Abstract
Background
Although neuraminidase inhibitors (NI) are the mainstay of treatment for influenza infection, prescribing practice for these agents is not well described. Additionally, benefit is contested.
Objectives
We examined provider prescriptions of NI during the 2009 pandemic and post-pandemic periods. We also evaluated the effectiveness of NI in reducing severity of influenza infection.
Study Design
Data on NI prescription and severity of influenza infection were compiled in healthy pediatric and adult beneficiaries enrolled in a prospective study of influenza like illness conducted at five military medical centers over five years. Subjects underwent nasal swabs to determine viral etiology of their infection. Demographic, medication and severity data were collected. Subjects who were influenza positive were included.
Results
Two hundred sixty three subjects were influenza positive (38%(H1N1)pdm09, 38.4% H3N2, and 20.5% B); 23.9% were treated with NI. NI were initiated within 48 hours in 63% of treated subjects. Although NI use increased over the five years of the study, early use declined. Most measures for severity of illness were not significantly reduced with NI; adults treated within 48 hours had only a modest reduction in duration and severity of some of their symptoms.
Conclusions
NI use in our population is increasing, but early use is not. NI use resulted in no reduction in complications of illness. Resolution of symptoms and reduction in severity of some symptoms was slightly better in adults who were treated early. These modest benefits do not support routine treatment with NI in otherwise healthy individuals with influenza.
Keywords: influenza, neuraminidase inhibitors, severity, prescription, antivirals
Background
Neuraminidase inhibitors (NI) are the only available prescription medications for treating both Influenza A and B. In the wake of the 2009 H1N1 pandemic, use of NI accelerated. The World Health Organization has endorsed NI for containment and interruption of pandemic spread.(1) Although NI have been licensed since 1999, data supporting their efficacy are underwhelming, and primarily derived from studies of seasonal, rather than pandemic, influenza. In general, the effectiveness of NI in treating seasonal influenza has been disappointing, with a median reduction in illness of 21 hours, and no reduction in complications.(2) Children treated with NI did show a 10% reduction in antibiotic use in one study, and a reduction in concurrent otitis media in another.(3)
The efficacy of NI treatment of (H1N1)pdm09 should not be impaired by resistance—such strains are nearly universally susceptible to NI.(4) NI use was quite high in the US during the H1N1 pandemic, with an estimated 18.3 million prescriptions,(5) 97.5% of which were oseltamivir.(6) However, the populations treated, and the timing of prescriptions related to illness onset are not well described. Further information on the outcomes and patterns of use of NI during and after the recent pandemic would be useful to predict the potential need for NI stockpiles in future pandemics.
Objectives
We evaluated the frequency of NI treatment in healthy military beneficiaries by reviewing NI use during a longitudinal, multi-site study focusing on influenza-like illness (ILI). We also evaluated the effectiveness of NI use in reducing severity of influenza infection in the setting of both seasonal and (H1N1)pdm09 outbreaks in a population with free and ready access to both healthcare and prescription medications.
Study Design
Procedures
Subjects were recruited from five military medical centers across the US. From October 2009 to May 2014, healthy subjects aged 0 to 65 years presenting within 72 hours after onset of influenza-like(ILI) symptoms were recruited. ILI was defined as a febrile illness accompanied by one of the following: cough, shortness of breath, chest pain, and/or sore throat, and not primarily due to a bacterial infection. Both inpatients and outpatients were eligible. Subjects with chronic medical illness were excluded.
Demographics, medical history, clinical symptoms, and physical exam findings were recorded using a standard questionnaire, and a nasopharyngeal (NP) swab (Nylon-flocked, Copan Diagnostics, Corona, CA) for respiratory viral detection was collected. After enrollment (day 0), participants returned for visits at days 3±1, and 7±2. Clinical symptoms and vital signs were recorded and a NP swab was collected. Subjects also completed symptom diaries for days -3 to 7 days after enrollment. Medications were recorded throughout the study. Use of NI was at the discretion of the provider. NI use was categorized as early (≤48 hours after onset of symptoms), late (>48 hours after onset), or none.
Severity measures
The severity of 20 symptoms were recorded at each visit and in the diaries. Severity was rated as 0: none; 1: mild; 2: moderate; and 3: severe. Research personnel trained participants on scoring. Symptoms included: GI (diarrhea, vomiting, anorexia, nausea and abdominal pain); upper respiratory (earache, runny nose, sore throat and sneezing); lower respiratory (cough, dyspnea, hoarseness and chest pain); systemic (myalgias, fatigue, headache and chills); and overall score including symptoms in upper respiratory, lower respiratory and systemic symptom groups. The latter four scores were modified from the severity measures outlined by Hayden et al. (7) Hospitalization, days of reduced activity, antipyretic use, secondary household cases and antibiotic use were also recorded. Secondary household cases were captured by asking subjects at enrollment and study visits if anyone else in the household was ill with similar symptoms, but cases were not confirmed by viral testing. Viral shedding at visits 1, 2 and 3 were documented from the NP swab results.
Specimen handling and detection of influenza virus
NP swabs were placed immediately into viral transport media, frozen at either at −70° or −80°F, and shipped on dry ice to the Naval Health Research Center (San Diego, CA). All specimens were cultured for influenza virus. Culture negative specimens also underwent detection for influenza virus by real-time reverse transcription polymerase chain reaction. We defined viral shedding as qualitative detection of virus by RT-PCR or culture on a given sample.
Statistical analysis
We examined the trend of antiviral use across seasons by performing Cochran-Armitage Trend tests. We performed univariate analysis to compare antiviral use (none vs. ever) and timing of antiviral use (early vs. late) against demographic and risk factors of having severe clinical outcomes using chi-square tests (exact test, as appropriate). We performed a multivariate logistic regression to examine the association between patient characteristics and receipt of antivirals. We then compared clinical severity scores between individuals using NI (vs. non-user) and timing of NI, respectively, using Wilcoxon rank-sum test for continuous variables, and chisquare test (exact test, as appropriate) for categorical variables. We performed Cochran-Armitage Trend tests to test whether NI use or early use is associated with shorter shedding of influenza. We consider a two-sided P value lower than 0.05 statistically significant. Analyses were performed using SAS software, Version 9.3 (SAS Institute, Cary, North Carolina).
Results
We enrolled 1,467 subjects from October 2009 to May 2014. Two hundred sixty three(17.9%) were influenza positive. Of these, 100(38%) were (H1N1)pdm09, 101(38.4%) H3N2, 54(20.5%) B, and 9(3.4 %) untypable. Figure 1 details subtype detection over the five seasons. Flu A predominated, accounting for 79.8% of all influenza infections. (H1N1)pdm09 predominated in 2009–10, and again in 2013–14, whereas H3N2 predominated in 2012–13. Overall, 63(23.9%) of influenza positive subjects received NI, with 100% receiving oseltamivir.
Figure 1.
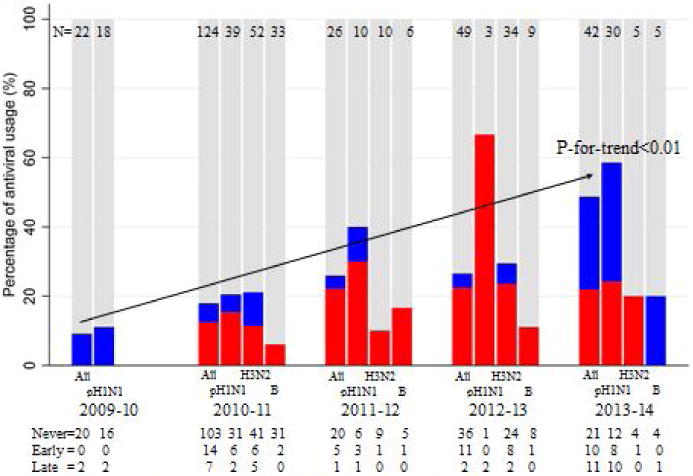
Proportion of antiviral usage among patients infected with three flu types, by season.
Red: Early antiviral prescription; Blue: Late antiviral prescription; Gray: No antiviral prescription
Figure 1 also depicts NI use by timing of initiation. Over the five seasons, NI use significantly increased from 9.1% to 50%(p<0.01). Although there were no differences in early initiation of NI according to subtypes, NI was prescribed more often for H1N1 than for the other viral subtypes(p=0.002). Also, despite increased use, early initiation of NI actually declined during the last season to 45% of treated subjects compared with 75% over the preceding 3 seasons(p=0.02). Overall, 40(63.5%) of treated subjects had NI initiated within 48 hours of symptom onset. However, only 9(12.5%) of the early treated group initiated NI within 24 hours. The majority of the late treated group initiated therapy between 48–72 hours(19, 82.6%).
Table 1 displays demographic characteristics. Subjects who were >18 years, non-obese, recipients of the current influenza season vaccine, or hospitalized were all more frequently given NI (Table 2). Subjects who received care at a US Navy medical institution (NMCSD and NMCP) as opposed to Army or Air Force (SAMMC and MAMC), were less likely to be given NI. However, no demographic group was more likely to have had NI initiated early, although there was a trend for non-hospitalized and pediatric patients to have NI initiated earlier than their counterparts.
Table 1.
Distribution of background characteristics between patients prescribed antivirals (early vs. late) and those with no prescription. Early vs. No means a comparison between those with early antiviral prescription and those with no prescription.
| Timing of antiviral prescription | Antiviral prescription | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| Early Rx (≤48 hrs) (n=40) |
Late Rx (>48 hrs) (n=23) |
Ever (n=63) |
Never (n=200) |
|||||||
| Variables | N | (%) | N | (%) | P | N | (%) | N | (%) | P |
|
|
|
|||||||||
| Age | ||||||||||
| 0–17 | 9 | (90.0) | 1 | (10.0) | 0.08 | 10 | (9.5) | 95 | (90.5) | <0.01 |
| 18–65 | 31 | (58.5) | 22 | (41.5) | 53 | (33.5) | 105 | (66.5) | ||
| Sex | ||||||||||
| Male | 23 | (60.5) | 15 | (39.5) | 0.55 | 38 | (25.3) | 112 | (74.7) | 0.55 |
| Female | 17 | (68.0) | 8 | (32.0) | 25 | (22.1) | 88 | (77.9) | ||
| Site | ||||||||||
| WRNMMC, MD | 2 | (66.7) | 1 | (33.3) | 0.90 | 3 | (33.3) | 6 | (66.7) | <0.01 |
| SAMHS, TX | 18 | (66.7) | 9 | (33.3) | 27 | (41.5) | 38 | (58.5) | ||
| NMCSD, CA | 11 | (57.9) | 8 | (42.1) | 19 | (19.2) | 80 | (80.8) | ||
| NMCP, VA | 2 | (100.0) | 0 | (0.0) | 2 | (3.4) | 57 | (96.6) | ||
| MAMC, WA | 7 | (58.3) | 5 | (41.7) | 12 | (38.7) | 19 | (61.3) | ||
| Race | ||||||||||
| White | 28 | (66.7) | 14 | (33.3) | 0.84 | 42 | (24.9) | 127 | (75.1) | 0.68 |
| African American | 6 | (54.5) | 5 | (45.5) | 11 | (23.4) | 36 | (76.6) | ||
| Asian | 2 | (66.7) | 1 | (33.3) | 3 | (14.3) | 18 | (85.7) | ||
| Others | 4 | (57.1) | 3 | (42.9) | 7 | (29.2) | 17 | (70.8) | ||
| Smoking status in patients aged 13 and older | ||||||||||
| Current Smoker | 2 | (25.0) | 6 | (75.0) | 0.16 | 16 | (66.7) | 8 | (33.3) | 0.89 |
| Former Smoker | 6 | (60.0) | 4 | (40.0) | 19 | (65.5) | 10 | (34.5) | ||
| Non-Smoker | 21 | (63.6) | 12 | (36.4) | 76 | (69.7) | 33 | (30.3) | ||
| Obesity (BMI>=30) | ||||||||||
| No | 29 | (63.0) | 17 | (37.0) | 0.66 | 46 | (30.3) | 106 | (69.7) | 0.01 |
| Yes | 3 | (50.0) | 3 | (50.0) | 6 | (12.2) | 43 | (87.8) | ||
| Morbid obesity (BMI>=40; not available in children) | ||||||||||
| No | 27 | (57.4) | 20 | (42.6) | 1.00 | 47 | (33.8) | 92 | (66.2) | 1.00 |
| Yes | 1 | (100.0) | 0 | (0.0) | 1 | (25.0) | 3 | (75.0) | ||
| Index attending daycare? (children only) | ||||||||||
| No | 6 | (100.0) | 0 | (0.0) | 0.91 | 6 | (8.5) | 65 | (91.5) | 0.19 |
| Yes | 3 | (75.0) | 1 | (25.0) | 4 | (18.2) | 18 | (81.8) | ||
| Any HH member attending Daycare? | ||||||||||
| No | 28 | (68.3) | 13 | (31.7) | 0.74 | 41 | (20.9) | 155 | (79.1) | 0.09 |
| Yes | 8 | (61.5) | 5 | (38.5) | 13 | (33.3) | 26 | (66.7) | ||
| Currently pregnant (only adult female <50 yrs) | ||||||||||
| No | 13 | (68.4) | 6 | (31.6) | 1.00 | 19 | (35.8) | 34 | (64.2) | 1.00 |
| Yes | 1 | (50.0) | 1 | (50.0) | 2 | (40.0) | 3 | (60.0) | ||
| Hospitalization | ||||||||||
| No | 35 | (71.4) | 14 | (28.6) | 0.07 | 49 | (21.3) | 181 | (78.7) | <0.01 |
| Yes | 4 | (40.0) | 6 | (60.0) | 10 | (62.5) | 6 | (37.5) | ||
| Receipt of Flu vaccine in the current season | ||||||||||
| No | 11 | (78.6) | 3 | (21.4) | 0.33 | 14 | (14.6) | 82 | (85.4) | <0.01 |
| Yes | 25 | (59.5) | 17 | (40.5) | 42 | (32.6) | 87 | (67.4) | ||
NOTE. *including monovalent (H1N1)pdm09 influenza vaccine
Table 2.
Odds ratio of patient characteristics in ever receiving an antiviral prescription among influenza-positive patients in ARIC study
| Variables | OR | 95% CI |
|---|---|---|
| Age | ||
| 0–17 | Ref | |
| 18–65 | 4.38 | (1.02, 18.88) |
| Site | ||
| WRNMMC, MD | NA | NA |
| SAMHS, TX | Ref | |
| NMCSD, CA | 0.29 | (0.10, 0.82) |
| NMCP, VA | 0.02 | (0.002, 0.21) |
| MAMC, WA | 0.58 | (0.14, 2.33) |
| Obesity (BMI>=30) | ||
| No | Ref | |
| Yes | 0.16 | (0.04, 0.66) |
| Hospitalization | ||
| No | Ref | |
| Yes | 11.21 | (1.14, 109.98) |
| Receipt of influenza vaccine* in the season of ILI onset | ||
| No | Ref | |
| Yes | 2.35 | (0.78, 7.13) |
NOTE. Ref. the reference group.
NA. Estimate in multivariate regression was not available due to very small number.
including monovalent pH1N1 influenza vaccine.
We did not find any differences in the following severity measures when comparing subjects treated with NI versus subjects never treated: antibiotic use, presumed secondary household cases, duration of hospitalization, oxygen use, and ICU admission (Data not shown). We also failed to detect any differences in these measures when comparing subjects who had early versus late initiation of NI. We did find a small difference in self-reported days of limited activity, with <1 less day of limited activity reported by the subjects who were treated early when compared to both subjects who were never treated (p=.05) and those who were treated late (p <.01).
Figure 2 displays differences in severity scores calculated from symptom diaries. Adults who were treated early had higher symptom scores in 2 categories on days 1 and 2: overall(p=.03 and p=.04) and GI(p=.03, p=.01) when compared to subjects who were never treated. Severity scores at baseline (day 1of illness) were similar between the early versus the late treated groups, and between the late treated and never treated groups, with the exception that the early treatment group had higher systemic scores at baseline compared to the late treatment group(p=.05). As their illness progressed, no difference was seen in resolution of any category of symptom when comparing adults who were never treated to those ever treated. However, adults who were treated early with NI had significantly lower severity scores over time in several categories than adults treated late. In early treated adults, upper respiratory scores were significantly lower on day 5(p=.02), systemic scores were lower on days 3 and 4(p=.02 and p=.05), and composite symptom severity scores were lower on day 2(p=.05). No differences were seen in GI or lower respiratory tract severity scores. Although we did not enroll enough children to determine differences in resolution of symptoms related to early versus late treatment, there was a significant difference in resolution of both systemic and composite symptoms by day 4 (systemic score= 2 vs. 0 and total symptom score=8 vs. 3, p<.05 for both) when comparing those children who were never treated to those children ever treated.
Figure 2.
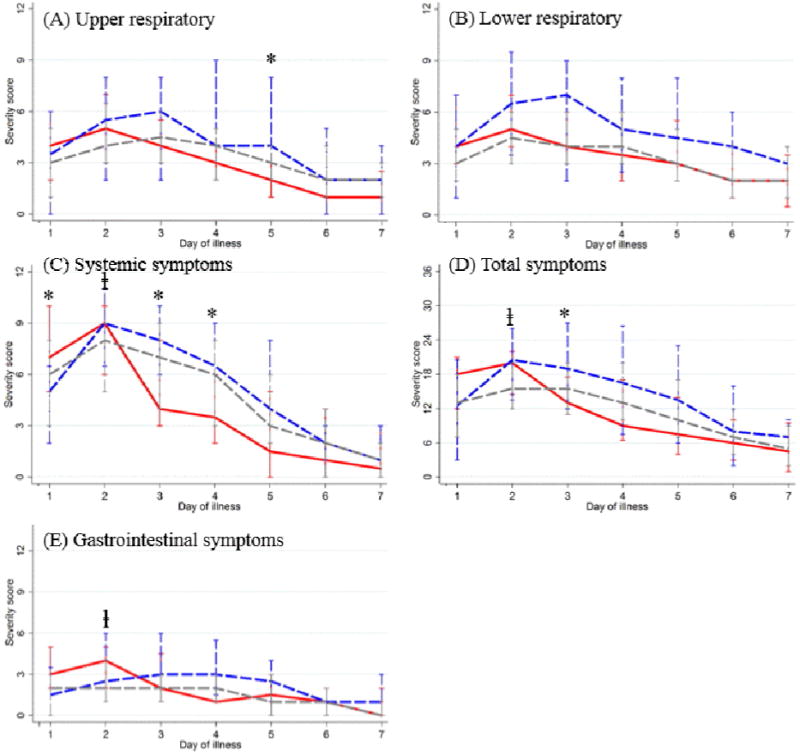
Daily median and interquartile range of composite symptom scores among influenza-positive adults in the first 7 days of illness, by timing of antiviral drug prescription
Red: Early antiviral prescription; Blue: Late antiviral prescription; Gray: No antiviral prescription
*: p<0.05 comparing EARLY to LATE use
ⱡ: p<0.05 comparing EVER to NEVER use
Due to the small number of subjects, there were no statistical differences in viral shedding from visit 1 to visits 2 or 3 when comparing treatment to non-treatment or early to late or no treatment. However, data trended toward a significant reduction in shedding amongst the early treated group, with no detectable virus in these subjects at visits 2 or 3. In comparison, 42.2% of subjects never treated and 42.9% of subjects treated late were shedding at visit 2, and 15.6% of never treated subjects and 14.3% of subjects treated late were shedding at visit 3.
Discussion
We found that NI use significantly increased in the years following (H1N1)pdm09, regardless of flu subtype, or demographic features. However, increased use did not translate into an improvement in early initiation of NI. Possibly practitioners and patients alike were more likely to consider NI treatment in the wake of the pandemic without regard to timing of initiation. However, if late treatment is not beneficial, an increase in late treatment, as seen in our population, would be a costly waste of resources.
Interestingly, providers caring for pediatric patients were less likely to prescribe NI, but more often prescribed it early. Likewise, Naval practitioners less often prescribed NI. Navy HCP are guided by an algorithm for NI therapy calling for treatment only in high risk patients, who were mostly excluded by our enrollment criteria. This policy could explain this difference, as neither the Army nor the Air Force have similar guidelines. Obese patients were less likely to be prescribed NI overall, representing a potential missed opportunity since some studies suggest that obese patients are at higher risk for complications of influenza.(8,9)
Our study accumulated information on NI treatment for (H1N1)pdm09, H3N2 and B subtypes. We found no differences in severity measures or outcomes related to NI use in (H1N1)pdm09 versus the other flu subtypes. Some previous studies had suggested that early treatment (≤48 hours) of high risk and pregnant adults infected with (H1N1)pdm09 resulted in lower hospitalization rates.(10,11) Other studies also indicated that high risk adult patients hospitalized with (H1N1)pdm09 had better outcomes when treated with NI.(12,13) A recent meta-analysis of the effectiveness of NI treatment on patients hospitalized with (H1N1)pdm09 showed a significant reduction in severe outcomes in patients treated early when compared to both late and no treatment.(14) However, all of these studies included a majority of patients with high risk co-morbidities, and few included children. Our study of adults and children without significant co-morbidities failed to document any differences in either hospitalization rates or outcomes such as duration of stay or PICU admission related to NI treatment overall, or early treatment in particular, for either (H1N1)pdm09 or other subtypes.
Recently, some authors have suggested that initiation of NI even beyond 48 hours may be of benefit. Fry et al(15) randomized 1190 influenza positive subjects to receive oseltamivir versus placebo, at < 48 hours after symptom onset and at > 48 hours. The authors found a median of one day reduction in symptoms in the treated group, including in those subjects who did not initiate treatment until the 3rd day.
In contrast, we found benefit to NI therapy only when it was initiated early, and these benefits were modest. If only early treatment provides any benefit to healthy patients, then providers should confine NI use to those who present within that time frame. Unfortunately, several studies have suggested that NI therapy is not started within 48 hours in a majority of patients.(13,16) Barriers to medical care or prescribed medications could be a factor in preventing early initiation of NI. However, we found a disturbing trend in our subjects, who do have access to both –by the last year, less than 50% had early initiation of treatment.
Our findings support those of a recent meta-analysis(17) which found that in treatment trials, oseltamivir reduced the time to alleviation of symptoms by 16.8 hours in adults, and 29 hours in healthy children. They did not find any differences in hospitalizations nor in respiratory complications in adults or children. They did find an increase in vomiting in both treated children and adults, in contrast to our findings of decreased GI scores in treated children, and no difference in GI severity scores between treated and untreated adults.
Shedding has been reported to be prolonged with (H1N1)pdm09 compared to seasonal influenza, (16, 18, 19) but there is little information on whether NI treatment reduces influenza shedding, and if such a reduction is dependent on the timing of NI initiation. Ling et al(20) reported prolonged shedding in 37% of their subjects hospitalized with H1N1 despite oseltamivir. However, treatment with oseltamivir within the first 3 days after illness onset did result in a significant decrease in prolonged shedding. We also found that subjects who were treated early had prompt resolution of shedding, regardless of viral subtype. Interestingly, late treatment did not have the same effect. Larger studies are needed to see if this effect on shedding is substantiated.
Our study had several limitations. Since the early treated group did have increased baseline severity by 2 different symptom scores, our results may have been biased to demonstrate less benefit of early treatment. We also found that the majority of the early treated group did not initiate therapy until 24–48 hours. Potentially, treatment at <24 hours would have had more dramatic effects, though this would be logistically difficult to achieve. We also had more subjects with H1N1 in the treated group compared to other subtypes, and severity of H1N1 may have been worse that the other subtypes, potentially biasing against the effect of treatment. Our study also excluded those subjects with significant co-morbidities, so our findings cannot be generalized to that population.
Our study provides evidence that NI treatment of previously healthy children and adults infected with influenza modestly accelerates symptom resolution and reduction in severity of some symptoms in adults if they are treated within 48 hours. We were unable to demonstrate any reduction in complications. NI therapy led to rapid resolution of shedding, but only when given early. This benefit could be an important means for reducing transmission, particularly during pandemics, but only if a mechanism is in place for provision of early therapy. Given the poor sensitivity of rapid influenza antigen assays, real-time PCR for rapid early detection of influenza virus could be a critical tool in identifying infected patients early enough to benefit from treatment. Provider education is needed to conserve resources and prevent inappropriate initiation of NI after 48 hours of illness.
Highlight Bullet Points.
-
-
We report NI prescription patterns and effects on influenza severity in healthy hosts
-
-
NI prescriptions are increasing, but early prescription within 48 hours is not
-
-
Early prescription of NI modestly reduces the severity of some symptoms in adults
-
-
Early prescription, but not late prescription, reduces the duration of viral shedding
Acknowledgments
We are indebted to the study team of clinical research coordinators, laboratory personnel, and data management staff for their dedication to the project. The views expressed are those of the authors and do not necessarily reflect the official views of the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., Uniformed Services University of the Health Sciences, the Department of Defense (DoD), or other federal agencies.
Funding: This work was supported by the Infectious Disease Clinical Research Program (IDCRP), a Department of Defense (DoD) program executed through the Uniformed Services University of the Health Sciences. This project has been funded in whole, or in part, with federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIH), under Inter-Agency Agreement [Y1-AI-5072].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST
Competing interests: All authors. None reported.
Ethical Approval: The study was approved by the Infectious Disease Institutional Review Board of the Uniformed Services University of the Health Sciences (IDCRP-045).
References
- 1.World Health Organization. Global agenda on influenza surveillance and control. 2002 www.who.int/csr/disease/influenza/csrinfluenzaglobalagenda/en/print.html.
- 2.Jefferson T, Jones MA, Doshi P, Del Mar CB, Heneghan CJ, Hama R, Thompson MJ. Neuraminidase inhibitors for preventing and treating influenza in healthy adults and children. Cochrane Database of Systematic Reviews. 2012;(1) doi: 10.1002/14651858.CD008965.pub3. Art. No.: CD008965. [DOI] [PubMed] [Google Scholar]
- 3.Wang K, Shun-Shin M, Gill P, Perera R, Harnden A. Neuraminidase inhibitors for preventing and treating influenza in children. Cochrane Database of Systematic Reviews. 2012;(1) doi: 10.1002/14651858.CD002744.pub3. Art. No.: CD002744. [DOI] [PubMed] [Google Scholar]
- 4.CDC. Antiviral agents for the treatment and chemoprophylaxis of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR. 2011;60 No. RRR-1. [PubMed] [Google Scholar]
- 5.Donner B, Bader-Weber S, Schwarz R, Peng MM, Smith JR, Niranjan V. Safety profile of Oseltamivir during the 2009 influenza pandemic. Pharmacoepidemiol Drug Saf. 2011;20:532–43. doi: 10.1002/pds.2136. [DOI] [PubMed] [Google Scholar]
- 6.Atkins CY, Patel A, Taylor TH, et al. Estimating effect of antiviral drug use during pandemic (H1N1) 2009 outbreak, United States. Emerg Infect Dis. 2011;17:1591–1598. doi: 10.3201/eid1709.110295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hayden FG, Fritz R, Lobo FC, Alvord W, Strober W, Strauss SE. Local and systemic cytokine responses during experimental human influenza A virus infection. Relation to symptom formation and host defense. J Clin Invest. 1998;101:643–9. doi: 10.1172/JCI1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cocoros NM, Lash TL, Demaria A, Klompos M. Obesity as a risk factor for severe influenza-like illness. Influenza Other Respir Viruses. 2014;8:25–32. doi: 10.1111/irv.12156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morgan OW, Bramley A, Freedman DS, et al. Morbid obesity as a risk factor for hospitalization and death due to 2009 pandemic influenza A (H1N1) disease. PLoS One. 2010;5:e9694. doi: 10.1371/journal.pone.0009694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Siston AM, Rasmussen SA, Honein MA, et al. Pandemic 2009 influenza A (H1N1) virus illness among pregnant women in the United States. JAMA. 2010;303:1517–25. doi: 10.1001/jama.2010.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grijalva-Otero I, Talavera JO, Solorzano-Santos F, et al. Critical analysis of deaths due to atypical pneumonia during the onset of the influenza A (H1N1) virus epidemic. Arch Med Res. 2009;40:662–8. doi: 10.1016/j.arcmed.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 12.Uyeki T, et al. Antiviral treatment for patients hospitalized with 2009 pandemic influenza A (H1N1) N Engl J Med. 2009;23:e110. doi: 10.1056/NEJMopv0910738. [DOI] [PubMed] [Google Scholar]
- 13.Louie Jk, et al. Treatment with Neuraminidase inhibitors for critically ill patients with influenza A (H1N1)pdm09. CID. 2012;55:1198–1204. doi: 10.1093/cid/cis636. [DOI] [PubMed] [Google Scholar]
- 14.Muthuri SG, Myles PR, Venkatesan S, Leonardi-Bee J, Nguyen-Van-Tam JS. Impact of neuraminidase inhibitor treatment on outcomes of public health importance during the 2009–2010 influenza A (H1N1) pandemic: a systemic review and meta-analysis in hospitalized patients. JID. 2013;207:553–563. doi: 10.1093/infdis/jis726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fry AM, Goswami D, Nahar K, et al. Efficacy of Oseltamivir treatment started within 5 days of symptom onset to reduce influenza illness duration and virus shedding in an urban setting in Bangladesh: a randomized placebo-controlled trial. Lancet. 2014;14:109–118. doi: 10.1016/S1473-3099(13)70267-6. [DOI] [PubMed] [Google Scholar]
- 16.Yu H, Liao Q, Yuan Y, et al. Effectiveness of oseltamivir on disease progression and viral RNA shedding in patients with mild pandemic 2009 influenza A H1N1: opportunistic retrospective study of medical charts in China. BMJ. 2010;341:c4779. doi: 10.1136/bmj.c4779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jefferson T, Jones M, Doshi P, Spencer EA, Onakpoya I, Heneghan CJ. Oseltamavir for influenza in adults and children: systemic review of clinical study reports and summary of regulatory documents. BMJ. 2014;348:1–18. doi: 10.1136/bmj.g2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Serres G, Roulea I, Hamelin ME, et al. Shedding of novel 2009 pandemic H1N1 (nH1N1) virus at one week post illness onset; Program and abstracts of the 49th Interscience Conference on Antimicrobial Agents and Chemotherapy; San Francisco, CA. 2009. Abstract K-1918a. [Google Scholar]
- 19.Witkop CT, Duffy MR, Macias EA, et al. Novel influenza A (H1N1) outbreak at the U.S. Air Force Academy: epidemiology and viral shedding duration. Am J Prev Med. 2010;38:121–126. doi: 10.1016/j.amepre.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 20.Ling LM, Chow AL, Lye DC, Tan AS, Krishnan, Cui L, Win NN, Chan M, Lim PL, Lee CC, Leo YS. Effects of early oseltamivir therapy on viral shedding in 2009 pandemic influenza A (H1N1) virus infection. CID. 2010;50:963–968. doi: 10.1086/651083. [DOI] [PubMed] [Google Scholar]


