Abstract
After peripheral nerve injury, Wallerian degeneration (WD) occurs in the distal nerve segment. During the process of degeneration, Schwann cells (SCs) dedifferentiate, proliferate and migrate to align in “bands of Büngner”, providing structural guidance and growth-promoting substrates to regenerating axons. The molecular signals that trigger SCs migration remain unclear. Here, we explored the molecular characteristics of the migration of cultured SCs prepared from rat sciatic nerves that had degenerated. The results revealed that elevated p-ERK1/2 was coupled with the migration of SCs, activated either by nerve degeneration or the addition of placenta growth factor. However, the inhibition of ERK1/2 activity, which activated the PI3K pathway, did not show a significant negative effect on SC migratory potential. Combined inhibition of ERK1/2 and AKT activity resulted in a significant decrease in SCs motility. These molecular characteristics suggest that both the ERK1/2 and AKT signals are involved in the migratory potential of SCs. It may be helpful to understand the process of nerve regeneration and perspective on promoting peripheral nerve regeneration.
Keywords: AKT, ERK, migration, nerve injury, Schwann cell
The incidence of injury or defect of peripheral nerves is frequent in hand surgery, and the effect of treatment is unsatisfactory. Wallerian degeneration (WD) is an active degeneration process of the nerve segment distal to the lesion or site of injury. As part of the regeneration and repair process following injury, quiescent Schwann cells (SCs) are activated, leading the SCs to dedifferentiate, proliferate and release a wide range of chemokines and cytokines including NGF, BDNF, GDNF, CNTF, LIF, IGF and FGF to remodel the extracellular matrix (ECM) and to promote axonal regeneration [1, 5, 6, 14, 17, 23]. After about 96 hr, proliferating SCs attain a migratory phenotype and align to form “bands of Büngner” to provide structural guidance and growth-promoting factors for regenerating axons [15, 20]. However, till now, the mechanism of nerve regeneration has remained unclear, and the kinetic or sensuous effect of nerve repair remains unsatisfactory. It is known that cytoskeleton dynamics contribute to cell migration and that they are also related to the MEK-ERK pathway [16, 19]. In addition, PI3K signalling was proved to be relevant to protection and pathology after central nerve system injuries [21]. Considering that ERK and AKT signals were involved in vital functions of nerve system, we wonder if SC mobility, which is influential in the process of WD, is related to the signals mentioned above. In this study, we attempted to explore the molecular mechanism contributing to SC motility in order to understand the process of alignment of Büngner bands in WD, which may be helpful in forming a favorable strategy for nerve regeneration.
MATERIALS AND METHODS
Animal model of sciatic nerve transection: Sprague-Dawley rats (10–12 weeks) were used for this study. The study was approved by the Shenyang Medical College Committee on Animal Research. The institution’s guidelines for the care and use of laboratory animals were followed. Rats were anesthetized by intraperitoneal (i.p.) injection of ketamine (75 mg/kg). Under aseptic conditions, sciatic nerves of the right hind legs were transected at the upper thigh level. Proximal and distal stumps were set in their original positions. Muscles and skin were carefully sutured. The contralateral nerve was left intact and served as the uninjured control. After 7 days, activated Schwann cells (aSCs) were obtained from the right hind leg, and quiescent Schwann cells (qSCs) were obtained from the left hind leg.
Primary Schwann cell culture: Culture of primary SCs was performed as previously described [20]. Briefly, SCs were obtained from sciatic nerves, and contaminating fibroblasts were removed from the culture by treating the cells with 10 µM cytosine arabinoside for 48 hr and by complement-mediated cytolysis using anti-Thy1.1 (Serotec, Oxford, U.K.) and rabbit complement (Cappel Laboratories, Cochranville, PA, U.S.A.). SCs were propagated on poly-L-lysine-coated plates in DMEM supplemented with 10% FBS. All cells were cultured at 37°C in a humidified CO2 incubator at 5%.
Immunostaining: Cells were fixed using 4% paraformaldehyde for 5 min and then incubated in a solution of 0.3% H2O2 and 0.1% Na azide in PBS for 20 min at room temperature (RT) to reduce endogenous peroxidase activity. Nonspecific binding was prevented by incubating the fixed cells for 1 hr in 3% normal serum and 1% bovine serum albumin (BSA) solutions in 0.1% Triton-PBS. Cells were then incubated overnight at RT with specific primary antibodies: anti-p75NGFr, specific to dedifferentiated SCs or anti-S100, specific to SCs. After washing 3 times in PBS, they were incubated with their respective secondary biotinylated antibodies (Vector Laboratories, Burlingame, CA, U.S.A.) for 1 hr at RT. Then, sections were incubated for another 1 hr with the avidin-biotin-peroxidase complex (Vector Laboratories), diluted 1/1,000 in PBS and subjected to immunostaining with 3, 3′-diaminobenzidine reagent (Vector Laboratories).
Cell migration assay (wound healing): Schwann cells were transplanted into six-well plates. After they grew to confluence in these plates, SCs were incubated with FBS-free medium for 24 hr. Then, a scratch was made on the monolayer of cells using a sterile P-200 pipette tip. The wound area was marked with marker pen. Immediately after scraping the monolayer of cells, the cell culture medium was changed to FBS-free medium containing indicated pharmacological inhibitors. Schwann cells were maintained for 24 hr. A phase-contrast microscope, (Eclipse TS100, Nikon, Tokyo, Japan) was used for capturing images during the time course (0–24 hr). Digital images were quantified using the image J program (NIH, Bethesda, MA, U.S.A.) to determine the movements of SCs by measuring the number of migrated cells at 0 and 24 hr. We compared the pictures obtained at 0 and 24 hr by overlapping them on a computer and then counted the number of cells present in the scratch area at 0 hr (Fig.1). The number of cells that moved to scratch area was considered to reflect its motility. Experiments were repeated at least three times.
Fig. 1.
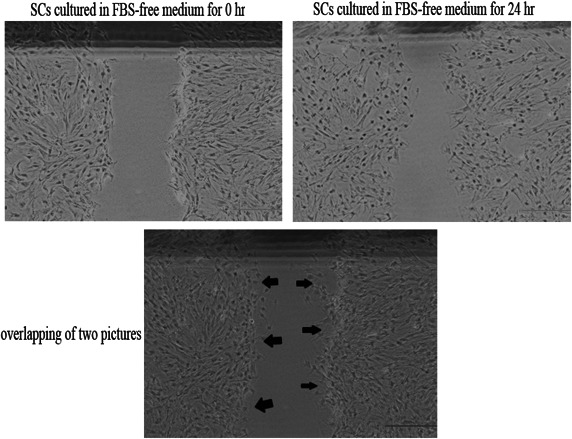
Measurement in the cell migration assay. The cells present in the scratch area are marked with arrows. Scale bar, 500 µm.
Western blotting: Before harvesting, the cells were incubated with FBS-free medium with different treatments for indicated durations. The harvested cells were lysed in a RIPA lysis buffer (50 mM Tris HCl, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate and 0.1% SDS) with a protease inhibitor cocktail containing phenylmethylsulfonyl fluoride (PMSF) and dithiothreitol (DTT) (Sigma Aldrich, St. Louis, MO, U.S.A.) for 60 min. The concentration of the extracted proteins was measured with a BCA protein assay (Kaiji, Nanjing, P.R. China). Equal amounts (50 µg) of proteins were separated using gel electrophoresis at 100V for 3 hr in SDS (sodium lauryl sulfate)-polyacrylamide gels and transferred to a Hybond-PVDF (polyvinylidene difluoride) membrane (Amersham, Arlington Heights, IL, U.S.A.). The membrane was incubated with the primary antibody diluted in TBS (PBS containing 0.5% BSA and 0.1% Tween 20) and then the horseradish peroxidase conjugated goat anti-rabbit secondary antibody (Pierce Biotechnology Inc., Rockford, IL, U.S.A.). ECL (enhanced chemiluminescence) reagents were used to detect the signals, according to the manufacturer’s instructions (Amersham).
Antibodies and reagents: The reagents used in this study with their sources are as follows:
Anti-S100 (ab868) and anti-p75 NGF receptor (ab8874) were from Abcam (Cambridge, MA, U.S.A.). Antibodies to ERK (4695), p-ERK (4370), AKT (4691), p-AKT (4060), U0126 (9903S) and LY294002 (9901S) were from Cell Signaling Technology (Danvers, MA, U.S.A.). Anti-PlGF (SRP4743) was from Sigma.
RESULTS
Increased migratory potential and phosphorylation status of ERK1/2 in aSCs compared with qSCs: The activated (aSCs) and quiescent (qSCs) phenotypes of SCs cultured from injured and intact nerves respectively were determined using immunostaining (Fig. 2). Micrographs show qSCs, which had a flat shape, stained positive for S100 (Fig. 2a) but not p75NGFr (Fig. 2c), whereas aSCs, which had a spindle shape, stained positive for both. (Fig. 2b and 2d). The migratory potentials of the two types of SCs were studied using a scratch wound assay. The results indicate that aSCs showed increased migratory potential compared with qSCs at the end of 24 hr (Fig. 3a). In addition, a significant increase in the phosphorylation of ERK1/2 but no of AKT was observed in aSCs compared with qSCs (Fig. 3b). No marked changes were observed in the total levels of ERK1/2 and AKT between the two cell types.
Fig. 2.
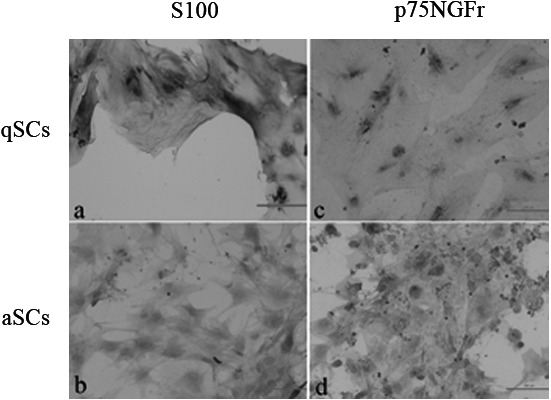
Determination of qSC and aSC phenotypes by immunostaining. qSCs and aSCs were identified by immunostaining for S100 and p75NGFr. Micrographs show qSCs stained for S100 (a) and p75NGFr (c) and aSCs stained for S100 (b) and p75NGFr (d). The flat shape and low density of the qSCs were in contrast to the spindle shape and high density of the aSCs. All experiments were repeated at least three times.
Fig. 3.
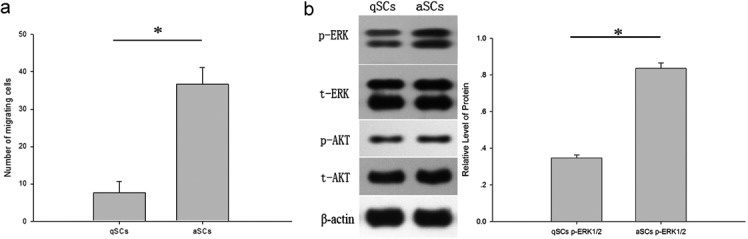
Higher motility of aSCs than qSCs with concurrent augmentation of phosphorylation of ERK1/2. qSCs and aSCs were respectively maintained with FBS-free DMEM at 0 and 24 hr. Cell motility of qSCs and aSCs was analyzed (a). Western blot analysis was performed to detect total ERK (t-ERK), total AKT (t-AKT) and phosphorylated forms of ERK (p-ERK) and AKT (p-AKT) in qSCs and aSCs at the assigned time points (0–24 hr). β-actin was used as a loading control (b). All experiments were repeated at least three times. Scale bar, 500 µm (*P<0.05).
Association of a higher level of phosphorylation of ERK1/2 with enhancement of aSC migration activated by PlGF: It is important to note that aSCs maintained in media containing 4 nM of PlGF for 24 hr subsequent to scratch wound creation showed an increased migratory capacity compared with those aSCs in media without PlGF (Fig. 4a). In addition, PlGF-treated aSCs also showed a significant increase in phospho-ERK1/2, but not phospho-AKT in a time-dependent manner. There was no increase in the levels of total ERK1/2 and AKT in aSCs following PlGF treatment (Fig. 4b).
Fig. 4.
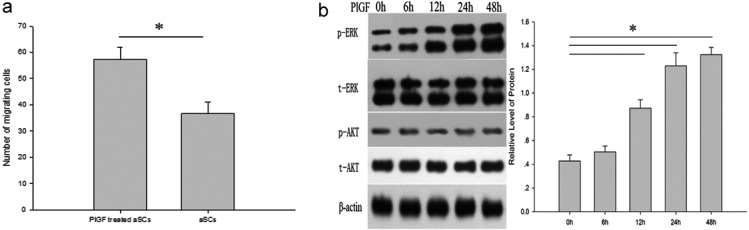
Association of PlGF-mediated enhancement of SCs motility with increased phosphorylation of ERK1/2. aSCs were maintained for 0 hr and 24 hr with 4 nM PlGF and FBS-free DMEM after being scratched. Untreated aSCs (control) were incubated with FBS-free DMEM for 0 hr and 24 hr after being scratched, and cell motility of aSCs maintained with PlGF was analyzed (a). aSCs in FBS-free DMEM were treated with 4 nM PlGF for indicated time points, and total ERK (t-ERK), total AKT (t-AKT) and phosphorylated forms of ERK (p-ERK) and AKT (p-AKT) were analyzed by western blotting. β-actin was used as a loading control (b). All experiments were repeated at least three times. Scale bar in micrograph, 500 µm (*P<0.05).
Coregulation of the enhanced migration potential of aSCs by ERK1/2 and AKT signals: The contribution of increased phosphorylation of ERK1/2 to the migration of aSCs in the scratch wound assay was investigated by incubating the cells for 24 hr with a MEK inhibitor, U0126 (30 µM), in the FBS-free medium after scratch wound creation. We found no significant reduction in aSC migration following inhibition of ERK1/2 activation (Fig. 5a). Meanwhile, phospho-AKT was significantly increased 12 hr after the inhibition of ERK1/2 activation (Fig. 5b). The enhancement of phospho-AKT suggested that the PI3K pathway is involved in aSC motility. Consequently, we examined the effects of a PI3K/AKT signaling inhibitor, LY294002 (10 µM), alone and in combination with U0126 (30 µM) on the migratory potential of aSCs using the scratch wound assay. aSCs incubated with LY294002 alone showed no significant reduction in motility, whereas the combination of U0126 and LY294002 resulted in a significant decrease in the migration of aSCs (Fig. 5c).
Fig. 5.
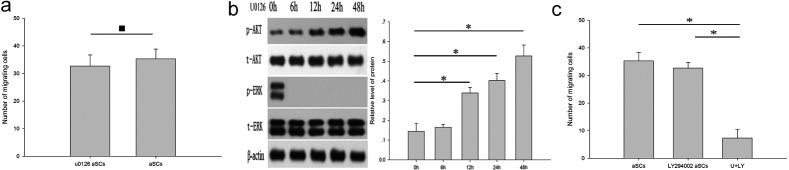
Involvement of the ERK and PI3K pathways in the enhanced motility of aSCs. aSCs were incubated with 30 µM U0126 for 0 hr and 24 hr after being scratched. Untreated aSCs incubated with FBS-free DMEM for 0 hr and 24 hr after being scratched were used as a control. Cell motility of aSCs maintained with different treatments was analyzed (a). aSCs in FBS-free DMEM were treated with 30 µM of U0126 for indicated time points and then assessed for total and phosphorylated levels of ERK1/2 and AKT by western blotting (b). aSCs were incubated with 10 µM LY294002 in FBS-free medium or both U0126 and LY294002 for 0 hr and 24 hr after being scratched. Untreated aSCs incubated with FBS-free DMEM for 0 hr and 24 hr after being scratched were used as a control (c). All experiments were repeated at least three times. Scale bar, 500 µm (*P<0.05).
DISCUSSION
In the present study, we provide evidences of the involvement of the ERK1/2 and AKT pathways in SCs’ motility. The ERK1/2 pathway may play a vital role in SC motility, and the AKT signal could be the alternate pathway that drives the motility of SCs when the ERK1/2 pathway is blocked.
In a previous study [3], p75NGFr expression in the distal stump peaked at day 7 after axotomy in the rat. Therefore, we adopted sciatic nerve transection in SD rats and harvested the distal stumps after 7 days post transection. The Wallerian degeneration process in the distal stump of the injured nerve is associated with activation of SCs, which form the bands of Büngner and express p75NGFr. In the current study, we showed the differences between qSCs and aSCs, including cell morphological disparity, cell motility and expression of key proteins (S100 and p75NGFr). Examination of SCs during cell culture suggested that after the SCs are activated, they begin to proliferate and dedifferentiate. The flat shape and low density characteristics of the quiescent SCs were in contrast to the characteristics of the activated SCs, which had a spindle shape and high density. Moreover, the enhancement of aSC motility may contribute to alignment of the bands of Büngner in order to provide structural guidance.
Considering the higher migratory potential of aSCs compared with qSCs, along with the augmentation of phosphorylation of ERK1/2, we investigated whether a similar tendency occurs after aSC motility is further elevated in order to prove the correlation between ERK1/2 and aSC motility. Therefore, PlGF was used as a trigger. PlGF, a member of the vascular endothelial growth factor (VEGF) family, participates actively in the angiogenesis of diverse cancers [2, 4, 9, 10, 22]. It was first cloned from the placenta [12], but it was later found to be normally expressed during wound healing and in the thyroid [7, 10, 11]. PlGF has been proven to have the ability to promote the migration of hematopoietic bone marrow progenitors, endothelial progenitor cells and breast cancer cells [8, 10, 18]. In our results, PlGF was demonstrated to enhance the motility of aSCs along with increased phosphorylation of ERK1/2.
To ascertain whether aSC motility is related to ERK1/2 signalling, aSCs were treated with U0126, a specific ERK inhibitor, and then, their motility was tested in a wound healing assay. The results suggested that aSCs migrated despite inhibition of ERK1/2. During the inhibition of ERK1/2, we also found that the p-AKT was activated. Subsequently, LY294002, a PI3K inhibitor, was used to evaluate the role of PI3K in the motility of aSCs, and LY294002 failed to inhibit the migratory potential of aSCs. aSCs were co-incubated with U0126 and LY294002 inhibitors to evaluate their synergistic inhibitory potential. The significant suppression of aSC motility after combination treatment with the ERK and PI3K inhibitors showed that both ERK1/2 and AKT signalling may contribute to the motility of aSCs. We noticed that no changes in p-AKT levels were observed between aSCs and qSCs or between untreated aSCs and PlGF-treated aSCs despite the differences in migratory potential between these groups. This suggests that PI3K signalling may not have an active regulatory role in the motility of aSCs in the presence of active ERK1/2 signalling and that the PI3K pathway can contribute to the migratory potential of aSCs in the absence of active ERK1/2 signalling. This indicates the possibility of a potential crosstalk between ERK1/2 and AKT signalling in SC motility, which remains to be explored in our further in vivo research.
Furthermore, we noticed that Napoli et al. found that ERK signalling may be central to dedifferentiation and demyelination of myelinating SCs [13]. According to the physiological process of WD, qSCs (myelinating Schwann cells) are activated, leading the SCs to dedifferentiate, proliferate and take on a role in physiological function. However, interestingly, we did not find aSCs were driven back to the state of differentiation and myelination when an inhibitor of ERK was used. We believe that the aSCs did not reach the time or dosage threshold required for cell state transition, and this requires further research.
In summary, our preliminarily investigation of the intracellular signalling events involved in SC motility showed that ERK1/2 signalling is an important factor in mediating migration of SCs and that AKT signalling is a potential alternate way of facilitating SC motility. It may be helpful to locate the specific cytokine or pathway involved in SC mobility and to further understand the mechanism of SC migration. Our further experiments in vivo should provide complementary information on the role of ERK and AKT in the formation of bands of Büngner, and they may help to form a favorable strategy for promoting nerve regeneration, which may contribute to clinical care.
Acknowledgments
The authors offer their thanks for the generous help provided by the Institute of Hand Surgery Research of Shenyang. This research was supported by funding from the Department of Education of Liaoning Province (grant number: L2011181) and Shenyang Technology Institute (grant number: F12-193-9-18). The authors report no conflicts of interest.
REFERENCES
- 1.Bampton E. T., Ma C. H., Tolkovsky A. M., Taylor J. S.2005. Osteonectin is a Schwann cell-secreted factor that promotes retinal ganglion cell survival and process outgrowth. Eur. J. Neurosci. 21: 2611–2623. doi: 10.1111/j.1460-9568.2005.04128.x [DOI] [PubMed] [Google Scholar]
- 2.Cao Y., O’Reilly M. S., Marshall B., Flynn E., Ji R. W., Folkman J.1998. Expression of angiostatin cDNA in a murine fibrosarcoma suppresses primary tumor growth and produces long-term dormancy of metastases. J. Clin. Invest. 101: 1055–1063. doi: 10.1172/JCI1558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chaballe L., Close P., Sempels M., Delstanche S., Fanielle J., Moons L., Carmeliet P., Schoenen J., Chariot A., Franzen R.2011. Involvement of placental growth factor in Wallerian degeneration. Glia 59: 379–396. doi: 10.1002/glia.21108 [DOI] [PubMed] [Google Scholar]
- 4.Donnini S., Machein M. R., Plate K. H., Weich H. A.1999. Expression and localization of placenta growth factor and PlGF receptors in human meningiomas. J. Pathol. 189: 66–71. doi: [DOI] [PubMed] [Google Scholar]
- 5.Heumann R., Korsching S., Bandtlow C., Thoenen H.1987. Changes of nerve growth factor synthesis in nonneuronal cells in response to sciatic nerve transection. J. Cell Biol. 104: 1623–1631. doi: 10.1083/jcb.104.6.1623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Höke A., Cheng C., Zochodne D. W.2000. Expression of glial cell line-derived neurotrophic factor family of growth factors in peripheral nerve injury in rats. Neuroreport 11: 1651–1654. doi: 10.1097/00001756-200006050-00011 [DOI] [PubMed] [Google Scholar]
- 7.Kagawa S., Matsuo A., Yagi Y., Ikematsu K., Tsuda R., Nakasono I.2009. The time-course analysis of gene expression during wound healing in mouse skin. Leg Med (Tokyo) 11: 70–75. doi: 10.1016/j.legalmed.2008.09.004 [DOI] [PubMed] [Google Scholar]
- 8.Kaplan R. N., Riba R. D., Zacharoulis S., Bramley A. H., Vincent L., Costa C., MacDonald D. D., Jin D. K., Shido K., Kerns S. A., Zhu Z., Hicklin D., Wu Y., Port J. L., Altorki N., Port E. R., Ruggero D., Shmelkov S. V., Jensen K. K., Rafii S., Lyden D.2005. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 438: 820–827. doi: 10.1038/nature04186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lacal P. M., Failla C. M., Pagani E., Odorisio T., Schietroma C., Falcinelli S., Zambruno G., D’Atri S.2000. Human melanoma cells secrete and respond to placenta growth factor and vascular endothelial growth factor. J. Invest. Dermatol. 115: 1000–1007. doi: 10.1046/j.1523-1747.2000.00199.x [DOI] [PubMed] [Google Scholar]
- 10.Li B., Sharpe E. E., Maupin A. B., Teleron A. A., Pyle A. L., Carmeliet P., Young P. P.2006. VEGF and PlGF promote adult vasculogenesis by enhancing EPC recruitment and vessel formation at the site of tumor neovascularization. FASEB J. 20: 1495–1497. doi: 10.1096/fj.05-5137fje [DOI] [PubMed] [Google Scholar]
- 11.Maes C., Coenegrachts L., Stockmans I., Daci E., Luttun A., Petryk A., Gopalakrishnan R., Moermans K., Smets N., Verfaillie C. M., Carmeliet P., Bouillon R., Carmeliet G.2006. Placental growth factor mediates mesenchymal cell development, cartilage turnover, and bone remodeling during fracture repair. J. Clin. Invest. 116: 1230–1242. doi: 10.1172/JCI26772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maglione D., Guerriero V., Viglietto G., Delli-Bovi P., Persico M. G.1991. Isolation of a human placenta cDNA coding for a protein related to the vascular permeability factor. Proc. Natl. Acad. Sci. U.S.A. 88: 9267–9271. doi: 10.1073/pnas.88.20.9267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Napoli I., Noon L. A., Ribeiro S., Kerai A. P., Parrinello S., Rosenberg L. H., Collins M. J., Harrisingh M. C., White I. J., Woodhoo A., Lloyd A. C.2012. A central role for the ERK-signaling pathway in controlling Schwann cell plasticity and peripheral nerve regeneration in vivo. Neuron 73: 729–742. doi: 10.1016/j.neuron.2011.11.031 [DOI] [PubMed] [Google Scholar]
- 14.Naveilhan P., ElShamy W. M., Ernfors P.1997. Differential regulation of mRNAs for GDNF and its receptors Ret and GDNFR alpha after sciatic nerve lesion in the mouse. Eur. J. Neurosci. 9: 1450–1460. doi: 10.1111/j.1460-9568.1997.tb01499.x [DOI] [PubMed] [Google Scholar]
- 15.Nguyen Q. T., Sanes J. R., Lichtman J. W.2002. Pre-existing pathways promote precise projection patterns. Nat. Neurosci. 5: 861–867. doi: 10.1038/nn905 [DOI] [PubMed] [Google Scholar]
- 16.Pawlak G., Helfman D. M.2002. Post-transcriptional down-regulation of ROCKI/Rho-kinase through an MEK-dependent pathway leads to cytoskeleton disruption in Ras-transformed fibroblasts. Mol. Biol. Cell 13: 336–347. doi: 10.1091/mbc.01-06-0302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sahenk Z., Seharaseyon J., Mendell J. R.1994. CNTF potentiates peripheral nerve regeneration. Brain Res. 655: 246–250. doi: 10.1016/0006-8993(94)91621-7 [DOI] [PubMed] [Google Scholar]
- 18.Taylor A. P., Leon E., Goldenberg D. M.2010. Placental growth factor (PlGF) enhances breast cancer cell motility by mobilising ERK1/2 phosphorylation and cytoskeletal rearrangement. Br. J. Cancer 103: 82–89. doi: 10.1038/sj.bjc.6605746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vial E., Sahai E., Marshall C. J.2003. ERK-MAPK signaling coordinately regulates activity of Rac1 and RhoA for tumor cell motility. Cancer Cell 4: 67–79. doi: 10.1016/S1535-6108(03)00162-4 [DOI] [PubMed] [Google Scholar]
- 20.Wakatsuki S., Araki T., Sehara-Fujisawa A.2014. Neuregulin-1/glial growth factor stimulates Schwann cell migration by inducing α5 β1 integrin-ErbB2-focal adhesion kinase complex formation. Genes Cells 19: 66–77. doi: 10.1111/gtc.12108 [DOI] [PubMed] [Google Scholar]
- 21.Walker C. L., Liu N. K., Xu X. M.2013. PTEN/PI3K and MAPK signaling in protection and pathology following CNS injuries. Front Biol. (Beijing) 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wei S. C., Tsao P. N., Yu S. C., Shun C. T., Tsai-Wu J. J., Wu C. H., Su Y. N., Hsieh F. J., Wong J. M.2005. Placenta growth factor expression is correlated with survival of patients with colorectal cancer. Gut 54: 666–672. doi: 10.1136/gut.2004.050831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang J. Y., Luo X. G., Xian C. J., Liu Z. H., Zhou X. F.2000. Endogenous BDNF is required for myelination and regeneration of injured sciatic nerve in rodents. Eur. J. Neurosci. 12: 4171–4180. [PubMed] [Google Scholar]


