Abstract
Chordomas of the tip of the tail in 6 ferrets were examined using histopathological, histochemical and immunohistochemical procedures. Histopathologically, round neoplastic cells containing numerous cytoplasmic vacuoles of varying sizes, categorized as “physaliphorous cells”, were observed in the amorphous eosinophilic or pale basophilic myxoid stroma. Physaliphorous cells were arranged in lobules and in a “chordoid” or “cobblestone” manner. The neoplasms were diagnosed as benign chordoma without local invasion and metastasis. Histochemically, the cytoplasm of small neoplastic cells was positive for periodic acid-Schiff stain and alcian blue (AB) pH 2.5 and pH 1.0 stains, but negative for hyaluronidase digestion-AB pH 2.5 stain. All neoplastic cells were strongly stained with colloidal ion, negative for high iron diamine AB pH 2.5 and toluidine blue pH 2.5 stains, and positive for Mayer’s mucicarmine stain. Immunohistochemistry using antibodies directed against low-molecular-weight cytokeratins (CK18, CK19 and CK20), vimentin and mucin core protein (MUC5AC) revealed that neoplastic cells had both epithelial and mesenchymal elements. The expression of low-molecular-weight cytokeratins suggests that neoplastic cells acquired the properties of glandular epithelial cells and produced epithelial mucus. Furthermore, the expression of cytokeratins, vimentin, S100 protein, brachyury and epithelial membrane antigen indicates that the neoplasms were equivalent to the classic type of human chordoma. Therefore, immunohistochemistry using these antibodies can be useful for the characterization of ferret chordoma.
Keywords: brachyury, chordoma, ferret, histochemistry, immunohistochemistry
Chordomas in animals and humans are considered to originate from remnants of the notochord in the nucleus pulposus of the intervertebral disk [4, 5, 15]. Chordomas have been reported in dogs, rats, mink, cats and predominantly in ferrets [1, 2, 4, 7, 8, 10, 18, 26, 32]. Histopathologically, neoplastic cells containing multiple, polygonal, cytoplasmic vacuoles are arranged in lobules surrounded by the thin to thick myxoid stroma (physaliphorous cells) [7, 15, 18]; these cells are arranged in a “chordoid” or “cobblestone” manner. The most characteristic aspect of this neoplasm is the accumulation of mucus in the extracellular myxoid stroma. The composition of mucus has been determined for several cases of human chordoma [22, 23, 28]. Because few studies are available that describe the mucus components of chordomas in animals, including ferrets, the nature of the mucus components in ferret chordomas is unclear [1, 8]. Immunohistochemical studies on the nature of human and animal chordomas have revealed the dual expression of cytokeratin and vimentin in intermediate filaments [2, 4, 7, 17, 18, 32]. However, such studies have not been conducted on animal chordomas. Therefore, histochemical and immunohistochemical analyses to characterize the neoplastic cells of ferret chordomas were conducted in this study.
MATERIALS AND METHODS
Histopathology: Tissue samples of tail-tip chordomas in 6 ferrets (3 males and 3 females, aged 3–5 years) were examined histopathologically. Neoplastic tissues fixed in 10% formalin solution were decalcified as necessary and then embedded in paraffin after dehydration with an ethanol solution. The 4-µm-thick tissue sections were stained with hematoxylin and eosin (HE).
Histochemistry: Mucus components, such as mucopolysaccharides in neoplastic cells and the extracellular myxoid stroma, were analyzed using the stains as follows: periodic acid-Schiff (PAS), alcian blue (AB) pH 2.5 and pH 1.0, hyaluronidase digestion-AB (HD-AB) pH 2.5, colloidal iron (CI), high iron diamine-AB (HID-AB) pH 2.5 and toluidine blue (TB) pH 2.5. Mayer’s mucicarmine stain was used to detect epithelial mucin and neutral mucopolysaccharides. After diastase digestion, PAS (PAS-D) staining was performed to detect glycogen deposition in neoplastic cells. The details of histochemistry are shown in Table 1.
Table 1. Results from histochemistry of mucus components in neoplastic cells.
| Stains | Mucus components | Cases | |||||
|---|---|---|---|---|---|---|---|
| No. 1 | No. 2 | No. 3 | No. 4 | No. 5 | No. 6 | ||
| PAS | Glycoproteins [neutral mucin and acidic glycoproteins (sulfomucin and sialomucin)] | +++ | +++ | +++ | +++ | +++ | +++ |
| PAS-D | Glycogen | + | + | ++ | + | + | ++ |
| AB pH 2.5 | Acidic glycoproteins (sulfomucin and sialomucin) | +++ | +++ | +++ | +++ | +++ | +++ |
| Acidic mucopolysaccharides (hyaluronic acid, chondroitin, heparin, keratan and dermatan) | |||||||
| AB pH 1.0 | Acidic glycoprotein (sulfomucin) | + | + | + | + | + | + |
| AB pH 2.5 after hyaluronidase treatment | Acidic mucopolysaccharide (hyaluronic acid) | ++ | ++ | ++ | ++ | ++ | ++ |
| CI | Acidic glycoprotein (sialomucin), acidic mucopolysaccharides (hyaluronic acid, chondroitin, heparin, keratan and dermatan) | + | + | + | + | + | + |
| HID-AB pH 2.5 | Acidic glycoprotein (sulfomucin) and acidic mucopolysaccharides | – | – | – | – | – | – |
| Mayer’s mucicarmine | Epithelial mucin and neutral mucopolysaccharides | +++ | ++ | ++ | ++ | ++ | +++ |
| TB pH 2.5 | Acidic mucopolysaccharides (chondroitin and mucoitinsulfuric acid) | – | – | – | – | – | – |
Staining rate: –, 0%; +, 1–30%; ++, 31–60%; +++, > 60%, PAS: periodic acid-Schiff, PAS-D: periodic acid-Schiff-diastase, AB: alcian blue, CI: colloidal iron, HID: high iron diamine, TB: toluidine blue.
Immunohistochemistry: The 4-µm-thick tissue sections were examined immunohistochemically by the avidin-biotin-peroxidase complex (ABC) technique. Each section was immersed in 0.5% periodic acid solution at room temperature for 15 min to inhibit endogenous peroxidase activity. All the primary antibodies were reacted with the sections at 4°C for 16 hr, and the secondary antibodies were reacted at room temperature for 30 min. The latter were then reacted at room temperature for 30 min with an avidin-peroxidase conjugate (Vectastain Elite ABC Kit; Vector Laboratories, Burlingame, CA, U.S.A.). The sections were immersed in 0.05% 3,3′-diaminobenzidine/H2O2 (DAB; Dojindo, Kamimashiki, Japan) solution for 10 min to visualize antigen-antibody complexes. Subsequently, the sections were counterstained with Mayer’s hematoxylin stain. Negative control sections were obtained by omission of the primary antibodies. Antibodies directed against mucin core proteins (MUC) (MUC1, MUC2 and MUC5AC) were used to detect mucin in neoplastic cells. The stomach and colon of ferrets were used as positive controls for expression of MUC1, MUC2 and MUC5AC. The characteristics of the primary antibodies used in immunohistochemistry are shown in Table 2.
Table 2. Characteristics of the primary antibodies used in immunohistochemistry.
| Antbodies | Clones | Dilutions | Pretreatments | Antibody suppliers |
|---|---|---|---|---|
| α1-antitrypsin | 1:1 | Cm | Nichirei, Tokyo, Japan | |
| Brachyury | D-10 | 1:100 | Ca | Santa Cruz Biotechnology, Santa Cruz, U.S.A. |
| CK AE1/AE3 | AE1/AE3 | 1:1 | PK | Nichirei Corp, Tokyo, Japan |
| CK7 | VO-TL-12/30 | 1:50 | PK | Dakopatts, Glostrup, Denmark |
| CK9 | ks9.7 | 1:10 | Cm | Progen, Heidelberg, German |
| CK13 | Ks13.1 | 1:10 | Cm | Dakopatts, Glostrup, Denmark |
| CK14 | LL002 | 1:20 | Cm | Biomeda, Foster City, U.S.A. |
| CK18 | RGE53 | 1:50 | Cm | Progen, Heidelberg, German |
| CK19 | BA17 | 1:1 | Cm | Thermo Fisher Scientific, U.S.A. |
| CK20 | Ks20.8 | 1:50 | PK | Dakopatts, Glostrup, Denmark |
| E-cadherin | 4A2C7 | 1:100 | Cm | Invitrogen, Camarillo, U.S.A. |
| EMA | E29 | 1:50 | Cm | Dakopatts, Glostrup, Denmark |
| MUC1 | Ma695 | 1:100 | Cm | Novocastra, Newcaslle, U.K. |
| MUC2 | Ccp58 | 1:100 | Cm | Novocastra, Newcaslle, U.K. |
| MUC5AC | CLH2 | 1:100 | Cm | Novocastra, Newcaslle, U.K. |
| NSE | BBS/NC/VI-H14 | 1:100 | Cm | Dakopatts, Glostrup, Denmark |
| PCNA | PC10 | 1:100 | Ca | Dakopatts, Glostrup, Denmark |
| S100 protein | 1:1000 | Cm | Dakopatts, Glostrup, Denmark | |
| Vimentin | V9 | 1:100 | Cm | Dakopatts, Glostrup, Denmark |
CK: cytokeratin, EMA: epithelial membrane antigen, NSE: neuron-specific enolase, PCNA: proliferating cell nuclear antigen, MUC: mucin core protein, Cm: citrate buffer pH 6.0 at 15 min in microwave, Ca: citrate buffer pH 6.0 at 15 min in autoclave, PK: protein kinase.
RESULTS
Histopathologic findings: Neoplastic tissues resected from 6 ferrets were composed of small to large neoplastic cell lobules surrounded by thin to thick myxoid stroma. The cytoplasm of neoplastic cells was irregularly shaped, containing multiple vacuoles of varying sizes in the amorphous eosinophilic and/or pale basophilic extracellular myxoid stroma (Fig. 1). Small and round neoplastic cells had a fine, vacuolated cytoplasm and a small central nucleus. Medium-sized neoplastic cells had fine to larger vacuoles and a peripheral nucleus. Large neoplastic cells had one to three large cytoplasmic vacuoles and a crescent-shaped nucleus displaced to a marginal region of the cytoplasm (Fig. 2). Mitotic figures were not observed. In some neoplastic tissues, cartilage and bone components were observed without evidence of malignancies, such as chondrosarcoma.
Fig. 1.
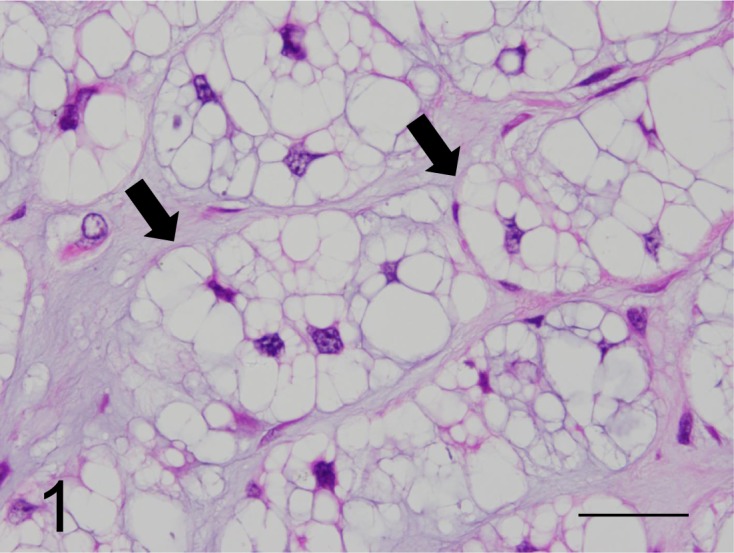
Neoplastic cells containing multiple cytoplasmic vacuoles of varying sizes (arrows) arranged in lobules, with abundant extracellular myxoid stroma (physaliphorous cells) (case No. 1). HE. Bar=200 µm.
Fig. 2.
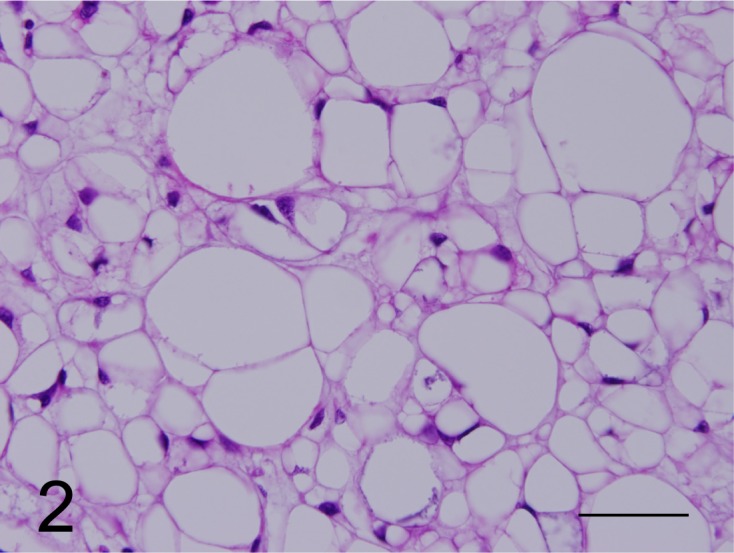
Cells with an entire vacuolated cytoplasm and with the nucleus located at the margin of the cytoplasm were categorized as vacuolated cells (case No. 1). HE. Bar=200 µm.
Histochemical findings: The cytoplasm of small neoplastic cells was positive for the PAS stain, and the glycogen granules in some neoplastic cells were eliminated by diastase digestion. Small cells were positive for AB pH 2.5 and pH 1.0 stains, while slightly positive for the HD-AB pH 2.5 stain. The cells were strongly stained with the CI stain (Fig. 3), but were negative for HID-AB pH 2.5 and TB pH 2.5 stains. Neoplastic cells were positive for Mayer’s mucicarmine stain (Fig. 4); however, cytoplasmic vacuoles were not stained by these procedures. The cytoplasm was stained equivalently in small and medium-sized neoplastic cells. In contrast, the staining intensity of the cytoplasm was reduced substantially in neoplastic cells with larger cytoplasmic vacuoles. Almost all small or large cytoplasmic vacuoles of neoplastic cells were not stained by any method. Large neoplastic cells containing large vacuoles had scant mucus components in the cytoplasm with the same staining intensity compared with that of small neoplastic cells. The results of the histochemical analyses of neoplastic cells are shown in Table 1. The extracellular myxoid stroma of neoplasm lacking glycogen granules was positive for PAS, AB pH 1.0 and pH 2.5, HD-AB pH2.5, CI and Mayer’s mucicarmine stains (Fig. 4) and was negative for HID-AB pH 2.5 and TB pH 2.5 stains.
Fig. 3.
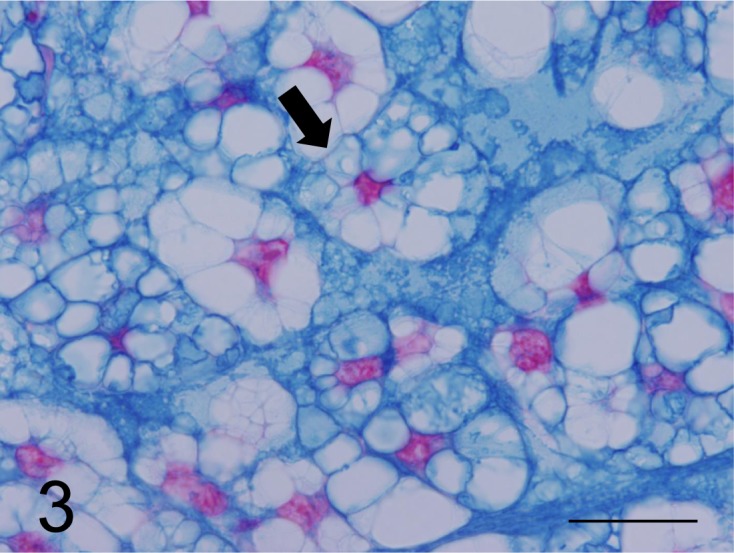
Neoplastic cells with foam in the cytoplasm (arrow) and extracellular matrix were strongly stained by the CI stain (case No. 1). Bar=125 µm.
Fig. 4.
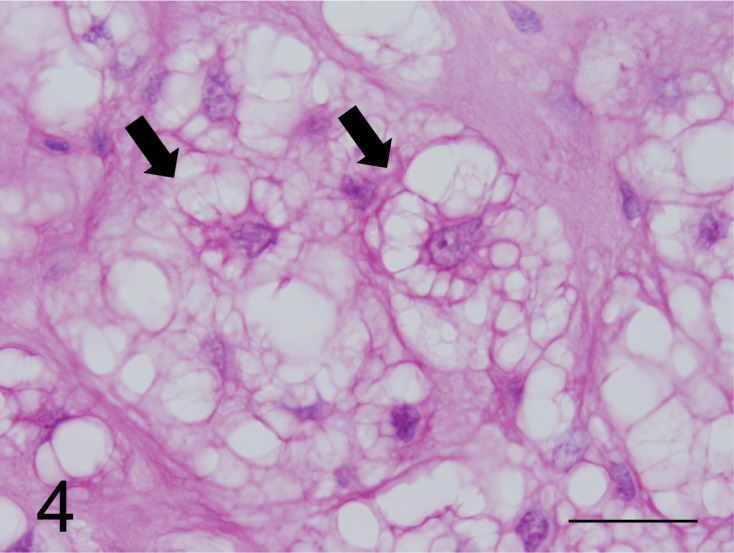
Neoplastic cells with foam in the cytoplasm (arrows) were strongly stained by the mucicarmine stain (case No. 1). Bar=125 µm.
Immunohistochemical findings: All neoplastic cells expressed CK AE1/AE3, CK18, CK19 and CK20 as well as vimentin (Figs. 5 and 6), but CK7, CK9, CK13 and CK14 were not detected. However, neoplastic cells expressed neuron specific enolase (NSE), S100 protein, α1-antitrypsin, epithelial membrane antigen (EMA) and proliferating cell nuclear antigen. Medium and large neoplastic cells expressions are decreased levels of these proteins. Neoplastic membranes expression of E-cadherin was not detected. Neoplastic cells expressed MUC5AC (Fig. 7), but not MUC1 and MUC2. In healthy ferrets used as positive controls, goblet cells of the large intestine expressed MUC1. Furthermore, chief cells of the gastric mucosa and goblet cells of the large intestine expressed MUC2, and gastric surface epithelial cells expressed MUC5AC. The nuclei of neoplastic cells expressed brachyury (Fig. 8). The cytoplasmic vacuoles of neoplastic cells did not express any antigens. The results are shown in Table 3.
Fig. 5.
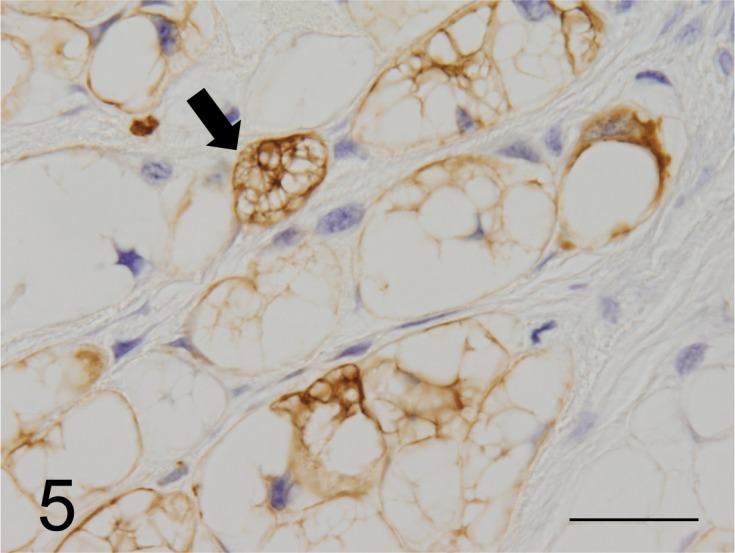
Neoplastic cells stained strongly by the anti-CK AE1/AE3 antibody (arrow). CK AE1/AE3 immunoreactivity is distributed widely throughout the cytoplasm (case No.1). Bar=125 µµm.
Fig. 6.
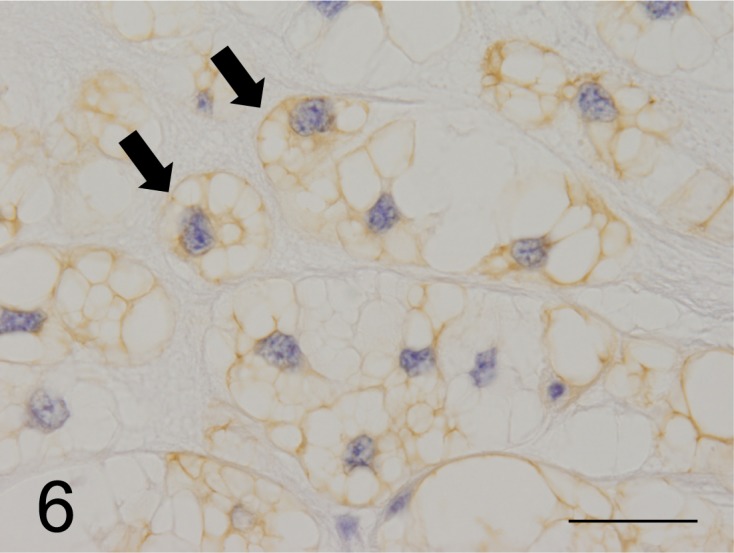
Most of the neoplastic cells were strongly stained by the anti-CK 19 antibody (arrows) (case No.1). Bar=125 µm.
Fig. 7.
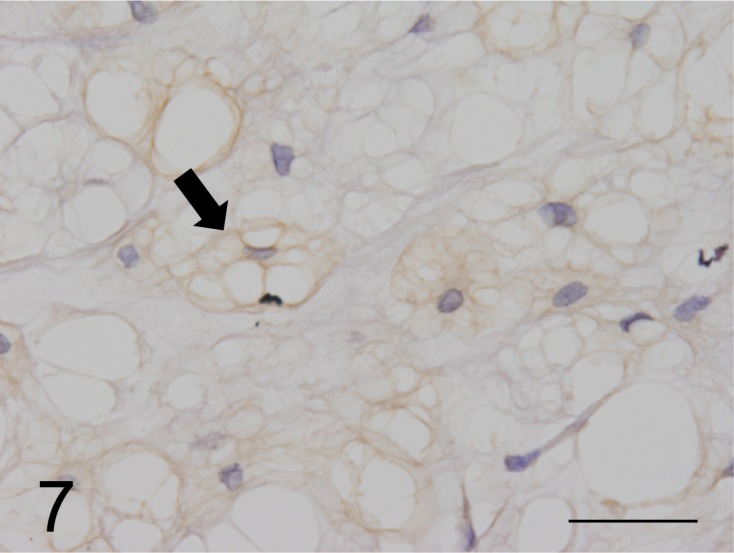
MUC5AC expressed on the surface of cell membranes and in the cytoplasm of neoplastic cells (arrow) (case No.1). Bar=125 µm.
Fig. 8.
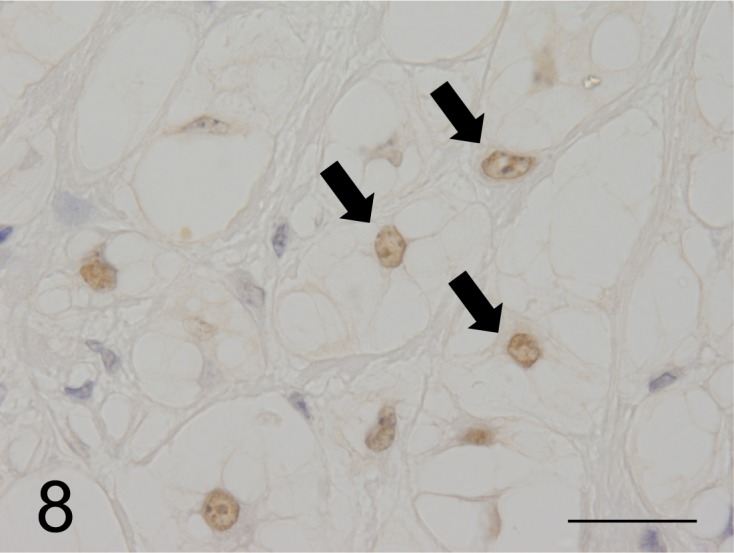
The nuclei of most neoplastic cells in the chordoma were stained by the anti-brachyury antibody (arrow), which is diagnostic for human chordomas (case No.1). Bar=125 µm.
Table 3. Characteristics of the primary antibodies used in immunohistochemistry.
| Antigens | Cases | Reference cases | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. 1 | No. 2 | No. 3 | No. 4 | No. 5 | No. 6 | Ferret [4] | Ferret [10] | Ferret [18] | Ferret [32] | Cat [2] | Dog [7] | Rat [26] | |
| CK AE1/AE3 | +++ | +++ | +++ | +++ | +++ | +++ | 20/20 | 2/4 | 1/1 | 2/2* | 1/1 | 1/1 | 9/9* |
| EMA | +++ | ++ | +++ | ++ | +++ | ++ | NT | 1/4 | NT | NT | NT | NT | NT |
| NSE | +++ | +++ | +++ | +++ | ++ | ++ | 18/20 | NT | NT | 2/2 | NT | 0/1 | 9/9 |
| S100 protein | +++ | +++ | +++ | +++ | ++ | ++ | 15/20 | 3/4 | NT | 2/2 | 1/1 | 1/1 | 7/9 |
| Vimentin | +++ | +++ | +++ | +++ | +++ | +++ | 20/20 | NT | 1/1 | 2/2 | 1/1 | 1/1 | NT |
| α1-antitrypsin | +++ | +++ | ++ | ++ | +++ | ++ | NT | NT | NT | NT | NT | NT | NT |
| Brachyury | +++ | +++ | +++ | +++ | +++ | +++ | NT | NT | NT | NT | NT | NT | NT |
| CK7 | — | — | — | — | — | — | NT | NT | NT | NT | NT | NT | NT |
| CK9 | — | — | — | — | — | — | NT | NT | NT | NT | NT | NT | NT |
| CK13 | — | — | — | — | — | — | NT | NT | NT | NT | NT | NT | NT |
| CK14 | — | — | — | — | — | — | NT | NT | NT | NT | NT | NT | NT |
| CK18 | +++ | +++ | ++ | +++ | +++ | +++ | NT | NT | NT | NT | NT | NT | NT |
| CK19 | +++ | +++ | +++ | ++ | +++ | +++ | NT | NT | NT | NT | NT | NT | NT |
| CK20 | +++ | +++ | +++ | +++ | +++ | +++ | NT | NT | NT | NT | NT | NT | NT |
| E-cadherin | — | — | — | — | — | — | NT | NT | NT | NT | NT | NT | NT |
| MUC1 | — | — | — | — | — | — | NT | NT | NT | NT | NT | NT | NT |
| MUC2 | — | — | — | — | — | — | NT | NT | NT | NT | NT | NT | NT |
| MUC5AC | + | + | ++ | + | + | ++ | NT | NT | NT | NT | NT | NT | NT |
| PCNA | + | + | + | ++ | + | + | NT | NT | NT | NT | NT | NT | NT |
—: nonimmunoreactive; +: slightly immunoreactive; ++: moderately immunoreactive; +++: strongly immunoreactive, Number of immunoreactive animals/total number of animals, CK: cytokeratin; EMA: epithelial membrane antigen; NSE: neuron-specific enolase; CK: cytokeratin; MUC: mucin core protein; PCNA: proliferating cell nuclear antigen, *: keratin, [ ]: reference number, NT: not tested.
DISCUSSION
The histopathological and immunohistochemical features of tail-tip chordoma were similar among 6 ferrets examined here and those described previously [1, 4, 10, 18]. Chordoma develops twice as frequently in female ferrets as in male ferrets [4]. However, no sex difference was observed in the present study. Chordoma in animals grows slowly and may cause local invasion and metastasis [4, 15, 18, 26, 32]. The chordomas studied here were composed of physaliphorous cells arranged in lobules surrounded by amorphous eosinophilic or pale basophilic myxoid stroma. Neoplastic cells tended to enlarge in proportion to the size of cytoplasmic vacuoles. Because neoplastic cell infiltrated local tissue, mitotic figures or metastasis was not observed, the neoplasms were diagnosed histopathologically as benign chordomas. In humans, chordoma is a rare malignant tumor that develops predominantly in the clivus and sacrococcygeal vertebrae, grows slowly and may recur or metastasize [5, 6]. Chordomas of ferrets develop predominantly in the tip of the tail and may be surgically resected at an early stage. Therefore, differences in the site of development may largely affect therapeutic outcomes. Cartilage and bone components are present in neoplastic tissues, which is attributable to metaplasia that is frequently observed in ferrets and dogs with chordomas [4, 7].
Human and animal chordomas are known to produce myxoid stroma. However, only a limited number of histochemical studies describe the composition of mucus in chordomas [1, 2, 7, 8]. The results of the present study suggest that the myxoid stroma contains a large amount of acidic mucopolysaccharides, such as chondroitin and hyaluronic acid [2, 8]. On the other hand, PAS and Mayer’s mucicarmine stains revealed that the mucus in neoplastic cells contains various macromolecules, such as mucopolysaccharides and glycoproteins. Both neoplastic cells and surrounding myxoid stroma were stained with PAS and Mayer’s mucicarmine stains, and therefore, in conclusion, the mucus in the myxoid stroma must have originated from neoplastic cells.
Immunohistochemical techniques have been used to analyze chordomas of animals [2, 4, 7, 10, 26] and humans [12, 13, 17, 20, 23, 28]. Cytokeratins are almost constantly expressed in neoplastic cells in ferrets, dogs, cats and rats (Table 3) [2, 4, 7, 10, 18, 26, 32]. Vimentin is expressed in neoplastic cells of chordomas in ferrets, dogs and cats (Table 3) [2, 4, 7, 18, 32]. The dual expression of cytokeratins and vimentin in animal chordomas [2, 4, 7, 10, 18, 32] suggests the sustained pluripotency of chordoma cells that originated from remnants of the notochord [27]. NSE is expressed in chordomas of ferrets (91%) and rats (100%) [4, 26, 32], and S100 protein is expressed in chordomas of ferrets (77%), dogs (100%), cats (100%) and rats (78%) [2, 4, 7, 10, 26, 32]. The expression of S100 protein and NSE in human chordomas is related to the composition of stromal glycosaminoglycans (chondroitin) [13]. The expression of NSE and S100 protein in the present study indicated the presence of glycosaminoglycans, such as chondroitin and hyaluronic acid, in the myxoid stroma and neoplastic cells. EMA is expressed in chordomas of ferrets (25%) [10] and in some cases of human chordomas [17, 23]. Neoplastic cells from all ferrets in this study expressed cytokeratins, vimentin, S100 protein, NSE and EMA (Table 3). These findings were considered to indicate that the immunohistochemical properties of neoplastic cells in ferrets in the present study are very similar to those reported in human chordomas [5].
There is no report of several sub-types of cytokeratin in animal chordomas, except for a report by Herron et al., using single monoclonal antibody for low-molecular-weight CK [10]. Therefore, little is known regarding the immunohistochemical properties of the cytoskeleton of animal chordomas. Hence, immunohistochemical analyses of ferret chordomas using antibodies directed against low-molecular-weight CKs (CK18, CK19 and CK20) and high-molecular-weight CKs (CK7, CK9, CK13 and CK14) were performed here. Consequently, the neoplastic cells expressed low-molecular-weight CKs (CK18, CK19 and CK20), but not high-molecular-weight CKs (CK14, CK7, CK9 and CK13). CK18, CK19 and CK20 are expressed primarily in mucosal and glandular epithelial cells of the gastrointestinal tract [9, 12, 19, 20, 25]. Neoplastic cells were suggested to differentiate into glandular epithelial cells. Furthermore, neoplastic cells are immunostained with an anti-MUC5AC antibody. MUC5AC is secreted by epithelial cells of the normal gastric mucosa and gallbladder epithelium of animals and humans [16, 24, 30]. The expression of MUC5AC suggests that neoplastic cells of ferret chordomas produce glycosylated mucin. Therefore, it can be concluded that neutral mucopolysaccharides, such as MUC5AC, of epithelial cell origin detected by histochemistry and immunohistochemistry were produced by cells that express cytokeratins. However, immunohistochemical analyses did not detect MUC1 and MUC2 in the neoplastic cells of any of the ferrets. The anti-MUC1 antibody is specific for the carbohydrate epitope of MUC1 (membrane-associated mucin) that is expressed by mucin-secreting epithelial cells of the endometrium, trachea, lung, pancreas and gastrointestinal tract of animals and humans [11, 16, 30]. The anti-MUC2 antibody is specific for cytoplasmic and extracellular membrane-associated MUC2 expressed in mucin-secreting epithelial cells of the gastrointestinal tract [30]. The different specificities of the antibodies used in the present study are considered to reflect the different results of the immunohistochemical classification of MUC expression in ferret chordomas. The lack of immunohistochemical detection of E-cadherin expression in tumor cells suggests that neoplastic cells do not differentiate into clear epithelial cells. However, the diversity of the immunohistochemical staining of neoplastic cells may represent the diversified differentiation potential of remnants of the notochord.
The brachyury protein encoded by the T gene is a transcription factor of more than 20 T-box family complex genes involved in the formation of the mesoderm [14, 21]. Brachyury mainly regulates the development of the notochord and has been well studied in frogs [3], zebrafish [33] and mice [14]. Brachyury, which is not expressed in normal adult tissues, is activated in human chordoma cells and is therefore extremely useful for the diagnosis of chordomas [31]. Positive results in the nuclei of tumor cells in all cases in ferrets suggest that immunohistochemical proof of brachyury expression is a useful method for the diagnosis of chordomas in animals as well as in human. The consistent presence of chondroid and osseous components in animal and human chordomas suggests that these components in ferrets are cartilaginous or indicates bone differentiation within the neoplasm [4, 29]. Brachyury was detected in the chondroid and osseous components of human chordomas, and its expression indicates that the chondroid component is of notochordal origin [31]. On the other hand, immunohistochemical analyses of neoplasms, such as chondromas and chondrosarcomas, of non-notochordal origin did not detect brachyury expression [31]. Consistent with these results, brachyury expression was undetectable in the cartilage and bone components of chordomas in this study. Although the reason is unknown, ferret chordomas are usually benign and highly differentiated in contrast to human chordomas that are generally malignant and poorly differentiated.
Human chordomas are classified into three types according to histopathological characteristics: classic, chondroid and dedifferentiated [5]. Immunohistochemical features differ among these types, and the chondroid components of chondroid chordomas express CKs and brachyury. The dedifferentiated cells in dedifferentiated chordomas do not express S100 protein, brachyury, CK and EMA. These differences in the immunohistochemical properties between neoplastic and nonneoplastic cells are used to specify the histopathological types of chordomas [5, 31]. Ferret chordomas are generally diagnosed as benign neoplasms without local invasion and metastasis [10] and are considered equivalent to the classic type of human chordoma [5, 23].
The histopathological features of all ferret chordomas characterized here are very similar to those of classic chordoma, but differ from those of chondroid chordomas and dedifferentiated chordomas [5]. Furthermore, the reactivity of these chordomas to antibodies directed against CKs, vimentin, S100 protein, brachyury and EMA indicates their equivalence to the classic type of human chordoma. Therefore, immunohistochemical analysis using these antibodies may be useful for the characterization of ferret chordomas.
The results of the present study are summarized as follows: (1) The myxoid stroma surrounding chordoma cell lobules contained mucus components of neoplastic origin. (2) The properties of mucus in neoplastic cells and the mucus components of neoplastic origin in the myxoid stroma were defined. (3) The pattern of expression of CKs, vimentin, S100 protein, brachyury and EMA suggests the tumor cell origin of the epithelial and mesenchymal elements in the myxoid stroma.
REFERENCES
- 1.Allison N., Rakich P.1988. Chordoma in two ferrets. J. Comp. Pathol. 98: 371–374. doi: 10.1016/0021-9975(88)90046-1 [DOI] [PubMed] [Google Scholar]
- 2.Carminato A., Marchioro W., Melchiotti E., Vascellari M., Mutinelli F.2008. A case of coccygeal chondroid chordoma in a cat: morphological and immunohistochemical features. J. Vet. Diagn. Invest. 20: 679–681. doi: 10.1177/104063870802000529 [DOI] [PubMed] [Google Scholar]
- 3.del Pino E. M.1996. The expression of Brachyury (T) during gastrulation in the marsupial frog Gastrotheca riobambae. Dev. Biol. 177: 64–72. doi: 10.1006/dbio.1996.0145 [DOI] [PubMed] [Google Scholar]
- 4.Dunn D. G., Harris R. K., Meis J. M., Sweet D. E.1991. A histomorphologic and immunohistochemical study of chordoma in twenty ferrets (Mustela putorius furo). Vet. Pathol. 28: 467–473. doi: 10.1177/030098589102800602 [DOI] [PubMed] [Google Scholar]
- 5.Flanagan A. M., Yamabuchi T.2013. Chordoma. pp. 328–329. In: WHO Classification of Tumours of Soft Tissue and Bone, 4th ed. (Christopher, D. M., Fletcher, Julia A., Bridge, Pancras C. W. Hogendoorn. and Fredrik, M. eds.), International Agency for Research on Cancer (IARC), Lyon. [Google Scholar]
- 6.Gay E., Sekhar L. N., Rubinstein E., Wright D. C., Sen C., Janecka I. P., Snyderman C. H.1995. Chordomas and chondrosarcomas of the cranial base: results and follow-up of 60 patients. Neurosurgery 36: 887–896, discussion 896–897. doi: 10.1227/00006123-199505000-00001 [DOI] [PubMed] [Google Scholar]
- 7.Gruber A., Kneissl S., Vidoni B., Url A.2008. Cervical spinal chordoma with chondromatous component in a dog. Vet. Pathol. 45: 650–653. doi: 10.1354/vp.45-5-650 [DOI] [PubMed] [Google Scholar]
- 8.Hadlow W. J.1984. Vertebral chordoma in two ranch mink. Vet. Pathol. 21: 533–536. [DOI] [PubMed] [Google Scholar]
- 9.Heikinheimo K., Persson S., Kindblom L. G., Morgan P. R., Virtanen I.1991. Expression of different cytokeratin subclasses in human chordoma. J. Pathol. 164: 145–150. doi: 10.1002/path.1711640208 [DOI] [PubMed] [Google Scholar]
- 10.Herron A. J., Brunnert S. R., Ching S. V., Dillberger J. E., Altman N. H.1990. Immunohistochemical and morphologic features of chordomas in ferrets (Mustela putorius furo). Vet. Pathol. 27: 284–286. [DOI] [PubMed] [Google Scholar]
- 11.Ho S. B., Niehans G. A., Lyftogt C., Yan P. S., Cherwitz D. L., Gum E. T., Dahiya R., Kim Y. S.1993. Heterogeneity of mucin gene expression in normal and neoplastic tissues. Cancer Res. 53: 641–651. [PubMed] [Google Scholar]
- 12.Horn K. D., Fowler J. C., Carrau R., Barnes E. L., Rao U. N.2001. Cytokeratin immunophenotyping of an unusual cervical vertebral chordoma with extensive chondroid foci and perilaryngeal recurrence: a case report with review of the literature. Am. J. Otolaryngol. 22: 428–434. doi: 10.1053/ajot.2001.28080 [DOI] [PubMed] [Google Scholar]
- 13.Karabela-Bouropoulou V., Kontogeorgos G., Papamichales G., Milas C., Roessner A., Vollmer E., Grundmann E.1988. S-100 protein and neuron specific enolase (NSE) expression by chordomas in relation to the composition of their stromal mucosubstances. Pathol. Res. Pract. 183: 256–261. doi: 10.1016/S0344-0338(88)80118-3 [DOI] [PubMed] [Google Scholar]
- 14.Kispert A., Koschorz B., Herrmann B. G.1995. The T protein encoded by Brachyury is a tissue-specific transcription factor. EMBO J. 14: 4763–4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koestner A., Higgins R. J.2002. Chordomas. pp. 728–729. In: Tumors in Domestic Animals, 4th ed. (Meuten, Donald, J. ed.), Iowa State Press, Ames. [Google Scholar]
- 16.Lacunza E., Bara J., Segal-Eiras A., Croce M. V.2009. Expression of conserved mucin domains by epithelial tissues in various mammalian species. Res. Vet. Sci. 86: 68–77. doi: 10.1016/j.rvsc.2008.05.011 [DOI] [PubMed] [Google Scholar]
- 17.Le Charpentier Y., Bellefqih S., Boisnic S., Roy-Camille R.1988. [Chordomas]. Ann. Pathol. 8: 25–32. [PubMed] [Google Scholar]
- 18.Munday J. S., Brown C. A., Richey L. J.2004. Suspected metastatic coccygeal chordoma in a ferret (Mustela putorius furo). J. Vet. Diagn. Invest. 16: 454–458. doi: 10.1177/104063870401600516 [DOI] [PubMed] [Google Scholar]
- 19.Naka T., Iwamoto Y., Shinohara N., Chuman H., Fukui M., Tsuneyoshi M.1997. Cytokeratin subtyping in chordomas and the fetal notochord: an immunohistochemical analysis of aberrant expression. Mod. Pathol. 10: 545–551. [PubMed] [Google Scholar]
- 20.O’Hara B. J., Paetau A., Miettinen M.1998. Keratin subsets and monoclonal antibody HBME-1 in chordoma: immunohistochemical differential diagnosis between tumors simulating chordoma. Hum. Pathol. 29: 119–126. doi: 10.1016/S0046-8177(98)90220-9 [DOI] [PubMed] [Google Scholar]
- 21.Packham E. A., Brook J. D.2003. T-box genes in human disorders. Hum. Mol. Genet. 12: R37–R44. doi: 10.1093/hmg/ddg077 [DOI] [PubMed] [Google Scholar]
- 22.Pelfrene A., Laumonier R.1976. [Histochemical study of the ground substance in chordoma]. Ann. Anat. Pathol. (Paris) 21: 357–364. [PubMed] [Google Scholar]
- 23.Persson S., Kindblom L. G., Angervall L.1991. Classical and chondroid chordoma. A light-microscopic, histochemical, ultrastructural and immunohistochemical analysis of the various cell types. Pathol. Res. Pract. 187: 828–838. doi: 10.1016/S0344-0338(11)80579-0 [DOI] [PubMed] [Google Scholar]
- 24.Reis C. A., David L., Nielsen P. A., Clausen H., Mirgorodskaya K., Roepstorff P., Sobrinho-Simões M.1997. Immunohistochemical study of MUC5AC expression in human gastric carcinomas using a novel monoclonal antibody. Int. J. Cancer 74: 112–121. doi: [DOI] [PubMed] [Google Scholar]
- 25.Scolyer R. A., Bonar S. F., Palmer A. A., Barr E. M., Wills E. J., Stalley P., Schatz J., Soper J., Li L. X., McCarthy S. W.2004. Parachordoma is not distinguishable from axial chordoma using immunohistochemistry. Pathol. Int. 54: 364–370. doi: 10.1111/j.1440-1827.2004.01633.x [DOI] [PubMed] [Google Scholar]
- 26.Stefanski S. A., Elwell M. R., Mitsumori K., Yoshitomi K., Dittrich K., Giles H. D.1988. Chordomas in Fischer 344 rats. Vet. Pathol. 25: 42–47. doi: 10.1177/030098588802500106 [DOI] [PubMed] [Google Scholar]
- 27.Stosiek P., Kasper M., Karsten U.1988. Expression of cytokeratin and vimentin in nucleus pulposus cells. Differentiation 39: 78–81. doi: 10.1111/j.1432-0436.1988.tb00083.x [DOI] [PubMed] [Google Scholar]
- 28.Suster S., Moran C. A.1995. Chordomas of the mediastinum: clinicopathologic, immunohistochemical, and ultrastructural study of six cases presenting as posterior mediastinal masses. Hum. Pathol. 26: 1354–1362. doi: 10.1016/0046-8177(95)90301-1 [DOI] [PubMed] [Google Scholar]
- 29.Tarik T., Scott E. K.2010. Mesenchymal tumors of the central nervous system. pp. 231–232. In: Practical Surgical Neuropathology: A Diagnostic Approach. (Perry, A. and Brat, D. J. eds.), Elsevier, Philadelphia. [Google Scholar]
- 30.Terada T.2013. An immunohistochemical study of primary signet-ring cell carcinoma of the stomach and colorectum: II. Expression of MUC1, MUC2, MUC5AC, and MUC6 in normal mucosa and in 42 cases. Int. J. Clin. Exp. Pathol. 6: 613–621. [PMC free article] [PubMed] [Google Scholar]
- 31.Vujovic S., Henderson S., Presneau N., Odell E., Jacques T. S., Tirabosco R., Boshoff C., Flanagan A. M.2006. Brachyury, a crucial regulator of notochordal development, is a novel biomarker for chordomas. J. Pathol. 209: 157–165. doi: 10.1002/path.1969 [DOI] [PubMed] [Google Scholar]
- 32.Williams B. H., Eighmy J. J., Berbert M. H., Dunn D. G.1993. Cervical chordoma in two ferrets (Mustela putorius furo). Vet. Pathol. 30: 204–206. doi: 10.1177/030098589303000214 [DOI] [PubMed] [Google Scholar]
- 33.Yabe T., Takada S.2012. Mesogenin causes embryonic mesoderm progenitors to differentiate during development of zebrafish tail somites. Dev. Biol. 370: 213–222. doi: 10.1016/j.ydbio.2012.07.029 [DOI] [PubMed] [Google Scholar]


