Highlights
-
•
The importance of Neospora caninum-associated disease in wildlife is reviewed.
-
•
There are only 12 reports of clinical neosporosis in wildlife species to date.
-
•
The best practice guidelines to follow for reporting wildlife cases of neosporosis are presented.
Keywords: Neospora caninum, Neosporosis, Wildlife, Nondomestic species, Clinical signs, Pathology
Graphical Abstract
Abstract
Neospora caninum is an apicomplexan parasite that is the etiologic agent of neosporosis, a devastating infectious disease regarded as a major cause of reproductive loss in cattle and neuromuscular disease in dogs worldwide. This protozoan pathogen is maintained in the environment by a heteroxenous life cycle that involves a definitive canid host and a wide range of intermediate hosts. In recent years, a number of wildlife species have been investigated for their possible involvement in the N. caninum life cycle and many have been implicated as intermediate hosts. However, in many instances these studies have utilized serological and molecular techniques to detect infection in clinically normal animals, and investigation of possible associated morbidity, mortality, and pathology has been neglected. As such, the occurrence and importance of Neospora-associated disease in wildlife species are unknown. In order to improve our understanding of the significance of N. caninum infection in nondomestic species, the present review provides an up-to-date summary of clinical neosporosis and N. caninum-associated pathologic lesions in naturally and experimentally infected wildlife species. We provide a list of all free-ranging and captive wildlife species identified with N. caninum infection to date using currently available diagnostic tools. The advantages and disadvantages of diagnostic methods in wildlife are addressed in order to recommend optimal diagnosis of confirming N. caninum infection and neosporosis in nondomestic species. Although current data would suggest that N. caninum infection does not adversely impact wildlife populations, there is a need for greater international uniformity in the diagnosis of N. caninum infection and neosporosis in nondomestic species in order to assess the true consequences of parasite infection.
1. Introduction
Neospora caninum (Apicomplexa: Coccidia), the etiologic agent of the polysystemic disease neosporosis, is an obligate intracellular tissue cyst-forming coccidian parasite of the phylum Apicomplexa (Dubey et al., 2007; Dubey and Schares, 2011). Neospora caninum shares many morphologic and biologic features with its close relative Toxoplasma gondii (Dubey et al., 2002, 2007; Dubey and Schares, 2011). Prior to its initial recognition in Norwegian dogs in 1984 (Bjerkas et al., 1984) and consequential classification as a distinct species in 1988 (Dubey et al., 1988), many N. caninum infections were misdiagnosed as toxoplasmosis (Dubey et al., 2002; Dubey and Schares, 2011). Key differences were subsequently identified that distinguish the two parasites with regard to their natural host range, antigenicity, virulence factors, and pathogenesis (for reviews, see Dubey and Lindsay, 1996; Dubey et al., 2002; Dubey et al., 2007). Differences between N. caninum and T. gondii have also been described using comparative genomics and transcriptomics analyses (Reid et al., 2012). In the past two decades N. caninum has been extensively investigated due to its importance as a veterinary pathogen. As a result of these studies, it is now known that N. caninum has a global distribution and causes severe neuromuscular disease in dogs, and abortion and neonatal mortality in cattle, resulting in devastating economic losses to the beef and dairy industries (Dubey et al., 2007; Dubey and Schares, 2011; Reichel et al., 2013).
Less is known about the epizootiology and impact of this parasite in wildlife (reviewed by Gondim, 2006; Dubey et al., 2007; Dubey and Schares, 2011; Almeria, 2013). Most studies of N. caninum infection in wildlife species report on the prevalence of infection using serologic and/or molecular diagnostic assays in asymptomatic animals. While helpful in documenting evidence of exposure to the pathogen amongst wildlife species, these studies do not provide insight into the nature of the host–pathogen interactions in these potential intermediate hosts. In some instances, these analyses are also limited by the uncertainty regarding the sensitivity and specificity of the assays used.
This review provides a critical analysis of clinical neosporosis and related pathologic findings in free-ranging and captive wildlife species for which postmortem analyses of gross and microscopic lesions have been described. Building upon the current literature, this paper aims to improve our knowledge of the host–pathogen interactions in wildlife by (1) reviewing the prevalence of clinical neosporosis as an outcome of infection with N. caninum in nondomestic species and the factors that predispose to pathologic sequelae, (2) examining our current understanding of the impact of N. caninum infection on wildlife populations, and (3) formulating best practice guidelines for documenting N. caninum infection and neosporosis in wildlife. Neospora caninum seroprevalence and molecular diagnostic studies in nondomestic species have been well reviewed (Gondim, 2006; Dubey et al., 2007; Dubey and Schares, 2011; Almeria, 2013) and, unless specifically associated with pathology or clinical disease, the details of these reviews will not be reiterated here.
2. Life cycle and transmission: domestic and sylvatic cycles
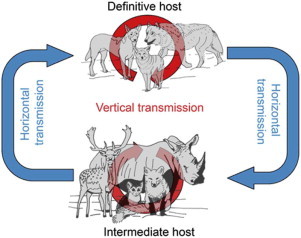
Neospora caninum is characterized by a complex facultative heteroxenous life cycle that involves a definitive canid host in which sexual replication occurs, and a range of intermediate hosts in which asexual replication takes place (Dubey and Lindsay, 1996; Dubey et al., 2006, 2007; Dubey and Schares, 2011). To date, the only confirmed definitive hosts of N. caninum are members of the Canis genus, including domestic and wild dogs (Canis familiaris) (McAllister et al., 1998), coyotes (Canis latrans) (Gondim et al., 2004b), gray wolves (Canis lupus lupus) (Dubey et al., 2011), and dingoes (Canis lupus dingo) (King et al., 2010). Cattle are the most common intermediate host of N. caninum; however, in recent years infection has been reported in many warm-blooded vertebrate species – some with the potential to serve as intermediate hosts in domestic and sylvatic transmission cycles (Gondim, 2006; Dubey et al., 2007; Dubey and Schares, 2011; Almeria, 2013). Neospora caninum is not considered to be zoonotic despite some serologic evidence of human exposure, particularly in immunocompromised populations (Tranas et al., 1999; Lobato et al., 2006; Barratt et al., 2010).
The N. caninum life cycle is characterized by three known infectious life stages: sporozoites within sporulated oocysts, rapidly dividing tachyzoites, and slowly proliferating bradyzoites within tissue cysts (Dubey et al., 2006). Light microscopic and ultrastructural morphology of these stages have been well described, with the notable exception of sporulated oocysts for which ultrastructural description is still lacking (Dubey and Lindsay, 1996; Speer et al., 1999; Dubey et al., 2002, 2006; Dubey, 2003). Oocysts are the environmentally resistant form of the parasite. They are presumably generated by sexual replication in the intestinal epithelial cells of the definitive host and expelled in the feces in an unsporulated (noninfectious) form, although entero-epithelial sexual stages in the canine alimentary tract have yet to be conclusively demonstrated (Dubey et al., 2004). Outside the host, oocysts undergo sporulation in 24–72 hours and develop two sporocysts, each of which contains four sporozoites, which render them orally infectious (Dubey et al., 2006, 2007; Reichel et al., 2007). Host infection may ensue when sporulated oocysts are ingested. In the gastrointestinal tract, sporozoites are released by excystation and parasitize the intestine, where they transform into tachyzoites (Hemphill et al., 2006). Tachyzoites are capable of infecting a wide range of nucleated host cells, including mononuclear cells that likely aid in parasite dissemination via leukocyte trafficking (Dubey et al., 2006; Hemphill et al., 2006). Within host cells, tachyzoites reside and replicate within a parasitophorous vacuole, an intracellular compartment formed from the host cell membrane with parasite modifications that preclude its fusion with endocytic vesicles (Buxton et al., 2002; Hemphill et al., 2006). During the acute phase of infection, tachyzoites may be found in virtually all host tissues and it is during this stage that progressive cycles of intracellular replication, infected host cell lysis, tachyzoite release and infection of surrounding cells, and related immunopathologic sequelae initiate lesion formation and, in some animals, clinical disease (Dubey et al., 2007). In an immunocompetent host, tachyzoites replicate for an estimated 20 divisions before they differentiate into bradyzoites, the quiescent life stage of the parasite that forms under host immune pressure and produces a tissue cyst (Goodswen et al., 2013).
Tissue cysts shelter bradyzoites from host immunological factors and facilitate long term parasite persistence and chronic asymptomatic infections (Hemphill et al., 2006; Dubey et al., 2007; Dubey and Schares, 2011). Recrudescence of infection may transpire with changes in host immune status (immunomodulation or immunosuppression) that may result in reactivation of bradyzoites and conversion to tachyzoites (Hemphill et al., 2006). This is well documented to occur in pregnant animals and permits tachyzoite spread to other tissues, including dissemination across the placenta and infection of the unborn fetus (Williams et al., 2009).
Natural N. caninum infections occur via horizontal or vertical transmission (Dubey et al., 2007). Vertical transmission (also referred to as transplacental or congenital transmission) is the predominant mode of transmission in cattle and other domesticated bovine species. Two forms of vertical transmission are recognized: exogenous and endogenous transplacental transmission (Trees and Williams, 2005; Williams et al., 2009). Exogenous transplacental transmission ensues following ingestion of sporulated oocysts by naïve cattle and is associated with epidemic abortion storms in a herd (Williams et al., 2009). Endogenous transplacental transmission follows recrudescence of infection in a persistently infected cow during pregnancy and is the principle mechanism responsible for maintaining the parasite within cattle populations, resulting in fetal transmission rates as high as 95% (Dubey et al., 2006; Williams et al., 2009; Reichel et al., 2013). Once infected, a cow remains infected for life and may pass infection to consecutive generations of offspring (Dubey and Schares, 2006; Dubey et al., 2006). Seropositive cattle have an increased risk of abortion estimated to be between 1.7 and 7.4 fold (Thurmond and Hietala, 1997). However, abortion risk diminishes with increasing parity, suggesting that some degree of host immunity develops over time, impeding the success of endogenous transplacental transmission (Thurmond and Hietala, 1997; Dubey et al., 2006, 2007). While vertical transmission of the pathogen dominates in cattle and horizontal (postnatal) transmission rates are largely considered infrequent, theoretical mathematical models indicate that some degree of horizontal transmission (ingestion of oocysts from the definitive host) is required to perpetuate N. caninum infection in a herd (French et al., 1999; Reichel et al., 2013).
In the definitive canid host and other carnivores, horizontal transmission may result from ingestion of tissue from infected intermediate hosts containing tissue cysts, or from sporulated oocyst-contaminated food or water (Dubey et al., 2006, 2007). Dogs have been shown to shed oocysts following consumption of infected offal or expelled placental membranes from infected cows (Williams et al., 2009). Vertical transmission also occurs in dogs. Subclinically infected bitches can transmit N. caninum parasites to their offspring in successive litters; however, transplacental infection on its own is considered less important in sustaining infection in canine populations (Barber and Trees, 1996; Dubey, 2003; Dubey et al., 2005, 2006, 2007; Reichel et al., 2007; Dubey and Schares, 2011; Goodswen et al., 2013).
Experimentally there is evidence that lactogenic transmission occurs in calves, but this mode of transmission has not been demonstrated under natural circumstances in calves or dogs (Davison et al., 2001; Dijkstra et al., 2001; Dubey et al., 2007). Neospora caninum DNA, but not viable organisms, has been found in semen collected from naturally exposed bulls and although theoretically possible, venereal transmission via contaminated sperm is considered unlikely (Dubey et al., 2007).
Worldwide, studies indicate that the N. caninum life cycle is maintained in a domestic cycle between dogs and cattle (Dubey et al., 2007). They show a strong correlation between the presence of domestic dogs and the incidence of neosporosis on cattle farms (McAllister et al., 1998; Sawada et al., 1998; Wouda et al., 1999; Basso et al., 2001; Moore et al., 2002; Otranto et al., 2003; Hobson et al., 2005; Wanha et al., 2005; Bartels et al., 2007; Dubey et al., 2007; Malmasi et al., 2007; Collantes-Fernandez et al., 2008). Compelling evidence suggests a sylvatic cycle involving domestic and wild canids, and ruminant and herbivore species occur in North America (McAllister et al., 1998; Barling et al., 2000; Gondim et al., 2004b; Gondim, 2006). This is supported by several studies that report high N. caninum seroprevalence in North American white-tailed deer, the isolation of viable N. caninum from this host, and that dogs fed brain from experimentally infected white-tailed deer shed oocysts infective to cattle (reviewed by Dubey et al., 2013). White-tailed deer are extensively hunted in North America and because most are eviscerated where they are killed, infected deer tissues (e.g., offal) may provide an important source of infection for domestic and wild canid definitive hosts (Dubey et al., 2007). Additionally, North American beef herds grazed in close proximity to coyotes have a higher risk of N. caninum infection, signifying that transmission may be occurring between these species (Barling et al., 2000; Gondim et al., 2004b; Dubey et al., 2007). Identification of seropositive cattle from areas where domestic dogs are not known to be present provides additional evidence to support the possibility of a sylvatic transmission cycle between wild canids and cattle (McAllister et al., 1998; Ferroglio et al., 2003; Gondim et al., 2004b).
Less is known about the existence of sylvatic cycles in other countries. In Australia, a sylvatic cycle between dingoes and macropod species has been postulated by King et al. (2011b). Indirect support for this hypothesis is provided by a recent serological survey of western grey kangaroos (Macropus fuliginosus ocydromus) from Western Australia that demonstrated a N. caninum seroprevalence of 18% (Mayberry et al., 2014), and by experimental N. caninum infection in the fat-tailed dunnart (Sminthopsis crassicaudata) showing it to be a highly susceptible intermediate host (King et al., 2011a). In Europe, similar sylvatic cycles involving wild ruminants and canids seem plausible but little evidence currently exists to support this hypothesis. One study conducted in Hungary showed farm dogs consuming potentially infected offal from aborted or dead calves, or raw game animals demonstrated a high rate of N. caninum seropositivity (Hornok et al., 2006). Furthermore, N. caninum antibodies and/or DNA have been detected in a number of European free-ranging carnivore and non-carnivore wildlife species (reviewed by Almeria, 2013).
3. What is the outcome of infection in experimental models and is it relevant to neosporosis in wildlife?
Reports of N. caninum infection in domestic and nondomestic animals appear to be common, yet there are relatively few reports of clinical neosporosis in any wildlife species (Dubey and Schares, 2011; Goodswen et al., 2013). The reason for this discrepancy is not well understood. Extrapolating from what is known about the more intensively studied T. gondii, multiple different host (species, genotype, age, and immune status) and parasite (dose, strain, life stage initiating infection, and route of infection) factors may account for the wide variation in clinical manifestations observed in N. caninum infection (Dubey, 2010). In recent years, there has been increasing interest in clarifying the role of the host immune response and parasite strain on infection outcome in order to better understand components influencing control of infection and disease pathogenesis.
3.1. Host immune response
In concordance with other intracellular pathogens, data from numerous in vitro and in vivo immunological studies in cattle and mice collectively indicate that protective host immunity induced by N. caninum infection is typified by a T helper 1 (Th1)-type response. In the acute stage of infection, this is mediated largely by interferon (IFN)-γ production from natural killer (NK) cells, and interleukin (IL)-12 primed CD4+ lymphocytes and CD8+ lymphocytes (for reviews, see Innes et al., 2002; Innes et al., 2005; Hemphill et al., 2006; Klevar et al., 2007; Monney and Hemphill, 2014). This pro-inflammatory Th1-type response appears to be essential for restricting parasite replication and induction of latent infection through stage conversion and formation of tissue cysts (Buxton et al., 2002; Innes et al., 2002; Dubey et al., 2006; Hemphill et al., 2006; Williams et al., 2009; Goodswen et al., 2013). In contrast, host immunomodulation, as may occur during pregnancy, favors a bias towards a Th2-type immune response characterized by increased expression of IL-4, and is associated with a bradyzoite to tachyzoite transformation (recrudescence), uncontrolled parasite replication, and corresponding pathologic effects (Buxton et al., 2002; Innes et al., 2002, 2005; Dubey et al., 2006; Hemphill et al., 2006; Williams et al., 2009; Goodswen et al., 2013). Humoral (antibody) immunity probably participates in controlling N. caninum infection via mechanisms involving antibody-neutralization of extracellular tachyzoites (Innes et al., 2002; Hemphill et al., 2006; Bartley et al., 2013a; Goodswen et al., 2013).
Immunological studies may be crucial to understanding the pathogenesis of neosporosis and outcome of parasite infection, but investigations of host immunity are challenging. The host immune response to N. caninum has been exclusively studied using bovine and murine experimental models, and while valuable for investigating the role of the host immune response, the true significance of findings obtained in these models can only be conclusively assessed in other host species. In many instances, by necessity, experiments have used tachyzoites to infect animals parenterally rather than the natural enteral route of infection by sporocysts or sporozoites because of the extreme difficulty in obtaining N. caninum oocysts from experimentally infected dogs (Dubey et al., 2007; Dubey and Schares, 2011) . While parasite life stage and mode of infection have been shown to impact the host immune response to T. gondii infection, whether or not these factors also modulate the host immune response to N. caninum infection is uncertain and necessitates additional investigation (Dubey, 2010). Some of the outstanding questions in the field of the N. caninum immunobiology involve understanding how various components of the host immune system interact with each other and with N. caninum infected cells to control parasite replication.
The understanding of why different hosts and individuals are more susceptible to neosporosis is growing. It has long been recognized that there is significant variation in species susceptibility to N. caninum infection. For example, gerbils are experimentally highly susceptible to developing clinical neosporosis while outbred mice are largely resistant to infection (Pipano et al., 2002). The reason for this difference is poorly understood, although presumably interspecies variation in immunity plays an important role because chemical immunosuppression (i.e., prednisolone treatment) of resistant mice produces disease (Lindsay and Dubey, 1989). Recent studies have provided evidence that intraspecific variations in host immunity can influence outcome of N. caninum infection. Differences in the humoral immune response have been described for different breeds of cattle (Santolaria et al., 2011) and strains of mice (Mols-Vorstermans et al., 2013) and, in the latter case, were linked to specific major histocompatibility complex (MHC) class I haplotypes. A retrospective study of Holstein cattle found MHC class II alleles DRB3*1001 and DRB3*2703 to be associated with pregnancy loss and resistance, respectively, regardless of serological status (Schwab et al., 2009). Advances in molecular techniques in recent years have enabled genetic characterization of genes associated with the host immune response, and consequently intra- and interspecific differences in innate and adaptive immune responses have been elucidated for a number of species (Litman et al., 2010; Belov et al., 2013; Buchmann, 2014). However, more research is needed to assess how these factors influence the host–pathogen interactions between N. caninum infection and wildlife.
3.2. Strain virulence and genetic variability
Significant variations in pathogenicity (virulence) and growth rates for different N. caninum isolates have been demonstrated in experimentally infected mice, gerbils, sheep and cattle (for review, see Al-Qassab et al., 2010). For example, Nc-Liverpool-infected mice exhibit more severe central nervous system inflammation and necrosis than mice infected with Nc-Nowra and Nc-SweB1 (Atkinson et al., 1999; Miller et al., 2002), and infection of pregnant cows with NC1 produces fetal death while no fetal death is observed in Nc-Spain 1H-infected cows (Rojo-Montejo et al., 2009). There is also evidence that different strains of N. caninum can influence the host immune response and subsequently infection outcome (Atkinson et al., 1999; Costa et al., 2008; Al-Qassab et al., 2010; Dellarupe et al., 2014; Regidor-Cerrillo et al., 2014). Some studies have shown differential immunoglobulin expression in mice and heifers infected with different parasite isolates and, although this appears to be linked to infection dynamics and clinical outcome, a direct causal relationship has yet to be identified (Atkinson et al., 1999; Costa et al., 2008; Rojo-Montejo et al., 2009; Dellarupe et al., 2014).
Interpretation of comparative N. caninum strain virulence studies is confounded by several factors which may affect pathogenicity including the parasite life stage used to initiate infection, route of inoculation, inoculum dose, and possible mitigation of virulence and other biological characteristics in cell culture derived parasites (Dubey et al., 2007; Al-Qassab et al., 2010; Dubey and Schares, 2011). As such, direct comparison between the information gleaned from these studies is often not possible, and whether or not virulence studies in mice and cattle will mimic the effect of N. caninum infection in other host species requires further investigation.
Most virulence studies have been conducted using isolates from clinically affected animals or neonatal infected animals, and little is known about the differences between virulent and avirulent strains, although as a result of genotyping technology this is beginning to change (Rojo-Montejo et al., 2009; Al-Qassab et al., 2010; Dubey and Schares, 2011; Regidor-Cerrillo et al., 2013). Increasingly, investigations are focusing on identifying genetic components that may impact strain pathogenicity and disease manifestations (Weiss et al., 1999; Gondim et al., 2004c; McInnes et al., 2006; Al-Qassab et al., 2010; Goodswen et al., 2013). In recent years, multilocus mini- and microsatellite analyses have proven to be useful tools for the genetic characterization of many protozoan organisms, including T. gondii, and are currently considered the gold standard for N. caninum genotyping (Al-Qassab et al., 2010; King et al., 2012; Goodswen et al., 2013). To date, over 100 different N. caninum strains have been analyzed using mini- and microsatellite technology, and data show that this parasite exists as a diverse heterogenous population and that different strains exhibit extensive genetic diversity worldwide despite sharing similar light microscopic and ultrastructure features (Regidor-Cerrillo et al., 2006, 2013; Basso et al., 2009, 2010; Al-Qassab et al., 2010; Goodswen et al., 2013). An association between this genetic diversity of N. caninum isolates and their pathogenicity has not been conclusively demonstrated and it remains unclear if the genetic structure of the parasite is a determinant of host clinical manifestations (Al-Qassab et al., 2010; Goodswen et al., 2013; Regidor-Cerrillo et al., 2013).
Mini- and microsatellite genotyping techniques have been successively applied to epidemiological and population genetic investigations of strains of N. caninum in domestic animals (Regidor-Cerrillo et al., 2006, 2013; Basso et al., 2009, 2010; Al-Qassab et al., 2010). Genotyping technology has rarely been applied to wildlife N. caninum strains but it has potential to provide valuable insight for future epidemiological investigations in captive and free ranging nondomestic species, establishing the importance of sylvatic and domestic cycles in neosporosis, and evaluating the emergence of new N. caninum isolates in wildlife populations.
4. Neosporosis in domestic animals
4.1. Overview of disease in cattle
Neospora caninum is a primary pathogen in cattles and abortion is the principal clinical manifestation (Dubey, 2003; Dubey et al., 2007; Dubey and Schares, 2011). Apart from fetal loss, morbidity and mortality are generally not observed in infected cows (Dubey et al., 2006, 2007). The majority of fetuses in seropositive dams will be infected via transplacental transmission and infection outcome is largely dependent on stage of gestation and therefore fetal age (Buxton et al., 2002; Innes et al., 2002; Dubey et al., 2006, 2007; Dubey and Schares, 2011; Goodswen et al., 2013). If infection occurs during the first 100 days of gestation when fetal lymphoid tissues are developing, survival is unlikely and fetal resorption or mummification results (Dubey, 2003; Dubey et al., 2006, 2007; Dubey and Schares, 2011). During the second and third trimesters, the fetus has an increasing capacity to mount an immune response but in many instances it appears insufficient as abortion is the most common sequela of infection in mid-gestation, particularly between 5 and 7 months (Buxton et al., 2002; Reichel et al., 2013). If infection occurs in the third trimester after fetal immunity is more developed, the birth of an infected but clinically normal calf is the most likely outcome (Buxton et al., 2002; Innes et al., 2005; Dubey et al., 2006), although birth of uninfected calves may infrequently occur (Gibney et al., 2008).
Aborted fetuses are predominately autolyzed but may also be expelled fresh or mummified and generally do not exhibit specific gross lesions (Dubey, 2003; Dubey et al., 2006). In some cases, fetal necropsy may reveal hydrocephalus, cerebellar and medulla hypoplasia, and scattered pale to dark foci in the brain, spinal cord, heart, and skeletal muscle that correlate histologically with areas of necrosis and inflammation (Dubey, 2003; Dubey et al., 2006; Schlafer and Miller, 2007). The most significant and distinctive histologic lesions of fetal neosporosis are found in the central nervous system (CNS) and consist of multifocal nonsuppurative encephalomyelitis with perivascular cuffs, necrosis, and microgliosis (Helman et al., 1998; Slapeta et al., 2003; Dubey et al., 2006). In addition, multifocal nonsuppurative myocarditis and myositis, and nonsuppurative periportal hepatitis with variable hepatic necrosis can be observed (Helman et al., 1998; Dubey, 2003; Dubey and Schares, 2006; Dubey et al., 2006). Nonsuppurative interstitial nephritis, interstitial pneumonia, and adrenal necrosis are reported less frequently (Barr et al., 1991; Dubey et al., 2006; Schlafer and Miller, 2007; Nishimura et al., 2013). The presence of intralesional tissue cysts and/or tachyzoites is inconsistent and may be challenging to detect with standard hematoxylin and eosin (H&E) stains; the use of N. caninum specific immunohistochemistry (IHC) greatly facilitates parasite identification (Barr et al., 1991; Dubey and Schares, 2006; Dubey et al., 2006). Protozoan cysts and tachyzoites are most likely to be found in the brain and spinal cord, if present (Dubey et al., 2006; Nishimura et al., 2013). Intracellular tachyzoites can also be recognized in myocytes and myocardial Purkinje fibers (Dubey et al., 1990, 2006; Barr et al., 1991). The placenta may be histologically normal or contain areas of cotyledonary necrosis associated with mononuclear inflammation and occasional clusters of tachyzoites within trophoblasts (Dubey et al., 1990, 2006; Schlafer and Miller, 2007).
Clinically affected congenitally infected calves may exhibit one or more of the following features: small size for gestational age, ataxia, proprioceptive deficits, inability to stand, hyperextension or flexion of forelimbs and/or hindlimbs, exophthalmia, scoliosis, hydrocephalus, and spinal cord narrowing (Dubey, 2003; Dubey et al., 2006; Schlafer and Miller, 2007). Mononuclear encephalomyelitis is the predominant histologic finding (Dubey and Schares, 2006; Dubey et al., 2006). CNS lesions are more frequently detected in the spinal cord than the brain, and tissue cysts are more common than tachyzoites (Dubey et al., 2006). Extraneural lesions are rare but may include myositis, nephritis, and pneumonia (Dubey et al., 2006).
4.2. Overview of disease in dogs
Neosporosis most frequently manifests as neuromuscular disease in young dogs, but dogs of any age may be affected (Ruehlmann et al., 1995; Barber and Trees, 1996; Buxton et al., 2002; Dubey, 2003; Reichel et al., 2007). Two main neurological presentations are recognized: polymyositis-polyradiculoneuritis and encephalomyelitis (Barber and Trees, 1996; Reichel et al., 2007).
Protozoal polymyositis-polyradiculoneuritis is most commonly seen in transplacentally infected young dogs 5 weeks to 6 months of age and may involve multiple littermates (Barber and Trees, 1996; Buxton et al., 2002; Reichel et al., 2007). Neospora caninum has a predilection for the lumbosacral spinal nerve roots in young dogs, and consequently pelvic limb muscle atrophy and arthrogryposis develop (Reichel et al., 2007; Garosi et al., 2010). Clinical signs include ascending hindlimb paralysis, muscle atrophy, rigid hyperextension of the pelvic limb, cervical weakness, and dysphagia (Buxton et al., 2002; Dubey, 2003; Reichel et al., 2007; Garosi et al., 2010). Histopathology may reveal nonsuppurative polyradiculoneuritis and polymyositis with intralesional tachyzoites and cysts (Barber and Trees, 1996; Dubey, 2003; Reichel et al., 2007).
In contrast, protozoal encephalomyelitis is more likely to affect adult dogs and is characterized by a more variable clinical presentation that ranges from localized CNS signs to widespread CNS involvement and disseminated disease (Barber and Trees, 1996; Reichel et al., 2007). Reported neurological clinical signs include hind limb paresis and paralysis, head tilt, seizures, ataxia, dysphagia, incontinence, seizures, and ocular abnormalities including miosis, diminished pupillary light reflexes, anisocoria, and enophthalmos (Barber and Trees, 1996; Buxton et al., 2002; Garosi et al., 2010). Gross necropsy lesions are infrequently described, although cerebellar atrophy has been noted in a few cases (Jackson et al., 1995; Cantile and Arispici, 2002; Lorenzo et al., 2002; Garosi et al., 2010). The most characteristic histopathologic lesion of protozoal encephalomyelitis is a multifocal nonsuppurative variably necrotizing meningoencephalomyelitis with tachyzoites and tissue cysts occasionally identified in neurons and neuropil (Barber and Trees, 1996; Dubey, 2003; Dubey et al., 2006; Brown et al., 2007; Reichel et al., 2007).
Although principally recognized as a neuromuscular disease in dogs, neosporosis can induce a variety of less common lesions including myocarditis, polymyositis, pancreatitis, and interstitial pneumonia with pulmonary edema and alveolitis, depending on the cells parasitized (Barber and Trees, 1996; Buxton et al., 2002; Garosi et al., 2010). Additionally, dermal neosporosis is becoming increasingly recognized as a unique presentation of N. caninum infection (Dubey and Lindsay, 1996; La Perle et al., 2001; McInnes et al., 2006; Dubey and Schares, 2011; Dubey et al., 2014b). It has been postulated that the cutaneous manifestation of disease may be precipitated by underlying immunosuppression, possibly related to drug therapy or concurrent disease (La Perle et al., 2001; Buxton et al., 2002; Ordeix et al., 2002; Boyd et al., 2005; McInnes et al., 2006; Dubey et al., 2014b). Macroscopic lesions are those of a multifocal to generalized ulcerative and nodular dermatitis characterized histologically by pyogranulomatous inflammatory infiltrates with variable admixed eosinophilic infiltrates, necrosis and hemorrhage (La Perle et al., 2001; Ordeix et al., 2002; Boyd et al., 2005). Myriad tachyzoites are observed in macrophages, keratinocytes, and neutrophils, and to a lesser extent, endothelial cells and fibroblasts (La Perle et al., 2001; Boyd et al., 2005; Ginn et al., 2007). Tissue cysts are not recognized in lesions of cutaneous neosporosis (La Perle et al., 2001; Ordeix et al., 2002; Boyd et al., 2005; Ginn et al., 2007).
5. Naturally acquired neosporosis and pathology in wildlife species
5.1. Eutherian mammals
5.1.1. Carnivora
Carnivores have been extensively investigated for shedding N. caninum oocysts, implying a potential role as definitive hosts of N. caninum. Naturally occurring oocysts of N. caninum have been identified only in North American coyotes (Canis latrans) (Gondim et al., 2004b; Wapenaar et al., 2006), domestic dog–dingo hybrids living in remote Aboriginal communities in Australia (King et al., 2010, 2012), and gray wolves (C. lupus lupus) (Dubey et al., 2011).
In addition to the above confirmed definitive hosts (Barber and Trees, 1996; Lindsay et al., 1996; Gondim et al., 2004a, 2004b; Dubey and Thulliez, 2005; Steinman et al., 2006; Wapenaar et al., 2007; Sobrino et al., 2008; Almberg et al., 2009; Bjorkman et al., 2010; Stieve et al., 2010; Bevins et al., 2013; Dubey et al., 2014a), N. caninum antibodies (Ab) and/or DNA have been detected in the following free-ranging carnivores: Iberian wolf (Canis lupus signatus) (Ab) (Sobrino et al., 2008), golden jackal (Canis aureus) (Ab) (Steinman et al., 2006), African wild dogs (Lycaon pictus) (Ab) (Woodroffe et al., 2012), red fox (Vulpes vulpes) (Ab, DNA) (Barber et al., 1997; Buxton et al., 1997; Simpson et al., 1997; Schares et al., 2001; Wolfe et al., 2001; Almeria et al., 2002; Hamilton et al., 2005; Hurkova and Modry, 2006; Steinman et al., 2006; Jakubek et al., 2007; Murphy et al., 2007; Wapenaar et al., 2007; Marco et al., 2008; Sobrino et al., 2008; De Craeye et al., 2011; Bartley et al., 2013b; Stuart et al., 2013; Dubey et al., 2014b), Culpeo fox (Dusicyon culpaeus) (Ab) (Martino et al., 2004), South American gray fox (Dusicyon griseus) (Ab) (Martino et al., 2004), North American gray fox (Urocyon cinereoenteus) (Ab) (Lindsay et al., 1996), Azara's fox (Lycalopex gymnocercus) (Ab) (Canon-Franco et al., 2004), crab-eating fox (Cerdocyon thous) (Ab) (Canon-Franco et al., 2004), European brown bear (Ursus arctos) (DNA) (Cobadiova et al., 2013), spotted hyena (Crocuta crocuta) (Ab) (Ferroglio et al., 2003), raccoon (Procyon lotor) (Ab, DNA) (Lindsay et al., 2001; Lemberger et al., 2005), raccoon dog (Nyctereute procyonoides) (Ab) (Kim et al., 2003), stone martin (Martes foina) (Ab) (Sobrino et al., 2008), pine martin (Martes martes) (Ab) (Sobrino et al., 2008), Eurasian badger (Meles meles) (Ab, DNA) (Sobrino et al., 2008; Bartley et al., 2013b), polecat (Mustella putorius) (Ab, DNA) (Sobrino et al., 2008; Bartley et al., 2013b), ferret (Mustela furo) (DNA) (Bartley et al., 2013b), American mink (Neovison vison) (Ab, DNA) (Bartley et al., 2013b; Stuart et al., 2013), European otter (Lutra lutra) (DNA) (Stuart et al., 2013), sea otter (Enhydra lutris neresis) (Dubey et al., 2003; Miller et al., 2010) common genet (Genetta genetta) (Ab) (Sobrino et al., 2008), Egyptian mongoose (Herpestes ichneumon) (Ab) (Sobrino et al., 2008; Millan et al., 2009), Eurasian wild cat (Felis silvestris silvestris) (Ab) (Sobrino et al., 2008), Iberian lynx (Lynx pardinus) (Ab) (Sobrino et al., 2008), cheetah (Acinonyx jubatus) (Ab) (Cheadle et al., 1999; Ferroglio et al., 2003), and lion (Panthera leo) (Ab) (Cheadle et al., 1999; Ferroglio et al., 2003). Viable N. caninum tachyzoites (isolates NcWolfMn1 and NcWolfMc2) have been recently isolated from the brains of two free-ranging gray wolves (Dubey et al., 2014a).
Neospora caninum antibodies have been detected in the following captive wild carnivores: European wolf (Canis lupus lupus) (Sedlak and Bartova, 2006; Andre et al., 2010), maned wolf (Chrysocyon brachyurus) (Vitaliano et al., 2004; Silva et al., 2005; Sedlak and Bartova, 2006; Andre et al., 2010), bush dog (Speothus venaticus) (Mattos et al., 2008; Andre et al., 2010), crab-eating fox (Cerdocyon thous) (Andre et al., 2010), hoary fox (Pseudalopex vetulus) (Andre et al., 2010), blue fox (Alopex lagopus) (Yu et al., 2009), Darwin's fox (Pseudalopex fulvipes) (Sedlak and Bartova, 2006), fennec fox (Vulpes zerda) (Sedlak and Bartova, 2006), fisher (Martes pennanti) (Sedlak and Bartova, 2006), red panda (Ailurus fulgens) (Qin et al., 2007), cheetah (Acinonyx jubatus) (Sedlak and Bartova, 2006), jaguarondi (Puma yagouaroundi) (Sedlak and Bartova, 2006; Andre et al., 2010), puma (Puma concolor) (Andre et al., 2010), jaguar (Panthera onca) (Andre et al., 2010), Indian lion (Panthera leo goojratensis) (Sedlak and Bartova, 2006), lion (Panthera leo) (Kamga-Waladjo et al., 2009; Andre et al., 2010), tiger (Panthera tigris) (Andre et al., 2010), Eurasian lynx (Lynx lynx) (Sedlak and Bartova, 2006), ocelot (Leopardus pardalis) (Andre et al., 2010), little spotted cat (Leopardus tigrinus) (Andre et al., 2010), Pampas cat (Oncilfelis colocolo) (Andre et al., 2010), caracal (Caracal caracal) (Andre et al., 2010), serval (Letailurus serval) (Andre et al., 2010), and fishing cat (Prionailurus viverrinus) (Andre et al., 2010).
There are only four reports of N. caninum infection in wild and/or wild captive carnivores where N. caninum parasites were detected histologically, three of which were associated with clinical neosporosis (van der Hage et al., 2002; Lemberger et al., 2005; Yu et al., 2009; Dubey et al., 2014b) (Table 1). Disease in nondomestic carnivores has only been described in young animals and although clinical manifestations and pathologic lesions vary among these cases, similar neurologic and dermatologic presentations have been reported in domestic dogs.
Table 1.
Reports of clinically significant neosporosis in wildlife species.
| Species | Wild or captive | Age, number | Con-current disease | Tests | Tachyzoites | Tissue-cysts | Animal location | Reference |
|---|---|---|---|---|---|---|---|---|
| Order Carnivora | ||||||||
| Family Mustelidae | ||||||||
| European Pine Marten (Martes martes) | Wilda | Juvenile, n = 1 | No | HP, IHC | Yes | No | The Netherlands (undisclosed location) | van der Hage et al., 2002 |
| Family Caniidae | ||||||||
| Red Fox (Vulpes vulpes) | Wildb | Juvenile, n = 1 | Yes c | HP,IHC, S, TEM, ISO (negd) | Yes | No | USA (New Jersey) |
Dubey et al., 2014a Dubey et al., 2014b |
| Blue Fox (Alopex lagupus) | Captive | Newborn, n = 1 | No | HP, IHC, S, PCR/Seq | Yes | Yes | China (Hebei province) | Yu et al, 2009 |
| Order Artiodactyla | ||||||||
| Family Cervidae | ||||||||
| California Black-Tailed Deer (Odocoileus hemionus columbianus) | Wild | Juvenile, n = 1 | No | HP, IHC | Yes | No | USA (California) | Woods et al, 1994 |
| Fallow deer (Dama dama) | Captive | Juvenile, n = 1 | No | HP, IHC, PCR/Seq | Yes | Yes | Switzerland (undisclosed location) | Soldati et al, 2004 |
| Eld's deer (Cervus eldi siamensis) | Captive | Stillborn (full-term), n = 1 | No | HP, IHC | No | Yes | France (Paris) | Dubey et al, 1996 |
| Axis deer (Axis axis) | Captive | Neonates, n = 5 | No | HP, S, PCR/Seq, ISO, MS | No/NR (1/4) | Yes/NR (1/4) | Argentina (La Plata) | Basso et al, 2014 |
| Family Bovidae | ||||||||
| Lesser kudu (Tragelaphus imberbis) | Captive | Stillborn (full term), n = 3 | No | HP, IHC (neg), S, PCR | No | No | Germany (Hannover) | Peters et al, 2001 |
| Order Perissodactyla | ||||||||
| Family Rhinocerotidae | ||||||||
| White rhinoceros (Ceratotherium simum) | Captive | Neonate, n = 1 | No | HP, TEM | Yes | Yes | South Africa (Lichtenburg) | Williams et al, 2002 |
| Captive | Adult, n = 1 | No | HP, IHC, PCR | Yes | No | Thailand (Chonbari) | Sommanustweechai et al, 2010 | |
| Captive | Fetus, n = 1 | No | HP, IHC, PCR/Seq, MS | Yes | No | Australia (New South Wales) | Sangster et al, 2010 | |
| Order Marsupialia | ||||||||
| Family Macropodidae | ||||||||
| Parma wallaby (Macropus parma) | Captive | Adult, n = 1 | No | HP, IHC, PCR/Seq | Yes | Yes | Austria (Vienna) | Cronstedt-Fell et al, 2012 |
NR, not reported; HP, histopathology; IHC, immunohistochemistry; S, serology; EM, electron microscopy; TEM, transmission electron microscopy; ISO, parasite isolation; PCR, polymerase chain reaction; PCR/Seq, PCR with sequencing; SUB, genotyping;; MS, microsatellite analysis; NAT, Neospora agglutination test (direct agglutination test); IFAT, indirect fluorescent antibody tests; IB, immunoblotting.
Free-ranging; maintained in a wildlife rehabilitation center for 3 weeks following dam abandonment.
Free-ranging; maintained 2 weeks in a wildlife rehabilitation center while recovering from vehicular trauma before development of disease.
Concurrent subclinical T. gondii infection.
Parasite isolation attempted 26d post clindamycin treatment.
Van der Hage et al. (2002) described a case of fatal neosporosis in a free-ranging juvenile European pine marten (Martes martes) (family Mustelidae) from the Netherlands (Table 1). The affected 7-week old pup was taken into care by wildlife rehabilitators following dam abandonment and initially presented with small body size, dehydration, diarrhea, and forelimb and hindlimb ataxia. Over the next two weeks, the pup developed a cough that progressed to dypsnea and tachycardia. Imaging demonstrated cardiomegaly, severely diminished myocardial contractility, and intra-abdominal fluid. The animal was euthanized, and a postmortem examination revealed cardiac biventricular dilation and multiple pale foci scattered throughout the liver. The heart, liver, and lung were evaluated histologically. Microscopic lesions included multifocal nonsuppurative myocarditis with cytoplasmic tachyzoites evident in some mononuclear cells, extensive bridging hepatic necrosis with hydropic degeneration of remaining hepatocytes (presumed sequelae of congestive heart failure), and increased numbers of pulmonary alveolar macrophages. Tissue cysts of N. caninum were not reported. The diagnosis of neosporosis was based on the detection of N. caninum antigen (disseminated tachyzoites) by IHC in all tissues evaluated; parasites were negative for T. gondii antigen by IHC. Details of antibodies used for IHC were not provided.
Yu et al. (2009) reported an outbreak of neosporosis in a group of newborn farm-bred blue foxes (Alopex lagopus) (family: Canidae) in China displaying a high rate of morbidity and mortality (Table 1). Of more than 200 pups born during the outbreak, over 60% were affected and of those, over 50% died. Signs included coarse fur, inappetence, emaciation, fever, depression, ataxia, and paralysis. Five animals euthanized for necropsy examination had consistent macroscopic lesions of hydrocephalus and coarse pia mater. Scattered splenic infarcts and foci of renal necrosis and capsular rugosity were also observed. The most significant histologic lesions consisted of multifocal nonsuppurative perivascular encephalitis and marked congestion, and small foci of necrosis in the cerebrum, cerebellum, and spinal cord. Inflammatory foci in the brain were often associated with protozoan cysts and less commonly clustered tachyzoites. Protozoan cysts presumed to be N. caninum tissue cysts were also found within renal tubules in two of four foxes with Periodic Acid Schiff (PAS) staining, although parasite identity was not confirmed by IHC. Additional changes were not described. Immunohistochemical analysis using canine and murine polyclonal antibodies raised against N. caninum and T. gondii, respectively, revealed the parasites in the brain were positive for N. caninum antigen and negative for T. gondii antigen. PCR of brain for the N. caninum specific Nc5 region was positive in four of five foxes and confirmed by sequencing. Some foxes (28/103 tested) were seropositive for N. caninum (c-ELISA, VMRD Laboratories, Pullman, Washington, USA).
Dubey et al. (2014b) reported a case of cutaneous neosporosis in a free-ranging juvenile red fox (Vulpes vulpes) (family: Canidae) with concurrent subclinical T. gondii infection from New Jersey, USA (Table 1). The fox was in care for two weeks while recovering from vehicular trauma before developing generalized crusty dermal lesions on the ventral thorax, face, and limbs. Histologic examination of a skin biopsy demonstrated marked granulomatous dermatitis with severe parakeratotic hyperkeratosis and neutrophilic crusting, necrotic keratinocytes associated with lymphocytic and neutrophilic infiltrates, epidermal hyperplasia, and myriad individual and clustered protozoan tachyzoites in the epidermis, particularly within keratinocytes. The fox had antibodies to both T. gondii (modified agglutination test (MAT), titer 1:3200) and N. caninum (Neospora agglutination test, NAT; titer 1:25). Transmission electron microscopy (TEM) of skin lesions revealed tachyzoites with electron dense rhoptries, consistent with N. caninum, and the tachyzoites were positive for N. caninum antigen and negative for T. gondii antigen by IHC using polyclonal rabbit anti-N. caninum and anti-T. gondii antibodies. Dermal lesions resolved with clindamycin treatment and parasites were not identified with histologic evaluation of skin biopsies after 4 weeks of treatment, although viable T. gondii (but not N. caninum) was isolated from skin biopsies at that time.
Lemberger et al. (2005) identified a single protozoan cyst in the brain of a juvenile free-ranging raccoon (Procyon lotor) (family: Procyonidae) euthanized due to canine distemper virus infection from Cook County, Illinois, USA (Table 2). Immunohistochemical staining was performed using polyclonal antibodies and the protozoan cyst was positively labeled with rabbit anti-N. caninum antibodies but not anti-T. gondii antibodies. Nc5 PCR of DNA derived from the brain confirmed the diagnosis of N. caninum infection. The protozoan cyst was considered an incidental finding.
Table 2.
Neospora caninum tissue cysts identified histologically in wildlife species without associated pathologic changes.
| Species | Wild or captive | Age | Concurrent disease | Tests | Cysts/Tachyzoites | Animal location | Reference |
|---|---|---|---|---|---|---|---|
| Order Carnivora | |||||||
| Family Procyonidae | |||||||
| Raccoon (Procyon lotor) | Wild | Juvenile | Yesa | HP, IHC (PC anti-Nc Ab of unreported sp.) | Yes/No | USA (Illinois) | Lemberger et al, 2005 |
| Order Rodentia | |||||||
| Family Muridae | |||||||
| Brown rat (Rattus norvegicus) | Wild | NR | No | HP, IHC, PCR | Yes/No | Mexico (Aguascalientes) | Medina-Esparza et al, 2013 |
| House mouse (Mus musculus) | Wild | NR | No | HP, IHC, PCR | Yes/No | Mexico (Aguascalientes) | Medina-Esparza et al, 2013 |
| Family Sciuridae | |||||||
| Rock squirrel (Spermophilus variegates) | Wild | NR | No | HP, IHC, PCR | Yes/No | Mexico (Aguascalientes) | Medina-Esparza et al, 2013 |
| Class Aves | |||||||
| Order Psittaciformes; family Psittidae | |||||||
| Red-and-green macaw (Ara chloropterus) | NR | NR | No | HP, IHC, Sb | Yes/No | Brazil | Mineo et al, 2011 |
| Blue-fronted Amazon parrot (Amazona aestiva) | NR | NR | No | HP, IHC, Sb | Yes/No | Brazil | Mineo et al, 2011 |
NR, not reported; HP, histopathology; IHC, immunohistochemistry; S, serology; PCR, polymerase chain reaction; IFAT, indirect fluorescent antibody test; Cyst, tissue cyst; Tachy, tachyzoites.
Canine distemper virus infection.
Seronegative by IFAT.
5.1.2. Artiodactyla
Neospora caninum antibodies and/or DNA have been detected in the following free-ranging nondomestic artiodactyls: white-tailed deer (Odocoileus virginianus) (Ab) (Dubey et al., 1999, 2009; Lindsay et al., 2002; Gondim et al., 2004a; Anderson et al., 2007; Gutierrez-Exposito et al., 2012; Olamendi-Portugal et al., 2012), black-tailed deer (Odocoileus hemionus columbianus) (Ab, DNA) (Woods et al., 1994; Dubey et al., 2008), mule deer (Odocoileus hemionus hemionus) (Ab) (Dubey et al., 2008; Myers et al., 2014), pampas deer (Ozotoceros bezoarticus) (Ab) (Tiemann et al., 2005a), roe deer (Capreolus capreolus) (Ab) (Bartova et al., 2006; Almeria et al., 2007; Panadero et al., 2010; De Craeye et al., 2011; Malmsten et al., 2011; Candela et al., 2014), fallow deer (Dama dama) (Ab) (Bartova et al., 2006; Marco et al., 2008), red deer (Cervus elaphus and C. elephus yarkandensis) (Ab) (Ferroglio and Rossi, 2001; Bregoli et al., 2006; Almeria et al., 2007; Gozdzik et al., 2010; Billinis, 2013), elk (Cervus canadensis) (Ab) (Gutierrez-Exposito et al., 2012), moose (Alces alces) (Ab) (Dubey and Thulliez, 2005; Stieve et al., 2010; Malmsten et al., 2011; Gutierrez-Exposito et al., 2012; Moskwa et al., 2014), caribou (Rangifer tarandus) (Ab) (Dubey and Thulliez, 2005; Stieve et al., 2010; Gutierrez-Exposito et al., 2012), North American bison (Bison bison) (Ab) (Dubey and Thulliez, 2005), European bison (Bison bonasus bonasus) (Ab) (Cabaj et al., 2005; Bien et al., 2010), African buffalo (Syncerus caffer) (Ab) (Ferroglio et al., 2003), Thompson's gazelle (Eudorcas thomsonii) (Ferroglio et al., 2003), impala (Aepyceros melampus) (Ab) (Ferroglio et al., 2003), eland (Taurotragus oryx) (Ab) (Ferroglio et al., 2003), chamois (Rupicapra rupicapra) (Ab, DNA) (Ferroglio and Rossi, 2001; Bregoli et al., 2006; Gaffuri et al., 2006; De Craeye et al., 2011), Alpine ibex (Capra ibex) (Ab) (Bregoli et al., 2006), Spanish ibex (Capra pyrenaica hispanica) (Ab) (Garcia-Bocanegra et al., 2012), European mouflon (Ovis musimon) (Bartova et al., 2006), Barbary sheep (Ammotragus lervia) (Ab) (Almeria et al., 2007), musk ox (Ovibos moschatus) (Ab) (Dubey and Thulliez, 2005), wild boar (Sus serofa) (Ab) (Bartova et al., 2006; Almeria et al., 2007), and warthog (Phacochoerus aethiopicus) (Ab) (Ferroglio et al., 2003).
Neospora caninum antibodies have also been detected in the following captive nondomestic artiodactyl species: Axis deer (Axis axis) (Basso et al., 2014), Eld's deer (Cervus eldi siamesis) (Dubey et al., 1996), Thorold's deer (Cervus albirostris) (Sedlak and Bartova, 2006), eastern elk (Cervus elaphus canadensis) (Sedlak and Bartova, 2006), Vietnam sitka deer (Cervus Nippon pseudaxis) (Bartova et al., 2006; Sedlak and Bartova, 2006), Brocket deer (Mazama spp.) (Tiemann et al., 2005b), Pere David's deer (Elaphurus davidianus) (Sedlak and Bartova, 2006), antelope (Tragelaphus iberbis) (Peters et al., 2001), blackbuck (Antilope cervicapra) (Sedlak and Bartova, 2006), lechwa (Kobus leche) (Sedlak and Bartova, 2006), African buffalo (Syncerus caffer caffer) (Sedlak and Bartova, 2006), eland (Taurotragus oryx) (Sedlak and Bartova, 2006), European bison (Bison bonasus bonasus) (Sedlak and Bartova, 2006), sitatunga (Tragelaphus spekei gratus) (Sedlak and Bartova, 2006), and vicuña (Vicugna vicugna mensalis) (Risco-Castillo et al., 2014).
Viable tachyzoites have been isolated from free-ranging white-tailed deer (isolates NC-WTDVA 1–3 (Vianna et al., 2005) and NcWTDMn1-2 (Dubey et al., 2013)) and European bison (isolates NC-PolBb1-2) (Bien et al., 2010) and captive axis deer (isolate Nc-Axis) (Yu et al., 2009). Transplacental transmission has been reported for free-ranging white-tailed deer (Dubey et al., 2013) and captive bred Eld's deer (Dubey et al., 1996), axis deer (Basso et al., 2014), and antelope (Peters et al., 2001).
There are five reports of N. caninum infection in artiodactyls (four cervids, one bovine), where parasites have been identified histologically and were associated with lesions (Woods et al., 1994; Dubey et al., 1996; Peters et al., 2001; Soldati et al., 2004; Basso et al., 2014). Disease in artiodactyls is associated with stillbirths and systemic disease in very young animals, and is more commonly described in captive animals. Multiple organ involvement is common and the most consistent lesion is nonsuppurative encephalitis.
Woods et al. (1994) described a case of systemic neosporosis in a free-ranging California black-tailed deer fawn (O. hemionus columbianus) (family: Cervidae) from Sacramento County, California (Table 1). The fawn was found dead. Macroscopic lesions included emaciation, pulmonary edema, and soft pulpy kidneys. Lung, liver, and kidney were evaluated histologically. The lungs were characterized by a diffuse moderate mononuclear interstitial pneumonia with intra-alveolar edema and macrophages, scattered fibrin thrombi, and clusters of interstitial and intra-alveolar tachyzoites were found. Hepatic lesions included moderate bridging portal and central fibrosis, random multifocal coagulation necrosis with mild neutrophilic infiltrates, and occasional moderate portal mononuclear infiltrates. The kidney displayed mild scattered tubular necrosis and mild mononuclear interstitial infiltrates. Tachyzoites were detected in renal tubular epithelial cells and tubule lumina. Parasite tachyzoites were positive for N. caninum antigen and negative for T. gondii antigen by IHC using polyclonal rabbit antibodies. Tachyzoites were most prevalent in renal medullary tubular epithelial cells with lesser numbers in renal tubules and endothelial cells. In the lungs, tachyzoites were distributed throughout alveolar spaces, alveolar epithelium, and alveolar septal capillary endothelium. In the liver, tachyzoites were concentrated adjacent and peripheral to areas of hepatic necrosis and were also detected within hepatocytes, sinusoidal endothelium, Kupffer cells, and free within sinusoidal spaces. Tissue cysts were not identified.
Soldati et al. (2004) reported neosporosis in a captive bred fallow deer fawn (Dama dama) (family: Cervidae) from Switzerland, displaying hindlimb paresis, opisthotonus, pyrexia, tachypnea, and tachycardia (Table 1). The fawn was euthanized. The main gross lesions consisted of patchy brown discoloration throughout the entire length of the spinal cord, multiple dark foci in the brain stem, and pulmonary congestion and edema. The most significant histologic finding was multifocal random necrotizing and granulomatous meningoencephalomyelitis. The most severe lesions were in thoracic segments of spinal cord and were comprised of extensive multifocal necrosis predominately affecting white matter associated with large numbers of gitter cells, microgliosis, astrocytosis, capillary endothelial proliferation, spheroids, and multifocal mineralization. Clustered tachyzoites and few protozoan cysts were detected within inflammatory foci and were positively labeled by IHC using polyclonal caprine anti-N. caninum antibodies. Nc5 PCR and sequencing were used to confirm the diagnosis of neosporosis. Parasites were negative for both T. gondii and Sarcocystis neurona by IHC using polyclonal caprine antibodies. A fawn from the same captive group had been euthanized, with similar clinical signs and histologic lesions one month previously but no tissues were available for further investigation.
Dubey et al. (1996) reported a case of congenital neosporosis in a captive bred full-term stillborn Eld's deer (Cervus eldi siamensis) (family: Cervidae) from a zoo in Paris, France. Macroscopic lesions were not detected (Table 1). Microscopic lesions were limited to the brain and included multifocal nonsuppurative encephalitis characterized by perivascular mononuclear infiltrates, microglial nodules with multinucleated macrophages, and necrosis. Lesions were most severe in the midbrain. Protozoan tissue cysts were detected in association with some lesions and in areas of inflammation elsewhere in the brain. Tissue cysts exhibited strong immunoreactivity to monoclonal murine anti-N. caninum antibodies and no immunoreactivity to polyclonal rabbit anti-T. gondii antibodies.
Basso et al. (2014) identified N. caninum as a cause of perinatal mortality in a captive herd of axis deer (Axis axis) (family: Cervidae) from a zoo in La Plata, Argentina (Table 1). One fawn died at two weeks of age following a presentation of congenital anal dilation, incontinence, weakness and ataxia. Megacolon and megarectum were noted on postmortem examination. Cytologic preparations of brain smears revealed the presence of protozoan cysts. Histologic lesions included nonsuppurative encephalitis and gliosis, suppurative bronchopneumonia, fibrinonecrotic enteritis, and hepatic degeneration. An additional four neonates were found dead in the same enclosure within a period of two months before or after the death of the fawn. All were weak at birth and died within one to two days of age. Macroscopic and microscopic lesions were not reported. The fawn and neonates were serologically positive for N. caninum and negative for T. gondii by IFAT (neonate N. caninum IFAT titers were 1:25, 1:400, 1:3200, and 1:6400 and the fawn N. caninum IFAT titer was 1:6400). Neospora-like cysts were observed in a fresh brain cytologic preparation (see Fig. 1, Soldati et al., 2004). No IHC with anti-N. caninum antibodies was reported. Detection of N. caninum antibodies in the three neonates that had not ingested colostrum was deemed most consistent with transplacental transmission. Neospora caninum DNA was detected by Nc5 PCR in brain samples from the fawn and one of the four neonates and viable N. caninum parasites were isolated from fawn brain samples. Multilocus microsatellite-DNA sequence and/or length determination analysis of 9 N. caninum microsatellite markers (MS1B, MS2, MS3, MS5, MS6A, MS6B, MS10, MS12 and MS21) revealed an identical microsatellite pattern in N. caninum DNA from the fawn, neonate, and isolated parasites, evidencing recent infection from a common point source. Serological analysis of 13 adult deer housed in the same enclosure revealed a 92% seroprevalence rate for N. caninum (IFAT titers varied from <1:25 to ≥1:6400).
Fig. 1.
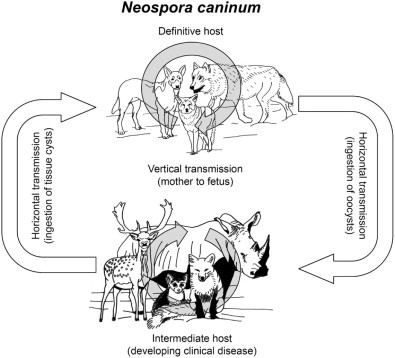
Life cycle of Neospora caninum.
Peters et al. (2001) reported congenital N. caninum infection in three full-term stillborn calves in a herd of captive lesser kudu (Tragelaphus imberbis) (family: Bovidae) from a zoo in Hanover, Germany (Table 1). Necropsies were performed on the calves, two of which were twins, and no macroscopic lesions were identified. The twin calves both had a moderate multifocal nonsuppurative meningoencephalitis with multiple granulomatous foci, gliosis, and mild mononuclear perivascular cuffs in the neuropil and leptomeninges and mild lymphohistiocytic periportal hepatitis. One calf also had mild lymphocytic myocarditis with focal myocardial mineralization. Parasites were not detected histologically. The twin calves and their dam were seropositive for N. caninum (IFAT titers were 1:16, 1:32, and 1:128, respectively; all were immunoblot (IB) positive). The third calf was histologically normal and seronegative by IFAT and IB for N. caninum (the dam was not tested). In this calf, N. caninum DNA was detected by Nc5 PCR in all tissues tested (brain, lung, heart, liver, and spleen) while in one of the twin calves, N. caninum DNA was found only in the brain and lung (the second twin was not tested). All tissue samples were negative for T. gondii DNA by B1 PCR. IHC using polyclonal rabbit anti-N. caninum antibodies failed to demonstrate N. caninum antigen in any of the three calves. The authors postulated that an N. caninum seronegative DNA positive calf might be explained by recent infection shortly before stillbirth or transplacental infection early in pregnancy prior to establishment of fetal immunocompetence. The absence of detectable histologic lesions in this calf evidenced that vertical transmission of N. caninum infection may result in birth of infected yet clinically normal calves, as is often the case in cattle.
5.1.3. Perissodactyla
Neospora caninum antibodies have been detected in free ranging zebra (Equus burchielli) (Ferroglio et al., 2003) and captive white rhinoceros (Ceratotherium simum) (Williams et al., 2002; Sangster et al., 2010; Sommanustweechai et al., 2010) with disease and transplacental infection only described in the latter species. White rhinoceros appear to be particularly susceptible to developing fatal neosporosis at any age. Of the three reported cases, all involved captive animals and liver lesions were common.
Williams et al. (2002) described neosporosis in a white rhinoceros calf (family: Rhinocerotidae) from a breeding center in Northern Province, South Africa, that was found dead at 16 days of age (Table 1). Necropsy findings were consistent with congestive heart failure and included marked cardiomegaly, generalized cyanosis, pulmonary congestion and edema, and hepatic congestion. Heart, liver, lung, and kidney were evaluated histologically, with the most significant lesions present in the heart including marked multifocal to diffuse myocarditis with myocardial degeneration and necrosis. Myocardial inflammatory infiltrates were predominately histiocytic with lesser numbers of lymphocytes, plasma cells, and neutrophils. Giemsa staining of heart sections highlighted intralesional protozoan cysts and tachyzoites that were not apparent on H&E. Parasites in the heart were strongly labeled with both monoclonal and polyclonal anti-N. caninum antibodies (origin not reported) and were negative for T. gondii (antibody details not provided). Additional lesions were marked hepatic centrilobular congestion and mild centrilobular hepatocellular necrosis and fibrosis, diffuse pulmonary congestion and edema with scattered intra-alveolar macrophages, and multifocal atelectasis. Transmission electron microscopy performed on myocardium demonstrated encysted bradyzoites and intracellular tachyzoites compatible with Neospora spp.
Sommanustweechai et al. (2010) reported neosporosis in a 16-year old white rhinoceros from a zoological institution in Chonbari, Thailand (Table 1). The animal was found in sternal recumbency shortly before death. Postmortem examination revealed hepatomegaly and extensive multifocal hepatic necrosis involving approximately 60% of the parenchyma. Pulmonary edema and hemorrhage, hemorrhagic splenic infarction, multifocal renal necrosis, mesenteric necrosis, mesenteric lymphadenomegaly, adrenomegaly with diffuse parenchymal ecchymoses, erosive and ulcerative gastritis, and black discoloration and focal hemorrhage of the jejunal mucosa were also observed. Brain, liver, lung, adrenal gland, kidney, spleen, and myocardium were evaluated histologically. Severe, widespread necrotic foci bounded by lymphocytes and plasma cells were present in the liver, adrenal cortex, kidney, and small intestine; intralesional tachyzoites were evident in all tissues except the latter. Additional findings included hepatic fibrosis and granulomas characterized by central necrosis and numerous giant cells and macrophages without surrounding fibrosis, necrotizing lymphadenitis and splenitis with lymphoid depletion and neutrophil infiltration, and pulmonary edema and hemorrhage. Parasites and lesions were not detected in sections of brain or heart. IHC using polyclonal rabbit anti-N. caninum antibodies positively labeled tachyzoites in the liver, adrenal gland, kidney and small intestine; immunopositive cells included Kupffer cells, hepatocytes, sinusoidal endothelium, and biliary and intestinal epithelium. Authors showed Neospora-like cysts in the liver and adrenal gland on H&E (see fig. 1BCD in Sommanustweechai et al., 2010). Nc5 PCR of liver was positive. Diagnostic tests to rule out T. gondii infection were not reported.
Sangster et al. (2010) identified N. caninum as the etiologic agent of abortion in a southern white rhinoceros fetus from a zoo in Dubbo, New South Wales, Australia (Table 1). Necropsy of the aborted fetus demonstrated hepatomegaly and herniation of a small segment of intestine through the umbilical opening. Microscopic lesions included multifocal hepatic necrosis with small numbers of mixed inflammatory cells and clustered extracellular and intrahepatocellular tachyzoites and cyst-like structures on N. caninum IHC (Figure 2). Similar tachyzoites were also present in the cerebellum with minimal inflammation. Polyclonal anti-N. caninum and anti-T. gondii antibodies (origin not reported) identified N. caninum but not T. gondii antigen in areas of hepatic necrosis. PCR of liver DNA targeting multiple markers (Nc5, SSU rDNA, LSU rDNA, ITS1, and ITS1-5.8S-ITS2 rDNA) and sequencing of the latter four PCR amplification products confirmed the presence of N. caninum DNA. Microsatellite analysis using the MS10 marker detected a unique pattern of trinucleotide repeats distinct from any characterized N. caninum isolates.
Fig. 2.
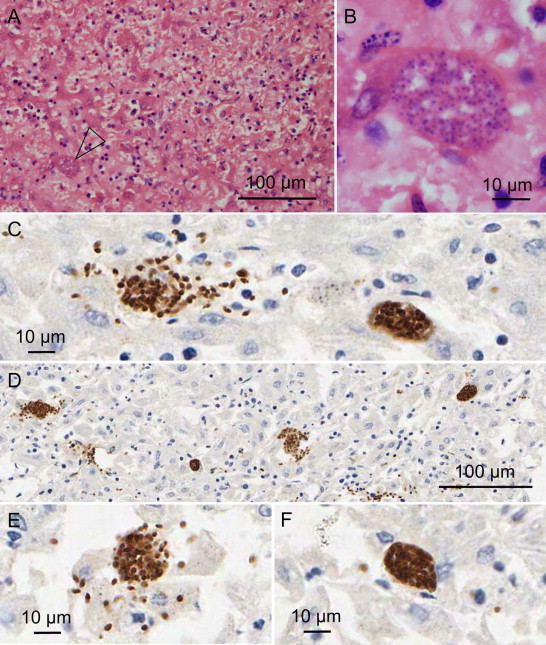
Liver from an aborted white rhinoceros fetus with naturally acquired congenital N. caninum infection. Multifocal hepatic necrosis with intralesional intracellular protozoan cyst-like structure (open arrow). H&E (A). Intracellular protozoan cyst-like structure. H&E (B). Immunohistochemistry (IHC) using polyclonal caprine anti-N. caninum showing clustered free and intracellular protozoal tachyzoites (C, D, E) and intracellular protozoan cyst-like structures (C, D, F). H&E photomicrographs (A, B) courtesy of Cheryl Sangster, Taronga Conservation Society Australia.
5.1.4. Rodentia
Neospora caninum antibodies and/or DNA have been detected in the following free ranging rodents: brown rat (Rattus norvegicus) (Ab, DNA) (Huang et al., 2004; Ferroglio et al., 2007; Jenkins et al., 2007; Medina-Esparza et al., 2013), house mouse (Mus musculus) (Ab, DNA) (Ferroglio et al., 2007; Jenkins et al., 2007; Barratt et al., 2008; Thomasson et al., 2011; Medina-Esparza et al., 2013), field mouse (Apodemus sylvaticus) (Ab, DNA) (Ferroglio et al., 2007; Thomasson et al., 2011), harvest mouse (Micromys minitus) (DNA) (Meerburg et al., 2012), common vole (Microtus arvalis) (DNA) (Fuehrer et al., 2010), water vole (Arvicola terrestris) (DNA) (Fuehrer et al., 2010), rock squirrel (Spermophilus variegates) (DNA) (Medina-Esparza et al., 2013), and capybara (Hydrochaeris hydrochaeris) (Ab, DNA) (Yai et al., 2008; Truppel et al., 2010; Valadas et al., 2010).
There are no reports of naturally occurring neosporosis in mice or other rodents; however, parasite tissue cysts have been identified in several species. These cases will be addressed briefly below. Some authors suggest that wild rodents contribute to the N. caninum sylvatic life cycle by serving as infected reservoir hosts for canid hosts (Huang et al., 2004; Ferroglio et al., 2007; Jenkins et al., 2007; Meerburg et al., 2012; Medina-Esparza et al., 2013). To our knowledge, viable N. caninum parasites have yet to be isolated from any species of wild rodents to date (Gondim, 2006; Dubey et al., 2007; Jenkins et al., 2007; Dubey and Schares, 2011; Medina-Esparza et al., 2013). As such, the putative role of rodents in the epidemiology of N. caninum requires further investigation.
Medina-Esparza et al. (2013) screened brown rats (R. norvegicus) (family: Muridae), house mice (M. musculus) (family: Muridae) and rock squirrels (S. variegates) (family: Sciuridae) for N. caninum infection by assaying samples of brain, spinal cord, and liver using N. caninum specific ITS1 PCR and IHC employing polyclonal caprine anti-N. caninum antibodies (Table 2). Liver was the tissue in which N. caninum cysts and parasite DNA were detected with greatest frequency and hepatitis (severity not reported) was evident histologically in all N. caninum positive animals, although it was also evident in some negative control animals. Neospora caninum immunopositive cysts were rarely found in the brain (1/13 mice, 0/6 rats, 0/14 squirrels) and heart (0/13 mice, 0/6 rats, 2/14 squirrels). Previous attempts to identify parasites by IHC in seropositive and DNA positive brown rats were unsuccessful (Hughes et al., 2006).
5.1.5. Lagomorpha
Neospora caninum antibodies and/or DNA have been detected in the following free-ranging lagomorphs: rabbit (Oryctolagus cuniculus) (Ab,DNA) (Ibrahim et al., 2009), Iberian hare (Lepus granatensis) (Ab) (Almeria et al., 2007; Hughes et al., 2008), brown hare (Lepus europaeus) (Ab) (Ezio and Anna, 2003; Bartova et al., 2010), and eastern cottontail rabbits (Sylvilagus floridanus) (DNA) (Zanet et al., 2013). Neosporosis has not been reported in lagomorphs.
5.1.6. Insectivora
Neospora caninum DNA has been detected in the common shrew (Sorex araneus) (Meerburg et al., 2012) and the white-toothed shrew (Crocidura russula) (Meerburg et al., 2012). There are no reports of neosporosis in insectivores.
5.1.7. Proboscidea
Neospora caninum antibodies have been detected in captive Indian elephants (Elephas maximus indicus) (Wiengcharoen et al., 2012). There are no reports of neosporosis in elephants.
5.1.8. Cetacea
The bottlenose dolphin (Tursiops truncatus) is the only cetacean species in which N. caninum antibodies have been detected (Dubey et al., 2003). Neosporosis in cetaceans has not been reported.
5.1.9. Pinnipedia
Neospora caninum antibodies have been detected in free-ranging and captive California sea lions (Zalophus californianus) (Dubey et al., 2003), and free-ranging walrus (Odobenus rosmarus) (Dubey et al., 2003), ringed seals (Phoca hispida) (Dubey et al., 2003), bearded seals (Erignathus barbatus) (Dubey et al., 2003), harbor seals (Phoca vitulina) (Dubey et al., 2003), ribbon seals (Phoca fasciata) (Dubey et al., 2003), spotted seals (Phoca largha) (Dubey et al., 2003; Fujii et al., 2007), and Kuril harbor seals (Phoca vitulina stejnergeri) (Fujii et al., 2007). Neosporosis in pinnipeds has not been reported.
5.2. Metatherian mammals
5.2.1. Marsupialia
Reports of naturally acquired N. caninum infection in marsupials are rare, with antibodies and/or DNA identified in just three marsupial species: free-ranging South American opossums (Didelphis marsupialis) (Ab) (Yai et al., 2003) and western grey kangaroos (Macropus fuliginosus ocydromus) (Ab) (Mayberry et al., 2014), and a captive Parma wallaby (Macropus parma) (DNA) (Cronstedt-Fell et al., 2012). Current dogma suggests that marsupials are prone to developing overwhelming toxoplasmosis and it has been proposed that they may be equally susceptible to pathologic sequelae of N. caninum infection (King et al., 2011a); however, this hypothesis does not appear to be supported by current data. Mayberry et al. (2014) recently investigated 102 asymptomatic western grey female kangaroos from Western Australia for N. caninum exposure using an indirect fluorescent-antibody test (IFAT) modified for use in macropods and reported an overall seroprevalence of 18% (IFAT titers not reported; cut-off 1:50). Importantly, this study did not indicate that N. caninum exposure negatively impacted the reproductive performance of any of these doe(s) is/are female kangaroos.
To date, there is only a single case of naturally occurring neosporosis in a marsupial. Cronstedt-Fell et al. (2012) reported a case of neosporosis in a captive adult Parma wallaby (M. parma) from a zoo in Vienna, Austria that was found dead. Postmortem examination revealed marked eccentric hypertrophy of the left side of the heart and moderate dilation of the right heart chambers. Significant histologic lesions were present in the heart and consisted of a multifocal interstitial lymphocytic myocarditis and moderate multifocal myocardial degeneration and necrosis associated with mild histiocytic infiltrates and minimal hemorrhage. Throughout the myocardium, cardiomyocytes occasionally contained clusters of protozoan zoites but these were not initially apparent in areas of inflammation and necrosis. Additional findings included moderate pulmonary edema, emphysema and congestion, moderate multifocal ulcerative gastritis and mild catarrhal colitis. The diagnosis of neosporosis was confirmed by IHC using polyclonal caprine anti-N. caninum and anti-T. gondii antibodies, which revealed numerous N. caninum immunopositive and T. gondii immunonegative free and intracellular protozoan tachyzoites and random cysts in areas of myocardial necrosis. Findings were supported by the detection of N. caninum DNA from cardiac tissue using ITS1 PCR followed by sequencing.
5.3. Birds
The role of birds in the N. caninum life cycle is uncertain. A positive correlation has been shown between the presence of birds on cattle farms and increased seroprevalence and risk of N. caninum-associated abortion storms, suggesting that birds may contribute to parasite transmission in sylvatic cycles either as mechanical vectors or as intermediate hosts (Bartels et al., 1999; Ould-Amrouche et al., 1999; Otranto et al., 2003). Until the recent identification of domestic chickens (Gallus domesticus) as intermediate hosts (Costa et al., 2008) and the discovery that domestic dogs fed N. caninum inoculated embryonated chicken eggs shed oocysts (Furuta et al., 2007), there was little evidence that avian species could function as natural hosts for the parasite. The putative role of birds as intermediate hosts is further supported by the identification of N. caninum antibodies and/or DNA in the following free-ranging birds: house sparrow (Passer domesticus) (DNA) (Gondim et al., 2010), common raven (Corvus corax) (Ab) (Molina-Lopez et al., 2012), magpie (Pica pica) (DNA) (Darwich et al., 2012), and common buzzard (Buteo buteo) (DNA) (Darwich et al., 2012).
Histologic evidence of N. caninum infection in birds is rare and naturally occurring neosporosis is not reported. South American parrots are the only birds in which protozoan cysts presumed to be N. caninum have been identified (Mineo et al., 2011). Histopathologic evaluation of a red-and-green macaw (Ara chloropterus) and a blue-fronted Amazon parrot (Amazona aestiva), both of which died from unrelated causes, revealed protozoan tissue cysts in peri-cloacal and cervical skeletal muscle, respectively (Table 2). IHC using polyclonal murine anti-N. caninum and anti-T. gondii antibodies and mAb 74.1.8, a monoclonal antibody directed against the bradyzoites specific antigen BAG1 (commonly expressed in both N. caninum and T. gondii tissue cysts (Weiss et al., 1999)) demonstrated that parasitic cysts were immunopositive for N. caninum and BAG1 and immunonegative for T. gondii. When the same antibodies were tested on pigeon tissues bearing Sarcocystis spp. cysts, a complete absence of staining was observed. Unfortunately, fresh tissue from the parrots was not available for further investigation and DNA extraction from formalin fixed paraffin embedded tissue was unsuccessful. Protozoan cysts in both cases were not associated with any significant histologic lesions and were considered incidental findings.
6. Experimentally acquired neosporosis and pathology in wildlife species
Experimentally induced N. caninum infections have been reported for a number of different species under a variety of experimental conditions. The purpose of this manuscript is not to detail the various experimental approaches used in these studies. For additional details, we encourage readers to seek out the references provided. Instead, we focus on (1) the pathologic changes associated with N. caninum infection, and (2) parasite detection in different wildlife laboratory models. By doing so, our aim is to better understand neosporosis in these species and ascertain optimal sample collection to facilitate diagnosis when N. caninum infection is suspected in wildlife cases.
Multiple species of rodents have been used to study the pathology of Neospora infection (Lindsay and Dubey, 1989; Dubey and Lindsay, 2000; Pipano et al., 2002; Uchida et al., 2003; Ramamoorthy et al., 2005; Hurkova-Hofmannova et al., 2007; Kang et al., 2009; Mols-Vorstermans et al., 2013; Bottari et al., 2014). Less traditional laboratory rodent species, considered here as wildlife species despite originating from breeding colonies, in which experimental infections were conducted and disease demonstrated, include the following: multimammate rat (Mastomys natalensis) (family muridae, subfamily murinae) (Hurkova-Hofmannova et al., 2007), sand rat (Psammomys obesus) (family muridae, subfamily gerbillinae) (Pipano et al., 2002), Tristam's jird (Meriones tristrami) (family muridae, subfamily gerbillinae) (Hurkova-Hofmannova et al., 2007), Wagner's gerbil (Gerbilus dasyuris) (family muridae, subfamily gerbillinae) (Hurkova-Hofmannova et al., 2007), Mongolian gerbils (Meriones unguiculatus) (family muridae, subfamily gerbillinae) (Cuddon et al., 1992; Gondim et al., 1999, 2001; Dubey and Lindsay, 2000; Ramamoorthy et al., 2005; Kang et al., 2009; Bottari et al., 2014), and Djungarian hamsters (Phodopus sungorus) (family cricetidae) (Uchida et al., 2003). Of these species, gerbiline rodents have proven to be highly susceptible to N. caninum infection and the Mongolian gerbil is currently the common immunocompetent experimental host used for the study of neosporosis (Dubey and Lindsay, 2000; Gondim et al., 2001; Ramamoorthy et al., 2005; Hurkova-Hofmannova et al., 2007; Kang et al., 2009). Clinical signs of disease are similar among different susceptible rodent genera and include roughened fur, apathy/depression, inappetence, weight loss, incoordination, and hindlimb paresis (Dubey and Lindsay, 2000; Gondim et al., 2001; Pipano et al., 2002; Uchida et al., 2003; Ramamoorthy et al., 2005; Hurkova and Modry, 2006). Although not always evident, the most commonly observed macroscopic lesions are fibrinous peritonitis with ascites containing tachyzoites (likely due to intraperitoneal method of inoculation), splenomegaly, and hepatomegaly (Dubey and Lindsay, 2000; Pipano et al., 2002; Ramamoorthy et al., 2005; Hurkova-Hofmannova et al., 2007; Bottari et al., 2014). Histopathologic changes are somewhat more variable and difficult to compare between studies due to inconsistencies in tissue evaluation and lesion description, differences in inoculum dose and route, and variations in disease chronicity in evaluated animals. Reported microscopic lesions include miliary hepatitis and hepatic necrosis, granulomatous hepatitis, splenic lymphoid and reticular cell hyperplasia, granulomatous splenitis, myocarditis, myositis, gastroenteritis (more severe in animals inoculated orally with sporulated oocysts), bronchopneumonia, and meningoencephalitis, with intralesional tachyzoites often evident in areas of inflammation (Dubey and Lindsay, 2000; Gondim et al., 2001; Hurkova-Hofmannova et al., 2007; Kang et al., 2009; Bottari et al., 2014). Of the species listed above, tissue cysts have only been described in Mongolian gerbils and Djungarian hamsters (Gondim et al., 2001; Uchida et al., 2003). Kang et al. (2009) used Nc5 PCR to investigate N. caninum tissue distribution in Mongolian gerbils during acute infection resulting from intraperitoneal inoculation with tachyzoites. Tissue screening showed tissues were targeted in the following order, from early to late: liver, spleen, and kidney; heart, skeletal muscle, spinal cord and blood; lung; and then brain. In this study, N. caninum DNA was detected from day six in the liver and spleen, and only from day eight onwards throughout the remainder of the body, suggesting that liver and spleen may be the optimal tissues for PCR detection of suspected infection (Kang et al., 2009).
Recent investigations of experimental neosporosis in the fat-tailed dunnart (Sminthopsis crassicaudata), a native Australian carnivorous marsupial, have demonstrated this species to be a susceptible intermediate host to N. caninum and that resultant disease is severe and dominated by tissue cyst production (King et al., 2011a). Dunnarts inoculated intraperitoneally with 105 tachyzoites were euthanized between 13 and 18 days post-inoculation due to severe clinical signs, which included roughened fur, lethargy, reduced activity, inappetence, progressive hindlimb paralysis, incontinence, and weight loss. The only consistent gross finding at necropsy was marked atrophy of adipose tissue. Microscopic lesions were consistent with disseminated protozoal infection and included severe necrosuppurative pancreatitis, widespread myonecrosis and myodegeneration of hindlimb skeletal muscle and urinary bladder detrusor muscle, suppurative cystitis, peritonitis, adrenal necrosis, and granulomatous to pyogranulomatous interstitial pneumonia. Intralesional tachyzoites and tissue cysts were common and displayed strong immunoreactivity to polyclonal caprine anti-N. caninum antibodies (Figure 3). Parasites were most numerous in the urinary bladder detrusor muscle, cardiac and skeletal striated muscle, and pancreas, with distinct replicating tachyzoites present in pancreatic acinar cells. IHC-positive parasites were also found in spleen, brain, accessory sex gland, mesenteric lymph node, gall bladder and gastric muscular tunics, and intestinal villi/enterocytes; parasites were not detected in the eye, kidney, or spinal cord. Bradyzoite specific IHC using monoclonal anti-T. gondii BAG5 (also referred to as BAG1) antibodies that cross react with their N. caninum equivalents confirmed the presence of N. caninum tissue cysts in the heart, lung, pancreas, adrenal gland, urinary bladder, and skeletal muscle. All dunnarts seroconverted to N. caninum by 14 days post inoculation as determined by cELISA, and N. caninum DNA was detected in the brain by Nc5 and ITS1 PCR. In contrast, dunnarts infected with 104 N. caninum tachyzoites did not demonstrate clinical signs or histologic lesions by 28 days post inoculation and N. caninum DNA was not detected in the brain, liver, and lung, although two animals had evidence of seroconversion. Dunnarts dosed orally with 20–40 N. caninum oocysts did not develop disease, histologic lesions, or seroconvert; N. caninum DNA was detected in the brain of one animal and in the other, N. caninum antigen was identified in the spleen by IHC.
Fig. 3.
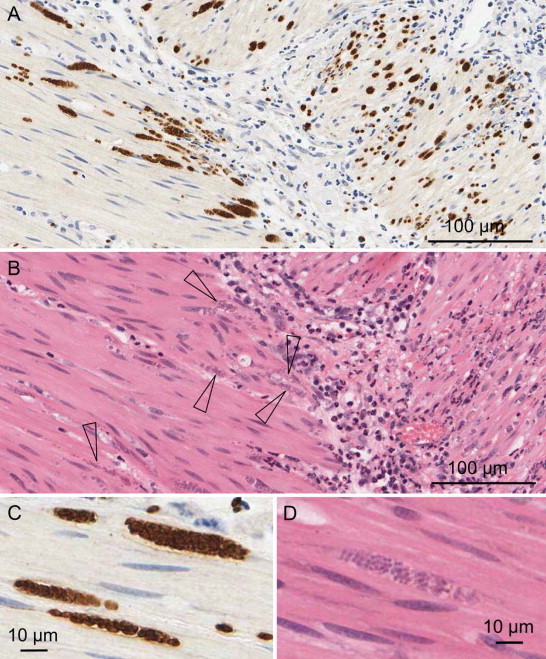
Urinary bladder from a fat-tailed dunnart (Sminthopsis crassicaudata) experimentally infected with N. caninum. IHC using polyclonal caprine anti-N. caninum showing marked necrotizing and widespread degeneration and necrosis of the detrusor muscle with many neutrophils and macrophages and intralesional protozoan cysts and tachyzoites (A). H&E of the same tissue; open arrows indicate intracellular protozoan organisms (B). N. caninum IHC (C) and H&E (D) of protozoan tissue cysts.
Barr et al. (1994) inoculated fetal and pregnant female rhesus macaques (Macaca mulatta) with N. caninum tachyzoites in order to assess primate susceptibility to infection. Two fetuses (experiment A) received direct intramuscular inoculations of 1 × 106 tachyzoites in utero on gestational day 65 and two pregnant dams (experiment B) received intravenous and intramuscular inoculations with a total of 1.6 × 107 tachyzoites on gestational day 43. This study confirmed that nonhuman primate fetuses could become infected by direct in utero inoculation and by transplacental transmission. Hysterotomy and fetal necropsy was conducted at 13–22 days (experiment A) and 67–70 days post inoculation (experiment B). Gross lesions in experiment A were oligohydramnios, placental edema, and complete absence of chorioamniotic fusion. Histologic lesions were consistent with systemic neosporosis and included pyogranulomatous deciduitis, fibrinous and pyogranulomatous variably necrotizing placentitis, multifocal superficial mixed dermatitis with small foci of parakeratotic hyperkeratosis, random focal pyogranulomatous to mononuclear encephalitis with occasional foci of necrosis, multifocal mononuclear interstitial pneumonia, and diffuse suppurative interstitial nephritis; intralesional tachyzoites were detected in all tissues but the latter. Additionally, rare infiltrates of mononuclear cells and/or protozoan tachyzoites were also noted in the skeletal muscle, liver, heart, spleen, and intestinal wall. Gross lesions in experiment B included multiple small cavitations and foci of discoloration in the cerebrum, and in one animal, the umbilical interstitium contained a small opaque pale tan focus. Microscopic lesions consisted of severe multifocal to coalescing granulomatous variably necrotizing encephalomyelitis, multifocal necrotizing and fibrinous placentitis, focal necrotizing omphalitis, and multifocal perivascular mononuclear deciduitis; intralesional tachyzoites were evident in all tissues except the latter. In all infected fetuses, Neospora-specific antibodies were detected by IFAT (IgM titers were <2 to 128, IgG titers were 160 – 5,120, and IgA titers were <40 to 1,280); tissue cysts were not identified; and tachyzoites were reisolated from fetal brain, placenta, and amniotic fluid. Parasites were stained immunohistochemically with polyclonal rabbit anti-N. caninum antibodies.
Over the years, birds have been intensively investigated as possible reservoir hosts for N. caninum. Evidence of N. caninum exposure in domestic fowl (Costa et al., 2008) and wild bird populations (Gondim et al., 2010; Mineo et al., 2011; Darwich et al., 2012; Molina-Lopez et al., 2012) has prompted questions about the role avian species may play in maintaining the parasite in the sylvatic life cycle. To address this gap in our knowledge of N. caninum epidemiology, experimental investigations in domestic pigeons (Columba livia) (McGuire et al., 1999; Mineo et al., 2009), quail (Coturnix coturnix japonica) (de Oliveira et al., 2013), and zebra finches (Poephila guttata) (McGuire et al., 1999) have aimed to examine the consequences of N. caninum infection in birds. These studies have shown that avian species differ in their susceptibility to N. caninum infection, similar to what is seen in mammals. Pigeons appear to be the most susceptible to infection while quail and zebra finches are more resistant.
Mineo et al. (2009) inoculated four pigeons intraperitoneally with 107 N. caninum tachyzoites and found that all birds rapidly seroconverted with peak N. caninum-specific IgG levels detected between 10 and 20 days post inoculation (IFAT titers varied from 1: < 200 to 1:640; cut-off 1:20). One pigeon died 25 days post inoculation, with evidence of widespread parasite dissemination; N. caninum (life stage not specified) was identified by immunohistochemical staining with polyclonal murine anti-N. caninum antibodies in the lungs, heart, central nervous system, and kidney. Additional light microscopic findings were not reported and whether or not parasites were associated with significant lesions is therefore unclear. Surviving pigeons were seronegative and IHC negative for N. caninum at 45 days post inoculation, suggesting that active infection may be resolved within several weeks and seroconversion is of limited duration.
These findings are in contrast to a previous study by McGuire et al. (1999) in which three pigeons were inoculated intraperitoneally with 104–106 tachyzoites, with the two birds receiving the higher inoculum dose being seropositive (IFAT titers 1:800 and 1:400) at 6 weeks post inoculation (study conclusion). All infected birds remained clinically normal, and had detectable N. caninum DNA in samples of brain with Nc5 PCR and viable tachyzoites reisolated from brain homogenates (McGuire et al., 1999). Histologic lesions were found in the brain (only tissue examined) of the pigeon infected with 106 tachyzoites and consisted of multifocal mononuclear perivascular cuffs in the cerebral cortex, midbrain, and meninges, and a moderate focal granulomatous and heterophilic neurohypophysitis (McGuire et al., 1999). Zebra finches receiving the same inoculum dose and route remained refractory and developed no evidence of infection (McGuire et al., 1999).
Quail inoculated subcutaneously with 3.5–5 × 105 N. caninum tachyzoites demonstrated no evidence of morbidity or mortality despite evidence of infection (de Oliveira et al., 2013). Tissues were screened for N. caninum at different time points throughout the experiment by histopathology, IHC using polyclonal bovine anti-N. caninum antibodies, and Nc5 PCR. In some birds, the liver contained mononuclear infiltrates associated with N. caninum antigen but this was detected only during the first week of infection (de Oliveira et al., 2013). Tissues that most frequently tested positive for N. caninum by IHC and PCR were the bursa of Fabricius, brain, spleen, liver, and lungs, demonstrating that parasite dissemination occurred post-inoculation. Tissue cysts were not detected. Infected birds seroconverted (IFAT titers not reported; cut-off 1:10) after a week, with peak titers detected between weeks two and three; however, rapid decline of the N. caninum antibody titer to undetectable levels by one month suggested that this species was able to adequately control infection.
Based on these studies, antibody production against N. caninum parasites in quail and pigeons, at least in some cases, may be sharply transient, and similar findings have also been reported for chickens (Furuta et al., 2007; Mineo et al., 2009). Speculatively, this may also be the case for other species of wild birds and thus should be considered when interpreting findings of serological surveys in avian species. Tissue cysts were not described in any of these reports and it remains unclear whether or not N. caninum establishes chronic infections in birds.
7. Animals with no evidence of naturally or experimentally acquired N. caninum exposure
To our knowledge, N. caninum exposure and/or infection have yet to be reported in the following orders of eutherian mammals: primates (primates), chiroptera (bats), dermoptera (colugos or flying lemurs), edentata (toothless mammals), tubulidentata (aardvarks), hyracoidae (hyraxes, dassies), pholidata (pangolins), and sirenia (dugongs and manatees).
There is no evidence to date of N. caninum exposure and/or infection in prototherian species (platypus and echidna) or any ectotherm species (reptiles, amphibians, and fish).
8. Diagnostic tools used for detection of N. caninum in wildlife
Several diagnostic assays have been employed with varying degrees of success to identify N. caninum infection in nondomestic species in order to better understand the potential role of wildlife in the epizootiology of N. caninum. Most of these reports have utilized serological techniques, with direct detection of parasite specific DNA using PCR-based molecular methods increasingly reported. Unfortunately, in many instances the interpretation and comparison of study results are confounded by many factors including variation in study design and methodology, sample size, and data interpretation. It is important to note that the identification of N. caninum antibodies, DNA, and/or tissue cysts is not necessarily analogous with the presence of viable parasites and data should be interpreted cautiously, particularly in those cases where only a single diagnostic test has been used (Dubey et al., 2007; Dubey and Schares, 2011). Specific procedures for many of the techniques described in this chapter may be found in “Protozoal abortion in farm ruminants: guidelines for diagnosis and control” (Ortega-Mora, 2007).
8.1. Isolation
Definitive identification of intermediate host species requires isolation of viable N. caninum parasites by bioassays in animals and/or cell culture. Unfortunately, N. caninum is notoriously difficult to isolate from naturally infected animals due to lack of parasite viability and/or low numbers, and it may take longer than a month to visualize parasite life stages in primary cultures (Conrad et al., 1993; Kim et al., 2000). Additionally, some isolates (e.g., white-tailed deer isolate NC-WTDVA-2) do not grow in cell culture (Vianna et al., 2005). Bioassays in rodents are costly, involve ethical considerations, and require colonies of immunosuppressed or susceptible species such as cortisone-treated outbred mice, IFN-γ gene knockout mice or gerbils (Dubey and Schares, 2006; Dubey et al., 2007). Consequently, parasite isolation is predominately used for research purposes when strain characterization is desired. That said, there is a clear need for the more frequent isolation of N. caninum globally, particularly from wildlife, because of existing uncertainties over virulence and sylvatic cycles as discussed above. To achieve this, national centers with infrastructure capable of culturing N. caninum are required to respond adequately to the unpredictable emergence of cases in wildlife.
Brain is the most common tissue for isolation studies but isolation has also been achieved using spinal cord, blood, heart or other infected tissues, as well as skin lesion biopsies (Dubey et al., 2007, 2014a; Dubey and Schares, 2011).
8.2. Histopathology and immunohistochemistry
Histopathology has a low sensitivity for detection of organisms compared to other available diagnostic tests because parasite life stages are not always present or readily observed in tissue section (Dubey and Schares, 2006). However, histopathology remains an extremely valuable diagnostic tool that is essential for improving our understanding of infection outcome in wildlife species. It enables lesion description, tissue distribution analysis, and identification of concurrent pathology with intralesional parasites, documenting an association between parasite presence and disease. Because it is not possible to definitively distinguish the different tissue-cyst forming coccidia using light microscopic examination of tissue alone, additional confirmatory diagnostic tests should be conducted in conjunction with histopathology.
IHC with N. caninum specific antibodies can facilitate diagnosis by identifying parasite-specific antigen (Dubey and Schares, 2006). In general, polyclonal antibodies are preferred over monoclonal antibodies for IHC diagnosis of neosporosis because they exhibit higher sensitivity in detecting N. caninum antigen when present (Dubey and Schares, 2006; Dubey et al., 2007). For this purpose, the commercial polyclonal antibodies (in our laboratory we use N. caninum antiserum, #PAB-NC, VMRD, Pullman, WA, USA) are globally distributed and suitable for protocol validation by individual histopathology laboratories. Polyclonal antibodies are less specific than monoclonal antibodies and may exhibit cross-reactivity with other closely related apicomplexan parasites (Dubey and Schares, 2006; Dubey et al., 2007). Although not used for routine diagnosis of N. caninum, bradyzoite specific antibodies (BAG1/BAG5) may be applied to differentiate tissue cysts from clustered tachyzoites (Dubey and Schares, 2006). The absence of commercially available bradyzoite/tachyzoite-specific antibodies makes these assays less suitable for routine analysis.
It is our opinion that postmortem examination should be conducted on (1) any wild animal euthanized for suspected neosporosis or (2) for investigation of N. caninum infection, and a variety of tissues be collected and fixed in 10% formalin for histopathology (processed into paraffin blocks within 7 days if IHC is considered). For that matter, best practice suggests that wherever possible, routine post mortem examination should be performed on any wild animal dying or euthanized for unknown cause, given the variability in signs associated with neosporosis. Based on our review of wildlife cases in which N. caninum parasites have been detected histologically, sampled tissues should include, at the minimum, brain and spinal cord (if possible), lung, heart, liver, skeletal muscle, and placenta (if applicable). For cases of suspected disease, spleen, lymph node, kidney, adrenal gland, urinary bladder, pancreas, representative sections of gastrointestinal tract, and reproductive structures should also be assessed to provide a complete picture. Even if all of these samples are not evaluated initially for a given research project, collection and proper sample storage will make future research projects possible and enable ancillary diagnostic tests to be conducted if warranted based on unusual health findings.
8.3. Ultrastructural analysis (transmission electron microscopy)
Transmission electron microscopy (TEM) can be used to identify features of different parasite species as well as differentiate between tachyzoites and cysts and identify infected cells. Due to advances in diagnostic techniques in recent years, it is no longer a method of choice for routine diagnosis of N. caninum infection.
8.4. Seroprevalence studies
Serological assays are useful diagnostic tools to assess wild animals for evidence of N. caninum exposure, and techniques may be applied antemortem or postmortem on sera and other biological fluids (e.g., CSF, thoracic fluid). In recent years, numerous investigations of N. caninum seroprevalence have been conducted in wildlife. Unfortunately, the data are not comparable between many studies due to the use of different techniques and lack of test validation in nondomestic species and results should be interpreted cautiously, particularly when only one serological method has been used (Gondim, 2006; Dubey et al., 2007; Dubey and Schares, 2011; Almeria, 2013). Many factors might influence whether or not antibodies are detected in wildlife by a given assay including postmortem degradation of immunoglobulins (if samples are obtained at necropsy), lack of appropriate species-specific secondary antibodies or conjugates, and potential for serological cross-reaction with closely related apicomplexan parasites (Dubey and Schares, 2006; Gondim, 2006; Almeria, 2013). Studies in cattle have shown that N. caninum specific antibodies can fluctuate during pregnancy and may fall below detectable levels (Dubey and Schares, 2011). Furthermore, infected animals do not always develop a detectable antibody response (De Marez et al., 1999; Lindsay et al., 1999). It is interesting to speculate that this may also be true for wild animals, especially given the above experimental results in birds, although additional research is required to identify if this is the case. Despite their limitations, serological studies have provided compelling evidence of N. caninum exposure in wildlife species.
Serological techniques for specific detection of N. caninum in domestic animals include the indirect fluorescent antibody test (IFAT), immunoblotting (IB), N. caninum direct agglutination test (NAT), and a variety of enzyme linked immunosorbent assays (ELISAs) (reviewed by Dubey and Schares, 2006). In wildlife, the most commonly used serological tests are the competitive enzyme-linked immunosorbent assay (cELISA) and N. caninum-agglutination test (NAT) because these techniques do not require the use of species specific secondary antibodies (Almeria, 2013). The usefulness and application of different serological techniques in wildlife species have been recently extensively reviewed (Almeria, 2013) and we encourage readers to seek out additional details from the provided reference. The limitation of these studies is that homologous true positive and negative sera are extremely difficult and often impossible to obtain, implying that cut-offs remain speculative.
8.5. Conventional and nested PCR
PCR is a highly sensitive and specific technique for detecting N. caninum DNA that may be applied to tissues and other clinical samples such as blood, CSF, and other body fluids. The N. caninum specific repetitive Nc5 gene and the internal transcribed spacer 1 (ITS1) region of the rRNA gene are the most common markers used for routine PCR-based N. caninum detection (Dubey and Schares, 2006). The ITS1 gene is a useful target for phylogenetic analysis between closely related taxa (e.g., Toxoplasma, Hammondia, and Neospora) because it is present at high copy number in the genome and exhibits interspecies variability and intraspecies conservation, allowing differentiation between N. caninum and other coccidian species (Holmdahl and Mattsson, 1996; Dubey et al., 2002; Gondim et al., 2004c; Al-Qassab et al., 2010). The D2 domain of the large subunit (D2-LSU) rDNA is also a phylogenetically important marker used to distinguish between closely related coccidian genera and species (Ellis et al., 1998, 1999; Slapeta et al., 2002). Amplification of ITS1/D2-LSU rDNA needs to be followed by direct bidirectional DNA sequencing to fully ascertain sequence identity. Since originally described by Muller et al. (1996), Holmdahl and Mattsson (1996), and Ellis et al. (1998), Nc5- and ITS1/D2-LSU rDNA-PCR protocols have been modified in many laboratories using a variety of primers and two-step nested PCR approaches targeting these markers have also been developed to enhance parasite detection in low level infections (Ellis et al., 1999; Slapeta et al., 2002, 2003; Dubey and Schares, 2006). Although some N. caninum isolates have minor sequence variations in the Nc5 and ITS1/D2-LSU rDNA regions, sufficient polymorphisms have not been identified to allow these genes to be useful for strain differentiation (Slapeta et al., 2002; Gondim et al., 2004c; Al-Qassab et al., 2010).
In low-level infections, the ability to detect N. caninum by PCR depends largely upon sampling strategy. Because N. caninum is assumed to encyst most commonly in the brain (Dubey et al., 2007; Dubey and Schares, 2011), it is the tissue used most frequently in molecular surveys of N. caninum infection in wildlife. Multiple studies have identified parasite DNA in other tissues, in some instances with greater frequency than the brain (Hughes et al., 2006; Ferroglio et al., 2007; Kang et al., 2009; Gondim et al., 2010; Sangster et al., 2010; Truppel et al., 2010; Bartley et al., 2013b; Medina-Esparza et al., 2013). Analysis of multiple tissues has been shown to increase the sensitivity of N. caninum detection and additional tissues in which N. caninum DNA has been commonly recognized include skeletal muscle, heart, liver, kidney, spinal cord, and lung (Hughes et al., 2006; Ferroglio et al., 2007; Kang et al., 2009; Gondim et al., 2010; Sangster et al., 2010; Truppel et al., 2010; Bartley et al., 2013b; Medina-Esparza et al., 2013), with skeletal muscle and liver in rodents (Ferroglio et al., 2007; Kang et al., 2009; Truppel et al., 2010; Medina-Esparza et al., 2013) and heart in sparrows (Gondim et al., 2010) identified by some studies as the tissues most likely to be PCR positive for N. caninum. Studies have also shown that testing more than one section of brain significantly increases the likelihood of finding N. caninum DNA (Hughes et al., 2008; Stuart et al., 2013). The tissue distribution of parasites in wildlife is not known, so to preclude underestimation of infection, we advise sampling multiple tissues (listed above) and at least two samples of brain to assess for N. caninum infection in molecular investigations. Tissues for molecular studies should be aseptically collected and stored frozen at −20 °C until used. It is important to note that in some studies, tissue samples were PCR positive for the ITS1 region but did not amplify the Nc5 region, possibly due to variation in copy number within the genome, which emphasizes the importance of using more than one marker for molecular investigations (Jenkins et al., 2007; Truppel et al., 2010).
8.6. Quantitative PCR
At present, quantitative PCR for N. caninum is only used in academic research but we mention it here because it has the potential to be a valuable tool for investigations of N. caninum infection and pathogenesis of neosporosis in wildlife. Compared with conventional and nested PCR, qPCR has greater sensitivity, does not require as much starting material, and allows both detection and quantitative estimation of N. caninum in biological samples. Several different qPCR techniques targeting the Nc5 gene are described (Collantes-Fernandez et al., 2002; Muller et al., 2002; Okeoma et al., 2005; Ghalmi et al., 2008). To our knowledge, qPCR has not been used in any wildlife species.
8.7. Mini- and microsatellite analysis – tools for genotyping
Mini- and microsatellites are short repetitive DNA elements ubiquitously distributed throughout the genomes of eukaryotic and prokaryotic organisms that are being used with increasing frequency for studies of genetic diversity in many organisms, including protozoa (Basso et al., 2009, 2010; Al-Qassab et al., 2010; Regidor-Cerrillo et al., 2013). These tandemly repetitive DNA motifs vary from 2–6 nucleotides (microsatellites) to 8–100 nucleotides (minisatellites) in length (Al-Qassab et al., 2010). Unique genetic patterns have been established for different N. caninum strains by analysis of length polymorphisms in microsatellite and minisatellite containing areas, and this technology is currently considered the gold standard for evaluating N. caninum genetic diversity (Al-Qassab et al., 2010; Goodswen et al., 2013). The microsatellite-containing region MS10 is a highly polymorphic locus that is particularly useful for characterizing divergence between different N. caninum strains and has shown excellent discriminatory power in detecting novel alleles (Basso et al., 2009; Al-Qassab et al., 2010). Recent description of multiplex microsatellite genotyping methods will enable the rapid assessment of N. caninum strain genetic diversity (Al-Qassab et al., 2010; Regidor-Cerrillo et al., 2013). Current applications include epidemiological investigation of abortion storms in cattle, differentiating between challenge and vaccine strains in vaccine development research, and population genetic diversity studies (Al-Qassab et al., 2010; Goodswen et al., 2013; Regidor-Cerrillo et al., 2013).
At present, most molecular investigations of N. caninum in wildlife have focused on detecting parasite DNA in tissue and little has been done to further characterize the parasite. Molecular genotyping techniques utilizing mini- and microsatellite technologies will be useful for epidemiological studies involving wildlife species and emerging new isolates and provide valuable information about the importance of sylvatic cycles in the biology of this parasite (Al-Qassab et al., 2010; Goodswen et al., 2013; Regidor-Cerrillo et al., 2013). Availability of the N. caninum draft genome sequence should be used to revisit the assay of markers and possibly develop a new array that may better define the N. caninum population structure.
8.8. Next generation sequencing (NGS)
Next generation sequencing (NGS) is an emerging high-throughput diagnostic technique that utilizes generic primers to amplify a conservative region of DNA found in all eukaryotic species followed by sequencing of numerous short strands of both host and microbial DNA (Sun et al., 2011; Fournier et al., 2013). In recent years, NGS technology has developed rapidly and become an important analytical tool used in many research investigations including human infectious disease investigations and assessments of microbial community composition in a variety of environments (Sun et al., 2011; Fournier et al., 2013; Slapeta and Linares, 2013). Advantages of this technology as compared to other diagnostic tests include identification of co-infections with other pathogens, and discriminatory capacity for genotyping and molecular epidemiologic analysis (Fournier et al., 2013; Slapeta and Linares, 2013). Disadvantages include cost of sequencing (although this is decreasing), unknown sensitivity of the generic primers for N. caninum and need for bioinformatics for sequence analysis of tens of thousands of sequence reads generated in a single assay (Fournier et al., 2013). NGS is not currently used in the standard diagnosis of N. caninum infection but it has been used successfully to identify the presence of apicomplexan protists (Slapeta and Linares, 2013) and represents a valuable diagnostic technique for investigations of wildlife disease and microbial community DNA analysis.
9. Neosporosis in wildlife: the right time for reflection
9.1. What is the impact of N. caninum infection on wildlife populations?
To date, an extensive number of wildlife species have been investigated for their possible role in the N. caninum life cycle and many have been implicated as intermediate hosts; however, the occurrence and importance of disease due to infection in nondomestic animals remain poorly understood. Most reports of N. caninum infection in wildlife species are based on serologic and/or molecular evidence in asymptomatic animals and, in many instances, investigations of possible associated morbidity, mortality, and pathology have been neglected. Histopathologic assessment to screen infected animals for evidence of pathologic changes and chronic infection (i.e., presence of tissue cysts) is a critical factor in building a complete picture of infection outcome and identifying wild animal intermediate hosts. Although current data would indicate that N. caninum does not appear to adversely impact wildlife populations, as evidenced by the low number of animals reported with clinical versus subclinical infection, additional investigation is warranted to clarify the significance of infection in nondomestic species and the role it may play in possible parasite transmission in natural conditions. Because N. caninum tissue cysts can easily be mistaken for other tissue cyst forming apicomplexan parasites, the detection of a protozoan tissue cyst should be followed up with IHC.
9.2. How often is N. caninum infection clinically relevant and what factors predispose to pathologic sequelae in nondomestic species?
There are only 12 reports of neosporosis in wildlife species (summarized in Table 1). White rhinoceros are over-represented and account for 25% (3/12) of reported cases. Of the additional nine reports, all involved different species. Five cases were described in artiodactyls (four cervids and one bovine), three reports were in carnivores (two canids and one mustelid), and one report was in a marsupial (macropod). With the exception of one case, all reports of neosporosis in nondomestic species were in captive animals (9/12 cases; 75%) or free-ranging wildlife species maintained in rehabilitation facilities at the time of diagnosis (2/12 cases; 12.7%). Captivity induced stress is believed to play a role in the development of toxoplasmosis (Dubey, 2010), and although it is tempting to conclude that captivity is also a risk factor for N. caninum-associated disease, these findings may be more reflective of sampling bias because captive animals are far more likely to receive a complete postmortem assessment. Similar to domestic dogs and cattle, most reports of neosporosis are in young or fetal animals, with only 2/12 (16.7%) cases described in adults (Dubey et al., 2006, 2007; Dubey and Schares, 2011). Concurrent disease, identified as a risk factor for neosporosis in cattle (Dubey et al., 2007; Dubey and Schares, 2011), was only reported in a fox (8.3%); however, additional diagnostic tests were inconsistently described and unidentified concurrent subclinical disease processes cannot be ruled out.
Clinical disease and pathologic changes associated with N. caninum infection in wildlife species are comparable to what has been reported for domestic dogs and cattle. In summary, affected nondomestic species are commonly found dead (7/12 cases; 58.3%) or exhibit neuromuscular signs (4/12 cases; 33%). Encephalitis (6/12 cases; 50%), myocarditis (4/12 cases; 33%), and hepatic necrosis (3/12; 25%) are frequently reported lesions.
9.3. What are the best practice guidelines for reporting wildlife cases of neosporosis?
At present, there are no generally accepted recommendations for the optimal tissues to collect or tests to perform in order to diagnosis N. caninum infection in nondomestic animals. With the goal of establishing greater international uniformity in making a diagnosis of neosporosis in wildlife, we propose that the following minimum criteria should be met whenever cases are suspected (Table 3).
Table 3.
Best practice protocol: criteria for reporting cases of neosporosis in wildlife species.
| Technique | Minimum | Optimum | Aspirational | Comments |
|---|---|---|---|---|
| Clinical history | X | X | X | A thorough clinical history and baseline health assessment on any animal exhibiting clinical disease or being handled for N. caninum screening purposes. Block submitted to public repository. |
| Histopathology | X | X | X | A necropsy and histopathology for animals with suspected neosporosis. Lesions and parasites are most commonly identified in the brain, spinal cord, heart, skeletal muscle, liver, and placenta. |
| IHC | X | X | X | IHC increases the likelihood of detecting parasites over histopathology alone. To rule out presence of closely related parasites such as Toxoplasma gondii and antibodies cross reactivity, IHC for T. gondii in conjunction with IHC for N. caninum is done.* |
| Serology | X | X | cELISA and NAT as they do not require species specific secondary antibodies.** | |
| PCR | X | X | N. caninum specific PCR (e.g., Nc5). PCR amplification of phylogenetically informative genes region (e.g., ITS1, D2 LSU rDNA) directly bidirectionally sequenced and submitted to public repository. Tissues in which N. caninum DNA is most commonly detected by PCR are brain, spinal cord, skeletal muscle, heart, liver, kidney, and lung. Due to uncertainty regarding parasite tissue distribution in wildlife, to maximize likelihood of parasite detection, PCR should be done on multiple tissues, including two sections or more of brain. | |
| Multilocus genotyping | X | Available mini- and microsatellite N. caninum genotyping PCR assay applied to asses if multiple genotypes are present in wildlife cases. | ||
| Isolation | X | Brain is most commonly used for parasite isolation studies. | ||
| TEM | X | Rarely done. | ||
| Microbiome / NGS | X | Not reported to date. |
For additional details on various diagnostic tests and sample collection, please refer to Section 8.
H&E, hematoxylin and eosin; IHC, immunohistochemistry; cELISA, competitive enzyme linked immunosorbent assay; NAT, Neospora agglutination test; TEM, transmission electron microscopy.
At the Veterinary Pathology Diagnostics Services, the following antibodies have been routinely utilized: for IHC-N.caninum – polyclonal caprine anti-N. caninum antibodies (Product Code PAB-NC, VMRD, Inc., Veterinary Medical Research and Development, Pullman, WA, USA); for IHC-T. gondii – polyclonal rabbit anti-T. gondii antibodies (Product Code RB-282-A, Thermo-Fisher Scientific Australia).
Commercially available N. caninum serological tests are reviewed by Dubey and Schares (2006).
A best practice diagnosis for N. caninum infection in wildlife should include the following:
-
(1)
A thorough clinical history and baseline health assessment should be obtained (whenever possible) on any animal exhibiting clinical disease or being handled for N. caninum screening purposes.
-
(2)
A necropsy including histopathology is always recommended for animals with suspected neosporosis. At necropsy, a variety of tissues should be collected and (a) stored frozen at −20 °C for future evaluation, and (b) fixed in 10% formalin and processed into paraffin blocks within 7 days. Tissue specimen collection should follow the guidelines outlined in section 8.2 and 8.5.
-
(3)
Diagnosis of N. caninum infection necessitates the detection of parasite antigen and DNA using both IHC and PCR, respectively. Molecular diagnostics based on PCR should include at least two independent markers. Primers for cyst forming coccidia should be used to rule out presence of similar parasites (e.g., Toxoplasma, Sarcocystis, Besnoitia, and Hammondia). Tissue cyst presence should be reported and cross-reactivity with anti-T. gondii antibody confirmed. Correlation of immunological status with additional diagnostic tests should be implemented whenever possible to strengthen the diagnosis of neosporosis.
-
(4)
Materials such as paraffin blocks, frozen material and full case documentation should be deposited in a public depository for wide dissemination and retrieval.
This best practice protocol for reporting cases of N. caninum infection will optimize our knowledge about N. caninum infection in nondomestic species and enable us to better assess the consequences of infection, incidence of clinical disease, and impact of this parasite on wildlife populations. While the above recommendations are mentioned as aspirational, we believe that those reports that do not include such data should be returned to researchers to complete appropriate analyses.
The incidence of neosporosis is low and consequently it may not be on top of mind in wildlife deaths. Wild animals, especially prey species, are renowned for dying without displaying clinical signs. Optimal sample collection and storage will facilitate ancillary diagnostic tests and confirmation of N. caninum infection even when it may not be initially suspected.
10. Conclusions
Investigations to date of N. caninum infection in free-ranging and captive wildlife have opened a productive research agenda. The present paper has highlighted multiple gaps in our knowledge base and raised questions about the effects of this parasite in wild animal health. Studies aimed at elucidating the role of wild animals in maintaining the parasite sylvatic cycle have identified many species as possible intermediate hosts and enhanced our knowledge about the parasite in wildlife. However, available data largely refer to detection of N. caninum antibodies and/or DNA, and there remain few reports of disease or associated pathological findings. It remains unclear whether or not N. caninum infection poses a substantial health risk to individuals within the nondomestic species, and the impact this has on a population basis. Furthermore, the disjoin between an expanding number of reports of N. caninum exposure and infection and the limited number (12) of studies reporting clinical neosporosis continue to increase. Reports must be interpreted conservatively when only one or a limited number of diagnostic tests are used to identify infection, particularly when histopathology is not available to assess host–parasite interaction and associated pathology. In order to ascertain the pathological impact of the parasite in wildlife populations and more accurately determine how often clinical disease attributable to N. caninum infection occurs, there is a need for greater uniformity in establishing a diagnosis of N. caninum infection. Therefore, our recommended protocol for reporting cases of neosporosis in nondomestic species offers an integrated approach for parasite detection and is intended to standardize future studies and allow comparisons between laboratories.
Unraveling the circumstances under which N. caninum infection triggers disease and chronic infections in nondomestic species continues to be a more difficult challenge. Immunological and pathogenicity investigations in wild animals are inherently challenging due to the lack of suitable experimental models. New animal models using susceptible animals are needed. Utilizing available N. caninum draft genome data and advanced diagnostic techniques in wildlife studies, such as microsatellite analysis and NGS technologies, will enhance our knowledge of N. caninum infection by facilitating epidemiological investigation, thereby allowing clinical manifestations of disease to be associated with parasite genotype and virulence, and the presence of co-infection with other microbial agents.
Conflict of interest
The authors declared that there is no conflict of interest.
Acknowledgements
The authors thank John Ellis, Milton McAllister, Bronwyn McAllen, Graeme Brown, Cheryl Sangster, Michelle Campbell-Ward, and Benn Bryant for discussion and valuable insight on this topic. The authors also acknowledge the Taronga Conservation Society Australia for the use of photomicrographs and the University of Sydney Veterinary Pathology Diagnostic Services for excellent technical assistance. This research was supported by funding from The Whitehead Bequest and the Dr. Williams Award in Veterinary Pathology (University of Sydney). Shannon L. Donahoe is supported by the International Postgraduate Research Scholarship (IPRS) and an Australian Postgraduate Award (APA) tenable at The University of Sydney. The authors extend their apologies to those authors whose work could not be cited owing to space limitations.
References
- Al-Qassab S., Reichel M.P., Ellis J. On the biological and genetic diversity in Neospora caninum. Diversity. 2010;2:411–438. [Google Scholar]
- Almberg E.S., Mech L.D., Smith D.W., Sheldon J.W., Crabtree R.L. A serological survey of infectious disease in Yellowstone National Park's canid community. PLoS ONE. 2009;4:e7042. doi: 10.1371/journal.pone.0007042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeria S. Neospora caninum and wildlife. ISRN Parasitol. 2013;2013:1–23. doi: 10.5402/2013/947347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeria S., Ferrer D., Pabon M., Castella J., Manas S. Red foxes (Vulpes vulpes) are a natural intermediate host of Neospora caninum. Vet. Parasitol. 2002;107:287–294. doi: 10.1016/s0304-4017(02)00162-0. [DOI] [PubMed] [Google Scholar]
- Almeria S., Vidal D., Ferrer D., Pabon M., Fernandez-de-Mera M.I., Ruiz-Fons F. Seroprevalence of Neospora caninum in non-carnivorous wildlife from Spain. Vet. Parasitol. 2007;143:21–28. doi: 10.1016/j.vetpar.2006.07.027. [DOI] [PubMed] [Google Scholar]
- Anderson T., DeJardin A., Howe D.K., Dubey J.P., Michalski M.L. Neospora caninum antibodies detected in Midwestern white-tailed deer (Odocoileus virginianus) by Western blot and ELISA. Vet. Parasitol. 2007;145:152–155. doi: 10.1016/j.vetpar.2006.11.012. [DOI] [PubMed] [Google Scholar]
- Andre M.R., Adania C.H., Teixeira R.H., Silva K.F., Jusi M.M., Machado S.T. Antibodies to Toxoplasma gondii and Neospora caninum in captive neotropical and exotic wild canids and felids. J. Parasitol. 2010;96:1007–1009. doi: 10.1645/GE-2502.1. [DOI] [PubMed] [Google Scholar]
- Atkinson R., Harper P.A., Ryce C., Morrison D.A., Ellis J.T. Comparison of the biological characteristics of two isolates of Neospora caninum. Parasitology. 1999;118(Pt 4):363–370. doi: 10.1017/s0031182098003898. [DOI] [PubMed] [Google Scholar]
- Barber J.S., Trees A.J. Clinical aspects of 27 cases of neosporosis in dogs. Vet. Rec. 1996;139:439–443. doi: 10.1136/vr.139.18.439. [DOI] [PubMed] [Google Scholar]
- Barber J.S., Gasser R.B., Ellis J., Reichel M.P., McMillan D., Trees A.J. Prevalence of antibodies to Neospora caninum in different canid populations. J. Parasitol. 1997;83:1056–1058. [PubMed] [Google Scholar]
- Barling K.S., Sherman M., Peterson M.J., Thompson J.A., McNeill J.W., Craig T.M. Spatial associations among density of cattle, abundance of wild canids, and seroprevalence to Neospora caninum in a population of beef calves. J. Am. Vet. Med. Assoc. 2000;217:1361–1365. doi: 10.2460/javma.2000.217.1361. [DOI] [PubMed] [Google Scholar]
- Barr B.C., Anderson M.L., Dubey J.P., Conrad P.A. Neospora-like protozoal infections associated with bovine abortions. Vet. Pathol. 1991;28:110–116. doi: 10.1177/030098589102800202. [DOI] [PubMed] [Google Scholar]
- Barr B.C., Conrad P.A., Sverlow K.W., Tarantal A.F., Hendrickx A.G. Experimental fetal and transplacental Neospora infection in the nonhuman primate. Lab. Invest. 1994;71:236–242. [PubMed] [Google Scholar]
- Barratt J., Al Qassab S., Reichel M.P., Ellis J.T. The development and evaluation of a nested PCR assay for detection of Neospora caninum and Hammondia heydorni in feral mouse tissues. Mol. Cell. Probes. 2008;22:228–233. doi: 10.1016/j.mcp.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Barratt J.L., Harkness J., Marriott D., Ellis J.T., Stark D. Importance of nonenteric protozoan infections in immunocompromised people. Clin. Microbiol. Rev. 2010;23:795–836. doi: 10.1128/CMR.00001-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels C.J., Wouda W., Schukken Y.H. Risk factors for Neospora caninum-associated abortion storms in dairy herds in the Netherlands (1995 to 1997) Theriogenology. 1999;52:247–257. doi: 10.1016/S0093-691X(99)00126-0. [DOI] [PubMed] [Google Scholar]
- Bartels C.J., Huinink I., Beiboer M.L., van Schaik G., Wouda W., Dijkstra T. Quantification of vertical and horizontal transmission of Neospora caninum infection in Dutch dairy herds. Vet. Parasitol. 2007;148:83–92. doi: 10.1016/j.vetpar.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Bartley P.M., Katzer F., Rocchi M.S., Maley S.W., Benavides J., Nath M. Development of maternal and foetal immune responses in cattle following experimental challenge with Neospora caninum at day 210 of gestation. Vet. Res. 2013;44:91. doi: 10.1186/1297-9716-44-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartley P.M., Wright S.E., Zimmer I.A., Roy S., Kitchener A.C., Meredith A. Detection of Neospora caninum in wild carnivorans in Great Britain. Vet. Parasitol. 2013;192:279–283. doi: 10.1016/j.vetpar.2012.10.001. [DOI] [PubMed] [Google Scholar]
- Bartova E., Sedlak K., Literak I. Prevalence of Toxoplasma gondii and Neospora caninum antibodies in wild boars in the Czech Republic. Vet. Parasitol. 2006;142:150–153. doi: 10.1016/j.vetpar.2006.06.022. [DOI] [PubMed] [Google Scholar]
- Bartova E., Sedlak K., Treml F., Holko I., Literak I. Neospora caninum and Toxoplasma gondii antibodies in European brown hares in the Czech Republic, Slovakia and Austria. Vet. Parasitol. 2010;171:155–158. doi: 10.1016/j.vetpar.2010.03.002. [DOI] [PubMed] [Google Scholar]
- Basso W., Venturini L., Venturini M.C., Moore P., Rambeau M., Unzaga J.M. Prevalence of Neospora caninum infection in dogs from beef-cattle farms, dairy farms, and from urban areas of Argentina. J. Parasitol. 2001;87:906–907. doi: 10.1645/0022-3395(2001)087[0906:PONCII]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Basso W., Schares S., Barwald A., Herrmann D.C., Conraths F.J., Pantchev N. Molecular comparison of Neospora caninum oocyst isolates from naturally infected dogs with cell culture-derived tachyzoites of the same isolates using nested polymerase chain reaction to amplify microsatellite markers. Vet. Parasitol. 2009;160:43–50. doi: 10.1016/j.vetpar.2008.10.085. [DOI] [PubMed] [Google Scholar]
- Basso W., Schares S., Minke L., Barwald A., Maksimov A., Peters M. Microsatellite typing and avidity analysis suggest a common source of infection in herds with epidemic Neospora caninum-associated bovine abortion. Vet. Parasitol. 2010;173:24–31. doi: 10.1016/j.vetpar.2010.06.009. [DOI] [PubMed] [Google Scholar]
- Basso W., More G., Quiroga M.A., Balducchi D., Schares G., Venturini M.C. Neospora caninum is a cause of perinatal mortality in axis deer (Axis axis) Vet. Parasitol. 2014;199:255–258. doi: 10.1016/j.vetpar.2013.10.020. [DOI] [PubMed] [Google Scholar]
- Belov K., Miller R.D., Old J.M., Young L.J. Marsupial immunology bounding ahead. Aust. J. Zool. 2013;61:24–40. [Google Scholar]
- Bevins S., Blizzard E., Bazan L., Whitley P. Neospora caninum exposure in overlapping populations of coyotes (Canis latrans) and feral swine (Sus scrofa) J. Wildl. Dis. 2013;49:1028–1032. doi: 10.7589/2013-02-034. [DOI] [PubMed] [Google Scholar]
- Bien J., Moskwa B., Cabaj W. In vitro isolation and identification of the first Neospora caninum isolate from European bison (Bison bonasus bonasus L.) Vet. Parasitol. 2010;173:200–205. doi: 10.1016/j.vetpar.2010.06.038. [DOI] [PubMed] [Google Scholar]
- Billinis C. Wildlife diseases that pose a risk to small ruminants and their farmers. Small Ruminant Res. 2013;110:67–70. [Google Scholar]
- Bjerkas I., Mohn S.F., Presthus J. Unidentified cyst-forming sporozoon causing encephalomyelitis and myositis in dogs. Z. Parasitenkd. 1984;70:271–274. doi: 10.1007/BF00942230. [DOI] [PubMed] [Google Scholar]
- Bjorkman C., Jakubek E.B., Arnemo J.M., Malmsten J. Seroprevalence of Neospora caninum in gray wolves in Scandinavia. Vet. Parasitol. 2010;173:139–142. doi: 10.1016/j.vetpar.2010.06.006. [DOI] [PubMed] [Google Scholar]
- Bottari N.B., Tonin A.A., Fighera R., Flores M.M., Franca R.T., Camillo G. Neospora caninum and Toxoplasma gondii: relationship between hepatic lesions, cytological and biochemical analysis of the cavitary liquid during the acute phase of the diseases in experimental models. Exp. Parasitol. 2014;136:68–73. doi: 10.1016/j.exppara.2013.11.004. [DOI] [PubMed] [Google Scholar]
- Boyd S.P., Barr P.A., Brooks H.W., Orr J.P. Neosporosis in a young dog presenting with dermatitis and neuromuscular signs. J. Small Anim. Pract. 2005;46:85–88. doi: 10.1111/j.1748-5827.2005.tb00298.x. [DOI] [PubMed] [Google Scholar]
- Bregoli M., Gioia C., Stefano N., Mariapia C., Claudio P. Serological survey of Neospora caninum in free-ranging wild ruminants. Vet. Arhiv. 2006;76:S111–S115. [Google Scholar]
- Brown C.B., Baker D.C., Barker I.K. The alimentary system. In: Maxie M.G., editor. Jubb, Kennedy, and Palmer's Pathology of Domestic Animals. Elsevier Saunders; Philadelphia, PA: 2007. pp. 272–273. [Google Scholar]
- Buchmann K. Evolution of innate immunity: clues from invertebrates via fish to mammals. Front Immunol. 2014;5:459. doi: 10.3389/fimmu.2014.00459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxton D., Maley S.W., Pastoret P.P., Brochier B., Innes E.A. Examination of red foxes (Vulpes vulpes) from Belgium for antibody to Neospora caninum and Toxoplasma gondii. Vet. Rec. 1997;141:308–309. doi: 10.1136/vr.141.12.308. [DOI] [PubMed] [Google Scholar]
- Buxton D., McAllister M.M., Dubey J.P. The comparative pathogenesis of neosporosis. Trends Parasitol. 2002;18:546–552. doi: 10.1016/s1471-4922(02)02414-5. [DOI] [PubMed] [Google Scholar]
- Cabaj W., Moskwa B., Pastusiak K., Gill J. Antibodies to Neospora caninum in the blood of European bison (Bison bonasus bonasus L.) living in Poland. Vet. Parasitol. 2005;128:163–168. doi: 10.1016/j.vetpar.2004.09.033. [DOI] [PubMed] [Google Scholar]
- Candela M.G., Serrano E., Sevila J., Leon L., Caro M.R., Verheyden H. Pathogens of zoonotic and biological importance in roe deer (Capreolus capreolus): seroprevalence in an agro-system population in France. Res. Vet. Sci. 2014;96:254–259. doi: 10.1016/j.rvsc.2014.02.003. [DOI] [PubMed] [Google Scholar]
- Canon-Franco W.A., Yai L.E., Souza S.L., Santos L.C., Farias N.A., Ruas J. Detection of antibodies to Neospora caninum in two species of wild canids, Lycalopex gymnocercus and Cerdocyon thous from Brazil. Vet. Parasitol. 2004;123:275–277. doi: 10.1016/j.vetpar.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Cantile C., Arispici M. Necrotizing cerebellitis due to Neospora caninum infection in an old dog. J. Vet. Med. A Physiol. Pathol. Clin. Med. 2002;49:47–50. doi: 10.1046/j.1439-0442.2002.00400.x. [DOI] [PubMed] [Google Scholar]
- Cheadle M.A., Spencer J.A., Blagburn B.L. Seroprevalences of Neospora caninum and Toxoplasma gondii in nondomestic felids from southern Africa. J. Zoo Wildl. Med. 1999;30:248–251. [PubMed] [Google Scholar]
- Cobadiova A., Vichova B., Majlathova V., Reiterova K. First molecular detection of Neospora caninum in European brown bear (Ursus arctos) Vet. Parasitol. 2013;197:346–349. doi: 10.1016/j.vetpar.2013.05.005. [DOI] [PubMed] [Google Scholar]
- Collantes-Fernandez E., Zaballos A., Alvarez-Garcia G., Ortega-Mora L.M. Quantitative detection of Neospora caninum in bovine aborted fetuses and experimentally infected mice by real-time PCR. J. Clin. Microbiol. 2002;40:1194–1198. doi: 10.1128/JCM.40.4.1194-1198.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collantes-Fernandez E., Gomez-Bautista M., Miro G., Alvarez-Garcia G., Pereira-Bueno J., Frisuelos C. Seroprevalence and risk factors associated with Neospora caninum infection in different dog populations in Spain. Vet. Parasitol. 2008;152:148–151. doi: 10.1016/j.vetpar.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Conrad P.A., Barr B.C., Sverlow K.W., Anderson M., Daft B., Kinde H. In vitro isolation and characterization of a Neospora sp. from aborted bovine foetuses. Parasitology. 1993;106(Pt 3):239–249. doi: 10.1017/s0031182000075065. [DOI] [PubMed] [Google Scholar]
- Costa K.S., Santos S.L., Uzeda R.S., Pinheiro A.M., Almeida M.A., Araujo F.R. Chickens (Gallus domesticus) are natural intermediate hosts of Neospora caninum. Int. J. Parasitol. 2008;38:157–159. doi: 10.1016/j.ijpara.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Cronstedt-Fell A., Richter B., Voracek T., Kubber-Heiss A. Neosporosis in a captive Parma wallaby (Macropus parma) J. Comp. Pathol. 2012;146:274–277. doi: 10.1016/j.jcpa.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Cuddon P., Lin D.S., Bowman D.D., Lindsay D.S., Miller T.K., Duncan I.D. Neospora caninum infection in English Springer Spaniel littermates. Diagnostic evaluation and organism isolation. J. Vet. Intern. Med. 1992;6:325–332. doi: 10.1111/j.1939-1676.1992.tb00364.x. [DOI] [PubMed] [Google Scholar]
- de Oliveira U.V., de Magalhaes V.C., Almeida C.P., Santos Idos A., Mota D.A., Macedo L.S. Quails are resistant to infection with Neospora caninum tachyzoites. Vet. Parasitol. 2013;198:209–213. doi: 10.1016/j.vetpar.2013.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darwich L., Cabezon O., Echeverria I., Pabon M., Marco I., Molina-Lopez R. Presence of Toxoplasma gondii and Neospora caninum DNA in the brain of wild birds. Vet. Parasitol. 2012;183:377–381. doi: 10.1016/j.vetpar.2011.07.024. [DOI] [PubMed] [Google Scholar]
- Davison H.C., Guy C.S., McGarry J.W., Guy F., Williams D.J., Kelly D.F. Experimental studies on the transmission of Neospora caninum between cattle. Res. Vet. Sci. 2001;70:163–168. doi: 10.1053/rvsc.2001.0457. [DOI] [PubMed] [Google Scholar]
- De Craeye S., Speybroeck N., Ajzenberg D., Darde M.L., Collinet F., Tavernier P. Toxoplasma gondii and Neospora caninum in wildlife: common parasites in Belgian foxes and Cervidae? Vet. Parasitol. 2011;178:64–69. doi: 10.1016/j.vetpar.2010.12.016. [DOI] [PubMed] [Google Scholar]
- De Marez T., Liddell S., Dubey J.P., Jenkins M.C., Gasbarre L. Oral infection of calves with Neospora caninum oocysts from dogs: humoral and cellular immune responses. Int. J. Parasitol. 1999;29:1647–1657. doi: 10.1016/s0020-7519(99)00154-x. [DOI] [PubMed] [Google Scholar]
- Dellarupe A., Regidor-Cerrillo J., Jimenez-Ruiz E., Schares G., Unzaga J.M., Venturini M.C. Clinical outcome and vertical transmission variability among canine Neospora caninum isolates in a pregnant mouse model of infection. Parasitology. 2014;141:356–366. doi: 10.1017/S0031182013001479. [DOI] [PubMed] [Google Scholar]
- Dijkstra T., Eysker M., Schares G., Conraths F.J., Wouda W., Barkema H.W. Dogs shed Neospora caninum oocysts after ingestion of naturally infected bovine placenta but not after ingestion of colostrum spiked with Neospora caninum tachyzoites. Int. J. Parasitol. 2001;31:747–752. doi: 10.1016/s0020-7519(01)00230-2. [DOI] [PubMed] [Google Scholar]
- Dubey J.P. Review of Neospora caninum and neosporosis in animals. Korean J. Parasitol. 2003;41:1–16. doi: 10.3347/kjp.2003.41.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey J.P. second ed. CRC Press; Boca Raton, FL: 2010. Toxoplasmosis of Animals and Humans. [Google Scholar]
- Dubey J.P., Lindsay D.S. A review of Neospora caninum and neosporosis. Vet. Parasitol. 1996;67:1–59. doi: 10.1016/s0304-4017(96)01035-7. [DOI] [PubMed] [Google Scholar]
- Dubey J.P., Lindsay D.S. Gerbils (Meriones unguiculatus) are highly susceptible to oral infection with Neospora caninum oocysts. Parasitol. Res. 2000;86:165–168. doi: 10.1007/s004360050027. [DOI] [PubMed] [Google Scholar]
- Dubey J.P., Schares G. Diagnosis of bovine neosporosis. Vet. Parasitol. 2006;140:1–34. doi: 10.1016/j.vetpar.2006.03.035. [DOI] [PubMed] [Google Scholar]
- Dubey J.P., Schares G. Neosporosis in animals–the last five years. Vet. Parasitol. 2011;180:90–108. doi: 10.1016/j.vetpar.2011.05.031. [DOI] [PubMed] [Google Scholar]
- Dubey J.P., Thulliez P. Prevalence of antibodies to Neospora caninum in wild animals. J. Parasitol. 2005;91:1217–1218. doi: 10.1645/GE-576R.1. [DOI] [PubMed] [Google Scholar]
- Dubey J.P., Carpenter J.L., Speer C.A., Topper M.J., Uggla A. Newly recognized fatal protozoan disease of dogs. J. Am. Vet. Med. Assoc. 1988;192:1269–1285. [PubMed] [Google Scholar]
- Dubey J.P., Miller S., Lindsay D.S., Topper M.J. Neospora caninum-associated myocarditis and encephalitis in an aborted calf. J. Vet. Diagn. Invest. 1990;2:66–69. doi: 10.1177/104063879000200113. [DOI] [PubMed] [Google Scholar]
- Dubey J.P., Rigoulet J., Lagourette P., George C., Longeart L., LeNet J.L. Fatal transplacental neosporosis in a deer (Cervus eldi siamensis) J. Parasitol. 1996;82:338–339. [PubMed] [Google Scholar]
- Dubey J.P., Hollis K., Romand S., Thulliez P., Kwok O.C., Hungerford L. High prevalence of antibodies to Neospora caninum in white-tailed deer (Odocoileus virginianus) Int. J. Parasitol. 1999;29:1709–1711. doi: 10.1016/s0020-7519(99)00142-3. [DOI] [PubMed] [Google Scholar]
- Dubey J.P., Barr B.C., Barta J.R., Bjerkas I., Bjorkman C., Blagburn B.L. Redescription of Neospora caninum and its differentiation from related coccidia. Int. J. Parasitol. 2002;32:929–946. doi: 10.1016/s0020-7519(02)00094-2. [DOI] [PubMed] [Google Scholar]
- Dubey J.P., Zarnke R., Thomas N.J., Wong S.K., Van Bonn W., Briggs M. Toxoplasma gondii, Neospora caninum, Sarcocystis neurona, and Sarcocystis canis-like infections in marine mammals. Vet. Parasitol. 2003;116:275–296. doi: 10.1016/s0304-4017(03)00263-2. [DOI] [PubMed] [Google Scholar]
- Dubey J.P., Sreekumar C., Knickman E., Miska K.B., Vianna M.C., Kwok O.C. Biologic, morphologic, and molecular characterisation of Neospora caninum isolates from littermate dogs. Int. J. Parasitol. 2004;34:1157–1167. doi: 10.1016/j.ijpara.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Dubey J.P., Knickman E., Greene C.E. Neonatal Neosproa caninum infections in dogs. Acta Parasitol. 2005;50:176–179. [Google Scholar]
- Dubey J.P., Buxton D., Wouda W. Pathogenesis of bovine neosporosis. J. Comp. Pathol. 2006;134:267–289. doi: 10.1016/j.jcpa.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Dubey J.P., Schares G., Ortega-Mora L.M. Epidemiology and control of neosporosis and Neospora caninum. Clin. Microbiol. Rev. 2007;20:323–367. doi: 10.1128/CMR.00031-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey J.P., Mansfield K., Hall B., Kwok O.C., Thulliez P. Seroprevalence of Neospora caninum and Toxoplasma gondii in black-tailed deer (Odocoileus hemionus columbianus) and mule deer (Odocoileus hemionus hemionus) Vet. Parasitol. 2008;156:310–313. doi: 10.1016/j.vetpar.2008.06.011. [DOI] [PubMed] [Google Scholar]
- Dubey J.P., Jenkins M.C., Kwok O.C., Zink R.L., Michalski M.L., Ulrich V. Seroprevalence of Neospora caninum and Toxoplasma gondii antibodies in white-tailed deer (Odocoileus virginianus) from Iowa and Minnesota using four serologic tests. Vet. Parasitol. 2009;161:330–334. doi: 10.1016/j.vetpar.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Dubey J.P., Jenkins M.C., Rajendran C., Miska K., Ferreira L.R., Martins J. Gray wolf (Canis lupus) is a natural definitive host for Neospora caninum. Vet. Parasitol. 2011;181:382–387. doi: 10.1016/j.vetpar.2011.05.018. [DOI] [PubMed] [Google Scholar]
- Dubey J.P., Jenkins M.C., Kwok O.C., Ferreira L.R., Choudhary S., Verma S.K. Congenital transmission of Neospora caninum in white-tailed deer (Odocoileus virginianus) Vet. Parasitol. 2013;196:519–522. doi: 10.1016/j.vetpar.2013.03.004. [DOI] [PubMed] [Google Scholar]
- Dubey J.P., Jenkins M.C., Ferreira L.R., Choudhary S., Verma S.K., Kwok O.C. Isolation of viable Neospora caninum from brains of wild gray wolves (Canis lupus) Vet. Parasitol. 2014;201:150–153. doi: 10.1016/j.vetpar.2013.12.032. [DOI] [PubMed] [Google Scholar]
- Dubey J.P., Whitesell L.E., Culp W.E., Daye S. Diagnosis and treatment of Neospora caninum–associated dermatitis in a red fox (Vulpes vulpes) with concurrent Toxoplasma gondii infection. J. Zoo Wildl. Med. 2014;45:454–457. doi: 10.1638/2013-0277.1. [DOI] [PubMed] [Google Scholar]
- Ellis J.T., Amoyal G., Ryce C., Harper P.A., Clough K.A., Homan W.L. Comparison of the large subunit ribosomal DNA of Neospora and Toxoplasma and development of a new genetic marker for their differentiation based on the D2 domain. Mol. Cell. Probes. 1998;12:1–13. doi: 10.1006/mcpr.1997.0143. [DOI] [PubMed] [Google Scholar]
- Ellis J.T., Morrison D.A., Liddell S., Jenkins M.C., Mohammed O.B., Ryce C. The genus Hammondia is paraphyletic. Parasitology. 1999;118(Pt 4):357–362. doi: 10.1017/s0031182098003801. [DOI] [PubMed] [Google Scholar]
- Ezio F., Anna T. Antibodies to Neospora caninum in European brown hare (Lepus europaeus) Vet. Parasitol. 2003;115:75–78. doi: 10.1016/s0304-4017(03)00201-2. [DOI] [PubMed] [Google Scholar]
- Ferroglio E., Rossi L. Prevalence of Neospora caninum antibodies in wild ruminants from the Italian Alps. Vet. Rec. 2001;148:754–755. doi: 10.1136/vr.148.24.754. [DOI] [PubMed] [Google Scholar]
- Ferroglio E., Wambwa E., Castiello M., Trisciuoglio A., Prouteau A., Pradere E. Antibodies to Neospora caninum in wild animals from Kenya, East Africa. Vet. Parasitol. 2003;118:43–49. doi: 10.1016/j.vetpar.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Ferroglio E., Pasino M., Romano A., Grande D., Pregel P., Trisciuoglio A. Evidence of Neospora caninum DNA in wild rodents. Vet. Parasitol. 2007;148:346–349. doi: 10.1016/j.vetpar.2007.06.031. [DOI] [PubMed] [Google Scholar]
- Fournier P.-E., Drancourt M., Colson P., Rolain J.-M., Scola B.L., Raoult D. Modern clinical microbiology: new challenges and solutions. Nat. Rev. Microbiol. 2013;11:574–585. doi: 10.1038/nrmicro3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French N.P., Clancy D., Davison H.C., Trees A.J. Mathematical models of Neospora caninum infection in dairy cattle: transmission and options for control. Int. J. Parasitol. 1999;29:1691–1704. doi: 10.1016/s0020-7519(99)00131-9. [DOI] [PubMed] [Google Scholar]
- Fuehrer H.P., Bloschl I., Siehs C., Hassl A. Detection of Toxoplasma gondii, Neospora caninum, and Encephalitozoon cuniculi in the brains of common voles (Microtus arvalis) and water voles (Arvicola terrestris) by gene amplification techniques in western Austria (Vorarlberg) Parasitol. Res. 2010;107:469–473. doi: 10.1007/s00436-010-1905-z. [DOI] [PubMed] [Google Scholar]
- Fujii K., Kakumoto C., Kobayashi M., Saito S., Kariya T., Watanabe Y. Seroepidemiology of Toxoplasma gondii and Neospora caninum in seals around Hokkaido, Japan. J. Vet. Med. Sci. 2007;69:393–398. doi: 10.1292/jvms.69.393. [DOI] [PubMed] [Google Scholar]
- Furuta P.I., Mineo T.W., Carrasco A.O., Godoy G.S., Pinto A.A., Machado R.Z. Neospora caninum infection in birds: experimental infections in chicken and embryonated eggs. Parasitology. 2007;134:1931–1939. doi: 10.1017/S0031182007003344. [DOI] [PubMed] [Google Scholar]
- Gaffuri A., Giacometti M., Tranquillo V.M., Magnino S., Cordioli P., Lanfranchi P. Serosurvey of roe deer, chamois and domestic sheep in the central Italian Alps. J. Wildl. Dis. 2006;42:685–690. doi: 10.7589/0090-3558-42.3.685. [DOI] [PubMed] [Google Scholar]
- Garcia-Bocanegra I., Cabezon O., Pabon M., Gomez-Guillamon F., Arenas A., Alcaide E. Prevalence of Toxoplasma gondii and Neospora caninum antibodies in Spanish ibex (Capra pyrenaica hispanica) Vet. J. 2012;191:257–260. doi: 10.1016/j.tvjl.2010.11.011. [DOI] [PubMed] [Google Scholar]
- Garosi L., Dawson A., Couturier J., Matiasek L., de Stefani A., Davies E. Necrotizing cerebellitis and cerebellar atrophy caused by Neospora caninum infection: magnetic resonance imaging and clinicopathologic findings in seven dogs. J. Vet. Intern. Med. 2010;24:571–578. doi: 10.1111/j.1939-1676.2010.0485.x. [DOI] [PubMed] [Google Scholar]
- Ghalmi F., China B., Kaidi R., Daube G., Losson B. Detection of Neospora caninum in dog organs using real time PCR systems. Vet. Parasitol. 2008;155:161–167. doi: 10.1016/j.vetpar.2008.04.007. [DOI] [PubMed] [Google Scholar]
- Gibney E.H., Kipar A., Rosbottom A., Guy C.S., Smith R.F., Hetzel U. The extent of parasite-associated necrosis in the placenta and foetal tissues of cattle following Neospora caninum infection in early and late gestation correlates with foetal death. Int. J. Parasitol. 2008;38:579–588. doi: 10.1016/j.ijpara.2007.09.015. [DOI] [PubMed] [Google Scholar]
- Ginn P.E., Mansell J.E.K., Rakich P.M. The skin and appendages. In: Maxie M.G., editor. Jub, Kennedy, and Palmer's Pathology of Domestic Animals. Elsevier Saunders; Philadelphia, PA: 2007. p. 711. [Google Scholar]
- Gondim L.F. Neospora caninum in wildlife. Trends Parasitol. 2006;22:247–252. doi: 10.1016/j.pt.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Gondim L.F., Saeki H., Onaga H., Haritani M., Yamane I. Maintenance of Neospora caninum tachyzoites using Mongolian gerbils (Meriones unguiculatus) N. Z. Vet. J. 1999;47:36. doi: 10.1080/00480169.1999.36107. [DOI] [PubMed] [Google Scholar]
- Gondim L.F., Pinheiro A.M., Santos P.O., Jesus E.E., Ribeiro M.B., Fernandes H.S. Isolation of Neospora caninum from the brain of a naturally infected dog, and production of encysted bradyzoites in gerbils. Vet. Parasitol. 2001;101:1–7. doi: 10.1016/s0304-4017(01)00493-9. [DOI] [PubMed] [Google Scholar]
- Gondim L.F., McAllister M.M., Mateus-Pinilla N.E., Pitt W.C., Mech L.D., Nelson M.E. Transmission of Neospora caninum between wild and domestic animals. J. Parasitol. 2004;90:1361–1365. doi: 10.1645/GE-341R. [DOI] [PubMed] [Google Scholar]
- Gondim L.F., McAllister M.M., Pitt W.C., Zemlicka D.E. Coyotes (Canis latrans) are definitive hosts of Neospora caninum. Int. J. Parasitol. 2004;34:159–161. doi: 10.1016/j.ijpara.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Gondim L.F., Laski P., Gao L., McAllister M.M. Variation of the internal transcribed spacer 1 sequence within individual strains and among different strains of Neospora caninum. J. Parasitol. 2004;90:119–122. doi: 10.1645/GE-134R. [DOI] [PubMed] [Google Scholar]
- Gondim L.S., Abe-Sandes K., Uzeda R.S., Silva M.S., Santos S.L., Mota R.A. Toxoplasma gondii and Neospora caninum in sparrows (Passer domesticus) in the Northeast of Brazil. Vet. Parasitol. 2010;168:121–124. doi: 10.1016/j.vetpar.2009.09.055. [DOI] [PubMed] [Google Scholar]
- Goodswen S.J., Kennedy P.J., Ellis J.T. A review of the infection, genetics, and evolution of Neospora caninum: from the past to the present. Infect. Genet. Evol. 2013;13:133–150. doi: 10.1016/j.meegid.2012.08.012. [DOI] [PubMed] [Google Scholar]
- Gozdzik K., Jakubek E.B., Bjorkman C., Bien J., Moskwa B., Cabaj W. Seroprevalence of Neospora caninum in free living and farmed red deer (Cervus elaphus) in Poland. Pol. J. Vet. Sci. 2010;13:117–120. [PubMed] [Google Scholar]
- Gutierrez-Exposito D., Miguel Ortega-Mora L., Gajadhar A.A., Garcia-Lunar P., Dubey J.P., Alvarez-Garcia G. Serological evidence of Besnoitia spp. infection in Canadian wild ruminants and strong cross-reaction between Besnoitia besnoiti and Besnoitia tarandi. Vet. Parasitol. 2012;190:19–28. doi: 10.1016/j.vetpar.2012.06.017. [DOI] [PubMed] [Google Scholar]
- Hamilton C.M., Gray R., Wright S.E., Gangadharan B., Laurenson K., Innes E.A. Prevalence of antibodies to Toxoplasma gondii and Neospora caninum in red foxes (Vulpes vulpes) from around the UK. Vet. Parasitol. 2005;130:169–173. doi: 10.1016/j.vetpar.2005.03.020. [DOI] [PubMed] [Google Scholar]
- Helman R.G., Stair E.L., Lehenbauer T.W., Rodgers S., Saliki J.T. Neosporal abortion in Oklahoma cattle with emphasis on the distribution of brain lesions in aborted fetuses. J. Vet. Diagn. Invest. 1998;10:292–295. doi: 10.1177/104063879801000314. [DOI] [PubMed] [Google Scholar]
- Hemphill A., Vonlaufen N., Naguleswaran A. Cellular and immunological basis of the host-parasite relationship during infection with Neospora caninum. Parasitology. 2006;133:261–278. doi: 10.1017/S0031182006000485. [DOI] [PubMed] [Google Scholar]
- Hobson J.C., Duffield T.F., Kelton D., Lissemore K., Hietala S.K., Leslie K.E. Risk factors associated with Neospora caninum abortion in Ontario Holstein dairy herds. Vet. Parasitol. 2005;127:177–188. doi: 10.1016/j.vetpar.2004.09.025. [DOI] [PubMed] [Google Scholar]
- Holmdahl O.J., Mattsson J.G. Rapid and sensitive identification of Neospora caninum by in vitro amplification of the internal transcribed spacer 1. Parasitology. 1996;112(Pt 2):177–182. doi: 10.1017/s0031182000084742. [DOI] [PubMed] [Google Scholar]
- Hornok S., Edelhofer R., Fok E., Berta K., Fejes P., Repasi A. Canine neosporosis in Hungary: screening for seroconversion of household, herding and stray dogs. Vet. Parasitol. 2006;137:197–201. doi: 10.1016/j.vetpar.2006.01.030. [DOI] [PubMed] [Google Scholar]
- Huang C.C., Yang C.H., Watanabe Y., Liao Y.K., Ooi H.K. Finding of Neospora caninum in the wild brown rat (Rattus norvegicus) Vet. Res. 2004;35:283–290. doi: 10.1051/vetres:2004010. [DOI] [PubMed] [Google Scholar]
- Hughes J.M., Williams R.H., Morley E.K., Cook D.A., Terry R.S., Murphy R.G. The prevalence of Neospora caninum and co-infection with Toxoplasma gondii by PCR analysis in naturally occurring mammal populations. Parasitology. 2006;132:29–36. doi: 10.1017/S0031182005008784. [DOI] [PubMed] [Google Scholar]
- Hughes J.M., Thomasson D., Craig P.S., Georgin S., Pickles A., Hide G. Neospora caninum: detection in wild rabbits and investigation of co-infection with Toxoplasma gondii by PCR analysis. Exp. Parasitol. 2008;120:255–260. doi: 10.1016/j.exppara.2008.07.011. [DOI] [PubMed] [Google Scholar]
- Hurkova L., Modry D. PCR detection of Neospora caninum, Toxoplasma gondii and Encephalitozoon cuniculi in brains of wild carnivores. Vet. Parasitol. 2006;137:150–154. doi: 10.1016/j.vetpar.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Hurkova-Hofmannova L., Vaclavek P., Skoric M., Fictum P., Modry D. Multimammate rat (Mastomys natalensis), Tristram's jird (Meriones tristrami) and Wagner's gerbil (Gerbillus dasyurus) as laboratory models of acute neosporosis. Res. Vet. Sci. 2007;82:377–381. doi: 10.1016/j.rvsc.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Ibrahim H.M., Huang P., Salem T.A., Talaat R.M., Nasr M.I., Xuan X. Short report: prevalence of Neospora caninum and Toxoplasma gondii antibodies in northern Egypt. Am. J. Trop. Med. Hyg. 2009;80:263–267. [PubMed] [Google Scholar]
- Innes E.A., Andrianarivo A.G., Bjorkman C., Williams D.J., Conrad P.A. Immune responses to Neospora caninum and prospects for vaccination. Trends Parasitol. 2002;18:497–504. doi: 10.1016/s1471-4922(02)02372-3. [DOI] [PubMed] [Google Scholar]
- Innes E.A., Wright S., Bartley P., Maley S., Macaldowie C., Esteban-Redondo I. The host-parasite relationship in bovine neosporosis. Vet. Immunol. Immunopathol. 2005;108:29–36. doi: 10.1016/j.vetimm.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Jackson W., de Lahunta A., Adaska J., Cooper B., Dubey J.P. Neospora caninum in an adult dog with progressive cerebellar signs. Prog. Vet. Neurol. 1995;6:124–127. [Google Scholar]
- Jakubek E.B., Farkas R., Palfi V., Mattsson J.G. Prevalence of antibodies against Toxoplasma gondii and Neospora caninum in Hungarian red foxes (Vulpes vulpes) Vet. Parasitol. 2007;144:39–44. doi: 10.1016/j.vetpar.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Jenkins M.C., Parker C., Hill D., Pinckney R.D., Dyer R., Dubey J.P. Neospora caninum detected in feral rodents. Vet. Parasitol. 2007;143:161–165. doi: 10.1016/j.vetpar.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Kamga-Waladjo A.R., Gbati O.B., Kone P., Lapo R.A., Dombou E., Chatagnon G. Neospora caninum and Toxoplasma gondii in lion (Panthera leo) from Senegal, West Africa. Asian J. Anim. Vet. Adv. 2009;4:346–349. [Google Scholar]
- Kang S.W., Park S.S., Choe S.E., Jean Y.H., Jung S.C., Kim K. Characterization of tissue distribution and histopathological lesions in Neospora caninum experimentally infected gerbils. Parasitol. Res. 2009;104:1261–1268. doi: 10.1007/s00436-008-1322-8. [DOI] [PubMed] [Google Scholar]
- Kim J.H., Sohn H.J., Hwang W.S., Hwang E.K., Jean Y.H., Yamane I. In vitro isolation and characterization of bovine Neospora caninum in Korea. Vet. Parasitol. 2000;90:147–154. doi: 10.1016/s0304-4017(00)00224-7. [DOI] [PubMed] [Google Scholar]
- Kim J.H., Kang M.S., Lee B.C., Hwang W.S., Lee C.W., So B.J. Seroprevalence of antibodies to Neospora caninum in dogs and raccoon dogs in Korea. Korean J. Parasitol. 2003;41:243–245. doi: 10.3347/kjp.2003.41.4.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King J.S., Slapeta J., Jenkins D.J., Al-Qassab S.E., Ellis J.T., Windsor P.A. Australian dingoes are definitive hosts of Neospora caninum. Int. J. Parasitol. 2010;40:945–950. doi: 10.1016/j.ijpara.2010.01.008. [DOI] [PubMed] [Google Scholar]
- King J.S., McAllan B., Spielman D.S., Lindsay S.A., Hurkova-Hofmannova L., Hartigan A. Extensive production of Neospora caninum tissue cysts in a carnivorous marsupial succumbing to experimental neosporosis. Vet. Res. 2011;42:75. doi: 10.1186/1297-9716-42-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King J.S., Jenkins D.J., Ellis J.T., Fleming P., Windsor P.A., Slapeta J. Implications of wild dog ecology on the sylvatic and domestic life cycle of Neospora caninum in Australia. Vet. J. 2011;188:24–33. doi: 10.1016/j.tvjl.2010.03.002. [DOI] [PubMed] [Google Scholar]
- King J.S., Brown G.K., Jenkins D.J., Ellis J.T., Fleming P.J., Windsor P.A. Oocysts and high seroprevalence of Neospora caninum in dogs living in remote Aboriginal communities and wild dogs in Australia. Vet. Parasitol. 2012;187:85–92. doi: 10.1016/j.vetpar.2011.12.027. [DOI] [PubMed] [Google Scholar]
- Klevar S., Kulberg S., Boysen P., Storset A.K., Moldal T., Bjorkman C. Natural killer cells act as early responders in an experimental infection with Neospora caninum in calves. Int. J. Parasitol. 2007;37:329–339. doi: 10.1016/j.ijpara.2006.11.002. [DOI] [PubMed] [Google Scholar]
- La Perle K.M., Del Piero F., Carr R.F., Harris C., Stromberg P.C. Cutaneous neosporosis in two adult dogs on chronic immunosuppressive therapy. J. Vet. Diagn. Invest. 2001;13:252–255. doi: 10.1177/104063870101300312. [DOI] [PubMed] [Google Scholar]
- Lemberger K.Y., Gondim L.F., Pessier A.P., McAllister M.M., Kinsel M.J. Neospora caninum infection in a free-ranging raccoon (Procyon lotor) with concurrent canine distemper virus infection. J. Parasitol. 2005;91:960–961. doi: 10.1645/GE-407R.1. [DOI] [PubMed] [Google Scholar]
- Lindsay D.S., Dubey J.P. Neospora caninum (Protozoa: apicomplexa) infections in mice. J. Parasitol. 1989;75:772–779. [PubMed] [Google Scholar]
- Lindsay D.S., Kelly E.J., McKown R.D., Stein F.J., Plozer J., Herman J. Prevalence of Neospora caninum and Toxoplasma gondii antibodies in coyotes (Canis latrans) and experimental infections of coyotes with Neospora caninum. J. Parasitol. 1996;82:657–659. [PubMed] [Google Scholar]
- Lindsay D.S., Dubey J.P., Duncan R.B. Confirmation that the dog is a definitive host for Neospora caninum. Vet. Parasitol. 1999;82:327–333. doi: 10.1016/s0304-4017(99)00054-0. [DOI] [PubMed] [Google Scholar]
- Lindsay D.S., Spencer J., Rupprecht C., Blagburn B.L. Prevalence of agglutinating antibodies to Neospora caninum in raccoons, Procyon lotor. J. Parasitol. 2001;87:1197–1198. doi: 10.1645/0022-3395(2001)087[1197:POAATN]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Lindsay D.S., Little S.E., Davidson W.R. Prevalence of antibodies to Neospora caninum in white-tailed deer, Odocoileus virginianus, from the southeastern United States. J. Parasitol. 2002;88:415–417. doi: 10.1645/0022-3395(2002)088[0415:POATNC]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Litman G.W., Rast J.P., Fugmann S.D. The origins of vertebrate adaptive immunity. Nat. Rev. Immunol. 2010;10:543–553. doi: 10.1038/nri2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobato J., Silva D.A., Mineo T.W., Amaral J.D., Segundo G.R., Costa-Cruz J.M. Detection of immunoglobulin G antibodies to Neospora caninum in humans: high seropositivity rates in patients who are infected by human immunodeficiency virus or have neurological disorders. Clin. Vaccine Immunol. 2006;13:84–89. doi: 10.1128/CVI.13.1.84-89.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo V., Pumarola M., Siso S. Neosporosis with cerebellar involvement in an adult dog. J. Small Anim. Pract. 2002;43:76–79. doi: 10.1111/j.1748-5827.2002.tb00033.x. [DOI] [PubMed] [Google Scholar]
- Malmasi A., Hosseininejad M., Haddadzadeh H., Badii A., Bahonar A. Serologic study of anti-Neospora caninum antibodies in household dogs and dogs living in dairy and beef cattle farms in Tehran, Iran. Parasitol. Res. 2007;100:1143–1145. doi: 10.1007/s00436-006-0385-7. [DOI] [PubMed] [Google Scholar]
- Malmsten J., Jakubek E.B., Bjorkman C. Prevalence of antibodies against Toxoplasma gondii and Neospora caninum in moose (Alces alces) and roe deer (Capreolus capreolus) in Sweden. Vet. Parasitol. 2011;177:275–280. doi: 10.1016/j.vetpar.2010.11.051. [DOI] [PubMed] [Google Scholar]
- Marco I., Ferroglio E., Lopez-Olvera J.R., Montane J., Lavin S. High seroprevalence of Neospora caninum in the red fox (Vulpes vulpes) in the Pyrenees (NE Spain) Vet. Parasitol. 2008;152:321–324. doi: 10.1016/j.vetpar.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Martino P.E., Montenegro J.L., Preziosi J.A., Venturini C., Bacigalupe D., Stanchi N.O. Serological survey of selected pathogens of free-ranging foxes in southern Argentina, 1998–2001. Rev. Sci. Tech. 2004;23:801–806. doi: 10.20506/rst.23.3.1521. [DOI] [PubMed] [Google Scholar]
- Mattos B.C., Patricio L.L., Plugge N.F., Lange R.R., Richartz R.R., Dittrich R.L. [Seroprevalence of antibodies anti-Neospora caninum and anti-Toxoplasma gondii in captive wild canids] Rev. Bras. Parasitol. Vet. 2008;17(Suppl. 1):267–272. [PubMed] [Google Scholar]
- Mayberry C., Maloney S.K., Mitchell J., Mawson P.R., Bencini R. Reproductive implications of exposure to Toxoplasma gondii and Neospora caninum in western grey kangaroos (Macropus fuliginosus ocydromus) J. Wildl. Dis. 2014;50:364–368. doi: 10.7589/2013-03-064. [DOI] [PubMed] [Google Scholar]
- McAllister M.M., Dubey J.P., Lindsay D.S., Jolley W.R., Wills R.A., McGuire A.M. Dogs are definitive hosts of Neospora caninum. Int. J. Parasitol. 1998;28:1473–1478. [PubMed] [Google Scholar]
- McGuire A.M., McAllister M., Wills R.A., Tranas J.D. Experimental inoculation of domestic pigeons (Columbia livia) and zebra finches (Poephila guttata) with Neospora caninum tachyzoites. Int. J. Parasitol. 1999;29:1525–1529. doi: 10.1016/s0020-7519(99)00103-4. [DOI] [PubMed] [Google Scholar]
- McInnes L.M., Irwin P., Palmer D.G., Ryan U.M. In vitro isolation and characterisation of the first canine Neospora caninum isolate in Australia. Vet. Parasitol. 2006;137:355–363. doi: 10.1016/j.vetpar.2006.01.018. [DOI] [PubMed] [Google Scholar]
- Medina-Esparza L., Macias L., Ramos-Parra M., Morales-Salinas E., Quezada T., Cruz-Vazquez C. Frequency of infection by Neospora caninum in wild rodents associated with dairy farms in Aguascalientes, Mexico. Vet. Parasitol. 2013;191:11–14. doi: 10.1016/j.vetpar.2012.08.007. [DOI] [PubMed] [Google Scholar]
- Meerburg B.G., De Craeye S., Dierick K., Kijlstra A. Neospora caninum and Toxoplasma gondii in brain tissue of feral rodents and insectivores caught on farms in the Netherlands. Vet. Parasitol. 2012;184:317–320. doi: 10.1016/j.vetpar.2011.09.001. [DOI] [PubMed] [Google Scholar]
- Millan J., Candela M.G., Palomares F., Cubero M.J., Rodriguez A., Barral M. Disease threats to the endangered Iberian lynx (Lynx pardinus) Vet. J. 2009;182:114–124. doi: 10.1016/j.tvjl.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C.M., Quinn H.E., Windsor P.A., Ellis J.T. Characterisation of the first Australian isolate of Neospora caninum from cattle. Aust. Vet. J. 2002;80:620–625. doi: 10.1111/j.1751-0813.2002.tb10967.x. [DOI] [PubMed] [Google Scholar]
- Miller M.A., Conrad P.A., Harris M., Hatfield B., Langlois G., Jessup D.A. A protozoal-associated epizootic impacting marine wildlife: mass-mortality of southern sea otters (Enhydra lutris nereis) due to Sarcocystis neurona infection. Vet. Parasitol. 2010;172:183–194. doi: 10.1016/j.vetpar.2010.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineo T.W., Carrasco A.O., Marciano J.A., Werther K., Pinto A.A., Machado R.Z. Pigeons (Columba livia) are a suitable experimental model for Neospora caninum infection in birds. Vet. Parasitol. 2009;159:149–153. doi: 10.1016/j.vetpar.2008.10.024. [DOI] [PubMed] [Google Scholar]
- Mineo T.W., Carrasco A.O., Raso T.F., Werther K., Pinto A.A., Machado R.Z. Survey for natural Neospora caninum infection in wild and captive birds. Vet. Parasitol. 2011;182:352–355. doi: 10.1016/j.vetpar.2011.05.022. [DOI] [PubMed] [Google Scholar]
- Molina-Lopez R., Cabezon O., Pabon M., Darwich L., Obon E., Lopez-Gatius F. High seroprevalence of Toxoplasma gondii and Neospora caninum in the common raven (Corvus corax) in the Northeast of Spain. Res. Vet. Sci. 2012;93:300–302. doi: 10.1016/j.rvsc.2011.05.011. [DOI] [PubMed] [Google Scholar]
- Mols-Vorstermans T., Hemphill A., Monney T., Schaap D., Boerhout E. Differential effects on survival, humoral immune responses and brain lesions in inbred BALB/C, CBA/CA, and C57BL/6 mice experimentally infected with Neospora caninum tachyzoites. ISRN Parasitol. 2013;2013:11. doi: 10.5402/2013/830980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monney T., Hemphill A. Vaccines against neosporosis: what can we learn from the past studies? Exp. Parasitol. 2014;140:52–70. doi: 10.1016/j.exppara.2014.02.015. [DOI] [PubMed] [Google Scholar]
- Moore D.P., Campero C.M., Odeon A.C., Posso M.A., Cano D., Leunda M.R. Seroepidemiology of beef and dairy herds and fetal study of Neospora caninum in Argentina. Vet. Parasitol. 2002;107:303–316. doi: 10.1016/s0304-4017(02)00129-2. [DOI] [PubMed] [Google Scholar]
- Moskwa B., Gozdzik K., Bien J., Kornacka A., Cybulska A., Reiterova K. Detection of antibodies to Neospora caninum in moose (Alces alces): the first report in Europe. Folia Parasitol. (Praha) 2014;61:34–36. [PubMed] [Google Scholar]
- Muller N., Zimmermann V., Hentrich B., Gottstein B. Diagnosis of Neospora caninum and Toxoplasma gondii infection by PCR and DNA hybridization immunoassay. J. Clin. Microbiol. 1996;34:2850–2852. doi: 10.1128/jcm.34.11.2850-2852.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller N., Vonlaufen N., Gianinazzi C., Leib S.L., Hemphill A. Application of real-time fluorescent PCR for quantitative assessment of Neospora caninum infections in organotypic slice cultures of rat central nervous system tissue. J. Clin. Microbiol. 2002;40:252–255. doi: 10.1128/JCM.40.1.252-255.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy T.M., Walochnik J., Hassl A., Moriarty J., Mooney J., Toolan D. Study on the prevalence of Toxoplasma gondii and Neospora caninum and molecular evidence of Encephalitozoon cuniculi and Encephalitozoon (Septata) intestinalis infections in red foxes (Vulpes vulpes) in rural Ireland. Vet. Parasitol. 2007;146:227–234. doi: 10.1016/j.vetpar.2007.02.017. [DOI] [PubMed] [Google Scholar]
- Myers W.L., Foreyt W.J., Talcott P.A., Evermann J.F., Chang W.Y. Serologic, trace element, and fecal parasite survey of free-ranging, female mule deer (Odocoileus hemionus) in Eastern Washington, USA. J. Wildl. Dis. 2014;51:125–136. doi: 10.7589/2014-05-119. [DOI] [PubMed] [Google Scholar]
- Nishimura M., Kohara J., Hiasa J., Muroi Y., Yokoyama N., Kida K. Tissue distribution of Neospora caninum in experimentally infected cattle. Clin. Vaccine Immunol. 2013;20:309–312. doi: 10.1128/CVI.00556-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okeoma C.M., Stowell K.M., Williamson N.B., Pomroy W.E. Neospora caninum: quantification of DNA in the blood of naturally infected aborted and pregnant cows using real-time PCR. Exp. Parasitol. 2005;110:48–55. doi: 10.1016/j.exppara.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Olamendi-Portugal M., Caballero-Ortega H., Correa D., Sanchez-Aleman M.A., Cruz-Vazquez C., Medina-Esparza L. Serosurvey of antibodies against Toxoplasma gondii and Neospora caninum in white-tailed deer from Northern Mexico. Vet. Parasitol. 2012;189:369–373. doi: 10.1016/j.vetpar.2012.04.011. [DOI] [PubMed] [Google Scholar]
- Ordeix L., Lloret A., Fondevila D., Dubey J.P., Ferrer L., Fondati A. Cutaneous neosporosis during treatment of pemphigus foliaceus in a dog. J. Am. Anim. Hosp. Assoc. 2002;38:415–419. doi: 10.5326/0380415. [DOI] [PubMed] [Google Scholar]
- Ortega-Mora L. CABI; Wallingford, England: 2007. Protozoal Abortion in Farm Ruminants: Guidelines for Diagnosis and Control. [Google Scholar]
- Otranto D., Llazari A., Testini G., Traversa D., Frangipane di Regalbono A., Badan M. Seroprevalence and associated risk factors of neosporosis in beef and dairy cattle in Italy. Vet. Parasitol. 2003;118:7–18. doi: 10.1016/j.vetpar.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Ould-Amrouche A., Klein F., Osdoit C., Mohammed H.O., Touratier A., Sanaa M. Estimation of Neospora caninum seroprevalence in dairy cattle from Normandy, France. Vet. Res. 1999;30:531–538. [PubMed] [Google Scholar]
- Panadero R., Painceira A., Lopez C., Vazquez L., Paz A., Diaz P. Seroprevalence of Toxoplasma gondii and Neospora caninum in wild and domestic ruminants sharing pastures in Galicia (Northwest Spain) Res. Vet. Sci. 2010;88:111–115. doi: 10.1016/j.rvsc.2009.05.010. [DOI] [PubMed] [Google Scholar]
- Peters M., Wohlsein P., Knieriem A., Schares G. Neospora caninum infection associated with stillbirths in captive antelopes (Tragelaphus imberbis) Vet. Parasitol. 2001;97:153–157. doi: 10.1016/s0304-4017(01)00401-0. [DOI] [PubMed] [Google Scholar]
- Pipano E., Shkap V., Fish L., Savitsky I., Perl S., Orgad U. Susceptibility of Psammomys obesus and Meriones tristrami to tachyzoites of Neospora caninum. J. Parasitol. 2002;88:314–319. doi: 10.1645/0022-3395(2002)088[0314:SOPOAM]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Qin Q., Wei F., Li M., Dubovi E.J., Loeffler I.K. Serosurvey of infectious disease agents of carnivores in captive red pandas (Ailurus fulgens) in China. J. Zoo Wildl. Med. 2007;38:42–50. doi: 10.1638/06-048.1. [DOI] [PubMed] [Google Scholar]
- Ramamoorthy S., Sriranganathan N., Lindsay D.S. Gerbil model of acute neosporosis. Vet. Parasitol. 2005;127:111–114. doi: 10.1016/j.vetpar.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Regidor-Cerrillo J., Pedraza-Diaz S., Gomez-Bautista M., Ortega-Mora L.M. Multilocus microsatellite analysis reveals extensive genetic diversity in Neospora caninum. J. Parasitol. 2006;92:517–524. doi: 10.1645/GE-713R.1. [DOI] [PubMed] [Google Scholar]
- Regidor-Cerrillo J., Diez-Fuertes F., Garcia-Culebras A., Moore D.P., Gonzalez-Warleta M., Cuevas C. Genetic diversity and geographic population structure of bovine Neospora caninum determined by microsatellite genotyping analysis. PLoS ONE. 2013;8:e72678. doi: 10.1371/journal.pone.0072678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regidor-Cerrillo J., Arranz-Solis D., Benavides J., Gomez-Bautista M., Castro-Hermida J.A., Mezo M. Neospora caninum infection during early pregnancy in cattle: how the isolate influences infection dynamics, clinical outcome and peripheral and local immune responses. Vet. Res. 2014;45:10. doi: 10.1186/1297-9716-45-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel M.P., Ellis J.T., Dubey J.P. Neosporosis and hammondiosis in dogs. J. Small Anim. Pract. 2007;48:308–312. doi: 10.1111/j.1748-5827.2006.00236.x. [DOI] [PubMed] [Google Scholar]
- Reichel M.P., Alejandra Ayanegui-Alcerreca M., Gondim L.F., Ellis J.T. What is the global economic impact of Neospora caninum in cattle – the billion dollar question. Int. J. Parasitol. 2013;43:133–142. doi: 10.1016/j.ijpara.2012.10.022. [DOI] [PubMed] [Google Scholar]
- Reid A.J., Vermont S.J., Cotton J.A., Harris D., Hill-Cawthorne G.A., Konen-Waisman S. Comparative genomics of the apicomplexan parasites Toxoplasma gondii and Neospora caninum: coccidia differing in host range and transmission strategy. PLoS Pathog. 2012;8:e1002567. doi: 10.1371/journal.ppat.1002567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risco-Castillo V., Wheeler J.C., Rosadio R., Garcia-Pena F.J., Arnaiz-Seco I., Hoces D. Health impact evaluation of alternative management systems in vicuna (Vicugna vicugna mensalis) populations in Peru. Trop. Anim. Health Prod. 2014;46:641–646. doi: 10.1007/s11250-014-0543-3. [DOI] [PubMed] [Google Scholar]
- Rojo-Montejo S., Collantes-Fernandez E., Regidor-Cerrillo J., Alvarez-Garcia G., Marugan-Hernandez V., Pedraza-Diaz S. Isolation and characterization of a bovine isolate of Neospora caninum with low virulence. Vet. Parasitol. 2009;159:7–16. doi: 10.1016/j.vetpar.2008.10.009. [DOI] [PubMed] [Google Scholar]
- Ruehlmann D., Podell M., Oglesbee M., Dubey J.P. Canine neosporosis: a case report and literature review. J. Am. Anim. Hosp. Assoc. 1995;31:174–183. doi: 10.5326/15473317-31-2-174. [DOI] [PubMed] [Google Scholar]
- Sangster C., Bryant B., Campbell-Ward M., King J.S., Slapeta J. Neosporosis in an aborted southern white rhinoceros (Ceratotherium simum simum) fetus. J. Zoo Wildl. Med. 2010;41:725–728. doi: 10.1638/2009-0250.1. [DOI] [PubMed] [Google Scholar]
- Santolaria P., Almeria S., Martinez-Bello D., Nogareda C., Mezo M., Gonzalez-Warleta M. Different humoral mechanisms against Neospora caninum infection in purebreed and crossbreed beef/dairy cattle pregnancies. Vet. Parasitol. 2011;178:70–76. doi: 10.1016/j.vetpar.2010.12.018. [DOI] [PubMed] [Google Scholar]
- Sawada M., Park C.H., Kondo H., Morita T., Shimada A., Yamane I. Serological survey of antibody to Neospora caninum in Japanese dogs. J. Vet. Med. Sci. 1998;60:853–854. doi: 10.1292/jvms.60.853. [DOI] [PubMed] [Google Scholar]
- Schares G., Wenzel U., Muller T., Conraths F.J. Serological evidence for naturally occurring transmission of Neospora caninum among foxes (Vulpes vulpes) Int. J. Parasitol. 2001;31:418–423. doi: 10.1016/s0020-7519(01)00118-7. [DOI] [PubMed] [Google Scholar]
- Schlafer D.H., Miller R.B. Jubb, Kennedy, and Palmer's Pathology of Domestic Animals. fifth ed. Elsevier Saunders; Philadelphia, PA: 2007. The female genital system; pp. 514–516. [Google Scholar]
- Schwab A.E., Geary T.G., Baillargeon P., Schwab A.J., Fecteau G. Association of BoLA DRB3 and DQA1 alleles with susceptibility to Neospora caninum and reproductive outcome in Quebec Holstein cattle. Vet. Parasitol. 2009;165:136–140. doi: 10.1016/j.vetpar.2009.07.004. [DOI] [PubMed] [Google Scholar]
- Sedlak K., Bartova E. Seroprevalences of antibodies to Neospora caninum and Toxoplasma gondii in zoo animals. Vet. Parasitol. 2006;136:223–231. doi: 10.1016/j.vetpar.2005.11.021. [DOI] [PubMed] [Google Scholar]
- Silva D.A., Vitaliano S.N., Mineo T.W., Ferreira R.A., Bevilacqua E., Mineo J.R. Evaluation of homologous, heterologous, and affinity conjugates for the serodiagnosis of Toxoplasma gondii and Neospora caninum in maned wolves (Chrysocyon brachyurus) J. Parasitol. 2005;91:1212–1216. doi: 10.1645/GE-527R.1. [DOI] [PubMed] [Google Scholar]
- Simpson V.R., Monies R.J., Riley P., Cromey D.S. Foxes and neosporosis. Vet. Rec. 1997;141:503. [PubMed] [Google Scholar]
- Slapeta J., Linares M.C. Combined amplicon pyrosequencing assays reveal presence of the apicomplexan “type-N” (cf. Gemmocystis cylindrus) and Chromera velia on the Great Barrier Reef, Australia. PLoS ONE. 2013;8:e76095. doi: 10.1371/journal.pone.0076095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slapeta J.R., Modry D., Kyselova I., Horejs R., Lukes J., Koudela B. Dog shedding oocysts of Neospora caninum: PCR diagnosis and molecular phylogenetic approach. Vet. Parasitol. 2002;109:157–167. doi: 10.1016/s0304-4017(02)00273-x. [DOI] [PubMed] [Google Scholar]
- Slapeta J.R., Modry D., Votypka J., Jirku M., Lukes J., Koudela B. Evolutionary relationships among cyst-forming coccidia Sarcocystis spp. (Alveolata: Apicomplexa: Coccidea) in endemic African tree vipers and perspective for evolution of heteroxenous life cycle. Mol. Phylogenet. Evol. 2003;27:464–475. doi: 10.1016/s1055-7903(03)00018-6. [DOI] [PubMed] [Google Scholar]
- Sobrino R., Dubey J.P., Pabon M., Linarez N., Kwok O.C., Millan J. Neospora caninum antibodies in wild carnivores from Spain. Vet. Parasitol. 2008;155:190–197. doi: 10.1016/j.vetpar.2008.05.009. [DOI] [PubMed] [Google Scholar]
- Soldati S., Kiupel M., Wise A., Maes R., Botteron C., Robert N. Meningoencephalomyelitis caused by Neospora caninum in a juvenile fallow deer (Dama dama) J. Vet. Med. A Physiol. Pathol. Clin. Med. 2004;51:280–283. doi: 10.1111/j.1439-0442.2004.00646.x. [DOI] [PubMed] [Google Scholar]
- Sommanustweechai A., Vongpakorn M., Kasantikul T., Taewnean J., Siriaroonrat B., Bush M. Systemic neosporosis in a white rhinoceros. J. Zoo Wildl. Med. 2010;41:165–168. doi: 10.1638/2009-0048.1. [DOI] [PubMed] [Google Scholar]
- Speer C.A., Dubey J.P., McAllister M.M., Blixt J.A. Comparative ultrastructure of tachyzoites, bradyzoites, and tissue cysts of Neospora caninum and Toxoplasma gondii. Int. J. Parasitol. 1999;29:1509–1519. doi: 10.1016/s0020-7519(99)00132-0. [DOI] [PubMed] [Google Scholar]
- Steinman A., Shpigel N.Y., Mazar S., King R., Baneth G., Savitsky I. Low seroprevalence of antibodies to Neospora caninum in wild canids in Israel. Vet. Parasitol. 2006;137:155–158. doi: 10.1016/j.vetpar.2005.12.022. [DOI] [PubMed] [Google Scholar]
- Stieve E., Beckmen K., Kania S.A., Widner A., Patton S. Neospora caninum and Toxoplasma gondii antibody prevalence in Alaska wildlife. J. Wildl. Dis. 2010;46:348–355. doi: 10.7589/0090-3558-46.2.348. [DOI] [PubMed] [Google Scholar]
- Stuart P., Zintl A., De Waal T., Mulcahy G., Hawkins C., Lawton C. Investigating the role of wild carnivores in the epidemiology of bovine neosporosis. Parasitology. 2013;140:296–302. doi: 10.1017/S0031182012001588. [DOI] [PubMed] [Google Scholar]
- Sun Y., Wolcott R., Dowd S. Tag-encoded FLX amplicon pyrosequencing for the elucidation of microbial and functional gene diversity in any environment. In: Kwon Y.M., Ricke S.C., editors. High-Throughput Next Generation Sequencing. Humana Press; New York, USA: 2011. pp. 129–141. [DOI] [PubMed] [Google Scholar]
- Thomasson D., Wright E.A., Hughes J.M., Dodd N.S., Cox A.P., Boyce K. Prevalence and co-infection of Toxoplasma gondii and Neospora caninum in Apodemus sylvaticus in an area relatively free of cats. Parasitology. 2011;138:1117–1123. doi: 10.1017/S0031182011000904. [DOI] [PubMed] [Google Scholar]
- Thurmond M.C., Hietala S.K. Effect of congenitally acquired Neospora caninum infection on risk of abortion and subsequent abortions in dairy cattle. Am. J. Vet. Res. 1997;58:1381–1385. [PubMed] [Google Scholar]
- Tiemann J.C., Souza S.L., Rodrigues A.A., Duarte J.M., Gennari S.M. Environmental effect on the occurrence of anti-Neospora caninum antibodies in pampas-deer (Ozotoceros bezoarticus) Vet. Parasitol. 2005;134:73–76. doi: 10.1016/j.vetpar.2005.07.015. [DOI] [PubMed] [Google Scholar]
- Tiemann J.C., Rodrigues A.A., de Souza S.L., Duarte J.M., Gennari S.M. Occurrence of anti-Neospora caninum antibodies in Brazilian cervids kept in captivity. Vet. Parasitol. 2005;129:341–343. doi: 10.1016/j.vetpar.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Tranas J., Heinzen R.A., Weiss L.M., McAllister M.M. Serological evidence of human infection with the protozoan Neospora caninum. Clin. Diagn. Lab. Immunol. 1999;6:765–767. doi: 10.1128/cdli.6.5.765-767.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trees A.J., Williams D.J. Endogenous and exogenous transplacental infection in Neospora caninum and Toxoplasma gondii. Trends Parasitol. 2005;21:558–561. doi: 10.1016/j.pt.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Truppel J.H., Montiani-Ferreira F., Lange R.R., Vilani R.G., Reifur L., Boerger W. Detection of Neospora caninum DNA in capybaras and phylogenetic analysis. Parasitol. Int. 2010;59:376–379. doi: 10.1016/j.parint.2010.05.001. [DOI] [PubMed] [Google Scholar]
- Uchida Y., Ike K., Kurotaki T., Takeshi M., Imai S. Susceptibility of Djungarian hamsters (Phodopus sungorus) to Neospora caninum infection. J. Vet. Med. Sci. 2003;65:401–403. doi: 10.1292/jvms.65.401. [DOI] [PubMed] [Google Scholar]
- van der Hage M.H., Kik M.J.L., Dorrestein G.M. 4th Scientific Meeting of the European Association of Zoo and Wildlife Veterinarians and European Wildlife Disease Association. European Wildlife Disease Association; Heidelberg, German: 2002. Neospora caninum: myocarditis in a European pine marten; pp. 217–220. [Google Scholar]
- Valadas S., Gennari S.M., Yai L.E., Rosypal A.C., Lindsay D.S. Prevalence of antibodies to Trypanosoma cruzi, Leishmania infantum, Encephalitozoon cuniculi, Sarcocystis neurona, and Neospora caninum in capybara, Hydrochoerus hydrochaeris, from Sao Paulo State, Brazil. J. Parasitol. 2010;96:521–524. doi: 10.1645/GE-2368.1. [DOI] [PubMed] [Google Scholar]
- Vianna M.C., Sreekumar C., Miska K.B., Hill D.E., Dubey J.P. Isolation of Neospora caninum from naturally infected white-tailed deer (Odocoileus virginianus) Vet. Parasitol. 2005;129:253–257. doi: 10.1016/j.vetpar.2004.02.031. [DOI] [PubMed] [Google Scholar]
- Vitaliano S.N., Silva D.A., Mineo T.W., Ferreira R.A., Bevilacqua E., Mineo J.R. Seroprevalence of Toxoplasma gondii and Neospora caninum in captive maned wolves (Chrysocyon brachyurus) from southeastern and midwestern regions of Brazil. Vet. Parasitol. 2004;122:253–260. doi: 10.1016/j.vetpar.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Wanha K., Edelhofer R., Gabler-Eduardo C., Prosl H. Prevalence of antibodies against Neospora caninum and Toxoplasma gondii in dogs and foxes in Austria. Vet. Parasitol. 2005;128:189–193. doi: 10.1016/j.vetpar.2004.11.027. [DOI] [PubMed] [Google Scholar]
- Wapenaar W., Jenkins M.C., O'Handley R.M., Barkema H.W. Neospora caninum-like oocysts observed in feces of free-ranging red foxes (Vulpes vulpes) and coyotes (Canis latrans) J. Parasitol. 2006;92:1270–1274. doi: 10.1645/GE-913R.1. [DOI] [PubMed] [Google Scholar]
- Wapenaar W., Barkema H.W., Schares G., Rouvinen-Watt K., Zeijlemaker L., Poorter B. Evaluation of four serological techniques to determine the seroprevalence of Neospora caninum in foxes (Vulpes vulpes) and coyotes (Canis latrans) on Prince Edward Island, Canada. Vet. Parasitol. 2007;145:51–58. doi: 10.1016/j.vetpar.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Weiss L.M., Ma Y.F., Halonen S., McAllister M.M., Zhang Y.W. The in vitro development of Neospora caninum bradyzoites. Int. J. Parasitol. 1999;29:1713–1723. doi: 10.1016/s0020-7519(99)00130-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiengcharoen J., Nokkaew W., Prasithpon S., Prasomtong P., Sukthana Y. Neospora caninum and Toxoplasma gondii antibodies in captive elephants (Elephaus maximus indicus) in Kanchanaburi Province. Thai. J. Vet. Med. 2012;42:235–240. [Google Scholar]
- Williams D.J., Hartley C.S., Bjorkman C., Trees A.J. Endogenous and exogenous transplacental transmission of Neospora caninum – how the route of transmission impacts on epidemiology and control of disease. Parasitology. 2009;136:1895–1900. doi: 10.1017/S0031182009990588. [DOI] [PubMed] [Google Scholar]
- Williams J.H., Espie I., van Wilpe E., Matthee A. Neosporosis in a white rhinoceros (Ceratotherium simum) calf. J. S. Afr. Vet. Assoc. 2002;73:38–43. doi: 10.4102/jsava.v73i1.547. [DOI] [PubMed] [Google Scholar]
- Wolfe A., Hogan S., Maguire D., Fitzpatrick C., Vaughan L., Wall D. Red foxes (Vulpes vulpes) in Ireland as hosts for parasites of potential zoonotic and veterinary significance. Vet. Rec. 2001;149:759–763. [PubMed] [Google Scholar]
- Woodroffe R., Prager K.C., Munson L., Conrad P.A., Dubovi E.J., Mazet J.A. Contact with domestic dogs increases pathogen exposure in endangered African wild dogs (Lycaon pictus) PLoS ONE. 2012;7:e30099. doi: 10.1371/journal.pone.0030099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods L.W., Anderson M.L., Swift P.K., Sverlow K.W. Systemic neosporosis in a California black-tailed deer (Odocoileus hemionus columbianus) J. Vet. Diagn. Invest. 1994;6:508–510. doi: 10.1177/104063879400600425. [DOI] [PubMed] [Google Scholar]
- Wouda W., Dijkstra T., Kramer A.M., van Maanen C., Brinkhof J.M. Seroepidemiological evidence for a relationship between Neospora caninum infections in dogs and cattle. Int. J. Parasitol. 1999;29:1677–1682. doi: 10.1016/s0020-7519(99)00105-8. [DOI] [PubMed] [Google Scholar]
- Yai L.E., Canon-Franco W.A., Geraldi V.C., Summa M.E., Camargo M.C., Dubey J.P. Seroprevalence of Neospora caninum and Toxoplasma gondii antibodies in the South American opossum (Didelphis marsupialis) from the city of Sao Paulo, Brazil. J. Parasitol. 2003;89:870–871. doi: 10.1645/GE-83R. [DOI] [PubMed] [Google Scholar]
- Yai L.E., Ragozo A.M., Canon-Franco W.A., Dubey J.P., Gennari S.M. Occurrence of Neospora caninum antibodies in capybaras (Hydrochaeris hydrochaeris) from Sao Paulo State, Brazil. J. Parasitol. 2008;94:766. doi: 10.1645/GE-1468.1. [DOI] [PubMed] [Google Scholar]
- Yu X., Chen N., Hu D., Zhang W., Li X., Wang B. Detection of Neospora caninum from farm-bred young blue foxes (Alopex lagopus) in China. J. Vet. Med. Sci. 2009;71:113–115. doi: 10.1292/jvms.71.113. [DOI] [PubMed] [Google Scholar]
- Zanet S., Palese V., Trisciuoglio A., Canton Alonso C., Ferroglio E. Encephalitozoon cuniculi, Toxoplasma gondii and Neospora caninum infection in invasive Eastern cottontail rabbits Sylvilagus floridanus in Northwestern Italy. Vet. Parasitol. 2013;197:682–684. doi: 10.1016/j.vetpar.2013.05.014. [DOI] [PubMed] [Google Scholar]


