; Murugan and Subramanian, 2002, 2006). Apart from these techniques, reports also indicate the utility of plant products, for example tamarind fruit pulp, seeds of Moringa oleifera, bark extracts of Terminalia arjuna and black berry juice as possible therapeutic agents in amelioration of fluoride toxicity (Hassan and Yousef, 2009; Ranjan et al., 2009; Sinha et al., 2007). Additionally, plant metabolites such as a 43 kD protein isolated from Cajanus indicus, quercetin and curcumin have been shown to ameliorate the fluoride induced oxidative stress and improve the functions of the liver, kidney and erythrocytes (Manna et al., 2007; Nabavi et al., 2012a, 2012b, c).
Averrhoa carambola L., (F: Oxalidaceae; star fruit/carambola) is believed to have originated in Sri Lanka. The plant has been cultivated in Southeast Asia and Malaysia for many centuries (Morton, 1987). In Ayurveda, the ripe fruit is considered digestive and tonic. In India, the ripened fruits are also used to halt hemorrhages and to relieve bleeding hemorrhoids, useful as a treatment for fever, eczema, hemorrhages, hemorrhoids and diarrhea (Morton, 1987). Phytochemical investigation revealed the presence of flavonoids, proanthocyanidins, (–)-epicatechin and vitamin C content in the fruit (Leong and Shui, 2002; Shui and Leong, 2004; Tiwari et al., 1979). In vitro and in vivo studies indicated that star fruit inhibited cytochrome P450 3A activity (Hidaka et al., 2004, 2006). Besides, the insoluble fibers isolated from the pomace of star fruit exhibited potent hypoglycemic activities in vitro: the insoluble fibers adsorbed glucose efficiently, retarded glucose diffusion and delayed the release of glucose from starch and inhibited the activity of α-amylase (Chau et al., 2004a). Further, consumption of water-insoluble fiber-rich fractions of star fruit pomace elevated fecal total lipids, fecal cholesterol and fecal bile acids excretion along with a reduction in serum triacyglycerol, serum and hepatic total cholesterol in hypercholesterolemic hamsters (Chau et al., 2004b). The phytochemical analyses and medicinal properties of star fruit are well documented but the role of star fruit in cases of fluoride toxicity has not been studied.
Materials and methods
Averrhoa carambola fruit powder preparation and analysis
Fruits of Averrhoa carambola were procured from the local market of Vallabh Vidyanagar and authenticated by our faculty taxonomist Dr. A. S. Reddy. The powder of star fruit was prepared by Aum Agri Pvt. Ltd (Baroda, Gujarat) by freeze drying method and stored in an air-tight container. The fiber content of the fruit powder was determined (Thimmaiah, 1999) after petroleum ether extraction followed by acid and alkaline treatment. Saponin and phytosterol contents of the fruit powder were determined using ferric chloride-sulfuric acid and vanillin-sulfuric acid methods, respectively (Ebrahimzadeh & Niknam, 1998; Goad & Akihisha, 1997). The polyphenol and flavonoid contents were measured using Folin-Ciocalteu and Vanillin sulfuric acid reagents (Thimmaiah, 1999). The total ascorbic acid content was estimated using 2, 4-dinitrophenyl hydrazine reagent (Schaffert & Kingsley, 1955). Total antioxidant power in terms of FRAP value was determined using 2, 4, 6- tripyridyl-s-triazine (TPTZ) reagent (Benzie & Strain, 1996).
Animals and diet
Colony bred male Albino rats (Charles Foster; 200–250 g bw) housed individually in a well-ventilated animal unit (26±2 °C, humidity 62%, and 12-h light/dark cycle) were supplied water ad libitum. The control animals were fed standard (commercial) diet (Pranav Agro Industries, Vadodara, India) and the experimental groups were provided diets with Ac fruit powder replacing the required amounts of standard (commercial) diet. The research protocol followed the guidelines of Institutional Animal Ethics Committee (MoEF/CPCSEA/Reg. 337) and was approved by the Institutional Committee for animal research.
After a 10-day adaptation period, 30 animals were randomly segregated into 5 groups of 6 animals each as follows: Normal control (NC) - normal animals without any treatment; Fluoride control (FC) - 100 ppm sodium fluoride administered through drinking water; FAc I - fluoride administered animals fed diet with 2.5 g/100 g of A. carambola fruit powder; FAc II - fluoride administered animals fed diet with 5 g/100 g of A. carambola fruit powder; FAc III - fluoride administered animals fed diet with 10 g/100 g of A. carambola fruit powder.
At the end of 30 days, the animals were fasted overnight and sacrificed under mild ether anesthesia. Blood was collected by cardiac puncture and plasma was separated and stored at low temperature. Liver and kidneys were excised and kept frozen until analyzed. Fecal matter was collected for biochemical analyses.
Analytical procedures
Plasma glucose, hepatic glycogen, hepatic hexokinase and G-6-Pase activities
Plasma glucose levels were measured by standard kit (Eve's Inn Diagnostics, Baroda, India). Hepatic glycogen was extracted with 30% KOH, and the yield was estimated by anthrone-sulfuric acid method (Seifter et al., 1950). The hepatic hexokinase (EC 2.7.1.1) was determined, based on the reduction of NAD+ through a coupled reaction with glucose-6-phosphate dehydrogenase (Brandstrup et al., 1957). Glucose-6-phosphatase (EC 3.1.3.9) activity was assayed by measuring the inorganic phosphate liberated from glucose-6-phosphate (Baginsky et al., 1974).
SGOT, SGPT, ACP, ALP activities and FRAP
Serum glutamate oxaloacetate (SGOT) and pyruvate (SGPT) transaminases, acid and alkaline phosphatases (ACP, ALP) levels were determined using standard kits (Eve's Inn Diagnostics, Baroda, India). The FRAP (ferric reducing ability of plasma) value of the animals was measured by the method of Benzie and Strain (1996).
Plasma and hepatic lipid profiles
Plasma total cholesterol (TC), HDL cholesterol (HDL-C) and triglycerides (TG) were measured by standard kits (Eve's Inn Diagnostics, Baroda, India) and the plasma total lipid (TL) content was estimated by sulphophosphovanillin method (Frings et al., 1972). Low-density lipoprotein cholesterol (LDL-C), very low-density lipoprotein cholesterol (VLDL-C), and atherogenic index of plasma (AIP) were calculated (Friedewald et al., 1972).
The total lipid content (TL) was determined gravimetrically using chloroform/methanol (2:1) liver extracts (Folch et al., 1957). The same extracts were also used for estimation of total cholesterol (TC) and triglycerides (TG) using standard kits (Eve's Inn Diagnostics, Baroda, India).
Hepatic HMG-CoA reductase and bile acid profile
Hepatic HMG-CoA reductase (EC 1.1.1.34) activity was measured in terms of the ratio of HMG-CoA to mevalonate (Rao & Ramakrishnan, 1975) as HMG-CoA reductase activity is inversely proportional to the ratio of HMG-CoA / mevalonate. Bile acid content was estimated using vanillin-phosphoric acid reagent (Snell & Snell, 1953).
Fecal cholesterol and bile acid content
The fecal cholesterol and bile acids were extracted using alkaline-methanol medium and cholesterol was estimated (Kaiek et al., 1984). A portion of the extract was acidified and used for bile acid estimation (Snell & Snell, 1953).
Hepatic and renal tissue lipid peroxidation and antioxidant profiles
The hepatic and renal lipid peroxidation was determined by the thiobarbituric acid (TBA) assay (Ohkawa et al., 1979). The hepatic and renal total ascorbic acid and reduced glutathione contents were estimated using methods of Schaffert and Kingsley (1955) and Jollow et al., (1984). Catalase (CAT; EC 1.11.1.6), glutathione peroxidase (GPx; EC 1.11.1.9) and superoxide dismutase (SOD; EC 1.15.1.1) activities were measured in both hepatic and renal tissues following the standard methods (Aebi, 1974; Flohe & Gunzler, 1984; Kakkar et al., 1984).
All chemicals used were of analytical grade (SISCO Research Laboratories, Mumbai, India) and all the measurements were taken using Elico ® SL 171 Mini Spec (Hyderabad, India).
Statistical Evaluation
Data are presented as mean ± SEM (n=6). One-way analysis of variance (ANOVA) with Tukey's significant difference post hoc test was used to compare differences among groups. Data were statistically analyzed using Graph Pad Prism 3.0 statistical software. The p-values <0.05 were considered statistically significant.
Results
The phytochemical analysis of the fruit powder indicated the presence of fiber (3.8 g%), phytosterols (5.06 g%), saponins (3.77 mg%), polyphenols (1.76 g%), flavonoids (0.277 g%) and ascorbic acid (0.088 g%).
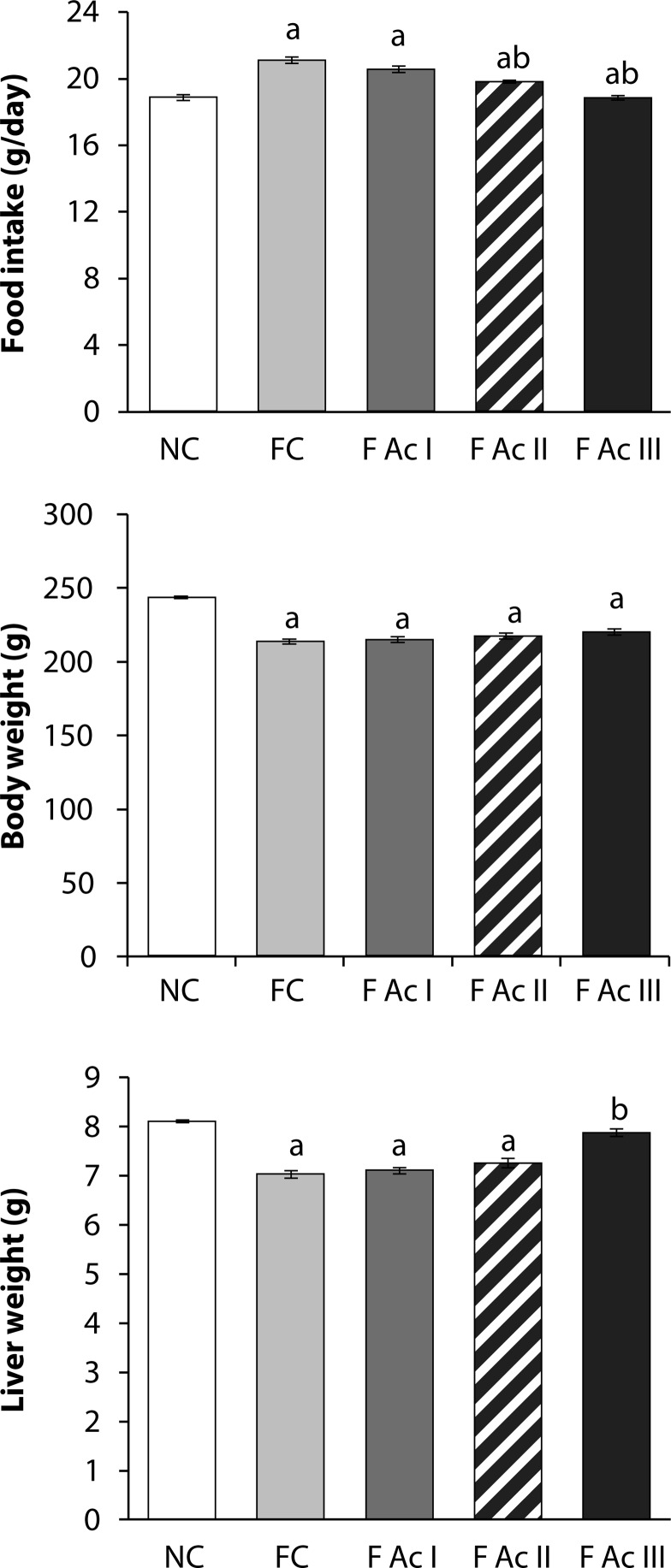
Fluoride exposed animals lost their body and liver weights significantly although the food intake increased. Addition of A. carambola fruit powder to the diet of these animals elevated the body and liver weights (3 and 12% respectively) and reduced the food intake (11%) (Figure 1).
Figure 1.
Food intake, body and liver weights. Values are mean ± SEM (n=6); a - compared with NC, b - compared with FC group
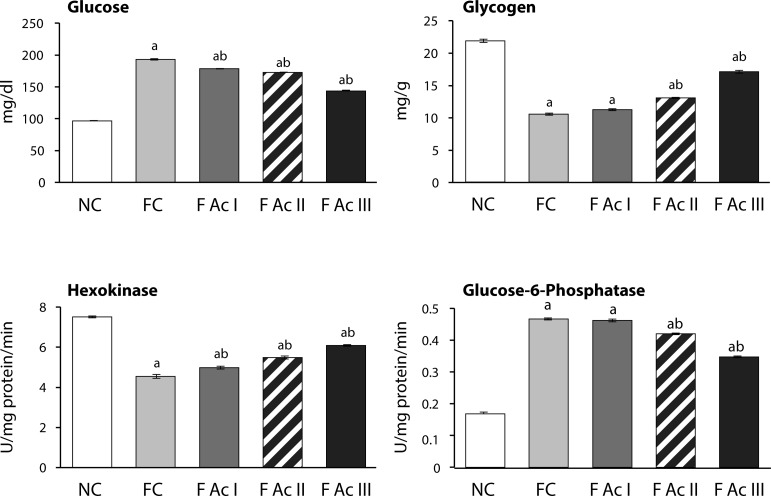
The FC group registered significant elevation in plasma glucose levels (99%), hepatic G-6-Pase activity (175%) and decline in hepatic glycogen content and hepatic hexokinase activity (52% and 40% respectively). In the FAc groups both fasting blood glucose levels and G-6-Pase activity decreased while the hepatic glycogen content and hexokinase activity increased in a dose-dependent manner (Figure 2).
Figure 2.
Plasma glucose and hepatic carbohydrate profiles. Values are mean ± SEM (n=6); a - compared with NC, b - compared with FC group
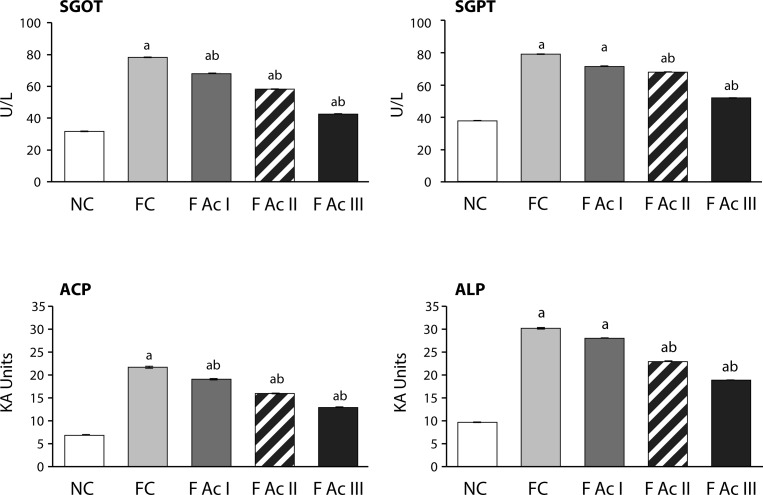
While fluoride exposure significantly increased the activities of SGOT, SGPT, ACP, and ALP and decreased the ferric reducing ability of plasma (FRAP) in experimental animals, addition of Ac fruit powder to the diet improved the hepatic functions (Figure 3; Table 1).
Figure 3.
Serum enzymatic profiles. Values are mean ± SEM (n=6); a - compared with NC, b - compared with FC group
Table 1.
Effect of A. carambola plasma total lipids, atherogenic index, Hepatic HMG-CoA and FRAP values.
| Parameters | NC | FC | F Ac I | F Ac II | F Ac III |
|---|---|---|---|---|---|
| TL (mg/dl) | 317.37±0.76 | 470.17±0.63a (+48.14) | 445.60±1.08ab (-5.22) | 390.31±1.81ab (-16.98) | 347.77±0.81ab (-26.03) |
| AIP (mg/dl) | 1.58±0.01 | 3.33±0.03a (+110.76) | 3.04±0.02ab (-8.71) | 2.31±0.02ab (-30.63) | 1.65±0.01b (-50.45) |
| HMG-CoA reductase* | 3.87±0.02 | 8.10±0.86a (-109.30) | 7.68±0.14a (+5.18) | 6.93±0.10a (+14.44) | 4.98±0.45b (+38.52) |
| FRAP (µmole/L) | 286.12±0.35 | 159.49±0.33a (-44.26) | 195.67±0.38ab (+22.68) | 219.96±0.20ab (+37.91) | 246.46±0.38ab (+54.53) |
Values are represented as mean ± SEM (n=6).
Indicates the comparison with normal control group
Denotes the comparison with fluoride control group at p<0.05; Percent changes (figures in parenthesis) in fluoride control group were in comparison with normal control and in those treatment groups were in comparison with fluoride control group
HMG-CoA reductase activity is inversely proportional to the ratio of HMG-CoA/ mevalonate.
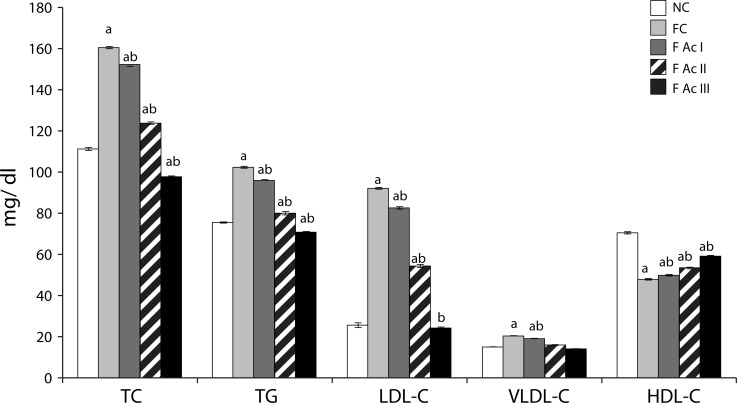
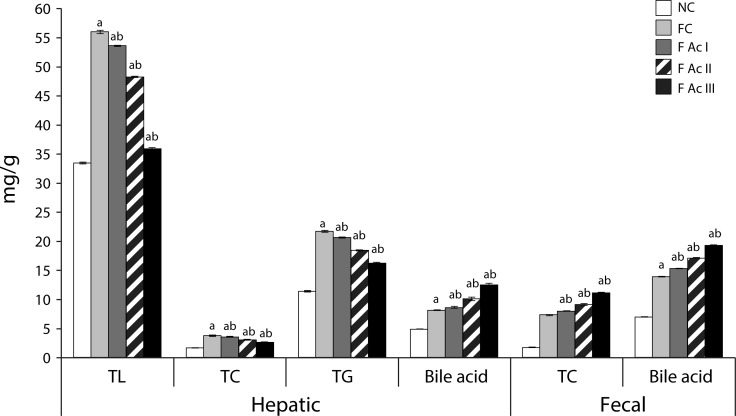
The FC group exhibited significantly high levels of plasma TL, TC, TG, LDL-C, VLDL-C, atherogenic index of plasma (AIP) and, hepatic lipids-TL, TC and TG with reduced HDL-C levels. Ac supplemented groups registered significantly lowered levels of plasma and hepatic lipid profiles and improved HDL-C levels (Table 1; Figures 4 & 5).
Figure 4.
Plasma lipid profiles. Values are mean ± SEM (n=6); a - compared with NC, b - compared with FC group
Figure 5.
Hepatic and fecal lipid profiles. Values are mean ± SEM (n=6); a - compared with NC, b - compared with FC group
Exposure to fluoride significantly suppressed the activity of HMG-CoA reductase, as indicated by increased HMG CoA-mevalonate ratio (NC vs FC, FAcI – FAcIII). However, addition of Ac powder to the diet caused increased HMG-CoA activity (5%; 14%; 39%). The bile acid production in hepatic tissue and its excretion in fluoride exposed animals increased significantly compared to that in the NC group and further increases were noted in the FAc groups (Table 1; Figure 5).
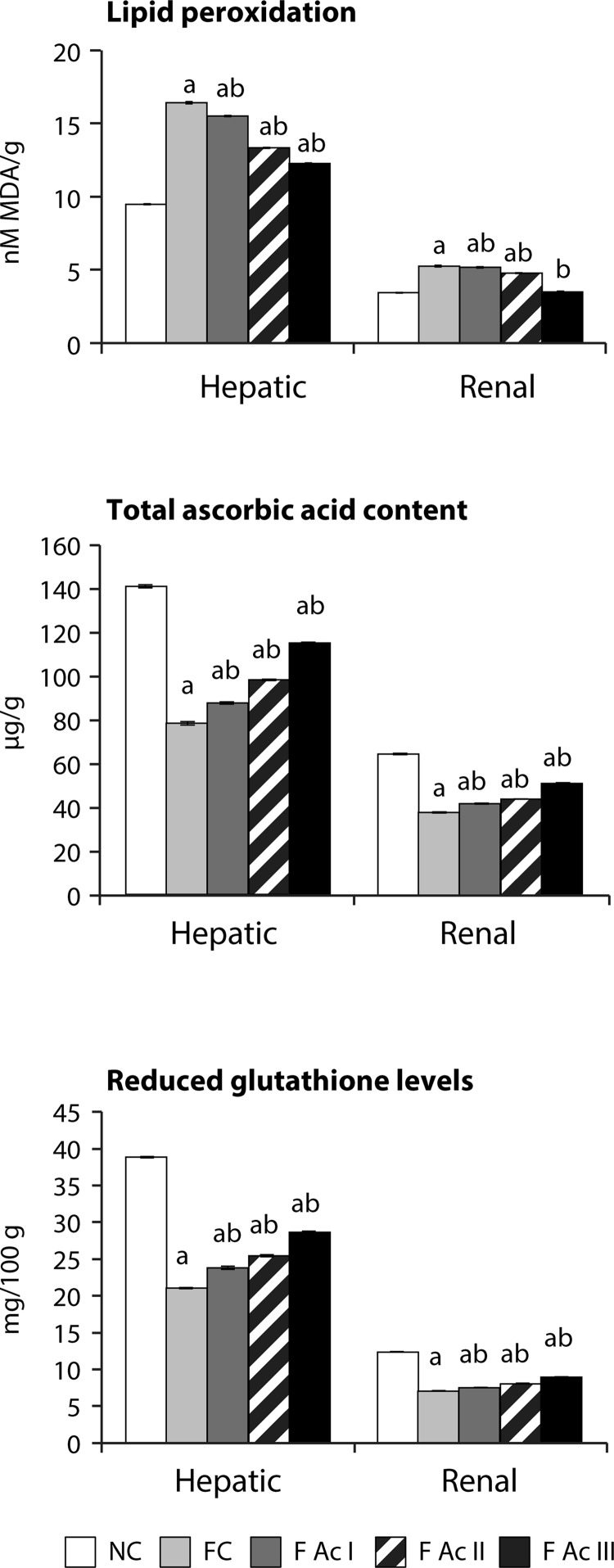
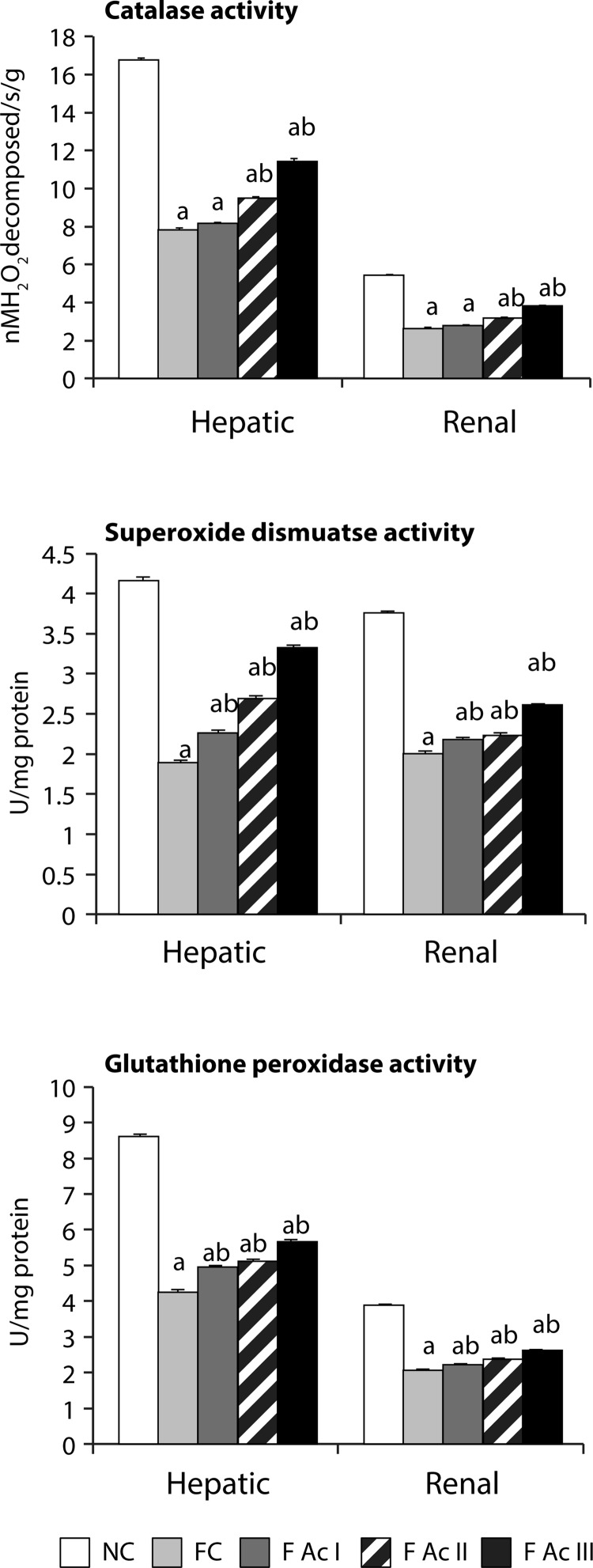
Both hepatic and renal tissue lipid peroxidation increased (73% and 53% respectively) significantly in fluoride exposed groups. In these groups, the hepatic and renal antioxidant profiles also declined significantly. However, addition of Ac fruit powder to the diet (FAc I – FAc III) improved the antioxidant profiles and decreased the lipid peroxidation in fluoride exposed animals (Figures 6 & 7).
Figure 6.
Hepatic and renal lipid peroxidation and non-enzymatic antioxidants. Values are mean ± SEM (n=6); a - compared with NC, b - compared with FC group.
Figure 7.
Hepatic and renal enzymatic antioxidants. Values are mean ± SEM (n=6); a - compared with NC, b - compared with FC group.
Discussion
The present investigation reveals for the first time the beneficial effects of Averrhoa carambola (Ac) fruit when used as a dietary supplement to regulate fluoride induced alterations in body carbohydrate, lipid and antioxidant metabolism. The positive effects of Ac fruit powder as a food supplement to fluoride exposed rats were found to be dose dependent, i.e., 10 g/100 g% dose was more potent in ameliorating fluoride induced metabolic alterations when compared to 2.5 and 5 g/100 g% doses.
Fluoride exposed animals lost their body and liver weights significantly although the food intake increased. Addition of A. carambola fruit powder to the diet of these animals elevated the body and liver weights and reduced the food intake. The reduction in body and liver weights of fluoride exposed rats could be due to the non-availability of immediate energy resources (carbohydrates) and thus an increase in food intake. These observations are in line with an earlier reported loss of body and liver weights in spite of increased food intake (Yadav et al., 2005). Further, the increased food intake in fluoride exposed animals could also be due to a possible suppression of the hunger/satiety center without increasing the energy assimilation, resulting in reduced body and liver weights. With A. carambola fruit powder addition, the fluoride induced hunger/satiety-center-inhibition could have been removed and regulated the food intake resulting in increases in body and liver weights.
Exposure to fluoride resulted in significant elevation of plasma glucose levels in FC groups indicating the hyperglycemic activity of fluoride, which is in confirmation with earlier findings (Chlubek et al., 2003; Grucka-Mamczar et al., 2007; Garcia-Montalvo et al., 2009). The increase in hepatic G-6-Pase activity and decline in hepatic glycogen content and hepatic hexokinase activity were countered with A. carambola fruit powder addition to the diet. These observations clearly show the antihyperglycemic potential of A. carambola to restore the alterations in carbohydrate metabolism caused by fluoride in a highly comparable way as observed in in-vitro induced hyperglycemic conditions (Chau et al., 2004a). The observed antihyperglycemic activities in fluoride exposed animals administered with A. carambola fruit powder supplemented feed could be attributed to the presence of polyphenols, flavonoids, ascorbic acid and saponins in the fruit since these phytochemicals are reported to be antihyperglycemic (Francis et al., 2002; Meydani & Hasan, 2010; Oguntibeju, 2008; Yao et al., 2004; Zunino et al., 2007). Similarly, the altered activities of SGOT, SGPT, ACP, ALP and the declined plasma antioxidant capacity (in terms of FRAP value) in fluoride exposed animals were improved upon the addition of Ac fruit powder to the diet, indicating the restoratory effects of Ac fruit on hepatic tissue.
A long term consumption of fluoride is known to cause hyperlipidemia and hypercholesterolemia (Grucka-Mamczar et al., 2004; Strunecka et al., 2007) and is also seen in the present study, as especially the FC group exhibited significantly increased lipid profiles both in plasma and hepatic tissue. The substantial decreases in the lipid profiles with an increase in HDL-C and improvement in AI with addition of A. carambola fruit powder to the diet clearly indicate the antihyperlipidemic and antiatherogenic properties of A. carambola fruit. The increased synthesis of hepatic bile acid and excretion of bile acid and cholesterol (through fecal matter) in fluoride exposed animals supplemented with Ac fruit powder also support the contention that A. carambola fruit is antihyperlipidemic and antiatherogenic; an observation similar to the purported effects of fiber-rich fractions of star fruit pomace in hypercholesterolemic hamsters (Chau et al., 2004b). The increases in HMG-CoA activity in these animals could be a feedback response to lowered cholesterol levels.
Phytometabolites such as phytosterols, saponins, polyphenols, flavonoids, ascorbic acid and fibers are known to influence the lipid metabolism. For instance, the dietary fibers increase the excretion of cholesterol by interfering with enterohepatic circulation of cholesterol (Arjamandi et al., 1992; Moundras et al., 1997). Polyphenols, flavonoids and ascorbic acid are reported to be antihyperlipidemic agents as they aid in cholesterol excretion through bile acids (Meydani & Hasan, 2010; Oguntibeju, 2008; Yao et al., 2004; Zunino et al., 2007). Further, both phytosterols and saponins (present in Ac fruit) could also be responsible for the cholesterol lowering effects of Ac fruit. Phytosterols are involved in inhibition of cholesterol absorption from the intestine due to their greater hydrophobicity and affinity for micelles than cholesterol and displace the intestinal cholesterol (Kritchevsky & Chen, 2005). Saponins precipitate cholesterol from micelles and interfere with enterohepatic circulation of bile acids leading to a reduction in plasma and hepatic cholesterol levels (Francis et al., 2002).
The relationship between fluoride intake and oxidative stress is well established (Barbier et al., 2010; Strunecka et al., 2007). Fluoride generates free radicals like superoxides (O2), hydrogen peroxides, peroxynitrites, hydroxyl radicals and other radicals leading to the chemical injury of lipids, proteins and DNA. In the present context, not only the hepatic and renal tissue lipid peroxidation increased significantly but the enzymatic and non-enzymatic antioxidant profiles in liver and renal tissues were found to decrease substantially. A. carambola fruit powder inclusion in the diet significantly reduced the lipid peroxidation and enhanced both enzymatic and non-enzymatic antioxidants in a dose-dependent manner. These antiperoxidative effects of A. carambola could be due to the inherent antioxidant capacity of the star fruit, i.e. FRAP, which is in agreement with the findings of Shui and Leong (2006).
Therefore the results of the present study clearly indicate that the fruits of A. carambola are useful as a dietary supplement in regulation of fluoride induced hyperglycemia, hyperlipemia and oxidative stress. Further, this work also suggests that A. carambola fruits could be used and promoted as alternative food supplements in fluoride endemic areas.
Acknowledgement
Financial assistance to RAV (Research Associateship, DST-PURSE Program, Sardar Patel University, Vallabh Vidyanagar, India) is gratefully acknowledged.
Glossary
ABBREVIATIONS
- NC
Normal control
- FC
fluoride exposed animals
- Ac
A. carambola
- FAc I
fluoride administered animals fed diet with 2.5% of A. carambola fruit powder
- FAc II
fluoride administered animals fed diet with 5% of A. carambola fruit powder
- FAc III
fluoride administered animals fed diet with 10% of A. carambola fruit powder
- G-6-Pase
glucose- 6-phosphatase
- TL
total lipids
- TC
total cholesterol
- TG
triglycerides
- LDL-C
low-density lipoprotein cholesterol
- VLDL-C
very low-density lipoprotein cholesterol
- HDL-C
high-density lipoprotein cholesterol
- AIP
atherogenic index of plasma
- SGOT
serum glutamate oxaloacetate transaminase
- SGPT
serum pyruvate transaminase
- ACP
acid phosphatase
- ALP
alkaline phosphatase
- FRAP
ferric reducing ability of plasma
- TAA
total ascorbic acid
- SOD
superoxide dismutase
- CAT
catalase
- GsH
reduced glutathione
- GPx
glutathione peroxidase
Conflict of interest
The authors declare no conflict of interest.
REFERENCES
- Aebi H. Catalase. In: Bergmeyer, editor. Methods of Enzymatic Analysis. Second Edition. New York: Academic Press; 1974. pp. 673–674. [Google Scholar]
- Arjmandi BH, Ahn J, Nathani S, Reeves RD. Dietary soluble fiber and cholesterol affect serum cholesterol concentration, hepatic portal venous short-chain fatty acid concentrations and fecal sterol excretion in rats. J Nutr. 1992;122:246–253. doi: 10.1093/jn/122.2.246. [DOI] [PubMed] [Google Scholar]
- Baginsky ES, Foa PP, Zad B. Glucose-6-phosphatase. In: Bergmeyer HU, editor. Methods of Enzymatic Analysis. New York: Academic Press; 1974. pp. 788–792. [Google Scholar]
- Barbier O, Arreola-Mendoza L, Razo LMD. Molecular mechanisms of fluoride toxicity. Chem-Biol Interact. 2010;188:319–333. doi: 10.1016/j.cbi.2010.07.011. [DOI] [PubMed] [Google Scholar]
- Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: The FRAP assay. Ann Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Brandstrup N, Kirk JE, Bruni C. The hexokinase and phosphoglucoisomerase activities of aortic and pulmonary artery tissue in individuals of various ages. J Gerontol. 1957;12:166–171. doi: 10.1093/geronj/12.2.166. [DOI] [PubMed] [Google Scholar]
- Chau CF, Chen CH, Lin CY. Insoluble fiber-rich fractions derived from Averrhoa carambola: hypoglycemic effects determined by in vitro methods. LWT Food Sci Technol. 2004a;37:331–335. [Google Scholar]
- Chau CF, Chen CH, Lin CY. Effects of a novel pomace fiber on lipid and cholesterol metabolism in the hamster. Nutr Res. 2004b;24:337–345. [Google Scholar]
- Chidambaram S, Ramanathan AL, Vasudevan S. Fluoride removal studies in water using natural materials. Water SA. 2003;29:339–343. [Google Scholar]
- Chlubek D, Grucka-Mamczar E, Birkner E, Polaniak R, Stawiarska-Pieta B, Duliban H. Activity of pancreatic antioxidative enzymes and malondialdehyde concentrations in rats with hyperglycemia caused by fluoride intoxication. J Trace Elem Med Biol. 2003;17:57–60. doi: 10.1016/S0946-672X(03)80047-0. [DOI] [PubMed] [Google Scholar]
- Ebrahimzadeh H, Niknam V. A revised spectrophotometric method for determination of triterpenoid saponins. Indian Drugs. 1998;35:379–381. [Google Scholar]
- Flohe L, Gunzler WA. Assay of glutathione peroxidase. Methods Enzymol. 1984;105:114–121. doi: 10.1016/s0076-6879(84)05015-1. [DOI] [PubMed] [Google Scholar]
- Folch J, Lees MS, Stanley GH. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- Francis G, Kerem Z, Makkar HPS, Becker K. The biological action of saponins in animal systems: a review. Br J Nutr. 2002;88:587–605. doi: 10.1079/BJN2002725. [DOI] [PubMed] [Google Scholar]
- Friedewald WT, Levy RT, Fredrickson DS. Estimation of the concentration of LDL in plasma without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- Frings CS, Fendley TW, Dunn RR, Queen CA. Improved determination of total serum lipid by sulphovanillin reaction. Clin Chem. 1972;8:673–674. [PubMed] [Google Scholar]
- Garcia-Montalvo EA, Reyes-Perez H, Del-Razo LM. Fluoride exposure impairs glucose tolerance via decreased insulin expression and oxidative stress. Toxicology. 2009;263:75–83. doi: 10.1016/j.tox.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Goad LJ, Akihisa T. Analysis of Sterols, Blackie Academic and Professional. 1st edition. London: 1997. pp. 423–425. [Google Scholar]
- Grucka-Mamczar E, Birkner E, Kasperczyk S, Kasperczyk A, Chlubek D, Samujlo D, Ceglowska A. Lipid balance in rats with fluoride induced hyperglycemia. Fluoride. 2004;37:195–200. [Google Scholar]
- Grucka-Mamczar E, Birkner E, Zalejska-Fiolka J, Machoy Z, Kasperczyk S, Blaszczyk I. Influence of extended exposure to sodium fluoride and caffeine on the activity of carbohydrate metabolism enzymes in rat blood serum and liver. Fluoride. 2007;40:62–66. [Google Scholar]
- Hassan HA, Yousef MI. Mitigating effects of antioxidant properties of black berry juice on sodium fluoride induced hepato-toxicity and oxidative stress in rats. Food Chem Toxicol. 2009;47:2332–2337. doi: 10.1016/j.fct.2009.06.023. [DOI] [PubMed] [Google Scholar]
- Hidaka M, Fujita K, Ogikubo T, Yamasaki K, Iwakiri T, Okumura M, Kodama H, Arimori K. Potent inhibition by star fruit of human cytochrome P4503A (CYP3A) activity. Drug Metab Dispos. 2004;32:581–583. doi: 10.1124/dmd.32.6.581. [DOI] [PubMed] [Google Scholar]
- Hidaka M, Okumura M, Ogikubo T, Kai H, Fujita K, Iwakiri T, Yamasaki K, Setoguchi N, Matsunga N, Arimari K. Transient inhibition of CYP3A in rats by star fruit juice. Drug Metab Dispos. 2006;34:343–345. doi: 10.1124/dmd.105.006486. [DOI] [PubMed] [Google Scholar]
- Jagtap S, Yenkie MK, Labhsetwar N, Rayalu S. Fluoride in drinking water and defluoridation of water. Chem Rev. 2012;112:2454–2466. doi: 10.1021/cr2002855. [DOI] [PubMed] [Google Scholar]
- Jamode AV, Sapkal VS, Jamode VS. Defluoridation of water using inexpensive adsorbents. J Ind Inst Sci. 2004;84:163–171. [Google Scholar]
- Jollow DJ, Mitchell JR, Zampaglione N, Gillette JR. Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3, 4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology. 1974;11:151–169. doi: 10.1159/000136485. [DOI] [PubMed] [Google Scholar]
- Kaiek HD, Stellaard F, Kruis W, Paumgartner G. Detection of increased bile acid content in simple stool samples. Clin Chim Acta. 1984;140:85–90. doi: 10.1016/0009-8981(84)90154-2. [DOI] [PubMed] [Google Scholar]
- Kakkar P, Das B, Viswanathan PN. A modified spectro-photometric assay of superoxide dismutase. Ind J Biochem Biophys. 1984;21:130–132. [PubMed] [Google Scholar]
- Kritchevsky D, Chen SC. Phytosterols-health benefits and potential concerns: a review. Nutr Res. 2005;25:413–428. [Google Scholar]
- Leong LP, Shui G. An investigation of the antioxidant capacity of fruits in Singapore markets. Food Chem. 2002;76:69–75. [Google Scholar]
- Manna P, Sinha M, Sil PC. A 43 kD protein isolated from the herb Cajanus indicus L attenuates sodium fluoride induced hepatic and renal disorders in vivo. J Biochem Mol Biol. 2007;40:382–395. doi: 10.5483/bmbrep.2007.40.3.382. [DOI] [PubMed] [Google Scholar]
- Meydani M, Hasan ST. Dietary polyphenols and obesity. Nutrients. 2010;2:737–751. doi: 10.3390/nu2070737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton JF. Fruits of Warm Climates. Miami; 1987. pp. 125–128. [Google Scholar]
- Moundras C, Behr SR, Remesy C, Demigne C. Fecal losses of sterols and bile acids induced by feeding rats guar gum are due to greater pool size and liver bile acid secretion. J Nutr. 1997;127:1068–1076. doi: 10.1093/jn/127.6.1068. [DOI] [PubMed] [Google Scholar]
- Murugan M, Subramanian E. Application of Aloe vera (Indian Aloe) a plant material for defluoridation. Ind J Environ Protect. 2002;22:1034–1039. [Google Scholar]
- Murugan M, Subramanian E. Studies on defluoridation of water by tamarind seed, an unconventional biosorbent. J Water Health. 2006;4:453–461. [PubMed] [Google Scholar]
- Nabavi SF, Moghaddam AH, Eslami S, Nabavi SM. Protective effects of curcumin against sodium fluoride-induced toxicity in rat kidneys. Biol Trace Elem Res. 2012a;145:369–374. doi: 10.1007/s12011-011-9194-7. [DOI] [PubMed] [Google Scholar]
- Nabavi SF, Nabavi SM, Abolhasani F, Moghaddam AH, Eslami S. Cytoprotective effects of curcumin on sodium fluoride-induced intoxication in rat erythrocytes. Bull Environ Contam Toxicol. 2012b;88:486–490. doi: 10.1007/s00128-011-0495-5. [DOI] [PubMed] [Google Scholar]
- Nabavi SM, Nabavi SF, Eslami S, Moghaddam AH. In vivo protective effects of quercetin against sodium fluoride-induced oxidative stress in the hepatic tissue. Food Chem. 2012c;132:931–935. [Google Scholar]
- Oguntibeju OO. The biochemical, physiological and therapeutic roles of ascorbic acid. Afr J Biotechnol. 2008;7:4700–4705. [Google Scholar]
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Ann Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- Ranjan R, Swarup D, Patra RC, Chandar V. Tamarindus indica L. and Moringa oleifera M. extract administration ameliorates fluoride toxicity in rabbits. Ind J Exp Biol. 2009;47:900–905. [PubMed] [Google Scholar]
- Rao AV, Ramakrishnan S. Indirect assessment of hydroxymethylglutaryl-Co-A reductase (NADPH) activity in liver tissue. Clin Chem. 1975;21:1523–1525. [PubMed] [Google Scholar]
- Schaffert RR, Kingsley GR. A rapid, simple method for the determination of reduced, dehydro-, and total ascorbic acid in biological material. J Biol Chem. 1955;212:59–68. [PubMed] [Google Scholar]
- Seifter S, Dayton S, Novic B, Muntwyler E. Estimation of glycogen with anthrone reagent. Arch Biochem. 1950;25:191–200. [PubMed] [Google Scholar]
- Shui GH, Leong LP. Analysis of polyphenolic antioxidants in star fruit using liquid chromatography and mass spectrophotometry. J Chromatogr A. 2004;1022:67–75. doi: 10.1016/j.chroma.2003.09.055. [DOI] [PubMed] [Google Scholar]
- Sinha M, Manna P, Sil PC. Aqueous extract of the bark of Terminalia arjuna plays a protective role against sodium-fluoride induced hepatic and renal oxidative stress. J Nat Med. 2007;61:251–260. [Google Scholar]
- Snell FD, Snell CT. Colorimetric Methods of analysis. Inc., New York: D. Van Nostrand Company; 1953. pp. 371–377. [Google Scholar]
- Strunecka A, Patocka J, Blaylock RL, Chinoy NJ. Fluoride interactions: from molecules to disease. Curr Sig Transd T. 2007;2:190–213. [Google Scholar]
- Susheela AK. A Treatise on Fluorosis. 3rd ed. New Delhi: Fluorosis Research and Rural Development Foundation; 2007. [Google Scholar]
- Thimmaiah SK. Standard Methods of Biochemical Analysis. New Delhi, India: Kalyani Publishers; 1999. pp. 264 & 293–298. [Google Scholar]
- Tiwari KP, Masood M, Minocha PK. Chemical constituents of Gmelina phillipinensis, Adenocalymna nitida, Allamanda cathartica, Averrhoa carambola and Maba buxifolia . J Ind Chem Soc. 1979;56:944. [Google Scholar]
- Yadav UC, Moorthy K, Baquer NZ. Combined treatment of sodium orthovanadate and Momordica charatia fruit extract prevents alterations in lipid profile and lipogenic enzymes in alloxan diabetic rats. Mol Cell Biochem. 2005;268:111–120. doi: 10.1007/s11010-005-3703-y. [DOI] [PubMed] [Google Scholar]
- Yao LH, Jiang YM, Shi J, Tomás-Barberán FA, Datta N, Singanusong R, Chen SS. Flavonoids in food and their health benefits. Plant Foods Hum Nutr. 2004;59:113–122. doi: 10.1007/s11130-004-0049-7. [DOI] [PubMed] [Google Scholar]
- Zunino ZS, David HS, Stephensen CB. Diets rich in polyphenols and vitamin A inhibit the development of type I autoimmune diabetes in non-obese diabetic mice. J Nutr. 2007;137:1216–1221. doi: 10.1093/jn/137.5.1216. [DOI] [PubMed] [Google Scholar]