Abstract
Our previous study showed that the novel proteasome inhibitor NPI-0052 induces apoptosis in multiple myeloma (MM) cells resistant to conventional and bortezomib (Velcade™) therapies. In vivo studies using human MM-xenografts demonstrated that NPI-0052 is well tolerated, prolongs survival, and reduces tumor recurrence. These preclinical studies provided the basis for an ongoing phase-1 clinical trial of NPI-0052 in relapsed/refractory MM patients. Here we performed pharmacodynamic (PD) studies of NPI-0052 using human MM xenograft murine model. Our results show that NPI-0052: 1) rapidly leaves the vascular compartment in an active form after intravenous (i.v) administration; 2) inhibits 20S proteasome chymotrypsin-like (CT-L, β5), trypsin-like (T-L, β2), and caspase-like (C-L, β1) activities in extra-vascular tumors, packed whole blood (PWB), lung, liver, spleen, and kidney, but not brain; and 3) triggers a more sustained (>24h) proteasome inhibition in tumors and PWB than in other organs (< 24h). Tissue distribution analysis of radiolabeled compound (3H-NPI-0052) in mice demonstrated that NPI-0052 leaves the vascular space and enters organs as the parent compound. Importantly, treatment of MM.1S bearing mice with NPI-0052 showed reduced tumor growth without significant toxicity, which was associated with prolonged inhibition of proteasome activity in tumors and PWB but not normal tissues.
Introduction
Protein degradation regulates normal cellular homeostasis through a multi-subunit complex called the proteasome(Adams 2004, Goldberg 2003) Pioneering studies by Ciechanover, Hershko and Rose et al. showed that ATP-dependent conjugation of proteins with polypeptide (ubiquitin) mediates protein degradation(Ciechanover, et al 1980a, Ciechanover, et al 1984, Ciechanover, et al 1980b, Ciehanover, et al 1978, Hershko, et al 1980). The 26S proteasome complex (Hough, et al 1987, Waxman, et al 1987, Wilk and Orlowski 1983) consists of 19S units and a 20S proteasome core(Arrigo, et al 1988, Ganoth, et al 1988); the 19S units regulate entry of ubiquitinated proteins into the 20S core chamber(Goldberg 2003, Peters, et al 1994). Protein ubiquitination occurs via ATP dependent complex enzymatic reactions involving E1 and E2 ubiquitin enzymes, as well as E3 ubiquitin ligases. Recognition of ubiquitinated proteins by the 19S regulatory subunits allows for entry and degradation of proteins into small peptides of 4-20 amino acids. Proteasomal activities residing within the 20S core complex i.e., chymotrypsin-like (CT-L, β5), trypsin-like (T-L, β2), and caspase-like (C-L, β1), are responsible for protein degradation(Arendt and Hochstrasser 1997). Importantly, to date, many human diseases have been linked to defects in the Ubiquitin-Proteasome Signaling (UPS) pathway(Adams 2004), suggesting the clinical utility of targeting proteasomes.
Our preclinical and clinical studies provided the basis for the FDA approval of the first in class proteasome inhibitor bortezomib (Velcade™), for the treatment of relapsed/refractory, relapsed, and newly diagnosed MM(Adams 2004, Hideshima, et al 2001, Kane, et al 2003, Richardson, et al 2003, Richardson, et al 2005, San Miguel, et al 2008). As with other agents, however, dose-limiting toxicities and the development of resistance limits its long-term utility(Lonial, et al 2005, Richardson, et al 2006, Richardson, et al 2007). Our recent study demonstrated that a novel proteasome inhibitor NPI-0052 (Feling, et al 2003) (USAN name marizomib) is distinct from bortezomib; and triggers apoptosis in MM cells resistant to bortezomib therapies (Chauhan, et al 2005). These preclinical data provided the basis for an ongoing phase-1 clinical trial of NPI-0052 in relapsed/refractory MM patients.
In the present study, we utilized our human MM xenograft mouse model to define the distribution profile of NPI-0052 in various tissues and tumor, and in parallel analyzed the kinetics of proteasome inhibition. Our data show that NPI-0052 rapidly distributes from the vascular compartment into tumor and organs, associated with both prolonged proteasome inhibition in tumors and PWB and a marked delay in tumor growth.
Material/subjects and Methods
Cell culture
Human MM.1S MM cells were kindly provided by Dr Steven Rosen (Northwestern University, Chicago, IL), and grown in RPMI-1640 media supplemented with 10% heat inactivated fetal-bovine serum, 100 units/ml penicillin, 100 μg/ml streptomycin, 2 mM L-glutamine, and plasmocin™ (10 μg/ml), as previously described(Chauhan, et al 2005). Prior to inoculation of MM.1S cells in SCID mice, cell viability was assessed by trypan blue exclusion assay, as previously described(Chauhan, et al 2008).
Reagents
NPI-0052 and 3H-NPI-0052 (5Ci/mMol) were provided by Nereus Pharmaceuticals, Inc. For the efficacy and proteasome inhibition studies in mice, NPI-0052 was dissolved in 100% DMSO to generate a 10 mg/ml stock solution, aliquoted, and stored frozen at - 80°C. For intravenous (i.v) administration, NPI-0052 was dissolved in 100% DMSO and serially diluted with 5% Solutol (Solutol® HS 15; polyethylene glycol 660 12- hydroxystearate, BASF, Shreveport, LA) to yield a final concentration of 2% DMSO. The vehicle control consisted of 2% DMSO and 98% (5% Solutol). The pH of the dosing solutions is between 6-7. Flurogenic proteasome substrates succinyl-Leu-Leu-Val-Tyr-AMC and Z-Leu-Leu-Glu-AMC were obtained from Boston Biochem (Cambridge, MA), and BZ-VGR-AMC from Biomol (Plymouth, PA). Purified 20S proteasome was purchased from Boston Biochem. Culture medium was obtained from Mediatech Inc (Manassas, VA), and Fetal Bovine Serum (FBS) was purchased from Lonza (Walkersville, MD). Immunoblot analysis was performed using the following primary antibodies: anti-caspase-3, anti-PARP, anti-BIP, anti-pEIF2α, pIκb and anti-Hsp70 (Cell signaling, Beverly MA); anti-CHOP/GADD153 (Abcam, Cambridge, MA); and anti-GAPDH (BD Bioscience Pharmingen, San Diego, CA). Horseradish peroxidase (HRP)-conjugated secondary Ab was purchased from Biosource, and enhanced chemiluminesence kit (ECL) was obtained from Amersham (Arlington Heights, IL).
Human plasmacytoma xenograft model
Animal studies were approved by the DFCI Institutional Animal Care and Use Committee. CB-17 SCID-male mice (4-6 weeks old) were purchased from Charles River Laboratories (Willminghton, MA) and housed for 1-2 weeks before starting experiments. Tumors were established by subcutaneous injection of MM.1S cell line (5.0 × 106 MM.1S cells in 100 microliter of serum free RPMI-1640 medium, viability > 99%) in the flank region of CB-17 SCID-male mice. The mice were divided into three different groups: Groups 1 and 2 (25 mice per group) for PD studies (time course) and Group 3 (10 mice) for drug efficacy studies. Tumor size was measured every third day in two dimensions using calipers, and tumor volume was calculated using the formula V = 0.5 a × b2, where a and b are the long and short diameter of the tumor respectively.
Treatment and dose schedule for pharmacodynamics (PD) and efficacy studies
The PD effects of NPI-0052 were assessed by measuring alterations in proteasome activity as a function of increasing exposure times and dose levels of NPI-0052. For the PD studies, mice were treated with vehicle or MTD dose of NPI-0052 (i.v) when tumors were ∼200-300 mm3. Our prior study (Chauhan, et al 2005) established the MTD of NPI-0052 and we have used both single and repeated dosing (three dose) in the present study. For the measurement of proteasome activity, mice were euthanized at 10 min, 1h, 4h, and 24h after NPI-0052 dosing. Liver, spleen, lung, kidney, brain and tumor were removed, immediately frozen on dry ice, and subsequently processed for determination of ex vivo proteasome activity. PWB was collected into tubes containing heparin from cardiac puncture. For efficacy studies, mice were treated i.v with vehicle or NPI-0052 (0.15 mg/kg) twice a week for three weeks. NPI-0052 was administered via the i.v route at a dose of 0.15 mg/kg either once (D1 for proteasome inhibition), three times (D1, D4, D8 for proteasome inhibition), or six times (D1, D4, D8, D11, D15, D18, for efficacy).
Treatment and dose schedule
For the administration of 3H-NPI-0052 to mice and rats (studies performed under the direction of Nereus at MPI Research, Mattawan, MI), the compound (0.0313 mg, or 5 mCi/mL) was stored at -80°C in 0.1 mL of ethanol. For use the material was formulated to 0.06 mg/mL in 40% propylene glycol/10% ethanol/50% citrate buffer (5mM final concentration, pH 5.0, stored on ice and used within five hours of preparation. MM.1S tumor-bearing mice were injected i.v with 3H-NPI-0052 (0.15 mg/kg; 0.45 mg/m2) (n=15 mice per group; experiments were performed at MPI Research, Mattawan, MI). Immediately prior to euthanasia, blood was collected from anesthetized mice via cardiac puncture following which the mice were euthanized by carbon dioxide inhalation at predetermined time points. Selected organs were removed, weighed and homogenized (5:1, w/v) in methanol, after which a 10:1 (v/w) ratio of acetonitrile was added to the tissue. This preparation was sonicated for 30 seconds and subsequently centrifuged at 3K rpm for 10 mins at 4°C. The supernatant was decanted, and formic acid (45%) added to the extracts, frozen at < -60°C, and then analyzed for total radioactivity and NPI-0052 concentrations. Based on the total radioactivity content, the extracts were pooled to obtain a minimum of 15,000 dpm for injection onto the YMC Pro C18 HPLC column. Using the relative retention time for authentic 3H-NPI-0052, the chromatographic peak signal was integrated as a percent of total radioactivity chromatographed. Using the specific activity of the dose, the ng-equivalents percent of 3H-NPI-0052 was calculated. Male Sprague-Dawley rats were administered 3H-NPI-0052 (0.1 mg/kg or 0.6 mg/m2) by i.v injection and at predetermined time points (n=1 per time point) euthanized by deep isoflurane anesthesia and subsequent submergence in a hexane/dry ice bath for 15 minutes, then removed and placed on dry ice (experiments performed at MPI Research, Mattawan, MI).
Quantitative Whole Body Autoradiography (QWBA) (QPS, Newark, DE), the frozen rat carcass was embedded in 2% carboxymethylcellulose, mounted on a microtome stage (Leica CM3600 Crymacrocut) and sections (approximately 40 μm thick) were prepared in the sagittal plane. The sections were placed on Scotch tape, dried, mounted on cardboard, and exposed to a 3H-sensitive phosphor imaging plate (Fuji Biomedical, Stamford, CT) for seven days in light-tight cassettes, followed by scanning using the Typhoon 9410™ image acquisition system (GE Healthcare, Sunnyvale, CA).
Protein extract preparation
After thawing, tissue samples (liver, lung, brain, kidney, tumor) were homogenized in 1 ml of lysis buffer (0.5 mM EDTA, pH 7.3, 20 mM HEPES, Triton X 100 0.05%) and centrifuged at 14k rpm for 30 min at 4°C. Supernatants were collected, and protein concentrations were determined using Bradford assay kit (Pierce, Rockford, IL). Whole blood and splenocytes (obtained after slow mechanical disruption) were first washed with PBS; RBCs were then lysed in 5 ml of red blood cells lysis buffer (Sigma, St. Luis, MO) for 5 min at room temperature, followed by washing twice with PBS and lysis in lysis buffer (0.5 mM EDTA, pH 8.0, 20 mM HEPES, Triton X 100 0.05%). Supernatants were collected, and protein concentrations were determined using Bradford assay kit (Pierce, Rockford, IL).
Proteasome activity assays
20S proteasome activity assays were performed using fluorogenic peptide substrates, as previously described(Chauhan, et al 2005, Lightcap, et al 2000). 20μg of protein was used in a total volume of 200μl for determination of 20S proteasome CT-L, T-L or C-L activity, as described for the in vitro 20S proteasome activity assays with these exceptions: 1) the assay buffer consisted of 20mM HEPES, 0.5mM EDTA, pH. 8.0; 2) the substrate used for determination of T-L activity was BZ-VGR-AMC; 3) the final substrate concentration was 60μM; and 4) the assay buffer was supplemented with a final concentration of 0.05% SDS for the evaluation of CT-L and the C-L activities. Data are presented as % inhibition compared to vehicle control treated animals.
In situ detection of apoptosis, and immunohistochemical (IHC) assessment of microvessel density (MVD)
Apoptotic cells in xenografted MM.1S tumors were identified by IHC staining for caspase-3 activation(Chauhan, et al 2008). Microvessel density (MVD), a measure of tumor angiogenesis, was assessed by IHC staining for Factor VIII, as previously described(Chauhan, et al 2005, Chauhan, et al 2008).
Western blotting
Total protein lysates were prepared and immunoblot blot analysis was performed, as previously described(Chauhan, et al 2005). Briefly, equal amounts of proteins were resolved by 10% or 12.5% SDS-PAGE, and transferred onto nitrocellulose membranes. Filters were blocked by incubation in 5% dry milk in PBST (0.05% Tween-20 in PBS) and probed with anti-Caspase-3, anti-PARP, anti-BIP, anti-pEIF2α, Hsp70, CHOP/GADD153, or anti-GAPDH Abs. Specific immunoreactive bands were detected by staining with HRP-conjugated secondary Abs, and blots were developed by enhanced chemiluminesence (ECL; Amersham, Arlington Heights, IL).
Statistical Analysis
Statistical significance of differences observed in untreated mice versus mice treated with NPI-0052 for various time perods was determined using a Student's t test. The minimal level of significance was P < 0.05.
Results
Pharmacodynamic (PD) effect of NPI-0052
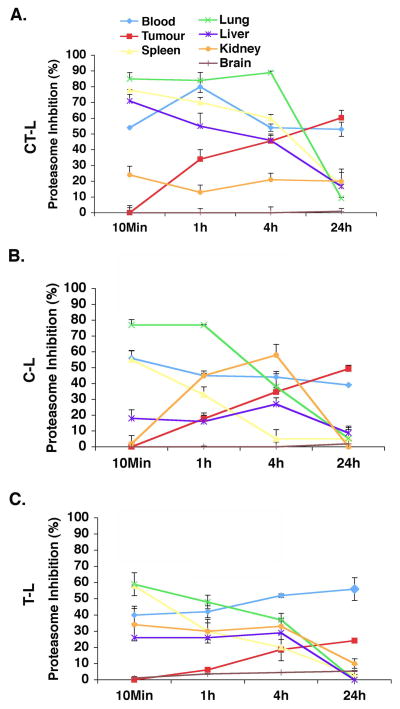
MM.1S tumor-bearing mice were treated with either a single (D1) or three (D1, D4, and D8) doses of NPI-0052 (0.15 mg/kg, i.v), and euthanized at 10 min, 1, 4 and 24h after dosing. PWB, liver, spleen, lung, kidney, brain and tumors were havested, and analyzed for CT-L, C-L, and T-L proteasome activities. Results showed significant inhibition of CT-L, C-L, and T-L proteasome activities in tumors and in these tissues, albeit with differential kinetics (Fig 1). A time course analysis of proteasome activity in response to a single dose treatment of mice showed that: 1) NPI-0052 inhibits all three proteasome activities in PWB, liver, spleen, lung, and kidney within 10 mins; 2) inhibition of proteasome activities occurs in tumors by 1h and is maximal at 24h; 3) NPI-0052 does not inhibit proteasome activity in brain; and 4) proteasome activities return to baseline within 24h in all tissues examined, except PWB and tumors (Fig 1). These findings demonstrate that NPI-0052 rapidly leaves the vascular compartments, and targets liver, spleen, lung, and kidney as early as 10 mins. Importantly, NPI-0052-triggered a more sustained inhibition of proteasome activities in tumors and PWB than in other tissues.
Figure 1. Proteasome inhibition in vivo in various tissues and tumors after a single dose of NPI-0052.
MM.1S tumor-bearing mice were injected with a single (Day1) dose of NPI-0052 (0.15 mg/kg, IV); euthanized at 10 min, 1, 4 and 24 hr after dosing. PWB, liver, spleen, lung, kidney, brain, and tumor were harvested. Protein extracts were prepared and analyzed for CT-L (A), C-L (B), and T-L (C) proteasome activities. Results are presented as percent inhibition compared to vehicle control. Data presented are means plus or minus SD (n = 3, P < 0.05).
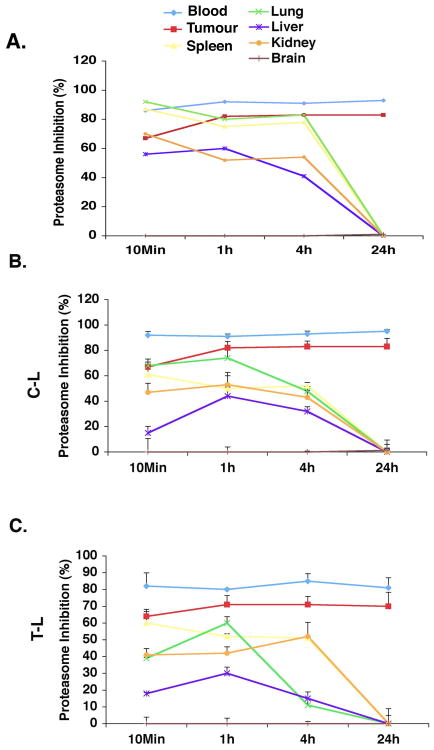
We next examined alterations in proteasome activity profiles in various tissues in response to the treatment with three doses of NPI-0052. As seen in Figure 2, our results showed that 1), all three proteasome activities were significantly inhibited (65-90%) in tumors and PWB, as well as in other tissues except brain within 10 mins following the third dose of NPI-0052; 2) all three proteasome activities in liver, kidney, lung, and spleen recovered within 24h after treatment with NPI-0052; and 3) inhibition of CT-L, C-L and T-L activities increased in tumor after the third compared to the first NPI-0052 treatment. For example, all three activities were inhibited approximately 70-80% at 24h post third dose versus 25-60% post single dose. In agreement with our prior studies(Chauhan, et al 2005), other normal tissues have the ability to recover proteasome activities even after exposure to three doses of NPI-0052. Importantly, no significant inhibition of proteasome activity was noted in brain tissue at any time point after either a single or three doses of NPI-0052, suggesting that NPI-0052 does not cross the blood-brain barrier at the efficacious dose tested.
Figure 2. Proteasome inhibition in vivo in various tissues and tumors after three doses of NPI-0052.
MM.1S tumor-bearing mice were injected with three doses (Day1, Day4 and Day8) of NPI-0052 (0.15 mg/kg, i.v.); euthanized at 10 min, 1, 4 and 24 hr after dosing; and PWB, liver, spleen, lung, kidney, brain and tumor were harvested. Protein extracts were prepared and analyzed for CT-L (A), C-L (B), and T-L (C) proteasome activities. Results are presented as percent inhibition compared to vehicle control. Data presented are means plus or minus SD (n = 3, P < 0.05).
Levels of 3H-NPI-0052 in tumor, blood and kidney of MM.1S-tumor bearing mice
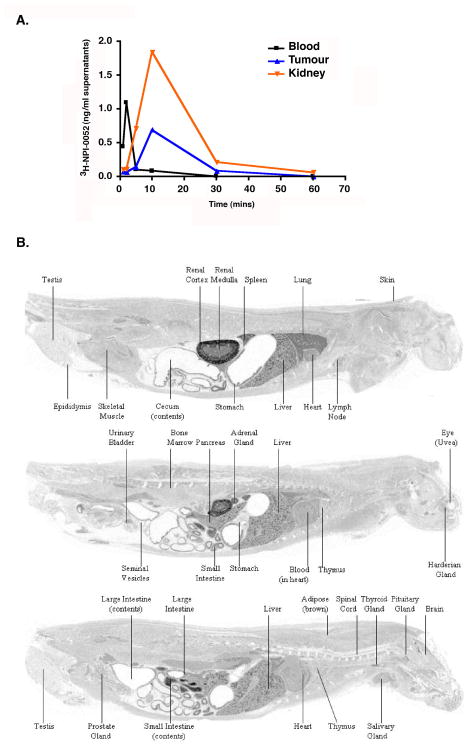
We next examined the distribution of 3H-NPI-0052 in selected organs of MM.1S-tumor bearing mice following the administration of 3H-NPI-0052 (0.15 mg/kg or 0.45 mg/m2; i.v). The maximal 3H-NPI-0052 levels occurred within 10 minutes after drug administration in the tumor, and exhibited a similar time-course in the kidney (Fig 3A). The mean drug level observed in the tumor supernatant equates to 2.924 ng/g tumor tissue (8.8 nM), which is greater than the fifty percent growth inhibitory concentrations of NPI-0052 for MM.1S cells (IC50: 7 nM), as previously reported(Chauhan, et al 2005). Our prior study also showed that these concentrations of NPI-0052 markedly block all three proteasome activities(Chauhan, et al 2005). Importantly, in the present study as well, these drug levels were sufficient to trigger proteasome inhibition in tumors (Figs 1,2, 5A) and induce anti-tumor activity (Fig 4A).
Figure 3. Distribution of radioactivity following i.v administration of 3H-NPI-0052.
(A) Distribution of 3H-NPI-0052 in MM.1S-tumor bearing mice following a single i.v dose of 0.15 mg/kg. (B) Whole-Body autoradiogram of the radioactivity distribution in a male albino rat at 10 min Post-dose following a single i.v administration of [3H]-NPI-0052 at a target dose of 0.1 mg/kg.
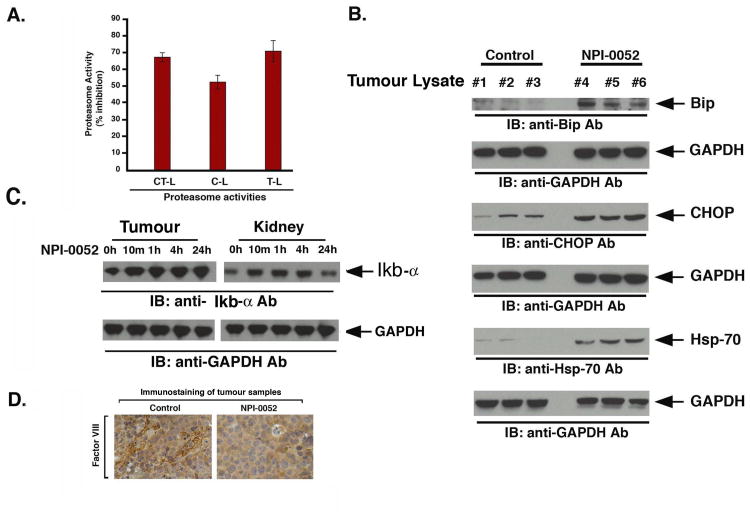
Figure 5. Effect of NPI-0052 on proteasome activities, endoplasmic reticulum stress signaling, and neovascularization, in vivo in xenografted MM.1S tumors.
(A) Mice bearing MM.1S subcutaneous xenografts were treated with vehicle (control) or NPI-0052 (as in panel A, Fig 4), and tumors were harvested 3h after the last dose injection (Day 21). Protein lysates were analyzed for CT-L, C-L, and T-L proteasome activities. The data represent % inhibition compared to vehicle control treated animals from two independent experiments. (B) Mice were treated with vehicle (control) or NPI-0052 (as in panel A, Fig 4), and tumors were harvested 3h after the last dose injection (Day21). Protein lysates were prepared, and subjected to immunoblot analysis with anti-Bip, anti-CHOP, anti-Hsp70, or anti-GAPDH Abs. Immunoblots show analysis of tumor lysates from 3 mice in each group (vehicle alone #1-#3 and NPI-0052-treated mice #4-#6). (C) Mice were injected with three doses (Day1, Day4 and Day8) of NPI-0052 (0.15 mg/kg, i.v.); euthanized at 10 min, 1h, 4h and 24h after last dosing; Tumors and kidneys were isolated, protein lysates were prepared and then subjected to immunoblot analysis using IκB or anti-GAPDH Abs. (D) Mice were treated with vehicle (control) or NPI-0052 (as in panel A, Fig 4), and tumors were harvested 3h after the last dose injection (Day 21). Paraffin-embedded tumor sections were subjected to immunostaining with Factor VIII, a marker for angiogenesis. Micrographs show markedly reduced blood vessel formation in tumor sections from NPI-0052-treated versus control mice. Photograph is representative of two mice receiving same treatment.
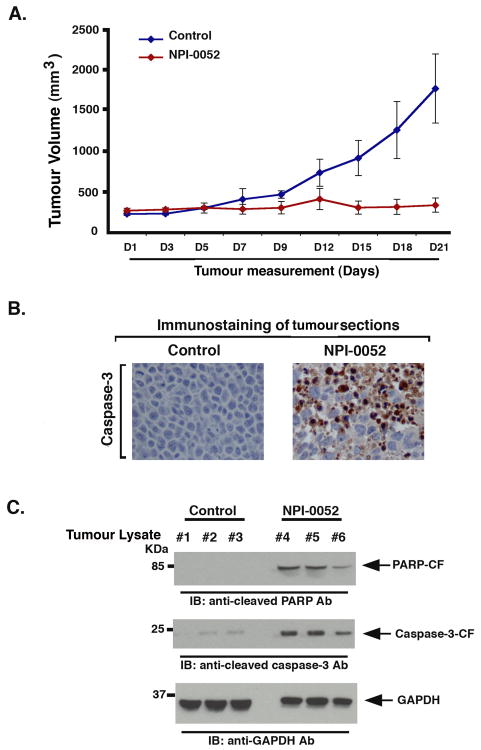
Figure 4. NPI-0052 inhibits human plasmacytoma growth in SCID mice.
(A) Mice (n=5 EA/group) received NPI-0052 (0.15 mg/kg; i.v.) on a twice weekly schedule for three weeks; and change in tumor volume was measured on the indicated days. A significant delay in tumor growth was noted in NPI-0052-treated mice compared to vehicle-treated control mice, as early as day 7 (P < 0.005). Bars indicate means ± SD. (B) Micrographs show apoptotic cells identified by caspase-3 cleavage (dark brown cells) in tumors harvested on day 21 (endpoint) from untreated- or NPI-0052 (0.15 mg/kg)-treated mice. Photographs are representative of two mice receiving same treatment. (C) Mice were treated with vehicle (control) or NPI-0052 (as in panel A), and tumors were harvested 3h after the last treatment (Day 21). Protein lysates were prepared and subjected to immunoblot analysis with anti-PARP, anti-cleaved caspase-3, or anti-GAPDH Abs. Immunoblots show analysis of tumor lysates from three mice in each group (vehicle alone #1-#3 and NPI-0052-treated mice #4-#6).
Distribution of 3H-NPI-0052 in Sprague-Dawley Rats
To confirm our findings in MM.1S tumor bearing mice, non-tumor bearing rats were injected with 3H-NPI-0052 (0.1 mg/kg or 0.6 mg/m2; i.v), and subjected to QWBA. In agreement with our data using MM.1S-tumor-bearing mice, high levels of 3H-NPI-0052 (total radioactivity) were noted in kidney (Fig 3B). Lung, liver and spleen also exhibited relatively high levels, whereas radioactivity was very low/undetectable in the brain, spinal cord and eye.
In vivo anti-MM activity of NPI-0052
Having shown that NPI-0052 targets the proteasome in xenografted tumors, we next determined whether the same dose and schedule of NPI-0052 triggered anti-tumor activity. MM.1S-tumor bearing mice were injected with NPI-0052 (0.15 mg/kg; i.v) twice a week for three weeks, and tumor volume was measured. In agreement with our prior studies(Chauhan, et al 2005), NPI-0052 treatment significantly decreased tumor growth relative to untreated vehicle-treated control mice (Fig 4A; P = 0.005). NPI-0052 treatment was not associated with any toxicity, since no differences in body weight and overall appearance were noted (data not shown). Importantly, the anti-MM activity of NPI-0052 was evident as early as day 5-7, when significant proteasome inhibition was observed in the tumors (Fig 4A).
To determine whether in vivo anti-MM activity of NPI-0052 is due to apoptosis, xenografted tumors were harvested from mice, and paraffin-embedded sections were examined for apoptosis by both immunostaining and immunoblot analysis for caspase-3 activation. Tumor sections from NPI-0052-treated mice showed increased numbers of caspase-3 positive cells compared to untreated control mice (Fig 4B). Moreover, immunoblot analysis of tumor lysates confirmed that NPI-0052 triggered caspase-3 cleavage in vivo (Fig 4C). Importantly, examination of tumor lysates from NPI-0052-treated mice showed robust cleavage of poly(ADP)-ribose polymerase (PARP), a hallmark of apoptosis (Fig 4C).
Our data therefore showed that either a single or three doses of NPI-0052 inhibit proteasome activity in MM xenografted tumors. For efficacy studies, we utilized a more frequent dosing schedule i.e., twice a week for three weeks, and we therefore next questioned whether proteasome activity is similarly affected by this dosing schedule. Tumors excised 3h after the last dose administration were examined for CT-L, C-L and T-L proteasome activity. Treatment with NPI-0052 (0.15 mg/kg), but not with vehicle alone, significantly inhibited all three proteasome activities (Fig 5A), confirming that NPI-0052-induced anti-MM activity in vivo (Fig 4) is associated with inhibition of proteasome activity in tumors.
Prior studies have shown a role for ER during bortezomib-induced apoptosis(Chauhan, et al 2008, Landowski, et al 2005, Mitsiades, et al 2002, Obeng, et al 2006). Analysis of tumor lysates from NPI-0052-treated mice showed induction of endoplasmaic stress-response, evidenced by upregulation of CHOP/GADD153, phospho-eIF2α, Bip, and Hsp70, providing in vivo evidence of ER stress-related signaling during NPI-0052-triggered cytotoxicity (Fig 5B).
Various prior studies have linked proteasome inhibition with alterations in nuclear factor-kappa B (NF-κB) (Adams, et al 1999, Chauhan, et al 2005, Mitsiades, et al 2002). We next examined whether NPI-0052-triggered proteasome inhibition in vivo is associated with changes in NF-κB. Analysis of tumor versus normal tissue (kidney) lysates from NPI-0052-treated mice showed stabilization of IκBα levels (Fig 5C). Importantly, the IκBα levels return to baseline in normal tissue, but not tumors, after 24h treatment with NPI-0052-the kinetics which correlates with recovery of proteasome activity in normal tissue vs. tumors (Fig 2). Together, these data provide evidence for a modulation of a proteasome substrate NF-κB by NPI-0052 in vivo.
Besides proteasome inhibition and induction of ER-Stress signaling, anti-angiogenic activity of NPI-0052 may also contribute to its anti-MM activity in vivo. We therefore next evaluated paraffin-embedded sections of xenografted tumors harvested from NPI-0052-treated and control mice for Factor VIII staining, a marker of angiogenesis. As seen in Fig 5D, NPI-0052 dramatically decreased the number of Factor VIII-positive cells compared to treatment with vehicle alone.
Discussion
Bortezomib (Velcade™) therapy has proven successful for the treatment of relapsed/refractory, relapsed and newly diagnosed multiple myeloma (MM)(Hideshima, et al 2001, Orlowski, et al 2002, Richardson, et al 2003, Richardson, et al 2005, San Miguel, et al 2008); however, prolonged treatment can be associated with toxicity and development of drug-resistance(Lonial, et al 2005, Richardson, et al 2006, Richardson, et al 2007). Our recent study showed that the novel proteasome inhibitor NPI-0052(Feling, et al 2003) induces apoptosis in MM cells resistant to conventional and bortezomib therapies(Chauhan, et al 2005). The mechanism whereby NPI-0052 overcomes bortezomib-resistance includes the ability to block all three proteasome activities (CT-L, T-L, and C-L) compared to bortezomib which predominantly inhibits CT-L activity. Additionally, NPI-0052-induced MM cell apoptosis is more dependent on caspase-8- than caspase-9-mediated signaling whereas, bortezomib-triggered cell death requires both caspase-8 and caspase-9 signaling pathways. Importantly, in contrast to bortezomib, NPI-0052-mediated proteasome inhibition is irreversible as evident in PWB but rapidly recovers in normal tissues including PBMC (Chauhan, et al 2005). These distinctions between NPI-0052 and bortezomib, in part, may account for the ability of NPI-0052 to overcome bortezomib-resistance. NPI-0052 is orally active (Chauhan, et al 2005). In murine tumor model studies, NPI-0052 is well tolerated and prolongs survival, with significantly reduced tumor recurrence. These findings provided the basis for an ongoing phase-1 clinical trial in MM. In the present study, we utilized our human MM xenograft mouse model to study the pharmacodynamic profile of NPI-0052, assessed by alterations in the proteasome activities as well as distribution of 3H-NPI-0052 in various tissues.
We show that NPI-0052 rapidly distributes from the vascular compartment to various organs and tumors, evident by inhibition of CT-L, C-L, and T-L proteasome activities. These findings are consistent with prior studies(Chauhan, et al 2005, Macherla, et al 2005) demonstrating the ability of NPI-0052 to target all three 20S proteasome activities. Of note, the kinetics of proteasome inhibition varied between tumors and other tissues. For example, the onset of NPI-0052-induced proteasome inhibition is rapid (within 10 min) in most tissues other than tumor, which occurs at 1h and is maximal at 24h. Intravenous injection of either single or three doses of NPI-0052 (0.15 mg/kg) inhibits proteasome activities in peripheral organs, whereas it does not inhibit proteasome activity in the brain, suggesting that NPI-0052 does not cross the blood-brain barrier at this dose and schedule, which is supported by the QWBA study in rats. Analytical studies demonstrated that after i.v injection, 3H-NPI-0052 rapidly enters the tumor as the parent compound and is present in concentrations consistent with proteasome inhibition and tumor cell death. Following i.v administration the distribution profile of 3H-NPI-0052 in rats appeared similar to that in MM.1S-tumor bearing mice. Previous studies using bortezomib have also shown rapid clearance and broad tissue distribution in a prostate xenograft tumor model(Adams, et al 1999).
Our data show that the duration of proteasome inhibition is more sustained in tumors and PWB (>24h) than in other tissues. Our prior report showed that a single dose of NPI-0052 (0.15 mg/kg) in rats led to significant proteasome inhibition in PWB, which slowly recovered by Day 7(Chauhan, et al 2005). This may be due to the irreversible covalent binding of NPI-0052 to active proteolytic sites and/or the inability of erythrocytes to generate new proteasomes. Studies using the reversible inhibitor bortezomib showed that proteasome activity recovers after 48h in blood(Chauhan, et al 2005), suggesting that reversible nature of proteasome inhibitor plays a role in proteasome activity recovery in blood.
In tumors, NPI-0052 inhibited all three proteasome activities by 24h after a single treatment (60% for CT-L; 24% for T-L, and 49% for C-L activity); moreover, administration of three doses enhanced inhibition of proteasome activities by 24h (83% for CT-L; 70% for C-L; and 70% for T-L). As noted above, sustained inhibition of proteasome activity may be due to the irreversible property of NPI-0052; however, a longer half-life of the proteasome in tumors versus normal tissues remains possible. Interestingly, NPI-0052-triggered inhibition of proteasome activity in liver, spleen, kidney, and lungs clearly recovers by 24h, suggesting that de novo and rapid proteasome synthesis may account for the recovery of proteasome activity in these tissues.
Our efficacy studies show that i.v injection of 0.15 mg/dose (0.45 mg/m2) given twice weekly for three weeks significantly reduces tumor growth, and is well tolerated. Importantly, this potent in vivo anti-MM activity of NPI-0052 was associated with significant inhibition of CT-L, C-L, and T-L proteasome activities (60-80% inhibition), suggesting that frequent dosing of NPI-0052 allows for sustained proteasome inhibition. Furthermore, these in vivo findings, coupled with our previously published in vitro data showing minimal toxicity of NPI-0052 against normal cells(Chauhan, et al 2005), confirm that MM cells are more sensitive to proteasome inhibition than normal cells.
Although the primary target for NPI-0052 is the proteasome, our in vitro studies showed that NPI-0052-induced apoptosis in MM cells is associated with loss of mitochondrial membrane potential, increase in O2- production, release of cytochrome-c/Smac, and activation of caspases–8, -9 and –3(Chauhan, et al 2005). Biochemical and genetic evidence showed that NPI-0052, in contrast to bortezomib, relies more on FADD-caspase-8-mediated cell death signaling pathway(Chauhan, et al 2005). In agreement with these in vitro results, IHC staining of xenografted tumors from NPI-0052-treated mice showed robust PARP cleavage and caspase-3 activation. Importantly, in the present study, we also show that NPI-0052 upregulated expresson of ER-stress related proteins (CHOP/GADD153, phospho-eIF2α, Bip), and Hsp70 in tumors, providing in vivo evidence for the role of ER signaling during NPI-0052-triggered cytotoxicity. Finally, results from the Factor VIII immunostaining of tumor sections confirmed the anti-angiogenic activity of NPI-0052 associated with anti-MM activity of NPI-0052 in vivo.
Collectively, our findings represent the first attempt to correlate the pharmacodynamic effects of NPI-0052 with its cytotoxic effects against MM in vivo in tumor and normal tissues. Importantly, NPI-0052 (i.v) is well tolerated at the dose and schedule examined. NPI-0052 blocks all three proteasome activities (CT-L, C-L, and T-L) by greater than 70% in most tissues without significant toxicity. Early results from a phase-1 clinical trial of NPI-0052 in patients with relapsed and relapsed/refractory MM have been reported to demonstrate potential clinical benefit at doses which are well tolerated(Richardson, et al 2008). Proteasome inhibition in PWB of treated patients showed a drug-dependent CT-L inhibition, with inhibition up to 28% observed at 0.025 mg/m2 dose. Complete inhibition of CT-L proteasome activity has been observed at MTD doses (0.7 mg/m2) in other clinical trials, without evidence of neuropathy or myelosuppression. In MM, dose escalation and scheduling effects are currently ongoing to define the dose-limiting toxicity and MTD, and in order to define a recommended phase-2 dose (RP2D) for evaluation in patients with advanced MM.
Acknowledgments
Grant support: This investigation was supported by NIH grants SPORE-P50100707, PO1-CA078378, and RO1CA050947; and The LeBow Family Fund to Cure Myeloma.
Footnotes
Authors' contributions and disclosure of Conflicts of Interest: DC designed research, analyzed data, and wrote the manuscript; AVS designed research, performed experiments and interpreted data; MP and KL provided H3-NPI-0052 and QWBA study data, and KCA analyzed data and wrote the manuscript. Disclosure-MP, GKL, and BP: Employees of Nereus Pharmaceuticals, Inc. DC and KC: Consultant to Nereus Pharmaceuticals, Inc.
References
- Adams J. The proteasome: a suitable antineoplastic target. Nat Rev Cancer. 2004;4:349–360. doi: 10.1038/nrc1361. [DOI] [PubMed] [Google Scholar]
- Adams J, Palombella VJ, Sausville EA, Johnson J, Destree A, Lazarus DD, Maas J, Pien CS, Prakash S, Elliott PJ. Proteasome inhibitors: a novel class of potent and effective antitumor agents. Cancer Res. 1999;59:2615–2622. [PubMed] [Google Scholar]
- Arendt C, Hochstrasser M. Identification of the yeast 20S proteasome catalytic centers and subunit interactions required for active-site formation. Proc Natl Acad Sci U S A. 1997;94:7156–7161. doi: 10.1073/pnas.94.14.7156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrigo AP, Tanaka K, Goldberg AL, Welch WJ. Identity of the 19S ‘prosome’ particle with the large multifunctional protease complex of mammalian cells (the proteasome) Nature. 1988;331:192–194. doi: 10.1038/331192a0. [DOI] [PubMed] [Google Scholar]
- Chauhan D, Catley L, Li G, Podar K, Hideshima T, Velankar M, Mitsiades C, Mitsiades N, Yasui H, Letai A, Ovaa H, Berkers C, Nicholson B, Chao TH, Neuteboom ST, Richardson P, Palladino MA, Anderson KC. A novel orally active proteasome inhibitor induces apoptosis in multiple myeloma cells with mechanisms distinct from Bortezomib. Cancer Cell. 2005;8:407–419. doi: 10.1016/j.ccr.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Chauhan D, Singh A, Brahmandam M, Podar K, Hideshima T, Richardson P, Munshi N, Palladino MA, Anderson KC. Combination of proteasome inhibitors bortezomib and NPI-0052 trigger in vivo synergistic cytotoxicity in multiple myeloma. Blood. 2008;111:1654–1664. doi: 10.1182/blood-2007-08-105601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciechanover A, Elias S, Heller H, Ferber S, Hershko A. Characterization of the heat-stable polypeptide of the ATP-dependent proteolytic system from reticulocytes. J Biol Chem. 1980a;255:7525–7528. [PubMed] [Google Scholar]
- Ciechanover A, Finley D, Varshavsky A. The ubiquitin-mediated proteolytic pathway and mechanisms of energy-dependent intracellular protein degradation. J Cell Biochem. 1984;24:27–53. doi: 10.1002/jcb.240240104. [DOI] [PubMed] [Google Scholar]
- Ciechanover A, Heller H, Elias S, Haas AL, Hershko A. ATP-dependent conjugation of reticulocyte proteins with the polypeptide required for protein degradation. Proc Natl Acad Sci U S A. 1980b;77:1365–1368. doi: 10.1073/pnas.77.3.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciehanover A, Hod Y, Hershko A. A heat-stable polypeptide component of an ATP-dependent proteolytic system from reticulocytes. Biochem Biophys Res Commun. 1978;81:1100–1105. doi: 10.1016/0006-291x(78)91249-4. [DOI] [PubMed] [Google Scholar]
- Feling RH, Buchanan GO, Mincer TJ, Kauffman CA, Jensen PR, Fenical W. Salinosporamide A: a highly cytotoxic proteasome inhibitor from a novel microbial source, a marine bacterium of the new genus salinospora. Angew Chem Int Ed Engl. 2003;42:355–357. doi: 10.1002/anie.200390115. [DOI] [PubMed] [Google Scholar]
- Ganoth D, Leshinsky E, Eytan E, Hershko A. A multicomponent system that degrades proteins conjugated to ubiquitin. Resolution of factors and evidence for ATP-dependent complex formation. J Biol Chem. 1988;263:12412–12419. [PubMed] [Google Scholar]
- Goldberg AL. Protein degradation and protection against misfolded or damaged proteins. Nature. 2003;426:895–899. doi: 10.1038/nature02263. [DOI] [PubMed] [Google Scholar]
- Hershko A, Ciechanover A, Heller H, Haas AL, Rose IA. Proposed role of ATP in protein breakdown: conjugation of protein with multiple chains of the polypeptide of ATP-dependent proteolysis. Proc Natl Acad Sci U S A. 1980;77:1783–1786. doi: 10.1073/pnas.77.4.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hideshima T, Richardson P, Chauhan D, Palombella VJ, Elliott PJ, Adams J, Anderson KC. The proteasome inhibitor PS-341 inhibits growth, induces apoptosis, and overcomes drug resistance in human multiple myeloma cells. Cancer Res. 2001;61:3071–3076. [PubMed] [Google Scholar]
- Hough R, Pratt G, Rechsteiner M. Purification of two high molecular weight proteases from rabbit reticulocyte lysate. J Biol Chem. 1987;262:8303–8313. [PubMed] [Google Scholar]
- Kane RC, Bross PF, Farrell AT, Pazdur R. Velcade: U.S. FDA approval for the treatment of multiple myeloma progressing on prior therapy. Oncologist. 2003;8:508–513. doi: 10.1634/theoncologist.8-6-508. [DOI] [PubMed] [Google Scholar]
- Landowski TH, Megli CJ, Nullmeyer KD, Lynch RM, Dorr RT. Mitochondrial-mediated disregulation of Ca2+ is a critical determinant of Velcade (PS-341/bortezomib) cytotoxicity in myeloma cell lines. Cancer Res. 2005;65:3828–3836. doi: 10.1158/0008-5472.CAN-04-3684. [DOI] [PubMed] [Google Scholar]
- Lightcap ES, McCormack TA, Pien CS, Chau V, Adams J, Elliott PJ. Proteasome inhibition measurements: clinical application. Clin Chem. 2000;46:673–683. [PubMed] [Google Scholar]
- Lonial S, Waller EK, Richardson PG, Jagannath S, Orlowski RZ, Giver CR, Jaye DL, Francis D, Giusti S, Torre C, Barlogie B, Berenson JR, Singhal S, Schenkein DP, Esseltine DL, Anderson J, Xiao H, Heffner LT, Anderson KC. Risk factors and kinetics of thrombocytopenia associated with bortezomib for relapsed, refractory multiple myeloma. Blood. 2005;106:3777–3784. doi: 10.1182/blood-2005-03-1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macherla VR, Mitchell SS, Manam RR, Reed KA, Chao TH, Nicholson B, Deyanat-Yazdi G, Mai B, Jensen PR, Fenical WF, Neuteboom ST, Lam KS, Palladino MA, Potts BC. Structure-activity relationship studies of salinosporamide A (NPI-0052), a novel marine derived proteasome inhibitor. J Med Chem. 2005;48:3684–3687. doi: 10.1021/jm048995+. [DOI] [PubMed] [Google Scholar]
- Mitsiades N, Mitsiades CS, Poulaki V, Chauhan D, Fanourakis G, Gu X, Bailey C, Joseph M, Libermann TA, Treon SP, Munshi NC, Richardson PG, Hideshima T, Anderson KC. Molecular sequelae of proteasome inhibition in human multiple myeloma cells. Proc Natl Acad Sci U S A. 2002;99:14374–14379. doi: 10.1073/pnas.202445099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obeng EA, Carlson LM, Gutman DM, Harrington WJ, Jr, Lee KP, Boise LH. Proteasome inhibitors induce a terminal unfolded protein response in multiple myeloma cells. Blood. 2006;107:4907–4916. doi: 10.1182/blood-2005-08-3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlowski RZ, Stinchcombe TE, Mitchell BS, Shea TC, Baldwin AS, Stahl S, Adams J, Esseltine DL, Elliott PJ, Pien CS, Guerciolini R, Anderson JK, Depcik-Smith ND, Bhagat R, Lehman MJ, Novick SC, O'Connor OA, Soignet SL. Phase I trial of the proteasome inhibitor PS-341 in patients with refractory hematologic malignancies. J Clin Oncol. 2002;20:4420–4427. doi: 10.1200/JCO.2002.01.133. [DOI] [PubMed] [Google Scholar]
- Peters J, Franke W, Kleinschmidt J. Distinct 19 S and 20 S subcomplexes of the 26 S proteasome and their distribution in the nucleus and the cytoplasm. J Biol Chem. 1994;269:7709–7718. [PubMed] [Google Scholar]
- Richardson P, Hofmeister C, Zimmerman T, Chanan-Khan A, Spear M, Palladino MA, Longenecker A, Cropp G, Lloyd KG, Wear S, Hannah AL, Anderson K. Phase 1 Clinical Trial of NPI-0052, a Novel Proteasome Inhibitor in Patients with Multiple Myeloma. Blood (ASH Annual Meeting Abstracts) 2008;2770 [Google Scholar]
- Richardson PG, Barlogie B, Berenson J, Singhal S, Jagannath S, Irwin D, Rajkumar SV, Srkalovic G, Alsina M, Alexanian R, Siegel D, Orlowski RZ, Kuter D, Limentani SA, Lee S, Hideshima T, Esseltine DL, Kauffman M, Adams J, Schenkein DP, Anderson KC. A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med. 2003;348:2609–2617. doi: 10.1056/NEJMoa030288. [DOI] [PubMed] [Google Scholar]
- Richardson PG, Briemberg H, Jagannath S, Wen PY, Barlogie B, Berenson J, Singhal S, Siegel DS, Irwin D, Schuster M, Srkalovic G, Alexanian R, Rajkumar SV, Limentani S, Alsina M, Orlowski RZ, Najarian K, Esseltine D, Anderson KC, Amato AA. Frequency, characteristics, and reversibility of peripheral neuropathy during treatment of advanced multiple myeloma with bortezomib. J Clin Oncol. 2006;24:3113–3120. doi: 10.1200/JCO.2005.04.7779. [DOI] [PubMed] [Google Scholar]
- Richardson PG, Sonneveld P, Schuster MW, Irwin D, Stadtmauer EA, Facon T, Harousseau JL, Ben-Yehuda D, Lonial S, Goldschmidt H, Reece D, San-Miguel JF, Blade J, Boccadoro M, Cavenagh J, Dalton WS, Boral AL, Esseltine DL, Porter JB, Schenkein D, Anderson KC. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med. 2005;352:2487–2498. doi: 10.1056/NEJMoa043445. [DOI] [PubMed] [Google Scholar]
- Richardson PG, Sonneveld P, Schuster MW, Irwin D, Stadtmauer EA, Facon T, Harousseau JL, Ben-Yehuda D, Lonial S, San Miguel JF, Cavenagh JD, Anderson KC. Safety and efficacy of bortezomib in high-risk and elderly patients with relapsed multiple myeloma. Br J Haematol. 2007;137:429–435. doi: 10.1111/j.1365-2141.2007.06585.x. [DOI] [PubMed] [Google Scholar]
- San Miguel JF, Schlag R, Khuageva NK, Dimopoulos MA, Shpilberg O, Kropff M, Spicka I, Petrucci MT, Palumbo A, Samoilova OS, Dmoszynska A, Abdulkadyrov KM, Schots R, Jiang B, Mateos MV, Anderson KC, Esseltine DL, Liu K, Cakana A, van de Velde H, Richardson PG. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med. 2008;359:906–917. doi: 10.1056/NEJMoa0801479. [DOI] [PubMed] [Google Scholar]
- Waxman L, Fagan JM, Goldberg AL. Demonstration of two distinct high molecular weight proteases in rabbit reticulocytes, one of which degrades ubiquitin conjugates. J Biol Chem. 1987;262:2451–2457. [PubMed] [Google Scholar]
- Wilk S, Orlowski M. Evidence that pituitary cation-sensitive neutral endopeptidase is a multicatalytic protease complex. J Neurochem. 1983;40:842–849. doi: 10.1111/j.1471-4159.1983.tb08056.x. [DOI] [PubMed] [Google Scholar]