Abstract
We examined the effects of chicken egg hydrolysate (also known as “bone peptide” or BP) on bone metabolism in 5- to 8-month-old orchidectomized dogs. The bone formation marker serum bone alkaline phosphatase (BAP) and the bone resorption marker urine deoxypyridinoline (DPD) were used as indicators to measure changes in bone metabolism. The following results were observed that Serum BAP was higher in dogs fed BP-enriched food throughout the clinical investigation. Serum BAP was statistically significantly higher in dogs fed BP-enriched food than in dogs fed non-BP-enriched food at 2 months after orchidectomy. This suggests that BP promoted bone formation immediately after orchidectomy.
Keywords: BAP, canine, DPD, egg yolk hydrolysate, orchidectomy
Bone density decreases rapidly in post-menopausal women, who are known to be at increased risk of osteoporosis [1]. This is because menopause decreases estrogen levels, causing bone resorption to outstrip bone formation and reducing bone density. Bone resorption also starts to outstrip bone formation in older men, and their bone density decreases [5]. This is also related to decreased testosterone production with increasing age. The close connection between human sex hormones and bone metabolism is thus well known. In dogs, osteoporosis is rare. However, numerous studies have found that orchidectomy and ovariectomy have an effect on bone metabolism [4, 6, 14].
Hori et al. investigated the value of a canine model of osteoporosis [6]. They performed ovariectomy on 15- to 18-month-old female beagles and subsequently fed them a low-calcium diet. They found that although there were no changes in body weight, serum calcium or serum inorganic phosphate, bone mass decreased. Fukuda et al. also measured parameters, such as calcium, phosphorus, testosterone, parathyroid hormone, calcitonin, osteocalcin and bone alkaline phosphatase (BAP) in blood, as well as bone mass, over time in orchidectomized male beagles with a mean age of 2 years [4]. They reported that their findings indicate an imbalance in bone metabolism (i.e. bone resorption>bone formation). Bone mass also declined significantly.
Salmeri et al. studied the effects of orchidectomy and ovariectomy at a young age on bone metabolism [12]. They reported that cessation of bone growth was delayed and that bone formation became unbalanced.
These previous studies suggest that orchidectomy and ovariectomy affect bone metabolism. Their findings also indicate that monitoring of bone metabolism is required after orchidectomy or ovariectomy.
As bone mass is known to decrease in humans after menopause, there have been numerous studies on how to prevent this decline [2]. There are also numerous commercially available food products and medications that aim to improve bone density. Among these, we focused on bone peptide (BP) (Bonepep®; Pharma Foods International Co., Ltd., Kyoto, Japan), a chicken egg hydrolysate produced by enzymatic treatment of the water-soluble component of defatted chicken eggs [10]. Kim et al. added BP to cultured osteocytes in order to investigate its effects on bone metabolism and discovered that BP promoted extension growth [7]. They also investigated bone density in ovariectomized rats and reported that bone density improved when they were fed BP.
We therefore conducted a study on 5- to 8-month-old dogs to determine in order to determine whether feeding with BP-enriched food after orchidectomy improves post-orchidectomy bone metabolism. The experimental procedures followed the Regulation for Animal Experments of Teikyo University of Science.
Experimental dogs: Ten healthy male dogs of ordinary household breeds were used. Table 1 shows the profiles of experimental dogs. The 10 experimental dogs were randomly allocated into two groups of five dogs each, one of which received food enriched with BP and the other received food without supplemented BP. Orchidectomy was carried out using standard methods.
Table 1. Profile of orchidectomized dogs.
| Breed | Age (mo) | Weight (kg) |
|---|---|---|
| Dogs fed non-BP-enriched food | ||
| Spitz | 8 | 6.6 |
| Mongrel | 8 | 3.8 |
| Miniature dachshund | 8 | 4.3 |
| Toy poodle | 7 | 1.9 |
| Labrador retriever | 8 | 25.5 |
| Dogs fed BP-enriched food | ||
| Welsh corgi | 6 | 4.8 |
| Golden retriever | 6 | 16.5 |
| Pomeranian | 8 | 3.2 |
| Boston terrier | 7 | 8.1 |
| Yorkshire terrier | 5 | 1.6 |
Rearing conditions: Commercially available dog food (Runfree Inc., Tokyo, Japan) was used as the experimental food. BP-enriched food was prepared by adding BP to the experimental food, which was fed to animals to give a dose of 2 mg/kg body weight. The BP dose of 2 mg/kg body weight was selected, because the ED 50 of BP is 1.6 mg/kg. As BP has little effect on nutritional composition, nothing was added to the non-BP-enriched food. Per 100 g, it contained 346 kcal of energy, 23 g of crude protein, 7 g of crude fat, 60 g of carbohydrate, 1 g of ash, 345 mg of calcium and 242 mg of phosphorus. Dogs were fed with the experimental food beginning immediately after orchidectomy. The amount of food was adjusted in accordance with increases in body weight. Blood and urine were sampled before orchidectomy and at 2, 4 and 6 months after orchidectomy. Blood and urine sampling was carried out at around 1 p.m. each time in order to minimize diurnal variations in marker levels.
Measurement of bone metabolism markers: Serum calcium, serum phosphorus, serum BAP and urine DPD were measured as bone metabolism markers. A Dri-Chem 7000 (Fujifilm, Tokyo, Japan) was used to measure serum calcium and serum phosphorus. Serum BAP and urine DPD were measured by ELISA. BAP was measured using a Canine BAP ELISA Kit (Biotang Inc., Lexington, MA, U.S.A.), and urine DPD was measured with the Canine DPD ELISA Kit (Biotang Inc.). Measurements were performed in accordance with the manufacturer’s instructions. Results are shown as changes over time and in terms of area under the curve (AUC).
Student t-test was used for statistical analysis, with P<0.05 being regarded as significant.
No health problems were evident during the experimental period.
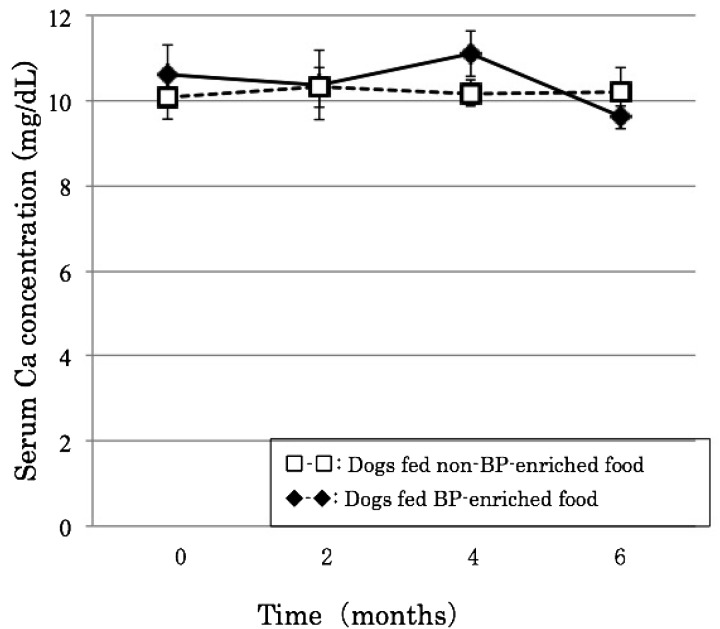
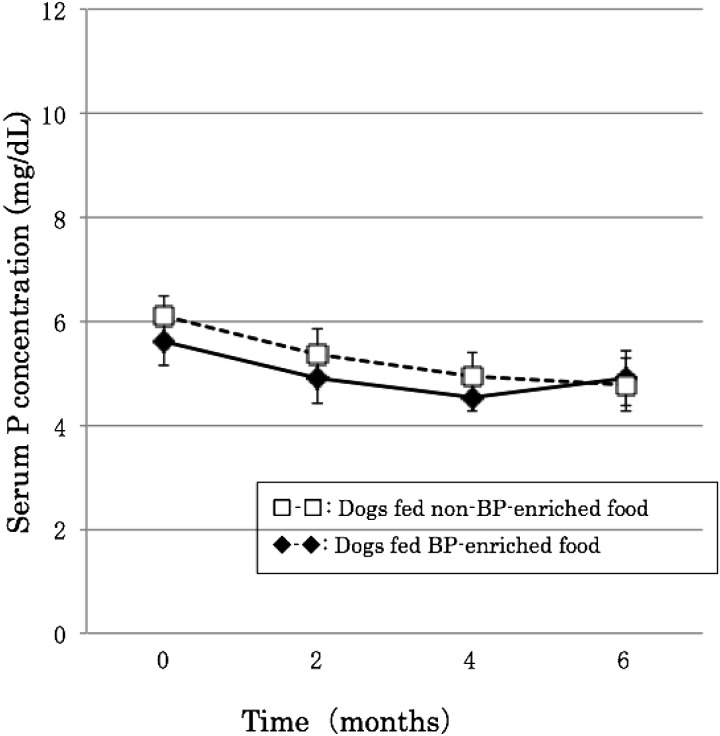
There was little change in calcium during the experimental period, which remained at around 11 mg/dl in dogs fed both BP-enriched and non-BP-enriched food, remaining within the normal range of 8–12 mg/dl. Serum potassium, on the other hand, gradually decreased after orchidectomy, although in both groups, it remained at 4–6 mg/dl, within the normal range of 2–10 mg/dl (Figs. 1 and 2).
Fig. 1.
Effects of BP on serum calcium concentration after orchidectomy.
Fig. 2.
Effects of BP on serum phosphorus concentration after orchidectomy.
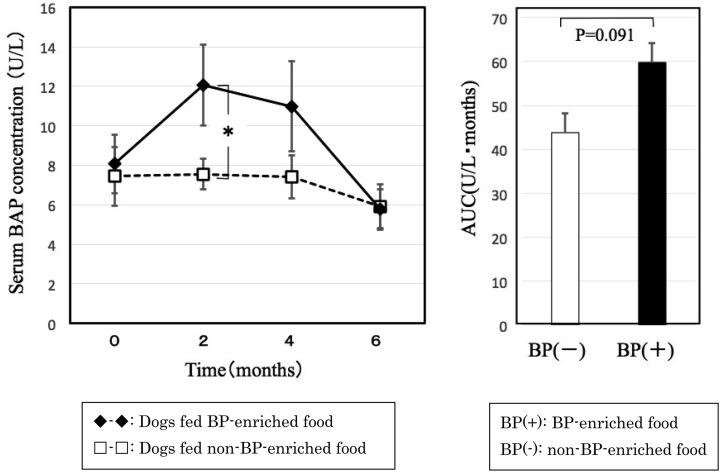
Serum BAP, a bone formation marker, was higher in dogs fed BP-enriched food than dogs fed non-BP-enriched food throughout the experimental period. In particular, serum BAP was significantly higher in dogs fed BP-enriched food than dogs fed non-BP-enriched food at 2 months after orchidectomy. However, there were no significant differences in any period when compared with pre-orchidectomy levels. The AUC was higher for dogs fed BP-enriched food than dogs fed non-BP-enriched food, but this difference was not significant, at P=0.091 (Fig. 3).
Fig. 3.
Changes in serum BAP concentration after orchidectomy. Graph on the right shows area under the curve.
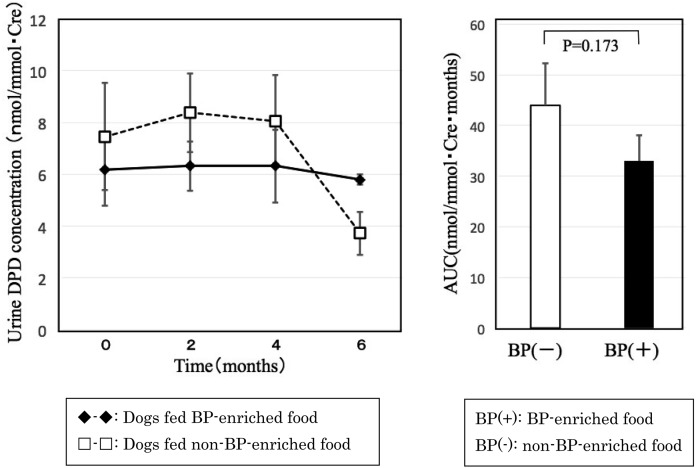
Urine DPD, a bone resorption marker, did not decrease below control level until 4 month after orchidectomy in dogs fed BP-enriched food and began decreasing below pre-orchidectomy levels at 6 months after orchidectomy. However, there were no significant differences in any period when compared with pre-orchidectomy levels. The AUC did not differ significantly between groups (Fig. 4). This suggests that adding BP to food has no effect on bone resorption.
Fig. 4.
Changes in urine DPD concentration after orchidectomy. Graph on the right shows area under the curve.
In this study, we measured serum calcium, serum phosphate, serum BAP and urine DPD as bone metabolism markers. Of these markers, BAP is an enzyme secreted by osteoblasts and released into the bloodstream as required. Its levels in blood are believed to be correlated with osteoblast count, and it is used in numerous studies as a bone formation marker [11, 15]. Urine DPD is released into the bloodstream as a result of collagen decomposition during bone breakdown, and as it is excreted in urine without having been metabolized in the body, it is used in many studies as a bone resorption marker [3, 13]. We used these markers to examine the improvements in bone metabolism in 5- to 8-month-old orchidectomized dogs given BP-enriched food.
In a previous study, Fukuda et al. orchidectomized dogs at a mean age of 2 years and investigated changes in their bone metabolism over a 1-year period [4]. They found that serum calcium increased significantly until 6 months after orchidectomy, after which it decreased. Serum phosphorus was unchanged until 6 months after orchidectomy, after which it decreased. In this study, we only monitored dogs for 6 months after orchidectomy and found no changes in calcium. Phosphorus decreased slightly, but remained within the normal range. Fukuda et al. also measured other bone formation markers, such as parathyroid hormone, calcitonin, osteocalcin and BAP, and reported that all these markers increased until 6 months after orchidectomy [4]. These findings suggest that orchidectomy enhances bone formation. We only measured BAP as a bone formation marker. We found that serum BAP did not increase in the six months after orchidectomy in dogs fed non-BP-enriched food. However, serum BAP was higher in dogs fed BP-enriched food than in dogs fed non-BP-enriched food. In particular, statistically significant differences were observed between the two groups at 2 months after orchidectomy. Our results thus differ from those of previous studies, particularly with regard to dogs fed non-BP-enriched food. The period for orchidectomy may be one factor causing this difference. The AUC for serum BAP was higher in dogs fed BP-enriched food than in dogs fed non-BP-enriched food, but this difference was not significant. Nevertheless, at P=0.091, this suggests the possibility that adding BP promotes bone formation.
The other side, the AUC for urine DPD did not differ significantly between groups. This suggests that adding BP to food has no effect on bone resorption.
Leem et al. investigated the effects of BP on bone metabolism in rats [8]. Because Mundy et al. suggested that BMP-2 is an important bone regulating factor [9], BMP-2 expression in four principal zones of the growth plate was assessed immunohistochemically in their study. They reported that both the number of BMP-2-positive cells and the intensity of BMP-2 expression in hypertrophic and ossified chondrocytes of the growth plates increased after administration of BP, as compared with the control group. Kim also reported that BP promotes the extension growth of cultured osteocytes in vitro[7]. Our results appear inconsistent with those of a rat study by Leem et al.[8]. However, as we used different markers, further studies are necessary to confirm whether the results are indeed inconsistent. The above findings suggest that BP is effective in suppressing the decline in bone formation in dogs a few months after orchidectomy.
We were unable to perform invasive measurements of bone density in this study, and it remains necessary to develop technology for non-invasive measurement of bone density.
REFERENCES
- 1.Albright F., Smith P. H., Richardson A. M.1941. Postmenopausal osteoporosis its clinical features. JAMA 116: 2465–2474. doi: 10.1001/jama.1941.02820220007002 [DOI] [Google Scholar]
- 2.Boonen S., Lips P., Bouillon R., Bischoff-Ferrari H. A., Vanderschueren D., Haentjens P.2007. Need for additional calcium to reduce the risk of hip fracture with vitamin d supplementation: evidence from a comparative metaanalysis of randomized controlled trials. J. Clin. Endocrinol. Metab. 92: 1415–1423. doi: 10.1210/jc.2006-1404 [DOI] [PubMed] [Google Scholar]
- 3.DeLaurier A., Jackson B., Ingham K., Pfeiffer D., Horton M. A., Price J. S.2002. Biochemical markers of bone turnover in the domestic cat: relationships with age and feline osteoclastic resorptive lesions. J. Nutr. 132Suppl 2: 1742S–1744S. [DOI] [PubMed] [Google Scholar]
- 4.Fukuda S., Iida H.2000. Effects of orchidectomy on bone metabolism in beagle dogs. J. Vet. Med. Sci. 62: 69–73. doi: 10.1292/jvms.62.69 [DOI] [PubMed] [Google Scholar]
- 5.Greendale G. A., Edelstein S., Barrett-Connor E.1997. Endogenous sex steroids and bone mineral density in older women and men: the Rancho Bernardo Study. J. Bone Miner. Res. 12: 1833–1843. doi: 10.1359/jbmr.1997.12.11.1833 [DOI] [PubMed] [Google Scholar]
- 6.Hori M., Takahashi H., Konno T., Inoue J., Haba T., Sakurada T., Noda T., Fujimoto K.1984. [Effect of elcatonin on experimental osteoporosis induced by ovariectomy and low calcium diet in beagles]. Nippon Yakurigaku Zasshi 84: 91–98(in Japanese). doi: 10.1254/fpj.84.91 [DOI] [PubMed] [Google Scholar]
- 7.Kim H. K., Lee S., Leem K. H.2011. Protective effect of egg yolk peptide on bone metabolism. Menopause 18: 307–313. doi: 10.1097/gme.0b013e3181f31b1f [DOI] [PubMed] [Google Scholar]
- 8.Leem K. H., Kim M. G., Kim H. M., Kim M., Lee Y. J., Kim H. K.2004. Effects of egg yolk proteins on the longitudinal bone growth of adolescent male rats. Biosci. Biotechnol. Biochem. 68: 2388–2390. doi: 10.1271/bbb.68.2388 [DOI] [PubMed] [Google Scholar]
- 9.Mundy G., Garrett R., Harris S., Chan J., Chen D., Rossini G., Boyce B., Zhao M., Gutierrez G.1999. Stimulation of bone formation in vitro and in rodents by statins. Science 286: 1946–1949. doi: 10.1126/science.286.5446.1946 [DOI] [PubMed] [Google Scholar]
- 10.Notoya K., Yoshida K., Tsukuda R., Taketomi S.1994. Effect of ipriflavone on expression of markers characteristic of the osteoblast phenotype in rat bone marrow stromal cell culture. J. Bone Miner. Res. 9: 395–400. doi: 10.1002/jbmr.5650090315 [DOI] [PubMed] [Google Scholar]
- 11.Ohishi T., Kushida K., Takahashi M., Kawana K., Yagi K., Kawakami K., Horiuchi K., Inoue T.1994. Urinary bone resorption markers in patients with metabolic bone disorders. Bone 15: 15–20. doi: 10.1016/8756-3282(94)90885-0 [DOI] [PubMed] [Google Scholar]
- 12.Salmeri K. R., Bloomberg M. S., Scruggs S. L., Shille V.1991. Gonadectomy in immature dogs: effects on skeletal, physical, and behavioral development. J. Am. Vet. Med. Assoc. 198: 1193–1203. [PubMed] [Google Scholar]
- 13.Sanecki R. K., Hoffmann W. E., Hansen R., Schaeffer D. J.1993. Quantification of bone alkaline phosphatase in canine serum. Vet. Clin. Pathol. 22: 17–23. doi: 10.1111/j.1939-165X.1993.tb00871.x [DOI] [PubMed] [Google Scholar]
- 14.Spain C. V., Scarlett J. M., Houpt K. A.2004. Long-term risks and benefits of early-age gonadectomy in dogs. J. Am. Vet. Med. Assoc. 224: 380–387. doi: 10.2460/javma.2004.224.380 [DOI] [PubMed] [Google Scholar]
- 15.Uebelhart D., Gineyts E., Chapuy M. C., Delmas P. D.1990. Urinary excretion of pyridinium crosslinks: a new marker of bone resorption in metabolic bone disease. Bone Miner. 8: 87–96. doi: 10.1016/0169-6009(91)90143-N [DOI] [PubMed] [Google Scholar]