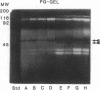
Abstract
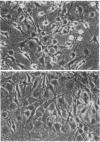
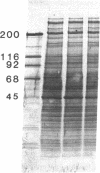
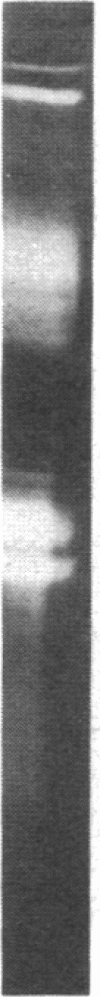
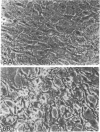
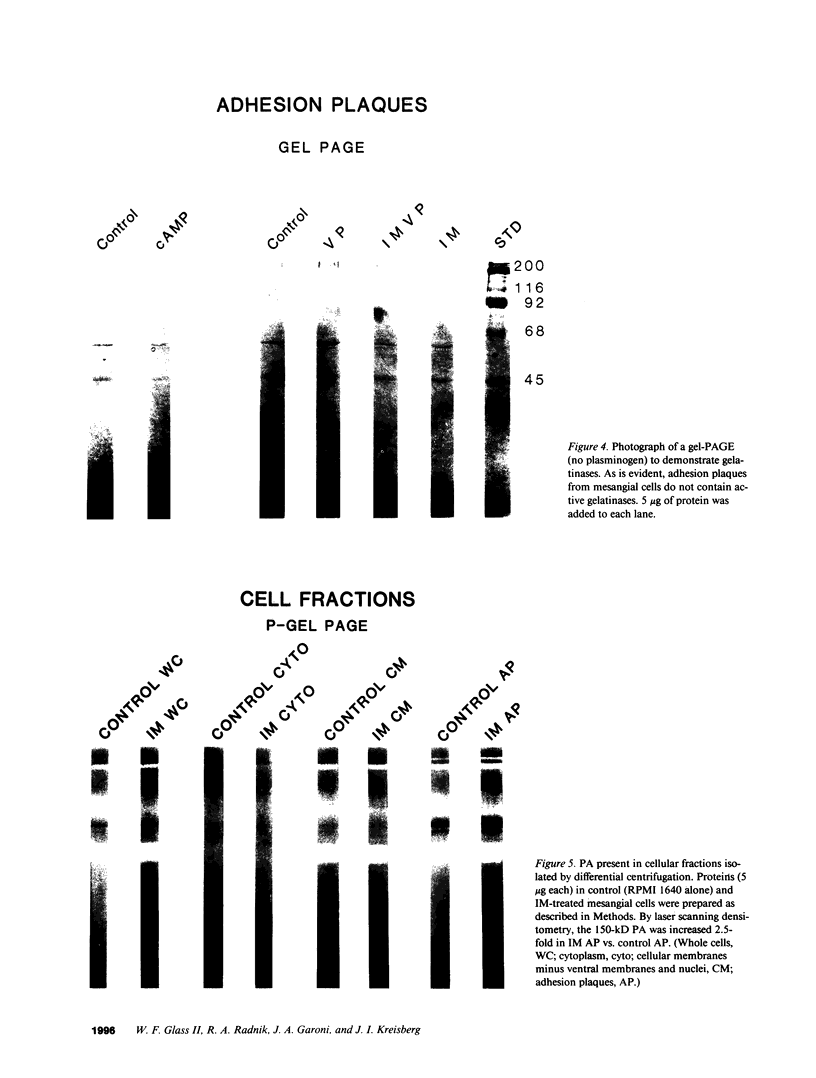
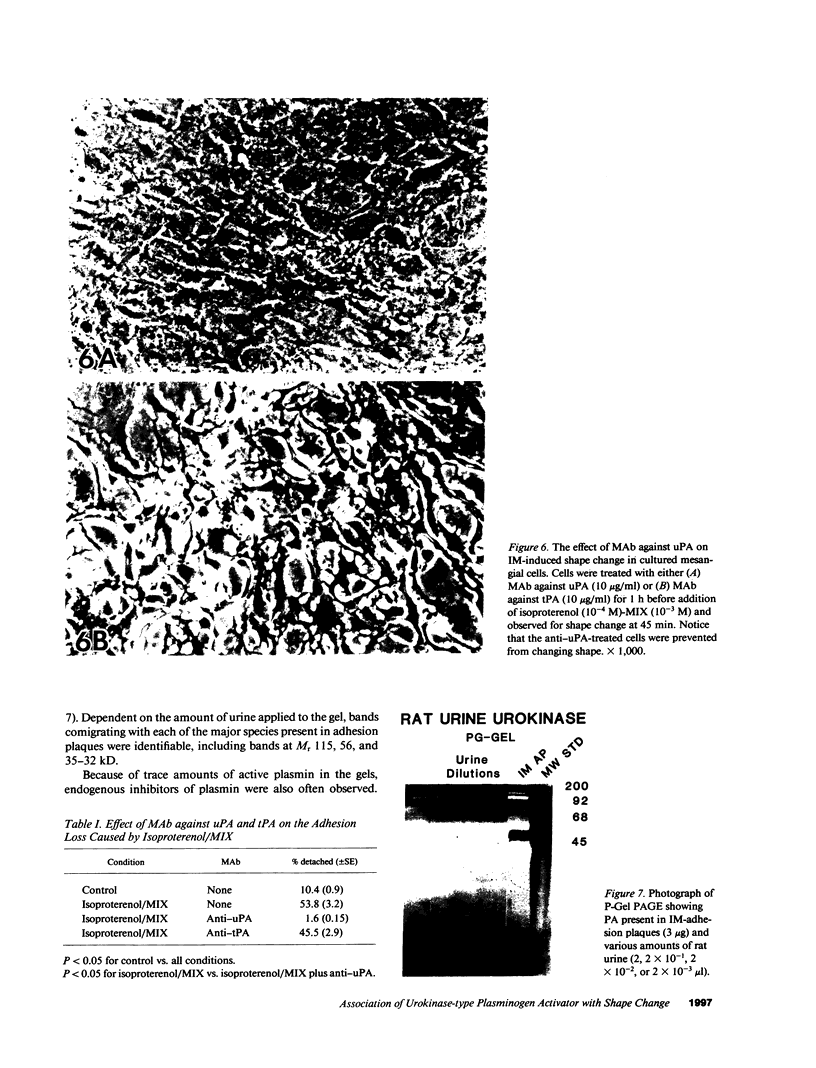
Mesangial cells in culture change shape and become less adhesive in response to cAMP elevation (e.g., treatment with isoproterenol plus isobutylmethylxanthine (IM). Inhibitors of serine proteases inhibit cellular shape change in response to IM. To further examine the role of cell surface proteases in shape change, adhesion plaque proteins (i.e., preparations of ventral membranes and extracellular matrix) were separated in SDS-polyacrylamide gels containing gelatin with and without plasminogen. Four discrete zones of lysis were evident in plasminogen gels (indicative of activation of plasminogen) from control adhesion plaques: one inconspicuous zone with a Mr approximately 150 kD, another at approximately 115 kD, and a doublet at approximately 35-32 kD. Another diffuse zone of lysis centered around Mr approximately 70 kD and contained a defined band of approximately 56 kD. Adhesion plaques contained most of the plasminogen activators (PA). 5 min after IM treatment, the Mr approximately 150- and approximately 115-kD PA were increased in activity. Vasopressin (VP), which prevented shape change and adhesion loss when added along with IM, inhibited the increase in these PA. Preincubation with monoclonal or polyclonal antibodies to urokinase-type plasminogen activator (uPA) totally inhibited the IM-inducible shape change and adhesion loss. Activation of plasminogen throughout the gels revealed multiple protease resistant bands that markedly increased with IM treatment (maximal at 45 min). These may represent focal control mechanisms. uPA thus may mediate focal proteolysis, which results in shape change and decreased adhesion.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler S., Striker L. J., Striker G. E., Perkinson D. T., Hibbert J., Couser W. G. Studies of progressive glomerular sclerosis in the rat. Am J Pathol. 1986 Jun;123(3):553–562. [PMC free article] [PubMed] [Google Scholar]
- Andreasen P. A., Pyke C., Riccio A., Kristensen P., Nielsen L. S., Lund L. R., Blasi F., Danø K. Plasminogen activator inhibitor type 1 biosynthesis and mRNA level are increased by dexamethasone in human fibrosarcoma cells. Mol Cell Biol. 1987 Aug;7(8):3021–3025. doi: 10.1128/mcb.7.8.3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ausiello D. A., Kreisberg J. I., Roy C., Karnovsky M. J. Contraction of cultured rat glomerular cells of apparent mesangial origin after stimulation with angiotensin II and arginine vasopressin. J Clin Invest. 1980 Mar;65(3):754–760. doi: 10.1172/JCI109723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avnur Z., Geiger B. Substrate-attached membranes of cultured cells isolation and characterization of ventral cell membranes and the associated cytoskeleton. J Mol Biol. 1981 Dec 5;153(2):361–379. doi: 10.1016/0022-2836(81)90283-7. [DOI] [PubMed] [Google Scholar]
- Blasi F., Vassalli J. D., Danø K. Urokinase-type plasminogen activator: proenzyme, receptor, and inhibitors. J Cell Biol. 1987 Apr;104(4):801–804. doi: 10.1083/jcb.104.4.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. M., Chen W. T. Fibronectin-degrading proteases from the membranes of transformed cells. Cell. 1987 Jan 30;48(2):193–203. doi: 10.1016/0092-8674(87)90423-5. [DOI] [PubMed] [Google Scholar]
- Chen W. T., Chen J. M., Parsons S. J., Parsons J. T. Local degradation of fibronectin at sites of expression of the transforming gene product pp60src. Nature. 1985 Jul 11;316(6024):156–158. doi: 10.1038/316156a0. [DOI] [PubMed] [Google Scholar]
- Chen W. T., Olden K., Bernard B. A., Chu F. F. Expression of transformation-associated protease(s) that degrade fibronectin at cell contact sites. J Cell Biol. 1984 Apr;98(4):1546–1555. doi: 10.1083/jcb.98.4.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman P. L., Patel P. D., Cwikel B. J., Rafferty U. M., Sznycer-Laszuk R., Gelehrter T. D. Characterization of the dexamethasone-induced inhibitor of plasminogen activator in HTC hepatoma cells. J Biol Chem. 1986 Mar 25;261(9):4352–4357. [PubMed] [Google Scholar]
- Couch C. B., Strittmatter W. J. Rat myoblast fusion requires metalloendoprotease activity. Cell. 1983 Jan;32(1):257–265. doi: 10.1016/0092-8674(83)90516-0. [DOI] [PubMed] [Google Scholar]
- Crutchley D. J., Conanan L. B., Maynard J. R. Human fibroblasts produce inhibitor directed against plasminogen activator when treated with glucocorticoids. Ann N Y Acad Sci. 1981;370:609–616. doi: 10.1111/j.1749-6632.1981.tb29767.x. [DOI] [PubMed] [Google Scholar]
- Danø K., Andreasen P. A., Grøndahl-Hansen J., Kristensen P., Nielsen L. S., Skriver L. Plasminogen activators, tissue degradation, and cancer. Adv Cancer Res. 1985;44:139–266. doi: 10.1016/s0065-230x(08)60028-7. [DOI] [PubMed] [Google Scholar]
- Danø K., Nielsen L. S., Møller V., Engelhart M. Inhibition of a plasminogen activator from oncogenic virus-transformed mouse cells by rabbit antibodies against the enzyme. Biochim Biophys Acta. 1980 Jun 5;630(1):146–151. doi: 10.1016/0304-4165(80)90146-4. [DOI] [PubMed] [Google Scholar]
- Glass R. H., Aggeler J., Spindle A., Pedersen R. A., Werb Z. Degradation of extracellular matrix by mouse trophoblast outgrowths: a model for implantation. J Cell Biol. 1983 Apr;96(4):1108–1116. doi: 10.1083/jcb.96.4.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey S., Minowada J., Takita H., Kover L., Markus G. Urokinase-like plasminogen activators of unusually high molecular weight secreted by a cell line derived from a human lung cancer case. J Biol Chem. 1982 May 25;257(10):5645–5651. [PubMed] [Google Scholar]
- Heussen C., Dowdle E. B. Electrophoretic analysis of plasminogen activators in polyacrylamide gels containing sodium dodecyl sulfate and copolymerized substrates. Anal Biochem. 1980 Feb;102(1):196–202. doi: 10.1016/0003-2697(80)90338-3. [DOI] [PubMed] [Google Scholar]
- Kalebic T., Garbisa S., Glaser B., Liotta L. A. Basement membrane collagen: degradation by migrating endothelial cells. Science. 1983 Jul 15;221(4607):281–283. doi: 10.1126/science.6190230. [DOI] [PubMed] [Google Scholar]
- Knudsen B. S., Harpel P. C., Nachman R. L. Plasminogen activator inhibitor is associated with the extracellular matrix of cultured bovine smooth muscle cells. J Clin Invest. 1987 Oct;80(4):1082–1089. doi: 10.1172/JCI113164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreisberg J. I. Cell biology and biochemistry of the glomerular mesangium. Miner Electrolyte Metab. 1988;14(2-3):167–175. [PubMed] [Google Scholar]
- Kreisberg J. I., Venkatachalam M. A., Patel P. Y. Cyclic AMP-associated shape change in mesangial cells and its reversal by prostaglandin E2. Kidney Int. 1984 Jun;25(6):874–879. doi: 10.1038/ki.1984.104. [DOI] [PubMed] [Google Scholar]
- Kreisberg J. I., Venkatachalam M. A., Radnik R. A., Patel P. Y. Role of myosin light-chain phosphorylation and microtubules in stress fiber morphology in cultured mesangial cells. Am J Physiol. 1985 Aug;249(2 Pt 2):F227–F235. doi: 10.1152/ajprenal.1985.249.2.F227. [DOI] [PubMed] [Google Scholar]
- Kreisberg J. I., Venkatachalam M. A. Vasoactive agents affect mesangial cell adhesion. Am J Physiol. 1986 Oct;251(4 Pt 1):C505–C511. doi: 10.1152/ajpcell.1986.251.4.C505. [DOI] [PubMed] [Google Scholar]
- Kreisberg J. I., Venkatachalam M., Troyer D. Contractile properties of cultured glomerular mesangial cells. Am J Physiol. 1985 Oct;249(4 Pt 2):F457–F463. doi: 10.1152/ajprenal.1985.249.4.F457. [DOI] [PubMed] [Google Scholar]
- Kruithof E. K., Vassalli J. D., Schleuning W. D., Mattaliano R. J., Bachmann F. Purification and characterization of a plasminogen activator inhibitor from the histiocytic lymphoma cell line U-937. J Biol Chem. 1986 Aug 25;261(24):11207–11213. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laiho M., Saksela O., Keski-Oja J. Transforming growth factor-beta induction of type-1 plasminogen activator inhibitor. Pericellular deposition and sensitivity to exogenous urokinase. J Biol Chem. 1987 Dec 25;262(36):17467–17474. [PubMed] [Google Scholar]
- Liotta L. A., Rao C. N., Barsky S. H. Tumor invasion and the extracellular matrix. Lab Invest. 1983 Dec;49(6):636–649. [PubMed] [Google Scholar]
- Lovett D. H., Sterzel R. B., Kashgarian M., Ryan J. L. Neutral proteinase activity produced in vitro by cells of the glomerular mesangium. Kidney Int. 1983 Feb;23(2):342–349. doi: 10.1038/ki.1983.25. [DOI] [PubMed] [Google Scholar]
- Nielsen L. S., Andreasen P. A., Grøndahl-Hansen J., Skriver L., Danø K. Plasminogen activators catalyse conversion of inhibitor from fibrosarcoma cells to an inactive form with a lower apparent molecular mass. FEBS Lett. 1986 Feb 17;196(2):269–273. doi: 10.1016/0014-5793(86)80261-7. [DOI] [PubMed] [Google Scholar]
- Pöllänen J., Saksela O., Salonen E. M., Andreasen P., Nielsen L., Danø K., Vaheri A. Distinct localizations of urokinase-type plasminogen activator and its type 1 inhibitor under cultured human fibroblasts and sarcoma cells. J Cell Biol. 1987 Apr;104(4):1085–1096. doi: 10.1083/jcb.104.4.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley J. P. Association of a protease (plasminogen activator) with a specific membrane fraction isolated from transformed cells. J Cell Biol. 1976 Nov;71(2):472–486. doi: 10.1083/jcb.71.2.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley J. P. Phorbol ester-induced morphological changes in transformed chick fibroblasts: evidence for direct catalytic involvement of plasminogen activator. Cell. 1979 May;17(1):131–141. doi: 10.1016/0092-8674(79)90301-5. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E. Fibronectin in cell adhesion and invasion. Cancer Metastasis Rev. 1984;3(1):43–51. doi: 10.1007/BF00047692. [DOI] [PubMed] [Google Scholar]
- Saksela O. Plasminogen activation and regulation of pericellular proteolysis. Biochim Biophys Acta. 1985 Nov 12;823(1):35–65. doi: 10.1016/0304-419x(85)90014-9. [DOI] [PubMed] [Google Scholar]
- Scott R. W., Bergman B. L., Bajpai A., Hersh R. T., Rodriguez H., Jones B. N., Barreda C., Watts S., Baker J. B. Protease nexin. Properties and a modified purification procedure. J Biol Chem. 1985 Jun 10;260(11):7029–7034. [PubMed] [Google Scholar]
- Sullivan L. M., Quigley J. P. An anticatalytic monoclonal antibody to avian plasminogen activator: its effect on behavior of RSV-transformed chick fibroblasts. Cell. 1986 Jun 20;45(6):905–915. doi: 10.1016/0092-8674(86)90565-9. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis J., Salvesen G. S. Human plasma proteinase inhibitors. Annu Rev Biochem. 1983;52:655–709. doi: 10.1146/annurev.bi.52.070183.003255. [DOI] [PubMed] [Google Scholar]