Abstract
Mesenchymal cells are natural tissue builders. They exhibit an extraordinary capacity to metamorphize into differentiated cells, using extrinsic spatial and temporal inputs and intrinsic algorithms, as well as to build and adapt their own habitat. In addition to providing a habitat for osteoprogenitor cells, tissues of the skeletal system provide mechanical support and protection for the multiple organs of vertebrate organisms. This review examines the role of mechanics on determination of cell fate during pre-, peri- and postnatal development of the skeleton as well as during tissue genesis and repair in postnatal life. The role of cell mechanics is examined and brought into context of intrinsic cues during mesenchymal condensation. Remarkable new insights regarding structure function relationships in mesenchymal stem cells, and their influence on determination of cell fate are integrated in the context of de novo tissue generation and postnatal repair. Key differences in the formation of osteogenic and chondrogenic condensations are discussed in relation to direct intramembranous and indirect endochondral ossification. New approaches are discussed to elucidate and exploit extrinsic cues to generate tissues in the laboratory and in the clinic.
Keywords: stem cell mechanics, cell fate, osteogenesis, chondrogenesis, skeletal development
1. Introduction and History
During embryonic development, cells respond to extrinsic and intrinsic signals to form the template of the complete organism. Extrinsic biochemical and biophysical signals comprise the dynamic environment of the cell. A body of literature documents the role of biochemical signals in determining cell fate during embryonic development [Thompson 1917, Turing 1952, Katagiri et al. 1990, Schofield and Wolpert 1990, Chen et al. 1991, Izumi et al. 1992, Freeman and Gurdon 2002, Shea et al. 2003, Derfoul et al. 2004, Bielby et al. 2004, Vogel and Sheetz 2006, Kawakami et al. 2006]. However, neither the biophysical signals experienced by multipotent progenitor cells during development nor the specific nature of biophysical signals conducive to guiding these cells toward specific cell lineages is well characterized [Anderson and Knothe Tate 2007a, Anderson and Knothe Tate 2007b, Falls 2007]. For more than a century, scientists have hypothesized that biophysical signals including mechanical stimuli modulate cell lineage commitment and the formation of specific tissue types during development [Thompson 1917, Carey et al. 1922, Pauwels 1960, Oster et al. 1983, Oster et al. 1985, Carter and Wong 1988, Estes et al. 2004]. In the past few decades, computational models have predicted the role of mechanical [Oster et al. 1983, Oster et al. 1985, Carter and Wong 1988] and biophysical forces [Oster et al. 1983, Oster et al. 1985, Munro unpublished] on morphogenesis, yet the mechanism by which these stimuli drive uncommitted stem cells to differentiate into specific cell lineages remains a conundrum. Recent studies have demonstrated that embryonic stem cells are more mechanosensitive than differentiated cells from the same species [Oberhofer 2005, Falls 2007, McBride 2007, Anderson and Knothe Tate 2007a, Anderson and Knothe Tate 2007b]. Furthermore, studies have demonstrated that biophysical mechanisms modulating fate decisions occur much earlier than previously thought [Hall and Miyake 2000]. Already during the earliest stage of tissue organization, i.e. gastrulation, mechanical forces (tensile) direct self assembly of the germ layers [Krieg et al. 2008]. Similarly, cell adhesion and cell tension modulate the patterning of the retina in drosophila [Kafer et al. 2007]. Hence, mechanical forces influence tissue phenotype throughout the pre- and perinatal time periods [Hall and Miyake 2000]. Elucidation of the mechanism by which forces inherent to life on Earth translate to self assembly of multicellular structures, including tissues and organs, is expected to provide an understanding of the etiology of pre- and perinatal developmental defects. In addition, once elucidated, these mechanisms can be exploited to deliver prophylactic or therapeutic biophysical stimuli for defect prevention as well as for guided repair or de novo generation of tissue during postnatal life. Hence, the goal of this review is to describe temporal and spatial aspects of biophysical cues that influence early stages of skeletal cell fate determination.
2. Epithelial-Mesenchymal Transitions and Interactions: The Basis of Positional Information
While all cells begin with the same genetic information, positional cues, including biochemical and biophysical factors, enable cells to determine their proper function within the multi-cellular, developing organism [Schofield and Wolpert 1990]. Changes in these positional cues over time further allow individual and groups of cells to adapt their behavior to the dynamic environment comprising the developing embryo. For example, at the eight cell stage of development, the first multicellular structures are epithelial in nature, i.e. the structures exhibit polarity as well as adherens junctions conferring permeability barrier function between cells and across epithelial sheets [Quinlan 1999]. Later, during gastrulation, the first epithelial-mesenchymal transition (EMT) results in the differentiation of the ectoderm and mesoderm as observed through the appearance of the primitive streak [Burdsal et al. 1993, Ohta et al. 2007]; the mesoderm derived from gastrulation is referred to as mesenchyme.
Already at this early developmental stage (EMT), biophysical signals play a role in providing a mechanically permissive site for egression of cells from a tight sheet. Namely, the expression of syndecan, a transmembrane proteoglycan that binds cells to the matrix, decreases at sites where cells leave the epithelium to form the mesenchyme [Bernfield et al. 1993]. A concomitant reduction of structural matrix proteins such as collagen, results in flattening of the cells at EMT sites. Studies in rabbit fibroblasts have shown that flattening of cell shape in synovial fibroblasts leads to increased gene expression and subsequent secretion of collagenase [Aggler et al. 1984], which would likely further exacerbate the decrease in structural proteins at EMT sites. Hence, by providing a site of structural weakening (or a “stress concentrator”), a path of least resistance is provided for egressing cells, e.g. incipient to bud formation in the chick lung [Abbott et al. 1991] or formation of the mesenchyme of the limb bud.
Cells within the mesoderm are influenced by inductive factors from the ectoderm [Burdsal et al. 1993]. Moreover, the ectoderm per se may provide a physical means for mesenchymal cells to determine their position in three dimensional space of the developing embryo, via absolute and referential markers (Figure 1) [Spiegel and Spiegel 1992, Burdsal et al. 1993]. As delineated in the example of the EMT above, positional cues are provided indirectly (referential markers of position) by the spatial presentation of extracellular matrix constituents, growth factors, proteases and anti-proteases which modulate cell shape, motility, adhesion, proliferation and differentiation during organ morphogenesis [Bernfield et al. 1993]. For instance, cell adhesion molecules, including fibronectin and laminin, pave a path from the basement membrane of the epithelial layer to the extracellular matrix of mesenchymal cells [Spiegel et al. 1983]. The ectoderm may also provide absolute positional markers like a global positioning device for cells; e.g. in the sea urchin, a subset of mesenchymal cells “inserts between the cells of the ectodermal wall in order to firmly anchor” and provide an absolute reference point for the skeletal template during differentiation [Spiegel and Spiegel 1992]. Such absolute reference points would provide pivotal positional information to multipotent mesenchymal cells during prenatal vertebrate development, when biophysical cues arising from ground reaction forces are not yet relevant [Anderson and Knothe Tate 2007a, Anderson and Knothe Tate 2007b].
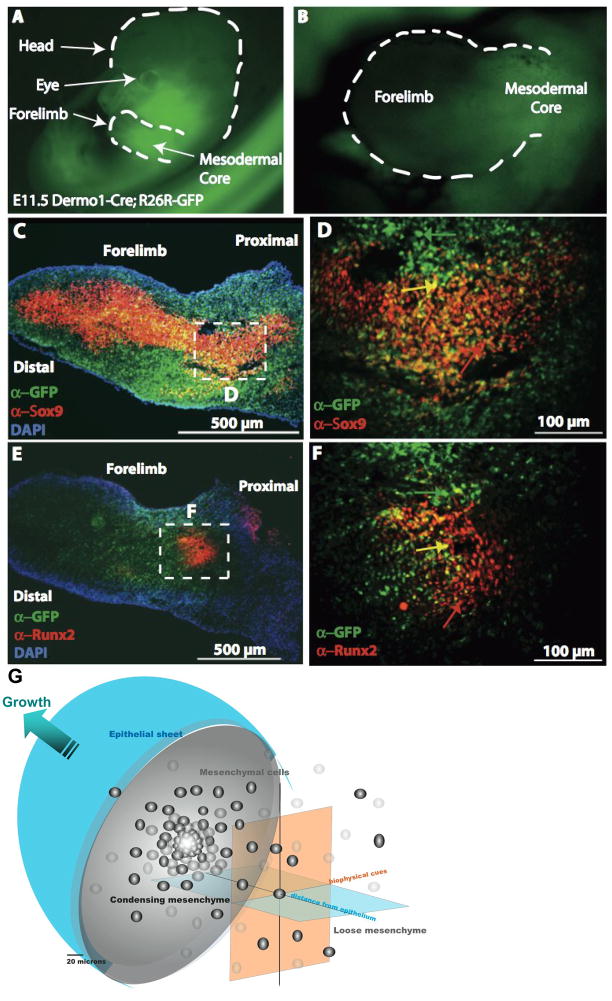
Figure 1. Key extrinsic factors and their role in mesenchyme condensations, e.g. in the murine embryonic limbud at E11.5.
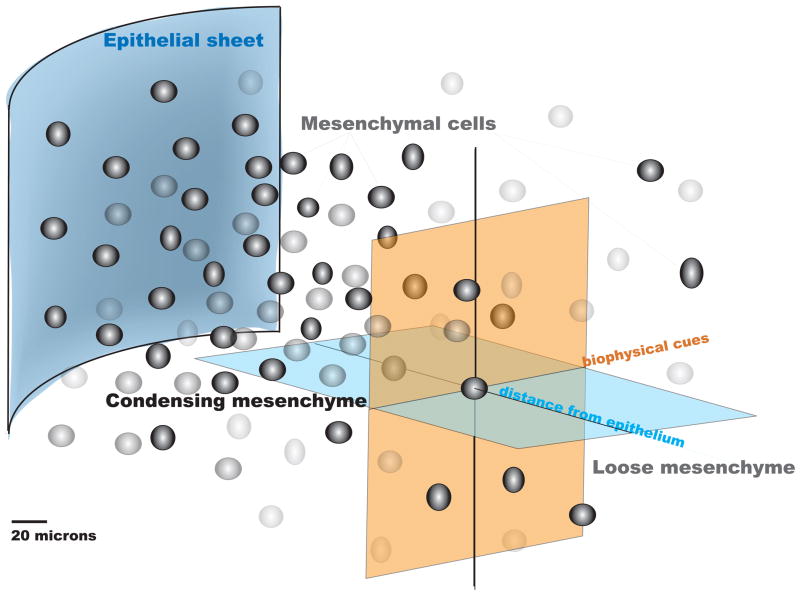
A–F: The ectoderm and mesenchmal core in limbbuds immunostained with antibodies against GFP for Dermo1 lineage (green), Sox9 (red: C,D), and Runx2 (red: E,F). D,F correspond to a high magnification enlargement of the respective dashed areas in the mesodermal core of C,E. In C–F, cells nuclei appear blue due to DAPI staining. G represents the bulbous limb bud schematically, demonstrating the spatial dependence of inductive biochemical cues from the ectoderm (depicted as “epithelial sheet”) as well as their interplay with biophysical cues deriving from the condensation as well as proximity to the ectoderm (epithelial sheet). Refer to 4. Genetic… for further details of gene activity associated with the condensation event. Fig. 1A–F, adapted from [Falls 2007]
The interaction between positional and genetic factors manifests itself particularly during mesenchymal condensation, which is the leading step of skeletogenesis or patterning of skeletal components [Dunlop and Hall 1995, Fujimaki et al. 2006]. In addition to the inherent positional cues provided by the epithelial-mesenchymal interactions described above, the condensation of the mesenchyme provides powerful biophysical and biochemical cues that modulate the differentiation of multipotent mesenchymal cells along specific lineages [Dunlop and Hall 1995]. In fact, the onset of proliferation followed by the formation of a condensation is referred to as the “earliest detectable cellular stage of chondrogenesis” (after [Dunlop and Hall 1995, Hall and Miyake 1992]) that “marks initiation of selective gene expression during skeletogenesis” [Dunlop and Hall 1995] (see 3.b. and 3.c. …Genetic Fingerprints). Although a century of work describes the role of biochemical signals in determination of mesenchymal stem cell fate, e.g. along osteo- and chondrogenic lineages (see review in [Ferguson et al. 1998, Mackie et al. 1998, Mackie et al. 2007] for further details), a dearth of information exists about the role of biophysical signals.
3. Mesenchymal Condensations – Leading Step in Skeletogenesis
Mesenchymal condensations are referred to as the cellular modules or units from which skeletal and many other tissues are built [Hall and Miyake 1992]. Mesenchymal condensations are the leading step in differentiation not only of skeletal tissues such as bone, cartilage, muscle, tendon and ligament, but also of teeth, feathers, hair follicles, scales, kidney and fat [Bard 1990, Dunlop and Hall 1995]. In this review we refer to them as the leading step in skeletogenesis, in reference to the fact that condensations represent the initiation of skeletal tissues in vertebrates at multiple time scales, e.g. embryonic development (time scale: hours to days), regeneration of skeletal tissues after trauma or clinical resection in postnatal life (time scale: days to weeks), as well as modification of morphology during evolution (time scale: centuries to millennia). Furthermore, in this review an emphasis will be placed on differences between osteogenic and chondrogenic condensations and the role of extrinsic factors in development, repair and regeneration of the skeleton in pre-, peri- and postnatal stages of vertebrate life.
Mesenchymal condensation refers both to the process of individual cells being compressed or brought together, as well as the multicellular entity comprised of cells that have gone or are going through the process of condensation (Figure 1) [Hall and Miyake 1992]. As evidenced by the presence of noncondensing mesenchyme, although all mesenchymal cells share the same genetic information, not all mesenchymal cells condense. Proximity to the epithelium is a putative prerequisite for mesenchymal condensation, as epithelial-mesenchymal inductive, and potentially mechanical, interactions are necessary for condensation to occur (see discussion of Notch expression in 3.b. …Genetic Fingerprints).
a. Cell Mechanics Perspective
An understanding of condensation mechanisms may yield insight into the earliest stages of cell and tissue specialization, yet the cues instructing cells to aggregate, dissipate, or condense are not known (Figures 1,2,5). Migration of mesenchymal cells to the limb and outgrowth of the limb by proliferation and differentiation occurs under the putative influence of mechanical stimuli [Oster et al. 1983, Oster et al. 1995]. The condensation event per se changes the distribution and equilibration of physical forces at the cellular level. In limb development and neural crest derived condensations, aggregation of cells toward a nexus is known to be important [Bee and von der Mark 1990]. Aggregation may be associated with a molecular “aggregation” cue that emanates from a central point [Epperlein and Lehmann 1975] or a “dissipation” cue that decreases about a central point [Gould et al. 1972]; this well studied signaling paradigm is used by Dictyostelium discoideum, a slime mold, to effect aggregation of independent amoeba into a multicellular organism exhibiting subspecialized structures [Keller and Segel 1970a,b]. Another plausible mechanism for condensation is the lack of dissipation from a nexus of cell division or increased mitotic activity, which is known to be important in osteogenic condensation of the scleral ossicles [Hall and Miyake 1992]; in this case, the aggregation cue may be colocalized with the area of increased mitotic activity. A third possible mechanism that may act in concert with the others is the effect of local (e.g.) through localized expression of cell adhesion molecules) and global cell traction (cells pulling cells) [Harris et al. 1980a, Harris et al. 1980b; Bard 1990], which at this stage of development would likely exert shear and tensile stresses at interfaces between cells [Oster 1983, Oster 1985].
Figure 2.
Cellular condensations depicted as an aggregation event, around a central locus for the case of a bulbous mesenchymal core (A), and as a core expanding about a center of mitotic activity (B). In the former case, the ectoderm or bounding sheath counteracts the compression of the mesoderm. In the latter case, the ectoderm resists the forces of expansion. Of note, each point in space within the condensing mesenchyme is subjected to a unique combination of stresses and mechanically modulated chemical gradients due to imperfect symmetry in boundary conditions and inhomogeneities in the mesenchyme.
Figure 5.
Schematic depiction of osteogenic condensation.
The ectoderm defines the outer edges or boundaries of the developing limb. As a tightly organized band of tissue that envelops the mesoderm, the ectoderm influences the balance of forces within the mesoderm. This tissue level force balance further influences force balances within individual cells, and ultimately transduces mechanical forces to the nucleus, where adaptation is manifested through modulation of gene activity. Interestingly, aggregation or lack of dissipation of cells around a common locus would likely result in compressive stresses within the condensation that would be equilibrated by the tensile stresses of the enveloping ectoderm (Fig. 2A). A mesenchymal condensation associated with increased cell division would result in a net expansion of tissue, creating a tensile force on the ectoderm, due to hoop stresses pushing the bounding envelope outwards (Fig. 2B). The differing effects of aggregation versus expansion may influence whether an osteogenic versus a chondrogenic condensation forms per se. Taking this a step further, the effect of aggregating cells versus expanding cell mass likely exert further influence on the subsequent persistence of epithelial mesenchymal transitions, perhaps influencing stem cell recruitment from the epithelium as well as definition of apoptotic zones during patterning and further morphogenetic events. Loci of condensation are bounded by zones of apopototic noncondensing mesenchyme, which delineate the individual elements of multi-element skeletal organs, e.g. the multiple bones and cartilages making up the hand and the vertebral column. In addition, areas of loose, noncondensing mesenchyme are areas observed to show increased vascularity [Ekblom et al. 1994], perhaps due to diminished cell viabilitiy in these areas or because these areas of less dense tissue provide less resistance to vascular invasion than the highly dense tissue of the condensed mesenchyme.
Hence, each point in space within the volume of the condensing mesenchyme is exposed to a unique combination of stresses and mechanically modulated chemical gradients, both of which make up the cell’s positional cues at a given point in time. In addition to the force equilibration effected by an increasing cell mass within the bounding envelope of the ectoderm, shear stresses due to relative movement between touching cells superimpose with the global stress regime imposed through the condensing mesenchyme bounded by the ectoderm. These physical forces are thus transduced between cells and across cell membranes via the cytoskeleton to the nucleus, thereby affecting cell morphology and fate, respectively.
Condensation is a metamorphizing event for the cell. The balance of forces in the cell during condensation plays a key role in determining cell shape and cell fate [McBeath et al. 2004]. Interacting factors that determine force distribution at the cell scale include i.a. cell motility and cell spreading via cell-cell (cadherins) and cell-matrix (integrins) contacts [Dunlop and Hall 1995], cytoskeletal tension (cytoskeletal proteins, and spatial distribution of multi- and single cell structures) [Chen et al. 1997, McBeath et al. 2004], the compliance of the surface to which the cell adheres (force equilibration in the cell) [Engler et al. 2006], and cell density (cell numbers, condensation volume) [Benya and Schaffer 1982].
During the epithelial-mesenchymal transition, cell-cell adhesions dissociate to allow the cell’s release from the epithelium and then movement through the mesenchyme, where cell-matrix adhesions dominate. Studies in epiblast explants demonstrate the role of cell-cell and cell-matrix adhesions in the EMT. Whereas cells within the epiblast exhibit the close packed morphology of epithelia, with tight, calcium dependent E-cadherin adhesion complexes between cells, loss of E-cadherin expression results in loss of cell-cell junctions. As the adhesive interactions between cells break down, cell morphology changes (to mesenchymal), and cells migrate [Burdsal et al. 1993]. As the epiblast cells migrate to form the mesoderm, cell-matrix adhesions play a more dominant role.
The change in the balance of cell-cell to cell-matrix interactions exerts a profound influence on cell and tissue mechanics and is likely influenced itself through the biochemical and physical interactions between the epithelium and mesenchyme, via the temporospatial presentation of matrix molecules including i.a. fibronectin, laminin, collagen and vitronectin [Burdsal et al. 1993]. Hence, tipping the balance of cell-cell to cell-matrix adhesions resulted in the EMT transition, allowing motile cells to form the mesenchyme. At some point, cell adhesion becomes less dynamic as the motile cell is integrated into the mesenchyme, establishing cell-cell and cell matrix adhesions to stabilize and adapt, until the balance of cell-matrix to cell-cell adhesions is tipped again, resulting in the condensation of mesenchymal cells through an increase in expression of N-cadherins (thereby increasing cell-cell adhesions, see 3.b. Chondrogenic Condensations…)
The cytoskeleton, anchored via the cell-cell (e.g. cadherins) and cell-matrix (e.g. integrins) adhesions, influences the shape of the cell through the degree of spreading in three dimensions. Shape is an intrinsic manifestation of form and function; while adipocytes are round storehouses for fat [Grigoriadis 1988, McBeath et al. 2004], osteoblasts spread themselves out to maximize contact with the substratum on which they deposit osteoid [Parfitt 1984, McBeath et al. 2004]. Groups of cells can form cell-matrix composite structures, including sheets, trusses, springs and dampers, that serve load bearing (osteogenic cells), load transfer (ligament- and tenogenic cells), mechanical energy transfer (myogenic), mechanical energy damping (chondrogenic), energy storage (lipogenic), and signal transfer (neurogenic) functions [Thompson 1917]. Hence, the empirical relationship between form and function not only holds for tissues but also for cells, which both build and serve as building blocks for the tissue. Differentiation is the culmination of phenotype to serve a specialized function.
Recent work underscores the role of cell shape, manifested physically through integration of all mechanical cues, as the driver of cell fate decisions [McBeath et al. 2004]. The relationship between shape, a measure of the degree of spreading, and lineage commitment to osteoblastic or adipocytic fate is intimately tied to the molecular switch of the RhoA and RhoA kinase (ROCK) signaling pathway and cytoskeletal tension. Namely, RhoA and RhoA kinase activity is higher in spread than in rounded cells and genetic modification of cells to turn off RhoA and ROCK activity results in commitment of mesenchymal stem cells to adipogenic lineage, even when cultured in osteogenic differentiation media; activation of this activity results in commitment of MSC’s to osteogenic lineage even when cultured in adipogenic differentiation media [McBeath et al. 2004]. Interestingly, while RhoA acts as a molecular switch through shape control, conditional activation of the RhoA effector, ROCK, induces osteogenic fate independent of cell shape, indicating that it is downstream of the RhoA and cytoskeletal tension signals [McBeath et al. 2004]. Furthermore, recent studies implicate RhoA/ROCK signaling in suppression of chondrogenesis via modulation of Sox9 expression and actin organization [Woods et al. 2005].
Furthermore, cell shape is highly modulated through the mechanical compliance of the cell’s immediate environment [Engler et al. 2006]. Just as the basis of cell motility lies in the coordinated generation of forces via formation and dissociation of adhesion complexes, the basis of cell spreading and adhesion lies in the balance of forces between the cell and the matrix in which it inhabits. Almost two decades ago it was recognized that the composition and organization of the extracellular matrix enforces cell shape and can modulate cell proliferation and differentiation in vitro [Tucker et al. 1985, Watt 1986, Abbott et al. 1991]. More recent work underscores how mismatch in cell and substrate compliance enforces cell shape and fate. Cells which inhabit more compliant (more elastic) matrices, deform the matrix more than cells which inhabit less compliant matrices (similar to the skeleton deforming soft versus hard mattresses). More compliant matrices, such as those found in adipogenic or neural tissues, are unable to effectively resist the cell’s forces. Thus, cell spreading results in local deformation of the matrix, causing it to “give way” around the cell and impose a more rounded shape. In addition, a very compliant matrix allows for the extension of filipodia, which in turn promotes the formation of cellular networks that maximize the efficiency of signal transfer (e.g. dendrites, osteocytes). Stiffer matrices effectively resist cell forces, allowing for increased cell spreading, which in turn promotes commitment to, e.g. osteogenic lineages. [Engler et al. 2006] Once again, cell shape embodies an integration of multiple mechanical cues, transducing these signals to the nucleus via the cytoskeletal-myosin network, a critical first step in the commitment process [McBeath et al. 2004, Engler et al. 2006].
Cell density also influences cell shape and is a permissive factor for cell differentiation in vitro [Benya and Shaffer 1982, McBeath et al. 2004]. When cultured at high density in osteogenic differentiation media, approximately 20% of human mesenchymal stem cells (hMSCs) commit to the osteogenic lineage; when cultured at low density in the same media, 80% of the cells commit to osteogenic lineage, due to the increased area available for cell spreading. These observations are reversed when hMSC’s are cultured at high density in adipogenic differentiation media, where 80% of cells commit to the adipocytic lineage, due to the enforcement of rounded cell shape due to space restrictions. When seeded at low density in an adipogenic differentiation media, a negligible percent of cells commit to the osteogenic lineage. Studies on single cells allow for control of spreading area without the potentially confounding effects of cell-cell contacts. These studies demonstrate that high density cultures leave little room for spreading, similar to seeding a single cell on a small island, thus enforcing a more rounded cell shape and a preference for adipocytic commitment [McBeath et al. 2004]. In contrast, low density leave ample room for spreading, similar to seeding a single cell on a large island, thus a preference for osteogenic commitment [McBeath et al. 2004]. Previous studies in differentiated (endothelial) cells demonstrated the influence of cell shape on cell proliferation and apoptosis, where single cells forced to assume a rounded morphology showed an increase in apoptotic markers and single cells allowed to spread show marked proliferation and no apoptotic markers [Chen et al. 1997, Ingber 1990]. In addition, bud formation in the early chick lung is associated with flattening of mesenchymal cells and thinning of the epithelial basement membrane, concomitant to loss in the structural protein collagen as well as tenascin [Abbott et al. 1991], an extracellular matrix molecule that can “interfere with the action of the ECM molecule fibronectin” [Chiquet-Ehrismann et al. 1988] and that is common in the mesenchymes of budding organs in embryos [Chiquet-Ehrismann et al. 1986]. Of all factors affecting bud formation, only cell shape change is correlated with initiation of the bud in the developing chick lung [Abbott et al. 1991]. Hence, cell shape regulates not only the fate of the cell but also the further fate of the tissue, through modulation of committed cell proliferation and apoptosis.
In the condensing mesenchyme, a mixed presentation of parameters likely influences cell shape and subsequent lineage commitment (as described below in b. Mechanobiology…). By combining parameters such as cell density and organization of multicellular structures in three dimensional space, cell shape and hence cell fate are likely determined through the combined influence of available room for spreading, compliance of the local environment (for a given volume, more dense environments would be less compliant), force equilibration through the cytoskeleton and cell-cell/cell/matrix contacts, as well as any global mechanical effects imposed through the bounding envelope of the epithelium [McBride 2007].
b. Chondrogenic condensations: mechanobiology and genetic fingerprints
As presented above, mechanical cues are inextricably tied to cellular and hence biological events occurring during condensation. Furthermore, they may provide intrinsic cues that determine whether the condensation follows a chondrogenic or osteogenic path (Figure 3).
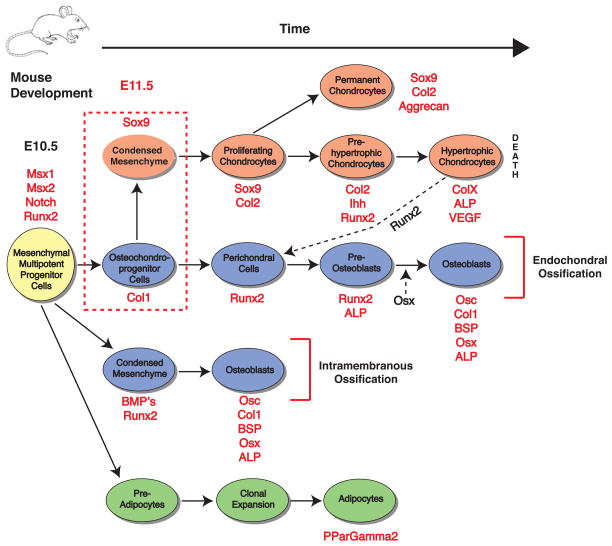
Figure 3. Mesenchymal multipotent progenitor cells have the capacity to differentiate into several lineages including chondrocytes (orange), osteoblasts (blue) and adipocytes (green), as well as myoblasts, tenocytes, stromal cells and endothelial cells (not shown).
The differentiation processes as well as markers found at each stage and select signaling pathways are shown. The dashed red box indicates the timepoint of chondrogenic condensation, which is presaged by Notch expression and initiated by Msx-1,2 (and whose expression continues in the condensation phase) [Hall and Miyake 1995]. Data points plotted after [Pittenger et al. 1999, Kronenberg 2003, Nakashima and de Crombrugghe 2003, Shum and Nuckolls 2002, Day et al. 2005, Rosen and MacDougald 2006].
A chemical gradient of bone morphogenetic proteins (BMP’s) [Kronenberg 2003] and fibronectin [Hall and Miyake 1992], resulting from epithelial-mesenchymal interactions, is thought to initiate condensations [Kronenberg 2003, Yoon and Lyons 2004]; mechanical stimuli modulate the spatiotemporal distribution of chemical gradients, within a given volume of developing tissue, by load-induced convective flow superimposed with diffusional transport [Shvartsman in press, Knothe Tate 2003, Knothe Tate et al. 1998a, b]. BMP signaling promotes condensation through the stimulation of N-cadherin production [Yoon and Lyons 2004]. N-cadherin is a part of the cadherin superfamily of molecules responsible for junctional cell-cell adhesions [Goodsell 2002] such as adherens junctions [Takeichi 1995]. During morphogenesis, cadherins guide the arrangement and rearrangement of cells and tissues by joining cells together in the appropriate configuration. While the mesenchyme is generally a loosely organized tissue, it expresses N-cadherin, which is implicated in the condensation of chondrocytes in the limbs. Catenin proteins, such as β-catenin, link the domains of cadherins together and stabilize the junction to the cytoskeleton, thus facilitating juxtacrine signaling between the cells, and potentially affecting adhesion, differentiation (by stabilizing cell shape during tissue formation) and migration [Klymkowski and Parr 1995]. If cells of mesenchymal tissues are dissociated, the individual cells reassociate with like cell types (self-recognition), but only if the cadherins are linked to the cytoskeleton via catenins [Morali et al. 2005, Barna and Niswander 2007]. β-catenin is a vital protein during both growth and development (as well as in the homeostasis of mature tissues) [Bellei et al. 2004]. In addition to stabilizing cadherin junctions to the cytoskeleton, β-catenin is the central transducer of the canonical Wnt signaling pathway, which has been implicated in the formation and mechanosensitivity of osteochondroprogenitor cells [Baksh et al. 2006, Falls 2007] and skeletal development in the embryo [Hill et al. 2005, Yu et al. 2003]. Complete loss of β-catenin results in early embryonic lethality during gastrulation [Morali et al. 2005]; hence the first epithelial to mesenchymal transition does not occur and no mesenchyme is formed. Due to the lethal ramifications of this mutation, conditional knockouts of β-catenin are used in experimental studies. Interestingly, the conditional β-catenin knockout of the limb mesenchyme renders the mesenchyme unable to transduce Wnt signaling during endochondral ossification [Hill et al. 2005]. This effect has been demonstrated during intramembranous ossification as well, in Dermo1Cre, conditional β-catenin mutants [Day et al. 2005]. β-catenin is critical for osteoblastogenesis, [Hu et al. 2005] and differentiation of the chondrocyte lineage to osteoblasts [Hill et al. 2005]; upstream effects on mesenchyme condensation are currently unknown. Hence, the condensation event provides an effective means for mechanical signals occurring through self-assembly of a tissue to be transduced downstream into delineation of cell fate; through increased cell-cell junctions (e.g. N-cadherins), transduction to individual cytoskeletons is increased via the catenins, such as β-catenin.
Interestingly, determination of cell fate is a process that is regulated by and itself regulates the pericellular environment, which constitutes the tissue on a macro scale. A genetic program marks the progression of unfated cells from mesenchymal to osteochondroprogenitor to osteoblast or chondrocyte (Figure 3). To assess cell fate at very early stages in the osteochondroprogenitor commitment process, it is important to understand the genetic regulators of osteoblastic and chondrocytic lineages; in the following section, these genetic regulators will be discussed in the context of condensation mechanobiology.
Recent studies of limb bud micromass cultures, designed to study limb chondrogenesis in vitro, show an upregulation in Notch signaling prior to condensation. Furthermore Notch expression peaks at some time between cell condensation and the formation of condensed nodules of cells, both of which occur prior to chondrogenic differentiation [Fujimaki et al. 2006]. Notch is expressed at earliest stages of chondrogenic lineage commitment. Expression of Notch inhibits differentiation and proliferation of cells and Notch expression decreases with increasing chondrogenic differentiation. Of note, the perichondrial cells sustain the expression of Notch throughout development of the limb [Watanabe et al. 2003]. Sometime after Notch signaling is upregulated (prior to mesenchymal progenitor cell condensation, reaching a peak during condensation), Sox9 expression is upregulated (prior to chondrogenic lineage commitment in the embryo) [Lefebvre et al. 2001]. Sox9 is one of the first transcription factors regulating the differentiation of osteochondroprogenitor cells and, as such, is often regarded as the master regulator of chondrocyte growth and differentiation.
In addition to increasing connectivity between cells and their cytoskeletons, the condensation event has been associated with other changes in cell morphology that may further influence downstream signaling events related to cell fate commitment [Barna and Niswander 2007]. In general, within the condensation, cells become more rounded; this is likely a consequence of biophysical factors including the natural compression around the locus of condensation, as well as the increase in cell-cell adhesion around the entire periphery of the cells within the condensation, resulting in maximal nuclear volume relative to cytoplasmic volume [Alexopolous et al. 2005]. These changes are accompanied by observations of small mitochondria, poorly developed endoplasmic reticulum and large nucleoli, all of which are likely to be associated with gene and subsequent cell fate modulation (see 8. Role of Other Biophysical Stimuli… below). [Hall and Miyake 1992]
Cell rounding is likely enhanced through the increasing cell density associated with the condensation process. In mesenchymal condensation at different locations in the chick, density increases of 0.7 – 4% per hour have been reported, e.g. yielding up to a 100% change in density per day (over two days) for the chick wingbud [Dunlop and Hall 1995]. Furthermore, condensation size is a nonlinear function of density, where density “increases from [circa] 2 to [circa] 5×104 cells/cm2 (near confluence)” translates to aggregate size increases of “ten to several hundred cells (diameter [circa] 500 μm” [Bard 1990]. Mitotic activity is stimulated through increasing density and through epithelial-mesenchymal interactions. In addition, cell-cell contact increases as a natural consequence of the increasing density of cells (increased interface area within the same volume). The increasing potential for cell signaling is a natural consequence of increased contact between cells and is further manifested via growth in signaling infrastructure, e.g. increased formation of gap junctions which confer the ability for direct cell-cell signaling through exchange of small molecules [Hall and Miyake 1992, Coelho and Kosher 1991].
The cartilaginous template is a precursor to bone and grows as a result of proliferation and differentiation of the chondrocytes (Figures 3, 4). Prior to differentiation of chondrocytes, there is an upregulation in expression of the Col1a1 gene [Pelttari et al. 2006]. Thereafter, the Col2a1 gene is upregulated concomitant to Col1a1 downregulation and the chondrocytes begin producing a matrix of type II collagen [Pelttari et al. 2006]. After initial proliferation, however, the chondrocytes at the center of the new template become hypertrophic (enlarged), cease to proliferate, and secrete type X collagen, a process regulated by the transcription factor Runx2, which readies the template for transformation into mature bone [Nakashima and de Crumbrugghe 2003]. Recent studies implicate both Runx1 and Runx2 in the process of endochondral (and intramembranous) bone formation, as both are expressed in the pre-chondrocytic mesenchyme at E12.5 in the murine embryo [Smith et al. 2005]. Given that Runx1 is not present in the hypertrophic cartilage and abrogration of the Runx2 gene allows formation of the cartilage template but no subsequent bone formation, Runx1 is a putative mediator of early chondrogenic events and Runx2 is a putative mediator of chondrogenic and osteogenic differentiation at later stages in development [Smith et al. 2005]. The hypertrophic chondrocytes change their genetic program and begin to function as the master regulatory cells for early endochondral ossification. They arrest cell division, change the type of extracellular matrix (ECM) they produce and change their morphology and pattern of gene expression [Shum and Nuckolls 2002]. The newly formed cartilaginous matrix begins to mineralize and vascular endothelial growth factor (VEGF) is secreted, which results in the infiltration of blood vessels. The remaining chondrocytes continue to proliferate, resulting in the lengthening of the growing bone template at each of its ends. The hypertrophic chondrocytes also directly control the differentiation of the first osteoblasts from the perichondral cells, that later become the bone collar, the first region of bone to mineralize [Kronenberg 2003].
Figure 4. Mesenchymal cells in chondrogenic condensations can assume either chondrogenic or osteogenic lineages.
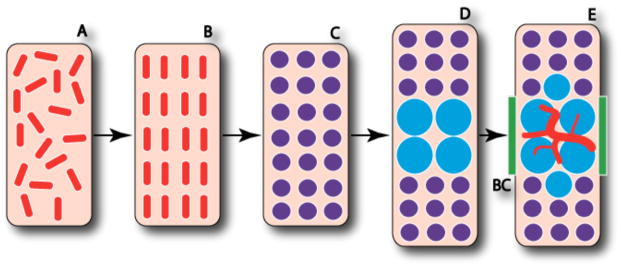
The process of endochondral ossification, from loose mesenchyme to initial osteoblast differentiation, entails intermediate chondrocyte differentiation. The loose mesenchymal progenitor cells (A) proliferate and organize into the condensed mesenchyme comprised of osteochondroprogenitor cells (B). The cells of the condensed mesenchyme differentiate into chondrocytes (C) and the cells at the center of the condensation stop proliferating and become hypertrophic (D). Perichondral cells adjacent to the hypertrophic chondrocytes differentiate into osteoblasts (E) and form the bone collar (BC) as the invasion of blood vessels begins along with continued osteoblast differentiation. After [Kronenberg, 2003].
Once the hypertrophic chondrocytes have started the cascade that leads to the differentiation of osteoblasts, the master transcription factor controlling the differentiation of osteoblasts, Runx2, begins to have a pronounced effect. Osteoblast differentiation begins around day 13.5 in the developing mouse embryo, and is primarily controlled by two transcription factors: Runx2 and Osterix (Osx). Runx2 is required for the differentiation of osteoblasts. The second transcription factor vital to proper osteoblast differentiation and maturation is Osx. It has a high degree of specificity compared with Runx2, and is expressed almost exclusively in osteoblasts. In the developing mouse embryo, the expression of Osx is first seen concurrently with the onset of osteoblastogenesis during the 13th day. Functionally, it is required for the process of osteoblast differentiation and therefore is required for overall bone formation [Nakashima and de Crombrugghe 2003]. Loss of this transcription factor causes cells in the periosteal region that should have differentiated into osteoblasts to differentiate into chondrocytes [Hill et al. 2005] (Figs. 2 – 3). Hence, tracking the up- and/or downregulation of genes coding for Sox9, Col1a1, Col2a1, Runx2, and Osx, allows one to map the determination of osteoblastic and chondrocytic fates (Fig. 4) in endochondral ossification.
c. Osteogenic “condensations”: mechanobiology and genetic fingerprints
The genetic markers of osteogenic differentiation via osteogenic condensations (intramembranous bone formation) are considered distinct from those of osteogenic differentiation via chondrogenic condensations (endochondral bone formation) [Dunlop and Hall 1995, Ferguson et al. 1998], but the molecular pathways of intramembranous bone formation are less well understood [Govindarajan and Overbeek 2006]. In intramembranous bone formation, mesenchymal cells differentiate directly into osteoblasts that secrete an extracellular matrix rich in Collagen I and initiate mineralization [Olsen et al. 2000]. Bone of the cranium and other flat bones are formed in this way and grow via further proliferation and differentiation of osteoblasts from multipotent cells derived from the margins (periosteum) and sutures. Intramembranous bone formation has been associated with expression of osteogenic gene markers including transcriptional factors for Collagen I (col1), Osteopontin, Bone Sialoprotein (BSP), and Osteocalcin (Oc), as well as well as BMPs (2, 4, 7) and inflammatory cytokines (TNF-alpha and IL-1alpha) [Lukic et al. 2005]. Given the tight coupling of angiogenesis and intramembranous bone formation, VEGF is expected to be detectable at earlier timepoints in intramembranous ossification via osteogenic condensations compared to endochondral ossification via chondrogenic condensations (after cartilage hypertrophy, Fig. 5).
Recent studies indicate that these processes may be under profound extrinsic control [Knothe Tate et al. 2007]. Interestingly, new studies show that fibroblast growth factor 9 (FGF9) is capable of switching the differentiation program of the cranial paraxial mesoderm from that of osteoblastic condensation (and subsequent intramembranous bone formation) to chondrogenic condensation (and subsequent endochondral ossification) [Govindarajan and Overbeek 2006] in the parietal bone. Given the previously mentioned role of Notch signaling in chondrogenic mesenchymal condensations (where a high level of Notch expression promotes cell proliferation and cell motility while inhibiting chondrogenic differentiation and given the fact that cells of the perichondrium sustain the expression of Notch throughout development of the limb [Watanabe et al. 2003]) it would be interesting to determine whether a sustained expression of Notch from epithelial sheets (that are flat like the cranium, when observed from the scale of a cell), combined with sustained expression of FGF 9, results in movement and proliferation of cells directly from the sheet, and then direct differentiation to osteogenic fate (Figure 5).
d. Linking Condensation Type to Ossification Mode
The condensation of the mesenchyme triggers the first gene activation specific to tissue differentiation. Already before first changes in gene activity indicative of cell fate and/or tissue specification can be measured, other environmental factors predispose the condensation to be osteogenic or chondrogenic. Hence, already at the time that mesenchymal condensations are forming the precursors to skeletal tissues, the fate of cells within is determined. Cells within endochondral condensations are osteochondroprogenitor cells, i.e. they are fated to follow the chondro- and/or osteogenic lineages. In contrast, cells within osteogenic condensations are osteoprogenitor cells prior to condensation; i.e. they are fated to follow the osteogenic lineage and the formation of the condensation “amplifies” the number of osteogenic cells [Dunlop and Hall 1995]. What drives the mesenchymal stem cells toward an osteogenic condensation versus a chondrogenic condensation is key for understanding developmental defects as well as for optimizing the genesis of tissue during postnatal healing and engineering of tissues. Presumably, these cells that share common genetic algorithms are not preprogrammed at such early timepoints. If not, extrinsic factors likely play an important role and cells “self assemble” the tissue according to positional cues.
Interestingly, osteogenic and chondrogenic condensations differ with respect to key biophysical parameters. One key difference is the distance between the cells comprising the condensation and the nearest epithelial surface. The transport efficiency of biochemical signals from the epithelial surface depends on the diffusion path length to the condensing cells [Schier 2003]. The effect of inductive factors influencing haptotaxis and chemotaxis is also geometry and path length dependent [Gurdon 1987, Ripamonti et al. 2006]. In addition, efficiency of mechanical signal transduction between the condensing mesenchyme and the epithelial sheath proximity between cells comprising the two structures. Osteogenic condensations occur only in close proximity to the epithelium; hence, cells within osteogenic condensations are likely to be influenced more (than cells in chondrogenic condensations) by both biochemical and biophysical cues arising from epithelial-mesenchymal interactions [Dunlop and Hall 1995]. It is well documented that intramembranous bone formation occurs in direct orchestration with angiogenesis [Carrington 1994, Gerber and Ferrara 2000], and close proximity to the epithelium would presumably favor such orchestration. Hall and Miyake [1992] refer to condensations in general as the “cellular products of epithelial-mesenchymal interactions”, where osteogenic condensations require a very close connection to EMT’s in comparison with chondrogenic condensations. Hall and colleagues’ seminal studies in the chick mandible showed that epithelial-mesenchymal interactions first cause mesenchymal cells to become preosteoblasts and then condensation formation increases the number of osteoprogenitor cells [Dunlop and Hall 1995]. Bone morphogenetic protein (BMP), present in the basement membrane of the epithelium at the time of the EM interaction, is likely to play a key role in commitment of the mesenchymal cells to osteogenic fate [Hall 1988]. In addition, the extracellular matrix molecule tenascin has been shown to regulate cell proliferation and is present at the time of the EM-interaction leading to osteogenic condensation [Hall 1988, Chiquet 1989, Hall and Miyake 1992]; more recent studies show that tenascin expression is regulated by mechanical stress in differentiated connective tissues [Chiquet-Ehrismann et al. 2004]. Hence, the spatial and temporal development of osteogenic and chondrogenic condensations result in distinct spatial and temporal patterns of biochemical and biophysical signals, which likely instruct the cells along appropriate lineages during tissue genesis. Direct osteogenic condensations without a cartilage template result in development of flat bones, such as the bones of the cranium [Dunlop and Hall 1995], as well as the rapid proliferation of woven bone during postnatal healing [Tami et al. 2003]. In contrast, chondrogenic condensations develop around a central locus, resulting in more bulbous tissue templates, such as the developing limb bud. It is likely not coincidental that the flat bones and trabecular like woven bone are generated with significant influence of epithelial sheet structures and the bones and cartilages derived from the more rounded chondrogenic condensations are less tightly coupled to the epithelium. The formation of intramembranous and endochondral bone may further reflect respective differences in how the condensation forms, e.g. recruitment of cells to a locus may be dominant in the case of osteogenic condensation whereas condensation about a locus through increase in cell division may dominate in the case of chondrogenic condensations. These different mechanisms themselves would have important implications for the biophysical factors defining the environment of individual cells in the condensation, i.e. cells in osteogenic condensations would be likely exposed to more shear stress than compressive or tensile stress and cells in chondrogenic condensations would be exposed to higher compressive or tensile stresses in comparison.
Furthermore, chondrogenic condensations involve interactions with extracellular matrix including glycosaminoglycans and proteoglycans. For instance, hyaluronan blocks chondrogenic condensations by increasing space between cells through imbibement of fluid (hydration). CD44, a cell surface glycoprotein prevalent at sites of mesenchymal condensations, removes hyaluronan through internalization [Underhill 1993, Toole 1997]. Increases in hyaladherins are also observed during condensation [Hall and Miyake 1995]. As mentioned previously, it has been documented that a minimum of 130 cells are required for a chondrogenic condensation, at a cell density of 5,000 cells/mm2. If greater densities (and/or hydrostatic forces) are imposed on the condensing mesenchyme through application of hypergravitational forces, chondrocytic condensations differentiate prematurely. [Hall and Miyake 1992]
4. Mesenchymal Condensations in Postnatal Repair and Regeneration of Tissue
There are redundant pathways provided by nature and evolution to produce bone. Although the most direct path to de novo production of bone involves osteogenic condensations that then differentiate to intramembranous bone, such as that forming the template of the cranium, the mandible and other flat bones, bone can also form through the process of endochondral ossification, which involves the ossification of a cartilaginous tissue template (Figure 4). Each of these pathways involves different extrinsic cues including biochemical and biophysical factors. The bone resulting from each pathway exhibits differences in its architecture (organization in space) as well as rate of formation (organization in time). In postnatal wound healing, the de novo regeneration of bone or rapid repair of a fracture via a rapid proliferative response (woven bone) more closely mimics the process of intramembranous bone formation via osteogenic condensations (Fig. 6) [Tami et al. 2003, Knothe Tate et al. 2007, Knothe Tate et al 2008], while the repair of bone via callus healing more closely mimics the process of endochondral ossification via chondrogenic condensations [Knothe Tate et al. 2007, Knothe Tate unpublished data]. Interestingly, just as tenascin was associated with the outgrowth of the lung bud in the developing chick, tenascin has also been implicated in healing of dermal wounds, providing a surface particularly conducive to the migration and proliferation of the epidermal sheet in dermal wounds. Recent data suggests that tenascin may play a role in the rapid proliferative response typical for healing via intramembranous bone formation; “TGF-β stimulates osteoblastic tenascin-C expression”, and “…tenascin-C may act as a mediator of TGF-β-induced new [woven] bone formation” [Alford and Hankenson 2006, Fujimoto et al. 1999, Mackie 1998]. Tenascin is present in the perichondrium during limb bud development [Chiquet-Ehrismann et al. 1986, Koyama et al. 1996] and likely exerts a role in epithelial-mesenchymal interactions during intramembranous bone healing via the periosteum [Chiquet 1999, Järvinen et al. 2000].
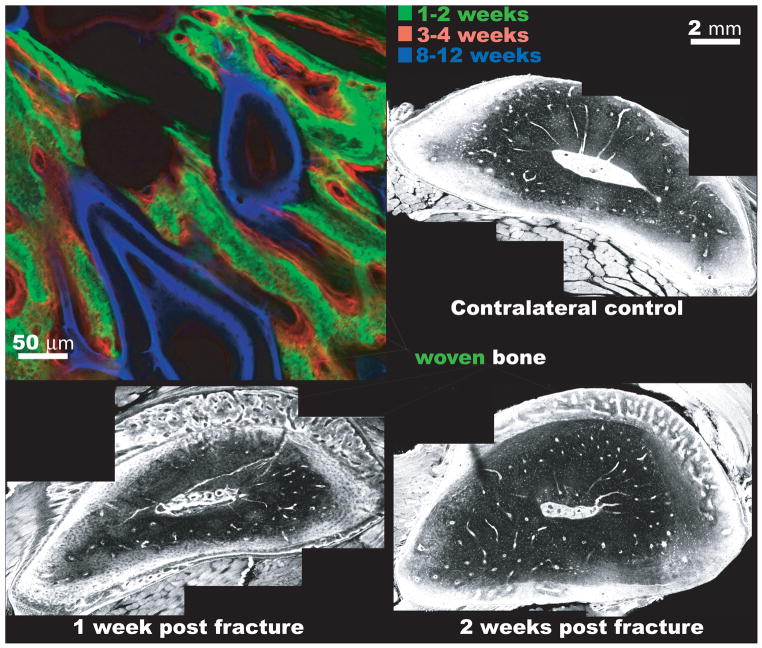
Figure 6. Bone generation via the periosteum during postnatal repair mimics that of de novo bone formation via osteogenic condensations during development.
(top left) In the adult sheep femur, a one step surgical procedure is carried out. First, a critical sized defect is filled with a cortical bone segment that is removed from the proximal bone length, leaving the periosteum intact around a new defect zone [Knothe and Springfield 2005]. An intramedullary nail fills the medullary canal, imparting mechanical stability. In absence of the bone marrow, the intramembranous bone fills the defect within two weeks of surgery (green). The intramembranous bone formation proceeds via the periosteum alone. Thereafter, bone is laid down on the woven bone scaffold and is remodeled at later timepoints. The time course of bone apposition is visible, because fluorochromes of different excitation and emission wavelengths are administered intramuscularly to the sheep during life. The fluorochromes chelate the mineral as it is incorporated into the matrix and any fluorochrome not incorporated into the mineral is excreted by the animal within days of administration. After conclusion of the experiment, the femur is removed in toto and processed for undecalcified histology and fluorescent imaging, at excitation and emission wavelengths corresponding to the fluorochromes administered at specific time points, using a laser scanning confocal microscope. (bottom left) In the adult rat, a rapid proliferative response with robust production of woven bone is observed, in a band between the cortex and the periosteum, within a week of fatigue fracture. The fatigue fracture is induced noninvasively through cyclic loading of the rat femur, where microdamage is incurred through subfailure loading. Microdamage accumulates and coalesces, mimicking the mode of fatigue failure that is prevalent in the metatarsals of dancers and army recruits. (bottom right) Two weeks after fracture, remodeling consolidates the woven bone, resulting in tissue that resembles the undamaged, mature bone within six weeks after fracture. Preterminal injection with 0.8% Procion Red solution allows for evaluation of vascular and pericellular perfusion at different time points during healing. After [Tami et al. 2003] All four micrographs are laser scanning confocal images at different respective length scales.
A rich body of literature documents the relationship between so-called primary bone healing (direct intramembranous bone formation) and secondary bone healing via a callus [Miclau et al. 2005, Aro and Chao 1993, Stoffel et al. 2000], where lack of stability is associated with formation of a callus, which then mineralizes via endochondral mechanisms. Interestingly, given sufficient mechanical stability, the periosteum alone is an efficient regenerator of bone in critical sized defects [Knothe Tate et al. 2007, Knothe Tate unpublished data]; within a week, the periosteum fills the 4 cm diameter and 2.54 cm long defect with woven bone resembling trabeculae (Figure 6) [Knothe Tate unpublished data]. Interestingly, similar observations have been reported for adult rat bone after fatigue fracture [Tami et al. 2003], where a rapid proliferative response is observed as woven bone within one week of fracture (Figure 6). In addition, bone regeneration after marrow ablation in rats has been shown to generate a similar network of trabecular like bone within ten days [Gerstenfeld et al. 2001].
5. Role of Mechanical Stimuli in Modulation of Cell Lineage Commitment During Pre- and Postnatal Life
Although postnatal wound healing is described as a recapitulation of embryonic development [Schotte and Smith 1959, Carter and Wong 1988, Ferguson et al. 1998] neither the mechanical forces prevalent during development of embryonic, postnatal nor engineered tissue are well characterized. All organisms on earth are imbibed with water and all organisms on earth are subject to the gravitational field of the planet, resulting in pressure gradients during movement that exude and imbibe fluid throughout our organs and tissues [Knothe Tate 1998a,b, Knothe Tate 2003, Anderson et al. 2007a,b, Anderson and Knothe Tate 2008, Anderson et al. 2008]. Interestingly, these gravitational effects are somewhat mitigated for aquamarine organisms as well as terrestrial vertebrates in utero, where gravitational forces are smaller than other body forces.
In addition to direct application of mechanical forces to surfaces of cells and tissues, microenvironmental changes can affect the differentiation of progenitor cells during the course of in utero development as well as postnatal tissue repair. Differentiating chondrocytes produce hyaluronic acid, which aggregates water and causes the matrix to swell, effectively imparting compressive forces to cells [Oster et al. 1983]. Mineralization of the matrix around hypertrophic chondrocytes is another endogenous change that can affect the differentiation state of surrounding cells. In mouse metatarsals, mineralization of the cartilage begins at around the same time as the first muscle contractions in the foot [Tanck et al. 2004]. This indicates that the first wave of mineralization may be stimulated by mechanical loading and/or mechanically modulated Ca2+ kinetics. As the matrix mineralizes, the cells experience a profound change in their surroundings, [Tanck et al. 2004] resulting in a two order of magnitude increase in the Young’s modulus (stiffness) of the tissue in a day’s time. It has been hypothesized that this literal change in state causes most hypertrophic chondrocytes to die. Hence, not only are chondrocytes capable of responding to their mechanical environment, but also a sudden change in that environment may constitute a key mechanotransduction event in development [Tanck et al. 2004]. Estimates for some loading parameters characterizing the in utero mechanical environment include static and hydrostatic compressive loading ranges from 0 – 13 kPa [Klein-Nulend et al. 1986, Klein-Nulend et al. 1987, Wong and Carter 1990, McBeath et al. 2004], with loading magnitudes as low as 1 – 2 kPa for promotion of chondrogenesis [Takahashi et al. 1998] (Figure 7). Of note, a hydrostatic compressive load of 1 – 2 kPa, is more than 1000 times smaller [Wong and Carter 1990] than the loading experienced in adult articular cartilage (10 to 20 MPa) [Adams 2006]. Loading parameters for shear stress are rarely reported, as the shear environment in utero is largely unknown. Nonetheless, one finite element model predicted the octahedral shear stress in a homogeneous tissue rudiment to be near zero [Wong and Carter 1990], and data from a recent study has demonstrated shear stresses as low as 0.2 dynes/cm2 (0.02 Pa) as effective mechanical signals for genetic regulation of embryonic multipotent progenitor cells [Oberhofer 2005, McBride 2007]. Hence, embryonic multipotent progenitor cells may be orders of magnitude more sensitive to shear stress than to compressive loading (1 – 2 kPa equals 10,000 – 20,000 dynes/cm2) and, furthermore, may demonstrate profoundly higher mechanosensitivity than mature committed cells.
Figure 7. Characteristic magnitudes and time domains of mechanical signals applied in studies of multipotent cell differentiation.
Each data point represents one study. The shape of the data point portrays the lineage to which the multipotent cell committed. Blue data points depict deviatoric, i.e. shear, stress magnitudes (abscissa, τ, dynes/cm2) and duration of signal over the time course of the study (ordinate, Time, days). Red data points depict dilatational stress (−σ: hydrostatic compression, +σ: tension, both depicted on a log10 scale, Pa). The yellow plane overlay (opaque to transparent reflecting respective likelihood of the stress ranges, orthogonal to two other planes) represents dilatational and deviatoric stress ranges predicted during cell fate determination in utero. Of note, there is little overlap between predicted in situ mechanical stress histories and the regimes applied in studies published to date. The points depicted in the figure represent both primary cells and cells lines, which have been demonstrated to have similar properties. 1 Pa = 10 dyn/cm2 (after Anderson and Knothe Tate 2007a) [McBeath et al. 2004, Meinel et al. 2004, Knippenberg et al. 2005, Wang et al. 2005, David et al. 2007, Akimoto et al. 2005, Baksh et al. 2006, Campbell et al. 2006, Takahashi et al. 1998, Miyhanishi et al. 2006, Hillsley and Frangos 1994, Billotte et al. 2001, Klein-Nulend et al. 1986, Wong and Carter 1990, Henderson et al. 2007, Angele et al. 2003, Roelofsen et al. 1995, Illi et al. 2005].
6. In Vitro Experimental Platforms to Elucidate Role of Extrinsic Cues in Fate Determination
A major challenge in understanding the role of mechanical signals in modulating mesenchymal stem cell fate is the lack of a common experimental platform to impart controlled forces to uncommitted cells. Mechanical forces have been imparted to multipotent cells using spinner flask [Meinel et al. 2004], perfused cartridge [Meinel et al. 2004], parallel flow chambers [Knippenberg et al. 2005, Wang et al. 2005, Datta et al. 2006], flexible membrane [Akimoto et al. 2005, Jakkaraju et al. 2005, Baksh et al. 2006, David et al. 2007], compressive gel [Takahashi et al. 1998, Campbell et al. 2006] as well as hydrostatic pressure based systems [Miyanishi et al. 2006] (Table 1). Furthermore, a recent study maps the range of mechanical signals, time scale of mechanical stress application and resultant cell fates (Figure 7) [Anderson and Knothe Tate 2007a] from studies published to date. Of note, the magnitudes and duration of mechanical stimuli applied previously do not coincide with the range of dilatational (producing a change in cell volume but not shape, e.g. hydrostatic compressive stress −σ) and deviatoric stresses (producing a change in cell shape but not volume, e.g. shear stress τ) predicted to occur in the embryo at the time that mesenchymal stem cells commit to a specific lineage [Anderson and Knothe Tate 2007a]. In addition, recent studies address the disparity between target and actual stresses imparted by devices that use fluid flow to impart shear stresses to cells seeded within [Anderson et al. 2004, Anderson et al. 2006, Anderson and Knothe Tate 2007b], introducing a further element of variability into experimental design. Finally, interpretation of data from studies designated to elucidate the role of mechanical signals in modulating fate is further confounded by inclusion of biochemical factors in the growth media, which introduces an additional independent extrinsic variable into the study design (Table 1).
Table 1.
Mechanical and biochemical cues reported in the literature to induce the differentiation of mesenchymal progenitor cells in vitro.
| Differentiation Type | Mechanical Cues | Biochemical Cues | Cell Type | Experimental System | Reference |
|---|---|---|---|---|---|
| Osteogenic | Shear stress, 1mL/min flow, ~1 dyne/cm2, 4, 8 or 16 days | +/− dexamethasone | Rat marrow stromal cells | Ti mesh scaffold, perfusion bioreactor, peristaltic pump | [Datta et al. 2006] |
| Endothelial | Shear stress, 15 dynes/cm2, 6 or 12 hours | None listed | C3H10T1/2 murine embryonic cell line | Parallel plate flow chamber | [Wang et al. 2005] |
| Osteogenic | Shear stress, mean shear: 6 dynes/cm2, amplitude: 2 dynes/cm2, shear rate: 84 dynes/cm2 | β-glycerophosphate, 1,25-dihydroxyvitamin D3 | Goat adipose tissue MSC’s | Parallel plate flow chamber | [Knippenberg et al. 2005] |
| Osteogenic | Shear stress; spinner flask: turbulent flow, ~0.084 dynes/cm2, perfused cartridge: <0.05 dynes/cm2, 5 weeks | Dexamethasone, β-glycerophosphate, ascorbic acid; spinner flask: convection at edges, diffusion interiorly, perfused cartridge: convection and diffusion throughout | Human MSC’s | Collagen, cross-linked collagen or silk substrate in a spinner flask; collagen substrate in a perfusion cartridge system | [Meinel et al. 2004] |
| Chondrogenic | See above | Dexamethasone, ascorbic acid, insulin; see above | See above | See above | See above |
| Chondrogenic | Intermittent hydrostatic pressure, 10 MPa (100,000,000 dynes/cm2), 1 Hz for 4 hours/day, 3, 7 or 14 days | TGF-β3, dexamethasone, ascorbic acid, insulin | Human MSC’s | Cell pellets in sterile bags, placed into MTS servo-hydraulic loading frame | [Miyanishi et al,. 2006] |
| Chondrogenic | Purely compressive loading, 20–30% gel deformation, 1–2 kPa (10,000 – 20,000 dynes/cm2), 3 – 7 days | None listed | Mouse embryonic limb bud cells | Compression of cells seeded onto compliant collagen gel | [Takahashi et al. 1998] |
| Chondrogenic | Compressive strain, 15% strain amplitude, 1 Hz, 8 days | +/− TGF-β3 | Human MSC’s | Alginate substrate, dynamic compression system | [Campbell et al. 2006] |
| Adipogenic versus osteogenic | Tensile loading, 0.4% strain, 1 Hz with 300 cycles daily, 7 or 14 days | Dexamethasone, β-glycerophosphate, retinoic acid, insulin, IBMX | C3H10T1/2 murine embryonic cell line | Flexcell, flexible membrane system, collagen coated rubber membrane | [David et al. 2007] |
| Adipogenic versus myogenic | Tensile loading, maximum strain: 20% elongation of cells, 6 cycles/minute | Dexamethasone, insulin, IBMX | C2C12 murine mesenchymal skeletal muscle precursor cell line | Flexcell, flexible membrane system, collagen coated rubber membrane | [Akimoto et al. 2005] |
| Adipogenic versus myogenic | Tensile loading, maximum strain: 5%, 0.5 Hz | Cycloheximide | Murine embryonic (E11) mesenchymal lung cells | Flexcell, flexible membrane system, poly-L-lysine coated flexible culture plates | [Jakkaraju et al. 2005] |
| Osteogenic | Tensile loading, maximum strain: 2%, 1 Hz for 1 hour/day, up to 21 days | Dexamethasone, β-glycerophosphate, ascorbic acid, Wnt 3a or 5a | Human MSC’s | Flexcell, flexible fibronectin coated flexible culture dish membrane system, | [Batesh et al. 2006] |
Recent studies demonstrate the promise of using in vitro models to elucidate skeletal development and to exploit nature’s tissue engineering paradigms in the research and clinical sector. One approach involves the engineering of osteogenic and/or chondrogenic condensations, from which target tissues can be cultivated in the lab or in situ after surgical implantion. Recently, in situ imaging of cellular events in chondrogenesis, using micromass pellet culture techniques, has led to the discovery that Sox9 controls cell morphology independent of ColII, which is the major downstream target of Sox9 and is a hallmark of chondrogenic condensation initiation [Barna and Niswander 2007]. The ability to observe specific cells’ activities, in real time, during and after condensation will likely provide new discoveries in the future.
Furthermore, studies with the embryonic murine stem cell line C3H10T1/2 show that not only the target seeding density [Watt 1988] but also the manner in which density is achieved exert a profound influence on the formation of multi-dimensional and multicellular tissue precursors [McBride 2007]. Seeding at very high density, as opposed to reaching the same density through population doublings, results in increased stacking of individual cells. Interestingly, the stochastic stacking resulting from seeding at density has different functional implications for cells than multidimensional structure achieved through population doublings. Namely, cells allowed to define their own multi-cellular structure, thus microenvironment, through population doublings exhibit a greater change in expression of osteogenic and chondrogenic gene markers than cells seeded at target density. In general, gene activity increases as a function of time and cell density [McBride 2007]. This may be due to the fact that cells exhibiting greatest up and down regulation of gene activity had been given adequate time to proliferate, which has been identified, together with condensation, as prerequisite to cell differentiation [Stein et al. 1990]. Future studies will enable the engineering of condensations in association with epithelial sheets to allow for further elucidation of EMT inductive factors in determining whether a condensation follows an osteogenic or chondrogenic path.
Another approach involves the delivery of mechanical signals directly to mesenchymal cells. Mechanical signals can be used to transduce biophysical forces as well as to define gradients of biochemical factors with the aim to modulate their mesenchymal stem cell differentiation and the subsequent formation of targeted tissue types. Mechanical signals can be controlled and delivered either within a perfusion chamber [Anderson and Knothe Tate 2007b] and/or within a scaffold structure [Anderson and Knothe Tate 2007a]. Application of shear stress via fluid drag over primary embryonic osteochondroprogenitor cells dissociated from the murine limb bud at E11.5 demonstrates a key role for β-catenin in transduction of mechanical signals during condensation [Falls 2007]. Exposure of these primary embryonic mesenchymal cells to 1 dyn/cm2 shear stress via fluid flow significantly upregulates Col1a1 transcription in the cells lacking β-catenin (Cre-loxP produced β-cateninc/c mice) and downregulates transcription in cells not lacking β-catenin. Transcription of Sox9, Runx2, Osx, AGC, and Ppar-γ are not significantly affected by exposure to shear stress [Falls 2007]. Other studies show that cells lacking β-catenin do not reassociate in culture to the same degree as normal cells after dissociation from the mesoderm [Morali et al. 1995]. Furthermore, computer models predict that isolated cells (lacking β-catenin) are exposed to higher levels of shear stress than reassociated normal cells [Falls 2007]. Increased production of type I collagen may provide a mechanism for β-cateninc/c cells to dampen flow-induced stress in absence of stress distribution via cell-cell junctions. These studies provide compelling experimental evidence that uncommitted primary embryonic cells exhibit mechanosensitivity prior to the determination of cell fate during development. [Falls 2007]
Interestingly, in continuing studies using the embryonic mesenchymal stem cell line C3H10T1/2, it has been shown that the duration of exposure to mechanical stress provides a more powerful stimulus for differentiation of multipotent cells than stress magnitude alone [McBride 2007]. In addition, the developmental context in which the cells are placed is a significant factor in modulation of gene activity important for musculoskeletal development. Developmental context was defined by the manner in which density was achieved, which itself exerts a profound influence on the formation of multi-dimensional and multi-cellular structures (see above) [McBride 2007]. Similar observations have been noted in in vitro studies of endothelial cells, whose activity is modulated more significantly by temporal gradients in shear stress compared to the spatial gradient in shear stress magnitudes [White and Frangos 2007]. One might expect that a mesenchymal stem cell within a condensation is exposed to stress magnitudes that vary with the cell’s position. However, the mechanical signals and mechanically modulated biochemical gradients to which the cell is subjected persist throughout the condensation event. These signals may be considered “short term” in context of the cell’s lifetime (approximately 12 hours of, e.g. a 25 year lifetime, in the case of an osteocyte [Knothe Tate et al. 2004]). However, a sustained, 12 hour mechanically modulated signal may constitute a metamorphosis in the context of the time period over which a pluripotent cell remains uncommitted.
Perhaps that is the crux of the challenge in understanding the extrinsic cues critical to modulating cell commitment, i.e. the time window is so comparatively short and the forces are so comparatively small, but they constitute the spatiotemporal scale of the pluripotent cell. Unraveling of the cues and mechanisms underlying fate commitment will require new approaches that are brought to bear at the time and length scale of the mesenchymal stem cell.
7. Discussion and Future Outlook
The capacity to self assemble is evident throughout nature, and self-assembly at vastly different time scales is a hallmark of so-called recapitulation events. For example, fracture healing via ossification of callus is a putative recapitulation of endochondral ossification during prenatal development of the skeleton. In contrast, periosteum mediated bone defect or fracture repair is a putative recapitulation of osteogenic ossification. Cells with same genetic algorithms self assemble tissues based on positional cues comprising biophysical and biochemical signals at different time scales. Within the context of this review, the condensation or aggregation of individual cells is the seminal event in development of the skeleton and in its repair or regeneration during postnatal healing.
Extrinsic cues appear to exert as much influence on instruction of the mesenchymal cells to commit to a lineage as intrinsic cues, given that all cells share genetic algorithms but interpret positional information to best adapt to their dynamic environment in the developing embryo. The biochemical cues and genetic hallmarks of precondensation events through commitment of osteochondroprogenitor cells to their ultimate fate are well understood compared to the role of biophysical forces on shaping cell fate. New approaches to study events early in skeletal development, including new imaging methods to visualize events as they take place and in vitro models to decipher multifactorial cues in a controlled fashion, are paving the way to elucidate the interplay between mechanics, transport, the formation of biochemical gradients, and manifestation of preprogrammed algorithms in four dimensional space (x,y,z,t). Perhaps the combination of these two approaches (integration of dynamic in situ imaging with state of the art delivery of controlled biophysical and biochemical signals) will provide the critical step. Finally, elucidation of the parameters controlling key aspects of development will provide a springboard for the extrinsic optimization of skeletal genesis (while preventing defects) during development, healing and engineering of tissues.
Acknowledgments
Parts of this work were supported by funding from the National Institutes of Health, the Presidential Research Initiative, National Science Foundation ADVANCE grant (Academic Career in Engineering and Science (ACES) program). This investigation was conducted in a facility constructed with support from Research Facilities Improvement Program Grant Number C06 RR12463-01 from the National Center for Research Resources, National Institutes of Health. Part of the research described in this review was supported by the Gene Expression and Genotyping Facility of the Case Comprehensive Cancer Center (P30 CA43703).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott LA, Lester SM, Erickson CA. Changes in mesenchymal cell-shape, matrix collagen and tenascin acoompany bud formation in the early chick lung. Anat Embryol. 1991;183:299–311. doi: 10.1007/BF00192217. [DOI] [PubMed] [Google Scholar]
- Adams MA. The mechanical environment of chondrocytes in articular cartilage. Biorheology. 2006;43:537–545. [PubMed] [Google Scholar]
- Aggler J, Risch SM, Werb Z. Changes in cell shape correlate with collagenase gene expression in rabbit synovial fibroblasts. J Cell Biol. 1984;98:1662–1671. doi: 10.1083/jcb.98.5.1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akimoto T, Ushida T, Miyaki S, Akaogi H, Tsuchiya K, Yan Z, Williams RS, Tateishi T. Mechanical stretch inhibits myoblast-to-adipocyte differentiation through Wnt signaling. Biochemical and Biophysical Research Communications. 2005;329:381–385. doi: 10.1016/j.bbrc.2005.01.136. [DOI] [PubMed] [Google Scholar]
- Alexopoulos LG, Setton LA, Guilak F. The biomechanical role of the chondrocyte pericellular matrix in articular cartilage. Acta Biomaterialia. 2005;1:317–325. doi: 10.1016/j.actbio.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Alford AI, Hankenson KD. Matricellular proteins: extracellular modulators of bone development, remodeling and regeneration. Bone. 2006;38:749–757. doi: 10.1016/j.bone.2005.11.017. [DOI] [PubMed] [Google Scholar]
- Anderson EJ, Sorkin AM, Knothe Tate ML. Performance evaluation of commercial cell flow chambers: how well is the stress controlled at a cellular level?. Proceedings of the IMECE04, 2004 ASME International Engineering Congress, BED TOC IMECE2004–61837.2004. [Google Scholar]
- Anderson EJ, Falls TD, Sorkin AM, Knothe Tate ML. The imperative for controlled mechanical stresses in unraveling cellular mechanisms of mechanotransduction. BioMedical Engineering OnLine. 2006;5:27. doi: 10.1186/1475-925X-5-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson EJ, Knothe Tate ML. Design of tissue engineering scaffolds as delivery devices for mechanical and mechanically modulated signals. Tissue Engineering. 2007a;13:2525–2538. doi: 10.1089/ten.2006.0443. [DOI] [PubMed] [Google Scholar]
- Anderson EJ, Knothe Tate ML. Open access to novel dual flow chamber technology for in vitro cell mechanotransduction, toxicity and pharmacokinetic studies. BioMedical Engineering OnLine. 2007b;6:46. doi: 10.1186/1475-925X-6-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson EA, Knothe Tate ML. The idealization of pericellular fluid space geometry and dimension results in a profound underprediction of nano-microscale stresses imparted by fluid drag on osteocytes. Journal of Biomechanics. 2008 doi: 10.1016/j.jbiomech.2008.02.035. Online First™. in press. [DOI] [PubMed] [Google Scholar]
- Anderson EA, Kreuzer SM, Small O, Knothe Tate ML. Pairing computational and scaled physical models to determine permeability as a measure of cellular communication in micro- and nano-scale pericellular spaces. Microfluidics and Nanofluidics. 2008;4:193–204. [Google Scholar]
- Angele P, Yoo JU, Smith C, Mansour J, Jepsen KJ, Nerlich M, Johnstone B. Cyclic hydrostatic pressure enhances the chondrogenic phenotype of human mesenchymal progenitor cells differentiated in vitro. Journal of Orthopaedic Research. 2003;21:451–7. doi: 10.1016/S0736-0266(02)00230-9. [DOI] [PubMed] [Google Scholar]
- Aro HT, Chao EY. Bone-healing patterns affected by loading, fracture fragment stability, fracture type and fracture site compression. Clin Orthop Relat Res. 1993;293:8–17. [PubMed] [Google Scholar]
- Baksh AR, Taboas DJ, Tuan R. Mechanical stimulation enhances canonical Wnt signaling and effects osteogenesis in human bone marrow-derived mesenchymal stem cells. Transactions of the 52nd Annual Meeting of the Orthopaedic Research Society. 2006;31:0131. [Google Scholar]
- Bard JBL. Traction and the formation of mesenchymal condensations in vivo. BioEssays. 1990;12:389–395. doi: 10.1002/bies.950120809. [DOI] [PubMed] [Google Scholar]
- Barna M, Niswander L. Visualization of cartilage formation: insight into cellular properties of skeletal progenitors and chondrodysplasia syndromes. Dev Cell. 2007;12:931–41. doi: 10.1016/j.devcel.2007.04.016. [DOI] [PubMed] [Google Scholar]
- Bee JA, von der Mark K. An analysis of chick limb bud intercellular adhesion underlying the establishment of cartilage aggregates in suspension culture. Journal of Cell Science. 1990;96:527–536. doi: 10.1242/jcs.96.3.527. [DOI] [PubMed] [Google Scholar]
- Bellei B, Pacchiarotti A, Perez M, Faraggiana T. Frequent beta-catenin overexpression without exon 3 mutation in cutaneous lymphomas. Mod Pathol. 2004;17:1275–1281. doi: 10.1038/modpathol.3800181. [DOI] [PubMed] [Google Scholar]
- Benya PD, Shaffer JD. Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell. 1982;30:215–224. doi: 10.1016/0092-8674(82)90027-7. [DOI] [PubMed] [Google Scholar]
- Bernfield M, Hinkes MT, Gallo RL. Developmental expression of the syndecans: possible function and regulation. Development Supplement. 1993:205–212. [PubMed] [Google Scholar]
- Bielby RC, Boccaccini AR, Polak JM, Buttery LD. In vitro differentiation and in vivo mineralization of osteogenic cells derived from human embryonic stem cells. Tissue Engineering. 2004;10:1518–1525. doi: 10.1089/ten.2004.10.1518. [DOI] [PubMed] [Google Scholar]
- Billotte WG, Dumas K, Hofmann MC. Transcriptional pathways induced by fluid shear stress in mouse preosteoblast cells. Biomedical Sciences Instrumentation. 2001;37:1–6. [PubMed] [Google Scholar]
- Burdsal CA, Damsky CH, Pedersen RA. The role of E-cadherin and integrins in mesoderm differentiation and migration at the mammalian primitive streak. Development. 1993;118:829–844. doi: 10.1242/dev.118.3.829. [DOI] [PubMed] [Google Scholar]
- Campbell JJ, Lee DA, Bader DL. Dynamic compressive strain influences chondrogenic gene expression in human mesenchymal stem cells. Biorheology. 2006;43:455–70. [PubMed] [Google Scholar]
- Carey E, Zeit W, McGrath B. Studies in the dynamics of histogenesis, XII. The regeneration of the patellae of dogs. American Journal of Anatomy. 1922;40:127–158. [Google Scholar]
- Carrington JL. Bone Morphogenetic Proteins and the induction of embryonic and adult bone. 1994. [Google Scholar]
- Carter DR, Wong M. Mechanical stresses and endochondral ossification in the chondroepiphysis. Journal of Orthopaedic Research. 1988;6:148–154. doi: 10.1002/jor.1100060120. [DOI] [PubMed] [Google Scholar]
- Chen P, Carrington JL, Hammonds RG, Reddi AH. Stimulation of chondrogenesis in limb bud mesoderm cells by recombinant human bone morphogenetic protein 2B (BMP-2B) and modulation by transforming growth factor beta 1 and beta 2. Experimental Cell Research. 1991;195:509–15. doi: 10.1016/0014-4827(91)90403-h. [DOI] [PubMed] [Google Scholar]
- Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Geometric control of cell life and death. Science. 1997;276:1425–1428. doi: 10.1126/science.276.5317.1425. [DOI] [PubMed] [Google Scholar]
- Chiquet-Ehrismann R, Mackie EJ, Pearson CA, Sakakura T. Tenascin: an extracellular matrix protein involved in tissue interactions during fetal development and oncogenesis. Cell. 1986;47:131–139. doi: 10.1016/0092-8674(86)90374-0. [DOI] [PubMed] [Google Scholar]
- Chiquet-Ehrismann R, Kalla P, Pearson CA, Beck K, Chiquet M. Tenascin interferes with fibronectin action. Cell. 1988;53:383–390. doi: 10.1016/0092-8674(88)90158-4. [DOI] [PubMed] [Google Scholar]
- Chiquet-Ehrismann R, Tucker RP. Connective tissues: signaling by tensacins. Int J Biochem Cell Biol. 2004;36:1085–1089. doi: 10.1016/j.biocel.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Chiquet M. Tenascin/J1/cytotactin: the potential function of heaxbarchion proteins in neural development. Dev Neurosci. 1989;11:266–275. doi: 10.1159/000111905. [DOI] [PubMed] [Google Scholar]
- Chiquet M. Regulation of extracellular matrix gene expression by mechanical stress. Matrix Biol. 1999;18:417–426. doi: 10.1016/s0945-053x(99)00039-6. [DOI] [PubMed] [Google Scholar]
- Coelho CN, Kosher RA. Gap junctional communication during limb cartilage differentiation. Developmental Biology. 1991;144:47–53. doi: 10.1016/0012-1606(91)90477-k. [DOI] [PubMed] [Google Scholar]
- Daniels K, Solursh M. Modulation of chondrogenesis by the cytoskeleton and extracellular matrix. Journal of Cell Science. 1991;100:249–254. doi: 10.1242/jcs.100.2.249. [DOI] [PubMed] [Google Scholar]
- Datta N, Pham QP, Sharma U, Sikavitsas VI, Jansen JA, Mikos AG. In vitro generated extracellular matrix and fluid shear stress synergistically enhance 3D osteoblastic differentiation. Proceedings of the National Academy of Science U S A. 2006;103:2488–2493. doi: 10.1073/pnas.0505661103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David V, Martin A, Lafage-Proust MH, Malaval L, Peyroche S, Jones DB, Vico L, Guignandon A. Mechanical loading down regulates PPARγ in bone marrow stromal cells and favours osteoblastogenesis at the expense of adipogenesis. Endocrinology. 2007;148:2553–2562. doi: 10.1210/en.2006-1704. [DOI] [PubMed] [Google Scholar]
- Day TF, Guo X, Garrett-Beal L, Yang Y. Wnt/beta-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Developmental Cell. 2005;8:739–750. doi: 10.1016/j.devcel.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Derfoul A, Carlberg AL, Tuan RS, Hall DJ. Differential regulation of osteogenic marker gene expression by Wnt-3a in embryonic mesenchymal multipotential progenitor cells. Differentiation. 2004;72:209–223. doi: 10.1111/j.1432-0436.2004.07205003.x. [DOI] [PubMed] [Google Scholar]
- Dunlop LL, Hall BK. Relationships between cellular condensation, preosteoblast formation and epithelial-mesenchymal interactions in initiation of osteogenesis. International Journal of Developmental Biology. 1995;39:357–371. [PubMed] [Google Scholar]
- Ekblom P, Ekblom M, Fecker L, Klein G, Zhang HY, Kadoya Y, Chu ML, Mayer U, Timpl R. Role of mesenchymal nidogen for epithelial morphogenesis in vitro. Development. 1994;20:2003–2014. doi: 10.1242/dev.120.7.2003. [DOI] [PubMed] [Google Scholar]
- Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–89. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- Epperlein HH, Lehmann R. The ectomesenchymal endodermal interaction system (EEIS) of Triturus alpestris in tissue culture. II. Observations on the differentiation of visceral cartilage. Differentiation. 1975;4:159–174. doi: 10.1111/j.1432-0436.1974.tb00349.x. [DOI] [PubMed] [Google Scholar]
- Estes BT, Gimble JM, Guilak F. Mechanical signals as regulators of stem cell fate. Current Topics in Developmental Biology. 2004;60:91–126. doi: 10.1016/S0070-2153(04)60004-4. [DOI] [PubMed] [Google Scholar]
- Falls TD. Thesis for the Master of Sciences (Engineering) Case Western Reserve University, Biomedical Engineering; 2007. Beta-catenin’s role in the mechanosensitivity of osteochondroprogenitor cells. Document number: 182630052. [Google Scholar]
- Ferguson C, Miclau T, Hu D, Alpern E, Helms JA. Common molecular pathways in skeletal morphogenesis and repair. Annals of the New York Academy of Sciences. 1998;857:33–42. doi: 10.1111/j.1749-6632.1998.tb10105.x. [DOI] [PubMed] [Google Scholar]
- Freeman M, Gurdon JB. Regulatory principles of developmental signaling. Annual Reviews in Cell Developmental Biology. 2002;18:515–539. doi: 10.1146/annurev.cellbio.18.012502.083458. [DOI] [PubMed] [Google Scholar]
- Fujimaki R, Toyama Y, Hozumi N, Tezuka K. Involvement of Notch signaling in initiation of prechondrogenic condensation and nodule formation in lumb bud micromass cultures. J Bone Miner Metab. 2006;24:191–198. doi: 10.1007/s00774-005-0671-y. [DOI] [PubMed] [Google Scholar]
- Fujimoto R, Tanizawa T, Nishida S, Yamamoto N, Soshi S, Endo N, Takahashi HE. Local effects of transforming growth factor-beta1 on rat calvaria: changes depending on the dose and the injection site. J Bone Miner Metab. 1999;17:11–17. doi: 10.1007/s007740050057. [DOI] [PubMed] [Google Scholar]
- Gerber HP, Ferrara N. Angiogenesis and bone growth. Trends Cardiovasc Med. 2000;10:223–228. doi: 10.1016/s1050-1738(00)00074-8. [DOI] [PubMed] [Google Scholar]
- Gerstenfeld LC, Cho TJ, Kon T, Aizawa T, Cruceta J, Graves BD, Einhorn TA. Impaired intramembranous bone formation during bone repair in the absence of tumor necrosis factor-alpha signaling. Cells Tissues Organs. 2001;169:285–294. doi: 10.1159/000047893. [DOI] [PubMed] [Google Scholar]
- Goodsell DS. The molecular perspective: cadherin. Stem Cells. 2002;20:583–584. doi: 10.1634/stemcells.20-6-583. [DOI] [PubMed] [Google Scholar]
- Gould RP, Day A, Wolpert L. Mesenchymal condensation and cell contact in early morphogenesis of the chick limb. Exp Cell Res. 1972;72:325–36. doi: 10.1016/0014-4827(72)90593-9. [DOI] [PubMed] [Google Scholar]
- Govindarajan V, Overbeek PA. FGF9 can induce endochondral ossification in cranial mesenchyme. BMC Developmental Biology. 2006;6:7. doi: 10.1186/1471-213X-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigoriadis AE, Heersche JN, Aubin JE. Differentiation of muscle, fat, cartilage, and bone from progenitor cells present in a bone-derived clonal cell population: effect of dexamethasone. J Cell Biol. 1988;106:2139–51. doi: 10.1083/jcb.106.6.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurdon JB. Embryonic induction – molecular prospects. Development. 1987;99:285–306. doi: 10.1242/dev.99.3.285. [DOI] [PubMed] [Google Scholar]
- Hall BK. The embryonic development of bone. Am Sci. 1988;76:174–181. [Google Scholar]
- Hall BK, Miyake T. The membranous skeleton: the role of cell condensations in vertebrate skeletogenesis. Anatomy and Embryology. 1992;186:107–124. doi: 10.1007/BF00174948. [DOI] [PubMed] [Google Scholar]
- Hall BK, Miyake T. Divide, accumulate, differentiate: cell condensation in skeletal development revisited. International Journal of Developmental Biology. 1995;39:881–893. [PubMed] [Google Scholar]
- Hall BK, Miyake T. All for one and one for all: condensations and the initiation of skeletal development. Bioessays. 2000;22:138–147. doi: 10.1002/(SICI)1521-1878(200002)22:2<138::AID-BIES5>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Harris AK, Wild P, Stopak D. Silicone rubber substrata: a new wrinkle in the study of cell locomotion. Science. 1980a;208:177–179. doi: 10.1126/science.6987736. [DOI] [PubMed] [Google Scholar]
- Harris AK, Stopak D, Wild P. Fibroblast traction as a mechanism for collagen morphogenesis. Nature. 1980b;290:249–251. doi: 10.1038/290249a0. [DOI] [PubMed] [Google Scholar]
- Henderson JH, de la Fuente L, Romero D, Colnot CI, Huang S, Carter DR, Helms JA. Rapid growth of cartilage rudiments may generate perichondrial structures by mechanical induction. Biomechanics and Modeling in Mechanobiology. 2007;6:127–37. doi: 10.1007/s10237-006-0038-x. [DOI] [PubMed] [Google Scholar]
- Hill TP, Spater D, Taketo MM, Birchmeier W, Hartmann C. Canonical Wnt/beta-catenin signaling prevents osteoblasts from differentiating into chondrocytes. Developmental Cell. 2005;8:727–38. doi: 10.1016/j.devcel.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Hillsley MV, Frangos JA. Bone tissue engineering: the role of interstitial fluid flow. Biotechnology and Bioengineering. 1994;43:573–581. doi: 10.1002/bit.260430706. [DOI] [PubMed] [Google Scholar]
- Hu H, Hilton MJ, Tu X, Yu K, Ornitz DM, Long F. Sequential roles of Hedgehog and Wnt signaling in osteoblast development. Development. 2005;132:49–60. doi: 10.1242/dev.01564. [DOI] [PubMed] [Google Scholar]
- Illi B, Scopece A, Nanni S, Farsetti A, Morgante L, Biglioli P, Capogrossi MC, Gaetano C. Epigenetic histone modification and cardiovascular lineage programming in mouse embryonic stem cells exposed to laminar shear stress. Circulation Research. 2005;96:501–508. doi: 10.1161/01.RES.0000159181.06379.63. [DOI] [PubMed] [Google Scholar]
- Ingber DE. Fibronectin controls capillary endothelial cell growth by modulating cell shape. (1990) Proceedings of the National Academy of Sciences U S A. 1990;87:3579–3583. doi: 10.1073/pnas.87.9.3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi T, Scully SP, Heydemann A, Bolander ME. Transforming growth factor beta 1 stimulates type II collagen expression in cultured periosteum-derived cells. Journal of Bone and Mineral Research. 1992;7:115–21. doi: 10.1002/jbmr.5650070116. [DOI] [PubMed] [Google Scholar]
- Jakkaraju S, Zhe X, Pan D, Choudhury R, Schuger L. TIPs are tension-responsive proteins involved in myogenic versus adipogenic differentiation. Developmental Cell. 2005;9:39–49. doi: 10.1016/j.devcel.2005.04.015. [DOI] [PubMed] [Google Scholar]
- Järvinen TA, Kannus P, Järvinin TL, Jozsa L, Kalimo H, Järvinen M. Tenascin-C in the pathobiology and healing process of musculoskeletal tissue injury. Scand J Med Sci Sports. 2000;10:376–382. doi: 10.1034/j.1600-0838.2000.010006376.x. [DOI] [PubMed] [Google Scholar]
- Kafer J, Hayashi T, Maree AFM, Carthew RW, Graner F. Cell adhesion and cortex contractility determine cell patterning in the drosophila retina. Proceedings of the National Academy of Sciences. 2007;104:18549–18554. doi: 10.1073/pnas.0704235104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katagiri T, Yamaguchi A, Ikeda T, Yoshiki S, Wozney JM, Rosen V, Wang EA, Tanaka H, Omura S, Suda T. The non-osteogenic mouse pluripotent cell line, C3H10T1/2, is induced to differentiate into osteoblastic cells by recombinant human bone morphogenetic protein-2. Biochemical and Biophysical Research Communications. 1990;172:295–299. doi: 10.1016/s0006-291x(05)80208-6. [DOI] [PubMed] [Google Scholar]
- Kawakami Y, Rodriguez-Leon J, Belmonte JC. The role of TGFbetas and Sox9 during limb chondrogenesis. Current Opinion in Cell Biology. 2006;18:723–729. doi: 10.1016/j.ceb.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Keller EF, Segel LA. Conflict between positive and negative feedback as an explanation for the initiation of aggregation in slime mould amoebae. Nature. 1970a;227:1365–1366. doi: 10.1038/2271365a0. [DOI] [PubMed] [Google Scholar]
- Keller EF, Segel LA. Initiation of slime mold aggregation viewed as an instability. J Theor Biol. 1970b;26:399–415. doi: 10.1016/0022-5193(70)90092-5. [DOI] [PubMed] [Google Scholar]
- Klein-Nulend J, Veldhuijzen JP, Burger EH. Increased calcification of growth plate cartilage as a result of compressive force in vitro. Arthritis Rheumatology. 1986;29:1002–1009. doi: 10.1002/art.1780290809. [DOI] [PubMed] [Google Scholar]
- Klein-Nulend J, Veldhuijzen JP, van de Stadt RJ, van Kampen GP, Kuijer R, Burger EH. Influence of intermittent compressive force on proteoglycan content in calcifying growth plate cartilage in vitro. Journal of Biological Chemistry. 1987;262:15490–15495. [PubMed] [Google Scholar]
- Klymkowski MW, Parr B. The body language of cells: the intimate connection between cell adhesion and behavior. Cell. 1995;83:5–8. doi: 10.1016/0092-8674(95)90226-0. [DOI] [PubMed] [Google Scholar]
- Knippenberg M, Helder MN, Doulabi BZ, Semeins CM, Wuisman PI, Klein-Nulend J. Adipose tissue-derived mesenchymal stem cells acquire bone cell-like responsiveness to fluid shear stress on osteogenic stimulation. Tissue Engineering. 2005;11:1780–8. doi: 10.1089/ten.2005.11.1780. [DOI] [PubMed] [Google Scholar]
- Knothe U, Springfield DSS. A novel surgical procedure for bridging of massive bone defects. World Journal of Surgical Oncology. 2005;3:7. doi: 10.1186/1477-7819-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate Knothe. unpublished data. [Google Scholar]
- Knothe Tate ML, Knothe U, Niederer P. Experimental elucidation of mechanical load-induced fluid flow and its potential role in bone metabolism and functional adaptation. The American Journal of the Medical Sciences. 1998a;316:189–95. doi: 10.1097/00000441-199809000-00007. [DOI] [PubMed] [Google Scholar]
- Knothe Tate ML, Niederer P. A theoretical FE-based model developed to predict the relative contribution of convective and diffusive transport mechanisms for the maintenance of local equilibria within cortical bone. In: Clegg S, editor. Advances in Heat and Mass Transfer in Biotechnology. The American Society of Mechanical Engineers; 1998b. pp. 133–142. HTD – Vol. 362/BED – Vol. 40. [Google Scholar]
- Knothe Tate ML. “Whither flows the fluid in bone?” An osteocyte’s perspective. Journal of Biomechanics. 2003;36:1409–24. doi: 10.1016/s0021-9290(03)00123-4. [DOI] [PubMed] [Google Scholar]
- Knothe Tate ML, Adamson JR, Tami AE, Bauer TW. Invited Review Article: Cells in Focus - The Osteocyte. The International Journal of Biochemistry and Cell Biology. 2004;36:1–8. doi: 10.1016/s1357-2725(03)00241-3. [DOI] [PubMed] [Google Scholar]
- Knothe Tate ML, Ritzman TF, Schneider E, Knothe U. Testing of a new one-stage bone-transport surgical procedure exploiting the periosteum for the repair of long-bone defects. Journal of Bone and Joint Surgery American Volume. 2007;89:307–316. doi: 10.2106/JBJS.E.00512. [DOI] [PubMed] [Google Scholar]
- Krieg M, Arboleda-Estudillo Y, Puech PH, Käfer J, Graner F, Müller DJ, Heisenberg CP. Tensile forces govern germ-layer organization in zebrafish. Nature Cell Biology. 2008;10:429–436. doi: 10.1038/ncb1705. [DOI] [PubMed] [Google Scholar]
- Koyama E, Shimazu A, Leatherman JL, Golden FB, Nah HD, Pacifici M. Expression of syndecan-3 and tenascin-C: possible involvement in periosteum development. J Orthop Res. 1996;14:403–412. doi: 10.1002/jor.1100140310. [DOI] [PubMed] [Google Scholar]
- Kronenberg HM. Developmental regulation of the growth plate. Nature. 2003;423:332–6. doi: 10.1038/nature01657. [DOI] [PubMed] [Google Scholar]
- Lefebvre V, Behringer RR, de Crombrugghe B. L-Sox5, Sox6 and Sox9 control essential steps of the chondrocyte differentiation pathway. Osteoarthritis and Cartilage. 2001;9:S69–75. doi: 10.1053/joca.2001.0447. [DOI] [PubMed] [Google Scholar]
- Lukic IK, Grcevic D, Kovacic N, Katavic V, Ivcevic S, Kalajzic I, Marusic A. Alteration of newly induced endochondral bone formation in adult mice without tumour necrosis factor receptor 1. Clinical and Experimental Immunology. 2005;139:236–244. doi: 10.1111/j.1365-2249.2005.02680.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie EJ, Abraham LA, Taylor SL, Tucker RP, Murphy LI. Regulation of tenascin-C expression in bone cells by transforming growth factor-beta. Bone. 1998;22:301–307. doi: 10.1016/s8756-3282(97)00297-4. [DOI] [PubMed] [Google Scholar]
- Mackie EJ, Ahmed YA, Tatarczuch L, Chen KS, Mirams M. Endochondral ossification: How cartilage is converted to bone in the developing skeleton. The International Journal of Biochemistry & Cell Biology. 2007;40:46–62. doi: 10.1016/j.biocel.2007.06.009. [DOI] [PubMed] [Google Scholar]
- McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Developmental Cell. 2004;6:483–95. doi: 10.1016/s1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- McBride SM. Thesis for the Master of Sciences (Engineering) Case Western Reserve University, Biomedical Engineering; 2007. Elucidating biophysical cues conducive to targeted mulipotent cell differentiation. Document number: case1196435881. http://rave.ohiolink.edu/etdc/view?acc_num=case1196435881. [Google Scholar]
- McCulloch CA, Tenenbaum HC. Dexamethasone induces proliferation and terminal differentiation of osteogenic cells in tissue culture. Anatomical Record. 1986;215:397–402. doi: 10.1002/ar.1092150410. [DOI] [PubMed] [Google Scholar]
- Meinel L, Karageorgiou V, Fajardo R, Snyder B, Shinde-Patil V, Zichner L, Kaplan D, Langer R, Vunjak-Novakovic G. Bone tissue engineering using human mesenchymal stem cells: effects of scaffold material and medium flow. Annals of Biomedical Engineering. 2004;32:112–22. doi: 10.1023/b:abme.0000007796.48329.b4. [DOI] [PubMed] [Google Scholar]
- Miclau T, Schneider RA, Eames BF, Helms JA. Common molecular mechanisms regulating fetal bone formation and adult fracture repair. In: Lieberman JR, Friedlaender GE, editors. Bone Regeneration and Repair: Biology and Clinical Applications. Tototwa, NJ: Humana Press; 2005. pp. 45–55. [Google Scholar]
- Miyanishi K, Trindade MC, Lindsey DP, Beaupre GS, Carter DR, Goodman SB, Schurman DJ, Smith RL. Effects of hydrostatic pressure and transforming growth factor-beta 3 on adult human mesenchymal stem cell chondrogenesis in vitro. Tissue Engineering. 2006;12:1419–28. doi: 10.1089/ten.2006.12.1419. [DOI] [PubMed] [Google Scholar]
- Moore KA, Lemischka IR. Stem cells and their niches. Science. 2006;311:1880–5. doi: 10.1126/science.1110542. [DOI] [PubMed] [Google Scholar]
- Morali O, Savagner P, Larue L. Epithelium-Mesenchyme Transitions are Crucial Morphogenetic Events Occurring During Early Development. In: Savagner P, editor. Rise and Fall of Epithelial Phenotype: Concepts of Epithelial-Mesenchymal Transition. Georgetown, TX: Landes Bioscience; 2005. pp. 12–15. [Google Scholar]
- Munro E. unpublished data. [Google Scholar]
- Nakashima K, de Crombrugghe B. Transcriptional mechanisms in osteoblast differentiation and bone formation. Trends in Genetics. 2003;19:458–66. doi: 10.1016/S0168-9525(03)00176-8. [DOI] [PubMed] [Google Scholar]
- Oberhofer K. Diploma thesis for the Dipl Bio ETH (Department of Biology, Sports- and Movement Science) Swiss Federal Institute of Technology (ETH); Zurich: 2005. Does mechanical stress promote bone cell fate in the murine embryonic mesenchymal stem cell line C3H/10T1/2? [Google Scholar]
- Ohta S, Suzuki K, Tachibana K, Tanaka H, Yamada G. Cessation of gastrulation is mediated by suppression of epithelial-mesenchymal transition at the ventral ectodermal ridge. Development. 2007;134:4315–4324. doi: 10.1242/dev.008151. [DOI] [PubMed] [Google Scholar]
- Olsen BR, Reginato AM, Wang W. Bone Development. Annual Review of Cell and Developmental Biology. 2000;16:191–220. doi: 10.1146/annurev.cellbio.16.1.191. [DOI] [PubMed] [Google Scholar]
- Oster GF, Murray JD, Harris AK. Mechanical aspects of mesenchymal morphogenesis. Journal of Embryology and Experimental Morphology. 1983;78:83–125. [PubMed] [Google Scholar]
- Oster GF, Murray JD, Maini PK. A model for chondrogenic condensations in the developing limb: the role of extracellular matrix and cell tractions. Journal of Embryology and Experimental Morphology. 1985;89:93–112. [PubMed] [Google Scholar]
- Parfitt AM. The cellular basis of bone remodeling: the quantum concept reexamined in light of recent advances in the cell biology of bone. Calcif Tissue Int. 1984;36:S37–45. doi: 10.1007/BF02406132. [DOI] [PubMed] [Google Scholar]
- Pauwels F. A new theory on the influence of mechanical stimuli on the differentiation of supporting tissue. The tenth contribution to the functional anatomy and causal morphology of the supporting structure. Zeitschrift fuer Anatomie und Entwicklungsgeschichte. 1960;121:478–515. [PubMed] [Google Scholar]
- Pelttari K, Winter A, Steck E, Goetzke K, Hennig T, Ochs BG, Aigner T, Richter W. Premature induction of hypertrophy during in vitro chondrogenesis of human mesenchymal stem cells correlates with calcification and vascular invasion after ectopic transplantation in SCID mice. Arthritis & Rheumatism. 2006;54:3254–66. doi: 10.1002/art.22136. [DOI] [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–7. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Quinlan MP. Rac regulates the stability of the adherens junction and its components, thus affecting epithelial cell differentiation and transformation. Oncogene. 1999;18:6434–42. doi: 10.1038/sj.onc.1203026. [DOI] [PubMed] [Google Scholar]
- Ripamonti U, Ferretti C, Heliotis M. Soluble and insoluble signals and the induction of bone formation: molecular therapeutics recapitulating development. J Anat. 209:447–468. doi: 10.1111/j.1469-7580.2006.00635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelofsen J, Klein-Nulend J, Burger EH. Mechanical stimulation by intermittent hydrostatic compression promotes bone-specific gene expression in vitro. Journal of Biomechanics. 1995;28:1493–503. doi: 10.1016/0021-9290(95)00097-6. [DOI] [PubMed] [Google Scholar]
- Rosen ED, MacDougald OA. Adipocyte differentiation from the inside out. Nature Reviews Molecular Cell Biology. 2006;7:885–96. doi: 10.1038/nrm2066. [DOI] [PubMed] [Google Scholar]
- Schier AF. Nodal signaling in vertebrate development. Annual Review of Cell and Developmental Biology. 2003;19:589–621. doi: 10.1146/annurev.cellbio.19.041603.094522. [DOI] [PubMed] [Google Scholar]
- Schofield JN, Wolpert L. Effect of TGF-beta 1, TGF-beta 2, and bFGF on chick cartilage and muscle cell differentiation. Experimental Cell Research. 1990;191:144–8. doi: 10.1016/0014-4827(90)90048-f. [DOI] [PubMed] [Google Scholar]
- Schotte OE, Smith CB. Wound Healing Process in Amputated Mouse Digits. Biological Bulletin. 1959;117:546–561. [Google Scholar]
- Shea CM, Edgar CM, Einhorn TA, Gerstenfeld LC. BMP treatment of C3H10T1/2 mesenchymal stem cells induces both chondrogenesis and osteogenesis. Journal of Cellular Biochemistry. 2003;90:1112–1127. doi: 10.1002/jcb.10734. [DOI] [PubMed] [Google Scholar]
- Shum L, Nuckolls G. The life cycle of chondrocytes in the developing skeleton. Arthritis Research & Therapy. 2002;4:94–106. doi: 10.1186/ar396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith N, Dong Y, Lian JB, Pratap J, Kingsley PD, van Wijnen AJ, Stein JL, Schwarz EM, O’Keefe RJ, Stein GS, Drissi MH. Overlapping expression of Runx1(Cbfa2) and Runx2(Cbfa1) transcription factors supports cooperative induction of skeletal development. J Cell Physiol. 2005;203:133–43. doi: 10.1002/jcp.20210. [DOI] [PubMed] [Google Scholar]
- Spiegel E, Burger MM, Spiegel M. Fibronectin and laminin in the extracellular matrix and basement membrane of sea urchin embryos. Exp Cell Res. 1983;144:47–55. doi: 10.1016/0014-4827(83)90440-8. [DOI] [PubMed] [Google Scholar]
- Spiegel E, Spiegel M. The insertion of mesenchyme cells into the ectoderm during differentiation of Sea urchin embryos. Development Genes and Evolution. 1992;201:383–388. doi: 10.1007/BF00365126. [DOI] [PubMed] [Google Scholar]
- Stein GS, Lian JB, Owen TA. Relationship of cell growth to the regulation of tissue-specific gene expression during osteoblast differentiation. FASEB Journal. 1990;4:3111–3123. doi: 10.1096/fasebj.4.13.2210157. [DOI] [PubMed] [Google Scholar]
- Stoffel K, Klaue K, Perren SM. Functional load of plates in fracture fixation in vivo and its correlate in bone healing. Injury. 2000;31:37–50. doi: 10.1016/s0020-1383(00)80042-x. [DOI] [PubMed] [Google Scholar]
- Takahashi I, Nuckolls GH, Takahashi K, Tanaka O, Semba I, Dashner R, Shum L, Slavkin HC. Compressive force promotes Sox9, type II collagen and aggrecan and inhibits IL-1β expression resulting in chondrogenesis in mouse embryonic limb bud mesenchymal cells. Journal of Cell Science. 1998;111:2067–76. doi: 10.1242/jcs.111.14.2067. [DOI] [PubMed] [Google Scholar]
- Takeichi M. Morphogenetic roles of classic cadherins. Current Opinion in Cell Biology. 1995;7:619–627. doi: 10.1016/0955-0674(95)80102-2. [DOI] [PubMed] [Google Scholar]
- Tami AE, Nasser P, Schaffler MB, Knothe Tate ML. Noninvasive fatigue fracture model of the rat ulna. Journal of Orthopaedic Research. 2003;21:1018–1024. doi: 10.1016/S0736-0266(03)00099-8. [DOI] [PubMed] [Google Scholar]
- Tanck E, Van Donkelaar CC, Jepsen KJ, Goldstein SA, Weinans H, Burger EH, Huiskes R. The mechanical consequences of mineralization in embryonic bone. Bone. 2004;35:186–90. doi: 10.1016/j.bone.2004.02.015. [DOI] [PubMed] [Google Scholar]
- Thompson DW. On Growth and Form. 1. Cambridge, UK: Cambridge University Press; 1917. [Google Scholar]
- Toole BP. Hyaluronan in morphogenesis. Journal of Internal Medicine. 1997;242:35–40. doi: 10.1046/j.1365-2796.1997.00171.x. [DOI] [PubMed] [Google Scholar]
- Tucker RP, Edwards BF, Erickson CA. Tension in the culture dish: microfilament organization and migratory behaviour of quail neural crest cells. Cell Motil. 1985;5:225–237. doi: 10.1002/cm.970050305. [DOI] [PubMed] [Google Scholar]
- Turing AM. The chemical basis of morphogenesis. Phil Trans R Soc. 1952;237B:37–72. [Google Scholar]
- Underhill CB. Hyaluronan is inversely correlated with the expression of CD44 in the dermal condensation of the embryonic hair follicle. J Invest Dermatol. 1993;101:820–826. doi: 10.1111/1523-1747.ep12371701. [DOI] [PubMed] [Google Scholar]
- Vogel V, Sheetz M. Local force and geometry sensing regulate cell functions. Nature Reviews Molecular Cell Biology. 2006;7:265–75. doi: 10.1038/nrm1890. [DOI] [PubMed] [Google Scholar]
- Wang H, Riha GM, Yan S, Li M, Chai H, Yang H, Yao Q, Chen C. Shear stress induces endothelial differentiation from a murine embryonic mesenchymal progenitor cell line. Arteriosclerosis, Thrombosis, and Vascular Biology. 2005;25:1817–1823. doi: 10.1161/01.ATV.0000175840.90510.a8. [DOI] [PubMed] [Google Scholar]
- Watanabe N, Tezuka Y, Matsuno K, Miyatani S, Morimura N, Yasuda M, Fujimaki R, Kuroda K, Hiraki Y, Hozumi N, Tezuka K. Suppression of differentiation and proliferation of early chondrogenic cells by Notch. J Bone Miner Metab. 2003;21:344–352. doi: 10.1007/s00774-003-0428-4. [DOI] [PubMed] [Google Scholar]
- Watt F. Effect of seeding density on stability of the differentiated phenotype of pig articular chondrocytes in culture. Journal of Cell Science. 89:373–378. doi: 10.1242/jcs.89.3.373. [DOI] [PubMed] [Google Scholar]
- White CR, Frangos JA. The shear stress of it all: the cell membrane and mechanochemical transduction. Philosophical Transactions of the Royal Society of London, Series B, Biological Sciences, 2007. 2007;362:1459–1467. doi: 10.1098/rstb.2007.2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong M, Carter DR. Theoretical stress analysis of organ culture osteogenesis. Bone. 1990;11:127–131. doi: 10.1016/8756-3282(90)90060-c. [DOI] [PubMed] [Google Scholar]
- Woods A, Wang G, Beier F. RhoA/ROCK signaling regulates Sox9 expression and actin organization during chondrogenesis. The Journal of Biological Chemistry. 2005;280:11626–11624. doi: 10.1074/jbc.M409158200. [DOI] [PubMed] [Google Scholar]
- Yoon BS, Lyons KM. Multiple functions of BMPs in chondrogenesis. Journal of Cellular Biochemistry. 2004;93:93–103. doi: 10.1002/jcb.20211. [DOI] [PubMed] [Google Scholar]
- Yu K, Xu J, Liu Z, Sosic D, Shao J, Olson EN, Towler DA, Ornitz DM. Conditional inactivation of FGF receptor 2 reveals an essential role for FGF signaling in the regulation of osteoblast function and bone growth. Development. 2003;130:3063–3074. doi: 10.1242/dev.00491. [DOI] [PubMed] [Google Scholar]