Abstract
Venous thromboembolism (VTE) is a leading cause of death among outpatient chemotherapy patients. However the VTE preventive measures for outpatients are not widely advocated. We did a meta-analysis to evaluate the outpatient VTE prevention's effectiveness and safety. We searched electronic databases until the end of December 2012 and reviewed the abstracts and manuscripts following the PRISMA guidelines. Occurrence of first VTE event was the efficacy outcome. The safety end point was major bleeding. We calculated Q statistic and a homogeneity formal test. The odds ratio (OR) estimates were pooled by using the Mantel–Haenszel fixed-effects method in the absence of heterogeneity. Data were analyzed using the R META package). We identified 1,485 articles and reviewed 37 articles based on initial screening. The number of patients included in 11 selected trials was 7,805. The odds of VTE was lower in the prophylaxis group (OR 0.56; 95 % CI 0.45–0.71) and improved when heparin-based prevention was analyzed (OR 0.53; 95 % CI 0.41–0.70). We found strong prevention among patients with lung cancer (OR 0.46; 95 % CI 0.29–0.74) and pancreatic cancer (OR 0.33; 95 % CI 0.16–0.67). Major bleeding events were frequent in the intervention group (OR 1.65; 95 % CI 1.12–2.44). Thromboprophylaxis reduced VTE episodes. The VTE events were reduced by 47 % in heparin-based prophylaxis trials compared to placebo. The patients receiving heparin-based prophylaxis had a 60 % increase in bleeding events. Improving risk stratification tools to personalize prevention strategies may enhance the VTE prevention applicability in cancer patients.
Keywords: Cancer, Thromboembolism, Prevention
Introduction
Venous thromboembolism (VTE) is one of the most common cardiovascular diseases in the United States. It is associated with active cancer in 20 % of the cases [1, 2]. VTE is a leading cause of death among patients receiving outpatient chemotherapy [3]. In a prospective observational study among 4,466 patients with cancer, VTE was associated to 9.2 % of the deaths, second only to progression of malignancy as cause of death [3]. The one-year survival rate of patients with cancer and VTE is a third of those without thrombosis [4]. Not only is VTE a potentially preventable costly disease, with an estimated cost per hospitalization over $20,000 [5]; the presence of VTE often delays adequate treatment for patients with malignancy.
While patients with cancer are frequently exposed to VTE risk factors such as surgery and prolonged hospital stay; chemotherapy is among the strongest risk factors for VTE [1, 6]. Using information from a population-based, case–control study of 625 patients with a first VTE, Heit et al. described that active cancer is associated with four times the risk of VTE (OR 4.1; 95 % CI 1.9–8.5). However those patients on chemotherapy have six times higher odds (OR 6.5; 95 % CI 2.1–20.2) of VTE than patients without malignancy [7, 8]. Despite this strong association current guidelines advocate VTE preventive measures in the inpatient setting and post operatively, but guidance regarding antithrombotic prophylaxis in patients receiving ambulatory chemotherapy is scarce [6]. Specifically, The National Comprehensive Cancer Network prophylactic anticoagulation guideline recommendations consist of providing pharmacological prophylaxis for inpatients [9]. Concordant with the European Society for Medical Oncology, the American Society of Clinical Oncology does not recommend routine prophylaxis except for patients with multiple myeloma receiving immunomodulatory agents [10, 11]. The American College of Chest Physicians recommends against routine prophylaxis in patients with no additional risk factors for VTE [12, 13]. The guidelines state that patients with solid tumor are likely to benefit from a primary thromboprophylactic approach in cases with high thrombosis risk and low bleeding risk, but these risk thresholds are not well standardized.
In the recent International guidelines on thrombosis and cancer prophylaxis was not recommended in patients undergoing chemotherapy; except for locally advanced or metastatic pancreatic or lung cancer being treated with chemotherapy [4]. To measure safety and efficacy of out-patient primary VTE prophylaxis in patients with solid tumors receiving chemotherapy, we have designed a meta-analysis of the most current literature.
Methods
Study selection, data extraction, and quality assessment
With the assistance of a master librarian, we conducted a systematic search in the electronic databases of Ovid MEDLINE (from 1946), EMBASE (from 1947), EBM reviews-Cochrane database of systematic reviews (from 2005), EBM reviews-ACP journal club (from 1991), EBM reviews-Database of abstracts of reviews of effects for articles that met our criteria. We searched for articles until December 31st 2012 and reviewed the candidate abstracts and manuscripts following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses [PRISMA] guidelines [14]. In addition, we manually searched for unpublished abstracts corresponding with our inclusion criteria presented at main national and international thrombosis meetings from 2010 to 2012.
We searched for articles that included pharmacological primary thromboprophylaxis in patients with solid tumors receiving chemotherapy in ambulatory clinics. We included randomized and non-randomized controlled trials, prospective trials, and retrospective trials which met our primary requirements. No language barriers were placed. Hematological malignancies including leukemia, lymphoma and multiple myeloma were excluded even if the chemotherapy was administered in the ambulatory setting. Anticoagulants used for prophylaxis could include unfractionated heparin (UFH), low molecular or ultra-low molecular heparin (ULMWH), direct factor Xa inhibitors or thrombin inhibitors. The MESH terms searched were: “venous thromboembolism”, thromboembolism, “venous thrombosis”, “thrombophlebitis”, “neoplasms”, “prevention”, “prophylaxis”, “cancer” (example search strategy on appendix Table 1). The articles were abstracted by two reviewers at all stages and disagreements were resolved by consensus with the assistance of two other co-authors. We summarized the study quality as high, medium or low likelihood of bias according to randomization technique, allocation concealment, comparability of groups at baseline, blinding, completeness of follow-up, assessment of incomplete data and validity of outcomes. We accounted for attrition, performance, and detection bias as recommended [15]. Low bias risk was equivalent to “unlikely to seriously alter results”; medium risk implied “bias that raises some doubt about results”; high risk was deemed to “seriously weaken confidence in results” [15].
Outcome definition
The primary efficacy outcome was first VTE. These events could be asymptomatic or symptomatic and included pulmonary embolism (PE) and deep vein thrombosis (DVT). DVT and PE occurring in the same patient were recorded as single event. Diagnosis of VTE could be made with Doppler imaging, ventilation/perfusion scan, CT angiography, venography, angiography or autopsy. The secondary efficacy outcome was all cause mortality. The primary safety end point was major bleeding, defined as those requiring transfusion of blood products, drop in hemoglobin >2 g, or bleeding in critical organs.
Statistical analysis
We calculated Q statistic and a formal test of homogeneity [16]. The I-squared (I2) index and corresponding 95 % confidence interval were used to summarize the proportion of the total variability in effect sizes due to between-study variation [17]. I2 values categorized by cut-points of 25, 50, and 75 % represented low, moderate, and high heterogeneity levels respectively [18]. The odds ratio (OR) and risk difference (RD) estimates from each study were pooled by using the Mantel–Haenszel fixed-effects method, the inverse-variance method and the random-effects model by DerSimonian and Laird [17, 19]. All pooled risk ratios were reported with the associated 95 % confidence intervals (CI). In the presence of significant heterogeneity (p < 0.1), the random-effects model results were presented over the fixed effects model. We performed an influence analysis estimating the pooled effect sizes after leaving each study out. Subgroup analyses for VTE and bleeding outcomes were performed using pre-specific subgroups including drug type, multiple types of tumors and catheter– based prophylaxis. Data were analyzed using R (R Development Core Team, www.R-project.org), R META package (Version 0.8-2, Author: Guido Schwarzer).
Results
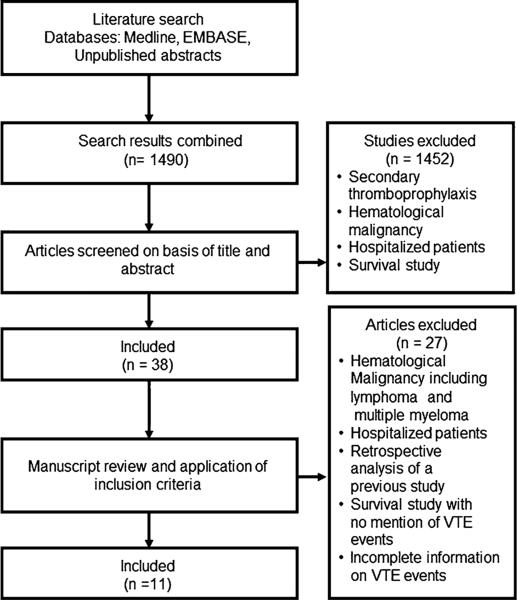
We identified 1,490 articles and considered 38 articles based on our initial screening of the title and the abstract (Fig. 1). After thorough review of these articles, 11 trials were included in our primary outcome meta-analysis. None of the included trials tested UFH, fondaparinux, or direct factor Xa inhibitors. Except for one (Young et al. [20]), the selected manuscripts were considered to be of low risk for bias. There were no unpublished papers included in the analysis.
Fig. 1.
Algorithm of manuscript selection
The total number of patients included was 7,805, the experimental and control groups were evenly divided. We analyzed TOPIC 1 (Breast Cancer) and TOPIC 2 (Non Small Cell Lung Cancer) as 2 independent trials, as originally designed, although they were finally published as a single manuscript [21]. The median ages of the participants in these trials ranged from 57 to 62 years, and were similar in the two groups. Two studies included only female participants [21, 22]. Malignancies included were those of the breast, lung, gastrointestinal tract (including pancreatic), ovary, head and neck, and brain. Chemotherapy regimens used in these studies differed. One trial reported catheter associated thrombosis (CAT) as the primary outcome measure, but included data on all VTE [20].
In two articles warfarin was used for primary thromboprophylaxis and the rest received heparin-based interventions [20, 22]. The heparin-based studies included dalteparin (3 studies) [23–25], nadroparin (3 studies) [26–28], semuloparin (1 study) [6] and certoparin (2 studies) [21]. The initiation of the prophylactic anticoagulant was not consistently timed or dosed, some trials using full dose and others prophylactic dose [23–25]. We found no consensus on optimal duration of prophylaxis (varied from 3 to 12 months).
VTE prevention
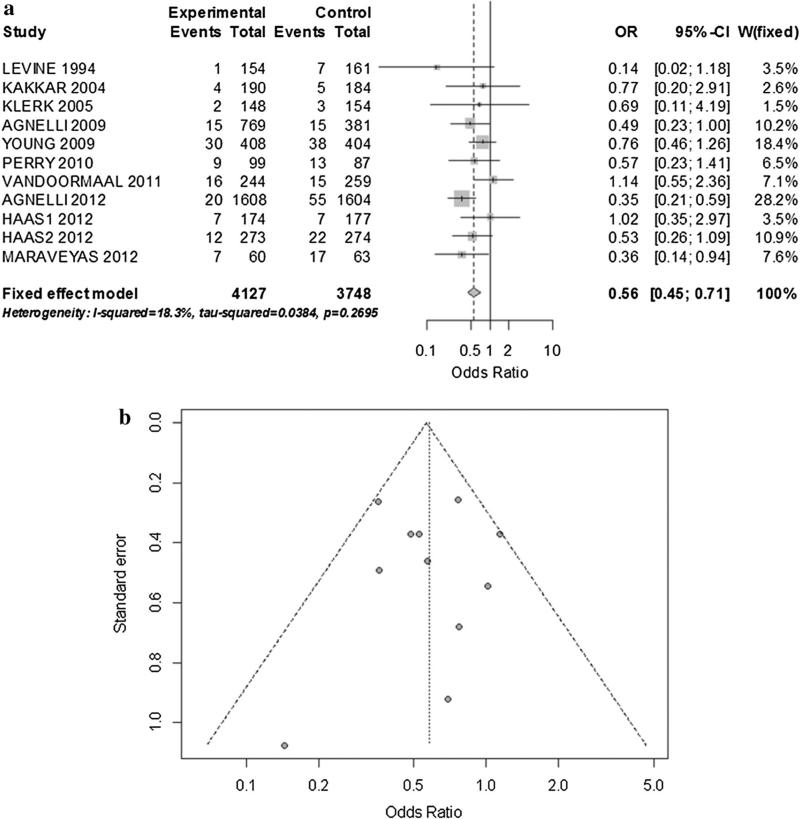
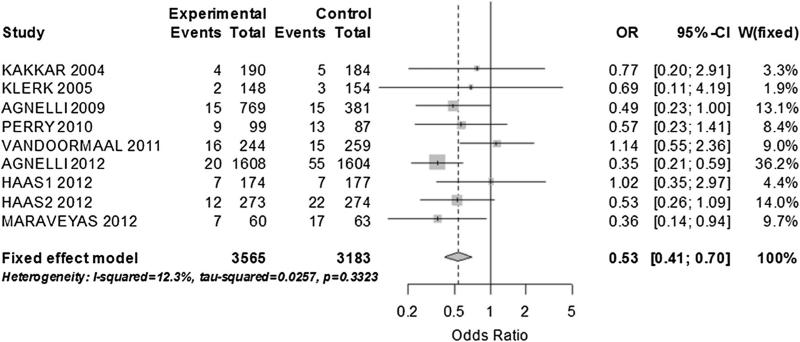
There were 320 VTE. There was a significant reduction of VTE in the prophylaxis group with low, non-significant heterogeneity (OR 0.56; 95 % CI 0.45–0.71; I2: 18.3 %) (Fig. 2a, b). The pooled risk difference was -0.02 [95 % CI -0.03 to -0.01; p < 0.001]. We did not detect a relevant publication bias (Fig. 2b). No single study influenced the pooled estimate and the result of a sensitivity analysis using only manuscripts with low risk of bias was not different to the main result (OR 0.54; 95 % CI 0.4–0.73; I2: 15 %). We analyzed pre-specified outcomes by drug: 9 eligible studies with heparin-based therapies, pooled analysis demonstrated low heterogeneity favoring the interventional group (OR 0.53; 95 % CI 0.41–0.70; I2 12.3 %) (RD -0.02; 95 % CI -0.03 to -0.01) (Fig. 3). The statistical significance of the findings was not impacted by the omission of any study. In this sub-analysis the most common type of cancers included were: lung (33 %), gastro intestinal (29 %) and breast (9 %). Two studies used warfarin; therefore we did not do a specific pooled analysis.
Fig. 2.
a Fixed effect model of VTE prevention in patients with cancer. b Funnel plot corresponding to primary analysis of VTE prevention in patients with cancer
Fig. 3.
Fixed effect model of VTE prevention in patients with solid tumor. Only trials with Heparin-based prevention
The second pre-specified analysis included studies not limited to a single type of malignancy. In the six included studies [6, 20, 23, 26–28] the estimate favored the interventional arm (OR 0.59; 95 % CI 0.45–0.78; I2: 40.4 %). However, we found moderate heterogeneity with a strong impact of the Agnelli 2009 [28] and 2012 [6] trials on the OR estimation. There was insufficient data to analyze thromboprophylaxis specific to patients with solid cancers and CAT. In the ten studies that were not restricted to CAT (excluding Young et al. [20].) we found a significant preventive effect (OR 0.52, 95 % CI 0.40–0.67) with no significant heterogeneity (I2: 15.4 %; p = 0.3).
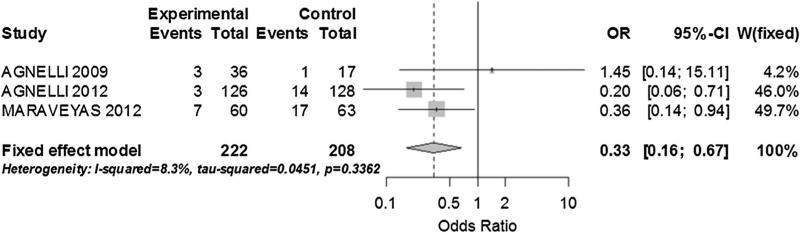
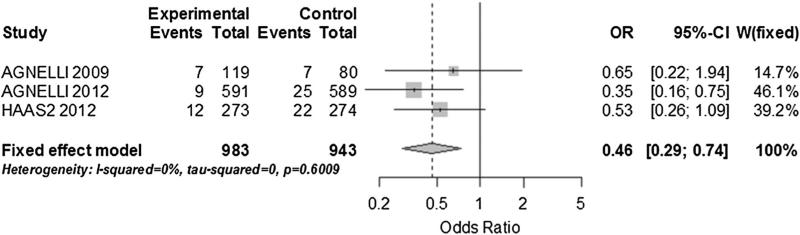
In a post hoc analysis of the trials with cancer type specific information we performed two subgroup analyses. For pancreatic cancer we grouped data on 3 studies with a total of 430 patients. There was no significant heterogeneity and heparin-based intervention was strongly associated with fewer VTE events (OR 0.33; 95 % CI 0.16–0.67; I2: 8.3 %) (Fig. 4) [6, 25, 28]. For lung cancer, we grouped 3 studies with a total of 1,926 patients [6, 21, 28]. We found no heterogeneity among the studies and a statistically significant reduction of VTE events in the heparin-based intervention arm (OR 0.46; 95 % CI 0.29–0.74; I2: 0 %) (Fig. 5).
Fig. 4.
Sub group analysis on VTE prevention in patients with pancreatic cancer
Fig. 5.
Sub group analysis on VTE prevention in patients with lung cancer
Safety
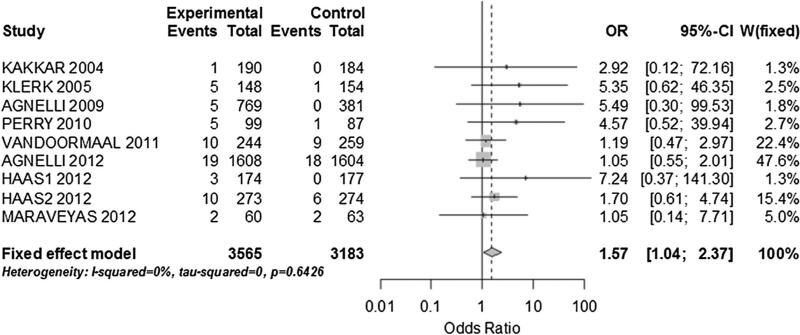
There were 68 major bleeding events among the 4,127 patients who received thromboprophylaxis and 40 major bleeding events in 3,748 patients who received placebo. When all the studies were included we found no heterogeneity, but a significant difference in bleeding events between the 2 groups was present (OR 1.65; 95 % CI 1.12–2.44; I2: 0 %). On a sensitivity analysis including only the studies with low risk of bias, the bleeding likelihood decreased (OR 1.41; 95 % CI 0.93–2.14; I2: 0 %) and was no longer statistically significant. When we grouped only studies with a low and ultralow molecular weight heparin intervention, the odd of major bleeding was measurably different between the 2 groups (OR 1.57; 95 % CI 1.04–2.37; I2: 0 %) (Fig. 6). There were no studies with high risk of bias included on this analysis. In addition, in the random effect model influence analysis, the omission of a trial by Agnelli et al. [6] using ULMWH, resulted in significantly increased major bleeding risk in the intervention group (OR 2.04; 95 % CI 1.19–2.37). When we combined only studies not limited to a single type of cancer, we found low heterogeneity and no significant difference in the major bleeding risk (OR 1.57; 95 % CI 0.98–2.49; I2: 14.6 %). In the influence analysis, there was a large variation in the pooled OR with the omission of the Agnelli 2012 study (OR 2.36; 95 % CI 1.18–4.71), but the likelihood of bleeding remained not significantly different between groups.
Fig. 6.
Bleeding events in patients with cancer. Only trial which provided heparin-based prophylaxis
The point estimate of the likelihood of major bleeding when only non-CAT studies were included was statistically significant [OR 1.52; 95 % CI 1.02–2.27; I2: 0 %]. The manuscripts did not provide enough information as to define heparin induced thrombocytopenia as a potential complication in this populaiton.
Mortality
We were unable to abstract adequate all-cause mortality in three of the studies, and therefore, our meta-analysis of mortality data was based on eight studies representing 6,374 patients, all with heparin-based intervention. The follow up was variable and only four studies provided mortality at more than 6 months, three of which included multiple types of malignancies [6, 24, 27, 28]. We found no statistically significant heterogeneity when the trials were combined and no difference in mortality between the treatment groups (OR 0.97; 95 % CI 0.87–1.08; I2: 17.8 %). We found similar results when we combined trials including multiple types of malignancy and follow-up of more than 6 months; again there was no significant mortality variation between groups (OR 0.97; 95 % CI 0.86–1.09; I2: 11.7 %).
Discussion
Overall, thromboprophylaxis reduced the episodes of VTE in patients with solid tumors. When we isolated trials in which heparin-based prophylaxis was used, there was a 47 % reduction in the VTE events compared to placebo. However, there was close to a 60 % increase in bleeding events among the patients who received heparin-based primary prophylaxis. We found no mortality benefit derived from VTE prophylaxis, but our analysis was limited by short follow up in the included studies.
This meta-analysis is larger than previously published and focuses on two key aspects; we only included solid tumor malignancies and we were able to pool data on outcomes specific to heparin-based prevention. Our results are similar to Di Nisio et al. [29] in which combining nine randomized controlled trials there was a 40 % reduction in the incidence of symptomatic VTE. Our meta-analysis included twice as many patients even though we excluded hematological malignancies. We believe that the differences in prevalence and treatment regimens for solid tumor and hematologic malignancies warrant separate evaluation. In addition, we did not include the trials by Sideras et al. and Altinbas et al. [30, 31] which focused on mortality and not on VTE prevention. Another meta-analysis by Akl et al. [32] focused on the evaluation of efficacy and safety of anticoagulants in patients with cancer with no therapeutic or prophylactic indication for anticoagulation and summarized five randomized controlled trials (1,656 participants). In contrast to the meta-analysis by Akl et al., the majority of the trials we found used heparin-based medications. Furthermore the primary focus of our meta-analysis was efficacy in prevention of VTE.
In a combined analysis on 811 patients with lung cancer using data from PROTECHT and TOPIC-2, published by Verso et al. [33], the authors reported a value of LMWH for primary VTE primary thromboprophylaxis (OR 0.54; 95 % CI 0.31–0.95). We replicated these results after adding patients with lung cancer form the SAVE-ONCO trial and we found a reduction by half of the VTE events in patients with lung cancer who used primary prophylaxis. Moreover, among 430 patients with pancreatic cancer the VTE incidence in the treatment arm was a third of the expected rate. Given the strong preventive effect in these 2 patient groups, the cost and bleeding risks of primary thromboprophylaxis is most likely to be justified in patients with lung or pancreatic malignancies. Based on simulated data, the value of primary anticoagulation would be cost effective if assumed that there is a 2 years relative mortality risk of 0.92 [34].
Weaknesses on the available data preclude wide utilization of primary thromboprophylaxis in patients with cancer. There is a paucity of data on the appropriate classification of thrombotic events in patients with cancer; therefore the outcomes we have analyzed are not homogeneous. To measure the value of thromboprophylaxis in future trials ideally there will be more discerning of symptoms, location (venous deep, venous superficial, arterial), and CAT as recently proposed by the international society of thrombosis and hemostasis [35]. With our meta-analysis we were not able to do a subgroup analysis accounting for these variables. Specifically, the incidence of CAT is a major problem in oncology which needs urgent attention [36]. Symptomatic catheter associated thrombosis present in 3–5 % of all patients with cancer who require central venous access [37–39] and it increased to as much as 30 % when asymptomatic cases are included [37]. To date, there is no conclusive evidence supporting the use of LMWH in prevention of CAT in patients with cancer [36].
We have concerns about the appropriateness of 2 g drop in hemoglobin as an adequate measure for significant bleed in patients receiving chemotherapy, since this may represent a side effect of chemotherapy itself. However, we only included randomized trials and expect the effect of a chemotherapy induced drop in hemoglobin to be balanced between the groups. We noted that in the influence analysis, the inclusion or exclusion of SAVE-ONCO caused a wide variation in the likelihood of a significant bleed [6]. Potential explanations include the utilization of a different agent which may have lower bleeding rates, the constricted exclusion criteria of the trial, or that it was an effect of the size of the trial which was the largest included. It remains unproven whether semuloparin has different safety profile when compared to LMWH in prevention of thromboembolism in patients with cancer. Novel anticoagulants may also have a different safety and efficacy profiles, but we did not include trials utilizing novel anticoagulants. We found a trial using apixaban, a novel Xa inhibitor, in thrombosis prophylaxis for patients with cancer. Since this trial was a small dose exploration study in which safety was the primary outcome we excluded it from our analysis [40]. Another relative weakness of the available data is that we cannot isolate the impact of specific chemotherapy agents on the incidence of VTE, which may change the relative efficacy of thromboprophylaxis. While bevacizumab, gemcitabine, and platinum agents seem to have a higher association of VTE, the value of thrombosis relative to a chemotherapy agent has not been prospectively tested [41, 42]. Similarly, some chemotherapy combinations may cause a greater degree of myelosupression which will put the patients at an unreasonable bleeding risk. It remains to be answered in future trials whether a reduced dose of anticoagulation or no anticoagulation at all is the best option in patients with expected thrombocytopenia and anemia.
Although there are several VTE risk factors and stratification scores specific to patients with cancer, a validated risk score was not used to stratify the randomization in any of the available trials [41, 43–47]. Specifically, Khorana et al. [44] has validated a model for predicting chemotherapy-associated VTE using: cancer site, hemoglobin, platelet count, body mass index, and leucocyte count. Barni et al., measured the Khorana score in a post hoc analysis of the PROTECHT trial and most of the recruited patients fell on an intermediate group (53.2 % in the nadroparin group and 52.1 % in the placebo group) [42]; this invites further exploration of risk factors that may reclassify these patients as low or high risk. In addition, there is a growing interest on the use of bio-markers as potential tools to risk stratify the VTE risk in patients with cancer, yet, the value of tailoring VTE prophylaxis based on novel biomarkers is not widely accepted [43, 48, 49]. Zwicker et al. recently tested tissue factor bearing microparticle levels as a discriminating factor for VTE in patients with cancer; only 23 patients received enoxaparin on this phase II trial. The patients with elevated microparticle levels were seven times more likely to have a VTE at 2 months of follow up without prophylaxis [47]. This supports the hypothesis of the value of better risk stratification as a tool to optimize VTE prevention in patients with cancer. We did not include this study in our analysis as it was published after the search criteria was closed, in addition the intervention group was intentionally different to the control patients regarding the thromboembolic risk.
Although there is a clearly measurable VTE rate reduction when primary thromboprophylaxis is given to patients with cancer, before anticoagulants are added to the conventional treatment of patients with cancer, we need more information on the value of risk stratification tools to personalize prevention strategies.
Appendix
See Table 1.
Table 1.
Example of search strategy used in our meta-analysis
| S. no. | Database: Ovid MEDLINE(R) < 1946 to June Week 2 2012 >, Ovid MEDLINE(R) In-Process & Other Non-Indexed Citations < June 22, 2012 >, Ovid OLDMEDLINE(R) < 1946–1965> |
|---|---|
| Search strategy | |
| 1 | Venous Thromboembolism/or thromboembolism/or venous thrombosis/or thrombophlebitis/(55500) |
| 2 | (Thromb$ and VTE).mp. or (venous$ adj3 thromb$).ti,ab. [mp = title, abstract, original title, nam0e of substance word, subject heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier] (31130) |
| 3 | 1 or 2 (67953) |
| 4 | Exp Neoplasms/(2369202) |
| 5 | Exp anticoagulants/or exp fibrinolytic agents/or exp platelet aggregation inhibitors/(284314) |
| 6 | (Prophyla$ or prevent$).ti,ab. (913626) |
| 7 | 5 or 6 (1166445) |
| 8 | Cancer$.ti,ab. (932981) |
| 9 | 4 or 8 (2540084) |
| 10 | 3 and 7 and 9 (2759) |
| 11 | (Etiology or prevention & control or therapy or drug therapy).fs. (4835450) |
| 12 | 10 and 11 (2431) |
| 13 | 1 and 12 (2093) |
| 14 | (Cohort studies or case reports or Letter or Historical Article or comment).pt. (2672141) |
| 15 | 13 not 14 (1702) |
| 16 | Exp Clinical Trial/or double-blind method/or (clinical trial$ or randomized controlled trial or multicenter study).pt. or exp Clinical Trials as Topic/or (randomi?ed adj7 trial$).mp. or (controlled adj3 trial$).mp. or (clinical adj2 trial$).mp. (992304) |
| 17 | 15 and 16 (597) |
| 18 | th.xs. (5045165) |
| 19 | (Venous adj3 thromb$).ti,ab. (30982) |
| 20 | 1 and 4 (5487) |
| 21 | 18 and 19 (16219) |
| 22 | 20 and 21 (1342) |
| 23 | 22 not 14 (1083) |
| 24 | 23 and 16 (383) |
| 25 | 17 or 24 (666) |
| 26 | Remove duplicates from 23 (1060) |
| 27 | Remove duplicates from 25 (645) |
Contributor Information
Minh Phan, Internal Medicine, University of Oklahoma Health Sciences Center, Oklahoma City, OK, USA.
Sonia John, Internal Medicine, University of Oklahoma Health Sciences Center, Oklahoma City, OK, USA.
Ana I. Casanegra, Cardiovascular Medicine Section, University of Oklahoma Health Sciences Center, 920 Stanton L Young Blvd., WP3010, Oklahoma City, OK 73104, USA
Suman Rathbun, Cardiovascular Medicine Section, University of Oklahoma Health Sciences Center, 920 Stanton L Young Blvd., WP3010, Oklahoma City, OK 73104, USA.
Aaron Mansfield, Department of Medical Oncology, Mayo Clinic, Rochester, MN, USA.
Julie A. Stoner, Department of Biostatistics and Epidemiology, University of Oklahoma Health and Sciences Center, Oklahoma City, OK, USA
Alfonso J. Tafur, Cardiovascular Medicine Section, University of Oklahoma Health Sciences Center, 920 Stanton L Young Blvd., WP3010, Oklahoma City, OK 73104, USA
References
- 1.Heit JA, Silverstein MD, Mohr DN, Petterson TM, O'Fallon WM, Melton LJ., 3rd Risk factors for deep vein thrombosis and pulmonary embolism: a population-based case-control study. Arch Int Med. 2000;160(6):809–815. doi: 10.1001/archinte.160.6.809. [DOI] [PubMed] [Google Scholar]
- 2.Roger VL, et al. Heart disease and stroke statistics–2012 update: a report from the American Heart Association. Circulation. 2012;125(1):e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khorana AA, Francis CW, Culakova E, Kudere NM, Lyman GH. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. J Thromb Haemost. 2007;5:632–634. doi: 10.1111/j.1538-7836.2007.02374.x. [DOI] [PubMed] [Google Scholar]
- 4.Sorensen HT, Mellemkjaer L, Olsen JH, Baron JA. Prognosis of cancers associated with venous thromboembolism. N Engl J Med. 2000;343(25):1846–1850. doi: 10.1056/NEJM200012213432504. [DOI] [PubMed] [Google Scholar]
- 5.Dobesh PP. Economic Burden of Venous Thromboembolism in Hospitalized Patients. Pharmacotherapy. 2009;29(8):943–953. doi: 10.1592/phco.29.8.943. [DOI] [PubMed] [Google Scholar]
- 6.Agnelli G, et al. Semuloparin for thromboprophylaxis in patients receiving chemotherapy for cancer. N Engl J Med. 2012;366(7):601–609. doi: 10.1056/NEJMoa1108898. [DOI] [PubMed] [Google Scholar]
- 7.Heit JA, et al. Risk factors for deep vein thrombosis and pulmonary embolism: a population-based case-control study. Arch Intern Med. 2000;160(6):809–815. doi: 10.1001/archinte.160.6.809. [DOI] [PubMed] [Google Scholar]
- 8.Heit JA, et al. Predictors of recurrence after deep vein thrombosis and pulmonary embolism: a population-based cohort study. Arch Intern Med. 2000;160(6):761–768. doi: 10.1001/archinte.160.6.761. [DOI] [PubMed] [Google Scholar]
- 9.Streiff M, et al. Venous thromboembolic disease. National Comprenesive Cancer Network; Washington: 2013. [Google Scholar]
- 10.Lyman GH, et al. American society of clinical oncology guideline: recommendations for venous thromboembolism prophylaxis and treatment in patients with cancer. J Clin Oncol. 2007;25:5490–5505. doi: 10.1200/JCO.2007.14.1283. [DOI] [PubMed] [Google Scholar]
- 11.Mandala M, Falanga A, Roila F. Management of venous thromboembolism (VTE) in cancer patients: ESMO clinical practice guidelines. Ann Oncol. 2011;22(6):vi85–vi92. doi: 10.1093/annonc/mdr392. [DOI] [PubMed] [Google Scholar]
- 12.Kahn SR, Lim W, Dunn AS, Cushman M, Dentali F, Akl EA, Cook DJ, Balekian AA, Klein RC, Le H. Schulman prevention of VTE in nonsurgical patients: antithrombotic therapy and prevention of thrombosis 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2):e195S–e226S. doi: 10.1378/chest.11-2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khorana AA, et al. Preventing VTE in outpatients with cancer. Chest. 2012;142(1):266–267. doi: 10.1378/chest.12-0423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liberati A, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. [Sept 1-27-2012];Cochrane Handbook for Systematic Reviews of Interventions 4.2.6. 2006 http://www.cochrane.org/resources/handbook/hbook.htm.
- 16.Egger M. Systematic reviews in health care. In: Altman D, editor. Meta-analysis in Contex. BMJ Books; London: 2001. [Google Scholar]
- 17.Huedo-Medina TB, et al. Assessing heterogeneity in meta-analysis: q statistic or I2 index? Psychol Methods. 2006;11(2):193–206. doi: 10.1037/1082-989X.11.2.193. [DOI] [PubMed] [Google Scholar]
- 18.Higgins JP, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22(4):719–748. [PubMed] [Google Scholar]
- 20.Young AM, et al. Warfarin thromboprophylaxis in cancer patients with central venous catheters (WARP): an open-label randomised trial. Lancet. 2009;373(9663):567–574. doi: 10.1016/S0140-6736(09)60205-1. [DOI] [PubMed] [Google Scholar]
- 21.Haas SK, et al. Low-molecular-weight heparin versus placebo for the prevention of venous thromboembolism in metastatic breast cancer or stage III/IV lung cancer. Clin Appl Thromb Hemost. 2012;18(2):159–165. doi: 10.1177/1076029611433769. [DOI] [PubMed] [Google Scholar]
- 22.Levine M, et al. Double-blind randomised trial of a very-low-dose warfarin for prevention of thromboembolism in stage IV breast cancer. Lancet. 1994;343(8902):886–889. doi: 10.1016/s0140-6736(94)90008-6. [DOI] [PubMed] [Google Scholar]
- 23.Kakkar AK, et al. Low molecular weight heparin, therapy with dalteparin, and survival in advanced cancer: the fragmin advanced malignancy outcome study (FAMOUS). J Clin Oncol. 2004;22(10):1944–1948. doi: 10.1200/JCO.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 24.Perry JR, et al. PRODIGE: a randomized placebo-controlled trial of dalteparin low-molecular-weight heparin thromboprophylaxis in patients with newly diagnosed malignant glioma. J Thromb Haemost. 2010;8(9):1959–1965. doi: 10.1111/j.1538-7836.2010.03973.x. [DOI] [PubMed] [Google Scholar]
- 25.Maraveyas A, et al. Gemcitabine versus gemcitabine plus dalteparin thromboprophylaxis in pancreatic cancer. Eur J Cancer. 2012;48(9):1283–1292. doi: 10.1016/j.ejca.2011.10.017. [DOI] [PubMed] [Google Scholar]
- 26.Klerk CPW, et al. The effect of low molecular weight heparin on survival in patients with advanced malignancy. J Clin Oncol. 2005;23(10):2130–2135. doi: 10.1200/JCO.2005.03.134. [DOI] [PubMed] [Google Scholar]
- 27.van Doormaal FF, et al. Randomized trial of the effect of the low molecular weight heparin nadroparin on survival in patients with cancer. J Clin Oncol. 2011;29(15):2071–2076. doi: 10.1200/JCO.2010.31.9293. [DOI] [PubMed] [Google Scholar]
- 28.Agnelli G, et al. Nadroparin for the prevention of thromboembolic events in ambulatory patients with metastatic or locally advanced solid cancer receiving chemotherapy: a randomised, placebo-controlled, double-blind study. Lancet Oncology. 2009;10(10):943–949. doi: 10.1016/S1470-2045(09)70232-3. [DOI] [PubMed] [Google Scholar]
- 29.Di Nisio M, et al. Primary prophylaxis for venous thromboembolism in ambulatory cancer patients receiving chemotherapy. Cochrane Database Syst Rev. 2012;15(2):CD008500. doi: 10.1002/14651858.CD008500.pub2. [DOI] [PubMed] [Google Scholar]
- 30.Altinbas M, et al. A randomized clinical trial of combination chemotherapy with and without low-molecular-weight heparin in small cell lung cancer. J Thromb Haemost. 2004;2(8):1266–1271. doi: 10.1111/j.1538-7836.2004.00871.x. [DOI] [PubMed] [Google Scholar]
- 31.Sideras K, et al. Low-molecular-weight heparin in patients with advanced cancer: a phase 3 clinical trial. Mayo Clin Proc. 2006;81(6):758–767. doi: 10.4065/81.6.758. [DOI] [PubMed] [Google Scholar]
- 32.Akl EA, et al. Anticoagulation for the long-term treatment of venous thromboembolism in patients with cancer. Cochrane Database Syst Rev. 2011;15(6):CD006650. doi: 10.1002/14651858.CD006650.pub3. [DOI] [PubMed] [Google Scholar]
- 33.Verso M, Gussoni G, Agnelli G. Prevention of venous thromboembolism in patients with advanced lung cancer receiving chemotherapy: a combined analysis of the PROTECHT and TOPIC-2 studies. J Thromb Haemost. 2010;8(7):1649–1651. doi: 10.1111/j.1538-7836.2010.03901.x. [DOI] [PubMed] [Google Scholar]
- 34.Pishko AM, Smith KJ, Ragni MV. Anticoagulation in ambulatory cancer patients with no indication for prophylactic or therapeutic anticoagulation: a cost-effectiveness analysis from a U.S. perspective. Thromb Haemost. 2012;108(2):303–310. doi: 10.1160/TH12-03-0185. [DOI] [PubMed] [Google Scholar]
- 35.Carrier M, et al. Venous thromboembolism in cancer clinical trials: recommendation for standardized reporting and analysis. J Thromb Haemost. 2012;10(12):2599–2601. doi: 10.1111/jth.12028. [DOI] [PubMed] [Google Scholar]
- 36.Debourdeau P, et al. International clinical practice guidelines for the treatment and prophylaxis of thrombosis associated with central venous catheters in patients with cancer. J Thromb Haemost. 2013;11(1):71–80. doi: 10.1111/jth.12071. [DOI] [PubMed] [Google Scholar]
- 37.Verso M, Agnelli G. Venous thromboembolism associated with long-term use of central venous catheters in cancer patients. J Clin Oncol. 2003;21(19):3665–3675. doi: 10.1200/JCO.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 38.Lee AY, et al. Incidence, risk factors, and outcomes of catheter-related thrombosis in adult patients with cancer. J Clin Oncol. 2006;24(9):1404–1408. doi: 10.1200/JCO.2005.03.5600. [DOI] [PubMed] [Google Scholar]
- 39.Evans RS, et al. Risk of symptomatic DVT associated with peripherally inserted central catheters. Chest. 2010;138(4):803–810. doi: 10.1378/chest.10-0154. [DOI] [PubMed] [Google Scholar]
- 40.Levine MN, et al. A randomized phase II trial of apixaban for the prevention of thromboembolism in patients with meta-static cancer. J Thromb Haemost. 2012;10(5):807–814. doi: 10.1111/j.1538-7836.2012.04693.x. [DOI] [PubMed] [Google Scholar]
- 41.Verso M, et al. A modified Khorana risk assessment score for venous thromboembolism in cancer patients receiving chemotherapy: the protecht score. Intern Emerg Med. 2012;7(3):291–292. doi: 10.1007/s11739-012-0784-y. [DOI] [PubMed] [Google Scholar]
- 42.Barni S, et al. Chemotherapy-associated thromboembolic risk in cancer outpatients and effect of nadroparin thromboprophylaxis: results of a retrospective analysis of the PROTECHT study. J Transl Med. 2011;9:179. doi: 10.1186/1479-5876-9-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ay C, et al. High plasma levels of soluble P-selectin are predictive of venous thromboembolism in cancer patients: results from the Vienna Cancer and Thrombosis Study (CATS). Blood. 2008;112(7):2703–2708. doi: 10.1182/blood-2008-02-142422. [DOI] [PubMed] [Google Scholar]
- 44.Khorana AA, et al. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008;111:4902–4907. doi: 10.1182/blood-2007-10-116327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Connolly GC, et al. Leukocytosis, thrombosis and early mortality in cancer patients initiating chemotherapy. Thromb Res. 2010;126(2):113–118. doi: 10.1016/j.thromres.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Date K, et al. Tumour and microparticle tissue factor expression and cancer thrombosis. Thromb Res. 2013;131(2):109–115. doi: 10.1016/j.thromres.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 47.Zwicker JI, et al. Prediction and prevention of thromboembolic events with enoxaparin in cancer patients with elevated tissue factor-bearing microparticles: a randomized-controlled phase II trial (the Microtec study). Br J Haematol. 2013;160(4):530–537. doi: 10.1111/bjh.12163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stender MT, et al. Preoperative plasma D-dimer is a predictor of postoperative deep venous thrombosis in colorectal cancer patients: a clinical, prospective cohort study with one-year follow-up. Dis Colon Rectum. 2009;52(3):446–451. doi: 10.1007/DCR.0b013e318197e2b2. [DOI] [PubMed] [Google Scholar]
- 49.Zwicker JI, et al. Tumor-derived tissue factor-bearing microparticles are associated with venous thromboembolic events in malignancy. Clin Cancer Res. 2009;15(22):6830–6840. doi: 10.1158/1078-0432.CCR-09-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]