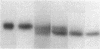
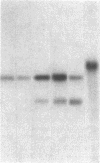
Abstract
The ability of mononuclear phagocytes to assemble and activate components of the fibrinolytic system on their surfaces may be crucial in effecting an efficient inflammatory response. Lys-plasminogen, the plasmin modified form of this zymogen, was found to bind specifically and with high affinity to murine peritoneal macrophages and to cells of the human monocytoid line U937. This modified plasminogen has been shown to be a more efficient substrate for plasminogen activators than native Glu-plasminogen. Binding was lysine binding site dependent, rapid and reversible. In contrast, although native Glu-plasminogen bound specifically to these cells, affinity was low. Lys-plasminogen inhibited the binding of Glu-plasminogen but the opposite was not true. Molecular analysis of the bound ligands indicated that Glu-plasminogen was converted to Lys-plasminogen and Lys-plasminogen to plasmin on the cell surface but not in the supernatant. Peritoneal macrophages from patients with indwelling catheters and tissue macrophages in chronic inflammatory lesions were shown to express immunologically identified Lys-plasminogen on their surfaces. Therefore binding and surface activation of kinetically favored Lys-plasminogen may provide an important physiological mechanism for localizing proteolytic activity on the surface of inflammatory cells.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Castellino F. J. Biochemistry of human plasminogen. Semin Thromb Hemost. 1984 Jan;10(1):18–23. doi: 10.1055/s-2007-1004404. [DOI] [PubMed] [Google Scholar]
- Cubellis M. V., Nolli M. L., Cassani G., Blasi F. Binding of single-chain prourokinase to the urokinase receptor of human U937 cells. J Biol Chem. 1986 Dec 5;261(34):15819–15822. [PubMed] [Google Scholar]
- Deutsch D. G., Mertz E. T. Plasminogen: purification from human plasma by affinity chromatography. Science. 1970 Dec 4;170(3962):1095–1096. doi: 10.1126/science.170.3962.1095. [DOI] [PubMed] [Google Scholar]
- Hajjar K. A., Harpel P. C., Jaffe E. A., Nachman R. L. Binding of plasminogen to cultured human endothelial cells. J Biol Chem. 1986 Sep 5;261(25):11656–11662. [PubMed] [Google Scholar]
- Harpel P. C. Alpha2-plasmin inhibitor and alpha2-macroglobulin-plasmin complexes in plasma. Quantitation by an enzyme-linked differential antibody immunosorbent assay. J Clin Invest. 1981 Jul;68(1):46–55. doi: 10.1172/JCI110253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattey E., Wojta J., Binder B. R. Monoclonal antibodies against plasminogen and alpha-2-antiplasmin: binding to native and modified antigens. Thromb Res. 1987 Mar 1;45(5):485–495. doi: 10.1016/0049-3848(87)90311-2. [DOI] [PubMed] [Google Scholar]
- Holvoet P., Lijnen H. R., Collen D. A monoclonal antibody specific for Lys-plasminogen. Application to the study of the activation pathways of plasminogen in vivo. J Biol Chem. 1985 Oct 5;260(22):12106–12111. [PubMed] [Google Scholar]
- Lucas M. A., Fretto L. J., McKee P. A. The binding of human plasminogen to fibrin and fibrinogen. J Biol Chem. 1983 Apr 10;258(7):4249–4256. [PubMed] [Google Scholar]
- Markus G., DePasquale J. L., Wissler F. C. Quantitative determination of the binding of epsilon-aminocaproic acid to native plasminogen. J Biol Chem. 1978 Feb 10;253(3):727–732. [PubMed] [Google Scholar]
- Markus G., Evers J. L., Hobika G. H. Comparison of some properties of native (Glu) and modified (Lys) human plasminogen. J Biol Chem. 1978 Feb 10;253(3):733–739. [PubMed] [Google Scholar]
- McConahey P. J., Dixon F. J. A method of trace iodination of proteins for immunologic studies. Int Arch Allergy Appl Immunol. 1966;29(2):185–189. doi: 10.1159/000229699. [DOI] [PubMed] [Google Scholar]
- Moonen G., Grau-Wagemans M. P., Selak I. Plasminogen activator-plasmin system and neuronal migration. Nature. 1982 Aug 19;298(5876):753–755. doi: 10.1038/298753a0. [DOI] [PubMed] [Google Scholar]
- Munson P. J., Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980 Sep 1;107(1):220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- Plow E. F., Freaney D. E., Plescia J., Miles L. A. The plasminogen system and cell surfaces: evidence for plasminogen and urokinase receptors on the same cell type. J Cell Biol. 1986 Dec;103(6 Pt 1):2411–2420. doi: 10.1083/jcb.103.6.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein R. L., Harpel P. C., Nachman R. L. Tissue plasminogen activator and urokinase enhance the binding of plasminogen to thrombospondin. J Biol Chem. 1986 Jul 25;261(21):9959–9965. [PubMed] [Google Scholar]
- Silverstein R. L., Leung L. L., Harpel P. C., Nachman R. L. Complex formation of platelet thrombospondin with plasminogen. Modulation of activation by tissue activator. J Clin Invest. 1984 Nov;74(5):1625–1633. doi: 10.1172/JCI111578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein R. L., Nachman R. L., Leung L. L., Harpel P. C. Activation of immobilized plasminogen by tissue activator. Multimolecular complex formation. J Biol Chem. 1985 Aug 25;260(18):10346–10352. [PubMed] [Google Scholar]
- Silverstein R. L., Nachman R. L. Thrombospondin binds to monocytes-macrophages and mediates platelet-monocyte adhesion. J Clin Invest. 1987 Mar;79(3):867–874. doi: 10.1172/JCI112896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickland S., Reich E., Sherman M. I. Plasminogen activator in early embryogenesis: enzyme production by trophoblast and parietal endoderm. Cell. 1976 Oct;9(2):231–240. doi: 10.1016/0092-8674(76)90114-8. [DOI] [PubMed] [Google Scholar]
- Sundström C., Nilsson K. Establishment and characterization of a human histiocytic lymphoma cell line (U-937). Int J Cancer. 1976 May 15;17(5):565–577. doi: 10.1002/ijc.2910170504. [DOI] [PubMed] [Google Scholar]
- Talle M. A., Rao P. E., Westberg E., Allegar N., Makowski M., Mittler R. S., Goldstein G. Patterns of antigenic expression on human monocytes as defined by monoclonal antibodies. Cell Immunol. 1983 May;78(1):83–99. doi: 10.1016/0008-8749(83)90262-9. [DOI] [PubMed] [Google Scholar]
- Unkeless J. C., Gordon S., Reich E. Secretion of plasminogen activator by stimulated macrophages. J Exp Med. 1974 Apr 1;139(4):834–850. doi: 10.1084/jem.139.4.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassalli J. D., Baccino D., Belin D. A cellular binding site for the Mr 55,000 form of the human plasminogen activator, urokinase. J Cell Biol. 1985 Jan;100(1):86–92. doi: 10.1083/jcb.100.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassalli J. D., Dayer J. M., Wohlwend A., Belin D. Concomitant secretion of prourokinase and of a plasminogen activator-specific inhibitor by cultured human monocytes-macrophages. J Exp Med. 1984 Jun 1;159(6):1653–1668. doi: 10.1084/jem.159.6.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallén P., Wiman B. Characterization of human plasminogen. II. Separation and partial characterization of different molecular forms of human plasminogen. Biochim Biophys Acta. 1972 Jan 26;257(1):122–134. doi: 10.1016/0005-2795(72)90261-9. [DOI] [PubMed] [Google Scholar]
- Wilson E. L., Dowdle E. Secretion of plasminogen activator by normal, reactive and neoplastic human tissues cultured in vitro. Int J Cancer. 1978 Oct 15;22(4):390–399. doi: 10.1002/ijc.2910220405. [DOI] [PubMed] [Google Scholar]
- Wiman B., Wallén P. Activation of human plasminogen by an insoluble derivative of urokinase. Structural changes of plasminogen in the course of activation to plasmin and demonstration of a possible intermediate compound. Eur J Biochem. 1973 Jul 2;36(1):25–31. doi: 10.1111/j.1432-1033.1973.tb02880.x. [DOI] [PubMed] [Google Scholar]