Abstract
Isoflurane is a representative inhalant anesthesia used in laboratory animals. However, isoflurane mediates respiratory depression and adverse clinical reactions during induction. In the present study, we established a novel balanced anesthesia method in mice that combined isoflurane anesthesia with midazolam and butorphanol (MB). Thirty-four male C57BL/6J mice received either isoflurane alone or isoflurane with an intra-peritoneal MB premedication (3 mg/kg midazolam and 4 mg/kg butorphanol). The minimum alveolar concentration (MAC) in each group was evaluated. Induction time and adverse clinical reactions were recorded in each group. Core body temperature, heart rate, respiratory rate, and oxygen saturation (SPO2) were assessed before and for 1 h after induction. Premedication with MB achieved a significant reduction in MAC compared with isoflurane monoanesthesia (isoflurane, 1.38 ± 0.15%; isoflurane with MB, 0.78 ± 0.10%; P<0.05). Induction time was significantly shortened with MB premedication, and adverse reactions such as excitement or incontinence were observed less frequently. Furthermore, isoflurane anesthesia with MB premedication caused increase of respiratory rates compared to isoflurane monoanesthesia. No significant decrease of SPO2 was observed in MBI anesthesia, while a decrease in SPO2 was apparent with isoflurane monoanesthesia (baseline, 98.3% ± 1.1; 10 min after induction, 91.8 ± 6.4%; P<0.05). In conclusion, premedication with MB was effective for the mitigation of respiratory depression induced by isoflurane in mice, with rapid induction and fewer adverse clinical reactions.
Keywords: balanced anesthesia, inhalant anesthesia, respiratory depression
Introduction
The individual distress and suffering of animals during experimentation procedures must be minimized in terms of the principle of laboratory animal welfare. It is also important to minimize the distress because it may affect the experimental data. Anesthesia has hypnotic and analgesic actions, and is a way of attenuating distress or pain during an experimental intervention such as surgery. However, anesthesia can induce systemic adverse reactions, including cardiac, respiratory, and neuronal disorders that may affect the data of experimentation [3, 36]. Consequently, anesthetic protocols with less influence on cardiorespiratory function may be required for the accomplishment of appropriate animal experimentation.
General anesthesia in rodents is accomplished by injection or inhalation of anesthetic compounds. The route of administration for injectable anesthesia is typically intra-peritoneal or subcutaneous. Several injectable anesthetic protocols have been established for rodents [1,2,3, 16, 29]. Representative injectable anesthetics in rodents include a combination of ketamine and a sedatives [3, 20], and medetomidine-based anesthesia [1, 16, 17]. Although these injectable anesthetic protocols are practical and simple, the time and depth of anesthesia is somewhat uncontrollable once the initial dose has been administered. In contrast, inhalant anesthesia enables real-time regulation of anesthetic depth by easy adjustment of the concentration [13]. Additionally, inhalant anesthesia can be used for both short and long durations of anesthesia. Furthermore, absorption and elimination of volatile agents occurs through the lungs, which leads to rapid induction and recovery. Volatile anesthetic agents available in laboratory animal experimentation include halothane, isoflurane, sevoflurane, and desflurane [4, 12, 13, 25]. The use of inhalant anesthetics has increased in rodents, especially following the development and commercial availability of ready-to-use inhalation anesthesia devices for small rodents [4, 9].
Isoflurane is one of the most commonly used inhalant anesthetic agents in mice [25]. Among the modern volatile agents, isoflurane has less influence on hepatic metabolism [13]. However, isoflurane induces respiratory depression [4, 34, 35]. Previous finding demonstrated that isoflurane induced marked respiratory rate decrease, compared to other injectable anesthesia such as combinational anesthesia of ketamine and xylazine [34]. In addition, high doses of isoflurane during induction may cause adverse clinical reactions. In larger animals such as dog, undesirable excitement may occur in response to the inhalation mask used during induction [23].
Balanced anesthesia is the administration of a mixture of sedatives, analgesics, and anesthetics to induce anesthesia using lower doses of each drug than would be required if each component were used alone [32]. In human and veterinary medicine, combinations of sedatives and analgesics are used as pre-anesthetics, which contribute to the stabilization of inhalant anesthesia, and conserve the use of gas during surgery. The drugs which used as pre-anesthetics include sedatives such as benzodiazepines and analgesics like opioid agents [14, 28]. Several combinations of pre-anesthetics for inhalant anesthesia have been reported in both human and veterinary medicine [20, 23, 24, 27, 28].
The aim of the present study was to establish an isoflurane-based balanced anesthesia for mice. The efficacy of midazolam and butorphanol premedication was evaluated when used in conjunction with isoflurane anesthesia.
Materials and Methods
Animals
All procedures in the current study were performed in accordance with the guidelines approved by the Azabu University Animal Experiment Committee. Thirty-four 8-week old male C57BL/6J mice were purchased from JAPAN SLC INC (C57BL/6JJmsSLC, Shizuoka, Japan). Animals were acclimated for 1 week before starting the experiment, and housed separately in polycarbonate cages (CL-0106–1; 310 mm × 360 mm × 175 mm; CLEA Japan, Tokyo, Japan) on wood shavings. Mice were maintained under a barrier system at the Azabu University Research Institute of Biosciences. The room temperature was 22 ± 1°C, with a humidity of 55 ± 5%, and a light cycle that included 14 h of light a day, from 06:00 to 20:00. Mice were fed a pelleted mouse diet (mouse and rat chow; MC-2, CLEA Japan), and had unrestricted access to sterilized drinking water provided in a water bottle. All experiments were performed when mice were 8 weeks of age. As circadian rhythm affects cardiovascular function [6], all experiments and weighing procedures were conducted between 14:00–17:00. After finishing the entire experiment, euthanasia was performed by intraperitoneal administration of pentobarbital at a concentration of 100 mg/kg followed by cervical dislocation.
Study procedure
Mice were divided into two groups. One group was anesthetized with isoflurane only (Isoflu, DS Pharma Animal Health Co., Ltd., Osaka, Japan). The second group was pre-medicated with a combination of butorphanol (Vetorphale, Meiji Seika Pharma Co., Ltd., Tokyo, Japan) and midazolam (Dormicum, Astellas Pharma Inc., Tokyo, Japan), prior to inhalant anesthesia with isoflurane (MBI anesthesia). In the present study, isoflurane anesthesia was maintained using a mask with room air. Mice in the MBI anesthetic group were administered intra-peritoneal injections of 3 mg/kg midazolam and 4 mg/kg butorphanol, 5 min prior to induction of anesthesia. Before the administration, mixture MB was prepared, and then diluted with saline. The concentration was adjusted to 3 ml/kg. Inhalant anesthesia with isoflurane was performed using a commercially available rodent inhalant anesthesia apparatus (SomnoSuite Small Animal Anesthesia System, Kent Scientific Corporation, Connecticut). Induction was performed in rodent induction chamber. The isoflurane concentration was set to 5% during induction. After induction, mice were rapidly transferred to a nose cone mask. Flow rate of isoflurane was set at 35 ml/min. The doses of all agents were determined according to a previous report in mice [12].
The minimum alveolar concentration (MAC) in each anesthetic protocol was firstly determined using groups of eight mice. Concurrently, adverse reactions during induction were recorded. The concentration of isoflurane used for maintenance of anesthesia in each group was based on the MAC determined in advance, and the influence of each anesthetic protocol on vital signs was evaluated (n=9). Additionally, induction time was recorded in each group.
MAC determination
MAC is indicator of the anesthetic concentration that is defined as the concentration for the prevention of motor response in 50% of individuals during inhalant anesthesia. In the present study, the MAC in each group was evaluated as previously described [30, 31]. In the assessment, mice were stimulated using forceps in the forelimb, hind limb, and tail under several isoflurane concentrations. Motor activity was considered a positive response. After induction, mice were initially maintained with 1.4% isoflurane. If an animal responded to the stimuli, the concentration of isoflurane was increased in steps of 0.2% until no response was observed. If an animal did not respond to the first stimuli, the isoflurane concentration was reduced in steps of 0.2%. In each test, an equilibration period of 10 min was applied. After the assessment, MAC was calculated in each animal using following formula: (the highest concentration that induces a response + the lowest concentration with no response)/2. All MAC assessments were performed by a single operator. Based on the MAC values measured in the individual mice, the mean MAC value was calculated for each group.
Assessment of induction time and adverse reactions
Induction time in each group was evaluated. Induction was achieved when the attitudinal reflex was lost, and no motor response was observed after transferring to anesthesia maintenance. The incidence of adverse reactions during induction in each group was likewise recorded. The adverse reactions investigated in the present study included head shaking, urination, defecation, and apnea, as previously described in mice [5].
Monitoring of vital signs
According to MAC determined in advance, we adjusted the isoflurane concentration to MAC × 1.5 for the monitoring of vital signs. This anesthetic concentration is assumed for the surgical anesthesia, according to previous report [5, 10, 15] Core body temperature, heart rate, respiratory rate, and oxygen saturation (SPO2) were evaluated in each animal. Core body temperature (°C) was measured using a commercial rectal temperature sensor (Right Temp, Kent Scientific Corporation), by inserting the sensor into rectum. Heart rate (beats/min) and SPO2 (%) were assessed using a rodent pulse oximeter and heart rate monitor (MouseSTAT Kent Scientific Corporation). Respiratory rate (breaths/min) was assessed by counting the number of thoracic movements per min by locating in mice holder. After starting anesthesia, the mice were positioned on a warm pad (RightTemp, Kent Scientific Corporation) to maintain a consistent surface temperature underneath them.
Statistical analysis
Results were expressed as mean ± SD. Differences in MAC and induction time between the groups were assessed using a Student’s t-test. Repeated measures analysis of variance (ANOVA) was used to analyze differences in vital sign measurements. When a significant difference was detected, a Dunnett’s multiple comparison t-test was performed to assess the differences between baseline and subsequent time points. The differences between groups at each time point were assessed using the Bonferroni’s test. Values of P<0.05 were considered statistically significant. All statistical analyses were performed using a commercial software program (Stat Mate IV; ATMS 160 Co., Ltd. Tokyo).
Results
MAC
The comparison of MAC in both groups was performed first. Mean ± SD of MAC following use of the two anesthetic protocols is shown in Table 1. There was a significant difference in MAC between the two groups (isoflurane, 1.38 ± 0.15; MBI, 0.78 ± 0.10). MBI anesthesia resulted in a 44% reduction in MAC compared to isoflurane alone.
Table 1. Results of MAC and induction time in both anesthethic groups.
| Isoflurane | MBI | P value | |
|---|---|---|---|
| MAC (%) | 1.38 ± 0.15 | 0.78 ± 0.10 | 0.00001 |
| Induction time (sec) | 282 ± 63 | 172 ± 33 | 0.001 |
Induction time and adverse reactions
The induction time was significantly reduced in the MBI anesthetic group, compared with the group that received only isoflurane (Table 1). The adverse clinical reactions observed during induction in both groups are shown Table 2. The percentage of mice that exhibited at least one adverse reaction in the isoflurane monoanesthetic and the MBI anesthetic groups were 100% and 25%, respectively. Specifically, the observance of head shaking and urination were reduced in the MBI anesthetic group. Apnea was not observed during anesthesia in either group. No fatal events were detected in the mice during the 1-week period after the anesthetic experiment.
Table 2. Incidence (%) of adverse reactions during induction.
| Isoflurane | MBI | |
|---|---|---|
| Head shaking | 50 | 25 |
| Urination | 37.5 | 0 |
| Defecation | 87.5 | 12.5 |
| Apnea | 0 | 0 |
Vital signs
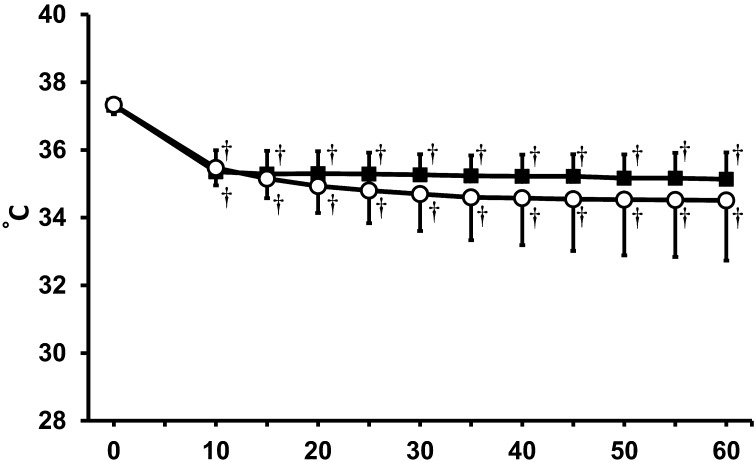
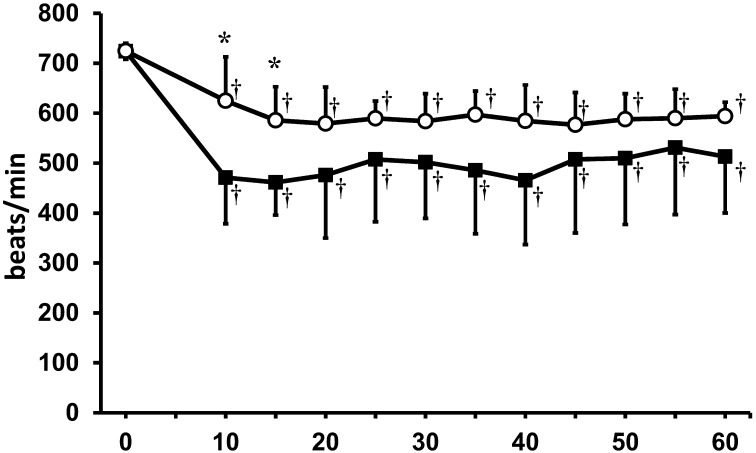
According to the MAC determined in the present study, the concentration of isoflurane in each group was set to 2.1% in the isoflurane monoanesthetic group and to 1.2% in the MBI anesthetic group. Baseline differences between the groups for each vital sign were not statistically significant. The time course of rectal temperatures is shown in Fig. 1. Both groups exhibited a significant decrease in rectal temperature during the anesthetic period, but no significant differences were observed between the groups. A decrease in heart rate was also observed in both groups (Fig. 2). In the MBI anesthetic group, there were significant decreases in heart rate at 10–15 min compared to heart rates observed during the same period in the isoflurane monoanesthetic group.
Fig. 1.
Rectal temperature (°C) in both anesthetic groups. ○: isoflurane anesthetic groups. ●: MBI anesthetic group. †Significant difference (P<0.05) from baseline. There was no significant difference between the groups. Data are presented as mean ± SD of 9 mice.
Fig. 2.
Heart rate (beats / min) in both anesthetic groups. ○: isoflurane anesthetic group. ●: MBI anesthetic group. *Significant difference between groups (P<0.05). †Significant difference (P<0.05) from baseline. Data are presented as mean ± SD of 9 mice.
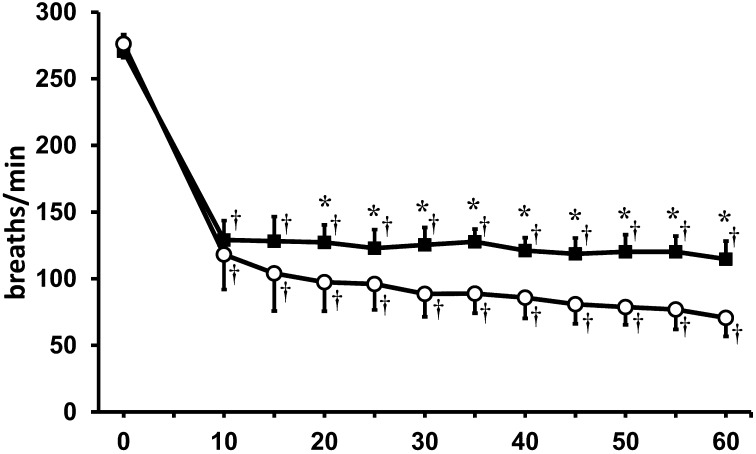
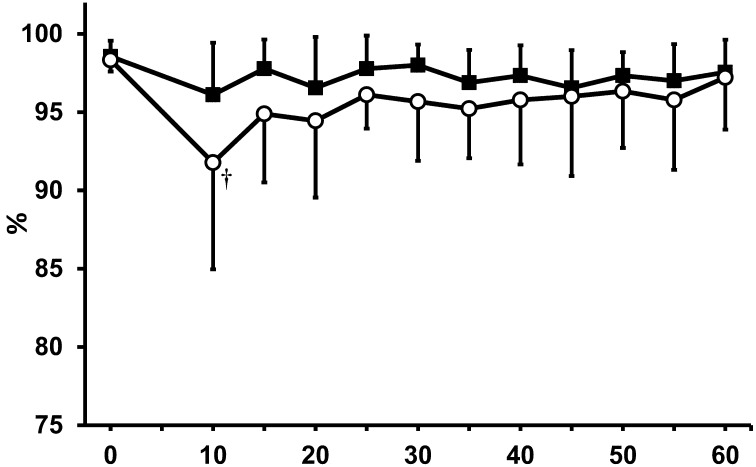
To assess respiratory function, respiratory rate and SPO2 were compared in both anesthetic groups. Although a significant decrease in respiratory rate was observed in both groups, mice in the MBI anesthetic group exhibited higher respiratory rates during the entire anesthetic period when compared with mice in the isoflurane monoanesthesia group (Fig. 3). The time course of SPO2 is shown in Fig. 4. In the isoflurane monoanesthetic group, a significant decrease in SPO2 was observed 10 min after initial induction (0 min, 98.3 ± 1.2%; 10 min, 91.8 ± 6.4%). However, no significant decreases in SPO2 were observed in the MBI anesthetic group.
Fig. 3.
Respiratory rate (breaths/min) in both anesthetic groups. ○: isoflurane anesthetic group. ●: MBI anesthetic group. *Significant difference between groups (P<0.05). †Significant difference (P<0.05) from baseline. Data are presented as mean ± SD of 9 mice.
Fig. 4.
SPO2 (%) in both anesthetic groups. ○: isoflurane anesthetic group. ●: MBI anesthetic group. †Significant difference (P<0.05) from baseline. Data are presented as mean ± SD of 9 mice.
Discussion
The present study demonstrated the efficacy of MB premedication when used in conjunction with inhalant isoflurane anesthesia in mice. Midazolam is classified as a benzodiazepine, which is commonly used as sedative in laboratory animals [12]. Midazolam is suitable for use as a pre-anesthetic due to its rapid onset of action [7, 12, 33]. Butorphanol is synthetic opioid analgesic that is routinely used as an analgesic agent in laboratory animals [12]. In humans, butorphanol is used as a pre-anesthetic for the control of perioperative pain [26]. In veterinary clinics, the combination of midazolam and butorphanol provides light to moderate sedation with minimal cardiovascular changes, and is often used clinically as a pre-anesthetic to isoflurane [14, 23, 27]. The combination of midazolam and butorphanol has also been used as a pre-anesthetic in human medicine during inhalant anesthesia [28]. In mice, combination of midazolam and butorphanol, along with medetomidine was used as injectable anesthesia in mice [16, 17]. Furthermore, recent rat study demonstrated that the combination of medetomidine, midazolam, and butorphanol can be used as anesthesia induction during tracheal intubation prior to inhalant anesthesia [18]. Considering these reports, we selected a combination of midazolam and butorphanol as the pre-anesthetic of isoflurane.
The MAC of each anesthetic protocol was assessed prior to the conduct of the full study. As a result, the mean MAC under isoflurane monoanesthesia was 1.38%, which was similar to previously reported MAC measurements in mice [19, 30]. In contrast, the MAC in MBI anesthesia was reduced to 0.78%. Therefore, the combination and dose of premedication agents described in the present study were effective for the reduction of isoflurane concentration required in mice. In the present study, the isoflurane concentration was set at 1.5 × MAC during vital sign monitoring. Previous findings have demonstrated that most surgical anesthesia planes were achieved when the gas concentration was set at MAC × 1.5 for maintenance [5, 10, 15]. Although anesthetic concentration requirements vary depending on gas-flow rate, anesthetic equipment, animal condition, or surgical invasiveness, it was suggested that maintaining an isoflurane concentration under MB premedication is approximately 1.2% for surgical anesthesia.
In the present study, isoflurane monoanesthesia mitigated adverse clinical reactions such as head shaking and urination in mice. The pathogenesis of the incontinence such as urination can be associated with muscle relaxant effect of isoflurane. Similar adverse reactions at induction have previously been reported with other volatile agents, sevoflurane in mice [5]. Additionally, previous canine studies have demonstrated that rapid induction of isoflurane anesthesia with a mask may cause adverse reactions such as breath holding, vocalization, ataxia, and excitement during induction [21, 22]. In contrast to isoflurane monoanesthesia, MBI anesthesia reduced the incidence of adverse events, and shortened the induction time, which might be associated with the sedative properties of midazolam during induction. Collectively, premedication with MB before isoflurane anesthesia contributed to the smooth induction of anesthesia in mice.
Following the dosage determination by the evaluation of MAC, the two anesthetic protocols were compared in terms of vital sings. As a result, significant decreases in rectal temperature, heart rate, and respiratory rate were observed in both anesthetic protocols. The decreases in rectal temperature and heart rate observed with both protocols were within allowable ranges during anesthesia [5]. Compared with isoflurane alone, MBI combinational anesthesia resulted in lower heart rates in the anesthetic period. A previous report described the association of isoflurane concentration and hemodynamic status [8]. In the report, mice anesthetized with 1% isoflurane exhibited lower heart rates and higher blood pressure compared to mice anesthetized with 2% isoflurane. In a human study, an increase in heart rate was observed following the delivery of a high concentration of inhalant anesthesia, while blood pressure decreased [11]. According to these findings, the relative decrease in heart rate observed under the MBI anesthesia may be associated with reduction of isoflurane concentration.
Respiratory depression is major adverse reaction of isoflurane [13]. In the present study, anesthesia with isoflurane alone caused remarkable decreases in respiratory rates as well as SPO2 instability. In contrast, relatively higher respiratory rates and stable SPO2 values were maintained during the entire anesthetic period when the MBI anesthetic protocol was used. One possible explanation for the current results was that the concentration of gas required for maintenance was markedly reduced by MB premedication. The percentage of decrease in MAC achieved by MB premedication in the present study was 44%. Another explanation may be the shortening of the induction period that expose to a high concentration of isoflurane. In dog study, it is indicated that isoflurane with MB premedication has less influence on respiratory functions [23]. Pre-medication with MB can be effective for the attenuation of respiratory depression induced by isoflurane in mice.
Appropriate anesthesia selection is essential for animal experimentation requiring surgical intervention. When using isoflurane anesthesia in respiratory experiments, consideration of respiratory depression might be a primary concern for the safe accomplishment of anesthesia. To attenuate the respiratory depression induced by isoflurane, O2 ventilation with artificial breathing, which requires tracheal intubation, can be effective. MBI combinational anesthesia established in the present study provides an alternative method for the attenuation of respiratory depression without intubation in mice. Further, because the concentration of isoflurane is decreased, the current protocol might be preferable when longer anesthetic periods are required. In addition to serving as perioperative analgesia, MB premedication may also augment post-surgical analgesia in mice since the analgesic actions of butorphanol last for a few hours [12]. Further studies are warranted to confirm the anesthetic depth and duration of action under various surgical conditions.
In summary, we have established an isoflurane-based balanced anesthesia method in mice. Further, the combination of MBI was effective for the attenuation of respiratory depression induced by isoflurane in mice, with smooth induction and low incidence of adverse reactions. The reported combination serves as a novel option for murine anesthesia, and could contribute to the accomplishment of appropriate animal experimentation.
References
- 1.Alves H.C., Valentim A.M., Olsson I.A., Antunes L.M.2009. Intraperitoneal anaesthesia with propofol, medetomidine and fentanyl in mice. Lab. Anim. 43: 27–33. doi: 10.1258/la.2008.007036 [DOI] [PubMed] [Google Scholar]
- 2.Arras M., Autenried P., Rettich A., Spaeni D., Rülicke T.2001. Optimization of intraperitoneal injection anesthesia in mice: drugs, dosages, adverse effects, and anesthesia depth. Comp. Med. 51: 443–456. [PubMed] [Google Scholar]
- 3.Buitrago S., Martin T.E., Tetens-Woodring J., Belicha-Villanueva A., Wilding G.E.2008. Safety and efficacy of various combinations of injectable anesthetics in BALB/c mice. J. Am. Assoc. Lab. Anim. Sci. 47: 11–17. [PMC free article] [PubMed] [Google Scholar]
- 4.Cesarovic N., Nicholls F., Rettich A., Kronen P., Hässig M., Jirkof P., Arras M.2010. Isoflurane and sevoflurane provide equally effective anaesthesia in laboratory mice. Lab. Anim. 44: 329–336. doi: 10.1258/la.2010.009085 [DOI] [PubMed] [Google Scholar]
- 5.Cesarovic N., Jirkof P., Rettich A., Nicholls F., Arras M.2012. Combining sevoflurane anesthesia with fentanyl-midazolam or s-ketamine in laboratory mice. J. Am. Assoc. Lab. Anim. Sci. 51: 209–218. [PMC free article] [PubMed] [Google Scholar]
- 6.Chaves A.A., Dech S.J., Nakayama T., Hamlin R.L., Bauer J.A., Carnes C.A.2003. Age and anesthetic effects on murine electrocardiography. Life Sci. 72: 2401–2412. doi: 10.1016/S0024-3205(03)00137-1 [DOI] [PubMed] [Google Scholar]
- 7.Chiba S., Nishiyama T., Yoshikawa M., Yamada Y.2009. The antinociceptive effects of midazolam on three different types of nociception in mice. J. Pharmacol. Sci. 109: 71–77. doi: 10.1254/jphs.08094FP [DOI] [PubMed] [Google Scholar]
- 8.Constantinides C., Mean R., Janssen B.J.2011. Effects of isoflurane anesthesia on the cardiovascular function of the C57BL/6 mouse. ILAR J. 52: e21–e31. [PMC free article] [PubMed] [Google Scholar]
- 9.Diven K.2003. Inhalation anesthetics in rodents. Lab. Anim. (NY) 32: 44–47. doi: 10.1038/laban0303-44 [DOI] [PubMed] [Google Scholar]
- 10.Doherty T.J., Geiser D.R., Frazier D.L.1997. Comparison of halothane minimum alveolar concentration and minimum effective concentration in ponies. J. Vet. Pharmacol. Ther. 20: 408–410. doi: 10.1046/j.1365-2885.1997.00086.x [DOI] [PubMed] [Google Scholar]
- 11.Ebert T.J., Harkin C.P., Muzi M.1995. Cardiovascular responses to sevoflurane: a review. Anesth. Analg. 81:(Suppl): S11–S22. doi: 10.1097/00000539-199512001-00003 [DOI] [PubMed] [Google Scholar]
- 12.Flecknell P.A.2010. General anesthesia. Laboratory Animal Anaesthesia. 3rd ed., Academic Press, London. [Google Scholar]
- 13.Gargiulo S., Greco A., Gramanzini M., Esposito S., Affuso A., Brunetti A., Vesce G.2012. Mice anesthesia, analgesia, and care, Part I: anesthetic considerations in preclinical research. ILAR J. 53: E55–E69. doi: 10.1093/ilar.53.1.55 [DOI] [PubMed] [Google Scholar]
- 14.Gross M.E., Smith J.A., Tranquilli W.J.1993. Cardiorespiratory effects of combined midazolam and butorphanol in isoflurane-anesthetized cats. Vet. Surg. 22: 159–162. doi: 10.1111/j.1532-950X.1993.tb01692.x [DOI] [PubMed] [Google Scholar]
- 15.de Jong R.H., Eger E.I., 2nd. 1975. MAC expanded: AD50 and AD95 values of common inhalation anesthetics in man. Anesthesiology 42: 384–389. doi: 10.1097/00000542-197504000-00003 [DOI] [PubMed] [Google Scholar]
- 16.Kawai S., Takagi Y., Kaneko S., Kurosawa T.2011. Effect of three types of mixed anesthetic agents alternate to ketamine in mice. Exp. Anim. 60: 481–487. doi: 10.1538/expanim.60.481 [DOI] [PubMed] [Google Scholar]
- 17.Kirihara Y., Takechi M., Kurosaki K., Kobayashi Y., Kurosawa T.2013. Anesthetic effects of a mixture of medetomidine, midazolam and butorphanol in two strains of mice. Exp. Anim. 62: 173–180. doi: 10.1538/expanim.62.173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Konno K., Shiotani Y., Itano N., Ogawa T., Hatakeyama M., Shioya K., Kasai N.2014. Visible, safe and certain endotracheal intubation using endoscope system and inhalation anesthesia for rats. J. Vet. Med. Sci. 76: 1375–1381. doi: 10.1292/jvms.14-0146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mogil J.S., Smith S.B., O’Reilly M.K., Plourde G.2005. Influence of nociception and stress-induced antinociception on genetic variation in isoflurane anesthetic potency among mouse strains. Anesthesiology 103: 751–758. doi: 10.1097/00000542-200510000-00013 [DOI] [PubMed] [Google Scholar]
- 20.Muir W.W., 3rd, Wiese A.J., March P.A.2003. Effects of morphine, lidocaine, ketamine, and morphine-lidocaine-ketamine drug combination on minimum alveolar concentration in dogs anesthetized with isoflurane. Am. J. Vet. Res. 64: 1155–1160. doi: 10.2460/ajvr.2003.64.1155 [DOI] [PubMed] [Google Scholar]
- 21.Mutoh T., Nishimura R., Kim H., Matsunaga S., Kadosawa T., Mochizuki M., Sasaki N.1995. Rapid inhalation induction of anesthesia by halothane, enflurane, isoflurane and sevoflurane and their cardiopulmonary effects in dogs. J. Vet. Med. Sci. 57: 1007–1013. doi: 10.1292/jvms.57.1007 [DOI] [PubMed] [Google Scholar]
- 22.Mutoh T., Nishimura R., Kim H.Y., Matsunaga S., Kadosawa T., Mochizuki M., Sasaki N.1995. Clinical application of rapid inhalation induction of anesthesia using isoflurane and sevoflurane with nitrous oxide in dogs. J. Vet. Med. Sci. 57: 1121–1124. doi: 10.1292/jvms.57.1121 [DOI] [PubMed] [Google Scholar]
- 23.Mutoh T., Kojima K., Takao K., Nishimura R., Sasaki N.2001. Comparison of sevoflurane with isoflurane for rapid mask induction in midazolam and butorphanol-sedated dogs. J. Vet. Med. A Physiol. Pathol. Clin. Med. 48: 223–230. doi: 10.1046/j.1439-0442.2001.00350.x [DOI] [PubMed] [Google Scholar]
- 24.Psatha E., Alibhai H.I., Jimenez-Lozano A., Armitage-Chan E., Brodbelt D.C.2011. Clinical efficacy and cardiorespiratory effects of alfaxalone, or diazepam/fentanyl for induction of anaesthesia in dogs that are a poor anaesthetic risk. Vet. Anaesth. Analg. 38: 24–36. doi: 10.1111/j.1467-2995.2010.00577.x [DOI] [PubMed] [Google Scholar]
- 25.Richardson C.A., Flecknell P.A.2005. Anaesthesia and post-operative analgesia following experimental surgery in laboratory rodents: are we making progress? Altern. Lab. Anim. 33: 119–127. [DOI] [PubMed] [Google Scholar]
- 26.Rosow C.E.1988. Butorphanol in perspective. Acute Care 12:(Suppl 1): 2–7. [PubMed] [Google Scholar]
- 27.Sano T., Nishimura R., Mochizuki M., Sasaki N.2003. Effects of midazolam-butorphanol, acepromazine-butorphanol and medetomidine on an induction dose of propofol and their compatibility in dogs. J. Vet. Med. Sci. 65: 1141–1143. doi: 10.1292/jvms.65.1141 [DOI] [PubMed] [Google Scholar]
- 28.Sinha C., Kaur M., Kumar A., Kulkarni A., Ambareesha M., Upadya M.2012. Comparative evaluation of midazolam and butorphanol as oral premedication in pediatric patients. J. Anaesthesiol. Clin. Pharmacol. 28: 32–35. doi: 10.4103/0970-9185.92431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith W.1993. Responses of laboratory animals to some injectable anaesthetics. Lab. Anim. 27: 30–39. doi: 10.1258/002367793781082377 [DOI] [PubMed] [Google Scholar]
- 30.Sonner J.M., Gong D., Li J., Eger E.I., 2nd, Laster M.J.1999. Mouse strain modestly influences minimum alveolar anesthetic concentration and convulsivity of inhaled compounds. Anesth. Analg. 89: 1030–1034. [DOI] [PubMed] [Google Scholar]
- 31.Tao F., Skinner J., Yang Y., Johns R.A.2010. Effect of PSD-95/SAP90 and/or PSD-93/chapsyn-110 deficiency on the minimum alveolar anesthetic concentration of halothane in mice. Anesthesiology 112: 1444–1451. doi: 10.1097/ALN.0b013e3181dcd3dc [DOI] [PubMed] [Google Scholar]
- 32.Tonner P.H.2005. Balanced anaesthesia today. Best Pract. Res. Clin. Anaesthesiol. 19: 475–484. doi: 10.1016/j.bpa.2005.02.005 [DOI] [PubMed] [Google Scholar]
- 33.Tranquilli W.J., Thurmon J.C., Grimm K.A., Lumb W.V. Lumb & Jones’ veterinary anesthesia and analgesia. 4th ed. Ames, Iowa: Blackwell Pub.; 2007. [Google Scholar]
- 34.Tsukamoto A., Serizawa K., Sato R., Yamazaki J., Inomata T.2015. Vital signs monitoring during injectable and inhalant anesthesia in mice. Exp. Anim. 64: 57–64. doi: 10.1538/expanim.14-0050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wiersema A.M., Dirksen R., Oyen W.J., Van der Vliet J.A.1997. A method for long duration anaesthesia for a new hindlimb ischaemia-reperfusion model in mice. Lab. Anim. 31: 151–156. doi: 10.1258/002367797780600125 [DOI] [PubMed] [Google Scholar]
- 36.Yu D., Liu B.2013. Developmental anesthetic neurotoxicity: from animals to humans? J. Anesth. 27: 750–756. doi: 10.1007/s00540-013-1609-5 [DOI] [PubMed] [Google Scholar]