Abstract
Mesenchymal stem (stromal) cells (MSCs) are multipotent cells with the ability to differentiate into several cell types, thus serving as a cell reservoir for regenerative medicine. Much of the current interest in therapeutic application of MSCs to various disease settings can be linked to their immunosuppressive and anti-inflammatory properties. One of the key mechanisms of MSC anti-inflammatory effects is the secretion of soluble factors with paracrine actions. Recently it has emerged that the paracrine functions of MSCs could, at least in part, be mediated by extracellular vesicles (EVs). EVs are predominantly released from the endosomal compartment and contain a cargo that includes miRNA, mRNA, and proteins from their cells of origin. Recent animal model-based studies suggest that EVs have significant potential as a novel alternative to whole cell therapies. Compared to their parent cells, EVs may have a superior safety profile and can be safely stored without losing function. In this article, we review current knowledge related to the potential use of MSC-derived EVs in various diseases and discuss the promising future for EVs as an alternative, cell-free therapy.
Introduction
Regenerative medicine focuses on the restoration of lost, damaged, or aging cells and tissues in the human body. Ferrari et al.1 demonstrated the value of a stem cell-based regenerative treatment for muscular dystrophies using bone marrow (BM)-derived myogenic progenitor cells. Since then numerous stem cell types have been investigated for use in tissue regeneration in both animal models and human clinical studies, with varying degrees of success.
Mesenchymal stem (or stromal) cells (MSCs) have emerged as a potential solution for tissue repair and wound healing.2 MSCs are multipotent, nonhematopoietic adult stem cells, which can be isolated from BM, umbilical cord,3,4 placental or adipose tissue. MSCs have the potential to differentiate into osteoblasts, chondrocytes, and adipocytes5 as well as endothelial, cardiovascular, and neurogenic cell types and are gaining credibility as a therapeutic agent because of their ex vivo expansion capacity and ethical acceptability.6 More recently, it has been discovered that, in addition to their direct role in tissue regeneration, MSCs have potent anti-inflammatory and/or immunosuppressive properties.7 Extensive research and clinical trials are currently underway for the use of MSCs as regenerative agents in many diseases including spinal cord injury, multiple sclerosis, Alzheimer's disease, liver cirrhosis and hepatitis, osteoarthritis, myocardial infarction, kidney disease, inflammatory bowel disease, diabetes mellitus, knee cartilage injuries, organ transplantation, and graft-versus-host disease (http://www.clinicaltrials.gov; accessed November 2014).
Paracrine Actions of MSCs
González et al.8 studied the contact-dependent mechanism of human adipose-derived MSCs in regulating inflammatory cytokines. In their study, they determined that human adipose-derived MSCs and macrophages both produce high levels of interleukin-10 (IL-10) only after cell-to-cell contact is maintained.8
Although potentially triggered by cell-to-cell contact events, the regenerative potential of MSC therapies has been found—at least in part—to be mediated via paracrine actions.9 For example, the paracrine effect of MSC-conditioned medium (CM) was observed to protect cardiomyocytes by interfering with the mitochondria-mediated apoptotic pathway. In this study, application of MSC-CM to cardiomyocytes exposed to hypoxia/reoxygenation reduced apoptosis through inhibition of the release of cytochrome C from mitochondria and reduction of caspase-3 activation.10 Similarly, renoprotective effects of human umbilical cord blood-derived MSCs (hUCB-MSCs) in streptozotocin-induced diabetic rats was reportedly mediated through paracrine action.11 In this case, the authors studied the effects of hUCB-MSC-CM on transforming growth factor (TGF)-β1-activated rat renal proximal tubular epithelia (NRK-52E) cells and observed attenuated expression of TGF-β1, α-smooth muscle actin, collagen I, and heat shock protein-47 mRNA and increased expression of E-cadherin and bone morphogenic protein-7 mRNA, thereby preventing diabetes kidney disease.11
Although it was initially believed that the potential of MSCs to differentiate into various cell types plays a crucial role in their therapeutic effects, the mechanism of action of transplanted MSCs does not predominantly include differentiating into a specific cell type for promoting or repairing the tissue damage in most disease settings.12,13,14 Several studies have demonstrated the predominance of short-lived paracrine mechanisms among the therapeutic actions of MSCs. In one such study, Toma et al.15 injected human MSCs (hMSCs) tagged with β-galactosidase into the left ventricle of immunodeficient mice. The majority of hMSCs were found in the spleen, lung, and liver, 4 days after injection. They also reported that only 0.44% of the injected hMSCs survived and, with time, they were morphologically indistinguishable from the surrounding cardiomyocytes. Other studies on systemically administered MSCs have also reported that <1% of the administered cells survive for more than 1 week and that the benefits of MSC therapy could be attributed to their secreted factors.16,17,18
In acute kidney injury (AKI), the protective effect of MSC administration was not attributed to MSCs differentiating into a tubular or endothelial cell phenotype, but to enhanced regulation of anti-inflammatory and organ-protective mediators such as IL-10, basic fibroblast growth factor, TGF-α, and B-cell lymphoma 2 (Bcl-2), reflecting primarily the paracrine function of MSCs.19 Tögel et al.20 reported the paracrine nature of cytoprotection in the immediate vicinity of administered MSCs in AKI. The authors demonstrated the production of renotropic factors—hepatocyte growth factor, and insulin-like growth factor 1 —that are known to decrease apoptosis and stimulate proliferation of renal epithelial cells.
Although these studies, and many others, provide strong evidence for the potency of MSC-secreted factors in mediating tissue repair and regeneration, the precise mechanisms by which MSCs act in a paracrine fashion are not fully understood. In addition to secreting an array of soluble factors, it has also been recognized that MSCs release large numbers of extracellular vesicles (EVs). Thus, it is of interest to consider the possibilities that the complex paracrine regenerative actions of exogenously administered MSCs and other stem cells communicate by transferring information and regulatory genes mediated, to some degree, by released EVs9,21,22 and that EVs derived from cultured MSCs have the potential to constitute a safe, effective cell-free therapy.
Extracellular Vesicles
EVs were first clearly described by Pan and Johnstone in 1983.23 Initially, the release of EVs was thought to represent a disposal mechanism by which cells eliminate unwanted proteins and other molecules. After years of subsequent research, however, EV release has emerged as an important mediator of cell-to-cell communication that is not only involved in normal physiological process but also plays a role in the development and progression of diseases. Among the subtypes of EV, the most numerous, referred to as exosomes, have a diameter of 40–100 nm, can be isolated by centrifugation at 100,000 ×g and can be concentrated at the interface of 0.8 and 2.7M sucrose layers. Preparations of EVs, typically a mixture of exosomes and other subtypes, can be isolated from all types of body fluids including blood, urine, bronchoalveolar lavage fluid, breast milk, amniotic fluid, synovial fluid, pleural effusions, and ascites.24 EVs can also be isolated from culture supernatants of many cell types, including T-cells, B-cells, dendritic cells, platelets, mast cells, epithelial cells, endothelial cells, neuronal cells, cancerous cells, and, as we describe in detail later, MSCs.25,26,27,28,29,30,31,32,33,34,35,36,37
Biogenesis of EVs
The modes of biogenesis for exosomes and microvesicles (MVs) are completely distinct and are described in this section.
Exosome biogenesis
Although the term “exosome” has been frequently used to describe all vesicles released by cells into the extracellular milieu, it is now known that there are multiple different types of EV. The major EV subtypes that are currently recognized are listed along with their basic characteristics in Table 1. Because of lack of specific markers it is very difficult to distinguish between different subtypes of vesicles within mixed preparations as they have overlapping composition, density, and size. Therefore, the International Society for Extracellular Vesicles suggested that the term EVs be used preferentially to describe preparations of vesicles from body fluids and cell cultures.38
Table 1. Different types of vesicles derived from various fluids and CM.
Exosomes are EVs of endosomal origin. The endosomal sorting complex required for transport and its associated proteins are involved in the formation of multivesicular bodies (MVBs) and intraluminal vesicles (ILV).39 Exosome membranes are enriched in lipids such as cholesterol, ceramide, and sphingolipids that are involved in the budding of ILVs into MVBs.40,41 As was first described during reticulocyte differentiation, ILVs are released from cells as a consequence of MVB fusion with the plasma membrane and, once released, are then termed as exosomes.23,42 Tan et al.41 further confirmed the endosomal origin of MSC-derived exosomes by detecting the components of lipid rafts. Table 2 provides additional details about proteins involved in MVB and exosome biogenesis. Exosomes may subsequently be internalized by other cells via direct membrane fusion, endocytosis or cell-type specific phagocytosis.43,44,45 Figure 1 illustrates the intracellular sources, release and uptake mechanisms associated with exosomes and other major subtypes of EV.
Table 2. Proteins associated with exosome biogenesis.
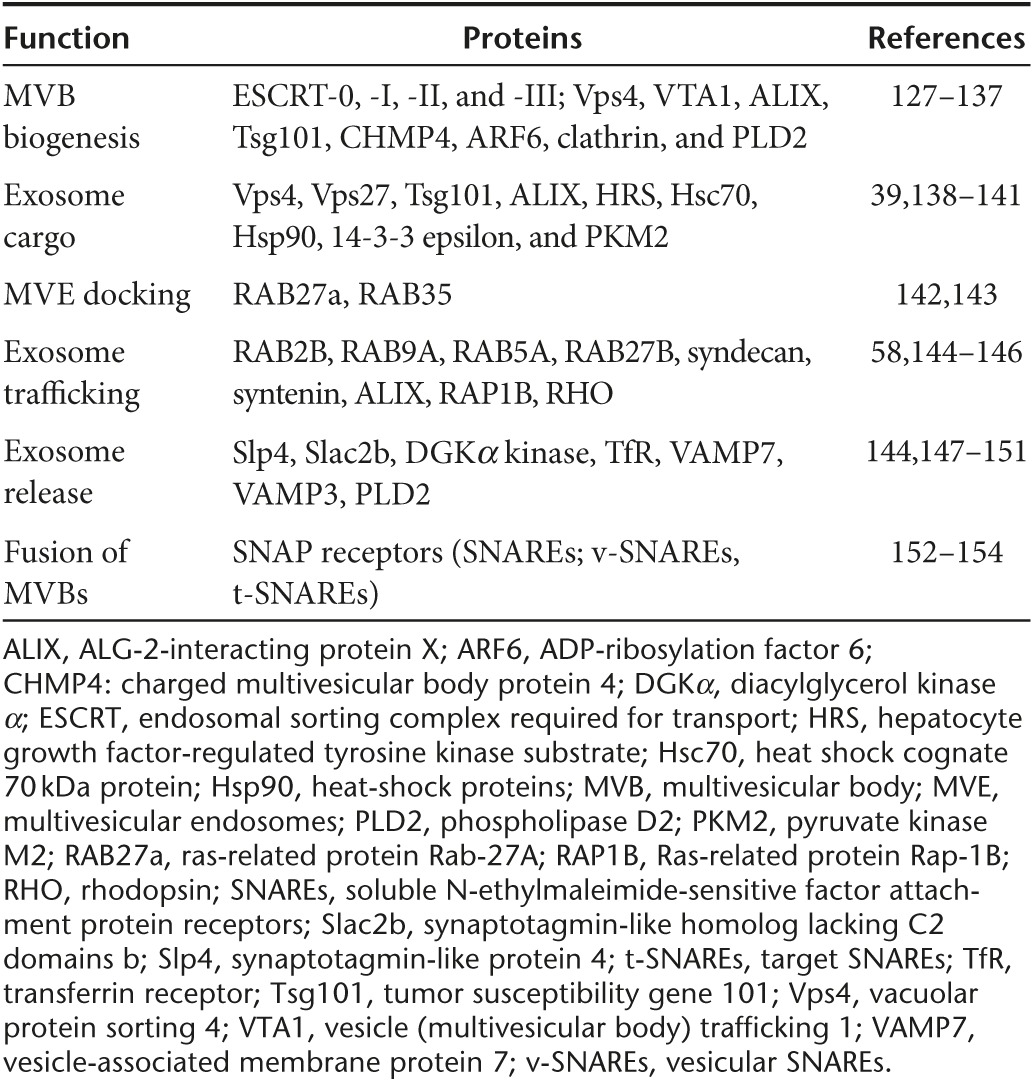
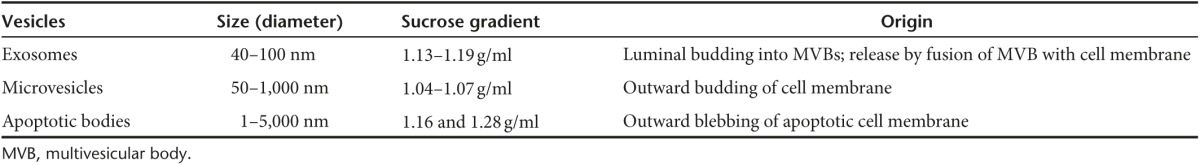
Figure 1.
EVs origin and internalization. Origin of EVs are generally via (a) endocytosis or inward budding of plasma membrane that consist of lipid rafts and is mediated by clathrin-dependent or caveolae-dependent pathway, This gives rise to (b) early endosomes leading to the formation of numerous ILVs within a membrane maturing to MVBs. Finally MVBs fuse with plasma membrane releasing ILVs as exosomes. (c) Ectosomes are vesicles shed from the cell surface and (d) apoptotic bodies are also known as apobodies and are released by cells undergoing apoptosis. EVs are internalized by the target cells through several pathways including (e) endocytosis, (f) fusion, and (g) phagocytosis.
Microvesicle biogenesis
MVs result from outward budding and fission of plasma membrane. Membrane budding initiated by the activity of aminophospholipid translocases to translocate phosphatidylserine to the outer membrane.46,47,48 ADP-ribosylation factor 6 plays an important role in enabling MV budding by stimulating phospholipase D activity, which in turn facilitates extracellular signal-regulated kinase activation.49,50 Contractile protein myosin light chain kinase 2 (which contracts cytoskeleton) is phosphorylated by extracellular signal-regulated kinase, which in turn stimulates serine phosphorylation of myosin II that ultimately triggers the release of MVs.46,50,51,52
Regulation of EV Biogenesis
Earlier literature has shown that MSCs release EVs differently depending on external stimulation suggesting that this process is likely to be regulated by cross-talk between MSCs and their surrounding microenvironment.53,54 For example, hypoxia or inflammatory conditioning of MSCs has been shown to regulate protein packaging into EVs and to affect their functional properties.53,54 Several pathways, which may be relevant to the microenvironment in which MSCs reside, have been reported to regulate biogenesis and secretion of EVs. Tumor suppressor-activated pathway 6 is found to regulate EV formation55 and is transcriptionally regulated by p53 thereby enhancing EV production.56,57 An alternative cross-talk pathway was suggested by Baietti et al.58 who described that syndecans interact with syntenin to regulate intraluminal budding of endosomal membrane domains containing CD63 and ALIX.
Therapeutic Effects of MSC-Derived EVs (MSC-EVs)
As described earlier, EVs facilitate cell-to-cell communication via the transfer of functionally relevant biomolecules59,60 (see Table 3) and thus, may be harnessed for therapeutic purposes in a similar fashion to their parent cells. From a translational perspective, EVs derived from MSCs have shown encouraging therapeutic effects in various animal models (see Figure 2), and their isolation from MSCs is potentially sustainable and reproducible. Furthermore, in comparison to whole cell-based therapies, MSC-EVs may offer specific advantages for patient safety such as lower propensity to trigger innate and adaptive immune responses61 and inability to directly form tumors. For example, it has been shown that MSC-derived EVs induced anti-inflammatory cytokines as well as triggering apoptosis in activated T-cells.62 MSC-EVs also carry mRNAs encoding immunoregulatory mediators including cytokine receptor-like factor 1, interleukin 1 receptor, and metallothionein 1X.63
Table 3. Molecular composition of EVs.
Figure 2.
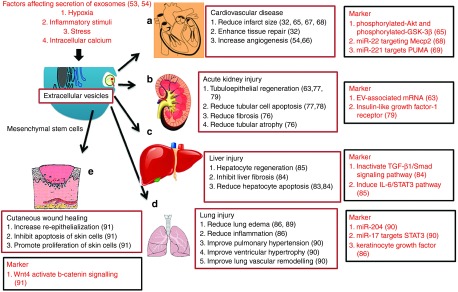
Potential clinical applications of EVs. Therapeutic benefits and mechanisms of action of MSC-derived EVs in: (a) various heart conditions, (b) kidney injury, (c) liver injury, (d) lung injury, and (e) wound healing.
In the remaining sections and in Table 4, we examine the evidence to-date for beneficial effects of MSC-EVs in several important disease areas and discuss some of the future needs and challenges that may be of critical importance to their successful clinical translation.
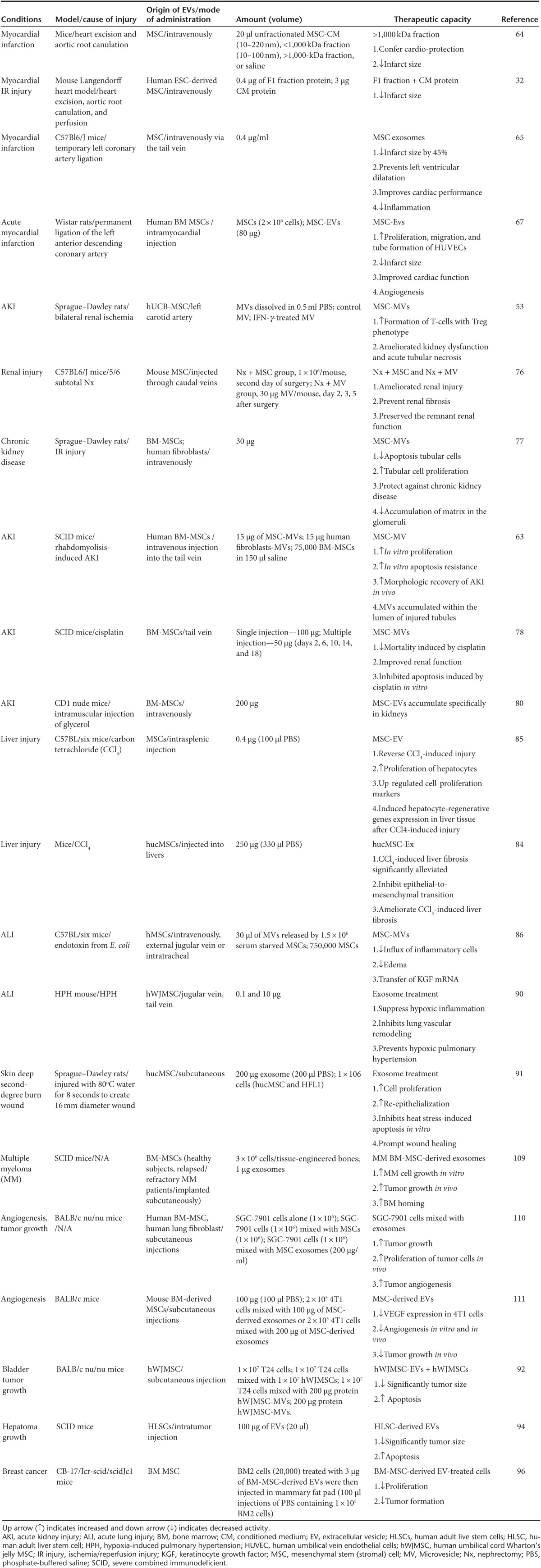
Table 4. Information of MSC-derived EVs in different studies.
MSC-EVs in cardiovascular disease
The CM obtained from hMSCs was shown by Timmers et al.64 to have the potential to reduce myocardial infarct size by 60% in a porcine model of cardiac ischemia/reperfusion (IR) injury. In this same study, fractionation of the CM revealed that the cardio-protective effect was confined to the fraction containing products >1,000 kDa (100–220 nm). In a mouse model of myocardial infarction, Lai et al.32 then directly demonstrated that the active, cardio-protective component of MSC-derived CM is, in fact, the EVs. In this study, administration of purified MSC-EVs reduced infarct size by ~40%.
Subsequently, Arslan et al.65 reported reduced infarct size following a single intravenous injection of MSC-EVs which could be attributed to the fact that EVs are internalized by target cells at the infarct site via endocytosis or phagocytosis. To further prove that intact MSC-EVs were required for therapeutic benefit, these authors demonstrated that homogenized EVs failed to reduce infarct size.65
Other studies have explored mechanisms by which the number and proangiogenic effects of EVs released by MSCs can be enhanced.66 For example, in a study of placental MSCs, under hypoxic conditions, Salomon et al.54 observed 3.3- and 6.7-fold increases in EV release in the presence of 1% and 3% O2 when compared with placental MSCs maintained at 8% O2. The resulting placental MSCs-derived EVs induced a significant, dose-dependent increase in tube formation by placental microvascular endothelial cells when compared with vehicle-treated cells.54 It was speculated that the increased proangiogenic effect of MSC-EVs derived under hypoxic conditions may be conferred by transcriptional activities of the hypoxia inducible factor family of proteins.54
Following on from the above result, Bian et al.67 isolated EVs from MSCs cultured under hypoxic conditions. In an in vitro angiogenesis assay, MSC-EVs at a concentration of 80 μg/ml, promoted human umbilical vein endothelial cell migration and tube formation that was comparable to that induced by vascular endothelial growth factor (VEGF). In vivo studies confirmed that intramyocardial injection of hypoxia-conditioned MSC-EVs significantly improved cardiac function and reduced myocardial infarct size with similar potency to that observed in a whole-cell MSC-treated group.67
Micro-RNAs associated with MSC-EVs also play an important role in cardio-protection. For instance, it was found that cardiac remodeling following myocardial infarction is regulated by miR-22-loaded EVs via targeting of methyl CpG binding protein 2.68 Similarly, the level of miR-221 is significantly higher in MSC-EVs when compared with their parent MSCs, and this miRNA was shown to enhance cardio-protection by reducing the expression of p53 upregulated modulator of apoptosis.69
MSC-EVs in AKI
AKI is a major cause of morbidity and mortality among hospitalized patients and is most commonly caused by IR injury, exposure to nephrotoxic compounds, and severe volume loss or obstruction to urine flow.70 It has been well established in animal models of renal IR and other forms of kidney injury that systemic or localized administration of MSCs results in amelioration of AKI.71,72,73 MSCs downregulate proinflammatory cytokines in T-cells and consequently induce regulatory T-cells (T-regs) in the spleen.71 Anti-inflammatory and immunoregulatory properties of MSCs have become one of the important mechanistic approaches to the treatment of AKI. A broad range of growth factors, cytokines, and chemokines secreted from MSCs have been identified including hepatocyte growth factor, insulin-like growth factor 1, VEGF, IL-1, IL-4, IL-5, IL-6, keratinocyte-derived chemokine, chemokine (C-X-C motif) ligand 16 (CXCL16), chemokine (C–C motif) ligand 2 (CCL2), CCL3, chemokine (C-X3-C motif) ligand 1 (CX3CL1), and CCL5.20,74 In experimental models, mediators such as these have been associated with enhanced cell proliferation and reduced cell apoptosis, identifying MSCs as uniquely providing multimodal therapeutic effects in AKI.75
Similar to MSCs, MSC-EVs are capable of modulating T-cell as well as innate immune cell functions.53 To date, there are few reported studies that directly compare the effect of MSCs and MSC-EVs in the setting of AKI. However, in a study involving mouse 5/6 subtotal nephrectomy (Nx)—a model of chronic kidney disease–He et al.76 reported that both MSC- and MSC-EV-treated mice showed strikingly similar benefits including reduced fibrosis and interstitial lymphocyte infiltration and reduced or absent tubular atrophy when compared with the untreated control group.
In the rat model of renal IR, Gatti et al.77 found that intravenous injection of 30 μg of MSC-EVs prevented AKI. The administered EVs were shown to transiently accumulate within glomeruli and injured tubules in association with increased proliferation and reduced apoptosis of tubular epithelial cells.77 This study also reported that the protective effect was specific to MSC-EVs as fibroblast-EVs were ineffective. Similarly, Bruno et al.63 also reported that human BM-derived MSC-EVs accelerated renal morphologic and functional recovery in glycerol-induced AKI in immunodeficient mice by inducing proliferation of tubular cells. In this study, they also reported that the effect of MSC-EVs on the recovery of AKI was similar to that of hMSCs.63
The effects of human MSC-EVs were also studied in severe combined immunodeficient (SCID) mice with AKI induced by the chemotherapeutic agent cisplatin.78 In this study, MSC-EVs significantly improved the survival (40% at day 21) by improving renal function and morphology, but were unable to prevent chronic tubular injury (see Table 4). Multiple injections of MSC-EVs, however, further decreased mortality in association with normal histology and renal function.78 MSC-EVs were found to upregulate antiapoptotic genes, including B-cell lymphoma-extra large, Bcl-2 and baculoviral IAP repeat containing 8, and downregulating cell apoptosis genes including, Caspase-1 (Casp1), Caspase-8 (Casp8) and lymphotoxin α in cisplatin-treated human tubular epithelial cells.78 Renoprotection was also conferred by horizontal transfer of insulin-like growth factor-1 receptor via BM–MSC–EV.79
Grange et al.80 studied the biodistribution of intravenously injected MSC-EVs in an AKI mouse model. They observed the specific accumulation of EVs at the site of injury as compared to healthy mice receiving the same quantity of MSC-EVs.80 Overall, of the disease areas studied, AKI, caused by a variety of clinically relevant insults, represents one of the most convincing examples of a distinct therapeutic benefit of systemic MSC-EV injection.78,80,81
MSC-EVs in liver disease
MSCs have been shown to be of benefit in a range of acute and chronic liver disease models and clinical translation of this work is currently underway in a number of centers.82 For example, injection of MSCs into the portal vein has been reported to protect the liver in a rat model of hepatic IR injury after partial hepatectomy. In this study, MSC administration was shown to reduce hepatocyte apoptosis and enhance liver regeneration.83
Fewer studies have addressed the potential benefits of MSC-EVs in chronic liver disease models. In one such study, human umbilical cord-MSC (hucMSC)-EVs were shown to specifically localize to the liver and to alleviate liver fibrosis in carbon tetrachloride (CCl4)-induced injury by reducing hepatocyte apoptosis and hepatic lobule destruction.84 MSC-EV administration suppressed epithelial to mesenchymal transdifferentiation via reduced TGF-β1 expression and Smad2 phosphorylation.84 Other in vivo studies have shown that MSC-EVs promote hepatocyte regeneration after CCl4-induced injury by inducing the IL-6/STAT3 pathway and cell cycle progression.85 In this case, the authors validated the direct hepatoprotective effects of MSC-EVs using the cell lines TAMH (an immortalized mouse hepatocyte line derived from transgenic MT42 male mice overexpressing TGF-α), THLE-2 (an immortalized primary human hepatocyte) and HuH-7 (a human hepatocarcinoma cell line) exposed in vitro to acetaminophen and hydrogen peroxide.85 Increased cytoprotection compared to control-treated cells was observed following treatment with 0.1 μg/ml MSC-EVs. Thus, both in vivo and in vitro studies have confirmed that MSC-EV therapy has the potential to promote liver regeneration following acute injury by directly enhancing hepatocyte survival and proliferation85 (see Table 4).
MSC-EVs in lung diseases
Endotoxin-induced acute lung injury (ALI) in mice results in increased lung protein permeability causing an inflammatory response in the alveoli that is commonly used as a model of human ALI associated with severe pneumonia or sepsis. In this model, it has recently been shown by Zhu et al.86 that administration of MSC-EVs decreased the influx of total inflammatory cells into the lung by 36% and influx of neutrophils by 73%. The suppression of lung inflammation was accompanied by reduced protein permeability, thereby preventing the formation of pulmonary edema. From a mechanistic perspective, keratinocyte growth factor (KGF) has been shown to reduce lung edema and inflammation in various ALI models.87,88 Lee et al. 89 reported that hMSCs produced KGF and that its secretion as a paracrine soluble factor mediated the restoration of alveolar fluid clearance in vivo. Thus, Zhu et al. 86 hypothesized that MSC-EVs transfer KGF mRNA to the injured alveolar epithelium and to verify this, they transfected the MSCs with KGF-specific small interfering RNA before isolating EVs. In keeping with this mechanism, the therapeutic effect of EVs from KGF-depleted MSCs was reduced compared to that of control MSC-EVs.
In a mouse model of hypoxia-induced pulmonary hypertension, the injection of MSC-EVs resulted in a delayed pulmonary influx of macrophages and reduced production of proinflammatory mediators compared to injection of EVs-derived from mouse lung fibroblasts.90 MSC-EVs, upon low dose multiple administration, also ameliorated pulmonary hypertension via increasing the levels of miR-204,90 ventricular hypertrophy, and lung vascular remodelling.90 The authors further tested the efficacy of two sequential injections of a higher dose of MSC-EVs and observed similar beneficial effects on early and later outcomes.90 Finally, MSC-EVs have been found to suppress hypoxic activation of signal transducer and activator of transcription 3 (STAT3) by up-regulating miR-17.90
MSC-EVs in cutaneous wound healing
In a recently reported study by Zhang et al. 91, the effects of locally injected hucMSC and hucMSC-EVs were studied in a rat deep second degree burn injury model. Using a range of histological and molecular indexes of healing, the authors found that injection of hucMSCs and hucMSC-EVs resulted in comparable and significant increase in re-epithelialization when compared with burn wounds that were treated with saline, human lung fibroblasts (HFL1) or HFL1-EVs. The epithelial healing effects were replicated in vitro in keratinocyte and dermal fibroblast cell lines in the form of increased cell proliferation and reduced apoptosis and were shown to be mediated by MSC-EV-delivered Wnt4 resulting in activation of β-catenin signaling and by activation of the AKT signaling pathway.91 Although additional studies are needed to confirm these striking observations in other preclinical models, the results suggest that cutaneous injury and ulceration represent one of the most promising clinical translational avenues for MSC-EV preparations.
Antitumor Activity of MSC-EVs
MSCs have also been shown to have anticancer activities. Wu et al.92 demonstrated that human umbilical cord Wharton's jelly MSC (hWJMSC)-derived EVs reduce the growth of T24 bladder carcinoma cells in vitro and in vivo. The authors reported that incubation of T24 cells with various concentration of hWJMSC-EVs (0, 50, 100, 200 µg/ml protein) resulted in cell-cycle arrest and tumor cell apoptosis.92 Similarly, Bruno et al.93 reported inhibited cell-cycle progression and induced apoptosis in HepG2 (liver) and Kaposi's cells, and necrosis in Skov-3 (ovarian cell line) when treated with MSC-EVs.
In a study carried out using human adult liver stem cell (HLSC)-EVs, Fonsato et al.94 reported induction of apoptosis in HepG2 hepatoma and primary hepatocellular carcinoma cells. Significant reduction in tumor growth was also observed in the presence of MV-HLSC in SCID mice inoculated with primary hepatocellular carcinoma cells.94 The authors concluded that the antitumor effects of HLSC-EVs could be because of selective delivery of miRNAs—a mechanism that may also explain the potential antitumor effects of MSC-EVs in some settings.
MicroRNA-9 has been associated with drug resistance via increasing the expression of P-glycoprotein.95 Munoz et al.95 reported that anti-miR-9-Cy5 was transferred from MSCs to glioblastoma multiforme cells via EVs, blocking the increase of P-glycoprotein and reversing the chemoresistance. Ono et al.96 reported that BM-MSC-EVs contributed to the dormant state of BM2 cells through EV-mediated transfer of miRNA.
MSC-EVs for Drug Delivery
EVs are natural transporters that may potentially reach a wide range of tissues following systemic administration, including the central nervous system as they have been reported to cross the blood–brain barrier.97 As EVs consist of a bilayered lipid membrane with an aqueous core they may potentially be loaded with both hydrophilic and lipophilic drugs.98 Furthermore, drugs could be either loaded into purified preparations of EVs99 or applied to parent cells and incorporated during EV biogenesis.100 Small molecules including siRNAs can also be loaded into the EVs either by electoporation or by chemical disruption.97,101 Although little explored to date, MSC-EVs may constitute a particularly promising vehicle for drug delivery given their inherent ability to exert disease-modulatory effects and the extensive literature documenting in vitro modification of MSCs using genetic and nongenetic approaches. As an example, Pascucci et al.102 observed that paclitaxel-treated MSCs mediated strong antitumorigenic effects because of their capacity to take up the drug and later release it in EVs. In this study, paclitaxel -treated MSC-EVs induced a dose-dependent inhibition of CFPAC-1 (human pancreatic adenocarcinoma) cell proliferation as well as 50% inhibition of tumor growth.
Clinical Translation of MSC-EVs: Unresolved Issues and Future Priorities
Tumorigenesis and other potential adverse effects of MSC-EVs
Despite reported antitumor effects in some settings, there is also theoretical potential for whole cell MSC therapy to directly or indirectly induce cancerous tumors or to accelerate the progression of pre-existing cancers. Although this concern has not, thus far, been borne out in human clinical trials, subpopulations of MSC-like cells have been found in the tumor microenvironment of several human cancers including gastric adenocarcinoma103 and osteosarcoma.104 Furthermore, some animal model studies have demonstrated preferential migration of intravenously administered MSCs to tumors.105,106 Although EVs clearly lack the potential to directly form tumors following in vivo administration, this does not imply that MSC-EV administration to human subjects is without any risk of promoting neoplasia. For instance, multiple myeloma (MM) cell proliferation has been shown to be increased in the presence of either autocrine or paracrine secretory factors of BM-MSCs.107,108 Roccaro et al.109 isolated EVs from BM-MSCs derived from both MM patients and healthy controls. In this study, the MM BM-MSC-derived EVs were found to promote MM tumor/cell growth, whereas normal BM-MSC-derived EVs inhibited the growth of MM tumor/cells both in vitro and in vivo. The MM BM-MSC-derived EVs were also found to induce cell dissemination and metastasis to distant BM niches.109
MSC-EVs have been found to modulate the tumor microenvironment, creating a niche for cancer cell metastasis and have been proven to mimic the effects of MSCs to promote tumor growth. Zhu et al.110 showed that MSC-EVs co-implanted with SGC-7901 (human gastric cancer) cells increased tumor growth and angiogenesis when compared with SGC-7901 cells alone. However, Lee et al.111 reported contradictory results suggesting that MSC-EVs suppress angiogenesis in vitro by downregulating the mRNA and protein levels of VEGF in tumor cells in a concentration-dependent manner. They speculated that this inconsistency could be because of different tumor types or MSC heterogeneity.111
Intravascular infusion of MSCs has been documented to cause embolism and death in experimental animals,112 whereas MSCs inoculated into infarcted myocardium were reported to induce adverse cellular growth such as cardiac sympathetic nerve sprouting.113 For adverse effects such as these, it appears likely that the risk associated with MSC-EV administration will be significantly lower or perhaps absent. However, as evidence of striking efficacy in a variety of disease settings now exists, it is incumbent on the research community to carefully evaluate the short- and long-term safety of biologically active EVs. Based on this limited information, it is clear that successful translation of MSC-EVs as a clinical therapy will require a significant amount of additional preclinical investigation of the interaction between MSC-EVs and tumor cells.
Large-scale EV production for clinical use
Although MSCs are relatively easy to expand using conventional tissue flasks and bioreactors, their growth in culture is finite and their biological properties may become altered with repeated passage. In order to facilitate large-scale MSC-EV production, new batches of MSCs will have to be periodically derived with significant impact on the costs of derivation, testing, and validation.114 Strategies such as MSC immortalization by natural selection or by genetic modification or clonal isolation could be used to overcome this limitation although this would also raise specific safety issues.115,116 Chen et al.117 proposed a robust scalable manufacturing process for therapeutic EVs through oncogenic immortalization of human embryonic stem cell (ESC)-derived MSCs. As EVs are isolated from media conditioned by cells, MSC culture in serum-free media would be of specific value to limit extraneous biological activity within the final therapeutic product. Other approaches to enhancing the purity of MSC-EVs preparations could include sequential centrifugation, filtration, and ultracentrifugation followed by sucrose density gradient to remove contaminating protein aggregates, cell debris, and genetic material.118,119,120 To scale up the amount of EVs isolated, bioreactors could be used to culture the MSCs.121 In this regard, a small number of studies have documented significant increases in EV yield from cells cultured in bioreactor systems when compared with conventional tissue culture flasks.122 It will be important, however, to also determine whether bioreactor culture conditions result in alterations to EV protein and RNA content that may impact on therapeutic efficacy.123,124 There are many challenges related to bioreactor culture including adequacy of oxygen supply, hydrodynamic shear stress, metabolic byproducts build-up, and pH balance.125,126 One should also be mindful that the impacts of such parameters are likely to differ for different cell types.
Conclusion
As we have summarized in this article, EVs can be readily isolated from MSCs of various origin and MSC-EVs are now known to have striking therapeutic benefits in a range of animal disease models. In some cases, these effects have been clearly shown to be of equal potency to those observed with whole cell MSC administration. The mechanisms underlying the anti-inflammatory and proregenerative effects of MSC-EVs have not yet been fully elucidated and are likely to vary from one disease target to another. Nonetheless, the fundamental basis for MSC-EV therapeutic effects lies in their ability to transmit biological information—in the form of proteins, glycoproteins, lipids, and ribonucleic acids—from stem cells to injured cells.
MSC-EVs have theoretical advantages over intact MSCs as a medicinal product and may, in the future, gain preference over whole cells in the discipline of regenerative medicine. However, in order for the field to advance to widespread clinical use of MSC-EVs for common human diseases, a range of important questions regarding their definition, standardization, cost-effective production, optimal dosing, and, most importantly, safety must be methodically addressed and answered.
Acknowledgments
The authors are supported by grants from the Health Research Board of Ireland (grant numbers HRA_POR/2013/341 (S.R., M.D.G., and T.R.) and HRA_HSR/2010/63 (M.D.G.)); the Irish Cancer Society (grant number CRF12RYA (A.E.R.)); Science Foundation Ireland [grant numbers 09/SRC/B1794 (M.D.G. and T.R.) and 12/IA/1624 (T.R.)); the European Union Framework 7 program (Health Collaborative Project VISICORT, grant number 602470 (M.D.G. and T.R.)); and the European Regional Development Fund.
References
- Ferrari G, Cusella-De Angelis G, Coletta M, Paolucci E, Stornaiuolo A, Cossu G, et al. Muscle regeneration by bone marrow-derived myogenic progenitors. Science. 1998;279:1528–1530. doi: 10.1126/science.279.5356.1528. [DOI] [PubMed] [Google Scholar]
- Barry FP, Murphy JM. Mesenchymal stem cells: clinical applications and biological characterization. Int J Biochem Cell Biol. 2004;36:568–584. doi: 10.1016/j.biocel.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Erices A, Conget P, Minguell JJ. Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol. 2000;109:235–242. doi: 10.1046/j.1365-2141.2000.01986.x. [DOI] [PubMed] [Google Scholar]
- Gang EJ, Jeong JA, Hong SH, Hwang SH, Kim SW, Yang IH, et al. Skeletal myogenic differentiation of mesenchymal stem cells isolated from human umbilical cord blood. Stem Cells. 2004;22:617–624. doi: 10.1634/stemcells.22-4-617. [DOI] [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Crapnell K, Blaesius R, Hastings A, Lennon DP, Caplan AI, Bruder SP. Growth, differentiation capacity, and function of mesenchymal stem cells expanded in serum-free medium developed via combinatorial screening. Exp Cell Res. 2013;319:1409–1418. doi: 10.1016/j.yexcr.2013.04.004. [DOI] [PubMed] [Google Scholar]
- Griffin MD, Ryan AE, Alagesan S, Lohan P, Treacy O, Ritter T. Anti-donor immune responses elicited by allogeneic mesenchymal stem cells: what have we learned so far. Immunol Cell Biol. 2013;91:40–51. doi: 10.1038/icb.2012.67. [DOI] [PubMed] [Google Scholar]
- González MA, Gonzalez-Rey E, Rico L, Büscher D, Delgado M. Adipose-derived mesenchymal stem cells alleviate experimental colitis by inhibiting inflammatory and autoimmune responses. Gastroenterology. 2009;136:978–989. doi: 10.1053/j.gastro.2008.11.041. [DOI] [PubMed] [Google Scholar]
- Xin H, Li Y, Chopp M. Exosomes/miRNAs as mediating cell-based therapy of stroke. Front Cell Neurosci. 2014;8:377. doi: 10.3389/fncel.2014.00377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang MX, He AN, Wang JA, Gui C. Protective paracrine effect of mesenchymal stem cells on cardiomyocytes. J Zhejiang Univ Sci B. 2009;10:619–624. doi: 10.1631/jzus.B0920153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JH, Hwang I, Hwang SH, Han H, Ha H. Human umbilical cord blood-derived mesenchymal stem cells prevent diabetic renal injury through paracrine action. Diabetes Res Clin Pract. 2012;98:465–473. doi: 10.1016/j.diabres.2012.09.034. [DOI] [PubMed] [Google Scholar]
- Karp JM, Leng Teo GS. Mesenchymal stem cell homing: the devil is in the details. Cell Stem Cell. 2009;4:206–216. doi: 10.1016/j.stem.2009.02.001. [DOI] [PubMed] [Google Scholar]
- Wagner J, Kean T, Young R, Dennis JE, Caplan AI. Optimizing mesenchymal stem cell-based therapeutics. Curr Opin Biotechnol. 2009;20:531–536. doi: 10.1016/j.copbio.2009.08.009. [DOI] [PubMed] [Google Scholar]
- Williams AR, Hare JM. Mesenchymal stem cells: biology, pathophysiology, translational findings, and therapeutic implications for cardiac disease. Circ Res. 2011;109:923–940. doi: 10.1161/CIRCRESAHA.111.243147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toma C, Pittenger MF, Cahill KS, Byrne BJ, Kessler PD. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation. 2002;105:93–98. doi: 10.1161/hc0102.101442. [DOI] [PubMed] [Google Scholar]
- Ringdén O, Uzunel M, Rasmusson I, Remberger M, Sundberg B, Lönnies H, et al. Mesenchymal stem cells for treatment of therapy-resistant graft-versus-host disease. Transplantation. 2006;81:1390–1397. doi: 10.1097/01.tp.0000214462.63943.14. [DOI] [PubMed] [Google Scholar]
- Lee RH, Pulin AA, Seo MJ, Kota DJ, Ylostalo J, Larson BL, et al. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009;5:54–63. doi: 10.1016/j.stem.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekkadan B, Milwid JM. Mesenchymal stem cells as therapeutics. Annu Rev Biomed Eng. 2010;12:87–117. doi: 10.1146/annurev-bioeng-070909-105309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tögel F, Hu Z, Weiss K, Isaac J, Lange C, Westenfelder C. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Renal Physiol. 2005;289:F31–F42. doi: 10.1152/ajprenal.00007.2005. [DOI] [PubMed] [Google Scholar]
- Tögel F, Weiss K, Yang Y, Hu Z, Zhang P, Westenfelder C. Vasculotropic, paracrine actions of infused mesenchymal stem cells are important to the recovery from acute kidney injury. Am J Physiol Renal Physiol. 2007;292:F1626–F1635. doi: 10.1152/ajprenal.00339.2006. [DOI] [PubMed] [Google Scholar]
- Li Y, Liu Z, Xin H, Chopp M. The role of astrocytes in mediating exogenous cell-based restorative therapy for stroke. Glia. 2014;62:1–16. doi: 10.1002/glia.22585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin H, Li Y, Liu Z, Wang X, Shang X, Cui Y, et al. MiR-133b promotes neural plasticity and functional recovery after treatment of stroke with multipotent mesenchymal stromal cells in rats via transfer of exosome-enriched extracellular particles. Stem Cells. 2013;31:2737–2746. doi: 10.1002/stem.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan BT, Johnstone RM. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell. 1983;33:967–978. doi: 10.1016/0092-8674(83)90040-5. [DOI] [PubMed] [Google Scholar]
- Simpson RJ, Jensen SS, Lim JW. Proteomic profiling of exosomes: current perspectives. Proteomics. 2008;8:4083–4099. doi: 10.1002/pmic.200800109. [DOI] [PubMed] [Google Scholar]
- Corcoran C, Rani S, O'Brien K, O'Neill A, Prencipe M, Sheikh R, et al. Docetaxel-resistance in prostate cancer: evaluating associated phenotypic changes and potential for resistance transfer via exosomes. PLoS One. 2012;7:e50999. doi: 10.1371/journal.pone.0050999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien K, Rani S, Corcoran C, Wallace R, Hughes L, Friel AM, et al. Exosomes from triple-negative breast cancer cells can transfer phenotypic traits representing their cells of origin to secondary cells. Eur J Cancer. 2013;49:1845–1859. doi: 10.1016/j.ejca.2013.01.017. [DOI] [PubMed] [Google Scholar]
- Blanchard N, Lankar D, Faure F, Regnault A, Dumont C, Raposo G, et al. TCR activation of human T cells induces the production of exosomes bearing the TCR/CD3/zeta complex. J Immunol. 2002;168:3235–3241. doi: 10.4049/jimmunol.168.7.3235. [DOI] [PubMed] [Google Scholar]
- Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, et al. B lymphocytes secrete antigen-presenting vesicles. J Exp Med. 1996;183:1161–1172. doi: 10.1084/jem.183.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Admyre C, Johansson SM, Paulie S, Gabrielsson S. Direct exosome stimulation of peripheral human T cells detected by ELISPOT. Eur J Immunol. 2006;36:1772–1781. doi: 10.1002/eji.200535615. [DOI] [PubMed] [Google Scholar]
- Li X, Li JJ, Yang JY, Wang DS, Zhao W, Song WJ, et al. Tolerance induction by exosomes from immature dendritic cells and rapamycin in a mouse cardiac allograft model. PLoS One. 2012;7:e44045. doi: 10.1371/journal.pone.0044045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heijnen HF, Schiel AE, Fijnheer R, Geuze HJ, Sixma JJ. Activated platelets release two types of membrane vesicles: microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and alpha-granules. Blood. 1999;94:3791–3799. [PubMed] [Google Scholar]
- Lai RC, Arslan F, Lee MM, Sze NS, Choo A, Chen TS, et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010;4:214–222. doi: 10.1016/j.scr.2009.12.003. [DOI] [PubMed] [Google Scholar]
- Raposo G, Tenza D, Mecheri S, Peronet R, Bonnerot C, Desaymard C. Accumulation of major histocompatibility complex class II molecules in mast cell secretory granules and their release upon degranulation. Mol Biol Cell. 1997;8:2631–2645. doi: 10.1091/mbc.8.12.2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Niel G, Raposo G, Candalh C, Boussac M, Hershberg R, Cerf-Bensussan N, et al. Intestinal epithelial cells secrete exosome-like vesicles. Gastroenterology. 2001;121:337–349. doi: 10.1053/gast.2001.26263. [DOI] [PubMed] [Google Scholar]
- van Balkom BW, de Jong OG, Smits M, Brummelman J, den Ouden K, de Bree PM, et al. Endothelial cells require miR-214 to secrete exosomes that suppress senescence and induce angiogenesis in human and mouse endothelial cells. Blood. 2013;121:3997–4006, S1. doi: 10.1182/blood-2013-02-478925. [DOI] [PubMed] [Google Scholar]
- Fauré J, Lachenal G, Court M, Hirrlinger J, Chatellard-Causse C, Blot B, et al. Exosomes are released by cultured cortical neurones. Mol Cell Neurosci. 2006;31:642–648. doi: 10.1016/j.mcn.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Février B, Raposo G. Exosomes: endosomal-derived vesicles shipping extracellular messages. Curr Opin Cell Biol. 2004;16:415–421. doi: 10.1016/j.ceb.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Gould SJ, Raposo G. As we wait: coping with an imperfect nomenclature for extracellular vesicles. J Extracell Vesicles. 2013;2 doi: 10.3402/jev.v2i0.20389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo M, Moita C, van Niel G, Kowal J, Vigneron J, Benaroch P, et al. Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J Cell Sci. 2013;126 Pt 24:5553–5565. doi: 10.1242/jcs.128868. [DOI] [PubMed] [Google Scholar]
- Mittelbrunn M, Sánchez-Madrid F. Intercellular communication: diverse structures for exchange of genetic information. Nat Rev Mol Cell Biol. 2012;13:328–335. doi: 10.1038/nrm3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan SS, Yin Y, Lee T, Lai RC, Yeo RW, Zhang B, et al. Therapeutic MSC exosomes are derived from lipid raft microdomains in the plasma membrane. J Extracell Vesicles. 2013;2 doi: 10.3402/jev.v2i0.22614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding C, Heuser J, Stahl P. Endocytosis and intracellular processing of transferrin and colloidal gold-transferrin in rat reticulocytes: demonstration of a pathway for receptor shedding. Eur J Cell Biol. 1984;35:256–263. [PubMed] [Google Scholar]
- Feng D, Zhao WL, Ye YY, Bai XC, Liu RQ, Chang LF, et al. Cellular internalization of exosomes occurs through phagocytosis. Traffic. 2010;11:675–687. doi: 10.1111/j.1600-0854.2010.01041.x. [DOI] [PubMed] [Google Scholar]
- Morelli AE, Larregina AT, Shufesky WJ, Sullivan ML, Stolz DB, Papworth GD, et al. Endocytosis, intracellular sorting, and processing of exosomes by dendritic cells. Blood. 2004;104:3257–3266. doi: 10.1182/blood-2004-03-0824. [DOI] [PubMed] [Google Scholar]
- Svensson KJ, Christianson HC, Wittrup A, Bourseau-Guilmain E, Lindqvist E, Svensson LM, et al. Exosome uptake depends on ERK1/2-heat shock protein 27 signaling and lipid Raft-mediated endocytosis negatively regulated by caveolin-1. J Biol Chem. 2013;288:17713–17724. doi: 10.1074/jbc.M112.445403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akers JC, Gonda D, Kim R, Carter BS, Chen CC. Biogenesis of extracellular vesicles (EV): exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. J Neurooncol. 2013;113:1–11. doi: 10.1007/s11060-013-1084-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergo MO, Gavino BJ, Steenbergen R, Sturbois B, Parlow AF, Sanan DA, et al. Defining the importance of phosphatidylserine synthase 2 in mice. J Biol Chem. 2002;277:47701–47708. doi: 10.1074/jbc.M207734200. [DOI] [PubMed] [Google Scholar]
- Zwaal RF, Schroit AJ. Pathophysiologic implications of membrane phospholipid asymmetry in blood cells. Blood. 1997;89:1121–1132. [PubMed] [Google Scholar]
- D'Souza-Schorey C, Chavrier P. ARF proteins: roles in membrane traffic and beyond. Nat Rev Mol Cell Biol. 2006;7:347–358. doi: 10.1038/nrm1910. [DOI] [PubMed] [Google Scholar]
- Muralidharan-Chari V, Clancy J, Plou C, Romao M, Chavrier P, Raposo G, et al. ARF6-regulated shedding of tumor cell-derived plasma membrane microvesicles. Curr Biol. 2009;19:1875–1885. doi: 10.1016/j.cub.2009.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen DH, Catling AD, Webb DJ, Sankovic M, Walker LA, Somlyo AV, et al. Myosin light chain kinase functions downstream of Ras/ERK to promote migration of urokinase-type plasminogen activator-stimulated cells in an integrin-selective manner. J Cell Biol. 1999;146:149–164. doi: 10.1083/jcb.146.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muralidharan-Chari V, Clancy JW, Sedgwick A, D'Souza-Schorey C. Microvesicles: mediators of extracellular communication during cancer progression. J Cell Sci. 2010;123 Pt 10:1603–1611. doi: 10.1242/jcs.064386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpinen L, Impola U, Sankkila L, Ritamo I, Aatonen M, Kilpinen S, et al. Extracellular membrane vesicles from umbilical cord blood-derived MSC protect against ischemic acute kidney injury, a feature that is lost after inflammatory conditioning. J Extracell Vesicles. 2013;2 doi: 10.3402/jev.v2i0.21927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon C, Ryan J, Sobrevia L, Kobayashi M, Ashman K, Mitchell M, et al. Exosomal signaling during hypoxia mediates microvascular endothelial cell migration and vasculogenesis. PLoS One. 2013;8:e68451. doi: 10.1371/journal.pone.0068451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lespagnol A, Duflaut D, Beekman C, Blanc L, Fiucci G, Marine JC, et al. Exosome secretion, including the DNA damage-induced p53-dependent secretory pathway, is severely compromised in TSAP6/Steap3-null mice. Cell Death Differ. 2008;15:1723–1733. doi: 10.1038/cdd.2008.104. [DOI] [PubMed] [Google Scholar]
- Feng Z. p53 regulation of the IGF-1/AKT/mTOR pathways and the endosomal compartment. Cold Spring Harb Perspect Biol. 2010;2:a001057. doi: 10.1101/cshperspect.a001057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Harris SL, Levine AJ. The regulation of exosome secretion: a novel function of the p53 protein. Cancer Res. 2006;66:4795–4801. doi: 10.1158/0008-5472.CAN-05-4579. [DOI] [PubMed] [Google Scholar]
- Baietti MF, Zhang Z, Mortier E, Melchior A, Degeest G, Geeraerts A, et al. Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat Cell Biol. 2012;14:677–685. doi: 10.1038/ncb2502. [DOI] [PubMed] [Google Scholar]
- Skog J, Würdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M, et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10:1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- Yu B, Zhang X, Li X. Exosomes derived from mesenchymal stem cells. Int J Mol Sci. 2014;15:4142–4157. doi: 10.3390/ijms15034142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokarizadeh A, Delirezh N, Morshedi A, Mosayebi G, Farshid AA, Mardani K. Microvesicles derived from mesenchymal stem cells: potent organelles for induction of tolerogenic signaling. Immunol Lett. 2012;147:47–54. doi: 10.1016/j.imlet.2012.06.001. [DOI] [PubMed] [Google Scholar]
- Bruno S, Grange C, Deregibus MC, Calogero RA, Saviozzi S, Collino F, et al. Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J Am Soc Nephrol. 2009;20:1053–1067. doi: 10.1681/ASN.2008070798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmers L, Lim SK, Arslan F, Armstrong JS, Hoefer IE, Doevendans PA, et al. Reduction of myocardial infarct size by human mesenchymal stem cell conditioned medium. Stem Cell Res. 2007;1:129–137. doi: 10.1016/j.scr.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Arslan F, Lai RC, Smeets MB, Akeroyd L, Choo A, Aguor EN, et al. Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Res. 2013;10:301–312. doi: 10.1016/j.scr.2013.01.002. [DOI] [PubMed] [Google Scholar]
- Sluijter JP, Verhage V, Deddens JC, van den Akker F, Doevendans PA. Microvesicles and exosomes for intracardiac communication. Cardiovasc Res. 2014;102:302–311. doi: 10.1093/cvr/cvu022. [DOI] [PubMed] [Google Scholar]
- Bian S, Zhang L, Duan L, Wang X, Min Y, Yu H. Extracellular vesicles derived from human bone marrow mesenchymal stem cells promote angiogenesis in a rat myocardial infarction model. J Mol Med (Berl) 2014;92:387–397. doi: 10.1007/s00109-013-1110-5. [DOI] [PubMed] [Google Scholar]
- Feng Y, Huang W, Wani M, Yu X, Ashraf M. Ischemic preconditioning potentiates the protective effect of stem cells through secretion of exosomes by targeting Mecp2 via miR-22. PLoS One. 2014;9:e88685. doi: 10.1371/journal.pone.0088685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu B, Gong M, Wang Y, Millard RW, Pasha Z, Yang Y, et al. Cardiomyocyte protection by GATA-4 gene engineered mesenchymal stem cells is partially mediated by translocation of miR-221 in microvesicles. PLoS One. 2013;8:e73304. doi: 10.1371/journal.pone.0073304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellomo R, Kellum JA, Ronco C. Acute kidney injury. Lancet. 2012;380:756–766. doi: 10.1016/S0140-6736(11)61454-2. [DOI] [PubMed] [Google Scholar]
- Hu J, Zhang L, Wang N, Ding R, Cui S, Zhu F, et al. Mesenchymal stem cells attenuate ischemic acute kidney injury by inducing regulatory T cells through splenocyte interactions. Kidney Int. 2013;84:521–531. doi: 10.1038/ki.2013.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys BD, Bonventre JV. Mesenchymal stem cells in acute kidney injury. Annu Rev Med. 2008;59:311–325. doi: 10.1146/annurev.med.59.061506.154239. [DOI] [PubMed] [Google Scholar]
- Imberti B, Morigi M, Benigni A. Potential of mesenchymal stem cells in the repair of tubular injury. Kidney Int Suppl (2011) 2011;1:90–93. doi: 10.1038/kisup.2011.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng K, Rai P, Plagov A, Lan X, Kumar D, Salhan D, et al. Transplantation of bone marrow-derived MSCs improves cisplatinum-induced renal injury through paracrine mechanisms. Exp Mol Pathol. 2013;94:466–473. doi: 10.1016/j.yexmp.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du T, Zhu YJ. The regulation of inflammatory mediators in acute kidney injury via exogenous mesenchymal stem cells. Mediators Inflamm. 2014;2014:261697. doi: 10.1155/2014/261697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Wang Y, Sun S, Yu M, Wang C, Pei X, et al. Bone marrow stem cells-derived microvesicles protect against renal injury in the mouse remnant kidney model. Nephrology (Carlton) 2012;17:493–500. doi: 10.1111/j.1440-1797.2012.01589.x. [DOI] [PubMed] [Google Scholar]
- Gatti S, Bruno S, Deregibus MC, Sordi A, Cantaluppi V, Tetta C, et al. Microvesicles derived from human adult mesenchymal stem cells protect against ischaemia-reperfusion-induced acute and chronic kidney injury. Nephrol Dial Transplant. 2011;26:1474–1483. doi: 10.1093/ndt/gfr015. [DOI] [PubMed] [Google Scholar]
- Bruno S, Grange C, Collino F, Deregibus MC, Cantaluppi V, Biancone L, et al. Microvesicles derived from mesenchymal stem cells enhance survival in a lethal model of acute kidney injury. PLoS One. 2012;7:e33115. doi: 10.1371/journal.pone.0033115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasoni S, Longaretti L, Rota C, Morigi M, Conti S, Gotti E, et al. Transfer of growth factor receptor mRNA via exosomes unravels the regenerative effect of mesenchymal stem cells. Stem Cells Dev. 2013;22:772–780. doi: 10.1089/scd.2012.0266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grange C, Tapparo M, Bruno S, Chatterjee D, Quesenberry PJ, Tetta C, et al. Biodistribution of mesenchymal stem cell-derived extracellular vesicles in a model of acute kidney injury monitored by optical imaging. Int J Mol Med. 2014;33:1055–1063. doi: 10.3892/ijmm.2014.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biancone L, Bruno S, Deregibus MC, Tetta C, Camussi G. Therapeutic potential of mesenchymal stem cell-derived microvesicles. Nephrol Dial Transplant. 2012;27:3037–3042. doi: 10.1093/ndt/gfs168. [DOI] [PubMed] [Google Scholar]
- Volarevic V, Nurkovic J, Arsenijevic N, Stojkovic M. Concise review: therapeutic potential of mesenchymal stem cells for the treatment of acute liver failure and cirrhosis. Stem Cells. 2014;32:2818–2823. doi: 10.1002/stem.1818. [DOI] [PubMed] [Google Scholar]
- Kanazawa H, Fujimoto Y, Teratani T, Iwasaki J, Kasahara N, Negishi K, et al. Bone marrow-derived mesenchymal stem cells ameliorate hepatic ischemia reperfusion injury in a rat model. PLoS One. 2011;6:e19195. doi: 10.1371/journal.pone.0019195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Yan Y, Wang B, Qian H, Zhang X, Shen L, et al. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate liver fibrosis. Stem Cells Dev. 2013;22:845–854. doi: 10.1089/scd.2012.0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan CY, Lai RC, Wong W, Dan YY, Lim SK, Ho HK. Mesenchymal stem cell-derived exosomes promote hepatic regeneration in drug-induced liver injury models. Stem Cell Res Ther. 2014;5:76. doi: 10.1186/scrt465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu YG, Feng XM, Abbott J, Fang XH, Hao Q, Monsel A, et al. Human mesenchymal stem cell microvesicles for treatment of Escherichia coli endotoxin-induced acute lung injury in mice. Stem Cells. 2014;32:116–125. doi: 10.1002/stem.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano T, Deterding RR, Simonet WS, Shannon JM, Mason RJ. Keratinocyte growth factor reduces lung damage due to acid instillation in rats. Am J Respir Cell Mol Biol. 1996;15:433–442. doi: 10.1165/ajrcmb.15.4.8879176. [DOI] [PubMed] [Google Scholar]
- Viget NB, Guery BP, Ader F, Nevière R, Alfandari S, Creuzy C, et al. Keratinocyte growth factor protects against Pseudomonas aeruginosa-induced lung injury. Am J Physiol Lung Cell Mol Physiol. 2000;279:L1199–L1209. doi: 10.1152/ajplung.2000.279.6.L1199. [DOI] [PubMed] [Google Scholar]
- Lee JW, Fang X, Gupta N, Serikov V, Matthay MA. Allogeneic human mesenchymal stem cells for treatment of E. coli endotoxin-induced acute lung injury in the ex vivo perfused human lung. Proc Natl Acad Sci USA. 2009;106:16357–16362. doi: 10.1073/pnas.0907996106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C, Mitsialis SA, Aslam M, Vitali SH, Vergadi E, Konstantinou G, et al. Exosomes mediate the cytoprotective action of mesenchymal stromal cells on hypoxia-induced pulmonary hypertension. Circulation. 2012;126:2601–2611. doi: 10.1161/CIRCULATIONAHA.112.114173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Wang M, Gong A, Zhang X, Wu X, Zhu Y, et al. HucMSC-exosome mediated-Wnt4 signaling is required for cutaneous wound healing. Stem Cells (Dayton, Ohio) 2014. [DOI] [PubMed]
- Wu S, Ju GQ, Du T, Zhu YJ, Liu GH. Microvesicles derived from human umbilical cord Wharton's jelly mesenchymal stem cells attenuate bladder tumor cell growth in vitro and in vivo. PLoS One. 2013;8:e61366. doi: 10.1371/journal.pone.0061366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno S, Collino F, Deregibus MC, Grange C, Tetta C, Camussi G. Microvesicles derived from human bone marrow mesenchymal stem cells inhibit tumor growth. Stem Cells Dev. 2013;22:758–771. doi: 10.1089/scd.2012.0304. [DOI] [PubMed] [Google Scholar]
- Fonsato V, Collino F, Herrera MB, Cavallari C, Deregibus MC, Cisterna B, et al. Human liver stem cell-derived microvesicles inhibit hepatoma growth in SCID mice by delivering antitumor microRNAs. Stem Cells. 2012;30:1985–1998. doi: 10.1002/stem.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz JL, Bliss SA, Greco SJ, Ramkissoon SH, Ligon KL, Rameshwar P. Delivery of functional anti-miR-9 by mesenchymal stem cell-derived exosomes to glioblastoma multiforme cells conferred chemosensitivity. Mol Ther Nucleic Acids. 2013;2:e126. doi: 10.1038/mtna.2013.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono M, Kosaka N, Tominaga N, Yoshioka Y, Takeshita F, Takahashi RU, et al. Exosomes from bone marrow mesenchymal stem cells contain a microRNA that promotes dormancy in metastatic breast cancer cells. Sci Signal. 2014;7:ra63. doi: 10.1126/scisignal.2005231. [DOI] [PubMed] [Google Scholar]
- Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011;29:341–345. doi: 10.1038/nbt.1807. [DOI] [PubMed] [Google Scholar]
- Lai RC, Yeo RW, Tan KH, Lim SK. Exosomes for drug delivery—a novel application for the mesenchymal stem cell. Biotechnol Adv. 2013;31:543–551. doi: 10.1016/j.biotechadv.2012.08.008. [DOI] [PubMed] [Google Scholar]
- Sun D, Zhuang X, Xiang X, Liu Y, Zhang S, Liu C, et al. A novel nanoparticle drug delivery system: the anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol Ther. 2010;18:1606–1614. doi: 10.1038/mt.2010.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Y, Wu N, Gan X, Yan W, Morrell JC, Gould SJ. Higher-order oligomerization targets plasma membrane proteins and HIV gag to exosomes. PLoS Biol. 2007;5:e158. doi: 10.1371/journal.pbio.0050158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlgren J, De L Karlson T, Brisslert M, Vaziri Sani F, Telemo E, Sunnerhagen P, et al. Plasma exosomes can deliver exogenous short interfering RNA to monocytes and lymphocytes. Nucleic Acids Res. 2012;40:e130. doi: 10.1093/nar/gks463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascucci L, Coccè V, Bonomi A, Ami D, Ceccarelli P, Ciusani E, et al. Paclitaxel is incorporated by mesenchymal stromal cells and released in exosomes that inhibit in vitro tumor growth: a new approach for drug delivery. J Control Release. 2014;192:262–270. doi: 10.1016/j.jconrel.2014.07.042. [DOI] [PubMed] [Google Scholar]
- Xu X, Zhang X, Wang S, Qian H, Zhu W, Cao H, et al. Isolation and comparison of mesenchymal stem-like cells from human gastric cancer and adjacent non-cancerous tissues. J Cancer Res Clin Oncol. 2011;137:495–504. doi: 10.1007/s00432-010-0908-6. [DOI] [PubMed] [Google Scholar]
- Brune JC, Tormin A, Johansson MC, Rissler P, Brosjö O, Löfvenberg R, et al. Mesenchymal stromal cells from primary osteosarcoma are non-malignant and strikingly similar to their bone marrow counterparts. Int J Cancer. 2011;129:319–330. doi: 10.1002/ijc.25697. [DOI] [PubMed] [Google Scholar]
- Beckermann BM, Kallifatidis G, Groth A, Frommhold D, Apel A, Mattern J, et al. VEGF expression by mesenchymal stem cells contributes to angiogenesis in pancreatic carcinoma. Br J Cancer. 2008;99:622–631. doi: 10.1038/sj.bjc.6604508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinagawa K, Kitadai Y, Tanaka M, Sumida T, Kodama M, Higashi Y, et al. Mesenchymal stem cells enhance growth and metastasis of colon cancer. Int J Cancer. 2010;127:2323–2333. doi: 10.1002/ijc.25440. [DOI] [PubMed] [Google Scholar]
- Chauhan D, Neri P, Velankar M, Podar K, Hideshima T, Fulciniti M, et al. Targeting mitochondrial factor Smac/DIABLO as therapy for multiple myeloma (MM) Blood. 2007;109:1220–1227. doi: 10.1182/blood-2006-04-015149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hideshima T, Chauhan D, Kiziltepe T, Ikeda H, Okawa Y, Podar K, et al. Biologic sequelae of I{kappa}B kinase (IKK) inhibition in multiple myeloma: therapeutic implications. Blood. 2009;113:5228–5236. doi: 10.1182/blood-2008-06-161505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roccaro AM, Sacco A, Maiso P, Azab AK, Tai YT, Reagan M, et al. BM mesenchymal stromal cell-derived exosomes facilitate multiple myeloma progression. J Clin Invest. 2013;123:1542–1555. doi: 10.1172/JCI66517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W, Huang L, Li Y, Zhang X, Gu J, Yan Y, et al. Exosomes derived from human bone marrow mesenchymal stem cells promote tumor growth in vivo. Cancer Lett. 2012;315:28–37. doi: 10.1016/j.canlet.2011.10.002. [DOI] [PubMed] [Google Scholar]
- Lee JK, Park SR, Jung BK, Jeon YK, Lee YS, Kim MK, et al. Exosomes derived from mesenchymal stem cells suppress angiogenesis by down-regulating VEGF expression in breast cancer cells. PLoS One. 2013;8:e84256. doi: 10.1371/journal.pone.0084256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furlani D, Ugurlucan M, Ong L, Bieback K, Pittermann E, Westien I, et al. Is the intravascular administration of mesenchymal stem cells safe? Mesenchymal stem cells and intravital microscopy. Microvasc Res. 2009;77:370–376. doi: 10.1016/j.mvr.2009.02.001. [DOI] [PubMed] [Google Scholar]
- Pak HN, Qayyum M, Kim DT, Hamabe A, Miyauchi Y, Lill MC, et al. Mesenchymal stem cell injection induces cardiac nerve sprouting and increased tenascin expression in a Swine model of myocardial infarction. J Cardiovasc Electrophysiol. 2003;14:841–848. doi: 10.1046/j.1540-8167.2003.03124.x. [DOI] [PubMed] [Google Scholar]
- Indira V, Corteling R, Stevanato L, Hicks C, Sinden J. The development of stem cell-derived exosomes as a cell-free regenerative medicine. J Circ Biomark. 2014;3 doi: 10.5772/58597. [DOI] [Google Scholar]
- Yeo RW, Lai RC, Zhang B, Tan SS, Yin Y, Teh BJ, et al. Mesenchymal stem cell: an efficient mass producer of exosomes for drug delivery. Adv Drug Deliv Rev. 2013;65:336–341. doi: 10.1016/j.addr.2012.07.001. [DOI] [PubMed] [Google Scholar]
- Pollock K, Stroemer P, Patel S, Stevanato L, Hope A, Miljan E, et al. A conditionally immortal clonal stem cell line from human cortical neuroepithelium for the treatment of ischemic stroke. Exp Neurol. 2006;199:143–155. doi: 10.1016/j.expneurol.2005.12.011. [DOI] [PubMed] [Google Scholar]
- Chen TS, Arslan F, Yin Y, Tan SS, Lai RC, Choo AB, et al. Enabling a robust scalable manufacturing process for therapeutic exosomes through oncogenic immortalization of human ESC-derived MSCs. J Transl Med. 2011;9:47. doi: 10.1186/1479-5876-9-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rani S, O'Brien K, Kelleher FC, Corcoran C, Germano S, Radomski MW, et al. Isolation of exosomes for subsequent mRNA, microRNA, and protein profiling. Methods Mol Biol. 2011;784:181–195. doi: 10.1007/978-1-61779-289-2_13. [DOI] [PubMed] [Google Scholar]
- Lamparski HG, Metha-Damani A, Yao JY, Patel S, Hsu DH, Ruegg C, et al. Production and characterization of clinical grade exosomes derived from dendritic cells. J Immunol Methods. 2002;270:211–226. doi: 10.1016/s0022-1759(02)00330-7. [DOI] [PubMed] [Google Scholar]
- Webber J, Clayton A. How pure are your vesicles. J Extracell Vesicles. 2013;2 doi: 10.3402/jev.v2i0.19861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hupfeld J, Gorr IH, Schwald C, Beaucamp N, Wiechmann K, Kuentzer K, et al. Modulation of mesenchymal stromal cell characteristics by microcarrier culture in bioreactors. Biotechnol Bioeng. 2014;111:2290–2302. doi: 10.1002/bit.25281. [DOI] [PubMed] [Google Scholar]
- Mitchell JP, Court J, Mason MD, Tabi Z, Clayton A. Increased exosome production from tumour cell cultures using the Integra CELLine Culture System. J Immunol Methods. 2008;335:98–105. doi: 10.1016/j.jim.2008.03.001. [DOI] [PubMed] [Google Scholar]
- de Jong OG, Verhaar MC, Chen Y, Vader P, Gremmels H, Posthuma G, et al. Cellular stress conditions are reflected in the protein and RNA content of endothelial cell-derived exosomes. J Extracell Vesicles. 2012;1 doi: 10.3402/jev.v1i0.18396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara AR, Galindo E, Ramírez OT, Palomares LA. Living with heterogeneities in bioreactors: understanding the effects of environmental gradients on cells. Mol Biotechnol. 2006;34:355–381. doi: 10.1385/MB:34:3:355. [DOI] [PubMed] [Google Scholar]
- King JA, Miller WM. Bioreactor development for stem cell expansion and controlled differentiation. Curr Opin Chem Biol. 2007;11:394–398. doi: 10.1016/j.cbpa.2007.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeatts AB, Choquette DT, Fisher JP. Bioreactors to influence stem cell fate: augmentation of mesenchymal stem cell signaling pathways via dynamic culture systems. Biochim Biophys Acta. 2013;1830:2470–2480. doi: 10.1016/j.bbagen.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henne WM, Buchkovich NJ, Emr SD. The ESCRT pathway. Dev Cell. 2011;21:77–91. doi: 10.1016/j.devcel.2011.05.015. [DOI] [PubMed] [Google Scholar]
- Roxrud I, Stenmark H, Malerød L. ESCRT & Co. Biol Cell. 2010;102:293–318. doi: 10.1042/BC20090161. [DOI] [PubMed] [Google Scholar]
- Wollert T, Wunder C, Lippincott-Schwartz J, Hurley JH. Membrane scission by the ESCRT-III complex. Nature. 2009;458:172–177. doi: 10.1038/nature07836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley JH, Hanson PI. Membrane budding and scission by the ESCRT machinery: it's all in the neck. Nat Rev Mol Cell Biol. 2010;11:556–566. doi: 10.1038/nrm2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malerød L, Stuffers S, Brech A, Stenmark H. Vps22/EAP30 in ESCRT-II mediates endosomal sorting of growth factor and chemokine receptors destined for lysosomal degradation. Traffic. 2007;8:1617–1629. doi: 10.1111/j.1600-0854.2007.00630.x. [DOI] [PubMed] [Google Scholar]
- Bowers K, Piper SC, Edeling MA, Gray SR, Owen DJ, Lehner PJ, et al. Degradation of endocytosed epidermal growth factor and virally ubiquitinated major histocompatibility complex class I is independent of mammalian ESCRTII. J Biol Chem. 2006;281:5094–5105. doi: 10.1074/jbc.M508632200. [DOI] [PubMed] [Google Scholar]
- Ghossoub R, Lembo F, Rubio A, Gaillard CB, Bouchet J, Vitale N, et al. Syntenin-ALIX exosome biogenesis and budding into multivesicular bodies are controlled by ARF6 and PLD2. Nat Commun. 2014;5:3477. doi: 10.1038/ncomms4477. [DOI] [PubMed] [Google Scholar]
- Tian T, Zhu YL, Zhou YY, Liang GF, Wang YY, Hu FH, et al. Exosome uptake through clathrin-mediated endocytosis and macropinocytosis and mediating miR-21 delivery. J Biol Chem. 2014;289:22258–22267. doi: 10.1074/jbc.M114.588046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norgan AP, Davies BA, Azmi IF, Schroeder AS, Payne JA, Lynch GM, et al. Relief of autoinhibition enhances Vta1 activation of Vps4 via the Vps4 stimulatory element. J Biol Chem. 2013;288:26147–26156. doi: 10.1074/jbc.M113.494112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuh AL, Audhya A. The ESCRT machinery: from the plasma membrane to endosomes and back again. Crit Rev Biochem Mol Biol. 2014;49:242–261. doi: 10.3109/10409238.2014.881777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musante L, Tataruch DE, Holthofer H. Use and isolation of urinary exosomes as biomarkers for diabetic nephropathy. Front Endocrinol (Lausanne) 2014;5:149. doi: 10.3389/fendo.2014.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amit I, Yakir L, Katz M, Zwang Y, Marmor MD, Citri A, et al. Tal, a Tsg101-specific E3 ubiquitin ligase, regulates receptor endocytosis and retrovirus budding. Genes Dev. 2004;18:1737–1752. doi: 10.1101/gad.294904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Q, Hope LW, Brasch M, Reinhard C, Cohen SN. TSG101 interaction with HRS mediates endosomal trafficking and receptor down-regulation. Proc Natl Acad Sci USA. 2003;100:7626–7631. doi: 10.1073/pnas.0932599100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conibear E. An ESCRT into the endosome. Mol Cell. 2002;10:215–216. doi: 10.1016/s1097-2765(02)00601-9. [DOI] [PubMed] [Google Scholar]
- Buschow SI, van Balkom BW, Aalberts M, Heck AJ, Wauben M, Stoorvogel W. MHC class II-associated proteins in B-cell exosomes and potential functional implications for exosome biogenesis. Immunol Cell Biol. 2010;88:851–856. doi: 10.1038/icb.2010.64. [DOI] [PubMed] [Google Scholar]
- Hoshino D, Kirkbride KC, Costello K, Clark ES, Sinha S, Grega-Larson N, et al. Exosome secretion is enhanced by invadopodia and drives invasive behavior. Cell Rep. 2013;5:1159–1168. doi: 10.1016/j.celrep.2013.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu C, Morohashi Y, Yoshimura S, Manrique-Hoyos N, Jung S, Lauterbach MA, et al. Regulation of exosome secretion by Rab35 and its GTPase-activating proteins TBC1D10A-C. J Cell Biol. 2010;189:223–232. doi: 10.1083/jcb.200911018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrowski M, Carmo NB, Krumeich S, Fanget I, Raposo G, Savina A, et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol. 2010;12:19–30; supp p 1. doi: 10.1038/ncb2000. [DOI] [PubMed] [Google Scholar]
- Ju R, Zhuang ZW, Zhang J, Lanahan AA, Kyriakides T, Sessa WC, et al. Angiopoietin-2 secretion by endothelial cell exosomes: regulation by the phosphatidylinositol 3-kinase (PI3K)/Akt/endothelial nitric oxide synthase (eNOS) and syndecan-4/syntenin pathways. J Biol Chem. 2014;289:510–519. doi: 10.1074/jbc.M113.506899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Curtis I, Meldolesi J. Cell surface dynamics - how Rho GTPases orchestrate the interplay between the plasma membrane and the cortical cytoskeleton. J Cell Sci. 2012;125 Pt 19:4435–4444. doi: 10.1242/jcs.108266. [DOI] [PubMed] [Google Scholar]
- Fader CM, Sánchez DG, Mestre MB, Colombo MI. TI-VAMP/VAMP7 and VAMP3/cellubrevin: two v-SNARE proteins involved in specific steps of the autophagy/multivesicular body pathways. Biochim Biophys Acta. 2009;1793:1901–1916. doi: 10.1016/j.bbamcr.2009.09.011. [DOI] [PubMed] [Google Scholar]
- Alonso R, Mazzeo C, Rodriguez MC, Marsh M, Fraile-Ramos A, Calvo V, et al. Diacylglycerol kinase α regulates the formation and polarisation of mature multivesicular bodies involved in the secretion of Fas ligand-containing exosomes in T lymphocytes. Cell Death Differ. 2011;18:1161–1173. doi: 10.1038/cdd.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laulagnier K, Grand D, Dujardin A, Hamdi S, Vincent-Schneider H, Lankar D, et al. PLD2 is enriched on exosomes and its activity is correlated to the release of exosomes. FEBS Lett. 2004;572:11–14. doi: 10.1016/j.febslet.2004.06.082. [DOI] [PubMed] [Google Scholar]
- Urbanelli L, Magini A, Buratta S, Brozzi A, Sagini K, Polchi A, et al. Signaling pathways in exosomes biogenesis, secretion and fate. Genes (Basel) 2013;4:152–170. doi: 10.3390/genes4020152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Record M, Subra C, Silvente-Poirot S, Poirot M. Exosomes as intercellular signalosomes and pharmacological effectors. Biochem Pharmacol. 2011;81:1171–1182. doi: 10.1016/j.bcp.2011.02.011. [DOI] [PubMed] [Google Scholar]
- Guo Z, Turner C, Castle D. Relocation of the t-SNARE SNAP-23 from lamellipodia-like cell surface projections regulates compound exocytosis in mast cells. Cell. 1998;94:537–548. doi: 10.1016/s0092-8674(00)81594-9. [DOI] [PubMed] [Google Scholar]
- Paumet F, Le Mao J, Martin S, Galli T, David B, Blank U, et al. Soluble NSF attachment protein receptors (SNAREs) in RBL-2H3 mast cells: functional role of syntaxin 4 in exocytosis and identification of a vesicle-associated membrane protein 8-containing secretory compartment. J Immunol. 2000;164:5850–5857. doi: 10.4049/jimmunol.164.11.5850. [DOI] [PubMed] [Google Scholar]
- Pelham HR. SNAREs and the specificity of membrane fusion. Trends Cell Biol. 2001;11:99–101. doi: 10.1016/s0962-8924(01)01929-8. [DOI] [PubMed] [Google Scholar]