Abstract
Recent studies have demonstrated that genetically modified hematopoietic stem cells (HSCs) can reduce HIV viremia. We have developed an HIV/AIDS-patient model in Simian/human immunodeficiency virus (SHIV)-infected pigtailed macaques that are stably suppressed on antiretroviral therapy (ART: raltegravir, emtricitabine and tenofovir). Following SHIV infection and ART, animals undergo autologous HSC transplantation (HSCT) with lentivirally transduced cluster of differentiation (CD)34+ cells expressing the mC46 anti-HIV fusion protein. We show that SHIV+, ART-treated animals had very low gene marking levels after HSCT. Pretransduction CD34+ cells contained detectable levels of all three ART drugs, likely contributing to the low gene transfer efficiency. Following HSCT recovery and the cessation of ART, plasma viremia rebounded, indicating that myeloablative total body irradiation cannot completely eliminate viral reservoirs after autologous HSCT. The kinetics of recovery following autologous HSCT in SHIV+, ART-treated macaques paralleled those observed following transplantation of control animals. However, T-cell subset analyses demonstrated a high percentage of C-C chemokine receptor 5 (CCR5)-expressing CD4+ T-cells after HSCT. These data suggest that an extended ART interruption time may be required for more efficient lentiviral transduction. To avoid complications associated with ART interruption in the context of high percentages of CD4+CCR5+T-cells after HSCT, the use of vector systems not impaired by the presence of residual ART may also be beneficial.
Introduction
Antiretroviral therapy (ART) has led to a significant decrease in AIDS-related cancers1; however, the incidence of non-Hodgkin lymphoma remains high, whereas an increase in non-AIDS-related cancers including Hodgkin lymphoma has been associated with high mortality of HIV-1-infected patients receiving ART.2,3 Recent studies demonstrating that HIV+ cancer patients are less likely to receive treatment make clear that this population is in need of more effective strategies to combat both infection and malignancy.4 Autologous hematopoietic stem cell transplantation (HSCT) has been used as a curative strategy for HIV-1-infected patients suffering from non-Hodgkin lymphoma and Hodgkin lymphoma for more than two decades.5,6 To date, only a limited number of studies have specifically examined the putative therapeutic benefit of conducting autologous HSCT in the context of reducing viral reservoirs in HIV-1-infected patients.7,8,9 Notably, although autologous HSCT has been the standard approach for treating HIV-1-infected patient with non-Hodgkin lymphoma and Hodgkin lymphoma, the elimination of viral reservoirs has yet to be reported.10
The potential to eliminate viral reservoirs following autologous transplantation of genetically modified hematopoietic stem cells (HSCs) has garnered a renewed optimism for the development of a curative strategy for HIV/AIDS.11,12 Allogeneic HSCT from a C-C chemokine receptor (CCR)5Δ32 donor to a HIV-1-infected patient with acute myelogenous leukemia was shown to induce a functional cure.7,10,13 It remains unclear what aspects of acute myelogenous leukemia treatment resulted in the complete elimination of replication competent virus in this patient. In a recent follow-up study examining two HIV-infected patients previously thought to have been cured of HIV following allogeneic HSCT from a wild-type CCR5 donor, viral rebound was observed 3 and 8 months, respectively, following ART interruption.14 These findings are a clear indication that the development of strategies to protect HSC-derived immune cells from further cycles of viral replication is a requirement for HSCT to lead to a functional cure.
Multiple approaches have been developed to genetically modify HIV-1 target cells (e.g., cluster of differentiation (CD)4+ T-cells) or self-renewing HSCs (e.g., CD34+ HSCs), which give rise to multiple infection-susceptible cell types.15,16 To date, several phase 1/2 clinical trials have been conducted in HIV-1-infected patients following infusion of genetically modified CD4+ T-cells or CD34+ HSCs (reviewed in ref. 17). For the most part, these studies have examined safety and feasibility of genetically modifying target cells prior to autologous transplantation; efficacy studies examining potential therapeutic benefits following the infusion of genetically modified cells have been limited, in part due to limited gene transfer.
Lentiviral vectors have emerged as an especially promising strategy to safely and easily modify HSCs.18 HIV-1-derived vector engineering is straightforward and widely practiced.19 In addition, the safety of lentiviral vectors is improved relative to previous generations of retroviral gene therapy vectors. Whereas gammaretroviral vectors are known to prefer insertion at genomic sites associated with oncogenesis, integration patterns of lentiviral vectors have demonstrated significantly less risk of oncogenic transformation.20,21 Alternative platforms, such as foamy virus,22 have also shown early promise in vitro and in vivo.23,24 Regardless of the specific format utilized, retrovirus-mediated gene therapy remains the best characterized and therefore, most translationally relevant strategy for HIV-1 remission/cure.
Our previous studies demonstrated that pigtailed macaques transplanted with gene-modified, autologous CD34+ HSCs maintain high levels of genetically modified CD4+ T-cells expressing the membrane bound HIV-1 fusion inhibitor, mC46, following challenge with an HIV-1 enveloped Simian/human immunodeficiency virus (SHIV).25 The primary goals of this study were to demonstrate the feasibilities of (i) autologous transplant in SHIV-infected pigtailed macaques on uninterrupted ART, and (ii) lentivirus-mediated gene modification strategies in this setting. Because HSCT and protected CD4+ T-cells are likely to significantly affect infection dynamics, we further sought to measure the effects of myeloablative total body irradiation (TBI) and infusion of mC46-expressing HSCs, on anti-SHIV immune responses, plasma viremia, and disease progression.
Results
HSCT following myeloablative irradiation does not eliminate viral reservoirs in ART-suppressed, SHIV-infected pigtailed macaques.
We sought to assess the effects of autologous HSCT in ART suppressed, SHIV-infected animals, and examine the feasibility and therapeutic benefit of conducting HSCT following lentiviral-mediated gene modification of CD34+ HSCs from animals that were maintained continuously on ART. Three cohorts of animals are outlined in Figure 1a. The first two pairs of pigtailed macaques (Cohorts 1 and 2), were transplanted while maintained on continuous ART throughout the transplant protocol; other than a 3-week staggering of HSCT, Cohorts 1 and 2 were identical. A third cohort utilized an extended ART washout period during transplant. Following engraftment in all three cohorts, ART was withheld and macaques were monitored for CD4+ T-cell depletion and viral rebound.
Figure 1.
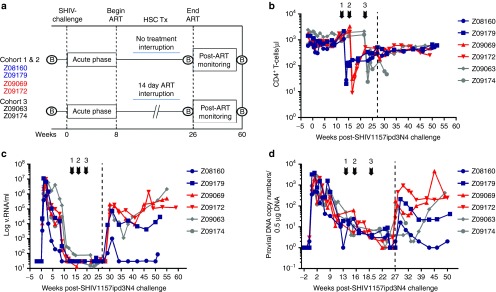
Effects of irradiation on CD4+ T-cells and plasma viremia. (a) Schematic outline of studies. Following baseline sample collections and 8 weeks following SHIV1157ipd3N4 challenge (“Acute phase”), macaques were placed on daily ART consisting of TFV, FTC and twice daily RAL (see Materials and Methods). Hematopoietic stem cell transplant (“HSC Tx”) was performed after stable suppression of plasma viremia was achieved. Following transplant recovery and cessation of ART, macaques were monitored for a period of ~6 months. A study time line for SHIV-naive control animals that underwent HSCT is also displayed. GI and lymph node biopsies (“B”) were collected at the indicated time points. (b) Total CD4+CD3+ T-cell levels were assessed by flow cytometry at the indicated time points over the course of these studies following SHIV1157ipd3N4 challenge and prior to and following HSCT. (c) Plasma viremia and (d) proviral DNA contents were determined by real-time PCR as indicated in the Methods section. Arrows indicate HSCT and dotted line indicates end of ART.
Preinfection CD4+ T-cell levels averaging 1,064 cells/μl (range 678–1,967) were observed in Cohorts 1–3 (Figure 1b). By week 8 following SHIV-1157ipd3N4 challenge, an average of 932 cells/μl (range 574–1,872) CD4+ T-cells was detected in peripheral blood equating to a decrease of ~12.4%. All animals were placed on ART consisting of daily PMPA, FTC, and twice daily RAL (see Materials and Methods for additional details). Each cohort, consisting of one control (transplanted with a lentiviral vector expressing green fluorescent protein (GFP)) and one experimental (lentiviral vector expressing GFP and mC46) underwent HSCT, including myeloablative TBI prior to cell infusion. Following TBI, CD4+ T-cell levels dropped to an average of 22 cells/μl (range 8–51). Animals were allowed to recover from HSCT prior to ART withdrawal; 2 weeks following the cessation of ART, plasma viremia rebounded to levels similar to pre-ART levels (1.2 × 105 cells/μl versus 1.1 × 105 cells/μl) in all but one macaque (Z08160, Cohort 1) that appeared to exhibit a natural controller phenotype prior to ART (Figure 1c). Similarly, cell-associated SHIV DNA levels initially decreased below the lower limit of detection of our assay following HSCT, but rebounded within the first few weeks following the end of ART (Figure 1d). Although plasma viremia and viral DNA content rebounded in the weeks following the end of ART, CD4+ T-cell levels continued to rise in the 6 months following the end of ART, averaging 488 cells/μl (Figure 1b). Our observations following autologous HSCT of ART-treated, SHIV-infected macaques strongly parallel to those found in other nonhuman primate studies,26 and in HIV-1-infected patients,27 suggesting that this animal model is an excellent surrogate for preclinical HSCT-based curative therapies for HIV/AIDS.
Effects of autologous transplantation on T-lymphocyte subsets
We did not observe measurable impairment in myeloid or lymphoid recovery following HSCT in our SHIV-infected animals, relative to our historical SHIV- and ART-naive control animals (see Supplementary Figures S1 and S2 and refs. 28,29). To examine the effects of autologous transplantation on T-lymphocytes subsets, we measured alterations in phenotypic and activation markers by flow cytometry. In the first 8 weeks postinfection, peripheral CD4+CCR5+ T-cells were reduced concordant with their low percentage in peripheral blood mononuclear cells (PBMCs) (Figure 2a). CD4+CCR5+ T-cell depletion was also observed in gut-associated-lymphoid tissue, but not in axillary lymph nodes (Figure 3). Interestingly, we observed a marked increase in CD4+CCR5+ T-cells following autologous transplant (38% versus ~5% preinfection, Figure 2a). Similar to findings in peripheral blood, the percentage of CD4+CCR5+ T-cells in axillary lymph nodes and gut-associated lymphoid tissue was highest in the weeks following HSCT. Interestingly, CD4+CCR5+ levels in the periphery dropped by ~50% following cessation of ART and virus rebound, whereas levels in lymph node were minimally changed, and a slight increase was observed in gut-associated lymphoid tissue (Figures 2a and 3).
Figure 2.
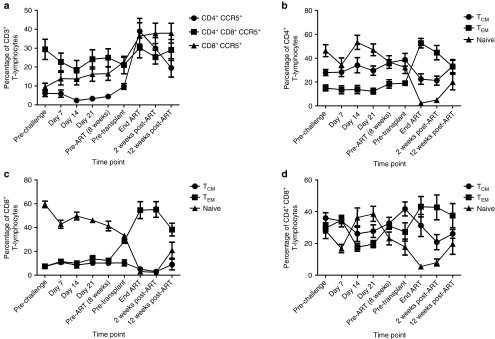
Phenotypic analysis of peripheral T-lymphocytes following SHIV-challenge. (a) The percentage of CD4+, CD4+CD8+, and CD8+ T-cells expressing CCR5+ was determined by flow cytometry throughout the course of these studies. (b–d) The percentage of central memory (TCM; CCR7+CD45RA–), effector memory (TEM; CCR7–CD45RA–), and naive cells (CCR7+CD45RA+) was determined in (b) CD4+CD3+, (c) CD8+CD3+, and (d) CD4+CD8+CD3+ T-cells.
Figure 3.
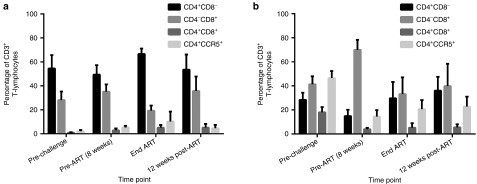
T-lymphocyte subset analysis in lymph node and GI biopsies. Single-cell suspensions were analyzed by flow cytometry to determine the percentage of CD4+ T-cells, CD8+ T-cells, double positive T-cells, and CD4+CCR5+ T-cells in (a) lymph nodes and (b) GI biopsies.
We next analyzed central memory, effector memory, and naive T-cell subsets following SHIV infection and autologous HSCT in CD4+ (Figure 2b), CD8+ (Figure 2c), and CD4+CD8+ double positive T-lymphocytes (Figure 2d). Peripheral blood CD4+ central memory T-cells (TCM: CD3+CD4+CCR7+CD45RA–) did not decline, as compared to minimal to modest declines observed in CD8+ and CD4+CD8+ TCM (Figure 2b–d). Peripheral blood effector memory cells (TEM: CD3+CD4+CCR7–CD45RA–) increased significantly following transplant (Figure 2b–d). Naive T-cell levels were reduced following infection, and more so following transplant (Figure 2b–d). In summary, our T-cell subset measurements in peripheral blood suggest preferential depletion of naive cells, and increase in effector memory cells, following transplant.
Immunological analysis
To determine the relative change in activation status of specific T-lymphocyte subsets following transplant in our animals, we measured Ki67+ and PD-1+ CD4+ T-cells, and also measured changes in the frequency of regulatory T-cells (Tregs). Examination of CD4+Ki67+ T-cells suggested that activation of CD4+ T-cells was relatively moderate during the initial 8 weeks following SHIV challenge, increased significantly following HSCT, and was further increased 2 weeks following the cessation of ART (see Supplementary Figure S3a). Following similar kinetics, the percentage of CD4+ T-cells expressing the immune exhaustion marker, PD-1, increased in the weeks following HSCT from ~10% at week 8 to ~64% prior to termination of ART (see Supplementary Figure S3b). Importantly, changes in the percentage of Tregs in peripheral blood did correlate with transplant (see Supplementary Figure S3c). These results suggest that productive SHIV viremia and myeloablative HSCT may play additive roles in increased T-cell activation post-transplant, in turn producing an enlarged pool of infection-susceptible target cells following withdrawal of ART.
We next examined the antibody response in SHIV1157ipd3N4-challenged macaques to gauge the ability of infected animals to generate an effective humoral response both prior to and following HSCT. Antibodies against HIV-envelope (Env) or SIV whole virus were readily detectable in all six macaques by week 3 postinfection, and plateaued at 8 weeks postinfection (see Supplementary Figure S4a–b). Neutralizing antibodies were observed in all six macaques at week 8 (Supplementary Figure S4c). Neutralization activity varied greatly, ranging from 18% to >60%. A decrease in antibody titers was observed following HSCT; however, antigen-specific antibodies remained detectable. As expected, in the weeks following cessation of ART and subsequent increase in plasma viremia, virus-specific antibodies increased by 103 enzyme immunoassay units. Our T-cell activation and B-cell functional data demonstrate that increased immune activation and anti-SHIV B-cell responses persist during hematopoietic recovery following HSCT.
Reduced transduction efficiency is observed in CD34+ HSCs isolated from ART-treated, SHIV-infected macaques
Using lentiviral transduction strategies, our previous studies indicated that gene-modified CD34+ HSCs expressing a membrane-bound fusion inhibitor, known as mC46, give rise to infection-resistant CD4+ T-cells following SHIV-challenge of pigtailed macaques.25 Although our initial findings demonstrated that HSCT in the setting of ART was safe and feasible, we further examined the efficiency of lentivirus-mediated gene transfer procedures in these SHIV-infected, ART-treated animals. We compared the efficiency of gene transfer in Cohorts 1–3 ex vivo and in vivo to our previously published results in SHIV- and ART-naive animals.25 Notably, animals in Cohort 3 underwent a 4-day interruption of ART prior to bone marrow harvest (Figure 1a). Ex vivo transduction efficiencies trended lower in Cohorts 1–3, compared to SHIV- and ART-naive controls, although this difference did not reach statistical significance (Figure 4a). However, following cell infusion into conditioned animals and hematopoietic recovery, steady state gene marking in total leukocytes was significantly impaired in Cohorts 1–3 relative to controls (Figure 4b). The ART interruption in Cohort 3 during transplant did not improve gene transfer efficiency. These results suggest that SHIV-infected, ART-treated animals display substantially lower efficiencies of lentivirus-mediated gene transfer in vivo relative to SHIV-and ART-naive controls, even when ART is temporarily interrupted during transplant.
Figure 4.
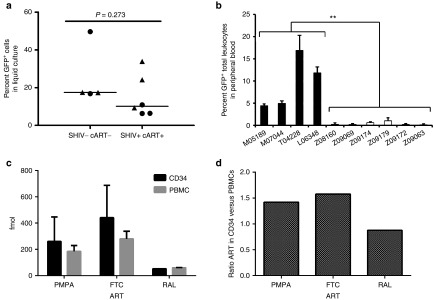
CD34+ HSCs from cART-treated animals display decreased transgene expression in vivo. (a) CD34+ HSCs isolated from four SHIV- and cART-naive macaques, and from six SHIV+, cART-treated animals (Cohorts 1–3, Figure 1) were transduced with lentiviral vectors expressing mC46 and GFP (circles) or GFP alone (triangles). The percentage of GFP+ HSCs was determined following 10 days in liquid culture. (b) Bulk transduced cells from (a) were infused into the autologous recipient. Following hematopoietic recovery (see Materials and Methods), steady state GFP expression was determined from total leukocytes. Data represent the average of multiple longitudinal blood draws from each animal plus standard deviation. (c–d) Using samples collected from animals in Cohorts 1–3 and prepared immediately prior to lentiviral transduction, the average absolute amounts of antiretroviral drugs in CD34+ HSCs and PBMCs (c) and the ratio of ART in CD34+ HSCs versus PBMCs (d) were determined by MASS-Spec/HPLC. All PBMC samples were collected in conjunction with bone marrow harvests.
ART reduces efficiency of lentivirus gene transfer ex vivo. We hypothesized that the reduced efficiency of lentiviral transduction in our SHIV-infected, ART-treated animals was due to the presence of ART in cells during transduction ex vivo, and in animals following cell infusion. To test this, we conducted experiments in cultured cell lines and primary CD34+ HSCs. As expected, our lentiviral vectors were efficiently inhibited by our ART regimen in vitro (Figure 5). A single pretransduction dose of ART significantly impaired transduction efficiencies of both HIV-based and foamy virus-based retroviral vectors. To address whether ART infection contributes to limitations on HSC transduction efficiency, CD34+ HSCs were isolated from a SHIV-infected, ART-treated macaque, and from a SHIV-naive and ART-naive macaque. Cells from the SHIV- and ART-naive animal were cultured in the presence and absence of ART, and compared to cells from the SHIV infected, ART-treated animal in PCR-based lentivirus integration assays. As shown in Figure 6, the absolute number of lentiviral copies per cell was determined by real-time PCR to be 5.5-fold less on average in samples from animals receiving ART ex vivo or in vivo. Increased 2-long tandem repeat circles (2-LTR) formation was observed 2 days following transduction in HSCs isolated from ART-treated macaques, suggesting poorer kinetics of lentiviral integration in these animals (Figure 6). In summary, ART-treated, SHIV-infected macaques displayed significantly decreased gene marking, with or without a limited treatment interruption during HSCT; low ex vivo transduction efficiencies subsequently led to minimal (<0.5%) in vivo gene modification (data not shown).
Figure 5.
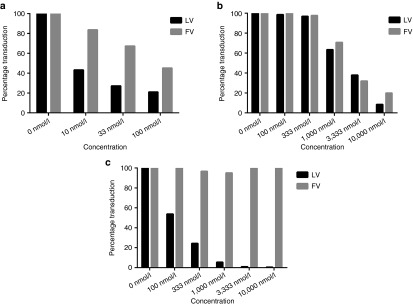
ART-mediated inhibition of lenti- and Foamy viral vectors. The effects of (a) Raltegravir, (b) PMPA and (c) FTC on lentiviral and foamy virus-based vectors was assessed. HT1080 cells were cultured in the presence of the indicated concentration of each respective antiretroviral drug. Next, transductions were performed with VSV-G pseudotyped, GFP-expressing lentiviral or foamy viral vectors. Transduction efficiencies were determined by measuring fluorescence 48 hours following transduction. Results are average of duplicate samples. VSV-G, vesicular stomatitis virus glycoprotein.
Figure 6.
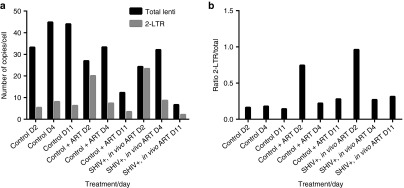
ART increases lentiviral 2-LTR circle formation in transduced macaque CD34+ cells in vitro. (a–b) CD34+ HSCs were harvested from a SHIV- and ART-naive animal, and were cultured in the absence (“Control”) or presence of ART (“Control + ART”). CD34+ HSCs were also harvested from a SHIV-infected, ART-treated animal (“SHIV+, in vivo ART”) (“SHIV+ART+). Cells were transduced with GFP lentivirus, and assayed by real-time PCR at 2, 4, and 11 days post-transduction for total lentiviral copies and 2-LTR forms of viral DNA (a) using previously described protocols (see Materials and Methods). (b) The ratio of 2-LTR circles to total viral copies was calculated. Results represent average of duplicate samples.
To understand the intracellular pharmacokinetics of our ART regimen, we directly measured ART levels in CD34+ HSCs and PBMCs from Cohorts 1–3 by MASS-spec/HPLC; measurements were made immediately prior to lentiviral transduction (Figure 4c–d). All three antiretroviral drugs were readily detectable in PBMCs, and importantly, in CD34+ HSCs. An average of 288.7 fmol PMPA, 441.4 fmol FTC, and 61.7 fmol RAL was detected in CD34+ HSCs ~16 hours following the most recent dose. Levels of PMPA, FTC, and RAL averaging 184.0, 279.6, and 59.8 fmol were detected, respectively, in PBMCs. The ratio of both PMPA (1.42) and FTC (1.58) in CD34+ HSCs versus PBMCs was slightly elevated, whereas RAL was nearly equivalent (0.9) (Figure 4d). The detection of all three ART drugs in CD34+ HSCs, at levels at or above those found in PBMCs, provides strong evidence that poor lentiviral gene transfer efficiency in our experiments is due to residual intracellular ART-mediated inhibition of lentiviral reverse transcription and/or integration.
Discussion
Although autologous HSCT has been performed routinely for HIV-1-infected patients with hematologic malignancies, large animal studies that model gene-modified, HSCT-based HIV-1 cure strategies are in urgent need. Here, we have demonstrated that SHIV-infected pigtailed macaques receiving ART can be successfully transplanted with genetically modified CD34+ HSCs. We have shown that similar to HIV-1-infected patients, autologous transplantation on its own is insufficient to induce functional cure: following treatment interruption, plasma viremia returned to pre-ART levels. Our extensive immunological analysis has demonstrated that while CD4+ T-cell counts are massively decreased following HSCT, responses that favor nascent infections arise due to heightened immune activation and massive hematopoietic recovery following myeloablative irradiation. This finding is particularly concerning if temporary discontinuation of ART becomes important to increase transduction efficiencies.
Limited transduction efficiency of CD34+ HSCs from SHIV-infected, ART-suppressed animals resulted in subpar gene-marking in the weeks following infusion of genetically modified cells. As we have shown earlier, the inclusion of an in vivo chemoselection cassette will likely increase the percentage of genetically modified cells.23,30 However, because of the low-level in vivo marking, it was deemed that multiple rounds of chemoselection would be required to achieve theoretical levels needed to assess gene modified CD34+ HSC transplantation as a curative therapy for HIV/AIDS. Hence, these studies represent the basal levels of gene marking attainable in the presence of ART when utilizing lentiviral vectors to genetically modify CD34+ HSC. Based on our earlier experience, initial marking levels ranging from 15 to 25% are typically expected.24 Our future studies will explore methods to improve the stability of genetically modified cells following infusion into SHIV-infected, ART-treated animals.
The transduction efficiencies achieved in these studies are similar to those previously observed in phase 1/2 clinical trials in HIV-1-infected patients.9,17 The majority, if not all clinical trials, conducted to date have utilized integrating viral vectors. As shown in these studies, lentiviral and gammaretroviral vectors are susceptible to inhibition by residual ART in CD34+ HSCs (see Supplementary Figure S1). Hence, to successfully achieve putative therapeutic thresholds of genetically modified immune cells in vivo, extended ART washout periods or modified protocols to achieve viral integration may be needed. Alternatively, it may be feasible to maintain patients on ART by temporarily substituting drug cocktails from those that inhibit reverse transcriptase and integrase to those that inhibit early in the infection (e.g., fusion inhibitors) and later stages of the viral life-cycle (e.g., protease inhibitors). In addition, switching to antiretroviral drugs with short half-lives in the weeks preceding HSCT may reduce and/or eliminate the requirement for a washout period.
Previous studies have indicated that both infection and/or the proliferative state of CD4+ T-cells alters the kinetic half-lives of antiretroviral drugs.31 Hence, SHIV-infection may alter the turnover rate of antiretroviral drugs in CD34+ HSCs while the minimal expansion observed during a 48-hour ex vivo transduction period may parallel findings in resting CD4+ T-cells, in which ART turnover is reduced.32 Previous findings have demonstrated estimated half lives of >39 hours for FTC, >60 hours for PMPA, and >9 hours for RAL in PBMCs33,34; however, to our knowledge this is the first study to examine intracellular ART concentrations in CD34+ HSCs isolated from ART-treated, SHIV-infected pigtailed macaques. Our findings strongly suggest that antiretroviral drugs targeting the early, postentry stages of the viral life-cycle (e.g., integrase, reverse transcriptase) will impair lentiviral-mediated transduction.
We hypothesized that an alternative vector platform might allow us to circumvent ART-dependent limits on gene transfer efficiency. Therefore, we evaluated the ART-dependent inhibition of foamy virus vectors, in comparison to HIV-1-based lentiviral vectors. In cell line-based in vitro assays, we found that foamy viral vectors were inhibited comparably to lentiviral vectors in the presence of two of our three ART drugs, PMPA and Raltegravir (Figure 5). Interestingly, our third ART component, FTC, was able to inhibit lentiviral vectors, but not foamy virus vectors. These results highlight the potential for alternate ART regimens to be selected not only on the basis of short intracellular half life, but also on the basis of specificity for inhibition of lentiviral species like HIV-1.
Other vector-based strategies may enable delivery of antiviral transgenes without the need to alter a patient's ART regimen. For example, natural occurring mutations that have previously been identified in ART-resistant HIV-1 clones may be introduced into the Gag-Pol packaging vector, which may enable the required single-round integration to occur. Retroviral-based genetic modification strategies may be circumvented entirely through the use of other strategies such as gene disruption using sequence specific nucleases, which are not limited by the presence of antiretroviral drugs (provided that the nucleases are not delivered by an ART susceptible viral vector). For example, CXCR4 and/or CCR5 targeting zinc-finger nucleases have been shown to yield a high percentage of gene disruption in primary CD4+ T-cells following transduction with adenoviral vectors.35,36
We monitored post-transplant recovery of lymphoid (see Supplementary Figure S1) and myeloid cell types (see Supplementary Figure S2) in our SHIV-infected, ART-suppressed animals and found no differences relative to kinetics in uninfected animals.28,29 The titer and time to detection of anti-SHIV antibodies paralleled those found in HIV-1-infected patients and nonhuman primate studies (see Supplementary Figure S4).37,38 HSCT did not measurably impact serum levels of antigen-specific antibodies (see Supplementary Figure S4), indicating that plasma cells may be relatively resistant to TBI, consistent with previous observations.39 Finally, we observed a marked increase in immune activation (see Supplementary Figure S3) and in CD4+CCR5+ cell counts (Figures 2 and 3) following transplant. These findings highlight the complex interplay between TBI-dependent immune responses that may increase or decrease viral replication. For example, it remains unclear whether the decrease in naive CD4+ T-cells following transplant results in an overall reduction of the viral reservoir,40 or if this reduction may be offset by increased CCR5 expression. The efficacy of HSCT-based cure strategies will require better understanding of the tradeoffs between HSCT-dependent antiviral effects with that of inflammation and immune activation, which may in turn increase nascent infection.
In the context of suppressive ART, autologous transplant is likely to impart a genetic bottleneck on virus diversity, by virtue of the stochastic loss of some, but not all, latently infected cells. Recent evidence suggests that the longevity of some such cells may be attributable to oncogenic transformation.41,42 We are well-positioned to further quantitate the extent to which stem cell transplant reduces the breadth and diversity of the residual latent reservoir in this model.29,43 Identification of specific cellular subsets or clones that persist through transplant may prove valuable in terms of developing novel strategies to eliminate this last vestule of the viral reservoir following irradiation and thereby improving HSCT-based therapies designed to cure HIV/AIDS.6
In summary, our findings indicate that curative HSCT strategies can be readily assessed in SHIV-infected, ART suppressed pigtailed macaques. The presence of ART in target cells may require an extended washout period as lentiviral integration is significantly impeded. Engraftment and hematopoietic recovery in SHIV-infected, ART-suppressed animals paralleled transplants in noninfected pigtailed macaques. Importantly, our findings suggest that the heightened immune responses that follow myeloablative irradiation, as well as the high percentage of CCR5-expressing CD4+ T-cells during HSCT, may promote nascent infection where continued ART does not fully suppress viral replication. Whether genetically modified CD4+ T-cells may limit or promote the elimination of newly infected cells at these sites remains to be determined.
Materials and Methods
Animals. Eight healthy juvenile pigtailed macaques (Macaca nemestrina) were housed at the University of Washington National Primate Research Center under conditions approved by the American Association for Accreditation of Laboratory Animal Care. Study protocols were approved by the Fred Hutchinson Cancer Research Center Institutional Review Board and the University of Washington Institutional Animal Care and Use Committee. Animals were monitored closely, and animal welfare was assessed on a daily basis and, if necessary, several times a day. If animals experienced pain, then they received pain medications. Animals were inoculated intravenously with a dose of 104 TCID50 SHIV1157ipd3N4. Tissue harvest and blood and serum collection were performed prior to and following SHIV challenge. Antiretroviral drugs were administered as described earlier.44
Hematopoietic stem cell transplant. HSCT was performed as described earlier.28,45 We defined hematopoietic recovery in these studies as the average percentage gene marking following stabilization of post-transplant platelet levels. We define “stabilization” as 8 consecutive weeks during which platelet counts vary by <10% or are above a “healthy minimum” value of 2.6 × 105 platelets/µl whole blood, in the absence of granulocyte colony-stimulating factor or blood transfusion support.
Peripheral sampling, lymph node, and gastrointestinal biopsies. Peripheral blood was collected at the indicated time points by venipuncture into heparin, EDTA, or SST tubes for isolation of PBMCs and the isolation of plasma and serum samples. PBMCs were isolated from whole blood using a hemolytic lysis solution (ammonium chloride). Gut biopsies were obtained using an 8.9-mm diameter video gastroscope. A maximum of 23 pinch biopsies were taken per indicated time point using a 2.0-mm biopsy forceps. Single-cell suspensions were isolated following treatment of biopsies with 60 U/ml collagenase (Sigma Aldrich, St. Louis, MO). For axillary lymph node isolation, a small incision is made in the skin (<1 inch), the lymph node excised, and the incision closed with nonabsorbable suture. Cells are isolated following mechanical sheering of lymph nodes and lysis of red blood cells in hemolytic lysis buffer.
Immunological analysis and intracellular ART detection. Antibody titer against disrupted whole SIV or HIV-Env was determined by ELISA.46 Neutralization assay was performed as described earlier.46 Briefly, on day 0, TZM-Bl obtained from the AIDS Research and Reference Reagent Program were seeded in 96-well white flat-bottomed tissue culture plates at 3 × 103 cells in 100 μl of complete medium (DMEM, 10% FBS, 1% penicillin/streptomycin) and cultured overnight at 37 °C. The following day, sera collected 2 weeks prior to SHIV1157ipd3N4 infection and sera collected at the indicated time points postinfection was diluted 1:50. Thirty microliters were incubated with an equal volume of SHIV1157ipd3N4 at 100 × TCID50 for 1.5 hours at 37 °C in 96-well U-bottomed tissue culture plates (Corning, Corning, NY). After incubation, 50 μl of the sera–virus mixture was transferred to TZM-bl cells and cultured for 72 hours at 37 °C. On day 4, the media was changed with fresh 100 μl DMEM 10/1 and an equal volume of luciferase assay substrate buffer (Promega Steady-Glo Luciferase Assay System, catalog #E2510, Madison, WI) was added to each well and mixed. Cells were incubated at room temperature for 10 minutes. The level of tat-induced firefly luciferase for all unknowns was determined with the Perkin Elmer TopCount NXT Luminescence Counter using the TopCount NXT program (Waltham, MA). Percentage neutralization was calculated from the reduction in virus entry in the presence of postinfection sera relative to the entry in the presence of the preinfection sera: ((RLUpre–RLUpost)/RLUpre) × 100. Intracellular ART was determined using slightly modified methods described earlier.47,48 Briefly, cryopreserved CD34+ HSCs and PBMCs from identical time points were thawed and washed two times in ice-cold PBS. Immediately thereafter, cell pellets from an equal number of cells were lysed in ice-cold methanol and kept on ice until loading onto a C18 Hypersil Gold aQ Column (Thermo Fisher Scientific, Waltham, MA). Standard curves were generated using known concentrations of each respective drug and the concentration within samples was extrapolated.
Plasma and cell-associated viral load assessment. Viral load was assayed as previously described.46,49 Briefly, viral RNA copy number was determined by real-time PCR following reverse transcription. Total viral DNA in PBMC was determined by real-time PCR and is expressed as the number of copies per 500 ng total DNA. Total lentiviral DNA and 2-LTR circles were determined as described previously.50
Lymphocyte immunophenotyping. The phenotyping panel consisted of the following antibodies: CD3-Ax700 (SP34-2), CD4-PerCP-Cy5.5 (L200), CD8-APC-Cy7 (SK1), CD45RA-APC (5H9), CCR5-PE (3A9), CCR7-PE-Cy7 (3D12). The activation panel consisted of the following antibodies: CD3-Ax700 (SP34-2), CD4-PerCP-Cy5.5 (L200), CD8-APC-Cy7 (SK1), CD25-APC (2A3), CD279-PE-Cy7 (EH12.1), FoxP3-PE (206D), and Ki67-Ax488 (B56). All antibodies were purchased from BD Biosciences (San Jose, CA) except for FoxP3, which was purchased from BioLegend (San Diego, CA). All samples were fixed with 1% paraformaldehyde prior to flow cytometric analysis on an LSR-II system.
SUPPLEMENTARY MATERIAL Figure S1. Lymphocyte analysis. Figure S2. ART-treated, SHIV-infected macaques exhibit normal hematopoiesis following HSCT. Figure S3. Activation status of peripheral T-lymphocytes. Figure S4. Antibody production and neutralizing activity is maintained following HSCT.
Acknowledgments
The authors are grateful for research funding from the National Institutes of Health, Bethesda, MD grants R01 AI080326, U19 AI096111, R01 HL098489, P30 DK056465, AI027757, and P51 RR00016. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The authors would also like to acknowledge Veronica Nelson, Erica Wilson, Kelvin Sze from the Fred Hutchison Cancer Research Center and Heather Mack at the University of Washington for their contributions to this study. In addition, the authors would like to thank Merck and Gilead for providing the antiretroviral drugs for these studies. The authors declare no competing interests. H.P.K. is the principal investigator and designed the outline of the study. P.Y. designed and conducted the studies. P.Y., C.P., and P.P. coordinated the animal studies. P.Y., J.K., H.M., W.O., and N.M. contributed substantially to data collection and analysis. P.G. and B.M. performed conducted intracellular ART analysis. S.D. and S.L. contributed substantially to the study conception and design and critically reviewed this manuscript. H.P.K. is a Markey Molecular Medicine Investigator and received support as the inaugural recipient of the José Carreras/E.D. Thomas Endowed Chair for Cancer Research.
Supplementary Material
References
- Little RF, Dunleavy K. Update on the treatment of HIV-associated hematologic malignancies. Hematology Am Soc Hematol Educ Program. 2013;2013:382–388. doi: 10.1182/asheducation-2013.1.382. [DOI] [PubMed] [Google Scholar]
- Chao C, Xu L, Abrams D, Leyden W, Horberg M, Towner W, et al. Survival of non-Hodgkin lymphoma patients with and without HIV infection in the era of combined antiretroviral therapy. AIDS. 2010;24:1765–1770. doi: 10.1097/QAD.0b013e32833a0961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson CA, Abramson JS. HIV-associated Hodgkin's lymphoma: prognosis and therapy in the era of cART. Adv Hematol. 2012;2012:507257. doi: 10.1155/2012/507257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suneja G, Shiels MS, Angulo R, Copeland GE, Gonsalves L, Hakenewerth AM, et al. Cancer treatment disparities in HIV-infected individuals in the United States. J Clin Oncol. 2014;32:2344–2350. doi: 10.1200/JCO.2013.54.8644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hütter G, Zaia JA. Allogeneic haematopoietic stem cell transplantation in patients with human immunodeficiency virus: the experiences of more than 25 years. Clin Exp Immunol. 2011;163:284–295. doi: 10.1111/j.1365-2249.2010.04312.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younan P, Kowalski J, Kiem HP. Genetic modification of hematopoietic stem cells as a therapy for HIV/AIDS. Viruses. 2013;5:2946–2962. doi: 10.3390/v5122946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hütter G, Thiel E. Allogeneic transplantation of CCR5-deficient progenitor cells in a patient with HIV infection: an update after 3 years and the search for patient no. 2. AIDS. 2011;25:273–274. doi: 10.1097/QAD.0b013e328340fe28. [DOI] [PubMed] [Google Scholar]
- DiGiusto DL, Stan R, Krishnan A, Li H, Rossi JJ, Zaia JA. Development of hematopoietic stem cell based gene therapy for HIV-1 infection: considerations for proof of concept studies and translation to standard medical practice. Viruses. 2013;5:2898–2919. doi: 10.3390/v5112898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiGiusto DL, Krishnan A, Li L, Li H, Li S, Rao A, et al. RNA-based gene therapy for HIV with lentiviral vector-modified CD34(+) cells in patients undergoing transplantation for AIDS-related lymphoma. Sci Transl Med. 2010;2:36ra43. doi: 10.1126/scitranslmed.3000931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hütter G, Ganepola S. Eradication of HIV by transplantation of CCR5-deficient hematopoietic stem cells. Scientific World Journal. 2011;11:1068–1076. doi: 10.1100/tsw.2011.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armijo E, Soto C, Davis BR. HIV/AIDS: modified stem cells in the spotlight. Cell Mol Life Sci. 2014;71:2641–2649. doi: 10.1007/s00018-014-1572-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaia JA, Forman SJ. Transplantation in HIV-infected subjects: is cure possible. Hematology Am Soc Hematol Educ Program. 2013;2013:389–393. doi: 10.1182/asheducation-2013.1.389. [DOI] [PubMed] [Google Scholar]
- Hütter G, Nowak D, Mossner M, Ganepola S, Müssig A, Allers K, et al. Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. N Engl J Med. 2009;360:692–698. doi: 10.1056/NEJMoa0802905. [DOI] [PubMed] [Google Scholar]
- Henrich TJ, Hu Z, Li JZ, Sciaranghella G, Busch MP, Keating SM, et al. Long-term reduction in peripheral blood HIV type 1 reservoirs following reduced-intensity conditioning allogeneic stem cell transplantation. J Infect Dis. 2013;207:1694–1702. doi: 10.1093/infdis/jit086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younan P, Kowalski J, Kiem HP. Genetically modified hematopoietic stem cell transplantation for HIV-1-infected patients: can we achieve a cure. Mol Ther. 2014;22:257–264. doi: 10.1038/mt.2013.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tebas P, Stein D, Tang WW, Frank I, Wang SQ, Lee G, et al. Gene editing of CCR5 in autologous CD4 T cells of persons infected with HIV. N Engl J Med. 2014;370:901–910. doi: 10.1056/NEJMoa1300662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bovolenta C, Porcellini S, Alberici L. Therapeutic genes for anti-HIV/AIDS gene therapy. Curr Pharm Biotechnol. 2013;14:488–500. doi: 10.2174/138920101405131111104009. [DOI] [PubMed] [Google Scholar]
- Kiem HP, Jerome KR, Deeks SG, McCune JM. Hematopoietic-stem-cell-based gene therapy for HIV disease. Cell Stem Cell. 2012;10:137–147. doi: 10.1016/j.stem.2011.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnock JN, Daigre C, Al-Rubeai M. Introduction to viral vectors. Methods Mol Biol. 2011;737:1–25. doi: 10.1007/978-1-61779-095-9_1. [DOI] [PubMed] [Google Scholar]
- Beard BC, Dickerson D, Beebe K, Gooch C, Fletcher J, Okbinoglu T, et al. Comparison of HIV-derived lentiviral and MLV-based gammaretroviral vector integration sites in primate repopulating cells. Mol Ther. 2007;15:1356–1365. doi: 10.1038/sj.mt.6300159. [DOI] [PubMed] [Google Scholar]
- Mitchell RS, Beitzel BF, Schroder AR, Shinn P, Chen H, Berry CC, et al. Retroviral DNA integration: ASLV, HIV, and MLV show distinct target site preferences. PLoS Biol. 2004;2:E234. doi: 10.1371/journal.pbio.0020234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trobridge GD, Allen J, Peterson L, Ironside C, Russell DW, Kiem HP. Foamy and lentiviral vectors transduce canine long-term repopulating cells at similar efficiency. Hum Gene Ther. 2009;20:519–523. doi: 10.1089/hum.2008.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiem HP, Wu RA, Sun G, von Laer D, Rossi JJ, Trobridge GD. Foamy combinatorial anti-HIV vectors with MGMTP140K potently inhibit HIV-1 and SHIV replication and mediate selection in vivo. Gene Ther. 2010;17:37–49. doi: 10.1038/gt.2009.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trobridge GD, Horn PA, Beard BC, Kiem HP. Large animal models for foamy virus vector gene therapy. Viruses. 2012;4:3572–3588. doi: 10.3390/v4123572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younan PM, Polacino P, Kowalski JP, Peterson CW, Maurice NJ, Williams NP, et al. Positive selection of mC46-expressing CD4+ T cells and maintenance of virus specific immunity in a primate AIDS model. Blood. 2013;122:179–187. doi: 10.1182/blood-2013-01-482224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavigner M, Watkins B, Lawson B, Lee ST, Chahroudi A, Kean L, et al. Persistence of virus reservoirs in ART-treated SHIV-infected rhesus macaques after autologous hematopoietic stem cell transplant. PLoS Pathog. 2014;10:e1004406. doi: 10.1371/journal.ppat.1004406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cillo AR, Krishnan A, Mitsuyasu RT, McMahon DK, Li S, Rossi JJ, et al. Plasma viremia and cellular HIV-1 DNA persist despite autologous hematopoietic stem cell transplantation for HIV-related lymphoma. J Acquir Immune Defic Syndr. 2013;63:438–441. doi: 10.1097/QAI.0b013e31828e6163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beard BC, Trobridge GD, Ironside C, McCune JS, Adair JE, Kiem HP. Efficient and stable MGMT-mediated selection of long-term repopulating stem cells in nonhuman primates. J Clin Invest. 2010;120:2345–2354. doi: 10.1172/JCI40767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts KL, Beard BC, Wood BL, Trobridge GD, Humphries RK, Adams AB, et al. No evidence of clonal dominance after transplant of HOXB4-expanded cord blood cells in a nonhuman primate model. Exp Hematol. 2014;42:497–504. doi: 10.1016/j.exphem.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trobridge GD, Wu RA, Beard BC, Chiu SY, Muñoz NM, von Laer D, et al. Protection of stem cell-derived lymphocytes in a primate AIDS gene therapy model after in vivo selection. PLoS One. 2009;4:e7693. doi: 10.1371/journal.pone.0007693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson PL, Kakuda TN, Lichtenstein KA. The cellular pharmacology of nucleoside- and nucleotide-analogue reverse-transcriptase inhibitors and its relationship to clinical toxicities. Clin Infect Dis. 2004;38:743–753. doi: 10.1086/381678. [DOI] [PubMed] [Google Scholar]
- Robbins BL, Srinivas RV, Kim C, Bischofberger N, Fridland A. Anti-human immunodeficiency virus activity and cellular metabolism of a potential prodrug of the acyclic nucleoside phosphonate 9-R-(2-phosphonomethoxypropyl)adenine (PMPA), Bis(isopropyloxymethylcarbonyl)PMPA. Antimicrob Agents Chemother. 1998;42:612–617. doi: 10.1128/aac.42.3.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LH, Begley J, Feng J, Quinn J, Rousseau F.2002Pharmacokinetic and pharmacodynamic characteristics of emtricitabine support its once daily dosing The XIV International AIDS Conference, International AIDS Society . http://www.iasociety.org/Default.aspx?pageId=11&abstractId=2431 (Abstract # TuPeB4546) (abstract ).
- Hawkins T, Veikley W, St Claire R, Hey A, Guyer B, Kearney BP.2004Intracellular pharmacokinetics of tenofovir-DP and carbovir-TP in patients receiving triple nucleoside regimensProgram and Abstracts of the 5th International Worksop on Clinical Pharmacology of HIV Therapy, April 1–3, 2004, Rome Italy Abstract #2.4 (abstract).
- Perez EE, Wang J, Miller JC, Jouvenot Y, Kim KA, Liu O, et al. Establishment of HIV-1 resistance in CD4+ T cells by genome editing using zinc-finger nucleases. Nat Biotechnol. 2008;26:808–816. doi: 10.1038/nbt1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J, Wang J, Crain K, Fearns C, Kim KA, Hua KL, et al. Zinc-finger nuclease editing of human cxcr4 promotes HIV-1 CD4(+) T cell resistance and enrichment. Mol Ther. 2012;20:849–859. doi: 10.1038/mt.2011.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomaras GD, Yates NL, Liu P, Qin L, Fouda GG, Chavez LL, et al. Initial B-cell responses to transmitted human immunodeficiency virus type 1: virion-binding immunoglobulin M (IgM) and IgG antibodies followed by plasma anti-gp41 antibodies with ineffective control of initial viremia. J Virol. 2008;82:12449–12463. doi: 10.1128/JVI.01708-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho O, Larsen K, Polacino P, Li Y, Anderson D, Song R, et al. Pathogenic infection of Macaca nemestrina with a CCR5-tropic subtype-C simian-human immunodeficiency virus. Retrovirology. 2009;6:65. doi: 10.1186/1742-4690-6-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor BP, Cascalho M, Noelle RJ. Short-lived and long-lived bone marrow plasma cells are derived from a novel precursor population. J Exp Med. 2002;195:737–745. doi: 10.1084/jem.20011626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wightman F, Solomon A, Khoury G, Green JA, Gray L, Gorry PR, et al. Both CD31(+) and CD31− naive CD4(+) T cells are persistent HIV type 1-infected reservoirs in individuals receiving antiretroviral therapy. J Infect Dis. 2010;202:1738–1748. doi: 10.1086/656721. [DOI] [PubMed] [Google Scholar]
- Wagner TA, McLaughlin S, Garg K, Cheung CY, Larsen BB, Styrchak S, et al. HIV latency. Proliferation of cells with HIV integrated into cancer genes contributes to persistent infection. Science. 2014;345:570–573. doi: 10.1126/science.1256304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldarelli F, Wu X, Su L, Simonetti FR, Shao W, Hill S, et al. HIV latency. Specific HIV integration sites are linked to clonal expansion and persistence of infected cells. Science. 2014;345:179–183. doi: 10.1126/science.1254194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beard BC, Adair JE, Trobridge GD, Kiem HP. High-throughput genomic mapping of vector integration sites in gene therapy studies. Methods Mol Biol. 2014;1185:321–344. doi: 10.1007/978-1-4939-1133-2_22. [DOI] [PubMed] [Google Scholar]
- Peterson CW, Younan P, Polacino PS, Maurice NJ, Miller HW, Prlic M, et al. Robust suppression of env-SHIV viremia in Macaca nemestrina by 3-drug ART is independent of timing of initiation during chronic infection. J Med Primatol. 2013;42:237–246. doi: 10.1111/jmp.12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trobridge GD, Beard BC, Gooch C, Wohlfahrt M, Olsen P, Fletcher J, et al. Efficient transduction of pigtailed macaque hematopoietic repopulating cells with HIV-based lentiviral vectors. Blood. 2008;111:5537–5543. doi: 10.1182/blood-2007-09-115022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Cleveland B, Klots I, Travis B, Richardson BA, Anderson D, et al. Removal of a single N-linked glycan in human immunodeficiency virus type 1 gp120 results in an enhanced ability to induce neutralizing antibody responses. J Virol. 2008;82:638–651. doi: 10.1128/JVI.01691-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Li H, Willson K, Breidinger S, Rizk ML, Wenning L, et al. Ultrasensitive liquid chromatography-tandem mass spectrometric methodologies for quantification of five HIV-1 integrase inhibitors in plasma for a microdose clinical trial. Anal Chem. 2012;84:8614–8621. doi: 10.1021/ac301581h. [DOI] [PubMed] [Google Scholar]
- King T, Bushman L, Kiser J, Anderson PL, Ray M, Delahunty T, et al. Liquid chromatography-tandem mass spectrometric determination of tenofovir-diphosphate in human peripheral blood mononuclear cells. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;843:147–156. doi: 10.1016/j.jchromb.2006.05.043. [DOI] [PubMed] [Google Scholar]
- Polacino P, Cleveland B, Zhu Y, Kimata JT, Overbaugh J, Anderson D, et al. Immunogenicity and protective efficacy of Gag/Pol/Env vaccines derived from temporal isolates of SIVmne against cognate virus challenge. J Med Primatol. 2007;36:254–265. doi: 10.1111/j.1600-0684.2007.00243.x. [DOI] [PubMed] [Google Scholar]
- Butler SL, Johnson EP, Bushman FD. Human immunodeficiency virus cDNA metabolism: notable stability of two-long terminal repeat circles. J Virol. 2002;76:3739–3747. doi: 10.1128/JVI.76.8.3739-3747.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


