Abstract
In preclinical trials, a sensitive functional test is required to detect changes in the motor behaviour of the SOD1G93A mouse model of amyotrophic lateral sclerosis (ALS). We evaluated changes in body weight and motor impairment in behavioural tests, such as the rotarod, the hanging-wire test and the treadmill, of transgenic and wild type mice. We found differences in detection of the onset of symptoms and progression of the disease between the different tests assessed. Moreover, the data showed significant gender differences in the motor behaviour of this mouse model. The rotarod and the hanging-wire test were more sensitive to detect early motor impairment. Moreover, the results suggested that the rotarod and hanging-wire became the most accurate tests rather than treadmill to characterise the ALS disease phenotype.
Keywords: Amyotrophic lateral sclerosis, hanging-wire test, rotarod, SOD1G93A mice, treadmill
Introduction
Amyotrophic lateral sclerosis (ALS) is a lethal neurodegenerative disorder that causes progressive paralysis and death within 3–5 years of diagnosis. During the last 20 years, the mouse model overexpressing SOD1 has been one of the most important research tools for scientists working in the field of ALS [18]. One of the best characterised SOD1 transgenic mouse strains carries the human SOD1G93A mutation. In this model, the SOD1 gene contains a glycine to alanine substitution at position 93, which results in a toxic gain of SOD1 function [10, 24]. The high copy mutant SOD1G93A mice are phenotypically healthy until approximately 80–90 days old, when they develop symptoms of motor neuron disease, such as shaking limbs when suspended [4]. After 120 days, most of these mice are in the end stages of disease and become completely paralysed.
The SOD1G93A mice develop a progressive paralysis starting in the hind limbs via denervation of the neuromuscular junction. This denervation prompts an active degeneration of ventral root axons and a significant loss of alpha motor neurons. The moment at which early motor deficits appear needs to be accurately defined, especially when therapeutic strategies are tested. A thorough understanding of the motor behaviour of the mouse model as well as the motor function tests available is vital for the correct development of a behavioural analysis. Therefore, several methods should be considered in order to select the approach that most accurately addresses the experimental questions.
Semi-quantitative or descriptive methods (e.g., observation or stride length analysis) were initially used by researchers for assessing motor function in SOD1G93A mice [10]. Various tests, such as rotarod, hanging-wire, foot print or treadmill tests [2, 5, 15] have been performed to detect the onset and progression of neurodegenerative diseases when an experimental treatment is being studied. The treadmill was first used as a training system to study and test the motor phenotype [12, 20]. Currently, complex and expensive systems based on computerised treadmill data are used to analyse walking pattern and to calculate standardised gait parameters [11, 16, 23]. This system has been less frequently emploied, and consequently, less information about it has been reported. Thus, the purpose of this work was to compare the conventional behavioural tests, such as rotarod or hanging-wire tests, to the treadmill. The experimental variables included changes in body weight, survival rate, and motor function assessed with the rotarod, hanging-wire and treadmill tests.
Materials and Methods
Animals
Animals were purchased from The Jackson Laboratory (B6SJL-Tg (SOD1-G93A) 1Gur/J) and were housed under a standard light:dark (12:12 h) cycle. Animals were provided with food and water ad libitum in the Servicio General de Apoyo a la Investigación-SAI of the Universidad de Zaragoza. Hemizygotic transgenic mice were obtained by mating transgenic males with F1 hybrid females (B6SJL). Transgenic mice were identified by PCR assay of DNA extracted from the tail with specific primers for human SOD1 (according to The Jackson Laboratory protocol). Wild type (WT) littermates were used as controls of the transgenic mice for behavioural tests. All of the procedures were approved by the in-house Ethics Committee for Animal Experiments of the Universidad de Zaragoza. Animal care and experimentation were performed according to the Spanish Policy for Animal Protection RD53/2013, which meets the European Union Directive 2010/63 on the protection of animals used for experimental and other scientific purposes.
During late-stage disease, access to food and water were provided. Euthanasia was performed when mice failed to right themselves within 30 s of being placed on their side (disease endpoint) [4].
Body weight and behaviour tests were performed weekly during the morning, beginning at postnatal (P) day 60 until the death of the animals.
Rotarod test
Motor coordination, strength and balance were assessed using a rotarod (ROTA-ROD/RS, LE8200, LSILETICA Scientific Instruments; Panlab, Barcelona, Spain). Mice started to train on the rotarod daily one week before recording the data. Animals were placed onto the cylinder at a constant speed of 14 rpm. The animals had three attempts to remain on the rotarod for a maximum of 180 s per trial, and the longest latency was recorded.
Hanging-wire test
Neuromuscular strength was tested by the hanging-wire test. Each mouse was placed on a wire lid of a conventional housing cage and the lid was turned upside down. The latency from the beginning of the test until the mouse stood with at least two limbs on the lid was timed. The animals had three attempts to stand for a maximum of 180 s per trial, and the longest latency was recorded.
Treadmill
Animals were subjected to involuntary exercise on a rodent treadmill (1055SDRM Columbus Instruments, Columbus, OH, USA). Motor activity was registered 3 days per week beginning at 45 days of age. To encourage the mice to run, the treadmill was equipped with an electrical shock grid at the rear of the treadmill. The shock grid was set to deliver 0.3 mA, which caused an uncomfortable shock but did not physically harm or injure the animals. Three speeds were analysed (11, 22 and 44 cm/s) during 10 mins at 5° incline. Performance was measured based on the time each mouse stayed on the shock grid. The more disease progression an animal had experienced, the more time it spent on the shock grid.
Statistical analysis
Statistical analysis for all behavioral data consisted of analyses of variance (ANOVA) to compare genotypes (SOD1G93A and WT) and gender (males and females). Significant differences were further evaluated using Bonferroni’s method for post hoc comparisons. Differences in body weight were examined using Student’s t-test. Survival and disease onset were plotted on Kaplan-Meier curves and analysed using the log-rank test. Statistical analyses were performed in SPSS version 19.0 (IBM). Data were presented as mean ± the standard error of the mean (SEM). Significance was set at a p-value of less than 0.05.
Results
Body weight and survival
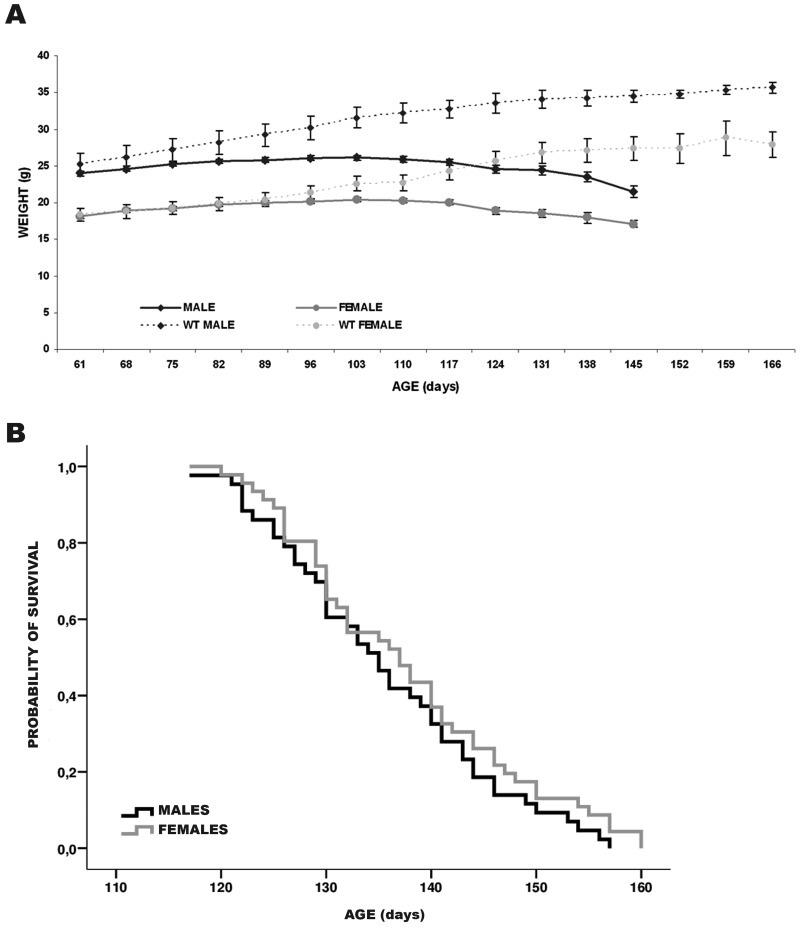
As shown in the Fig. 1A, wild type animals, or control group, increased their weight continuously and they never showed motor symptoms. From P61 to P142 the transgenic males always weighted more than females (P<0.01). Maximum weight was reached at P103 in males and females, from this time the weight started to decrease gradually. At the end of the experiment, transgenic animals lost up to 27% (males) and 16% (females) of his maximum weight. From P117 the weight loss was more important and fast in transgenic mice. Furthermore, comparing wild type mice to their transgenic mate of different sex separately, the weight loss began to be significant around P96 (P<0.05) in males while in females, this loss was found at P117 (P<0.05).
Fig. 1.
Body weight and survival time. (A) Changes over time in weight of male (black) and female (grey) wild type and transgenic mice (WT males n=10, WT females n=10, transgenic females n=43 and transgenic males n=43). Data are shown as mean ± SEM. (B) Kaplan-Meier survival comparison of the male and female transgenic mice. No significant differences were detected between transgenic male and female mice.
When survival time was analyzed, no statistical significant difference was observed between males and females (Fig. 1B). Transgenic males showed a mean life span of 135.6 ± 1.6 days, while the mean in females was observed at 137.7 ± 1.6 days.
Rotarod test
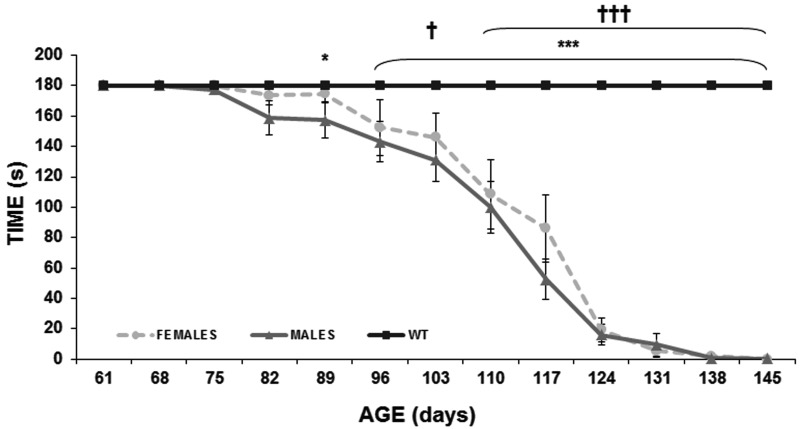
Significant differences between control and transgenic mice was observed at P89 (P<0.05) and P96 (P<0.001) until the endpoint. In particular, some transgenic males showed early symptoms around P75, although from P82 these symptoms became more evident (Fig. 2). In contrast females showed early symptoms at P82 and started to decline around P96, although the performance was worse from P103, which was coincident with the maximum weight observed (P<0.05, P<0.001). During disease progression significant differences were not detected between transgenic males and females, and in both sexes, a similar progression was observed, showing transgenic male mice more motor deficits than transgenic female mice.
Fig. 2.
Rotarod test. Changes over time in rotarod test performance of male (black) and female (grey) wild type and transgenic mice (WT males n=10, WT females n=10, transgenic females n=15 and transgenic males n=15). Data are shown as mean ± SEM. *P<0.05 and ***P<0.001 (male mice), †P<0.05 and †††P<0.001 (female mice).
Hanging-wire test
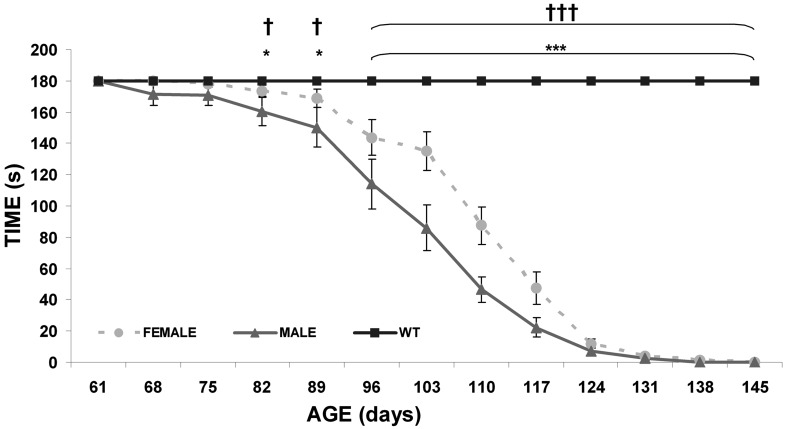
Motor symptoms appeared earlier in transgenic males (P68) than transgenic females (P82), although in both sexes a similar test evolution was observed (Fig. 3A). The decline began to be significant at P82 (P<0.05) and continued until the end (P<0.001) when control and transgenic mice, males and females, were compared. Significant differences between transgenic male and female mice were detected at P103, which was coincident with the maximum weight observed, P110 and P117 days of life, in which the most important and fast loss of weight was registered (P<0.01).
Fig. 3.
Hanging-wire test. Changes over time in hanging-wire test of male (black) and female (grey) wild type and transgenic mice (WT males n=10, WT females n=10, transgenic females n=15 and transgenic males n=15). Data are shown as mean ± SEM. *P<0.05 and ***P<0.001 (male mice), †P<0.05 and †††P<0.001 (female mice).
Treadmill
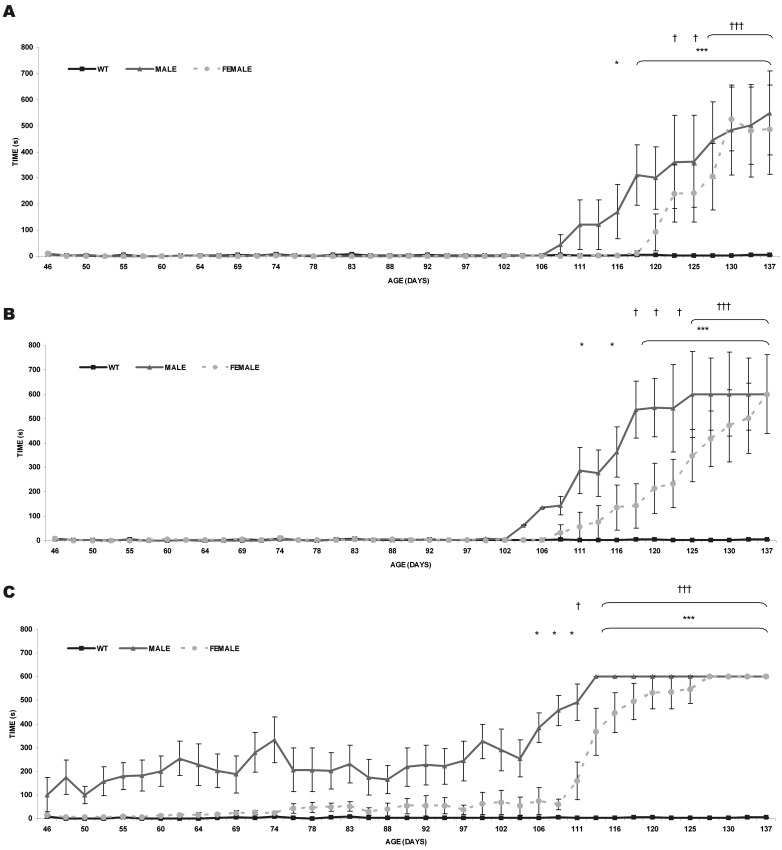
When the speed was set at 11 cm/s on the treadmill, the first motor deficits were detected at P109 in transgenic males and P120 in transgenic females (Fig. 4A). However, significant motor function began to get worse at P116 (P<0.05) in males and P123 in females (P<0.05), becoming gradually aggravated (P<0.001). At 22 cm/s alterations in motor function were detected earlier at P104 in males and at P108 in females (Fig. 4B). Significant differences were detected at P111 in males and at P118 in females and form these points significance increased (P<0.001). Finally, at 44 cm/s some animals had problems to reach the performance from the beginning, showing males more motor difficulties than females (Fig. 4C). Significant differences at P106 in males and P111 in females, comparing to wild-type, were visible (P<0.05) and motor dysfunction became more evident at P113 (P<0.001). At P74, transgenic mice (males and females) showed the first alteration in their motor behaviour.
Fig. 4.
Treadmill. The shock time (s) was registered at 3 speeds (11, 22 and 44 cm/s) from P45 to P137 (WT males n=10, WT females n=10, transgenic females n=10 and transgenic males n=10). Data are shown as mean ± SEM. (A) Results at 11 cm/s. (B) Results at 22 cm/s. (C) Results at 44 cm/s. *P<0.05 and ***P<0.001 (male mice), †P<0.05 and †††P<0.001 (female mice).
Discussion
The most objective and simple parameter to first characterize transgenic SOD1G93A mice is the body weight, which is also virtually observer independent. ALS, in fact, is accompanied by a significant weight loss in both human and mice.
Studies of SOD1G93A mice confirmed that changes in body weight occurred as a sign of motor neuron death over the course of the disease. This is a rather unspecific characteristic and it can be due to many conditions such as dehydratation or malnutrition, developmental abnormalities [6], background strain [23] or orolingual motor deficits [19]. However, the age at which these changes occur differs substantially among published studies. Our results are coincident with the ones described by Azari [3], Hayworth [12] or Smittkamp [19] who reported stable body weight in transgenic mice from P55 to P125 to finally decrease during end-stage disease. Furthermore, reported differences in body weight between transgenic mice and control mice emerging from P98 to P126 have been also described [1, 20, 22]. Therefore these results suggested that weight loss was not an accurate measurement of disease onset or progression in this animal model of ALS.
Differences between transgenic male and female mice have been described in several studies, not only about weight or survival, but also about onset and progression disease [7, 21]. In case of survival rate, genetic background studies in this model described longer survival in transgenic females than in transgenic males [1, 7, 13], albeit females survived two more days than males in our study, no significant gender differences were detected [12, 14]. Moreover we found that transgenic male mice exhibited more severe symptoms including earlier onset and motor deficits than transgenic female mice in each behaviour test assessed. In rotarod, hanging-wire test and treadmill, the male mice showed deficits before females, therefore the differences in disease progression between males and females are obvious. But only in the case of hanging-wire test significant differences appeared between transgenic male and female mice, so our results are consistent with previous reports that transgenic females displayed motor symptom later than transgenic male mice with no evidence of higher survival rates. These discrepancies may be related to the copy number of the transgene, the mouse background, the experimental protocol (food supplies in final stage) or estrogens effects [7]. On view of these results, we recommend that gender should be taken into account in experimental design since the behavioural differences seem to contribute to variability.
The most frequently used analysis of motor impairment in mice is the rotarod test which let us register how long the animals were able to run on a rotating cylinder [22]. The rotarod test is a complex task that requires good motor coordination and balance in addition to strength; a decline in motor strength is specific for ALS disease. Hence a decline in rotarod performance is a rather indirect measurement of the motor deficit. However, the lack of sensitivity of this test to detect motor deficits prior to the onset of clinical symptoms has been widely discussed and other tests have been also used to expand the time frame to detect early changes [3, 14]. Although the first motor dysfunction evidence appeared in the pre-symptomatic phase (P75 in males and P82 in females), transgenic male mice showed this motor dysfunction 2 weeks later while transgenic female mice displayed it 3 weeks later since the deficits began to be significant. The performance progression observed for this test was similar to that described by Alves [1] or Smittkamp [19]. The wide variety of results could be explained by differences in training days, protocols performance or genetic background of mice model. Overall, previous experiences in our group have demonstrated that animals must be training before, and the training should realize at least one week before starting the experiments in order to ensure proper test development if early symptoms want to be detected [17].
The hanging-wire test requires only balance and grip strength [6] and there is no need for any significant training of the mice. The simplicity (a conventional cage lid), reliability and improved diagnostic accuracy of this test are a great advantage. Our results are in agreement with Hayworth [12], and Smittkampp [19] which reported the first muscle limb weakness changes at P68 in transgenic females and P82 in transgenic males. Moreover we found that disease progression was better endured by transgenic female mice than transgenic male mice, which is in accordance to Choi [7], and this gender differences were significant during symptomatic stage (P103, P110 and P117). Therefore, we could be able to detect early motor performance deficits, suggesting this test as a good choice among the rest.
Treadmill-based systems have been suggested as a better way to monitor gait dysfunction and disease progression in models of genetic neurological disorders [11, 23] and as a training system to evaluate the benefit of physical activity in ALS [13, 21]. As compared to the methods that do not use computerized acquisition and analysis (footprints), the computerized gait analysis systems are marketed as a more efficient, sensitive and reliable way to measure motor deficits [9]. This system can incorporate a video camera for capture of paw placement, so parameters like stride, swing or stance time are able to be analyzed [16].
Among the speeds we applied in this test, we found that the slow and medium speeds (11 and 22 cm/s) were unable to detect motor deficits before P100, and as a consequence it was possible that mice could offset the loss of hind limb muscle strength, unlike the results observed in the rotarod test. When we worked at 44 cm/s the deficits were detected before (around P74), which is in agreement with Guillot [9] and Wooley [23]. At this point, the treadmill was able to detect early symptoms as well as the rotarod or the hanging-wire test. We therefore suggest, an intermediate speed between 22 and 44 cm/s, that could be enough to detect early motor symptoms and therefore it could not cause excessive attrition, especially in the case of transgenic male mice. Moreover, this system is also able to change the conditions of the test (belt inclination for example), so new protocols of resistance and strength are possible; and the training is not required. In addition, these results are in accordance with the speed range used in the same animal model [8] and highlight the importance of using a particular speed value in treadmill, especially when further molecular and cellular studies are needed. However, comparing treadmill to rotarod test we found a best sensitivity in rotarod test as we could detect the first significant motor deficit symptoms in transgenic mice at P90 in rotarod test while this detection was found since P106 in treadmill.
The results show gender differences in the motor impairment, so balanced groups must be taken into account in the experimental design in order to avoid any sex bias. The clinical phenotype in SOD1 transgenic mice is perfectly characterized by the conventional behavioural tests such as rotarod and hanging-wire, as shown by the data. Moreover, our results suggested that the use of rotarod and hanging-wire can represent the most accurate behavioural tests to monitor the disease progression in SOD1 transgenic mice. However, the sensitivity of treadmill in our experimental conditions was not comparable to the other tests, undermining its use when a precise monitoring of the disease is needed.
Conflict of Interest
The authors declare that they have no conflict of interest.
Acknowledgments
The authors would like to acknowledge the use of Servicios Científico-Técnicos del CIBA (Instituto Aragonés de Ciencias de la Salud-SAI Universidad de Zaragoza), especially to Miriam de la Torre, Silvia Garcés, Pilar Lierta, Susana Murillo and Eduardo Romanos for their expert technical assistance.
This work was supported by grants PI10/0178 from Fondo de Investigación Sanitaria of Spain, ALS Association nº 554406 and Ministerio de Ciencia e Innovación INNPACTO IPT-2011–1091-900000.
References
- 1.Alves C.J., de Santana L.P., dos Santos A.J., de Oliveira G.P., Duobles T., Scorisa J.M., Martins R.S., Maximino J.R., Chadi G.2011. Early motor and electrophysiological changes in transgenic mouse model of amyotrophic lateral sclerosis and gender differences on clinical outcome. Brain Res. 1394: 90–104. doi: 10.1016/j.brainres.2011.02.060 [DOI] [PubMed] [Google Scholar]
- 2.Aartsma-Rus A., van Putten M.2014. Assessing functional performance in the mdx mouse model. J. Vis. Exp. e51303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Azari M.F., Lopes E.C., Stubna C., Turner B.J., Zang D., Nicola N.A., Kurek J.B., Cheema S.S.2003. Behavioural and anatomical effects of systemically administered leukemia inhibitory factor in the SOD1(G93A G1H) mouse model of familial amyotrophic lateral sclerosis. Brain Res. 982: 92–97. doi: 10.1016/S0006-8993(03)02989-5 [DOI] [PubMed] [Google Scholar]
- 4.Azzouz M., Leclerc N., Gurney M., Warter J.M., Poindron P., Borg J.1997. Progressive motor neuron impairment in an animal model of familial amyotrophic lateral sclerosis. Muscle Nerve 20: 45–51. doi: [DOI] [PubMed] [Google Scholar]
- 5.Brooks S.P., Dunnett S.B.2009. Tests to assess motor phenotype in mice: a user’s guide. Nat. Rev. Neurosci. 10: 519–529Review. doi: 10.1038/nrn2652 [DOI] [PubMed] [Google Scholar]
- 6.Crawley J.N.2000. What’s wrong with my mouse? Behavioral Phenotyping of Transgenic and Knockout Mice. John Wiley & Sons, Inc., New York. [Google Scholar]
- 7.Choi C.I., Lee Y.D., Gwag B.J., Cho S.I., Kim S.S., Suh-Kim H.2008. Effects of estrogen on lifespan and motor functions in female hSOD1G93A transgenic mice. J. Neurol. Sci. 268: 40–47. doi: 10.1016/j.jns.2007.10.024 [DOI] [PubMed] [Google Scholar]
- 8.Gerber Y.N., Sabourin J.C., Hugnot J.P., Perrin F.E.2012. Unlike physical exercise, modified environment increases the lifespan of SOD1G93A mice however both conditions induce cellular changes. PLoS ONE 7: e45503. doi: 10.1371/journal.pone.0045503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guillot T.S., Asress S.A., Richardson J.R., Glass J.D., Miller G.W.2008. Treadmill gait analysis does not detect motor deficits in animal models of Parkinson’s disease or amyotrophic lateral sclerosis. J. Mot. Behav. 40: 568–577. doi: 10.3200/JMBR.40.6.568-577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gurney M.E., Pu H., Chiu A.Y., Dal Canto M.C., Polchow C.Y., Alexander D.D., Caliendo J., Hentati A., Kwon Y.W., Deng H.X. 1994. Motor neuron degeneration in mice that express a human Cu, Zn superoxide dismutase mutation. Science 264: 1772–1775. doi: 10.1126/science.8209258 [DOI] [PubMed] [Google Scholar]
- 11.Hampton T.G., Amende I.2010. Treadmill gait analysis characterizes gait alterations in Parkinson’s disease and amyotrophic lateral sclerosis mouse models. J. Mot. Behav. 42: 1–4. doi: 10.1080/00222890903272025 [DOI] [PubMed] [Google Scholar]
- 12.Hayworth C.R., Gonzalez-Lima F.2009. Pre-symptomatic detection of chronic motor deficits and genotype prediction in congenic B6.SOD1(G93A) ALS mouse model. Neuroscience 164: 975–985. doi: 10.1016/j.neuroscience.2009.08.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kirkinezos I.G., Hernandez D., Bradley W.G., Moraes C.T.2003. Regular exercise is beneficial to a mouse model of amyotrophic lateral sclerosis. Ann. Neurol. 53: 804–807. doi: 10.1002/ana.10597 [DOI] [PubMed] [Google Scholar]
- 14.Knippenberg S., Thau N., Dengler R., Petri S.2010. Significance of behavioural tests in a transgenic mouse model of amyotrophic lateral sclerosis (ALS). Behav. Brain Res. 213: 82–87. doi: 10.1016/j.bbr.2010.04.042 [DOI] [PubMed] [Google Scholar]
- 15.Ludolph A.C., Bendotti C., Blaugrund E., Chio A., Greensmith L., Loeffler J.P., Mead R., Niessen H.G., Petri S., Pradat P.F., Robberecht W., Ruegg M., Schwalenstöcker B., Stiller D., van den Berg L., Vieira F., von Horsten S.2010. Guidelines for preclinical animal research in ALS/MND: a consensus meeting. Amyotroph. Lateral Scler. 11: 38–45. doi: 10.3109/17482960903545334 [DOI] [PubMed] [Google Scholar]
- 16.Mancuso R., Oliván S., Osta R., Navarro X.2011. Evolution of gait abnormalities in SOD1(G93A) transgenic mice. Brain Res. 1406: 65–73. doi: 10.1016/j.brainres.2011.06.033 [DOI] [PubMed] [Google Scholar]
- 17.Miana-Mena F.J., Muñoz M.J., Yagüe G., Mendez M., Moreno M., Ciriza J., Zaragoza P., Osta R.2005. Optimal methods to characterize the G93A mouse model of ALS. Amyotroph. Lateral Scler. Other Motor Neuron Disord. 6: 55–62. doi: 10.1080/14660820510026162 [DOI] [PubMed] [Google Scholar]
- 18.Shibata N.2001. Transgenic mouse model for familial amyotrophic lateral sclerosis with superoxide dismutase-1 mutation. Neuropathology 21: 82–92. doi: 10.1046/j.1440-1789.2001.00361.x [DOI] [PubMed] [Google Scholar]
- 19.Smittkamp S.E., Brown J.W., Stanford J.A.2008. Time-course and characterization of orolingual motor deficits in B6SJL-Tg(SOD1-G93A)1Gur/J mice. Neuroscience 151: 613–621. doi: 10.1016/j.neuroscience.2007.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tankersley C.G., Haenggeli C., Rothstein J.D.2007. Respiratory impairment in a mouse model of amyotrophic lateral sclerosis. J. Appl. Physiol. 102: 926–932. doi: 10.1152/japplphysiol.00193.2006 [DOI] [PubMed] [Google Scholar]
- 21.Veldink J.H., Bär P.R., Joosten E.A., Otten M., Wokke J.H., van den Berg L.H.2003. Sexual differences in onset of disease and response to exercise in a transgenic model of ALS. Neuromuscul. Disord. 13: 737–743. doi: 10.1016/S0960-8966(03)00104-4 [DOI] [PubMed] [Google Scholar]
- 22.Weydt P., Hong S.Y., Kliot M., Möller T.2003. Assessing disease onset and progression in the SOD1 mouse model of ALS. Neuroreport 14: 1051–1054. [DOI] [PubMed] [Google Scholar]
- 23.Wooley C.M., Sher R.B., Kale A., Frankel W.N., Cox G.A., Seburn K.L.2005. Gait analysis detects early changes in transgenic SOD1(G93A) mice. Muscle Nerve 32: 43–50. doi: 10.1002/mus.20228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yim M.B., Kang J.H., Yim H.S., Kwak H.S., Chock P.B., Stadtman E.R.1996. A gain-of-function of an amyotrophic lateral sclerosis-associated Cu, Zn-superoxide dismutase mutant: an enhancement of free radical formation due to a decrease in Km for hydrogen peroxide. Proc. Natl. Acad. Sci. USA 93: 5709–5714. doi: 10.1073/pnas.93.12.5709 [DOI] [PMC free article] [PubMed] [Google Scholar]