Abstract
The chick embryo chorioallantoic membrane (CAM) is a preclinical model widely used for vascular and anti-vascular effects of therapeutic agents in vivo. In this study, we examine the suitability of CAM as a predictive model for acute toxicology studies of drugs by comparing it to conventional mouse and rat models for 10 FDA-approved anticancer drugs (paclitaxel, carmustine, camptothecin, cyclophosphamide, vincristine, cisplatin, aloin, mitomycin C, actinomycin-D, melphalan). Suitable formulations for intravenous administration were determined before the average of median lethal dose (LD50) and median survival dose (SD50) in the CAM were measured and calculated for these drugs. The resultant ideal LD50 values were correlated to those reported in the literature using Pearson’s correlation test for both intravenous and intraperitoneal routes of injection in rodents. Our results showed moderate correlations (r2=0.42 − 0.68, P<0.005–0.05) between the ideal LD50 values obtained using the CAM model with LD50 values from mice and rats models for both intravenous and intraperitoneal administrations, suggesting that the chick embryo may be a suitable alternative model for acute drug toxicity screening before embarking on full toxicological investigations in rodents in development of anticancer drugs.
Keywords: alternative predictive model, chick embryo chorioallantoic membrane (CAM), median lethal dose (LD50), preclinical toxicology, 3R’s approach
Introduction
In conventional drug discovery and development pipelines, preclinical animal models are used to investigate the toxicity, stability, pharmacokinetics, and mechanism of action of new bioactive molecules. In 1959, Russell and Burch published a practical “Three Rs” strategy in “The Principles of Humane Experimental Technique” to reduce the sufferings of animals caused by scientific experimentation [38]. The “first R” refers to “replacement” of the use of animals both absolutely by computer models or in vitro cultures, and relatively with lower animals having a lower potential for pain perception. In practice, replacement of animals with an in vitro cell culture system is not ideal because of the huge discrepancy between the two systems in terms of systemic environments such as the vascular system [43].
The chick embryo chorioallantoic membrane (CAM) refers to the outermost extra-embryonic membrane that is highly vascularized for gaseous exchange and calcium transportation between the embryo and its environment. The CAM provides a technically simple way of studying complex biological systems with well-developed vascular tissues. It also has high reproducibility; and a small footprint, and it is inexpensive and easy to handle. The CAM model is recognized as an intermediate model that can bridge the gap between cell-based and animal-based assays; other than showing similar patterns of cellular toxicity as in vitro models, the CAM also gives tissue responses similar to those in mammalian models [25, 45].
In biomedical research, the CAM has been widely used as a model for angiogenesis studies due to its thin, transparent, and vascularized structure [1, 35]; and in xenograft tumor studies for invasive human tumor cells due to its immature immune system [9, 25]. Moreover, the CAM has been accepted as a substitute for the Draize test on rabbits for the testing of irritation potential of chemicals [5, 21, 40, 48]. The Institutional Animal Care and Use Committee (IACUC), an Association of New England Medical Center and Tufts [19], as well as the National Institutes of Health, USA [27], mandated that a chick embryo that has not reached the 14th day of its gestation period would not experience pain and can therefore be used for experimentation without any ethical restrictions or prior protocol approval, simplifying the planning process. Using a model that minimizes pain and suffering of animals is also in line with the “second R,” which advocates “refinement” to minimize animal suffering and enhance animal welfare.
The “third R” focuses on “reduction” to minimize animal use by obtaining more information from the same number of animals. This principle can be challenging to adhere to in dose-range finding and maximum tolerated dose studies, which are the first steps in preclinical toxicology investigations in the development of new medicines. These studies often require many animals and take a long time, especially if the drug candidate studied is new in term of class and has no related literature to guide dose selection. Therefore, establishment of estimated dosages in a lower animal model that is strongly correlated with rodents would be helpful in reducing the number of animals required for subsequent studies in rodents. In our study, we selected ten FDA-approved anticancer drugs to examine the correlation of ideal LD50 values in both the CAM and rodents to assess the suitability of the CAM as a predictive model for testing toxicology and pharmacology of drugs in rodents.
Materials and Methods
Chick embryo chorioallantoic membrane (CAM) model
Fresh fertilized chicken eggs of the Lohmann Brown chicken variety were purchased from Hong Hing Sdn. Bhd, Selangor, Malaysia, and disinfected with ethanol. The eggs were then incubated with the narrow apex down in a 90° swinging incubator (Savimat MG 200, Chauffry, France) at 37°C and 65% relative humidity for embryogenesis (embryo development day 1 or EDD-1). On EDD-3, an opening on the apex of the eggs was made and then sealed with adhesive tape to avoid contamination and dessication of the egg contents. The eggs were then further incubated in a stagnant position with the apex upright until EDD-9. The viability of the embryos and the vasculature of the CAM were then visually inspected and were used for drug administration by needle injection.
Anticancer drugs and preparation
Anticancer agents (paclitaxel, carmustine, camptothecin, cyclophosphamide, vincristine, cisplatin, aloin, mitomycin C, actinomycin-D, melphalan) were purchased from Sigma-Aldrich (St. Louis, MO, USA). The solvents used for dissolving anticancer agents varied based on the drug physicochemical characteristics. Molecular grade solvents were used throughout our experiments and were purchased from Sigma-Aldrich (St. Louis, MO, USA). Sterile clinical grade saline with 0.9% NaCl at pH 7.4 (A.N.B. Laboratories Co., Ltd., Bangkok, Thailand) was purchased from a retail pharmacy. The drug formulations were selected based on each drug’s MSDS and datasheet, as well as from information in the literature, and were then independently verified in our laboratory to be suitable for the CAM blood system if intravenous injection of the formulated drugs did not result in precipitation. The final drug formulations are shown in Table 1. Drugs were further diluted with saline to the desired dosages before a final 20 µl solution was injected into the CAM vascular system.
Table 1. Formulations for FDA-approved anticancer drugs and the effect of the formulations only on chick embryos.
| Anticancer drugs | Solvents used in formulations# | No. of dead embryos (n=10)# |
|---|---|---|
| Cyclophosphamide | Saline | 0 |
| Cisplatin | Saline | 0 |
| Vincristine | Saline | 0 |
| Mitomycin C | Saline | 1 |
| Aloin | Saline | 0 |
| Camptothecin | 0.025% Sodium hydroxide in saline | 0 |
| Carmustine | 5% Ethanol in saline | 0 |
| Melphalan | 5% cocktail (19:1 of ethanol / 6 N hydrochloric acid) in saline | 1 |
| Actinomycin-D | 1.2% acetone in saline | 1 |
| Paclitaxel | 1.5% cocktail (1:1 of ethanol / Cremophor EL) in saline | 1 |
# Percentage of solvents used in formulations and no. of dead embryos were based on the highest dosages of the drugs administered to the CAM.
Drug administration
Drug experimentation on CAMs by IV administration was performed on EDD-9 when the blood vasculature has fully developed [34]. EDD-9 chick embryos are commonly utilized for testing of drug formulations and irritants [24, 44] while embryos of later stages, for example. EDD-11 and above, have been used for testing in vascular assays [34, 44, 47]. For project planning purposes, EDD-9 is advantageous, since experimentation on chick embryos younger than EDD-14 does not require approval from an animal ethics committee [19, 27]. On EDD-9, CAM development and vascular formation were macroscopically examined via the opened apex of the egg. The healthy developed embryos (n=10) were randomly grouped and injected intravenously at the main vasculature (CAM allantoic arteries) of the CAM, which is located just under the membrane, with desired dosages of drugs using a microliter capillary syringe with a 33-gauge needle (Hamilton, Reno, NV, USA). Eggs were then sealed with parafilm and placed in an incubator at 37°C for further observation at 24 h and 48 h, and mortality rate was recorded. Embryos were considered alive if there was no death 48 h post administration [28]. The embryos were considered dead if they were motionless and had cloudy contents when observed through the opening at the apex of the egg.
Acute toxicity and ideal LD50 determination
In the early stage of the experiment, six concentrations of drug doses covering the range extending to 10-fold the dose was selected based on literature reports were tested on the CAM. Once the dose at which 50% of the embryos died was approximately determined, a 3.2-fold (or half log) up-and-down dosing procedure based on OECD guidelines was used to determine the exact LD50 [28]. Each drug dosage and vehicle control solvent system (without drugs) was repeated for 10 embryos. Instead of using the “conventional” Reed and Muench method of LD50 estimation [33], the ideal LD50 was calculated from the results using the modified Reed and Muench method [39], which employs cumulative values of both% dead animals and% surviving animals.
Formulae for the “conventional” Reed and Muench method (Supplementary Table 1):
Calculating LD50
A=[ 50% − (% of mortality below 50%) ] / [ (% of mortality above 50%) − (% of mortality below 50%) ]
B=Log [(Dosage corresponding (to%) of mortality above 50%) / (Dosage corresponding (to%) of mortality below 50%)]
LD50 / embryo=Log−1 [Log (Dosage corresponding to% of mortality below 50%) + (A × B)]
Calculating SD50
A=[ 50% − (% of survival below 50%) ] / [ (% of survival above 50%) − (% of survival below 50%) ]
B=Log [(Dosage corresponding (to%) of survival below 50%) / (Dosage corresponding (to%) of survival above 50%)]
SD50 / embryo=Log−1 [Log (Dosage corresponding to% of survival below 50%) + (A × B)]
The values for % of mortality or survival above and below 50% were obtained from Table 2. The computation of LD50 and SD50 values is demonstrated in Supplementary Table 1.
Table 2. Toxicity pattern of FDA-approved anticancer drugs in the CAM model 48 hours post administration.
| Drugs | Dose (μg/embryo) |
Log dose | No. of deaths embryo (n=10) |
No. of surviving embryo (n=10) |
Cumulative # | % of mortality |
% of survival |
||
|---|---|---|---|---|---|---|---|---|---|
| Death | Survival | Total | |||||||
| Cyclophosphamide | 400 | 2.6 | 9 | 1 | 18 | 1 | 19 | 94.7 | 5.3 |
| 125 | 2.1 | 7 | 3 | 9 | 4 | 13 | 69.2 | 30.8 | |
| 40 | 1.6 | 2 | 8 | 2 | 12 | 14 | 14.3 | 85.7 | |
| Cisplatin | 48 | 1.68 | 10 | 0 | 18 | 0 | 18 | 100 | 0 |
| 15 | 1.18 | 8 | 2 | 8 | 2 | 10 | 80 | 20 | |
| 4.7 | 0.67 | 0 | 10 | 0 | 12 | 12 | 0 | 100 | |
| Vincristine | 0.96 | –0.02 | 10 | 0 | 24 | 0 | 24 | 100 | 0 |
| 0.3 | –0.5 | 10 | 0 | 14 | 0 | 14 | 100 | 0 | |
| 0.096 | –1 | 4 | 6 | 4 | 6 | 10 | 40 | 60 | |
| Carmustine | 120 | 2.08 | 9 | 1 | 17 | 1 | 18 | 94.4 | 5.6 |
| 37.5 | 1.57 | 5 | 5 | 8 | 6 | 14 | 57.1 | 42.9 | |
| 12 | 1.08 | 3 | 7 | 3 | 13 | 16 | 18.8 | 81.2 | |
| Camptothecin | 30 | 1.48 | 9 | 1 | 18 | 1 | 19 | 94.7 | 5.3 |
| 9.4 | 0.97 | 5 | 5 | 9 | 6 | 15 | 60 | 40 | |
| 3 | 0.48 | 4 | 6 | 4 | 12 | 16 | 25 | 75 | |
| Aloin | 200 | 2.3 | 7 | 3 | 17 | 3 | 20 | 85 | 15 |
| 62.6 | 1.8 | 4 | 6 | 10 | 9 | 19 | 52.6 | 47.4 | |
| 19.4 | 1.29 | 6 | 4 | 6 | 13 | 19 | 31.6 | 68.4 | |
| Mitomycin-C | 16.6 | 1.22 | 7 | 3 | 14 | 3 | 17 | 82.4 | 17.6 |
| 5.2 | 0.72 | 5 | 5 | 7 | 8 | 15 | 46.7 | 53.3 | |
| 1.62 | 0.21 | 2 | 8 | 2 | 16 | 18 | 11.1 | 88.9 | |
| Actinomycin-D | 0.006 | –2.22 | 9 | 1 | 19 | 1 | 20 | 95 | 5 |
| 0.002 | –2.69 | 6 | 4 | 10 | 5 | 15 | 66.7 | 33.3 | |
| 0.0006 | –3.22 | 4 | 6 | 4 | 11 | 15 | 26.7 | 73.3 | |
| Melphalan | 31 | 1.49 | 8 | 2 | 17 | 2 | 19 | 89.5 | 10.5 |
| 9.7 | 0.99 | 7 | 3 | 9 | 5 | 14 | 64.3 | 35.7 | |
| 3 | 0.48 | 2 | 8 | 2 | 13 | 15 | 13.3 | 86.7 | |
| Paclitaxel | 9.38 | 0.97 | 9 | 1 | 17 | 1 | 18 | 94.4 | 5.6 |
| 3 | 0.48 | 4 | 6 | 8 | 7 | 15 | 53.3 | 46.7 | |
| 0.92 | –0.04 | 4 | 6 | 4 | 13 | 17 | 23.5 | 76.5 | |
# The formulae for calculating cumulative death / survival were those reported by Reed and Muench [33].
Formula for the modified Reed and Muench method (Supplementary Table 1):
Ideal LD50=[Median% of mortality (LD50) + Median% of survival (SD50) ] / 2
Mouse and rat LD50 values were obtained from published data in the literature and the Toxicology Data Network (TOXNET).
Pearson’s correlation test
Pearson’s correlation test was determined using the GraphPad Prism 5 software (GraphPad Software Inc., San Diego, CA, USA). The value was considered significant if P<0.05.
Results
Drug formulations for the CAM model
First, suitable formulations for the ten FDA-approved anticancer drugs were determined based on the drug solubility and toxicity of the formulating agents in the CAM. As these drugs have all been well studied, the formulations reported in the literature for delivery in the blood system were selected for further optimization (Table 1). Among the ten drugs examined, 5 drugs (cyclophosphamide, cisplatin, vincristine, mitomycin C, and aloin) were dissolved in saline and did not result in precipitation in blood upon administration. For camptothecin, Hentze and colleagues [17] reported that it was soluble in 0.1 N NaOH as a stock solution, while Rajpal Deshmukh [32] observed that this concentration of NaOH caused hemorrhage in the CAM. Therefore in our study, camptothecin in 0.1 N NaOH was diluted with saline at pH 7.4 to final dosages containing 0.005 N (0.025%) or less NaOH for the various concentrations tested. Carmustine and melphalan had similar formulations, with 5% or less ethanol in the final injectable solutions, and this amount of ethanol was tolerated by the CAM [31, 44]. Actinomycin-D was reported to dissolve in acetone, and in our experimental protocol, 1.2% of acetone in the final solution of the most concentrated sample was acceptable in the CAM; as Chen et al. (2013) previously reported successful usage of a much higher amount (50%) of acetone for angiogenesis assays in the CAM [7]. Paclitaxel has poor water solubility and had to be first prepared as a stock cocktail in 1:1 cremophore/ethanol, before further dilution with saline to a final injectable solution containing up to 1.5% of the cocktail. The amount of potentially hazardous cremophore and ethanol in our experiments were very low (<1.5%) compared with that in previous reports that utilized up to 5% of cremophore and ethanol mixtures in their cocktails [24]. The vehicle solvent systems corresponding to the highest dosage of drugs administrated to the CAM showed 0 or 10% mortality (n=0 or 1 out of 10), suggesting that the vehicle solvents alone had minimal adverse effects on the CAM.
Ideal LD50 calculation in the CAM
In 1938, Reed and Muench (R & M) [33] proposed an arithmetic method of determining the median lethal dose (LD50). A recent paper by Saganuwan [39] suggested that animal toxicity can be more precisely and accurately determined by calculating the mean of the median percentage survival (SD50) and median percentage death (LD50), and he termed this the “ideal LD50”. Using the ideal LD50 method, the toxicity profile of the 10 anticancer drugs in the CAM 48 h post administration was determined (Table 2). In Table 2, the number of embryo surviving at 48 h after administration for all the drugs investigated was dose dependent, with the higher doses yielding lower survival. Two exceptions were aloin and paclitaxel when analyzed according to the two lower doses in the respective drugs, with the lowest doses resulting in fewer (for aloin) or the same (for paclitaxel) number of embryo surviving as the medium doses. The reason for not conforming to dose dependence was not known but could have been due to non-drug-related errors. Next, the LD50, SD50, and ideal LD50 values for each investigated drug in the CAM based on the R & M method are shown in Supplementary Table 1. The ideal LD50 values and corresponding log ideal LD50 values for the CAM were computed and presented in Table 3 together with the LD50 value and corresponding log LD50 value for rodents extracted from literature.
Table 3. Ideal LD50 values (mg/kg) for FDA-approved anticancer drugs in CAM and rodent models.
| Drugs | Model and route of drug administration | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ideal CAM IV | Mice IV | Mice IP | Rats IV | Rats IP | ||||||||||
| mg/kg | log (mg/kg) | mg/kg | log (mg/kg) | mg/kg | log (mg/kg) | mg/kg | log (mg/kg) | mg/kg | log (mg/kg) | |||||
| Cyclophosphamide | 1.79 | –0.32 | 140 | 2.15 | 110 | 2.04 | 148 | 2.17 | 40 | 1.6 | ||||
| Cisplatin | 0.21 | –0.62 | 11 | 1.04 | 6.6 | 0.82 | 8 | 0.9 | 6.4 | 0.81 | ||||
| Vincristine | 0.01 | –2.54 | 3 | 0.48 | 1.3 | 0.11 | 1 | 0 | 1.25 | 0.1 | ||||
| Carmustine | 0.57 | –0.12 | 45 | 1.65 | 21.26 | 1.33 | 13.8 | 1.14 | 17.42 | 1.24 | ||||
| Camptothecin | 0.14 | –0.87 | 38 | 1.58 | 64 | 1.81 | 234 | 2.38 | Not available | Not available | ||||
| Aloin | 0.96 | –0.02 | 200 | 2.3 | 218.00# | 2.34 | >15.00§ | 1.18 | >50.00§ | 1.7 | ||||
| Mitomycin-C | 0.26 | –0.58 | 4 | 0.6 | 4 | 0.6 | 3 | 0.48 | 2 | 0.3 | ||||
| Actinomycin-D | 0.00003 | –4.52 | 1.03 | 0.01 | 0.75 | –0.12 | 0.46 | –0.34 | 0.1 | –1 | ||||
| Melphalan | 0.14 | –0.85 | 20.8 | 1.32 | 10 | 1 | 4.1 | 0.61 | 4.48 | 0.65 | ||||
| Paclitaxel | 0.04 | –1.4 | 12 | 1.08 | 128 | 2.1 | 85 | 1.93 | 32.53 | 1.51 | ||||
Ideal CAM LD50 values were computed from the data in Table 2. The LD50 values for rodents were extracted from the Toxicology Data Network (TOXNET) from United States National Library of Medicine. (http://toxnet.nlm.nih.gov) except for those marked with superscript symbols. # Fahim et al. [12, 13]. § Anderson [4].
From Table 3, the ideal LD50 values of the drugs studied in the CAM models ranged from 0.00003–1.79 mg/kg, while those in mice and rats ranged from 0.1–218 mg/kg. The LD50 values for mice were similar to those for rats. Additionally, the LD50 values for intravenous (IV) administration in mice or rats were almost the same to those for intraperitoneal (IP) administration in the same species. Other than actinomycin-D, which had an exceptionally low LD50 in the CAM with IV administration, the ratio of LD50 in rodents (IP or IV) to the CAM with IV administration ranged from tenfold to over a thousandfold. The data in Table 3 were used to plot Figs 1 and 2) to examine the correlation between the CAM ideal LD50 and rodent LD50 values.
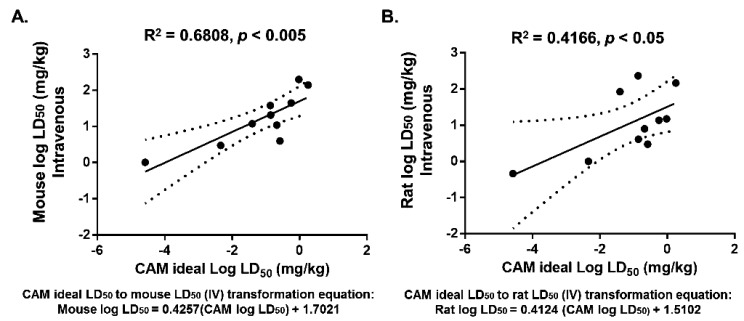
Fig. 1.
The ideal LD50 values of the drugs in the CAM significantly correlated with LD50 values in mice (A) and rats (B) for intravenous injection. The CAM ideal LD50 was determined based on the average of the LD50 and SD50 after 48 h of exposure to drugs. LD50 values for mice and rats were obtained from the literature and TOXNET. Solid lines indicate regression lines (best-fit lines), and dotted curves indicate 95% confidence bands for the best-fit lines. r2=coefficient of determination. Formulation represent the transformed CAM ideal LD50 to rodents.
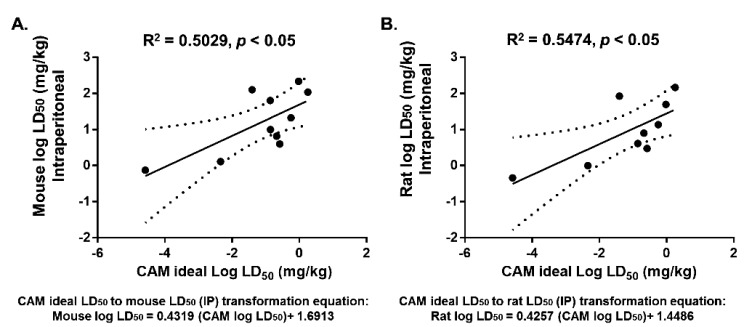
Fig. 2.
The ideal LD50 values of the drugs in the CAM correlated with LD50 values in mice (A) and rats (B) for intraperitoneal injection. The CAM ideal LD50 was determined based on the average of the LD50 and SD50 after 48 h of exposure to drugs. LD50 values of mice and rats for IP administration were obtained from the literature and TOXNET. Solid lines indicate regression lines (best-fit lines), and dotted curves indicate 95% confidence bands for the best-fit lines. r2=coefficient of determination. Formulation represent the transformed CAM ideal LD50 to rodents.
Significant correlation between the ideal LD50 for the CAM and LD50 for mice
We next examined the ability of the CAM model to replace rodents in acute toxicology studies by correlating the ideal LD50 values of our selected drugs in the CAM with the LD50 values in mice and rats for both the IV and IP routes of administration. As shown in Fig. 1, Pearson’s correlation test yielded highly significant correlation (r2=0.6808, P<0.005) between the CAM and mice treated with IV drugs (Fig. 1A). In addition, the CAM also significantly correlated with rats treated with IV injection of drugs (r2=0.4166, P<0.05), but the correlation was not as tight as with the mice treated with IV drugs (Fig. 1B). These values indicated that drug toxicity in the CAM is well correlated with that in rodents, suggesting that the CAM can be a good predictive model in toxicological studies involving IV administration. We next examined the correlations between the CAM with IV administration with rodents IP administration. Interestingly, we found that both rodent models were also significantly correlated (r2=0.5, P<0.05) with the CAM even though the routes of administration differed between the CAM and the rodents (Figs. 2A and 2B. An equations for transforming the CAM ideal LD50 to estimate LD50 values for rodents were generated based on the linear regression graphs in Figs. 1 and 2.
Discussion
The chemical and pharmaceutical industries have been highly criticized for their concerning use of experimental animals for toxicity and safety evaluation of substances. A report from the industry community in Europe stated that in 2011, the number of animals used for experimental and scientific purposes was 11.5 million, with rodents and rabbits representing 80% of the animals. Among the animals used, 64% were mice, and 14% were rats; most of these animals were used for toxicity and safety evaluations of human and veterinary medicines [8]. Thus there is a compelling need to replace the use of rodents as the first line organism for toxicity and safety evaluations for pharmaceutical and non-pharmaceutical substances.
The first example of using a lower nonmammalian animal to replace rodents and rabbits in toxicology testing was the use of brine shrimp larvae [22, 26]. In 2002, Parng and colleagues proposed the use of zebrafish embryos as another predictive model for rodents based on statistical correlation in toxicology data drug screening data between the two organisms [30]. Since then, zebrafish embryos have been used in large-scale screening of bioactive compounds [2, 3, 14]. As an alternative, the CAM would be expected to be a closer mimic to rodents compared with invertebrates and fish because of the similarities in physiological makeup between chicks and rodents such as in the vasculature system and organogenesis. On the other hand, fish embryos have poor drug absorption and limited metabolic capacity, which may result in underestimation of the toxic effects of the drugs tested [10]. In a study to test toxicity of crude snake venom, which consisted of complex mixtures of proteins and enzymes [6, 36], the data obtained from using the CAM was found to correlate strongly with that from mice [42]. Furthermore, a study by Gunnarsson et al. on orthologs for 1,318 human drug targets in 16 species; reported that the chicken has orthologs to 70% of the human drug targets, which is just less than the mouse, which has 87% orthology [15]. While their report suggests that different species have different sensitivity to drugs, it also implies that mouse and chicken have a high degree of orthology in their drug targets. In a separate study by Schrage et al., comparison between in vitro cytotoxicity and in vivo acute oral toxicity in rats of various substances including industrial chemicals, agrochemicals and drug formulations revealed more than 50% concordance with each other [41]. Compared with in vitro models, it is expected that the CAM model utilized in our study would yield a higher degree of concordance with in vivo acute toxicity models, since the CAM incorporates biotransformation processes such as metabolism and degradation that drugs often undergo in vivo. Furthermore, drug formulations for in vitro studies are different from those required by intravenous in vivo studies, especially because of the need to consider contact with blood plasma proteins in animal blood.
The conventional CAM assay administers drugs through implantation of a membrane or coverslip containing the investigated compounds in order to study neovascularization and/or to study corrosivity on the CAM. In this study, the selected drugs were administered intravenously via the CAM allantoic arteries, as this is the most common route of administration for anticancer drugs. For drugs developed for the IV route of administration, there are several advantages to using the CAM: Firstly, the CAM has an extensive capillary network that is well developed by EDD-9 [34] for researchers to test drug formulations and their effects on the vasculature system via the IV route of administration. Testing of drug effects on vasculature is possible through monitoring of blood flow under microscopic examination before and after drug injection [44]. Secondly, blood circulation in the CAM is minimally disrupted by puncture of the blood vessel, and this enables the drug to continue in the circulation to reach embryo organs [23]. Thirdly, drug compounds can be tested on the CAM using both intraperitoneal and intravenous systemic delivery methods, which are the same as the methods typically performed on rodents [46]. Also, since the ideal LD50 for the CAM with IV administration correlated well with those of rodents with both IP and IV routes of administration, this further extends the versatility of the CAM model as a replacement for toxicology work in rodents.
Studies by Hammer-Wilson et al. and Hornung et al. [16, 18] have shown that IP administration has effects similar to IV administration in the CAM based on the high drug level detectable in blood only mins after administration. Therefore, instead of comparing data from IP administered in the CAM and rodents, this study analyzed the correlation between data from IV administration in the CAM and IP administration in rodents. Based on Fig. 2, even though significant correlation was obtained in the comparison analysis between IV administration in the CAM and IP administration in rodents, the coefficient of determination (r2) value was not as high as the ones obtained for IV administration in rodents (Fig. 1). This is not surprising, since delivery of drugs to target organs is direct in IV administration, while the delivery through IP administration in the peritoneal region depends on diffusion to reach organs. As most chemotherapy drugs are developed for IV administration, the correlation with the IV-treated rodents suggests that the CAM model may have wide utility in the development of cancer therapeutics. Comparison of ideal LD50 values in the CAM to LD50 values in rats and mice with the oral route of administration was not conducted in this study because the oral route of administration was not available for many of the anticancer drugs selected. As orally administered drugs are eventually distributed systematically, upon overcoming adsorption and permeability barriers in the gastrointestinal system, the LD50 values for the oral route of administration are likely to be comparable to those of IP or IV routes [11].
Other than reducing in the number of animals used, the CAM model is also able to reduce the amount of drugs required for testing. According to Table 3, the ideal LD50 values in the CAM were more than ten to more than a thousand times lower than the LD50 values in rodents. Therefore, the overall amount of a drug that could be used in toxicity studies in the CAM is much less than that required in rodents. Hence, even though the weight of the CAM (40 g) is approximately twice that of a mouse, the overall drug required is still considerably lower. Furthermore, there is a reduction in the amount of the drug required later in toxicological studies in mice because more accurate starting dosages can be selected for dose-finding experiments; by transforming the ideal LD50 in the CAM to that in rodents using the linear regression equations. The predictive value of the correlation factors may be further fine-tuned with testing of more drugs and more chick embryos. Also, as the strength of the correlation between the ideal LD50 values in the CAM and rodents is only moderate (r2=0.5) with a big range in fold differences between the two, the ideal LD50 values in the CAM should only be utilized to guide selection of initial doses in rodents, and not to replace full investigations of rodent toxicity. Technically, IV administration of drugs into the CAM arteries is challenging, requires practice, and may cause non-drug-related deaths. In addition, the acute toxicity observed in this study only captured the overall toxicity to the embryos when it could actually be due to different forms of toxicity such as developmental toxicity, neurotoxicity or organ-specific toxicity.
This paper tested and compared the LD50 of FDA-approved anticancer drugs because toxicity data for these compounds in different species are readily available from the literature and databases. Moreover, as many anticancer drugs are potent cytotoxic agents, the LD50 values measured were more accurate because they were less likely due to non-pharmacological effects or noise. However, the regulatory requirement for acute toxicity studies in pharmaceutical drug development prior to initial clinical trials in humans has recently come under criticism. A European initiative reviewed acute toxicity studies for 74 compounds and concluded that such toxicology data are not needed prior to initial clinical trials in humans, as information can be obtained from other studies [37]. Therefore, the wider utility of our study may be in acute toxicity studies of other non-pharmaceuticals such as industrial chemicals, biomaterials and environmental samples.
In conclusion, the CAM is relatively simple, quick, and low-cost model that allows screening of a large number of pharmacological samples in a short time [29]. The results of this study show that there is significant correlation in the ideal LD50 values generated using the CAM verses mice, suggesting that acute toxicity studies in rodents could benefit from preliminary studies using the CAM as a way to save time, the amount of drug used, and number of animals used. Importantly, the CAM model conforms to the 3Rs approach in preclinical experimentation, as it can serve as a replacement for higher animals, provides refinement through the use of insensate embryos with no susceptibility to pain at embryonic development days prior to 14 (EDD-14) [20], and can reduce the number of animals required by providing estimated starting dosages for toxicology studies in rodents. Altogether, our results support the use of the CAM model as an alternative predictive model in acute toxicological studies for new drugs.
Conflict of Interest
The authors declare that there are no conflicts of interest.
Fundings
This work was funded by the donors of the Cancer Research Initiatives Foundation (CARIF) and University of Malaya-High Impact Research Grants (UM.C/625/1/HIR/MOHE/MED/17 & UM.C/625/1/HIR/MOHE/MED/33) from the Ministry of Higher Education, Malaysia.
Supplementary Material
References
- 1.Alavi A., Hood J.D., Frausto R., Stupack D.G., Cheresh D.A.2003. Role of Raf in vascular protection from distinct apoptotic stimuli. Science 301: 94–96. doi: 10.1126/science.1082015 [DOI] [PubMed] [Google Scholar]
- 2.Ali S., van Mil H.G., Richardson M.K.2011. Large-scale assessment of the zebrafish embryo as a possible predictive model in toxicity testing. PLoS ONE 6: e21076. doi: 10.1371/journal.pone.0021076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ali S., Champagne D.L., Spaink H.P., Richardson M.K.2011. Zebrafish embryos and larvae: a new generation of disease models and drug screens. Birth Defects Res. C Embryo Today 93: 115–133. doi: 10.1002/bdrc.20206 [DOI] [PubMed] [Google Scholar]
- 4.Andersen F.A.2007. Final report on the safety assessment of Aloe andongensis extract, Aloe andongensis leaf juice, Aloe arborescens leaf extract, Aloe arborescens leaf juice, Aloe arborescens leaf protoplasts, Aloe barbadensis flower extract, Aloe barbadensis leaf, Aloe barbadensis leaf extract, Aloe barbadensis leaf juice, Aloe barbadensis leaf polysaccharides, Aloe barbadensis leaf water, Aloe ferox ferox leaf extract. Int. J. Toxicol. 26: 1–50. doi: 10.1080/10915810701351186 [DOI] [PubMed] [Google Scholar]
- 5.Ardelean S., Feflea S., Ionescu D., Năstase V., Dehelean C.A.2011. Toxicologic screening of some surfactants using modern in vivo bioassays. Rev. Med. Chir. Soc. Med. Nat. Iasi 115: 251–258. [PubMed] [Google Scholar]
- 6.Calvete J.J., Fasoli E., Sanz L., Boschetti E., Righetti P.G.2009. Exploring the venom proteome of the western diamondback rattlesnake, Crotalus atrox, via snake venomics and combinatorial peptide ligand library approaches. J. Proteome Res. 8: 3055–3067. doi: 10.1021/pr900249q [DOI] [PubMed] [Google Scholar]
- 7.Chen Z., Wen Z., Bai X.2013. In vivo chick chorioallantoic membrane (CAM) angiogenesis assay. Bio-protocol 3 (18) (Abstract).
- 8.Commission of the European Community. Seven Report from the Commission to the Council and the European Parliament on the Statistics on the number of animals used for experimental and other scientific purposes in the member states of the European Union COM (2013) 859/final. 2013.
- 9.Durupt F., Koppers-Lalic D., Balme B., Budel L., Terrier O., Lina B., Thomas L., Hoeben R.C., Rosa-Calatrava M.2012. The chicken chorioallantoic membrane tumor assay as model for qualitative testing of oncolytic adenoviruses. Cancer Gene Ther. 19: 58–68. doi: 10.1038/cgt.2011.68 [DOI] [PubMed] [Google Scholar]
- 10.Eimon P.M., Rubinstein A.L.2009. The use of in vivo zebrafish assays in drug toxicity screening. Expert Opin. Drug Metab. Toxicol. 5: 393–401. doi: 10.1517/17425250902882128 [DOI] [PubMed] [Google Scholar]
- 11.Elas M., Parasca A., Grdina D.J., Halpern H.J.2003. Oral administration is as effective as intraperitoneal administration of amifostine in decreasing nitroxide EPR signal decay in vivo. Biochim. Biophys. Acta 1637: 151–155. doi: 10.1016/S0925-4439(02)00228-4 [DOI] [PubMed] [Google Scholar]
- 12.Fahim F., Abd-Allah N., Esmat A.1993. Some metabolic aspects in normal and tumor-bearing mice treated with a natural anthraquinone. J. Tumor Marker Oncol. 8: 25–41. [Google Scholar]
- 13.Fahim F., Esmat A., Essam A., Amin M.1997. Pharmacokinetic and tumor studies of aloin (A natural anthraquinone) on mice bearing solid Ehrlich carcinoma. J. Tumor Marker Oncol. 12: 127–134. [Google Scholar]
- 14.Gibert Y., Trengove M.C., Ward A.C.2013. Zebrafish as a genetic model in pre-clinical drug testing and screening. Curr. Med. Chem. 20: 2458–2466. doi: 10.2174/0929867311320190005 [DOI] [PubMed] [Google Scholar]
- 15.Gunnarsson L., Jauhiainen A., Kristiansson E., Nerman O., Larsson D.G.J.2008. Evolutionary conservation of human drug targets in organisms used for environmental risk assessments. Environ. Sci. Technol. 42: 5807–5813. doi: 10.1021/es8005173 [DOI] [PubMed] [Google Scholar]
- 16.Hammer-Wilson M.J., Cao D., Kimel S., Berns M.W.2002. Photodynamic parameters in the chick chorioallantoic membrane (CAM) bioassay for photosensitizers administered intraperitoneally (IP) into the chick embryo. Photochem. Photobiol. Sci. 1: 721–728. doi: 10.1039/b205471j [DOI] [PubMed] [Google Scholar]
- 17.Hentze H., Latta M., Künstle G., Dhakshinamoorthy S., Ng P.Y., Porter A.G., Wendel A.2004. Topoisomerase inhibitor camptothecin sensitizes mouse hepatocytes in vitro and in vivo to TNF-mediated apoptosis. Hepatology 39: 1311–1320. doi: 10.1002/hep.20174 [DOI] [PubMed] [Google Scholar]
- 18.Hornung R., Hammer-Wilson M.J., Kimel S., Liaw L.H., Tadir Y., Berns M.W.1999. Systemic application of photosensitizers in the chick chorioallantoic membrane (CAM) model: photodynamic response of CAM vessels and 5-aminolevulinic acid uptake kinetics by transplantable tumors. J. Photochem. Photobiol. B 49: 41–49. doi: 10.1016/S1011-1344(99)00014-7 [DOI] [PubMed] [Google Scholar]
- 19.IACUC Policy on Protocol Approval for Use of Chicken Embryos and Eggs. 2001. An Association of New England Medical Center and Tufts.
- 20.IACUC Policies and Procedures. 2012. The Use of Chicken / Avian Embryos. University of Louisville. May 2012. https://louisville.edu/research/iacuc/docs/policies/24%20Use%20of%20Chicken-Avian%20Embryos.pdf.
- 21.Kishore A.S., Surekha P.A., Sekhar P.V., Srinivas A., Murthy P.B.2008. Hen egg chorioallantoic membrane bioassay: an in vitro alternative to draize eye irritation test for pesticide screening. Int. J. Toxicol. 27: 449–453. doi: 10.1080/10915810802656996 [DOI] [PubMed] [Google Scholar]
- 22.Logarto Parra A., Silva Yhebra R., Guerra Sardiñas I., Iglesias Buela L.2001. Comparative study of the assay of Artemia salina L. and the estimate of the medium lethal dose (LD50 value) in mice, to determine oral acute toxicity of plant extracts. Phytomedicine 8: 395–400. doi: 10.1078/0944-7113-00044 [DOI] [PubMed] [Google Scholar]
- 23.Leng T., Miller J.M., Bilbao K.V., Palanker D.V., Huie P., Blumenkranz M.S.2004. The chick chorioallantoic membrane as a model tissue for surgical retinal research and simulation. Retina 24: 427–434. doi: 10.1097/00006982-200406000-00014 [DOI] [PubMed] [Google Scholar]
- 24.Lim S.H., Nowak-Sliwinska P., Kamarulzaman F.A., van den Bergh H., Wagnières G., Lee H.B.2010. The neovessel occlusion efficacy of 15-hydroxypurpurin-7-lactone dimethyl ester induced with photodynamic therapy. Photochem. Photobiol. 86: 397–402. doi: 10.1111/j.1751-1097.2009.00684.x [DOI] [PubMed] [Google Scholar]
- 25.Lokman N.A., Elder A.S., Ricciardelli C., Oehler M.K.2012. Chick chorioallantoic membrane (CAM) assay as an in vivo model to study the effect of newly identified molecules on ovarian cancer invasion and metastasis. Int. J. Mol. Sci. 13: 9959–9970. doi: 10.3390/ijms13089959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martins J., Oliva Teles L., Vasconcelos V.2007. Assays with Daphnia magna and Danio rerio as alert systems in aquatic toxicology. Environ. Int. 33: 414–425. doi: 10.1016/j.envint.2006.12.006 [DOI] [PubMed] [Google Scholar]
- 27.National Institute of Health1991. The Public Health Service Responds to Commonly Asked Questions.” ILAR News 33.4: 68–70. Office of Laboratory Animal Welfare. http://grants.nih.gov/grants/olaw/references/ilar91.htm.
- 28.OECD2008. OECD Guidelines For The Testing Of Chemicals. OECD Test Guideline (425). Acute Oral Toxicity − Up-And-Down-Procedure (UDP). [Google Scholar]
- 29.Ohno Y., Kaneko T., Inoue T., Morikawa Y., Yoshida T., Fujii A., Masuda M., Ohno T., Hayashi M., Momma J., Uchiyama T., Chiba K., Ikeda N., Imanishi Y., Itakagaki H., Kakishima H., Kasai Y., Kurishita A., Kojima H., Matsukawa K., Nakamura T., Ohkoshi K., Okumura H., Saijo K., Sakamoto K., Suzuki T., Takano K., Tatsumi H., Tani N., Usami M., Watanabe R.1999. Interlaboratory validation of the in vitro eye irritation tests for cosmetic ingredients. (1) Overview of the validation study and Draize scores for the evaluation of the tests. Toxicol. In Vitro 13: 73–98. doi: 10.1016/S0887-2333(98)00064-2 [DOI] [PubMed] [Google Scholar]
- 30.Parng C., Seng W.L., Semino C., McGrath P.2002. Zebrafish: a preclinical model for drug screening. Assay Drug Dev. Technol. 1: 41–48. doi: 10.1089/154065802761001293 [DOI] [PubMed] [Google Scholar]
- 31.Pushpanjali A.K., Pal R.L., Prasad A., Prasad S.K., Singh A., Kumar S.A., Jadhao S.B.2005. In ovo embryotoxicity of α-endosulfan adversely influences liver and brain metabolism and the immune system in chickens. Pestic. Biochem. Physiol. 82: 103–114. doi: 10.1016/j.pestbp.2004.09.004 [DOI] [Google Scholar]
- 32.Rajpal Deshmukh G., Hema Kumar K., Suresh Reddy P.V., Srinivasa Rao B., Venkata Satish Kumar C.2012. Evaluation of eye irritation potential of aqueous leaf extract of achyranthes aspera by in vitro and in vivo method. ISRN Toxicol 2012: 693489. doi: 10.5402/2012/693489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reed L.J., Muench H.1938. A simple method of estimating fifty percent end points. Am. J. Hyg. 27: 493–497. [Google Scholar]
- 34.Ribatti D., Nico B., Vacca A., Roncali L., Burri P.H., Djonov V.2001. Chorioallantoic membrane capillary bed: a useful target for studying angiogenesis and anti-angiogenesis in vivo. Anat. Rec. 264: 317–324. doi: 10.1002/ar.10021 [DOI] [PubMed] [Google Scholar]
- 35.Richardson M., Singh G.2003. Observations on the use of the avian chorioallantoic membrane (CAM) model in investigations into angiogenesis. Curr. Drug Targets Cardiovasc. Haematol. Disord. 3: 155–185. doi: 10.2174/1568006033481492 [DOI] [PubMed] [Google Scholar]
- 36.Risch M., Georgieva D., von Bergen M., Jehmlich N., Genov N., Arni R.K., Betzel C.2009. Snake venomics of the Siamese Russell’s viper (Daboia russelli siamensis) — relation to pharmacological activities. J. Proteomics 72: 256–269. doi: 10.1016/j.jprot.2009.01.006 [DOI] [PubMed] [Google Scholar]
- 37.Robinson S., Delongeas J.L., Donald E., Dreher D., Festag M., Kervyn S., Lampo A., Nahas K., Nogues V., Ockert D., Quinn K., Old S., Pickersgill N., Somers K., Stark C., Stei P., Waterson L., Chapman K.2008. A European pharmaceutical company initiative challenging the regulatory requirement for acute toxicity studies in pharmaceutical drug development. Regul. Toxicol. Pharmacol. 50: 345–352. doi: 10.1016/j.yrtph.2007.11.009 [DOI] [PubMed] [Google Scholar]
- 38.Russell W.M.S., Burch R.L.1959. The Principles of Humane Experimental Technique. Methuen, London UK. [Google Scholar]
- 39.Saganuwan A.2011. A modified arithmetical method of Reed and Muench for determination of a relatively ideal median lethal dose (LD50). Afr. J. Pharm. Pharmacol. 5: 1543–1546. doi: 10.5897/AJPP11.393 [DOI] [Google Scholar]
- 40.Saw C.L., Heng P.W., Liew C.V.2008. Chick chorioallantoic membrane as an in situ biological membrane for pharmaceutical formulation development: a review. Drug Dev. Ind. Pharm. 34: 1168–1177. doi: 10.1080/03639040801974295 [DOI] [PubMed] [Google Scholar]
- 41.Schrage A., Hempel K., Schulz M., Kolle S.N., van Ravenzwaay B., Landsiedel R.2011. Refinement and reduction of acute oral toxicity testing: a critical review of the use of cytotoxicity data. Altern. Lab. Anim. 39: 273–295. [DOI] [PubMed] [Google Scholar]
- 42.Sells P.G., Ioannou P., Theakston R.D.1998. A humane alternative to the measurement of the lethal effects (LD50) of non-neurotoxic venoms using hens’ eggs. Toxicon 36: 985–991. doi: 10.1016/S0041-0101(98)00004-X [DOI] [PubMed] [Google Scholar]
- 43.Straughan D.W., Fentem J.H., Balls M., Jose V.C., Maria Jose G.L.1996. Replacement Alternative and Complementary In Vitro Methods in Pharmaceutical Research. In vitro Methods in Pharmaceutical Research. San Diego, Academic Press. [Google Scholar]
- 44.Tay S.L., Heng P.W., Chan L.W.2012. The chick chorioallantoic membrane imaging method as a platform to evaluate vasoactivity and assess irritancy of compounds. J. Pharm. Pharmacol. 64: 1128–1137. doi: 10.1111/j.2042-7158.2012.01506.x [DOI] [PubMed] [Google Scholar]
- 45.Valdes T.I., Kreutzer D., Moussy F.2002. The chick chorioallantoic membrane as a novel in vivo model for the testing of biomaterials. J. Biomed. Mater. Res. 62: 273–282. doi: 10.1002/jbm.10152 [DOI] [PubMed] [Google Scholar]
- 46.Vargas A., Zeisser-Labouèbe M., Lange N., Gurny R., Delie F.2007. The chick embryo and its chorioallantoic membrane (CAM) for the in vivo evaluation of drug delivery systems. Adv. Drug Deliv. Rev. 59: 1162–1176. doi: 10.1016/j.addr.2007.04.019 [DOI] [PubMed] [Google Scholar]
- 47.Wilting J., Christ B., Weich H.A.1992. The effects of growth factors on the day 13 chorioallantoic membrane (CAM): a study of VEGF165 and PDGF-BB. Anat. Embryol. (Berl.) 186: 251–257. doi: 10.1007/BF00174147 [DOI] [PubMed] [Google Scholar]
- 48.Yan X., Piterski C., Nitka S.2007. Evaluation of the hen’s egg test-chorioallantonic membrane (CAM) method in prediction of the eye irritation potential formulated personal wash products. Cutan. Ocul. Toxicol. 26: 25–36. doi: 10.1080/15569520601183815 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.