Abstract
The Flinders Technology Associates filter paper cards (FTA® cards) can be used to store nucleic acid from various samples and are easily portable. However, RNA is physicochemically unstable compared with DNA, and appropriate methods have not been established for storage and extraction of RNA from FTA® cards. The present study investigated the optimum conditions for storage and elution of viral RNA (vRNA) using rabies virus (RABV) applied to FTA® cards. When TE buffer was used, the elution rates of vRNA increased with the length of the elution time. When the cards were stored at −80°C or −20°C, vRNA was stable over 3 months. Degradation of vRNAs occurred following storage at 4°C and room temperature, suggesting that RNA should be extracted from cards as soon as possible if no freezer is available. When we tried to amplify vRNA from RABV-infected animal brains applied to FTA® cards and stored at −80°C for 6 months, we did not detect any amplified products with the primer set for 964 bp of RABV N gene. However, we were able to detect amplified products by increasing the elution time of vRNA from FTA® cards from 30 min to 24 hr or by changing the primer sets to amplify 290 bp of N gene. Thus, we recommend extending the elution time for damaged or low concentration samples in FTA® cards.
Keywords: FTA card, rabies virus, storage, viral RNA
The newly developed Flinders Technology Associates (FTA®) Whatman filter paper cards can be used to dissolve the cells of directly applied blood and other samples and fix the nucleic acid in their fibers for storage. Also, it is claimed that FTA® cards protect nucleic acids from nucleases, oxidation and UV damage so that the DNA can be stored for a long time. For this reason, FTA® cards are widely used for storing nucleic acids from a variety of samples. In addition, since the cards are easily transported, they are also suitable for sampling in the field and are used in molecular biology and molecular epidemiology research [6]. FTA® cards can be used to store both DNA and RNA samples. They have been used for sampling many RNA viruses, including Newcastle disease [7], porcine reproductive and respiratory syndrome [1], infectious bursal disease [4], foot and mouth disease [5] and rabies [8]. However, RNA is physicochemically unstable compared with DNA. We thus investigated the optimum methods for storing and extracting viral RNA (vRNA) from FTA® cards [2, 3, 7].
MATERIALS AND METHODS
Common methods to determine the optimum conditions for storage and elution of vRNA on FTA® cards:
Preparation of FTA® card samples. Using a cutting mat (Whatman Japan, Tokyo, Japan) with RNase and DNA removed with RNase AWAY (Molecular BioProducts, San Diego, CA, U.S.A.) and a Harris Uni-Core punch, we cut 6 mm-diameter disks from FTA® classic cards (WB120306, Whatman Japan). Ten µl of RC-HL strain (The Chemo-Sero-Therapeutic Research Institute, >108.5 TCID50/ml; manufacturer’s serial number 148), a rabies TC vaccine for animals, was applied to the cut-out disks. The disks were placed in 1.5 ml micro centrifuge tubes for storage with silica gel after drying at room temperature for 24 hr. Four disks punch-out from the FTA® cards were used for the each condition to investigate the elution and storage of RNA on the FTA® cards.
Elution of vRNA from FTA®cards. 150 µl of eluent was added to the disks in microtubes so as to soak the disks, and the tubes were left to stand at room temperature. The disk was removed from the microtube using a sterile pipette tip, and the remaining solution was considered as RNA eluate. QIAamp® Viral RNA Mini Kit (QIAGEN Japan, Tokyo, Japan) was used to extract the vRNA from the eluates. In addition, RNA was extracted directly from the vaccine liquid using this kit for use as a positive control. The extracted RNA extract was kept at −80°C until use.
Preparation of standard RNA for the estimation of RNA copy number by Real-time PCR. The RABV full length N gene region (base position: 71–1,423) was amplified using RT-PCR with the primer pairs of RCHL-Nfull-F and RCHL-Nfull-R listed in Table 1. Recombinant plasmid DNA was prepared by incorporating the amplified product into pGEM-T Easy Vector (Promega Japan, Tokyo, Japan) and transformed into JM109 (Nippon Gene, Toyama, Japan). After extraction of the plasmids using the Wizard Plus SV Minipreps DNA Purification System (Promega Japan), the plasmids containing target sequence inserts were linearized with Sal I (Toyobo, Osaka, Japan) at 37°C for 1 hr and purified using Wizard SV Gel and PCR Clean-Up System (Promega Japan). In vitro transcription was performed using Riboprobe® In Vitro Transcription Systems (Promega Japan). Plasmid DNA was removed using the RNeasy Mini Kit (QIAGEN, Hilden, Germany) according to the RNA clean up protocol. The concentration of synthesized RNA was measured using a NanoDrop ND-1000 Spectrophotometer (NanoDrop Technologies, Wilmington, DE, U.S.A.), and the copy number of the RNA was calculated using the following formula; (Concentration of RNA in g / µl / [length of amplicon × 340]) × 6.022 × 1023=number of molecules / µl.
Table 1. Primers used in this study.
| Primer | Sequence (5′−3′) | Size of amplicon | Use |
|---|---|---|---|
| RCHL-Nfull-F | ATGGATGCCGACAGGATTG | 1,353 bp | RT-PCR |
| RCHL-Nfull-R | TTAAGAGTCGCTCGAATACGTCTTG | ||
| P1 | CTACAATGGATGCCGACAAGA | 964 bp | RT-PCR |
| P2 | CCCATATAACATCCAACAAAGTG | ||
| Nes-S | ATGGATGCCGACAAGATTGT | 290 bp | RT-PCR |
| Nes-C | GCWATCAGGATTCCATAGCT | ||
| realNgeneF1 | CGGCTGTTCCTCACTCTTATTTC | 133 bp | Real time-PCR |
| realNgeneR1 | CTGATTTGACCCATATAGCATCC |
Evaluation of elution rate of vRNA from FTA®cards. The RABV RNA yield from FTA® cards was measured by amplifying the N gene region 133 bp (base position: 836-968) with Power SYBR® Green RNA-to-CT 1-Step Kit (Applied Biosystems, Foster City, CA, U.S.A.) with the 7500 Fast Real-Time PCR System (Applied Biosystems). The primer pairs of realNgeneF1 and realNgeneR1 were used for the real-time PCR (Table 1). We added 2 µl RNA extract to 18 µl mixture containing 2 × RT-PCR Mix, RT Enzyme Mix, 1 µM forward and reverse primers and used the average value for the RNA yield of 3 wells to calculate the copy number. The RNA copy number in each disk was calculated from the calibration curve produced by tenfold dilution of the synthesized RNA, from 5 × 108 copies / µl to 5 × 102 copies / µl. In addition, the RNA elution efficiency was calculated from the ratio of the copy number of vRNA recovered from the FTA® cards and of vRNA extracted directly from the vaccine liquid.
Evaluation of the damage on vRNA. RT-PCR was conducted using the Super Script One Step RT-PCR System Kit (Invitrogen Japan, Tokyo, Japan) with the primer pairs of RCHL-Nfull-F and RCHL-Nfull-R for amplification of full length N gene (1,353 bp). Reactions were performed in a total of 25 µl mixture containing 2× Reaction Mix, SuperScript®III RT/Platinum®Taq High Fidelity Enzyme Mix (Invitrogen Japan), 10 µM forward and reverse primers, RNase-free sterile distilled water and RNA extract. In addition, an RT-PCR product with RNA extract replaced with nuclease-free water was used as a negative control. Amplified products were subjected to electrophoresis in a 1.5% agarose gel (Funakoshi, Tokyo, Japan) and checked under UV light after staining with ethidium bromide. The intensity of target bands of amplified products was compared with that of the control and evaluated semiquantitatively as integrated optical density. When the intensity of the control product was set at 100%, the integrated optical density of the samples was undetected and at 0–25% 25–50%, 50–75% and 75% or more, which were represented as –, ±, +, ++ and +++, respectively.
Requirement studies for storage and elution of vRNA on FTA® cards:
Comparison of elution rates among different eluents. We compared the elution rates of vRNA using five different RNA eluents: nuclease-free water, TE-buffer (10 mM Tris-HCI, 1 mM EDTA, pH8.0), RNA Rapid Extraction Solution (Ambion, Austin, TX, U.S.A.), Trizol and buffer AVL (QIAGEN Japan) that is supplied with the QIAamp® Viral RNA Mini Kit. Elution times of 5 min, 15 min, 30 min, 60 min and 24 hr were compared.
Evaluation of storage at different temperatures and time periods on vRNA damage. The FTA® cards were stored at temperatures of −80°C, −20°C, 4°C and room temperature, for periods of 1–4 weeks and 2–3 months. The degree of RNA damage was evaluated by comparing the amount of RT-PCR-amplified products between the RNA from FTA® cards stored in the different conditions and vRNA directly extracted from the vaccine liquid.
Influence of mouse brain emulsion on vRNA storage on FTA® cards. In order to investigate the impact of RNA damage in mouse brain emulsion, the vaccine liquid mixed with brain emulsion was applied to the FTA® cards and stored at −80°C. A 15 weeks old BALB/c mouse was used in this experiment, and euthanasia was performed in accordance with institutional guidelines. The mouse brain emulsion was prepared by adding 1 g of frozen mouse brain to 10 ml of PBS, and 10 µl of vaccine liquid was added to 90 µl of this emulsion. After mixing with a vortex, 10 µl of the centrifuged supernatant was applied to FTA® cards. For the control, we used samples without brain emulsion, which were prepared by diluting the vaccine liquid with sterile distilled water to the same concentration as the above samples. FTA® cards with the applied samples were dried for 24 hr at room temperature and kept at −80°C until use. The extraction of the RNA from FTA® cards was conducted using TE-buffer.
Detection of vRNA from RABV infections in field samples: Nine brain samples from cows and horses diagnosed as rabies positive in Brazil were applied to FTA® cards. After drying, the cards were sealed in protective pouches with silica gel (Whatman Japan) and transported by air from Brazil to Japan at room temperature. The cards were stored for 6 months at −80°C after arrival in Japan. We used nine specimens stored on FTA® cards in this study (BRhr1502, BRbv1503, BRbv1504, BRhr1505, BRbv1506, BRbv1507, BRhr1508, BRbv1509 and BRbv1510). RNA was extracted from the nine samples using the above methods. RT-PCR was conducted with the primer pairs for 964 bp (P1/P2, base position: 66−1,029) and 290 bp (Nes-S/Nes-C, base position: 71−360) of N gene. RNA was detected by electrophoresis and visual observation, with positive and negative represented as + and –.
Statistical analysis: The significance of differences between groups was calculated using one-tailed student’s t-test. P<0.05 was considered as the level of significance.
RESULTS
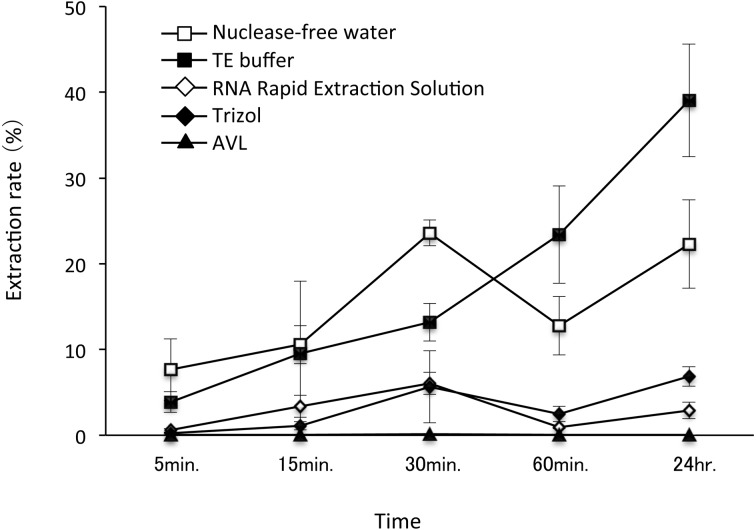
RNA elution conditions: Elution of RNA from FTA® cards was compared using nuclease-free water, TE-buffer, RNA Rapid Extraction Solution (Ambion), Trizol and buffer AVL (QIAGEN). The elution rate of vRNA was highest after 30 min in nuclease-free water compared to the other eluents, but it declined after 30 min. A similar pattern was observed with all of the other eluents, except for TE-buffer. The RNA elution rate in TE-buffer increased with longer elution times (Fig. 1). RNA detection using RT-PCR to amplify the full length N gene of RABV was successful with a 30 min elution, and there was no significant damage to the RNA even when the FTA® cards were immersed in TE-buffer for 24 hr (Table 2). The amount of amplified products following extraction with nuclease-free water, RNA Rapid Extraction Solution, Trizol or buffer AVL was relatively low compared with control RNA and RNA eluted using TE-buffer, suggesting that these eluents may affect the RNA quality during the elution step from the FTA® cards. From these results, we concluded that TE-buffer is the best eluent for RNA extraction from FTA® cards, and in subsequent tests, the RNA was eluted from FTA® cards soaked for 30 min in TE-buffer.
Fig. 1.
Comparison of elution rates of vRNA from FTA® cards using five different eluents. The symbols and vertical bars indicate the average values and standard deviations of extraction rates of vRNA from the FTA® card disks. Extraction rates of vRNA were estimated from the copy numbers of RNA by real-time PCR with the primer pairs of realNgeneF1 and realNgeneR1.
Table 2. Comparison of elution rates of vRNA from FTA® cards using different eluents.
| Eluent | Elution time | ||||
|---|---|---|---|---|---|
| 5 min | 15 min | 30 min | 60 min | 24 hr | |
| TE-buffer | + | + | ++ | ++ | ++ |
| Trizol | ± | ++ | ++ | + | + |
| Nuclease-free water | + | + | + | + | + |
| RNA Rapid Extraction Solution | ± | + | + | + | + |
| Buffer AVL | – | – | – | – | – |
When the intensity of the positive control band by RT-PCR with the primer pairs of RCHL-Nfull-F and RCHL-Nfull-R was set at 100%, the integrated optical density at the undetected (0%), 0–25%, 25–50% and 50–75% is represented as –, ±, + and ++ respectively.
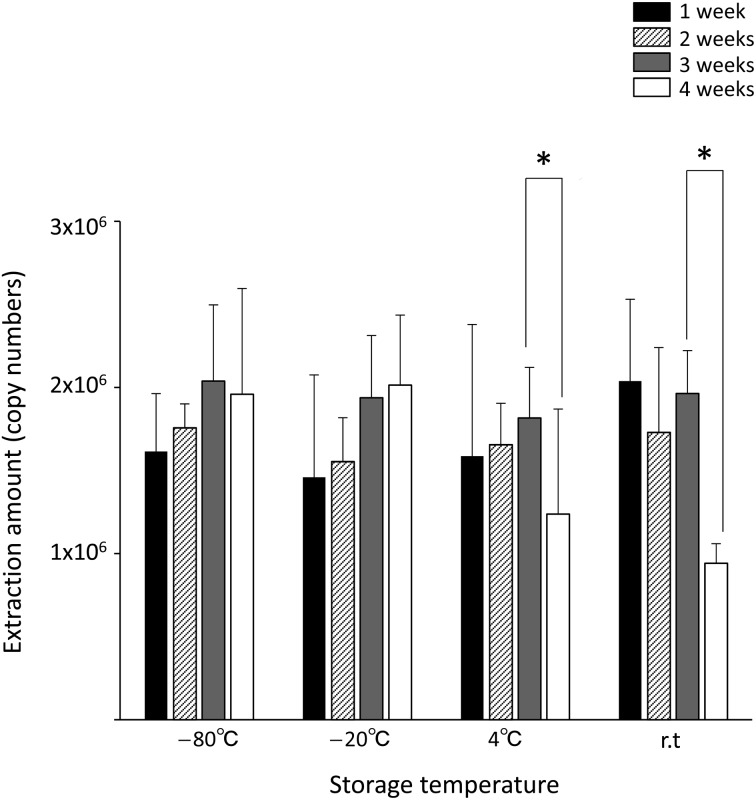
Storage conditions for vRNA on FTA® cards: When the FTA® cards were stored at −80°C and −20°C for 4 weeks, there was no significant decline in vRNA yield or damage to the RNA. Even when the storage period was extended to 3 months, there was no significant damage to the RNA (Fig. 2 and Table 3). However, when the FTA® cards were stored at 4°C or room temperature, RNA yield significantly declined between 3 and 4 weeks (one-tailed t-test: P<0.01) (Fig. 2). Significant RNA damage was observed after 1–2 weeks of cards storage at room temperature and 3–4 weeks of storage at 4°C, suggesting that the cards containing RNA samples should be stored under as cool as possible. Thus, the storage at −80°C or −20°C is optimal for retaining RNA samples on FTA® cards for long storage. In addition, since no obvious difference in RNA yield or RNA damage was observed between storage temperatures of −80°C and −20°C, deep freezing at −80°C is not absolutely necessary for storing FTA® cards.
Fig. 2.
Influence of storage temperatures and time periods of FTA® cards on vRNA yield from the cards. The graphs indicate the average values with standard deviations calculated from the extraction amounts of vRNAs from the FTA® card disks. *P<0.01.
Table 3. Effect of storage temperature and period on the stability of RNA on FTA® cards.
| Storage temperature | Storage period (weeks) | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 8 | 12 | |
| −80°C | ++ | ++ | +++ | ++ | +++ | ++ |
| −20°C | +++ | ++ | +++ | ++ | +++ | +++ |
| 4°C | ++ | + | ++ | ± | ± | – |
| r.t. | + | ± | ± | – | – | – |
When the intensity of the positive control band by RT-PCR with the primer pairs of RCHL-Nfull-F and RCHL-Nfull-R was set at 100%, the integrated optical density at the undetected (0%), 0–25%, 25–50%, 50–75% and 75% or more is represented as –, ±, +, ++ and +++, respectively.
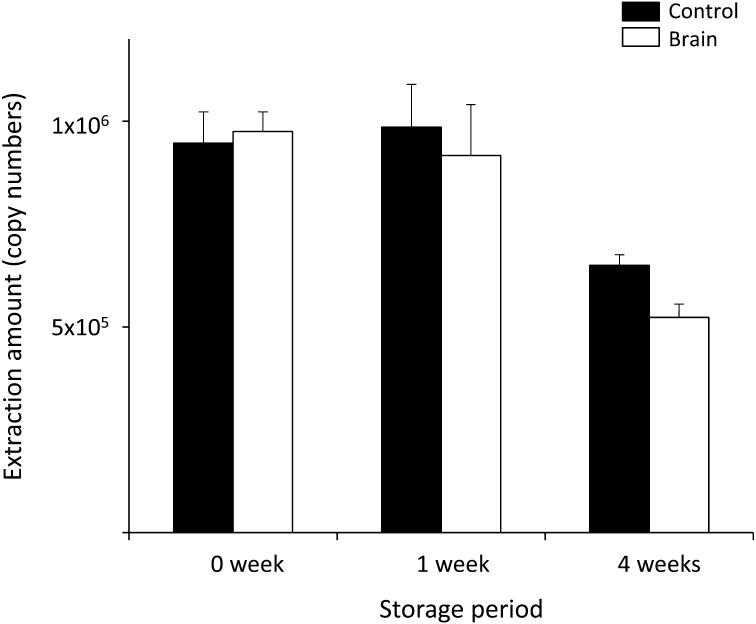
Influence of mouse brain emulsion on vRNA storage on FTA® cards: When we compared the samples with and without brain emulsion, no significant difference was observed in the amount of RNA eluted from the FTA® cards stored at −80°C (Fig. 3), suggesting that the presence of mouse brain emulsion did not influence on the RNA condition on FTA® cards. However, the significant reduction of vRNA yeild was observed at the both condition, with or without brain emulsion, in the FTA cards stored for 4 weeks (redulction rate: 34%–43%, P<0.03) (Fig. 3, Table 4), although no significant decline in the RNA quantity was observed when vaccine liquid was directly used as a sample with the same storage condition (one-tailed t-test: P>0.08) (Fig. 2). The vaccine liquids used for the test were 10 times diluted by PBS with or without brain emulsion, suggesting that RNA damage may be promoted under the low concentration of RNA on FTA® cards, not due to the existence of brain emulsion.
Fig. 3.
vRNA yield from FTA® cards with and without mouse brain emulsion. The FTA® cards with samples were stored at −80°C. The graphs indicate the average values with standard deviations calculated from the extraction amounts of vRNAs from the FTA® card disks.
Table 4. Influence of brain tissue on RNA stability on FTA® cards.
| Brain emulsion | Storage period (weeks) | ||
|---|---|---|---|
| 0 | 1 | 4 | |
| w/o | +++ | +++ | +++ |
| w/ | +++ | +++ | ++ |
When the intensity of the positive control band by RT-PCR with the primer pairs of RCHL-Nfull-F and RCHL-Nfull-R was set at 100%, the integrated optical density at 50–75% and 75% or more is represented as ++ and +++, respectively.
Detection of RNA from field rabies virus infection samples: Nine brain samples from animals diagnosed as rabies positive were applied to FTA® cards, and vRNA was extracted from them by eluting with TE-buffer for 30 min. RT-PCR was conducted for detection of extracted vRNA, but no amplification product was detected in any of the nine specimens with the primer pairs for 964 bp of N gene. RT-PCR products were detected in seven specimens when the primer pairs for 290 bp of N gene were used for amplification (Table 5). RT-PCR products of 964 bp were detected in three specimens when the elution time of FTA® cards was extended from 30 min to 24 hr. One additional specimen was detected with the primer pairs for 290 bp amplification. These results suggest that the elution rate of vRNA increased with elution time of RNA from FTA® cards from 30 min to 24 hr, although short-chain RNA was detected following a 30-min elution in TE-buffer.
Table 5. Detection of RABV RNA from brain samples applied to FTA® cards after 6 months storage.
| Sample | Host | Elution for 30 min | Elution for 24 hr | ||
|---|---|---|---|---|---|
| 964 bpa) | 290 bpb) | 964 bpa) | 290 bpb) | ||
| BRhr1502 | Equine | – | – | + | + |
| BRbv1503 | Bovine | – | + | – | + |
| BRbv1504 | Bovine | – | + | + | + |
| BRhr1505 | Equine | – | + | – | + |
| BRbv1506 | Bovine | – | + | – | + |
| BRbv1507 | Bovine | – | + | + | + |
| BRhr1508 | Equine | – | – | – | – |
| BRbv1509 | Bovine | – | + | – | + |
| BRbv1510 | Bovine | – | + | – | + |
| No. of detection/No. of samples | 0/9 | 7/9 | 3/9 | 8/9 | |
a) The primer pairs of P1 and P2 were used for the amplification of 964 bp of RABV N gene. b) The primer pairs of Nes-S and Nes-C were used for the amplification of 290 bp of RABV N gene.
DISCUSSION
In this research, we investigated the optimum conditions for storage and extraction of RABV RNA stored on FTA® cards in detail.
When the storage temperatures of the cards were compared, the most appropriate temperature for detection of RABV RNA following storage on FTA® cards was −80°C or −20°C because the RNA quality on the cards appeared to be kept for 3 months, suggesting that a freezer is useful to keep RNA samples on the cards for long time periods. At 4°C and room temperature, the significant decline in the extraction rates of vRNA from the cards was observed between 3 and 4 weeks. However, since little damage on vRNA was observed when the cards were stored at 4°C for 3 weeks, if no freezer is available, we recommend that the card should be kept as cold as possible and that RNA on the cards should be extracted within 3 weeks. As another factor that can promote RNA degradation, low concentration of the RNA on the cards can affect the RNA stability even when a freezer is used for card storage. It thus may be better to extract more earlier time in the samples of low RNA concentration. Since the concentration of vRNA in brain emulsion from field samples may not be uniform, it is recommended that FTA® cards are stored for as short a time as possible.
TE-buffer was found to be the most suitable eluent for RABV RNA from FTA® cards of those compared in this study, and elution time >30 min from FTA® cards soaked in TE-buffer appeared to be best. This result is supported by an earlier study where the vRNA yeild from FTA® Elute cards was investigated using different elution buffers. TE-1 buffer (10 mM Tris-HCI, 1 mM EDTA, pH10.0) was the best of the eluents tested, with an inferred elution efficiency of 6.1% [2]. Although the vRNA in our study was different, the elution rate obtained significantly exceeded this. This may be due to the differences in the type of FTA® card, the elution method and/or the pH of TE-buffer. In addition, Picard-Meyes et al. succeed the extraction of vRNA of RABV and other lyssaviruses from the FTA® card stored at room temperature for 35–48 days using DMEM as eluent [8], suggesting that DMEM may be also useful for RNA elution. Additional comparison of eluents including DMEM may be necessary to evaluate the utility of TE-buffer.
The long-chain nucleotide sequences are useful for high-resolution analysis of molecular evolution. Although it was difficult to obtain long-chain amplified products from field samples stored for a long time on FTA® cards, we were able to detect short-chain RNA. However, extending the elution time to 24 hr improved the detection efficiency of vRNA from FTA® cards. Thus, we recommend an extended elution time, particularly for the collection of degraded samples from FTA® cards.
All RNA elution steps in this study were conducted at room temperature, although, it is clear that damage to RNA occurs faster under ambient conditions. Consequently, it is possible that the RNA was damaged during elution. It is expected that determining the optimal temperature conditions for RNA elution will lead to further improvements in RNA recovery efficiency.
Acknowledgments
This study was supported in part by a Grant-in-Aid for Scientific Research (no. 24580453) from the Japan Society for the Promotion of Science and the Strategic Research Base Development Program for Private Universities sponsored by the Ministry of Education, Culture, Sports, Science and Technology of Japan (S0991023).
REFERENCES
- 1.Inoue R., Tsukahara T., Sunaba C., Itoh M., Ushida K.2007. Simple and rapid detection of the porcine reproductive and respiratory syndrome virus from pig whole blood using filter paper. J. Virol. Methods 141: 102–106. doi: 10.1016/j.jviromet.2006.11.030 [DOI] [PubMed] [Google Scholar]
- 2.Li Y., Yoshida H., Wang L., Tao Z., Wang H., Lin X., Xu A.2012. An optimized method for elution of enteroviral RNA from a cellulose-based substrate. J. Virol. Methods 186: 62–67. doi: 10.1016/j.jviromet.2012.08.016 [DOI] [PubMed] [Google Scholar]
- 3.Linhares D. C., Rovira A., Torremorell M.2012. Evaluation of Flinders Technology Associates cards for collection and transport of samples for detection of Porcine reproductive and respiratory syndrome virus by reverse transcription polymerase chain reaction. J. Vet. Diagn. Invest. 24: 328–332. doi: 10.1177/1040638711429492 [DOI] [PubMed] [Google Scholar]
- 4.Maw M. T., Yamaguchi T., Kasanga C. J., Terasaki K., Fukushi H.2006. A practical tissue sampling method using ordinary paper for molecular detection of infectious bursal disease virus RNA by RT-PCR. Avian Dis. 50: 556–560. doi: 10.1637/7537-032806R.1 [DOI] [PubMed] [Google Scholar]
- 5.Muthukrishnan M., Singanallur N. B., Ralla K., Villuppanoor S. A.2008. Evaluation of FTA cards as a laboratory and field sampling device for the detection of foot-and-mouth disease virus and serotyping by RT-PCR and real-time RT-PCR. J. Virol. Methods 151: 311–316. doi: 10.1016/j.jviromet.2008.05.020 [DOI] [PubMed] [Google Scholar]
- 6.Natarajan P., Trinh T., Mertz L., Goldsborough M., Fox D. K.2000. Paper-based archiving of mammalian and plant samples for RNA analysis. Biotechniques 29: 1328–1333. [DOI] [PubMed] [Google Scholar]
- 7.Perozo F., Villegas P., Estevez C., Alvarado I., Purvis L. B.2006. Use of FTA filter paper for the molecular detection of Newcastle disease virus. Avian Pathol. 35: 93–98. doi: 10.1080/03079450600597410 [DOI] [PubMed] [Google Scholar]
- 8.Picard-Meyer E., Barrat J., Cliquet F.2007. Use of filter paper (FTA) technology for sampling, recovery and molecular characterisation of rabies viruses. J. Virol. Methods 140: 174–182. doi: 10.1016/j.jviromet.2006.11.011 [DOI] [PubMed] [Google Scholar]