Abstract
Few studies have been conducted that have examined the long-term effect of different doses of physical activity (PA) on weight change in overweight adults without a prescribed reduction in energy intake. This study examined the effect of different prescribed doses of PA on weight change, body composition, fitness and PA in overweight adults. 278 overweight adults (BMI: 25.0 to 29.9 kg/m2; Age: 18 to 55 years) with no contraindications to PA were randomized to one of three intervention groups for a period of 18 months. MOD-PA was prescribed 150 min/wk and HIGH-PA 300 min/wk of PA. SELF was provided a self-help intervention to increase PA. There was no recommendation to reduce energy intake. MOD-PA and HIGH-PA was delivered in a combination of in-person and telephone contacts across 18 months. 18-month percent weight change was −0.7±4.6% in SELF, −0.9±4.7% in MOD-PA, and −1.2±5.6% in HIGH-PA. Subjects were retrospectively grouped as remaining within ±3% of baseline weight (WT-STABLE), losing >3% of baseline weight (WT-LOSS), or gaining >3% of baseline weight (WT-GAIN) for secondary analyses. 18-month weight change was 0.0±1.3% for WT-STABLE, +5.4±2.6% for WT-GAIN, and −7.4±3.6% for WT-LOSS. 18-month change in PA was 78.2±162.6 min/wk for WT-STABLE, 74.7±274.3 for WT-GAIN, and 161.9±252.6 min/wk for WT-LOSS. The weight change observed in WT-LOSS was a result of higher PA combined with improved scores on the Eating Behavior Inventory, reflecting the adoption of eating behaviors to facilitate weight loss. Strategies to facilitate the maintenance of these behaviors are needed to optimize weight control.
Keywords: exercise, overweight, fitness, weight control
INTRODUCTION
Estimates indicate that 65% to 70% of adults are overweight, with over 30% of adults classified as obese (1). Excess body weight has been shown to increase the risk of various health outcomes (2). While it is important to reduce body weight, there is also a need to implement interventions to curtail weight gain and the development of obesity, as well as elicit even modest reductions in body weight.
Physical activity (PA) when coupled with a reduction in energy intake enhances initial and long-term weight loss (3). PA may play a role in the primary prevention of weight gain, and the US Dietary Guidelines for Americans (4) recommends 60 minutes of moderate-intensity PA for the prevention of weight gain. More recent literature reviews conclude that PA ranging from approximately 150–250 min/wk results in weight loss of <3 percent of initial body weight (3, 5). As acknowledged in these reviews, few studies have used randomized designs with interventions implemented over an extended period (i.e., >12 months). Moreover, relatively recent claims in the popular media have suggested to the general public that physical activity has limited effects on body weight regulation, and has inferred that physical activity may actually contribute to weight gain and the increasing rates of obesity (6). Thus, it is important for research to quantify the effect of various doses of physical activity on change in body weight within the context of prospective intervention studies, as this information will inform clinical recommendations and clarify public health information regarding this matter.
The purpose of this study was to examine the effect of different prescribed doses of moderate-to-vigorous intensity PA on body weight in overweight adults (BMI = 25.0 to <30.0 kg/m2). Secondary outcomes included body composition, fitness, and PA. In addition, secondary analyses were performed with subjects classified as having remained within ±3% of baseline weight (WT-STABLE), lost weight of >3% compared to baseline weight (WT-LOSS), or gained weight of >3% compared to baseline weight (WT-GAIN). The criterion of ±3% of baseline weight being used to define weight stability is based on the published criteria proposed by Stevens et al. (7). Secondary analysis from prior studies conducted by our research group have provided additional insight into the effect of PA on weight change and the dose of PA that may be necessary to elicit significant long-term changes in weight (8, 9), and therefore we have taken a similar approach in this study.
METHODS
Subjects
Two-hundred seventy eight adults were recruited between 2003 and 2006 to participate in this study using newspaper, television, radio, and direct mail advertisements. Subjects were 18 to 55 years of age, had a BMI of 25.0 to 29.9 kg/m2, and reported being sedentary, which was defined as <3 days per week of <20 minutes per day of structured exercise. Exclusion criteria included a medical condition that prohibited participation in prescribed levels of PA, a history of coronary heart disease, a medical condition that may affect body weight, taking medication that would affect body weight (i.e., synthroid) or blood pressure (i.e., beta blocker), or recent weight loss of ≥10 pounds over the prior 12 months.
A medical history and PA readiness questionnaire (10) were completed prior to entry into this study and were used to determine eligibility. The participant’s physician provided written documentation that the proposed intervention was not contraindicated. Written informed consent was provided by participants prior to initiating this study, and the protocol was approved by the Institutional Review Board of the University of Pittsburgh (Pittsburgh, PA). Eligible subjects participated in baseline assessments as describe below prior to randomization to determine final eligibility.
Outcome Assessments
Subjects completed assessments of the primary outcomes at 0, 6, 12, and 18 months as described below. Subjects received $50 at 6, 12, and 18 months for completion of these assessments.
Body weight was assessed using a calibrated balance-beam scale to the nearest 0.1 kg (0.25 pounds) with the subject clothed in a cloth hospital gown. Height was assessed using a wall-mounted stadiometer to the nearest 0.1 cm. BMI was computed as kg/m2.
Body composition was assessed using bioelectrical impedance. The equation proposed by Segal et al. (11) was used to compute lean body mass (LBM), with percent body fat computed as: Percent Body Fat = [(weight – LBM)/weight]*100. Fat distribution was determined using anthropometry and included waist circumference measured horizontally at the level of the umbilicus, hip circumference measured at the widest observed aspect of the buttocks, and sagittal diameter measured at the iliac crests. Measurements were represented as the average of two measures that differed by ≤1.0 cm.
Fitness was assessed using a submaximal graded exercise on a treadmill as previously described (9). PA was assessed using a modified version of the questionnaire developed for the Harvard Alumni Study (12), with activity assessed for the prior 7-day period. Subjects reported periods of walking performed for exercise or purposeful activity, with household and occupational walking excluded. Additionally, subjects reported other fitness, sport, or recreational periods of PA. Energy expenditure (kcal/wk) and minutes of PA were computed as previously described (9). Briefly, energy expenditure was computed using previously published scoring algorithms (12) and classifications based on the compendium of physical activity (13). Minutes of activity was summed as the reported minutes of moderate-to-vigorous physical activity reported on the questionnaire.
Dietary intake was assessed using a food frequency questionnaire (14, 15). To determine if subjects were engaging in eating behaviors (i.e., weighing food, self-monitoring, self-weighing, etc.) consistent with weight control, the Eating Behavior Inventory (EBI) (16) was completed at each assessment period. A higher score is indicative of engagement in more eating behaviors recommended for weight control.
Intervention
Subjects were randomized to either a moderate PA dose intervention, high PA dose intervention, or a self-help intervention for a period of 18 months. The interventions were delivered in a closed-group format to minimize contamination between the intervention groups. Randomization was performed by the study statistician using a computer program with randomization blocked by gender.
Moderate PA dose intervention (MOD-PA)
MOD-PA participated in a behavioral intervention to promote progression and maintenance of 150 min/wk of structured PA by week 12, with the goal to sustain this dose of activity for the remainder of the 18 month intervention. Subjects were encouraged to spread the PA over a period of at least 5 days per week and to engage in bouts of PA that were at least 10 minutes in duration. Intensity was prescribed as moderate-to-vigorous, which was defined as 55–85% of age-predicted maximal heart rate or 11–15 on the 15-point rating of perceived exertion scale. For months 1–6, subjects attended weekly behavioral intervention sessions to promote the adoption of this dose of PA, with each month consisting of 3 weekly group sessions and 1 individual session with their assigned PA counselor. During months 7–18 subjects attended 2 group intervention sessions per month combined with 2 telephone calls per month with their assigned counselor so that weekly contact was sustained throughout the 18 month intervention session. The counselor followed a structured script for the telephone intervention calls, with the goal to complete this call in ≤10 minutes.
Each intervention session was supplemented with a written lesson that highlighted the key points of the behavioral session. During each session subjects were encouraged to exercise on-site with the intervention staff, with an additional supervised session offered on the weekends during months 1–3 to facilitate the initial adoption of the prescribed dose of PA. All remaining PA was performed under non-supervised conditions.
Subjects were provided guidance on healthy eating behaviors consistent with a balanced nutritional diet, but an energy restricted diet was not provided nor encouraged. Subjects received a monthly newsletter that included general health information along with pertinent information related to the logistics of the study.
High PA dose intervention (HIGH-PA)
HIGH-PA received an 18 month intervention that was virtually identical to the intervention described for MOD-PA. However, HIGH-PA was prescribed PA that progressed from 100 to 300 min/wk, with the dose of PA increasing by 25 min/wk at 4-week intervals.
Self-help group (SELF)
SELF only attended assessment visits at 0, 6, 12, and 18 months, with no additional intervention contact provided by the staff. Subjects received a PA self-help manual (Active Living Everyday) (17), along with the same monthly newsletter provided to the MOD-PA and HIGH-PA groups. This SELF intervention is modeled after prior intervention (18), and was also intended to maintain contact to enhance retention of subjects in this group across the 18 month study period.
Statistical Analysis
An a-priori power calculation was computed based on expected differences in body weight at 18 months between the randomized groups. Randomization of 78 subjects per group would provide 80% power at an alpha level of 0.05 to detect a moderate effect size of 0.45 for differences in weight loss between the groups. The intention-to-treat analyses for this study are based on samples of 84 subjects for SELF, 76 subjects for MOD-PA, and 88 subjects for HIGH-PA.
Statistical analyses were performed using SAS (version 9.2), with the type I error rate fixed at 0.05 (two-tailed). Normality of the outcome variables was checked using the Kolmogorov-Smirnov test. For dietary intake (kcal/d), PA (min/wk), and PA (kcal/wk), log-transformation was made as the results of the tests. Separate mixed effects models using the unstructured dependence structure were fit to the outcomes measured at baseline, 6, 12, and 18 months. Alternative mixed effects models with the three change scores: baseline to month 6, baseline to month 12, baseline to month 18 as the outcomes were fit with the baseline value of the outcome of interest being adjusted in the models as covariate.
Secondary analyses were performed with subjects classified as having remained within ±3% of baseline weight (WT-STABLE), lost weight of >3% compared to baseline weight (WT-LOSS), or gained weight of >3% compared to baseline weight (WT-GAIN). The criterion of ±3% of baseline weight being used to define weight stability is based on the published criteria proposed by Stevens et al. (7). All analyses were intention-to-treat analyses with baseline data carried forward for missing data at subsequent assessment periods.
RESULTS
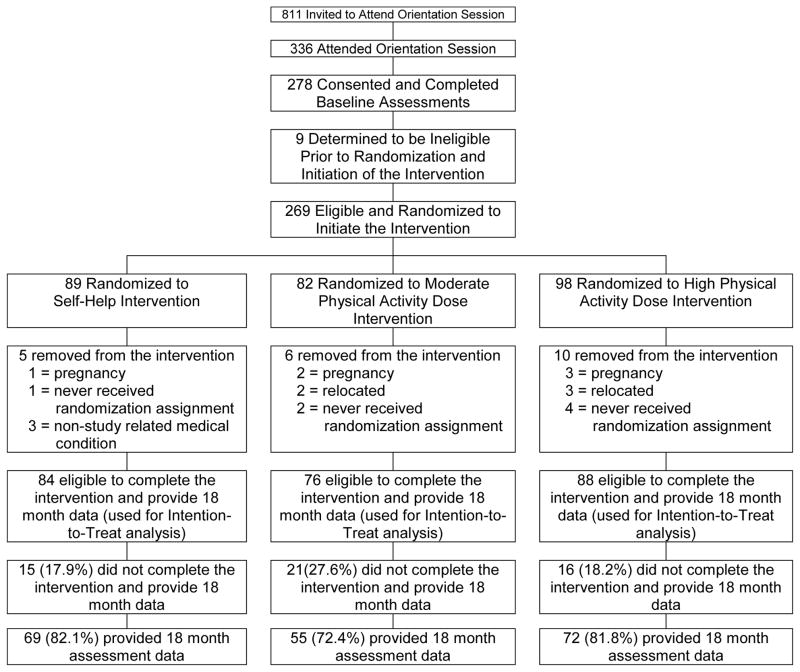
As shown in Figure 1, 278 individuals attended an orientation session, consented to participate in this study, and completed baseline assessments. This resulted in 269 subjects eligible to be randomized to the intervention phase of this study with 89, 82, and 98 subjects randomized to SELF, MOD-PA, and HIGH-PA, respectively. Prior to the completion of this study, 21 subjects were removed for reasons shown in Figure 1, which resulted in 248 subjects eligible to complete the study and 196 subjects (79% of eligible subjects) providing data at the 18 month assessment. Thus, the intention-to -treat analyses are based on the 248 subjects eligible to complete this study. The justification for not including the 21 subjects who did not receive their randomization assignment, became pregnant, had a non-study related medical condition, or relocated to a geographical region that did not allow them to attend the intervention or assessments visits is similar to the approach that we have used in previously published studies (8, 9, 19). Moreover, these factors were a prior exclusionary criteria. There was no significant difference in attrition rates between groups based on chi-square analysis. There were no significant unexpected serious adverse events reported. Demographic characteristics are shown in Table 1.
Figure 1.
Flow chart of participant recruitment and retention.
Table 1.
Summary of Demographic Characteristics of Subjects.
| Self-Help (SELF) | Moderate Physical Activity Group (MOD-PA) | High Physical Activity Group (HIGH-PA) | Total | |
|---|---|---|---|---|
| Number of Subjects | ||||
| Randomly Assigned | 89 | 82 | 98 | 269 |
| Intention-to-Treat Analysis | 84 | 76 | 88 | 248 |
| Completers Analysis | 69 | 55 | 72 | 196 |
| Gender (Females) | ||||
| Randomly Assigned | 82 (92.1%) | 74 (90.2%) | 90 (91.8%) | 246 (91.4%) |
| Intention-to-Treat Analysis | 77 (91.7%) | 69 (90.8%) | 81 (92.0%) | 227 (91.5%) |
| Completers Analysis | 62 (89.9%) | 50 (90.9%) | 66 (91.7%) | 178 (90.8%) |
| Age (years) | ||||
| Randomly Assigned | 44.7±7.9 | 43.5±8.8 | 45.0±8.4 | 44.4±8.4 |
| Intention-to-Treat Analysis | 44.8±7.8 | 44.4±8.2 | 45.6±7.9 | 44.9±7.9 |
| Completers Analysis | 45.4±7.3 | 44.6±8.3 | 46.0±8.1 | 45.4±7.9 |
| Weight (kg) | ||||
| Randomly Assigned | 73.9±7.9 | 74.1±8.3 | 74.5±8.3 | 74.2±8.2 |
| Intention-to-Treat Analysis | 73.7±8.0 | 74.2±8.4 | 74.3±8.2 | 74.1±8.2 |
| Completing Treatment | 73.8±8.3 | 74.6±8.2 | 73.6±7.6 | 74.0±8.0 |
| Body Mass Index (kg/m2) | ||||
| Randomly Assigned | 27.1±1.7 | 27.1±1.7 | 27.0±1.6 | 27.1±1.7 |
| Intention-to-Treat Analysis | 27.1±1.7 | 27.2±1.8 | 27.0±1.6 | 27.1±1.7 |
| Completing Treatment | 27.0±1.7 | 27.2±1.8 | 26.9±1.6 | 27.0±1.7 |
| Ethnicity* | ||||
| American Indian or Alaska Native | N=0, 0% (N=0, 0%) | N=0, 0% (N=0, 0%) | N=1, 1.1% (N=0, 0%) | N=1, 0.4% (N=0, 0%) |
| Asian | N=1, 1.2% (N=1, 1.5%) | N=2, 2.6% (N=2, 3.6%) | N=0, 0% (N=0, 0%) | N=3, 1.2% (N=3, 1.5%) |
| Black or African-American | N=13, 15.5% (N=11,15.9%) | N=11, 14.5% (N=7, 12.7%) | N=13, 14.8% (N=13, 18.1%) | N=37, 14.9% (N=31, 15.8%) |
| Hispanic, Latino, Portuguese, Cape Verdean | N=2, 2.4% (N=2, 2.9%) | N=1, 1.3% (N=1, 1.8%) | N=1, 1.1% (N=1, 1.4%) | N=4, 1.6% (N=4, 2.0%) |
| Native Hawaiian or Other Pacific Islander | N=0, 0% (N=0, 0%) | N=0, 0% (N=0, 0%) | N=0, 0% (N=0, 0%) | N=0, 0% (N=0, 0%) |
| White | N=66, 78.6% (N=55, 79.7%) | N=60, 78.9% (N=43, 78.2%) | N=72, 81.8% (N=57, 79.2%) | N=198, 79.8% (N=155, 79.1%) |
| Other | N=2, 2.4% (N=0, 0%) | N=1, 1.3 (N=1, 1.8%) | N=1, 1.1% (N=1, 1.4%) | N=4, 1.6% (N=2, 1.0%) |
| Not Specific – Missing | N=0, 0% (N=0, 0%) | N=1, 1.3% (N=1, 1.8%) | N=0, 0% (N=0, 0%) | N=1, 0.4% (N=1, 0%) |
indicates that numbers not in parentheses are based on the intent-to-treat analysis (N=248) and numbers in parentheses are based on subjects completing 18 months (N = 196).
Physical Activity
PA across 0, 6, 12 and 18 months revealed a significant Group X Time interaction effect (p<0.001). Post-hoc analyses with Bonferroni adjustment showed that the Group X Time interaction effect remained statistically significant for comparisons between HIGH-PA and both MOD-PA and SELF; however, the comparison of MOD-PA and SELF was not statistically significant. Increase in PA from baseline to 6, 12, and 18 months was 244.6±233.3, 188.7±216.3, and 154.8±237.5 min/wk in HIGH-PA compared to 131.4±150.6, 98.4±148.6, 66.1±169.5 min/wk in MOD-PA and 95.4±224.8, 71.0±236.4, 74.6±223.9 min/wk in SELF (Table 2).
Table 2.
Physical activity, weight, body composition, and fitness by randomized group assignment (mean ± standard deviation)
| Group | Baseline | 6 Months | 12 Months | 18 Months | Time | Group | Group X Time | |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Physical Activity (min/wk) | Self-Help (N=84) | 146.5±201.0 | 242.0±210.9 | 217.5±222.0 | 221.1±204.5 | <0.0001* | 0.1278* | 0.0421* |
| MOD-PA (N=76) | 110.8±123.8 | 242.3±157.5 | 209.2±140.7 | 176.9±151.3 | ||||
| HIGH-PA (N=88) | 83.1±122.2 | 327.7±220.9 | 271.8±199.4 | 237.9±216.7 | ||||
| Total (N=248) | 113.1±155.5 | 272.5±203.4 | 234.3±193.3 | 213.5±195.4 | ||||
| Physical Activity (kcal/wk) | Self-Help (N=84) | 1018.4±1070.2 | 1529.4±1101.1 | 1438.7±1200.9 | 1460.4±1126.3 | <0.0001* | 0.3096* | 0.0015* |
| MOD-PA (N=76) | 799.1±693.4 | 1504.4±856.5 | 1324.1±819.7 | 1147.4±814.0 | ||||
| HIGH-PA (N=88) | 694.8±669.6 | 2008.1±1250.6 | 1685.4±1053.6 | 1526.8±1170.2 | ||||
| Total (N=248) | 836.4±841.2 | 1691.6±1112.0 | 1491.1±1050.0 | 1388.0±1066.0 | ||||
| Weight (kg) | Self-Help (N=84) | 73.7±8.0 | 72.8±8.4 | 72.8±8.6 | 73.2±8.5 | <0.0001 | 0.960 | 0.539 |
| MOD-PA (N=76) | 74.2±8.4 | 72.9±8.4 | 73.5±8.5 | 73.5±8.7 | ||||
| HIGH-PA (N=88) | 74.3±8.2 | 72.9±8.5 | 73.0±8.7 | 73.5±9.2 | ||||
| Total (N=248) | 74.1±8.2 | 72.9±8.4 | 73.1±8.6 | 73.4±8.8 | ||||
| Body Mass Index (kg/m2) | Self-Help (N=84) | 27.1±1.7 | 26.7±2.0 | 26.7±2.2 | 26.9±2.1 | <0.0001 | 0.601 | 0.527 |
| MOD-PA (N=76) | 27.2±1.8 | 26.7±1.9 | 26.9±2.0 | 26.9±2.0 | ||||
| HIGH-PA (N=88) | 27.0±1.6 | 26.4±1.9 | 26.5±2.1 | 26.7±2.4 | ||||
| Total (N=248) | 27.1±1.7 | 26.6±1.9 | 26.7±2.1 | 26.8±2.2 | ||||
| Waist (cm) | Self-Help (N=84) | 89.3±8.8 | 88.0±8.5 | 88.1±8.6 | 88.4±8.8 | <0.0001 | 0.286 | 0.699 |
| MOD-PA (N=76) | 91.4±7.9 | 89.5±8.7 | 90.2±8.9 | 90.6±9.4 | ||||
| HIGH-PA (N=88) | 90.5±8.4 | 88.5±9.2 | 87.8±10.1 | 89.4±10.0 | ||||
| Total (N=248) | 90.4±8.4 | 88.6±8.8 | 88.7±9.3 | 89.4±9.4 | ||||
| Percent Body Fat | Self-Help (N=84) | 33.7±4.4 | 33.2±5.2 | 33.2±5.6 | 33.2±5.4 | <0.0001 | 0.328 | 0.313 |
| MOD-PA (N=76) | 33.5±4.1 | 32.7±4.8 | 33.3±4.9 | 33.3±4.8 | ||||
| HIGH-PA (N=88) | 33.0±4.1 | 31.8±4.7 | 32.3±4.9 | 32.3±5.3 | ||||
| Total (N=248) | 33.4±4.2 | 32.5±4.9 | 32.9±5.2 | 32.9±5.2 | ||||
| Fitness (minutes) | Self-Help (N=84) | 10.2±3.3 | 11.9±3.8 | 11.9±4.0 | 11.9±4.1 | <0.0001 | 0.1215 | 0.008 |
| MOD-PA (N=76) | 9.8±3.6 | 13.1±5.2 | 12.1±4.6 | 11.8±4.4 | ||||
| HIGH-PA (N=88) | 10.3±3.8 | 13.7±3.9 | 13.0±3.8 | 13.1±4.0 | ||||
| Total (N=248) | 10.1±3.6 | 12.9±4.4 | 12.4±4.1 | 12.3±4.2 | ||||
indicates p-values were obtained from the mixed effects models with log-transformed data.
Energy Intake
Changes from baseline to 18 months were −201.0±517.3 kcal/d for dietary intake, −0.7±6.7% for percent fat intake, +0.6±7.0% for percent carbohydrate intake, and +0.2±2.6% for percent protein intake. There was no group by time interaction for changes in these dietary intake parameters. Likewise there was a significant increase in the EBI score from 73.1±9.9 to 78.3±10.8 (p<0.001), suggesting an improvement in eating and weight loss behaviors, with no significant difference between groups.
Body Weight
Modest weight loss occurred in all treatment groups at 6, 12, and 18 months with no significant difference between groups (Table 3). Percent weight change at 6 months was −1.3±3.6%, −1.8±3.5%, and −1.9±3.8% in SELF, MOD-PA, and HIGH-PA, respectively. Percent weight change from 0 to 18 months was −0.7±4.6% in SELF, −0.9±4.7% in MOD-PA, and −1.2±5.6% in HIGH-PA with no significant difference between groups. Waist circumference decreased from 90.4±8.4 cm to 89.4±9.4 cm (p<0.0001) and percent body fat from 33.4±4.2% to 32.9±5.2% (p<0.0001), with no significant difference between groups (Table 2).
Table 3.
Physical activity, weight, body composition, and fitness by 18 month weight change classification (mean ± standard deviation)
| *18 Month Weight Change Classification | Baseline | 6 Months | 12 Months | 18 Months | Time | Group | Group X Time | |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Physical Activity (min/wk) | WT-GAIN (N=48) | 149.8±196.9 | 295.5±249.3 | 212.4±198.6 | 224.5±196.0 | <0.0001+ | 0.1588+ | 0.0296+ |
| WT-STABLE (N=132) | 107.5±151.7 | 236.5±181.7 | 213.9±184.6 | 185.7±178.6 | ||||
| WT-LOSS (N=68) | 97.9±125.4 | 326.1±196.1 | 289.1±198.1 | 259.9±218.3 | ||||
| Total (N=248) | 113.1±155.5 | 272.5±203.4 | 234.3±193.3 | 213.5±195.4 | ||||
| Physical Activity (kcal/wk) | WT-GAIN (N=48) | 1011.2±1038.9 | 1784.0±1238.7 | 1333.9±1018.4 | 1404.5±1069.8 | <0.0001+ | 0.0229+ | 0.0354+ |
| WT-STABLE (N=132) | 816.2±850.2 | 1491.4±1025.8 | 1382.4±1018.8 | 1233.1±965.2 | ||||
| WT-LOSS (N=68) | 752.0±638.9 | 2014.9±1110.7 | 1813.1±1077.7 | 1677.3±1196.7 | ||||
| Total (N=248) | 836.4±841.2 | 1691.6±1112.0 | 1491.1±1050.0 | 1388.0±1066.0 | ||||
| Weight (kg) | WT-GAIN (N=48) | 73.7±7.9 | 74.6±7.9 | 76.4±8.7 | 77.8±8.2 | <0.0001 | 0.005 | <0.0001 |
| WT-STABLE (N=132) | 74.2±8.2 | 73.3±8.3 | 73.6±8.0 | 74.2±8.1 | ||||
| WT-LOSS (N=68) | 74.1±8.5 | 70.7±8.6 | 69.7±8.6 | 68.7±8.6 | ||||
| Total (N=248) | 74.1±8.2 | 72.9±8.4 | 73.1±8.6 | 73.4±8.8 | ||||
| Body Mass Index (kg/m2) | WT-GAIN (N=48) | 27.1±1.7 | 27.4±1.9 | 28.1±2.0 | 28.6±1.8 | <0.0001 | <0.0001 | <0.0001 |
| WT-STABLE (N=132) | 27.1±1.6 | 26.8±1.7 | 26.9±1.7 | 27.1±1.6 | ||||
| WT-LOSS (N=68) | 26.9±1.8 | 25.7±2.1 | 25.3±2.1 | 25.0±2.1 | ||||
| Total (N=248) | 27.1±1.7 | 26.6±1.9 | 26.7±2.1 | 26.8±2.2 | ||||
| Waist (cm) | WT-GAIN (N=48) | 89.5±8.9 | 89.4±7.7 | 89.7±8.5 | 91.3±9.0 | <0.0001 | 0.006 | <0.0001 |
| WT-STABLE (N=132) | 90.9±8.4 | 89.5±8.9 | 90.0±8.7 | 91.1±8.6 | ||||
| WT-LOSS (N=68) | 90.1±8.2 | 86.3±9.0 | 85.3±10.3 | 84.9±9.9 | ||||
| Total (N=248) | 90.4±8.4 | 88.6±8.8 | 88.7±9.3 | 89.4±9.4 | ||||
| Percent Body Fat | WT-GAIN (N=48) | 33.6±4.7 | 34.2±4.6 | 35.4±4.5 | 36.1±4.2 | <0.0001 | <0.0001 | <0.0001 |
| WT-STABLE (N=132) | 33.9±3.7 | 33.4±4.0 | 33.9±4.0 | 33.9±4.0 | ||||
| WT-LOSS (N=68) | 32.0±4.5 | 29.7±5.5 | 29.2±5.7 | 28.7±5.4 | ||||
| Total (N=248) | 33.4±4.2 | 32.5±4.9 | 32.9±5.2 | 32.9±5.2 | ||||
| Fitness (minutes) | WT-GAIN (N=48) | 10.3±3.6 | 11.9±3.2 | 11.2±3.5 | 11.7±3.5 | <0.0001 | 0.001 | <0.0001 |
| WT-STABLE (N=132) | 10.3±3.4 | 12.5±4.2 | 11.7±3.9 | 11.5±3.9 | ||||
| WT-LOSS (N=68) | 9.7±3.8 | 14.3±5.1 | 14.7±4.2 | 14.4±4.6 | ||||
| Total (N=248) | 10.1±3.6 | 13.0±4.4 | 12.4±4.2 | 12.3±4.2 | ||||
WT-GAIN = >3% gain in weight from baseline to 18 months; WT-STABLE: ±3% change in weight from baseline to 18 months; WT-LOSS: >3% weight loss from baseline to 18 months.
indicates p-values were obtained from the mixed effects models with log-transformed data.
Fitness
There was a significant Group X Time (p<0.001) interaction for change in fitness between the groups. The increase in time to achieve 85% of age-predicted maximal heart rate from baseline to 6 months was 1.6, 3.3, and 3.4 minutes for SELF, MOD-PA, and HIGH-PA, respectively (Table 2). At 18 months the change from baseline was 1.6 minutes for SELF, 2.0 minutes for MOD-PA, and 2.8 minutes for HIGH-PA.
Analysis by Weight Change Categories
Subjects were classified as having remained within ±3% of baseline weight (WT-STABLE), lost weight of >3% compared to baseline weight (WT-LOSS), or gained weight of >3% compared to baseline weight (WT-GAIN). The criterion of ±3% of baseline weight being used to define weight stability is based on the published criteria proposed by Stevens et al. (7). Overall, 53.2% (N=132) of subjects remained weight stable, 27.4% (N=68) lost weight, and 19.4% (N=48) gained weight using these criteria at 18 months. Chi-square analysis showed no significant difference between randomized groups for the number of subjects in these weight categories (chi-square p-value = 0.80).
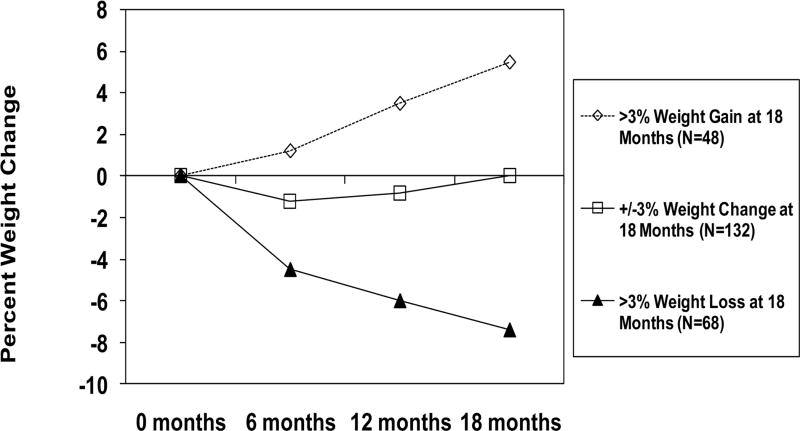
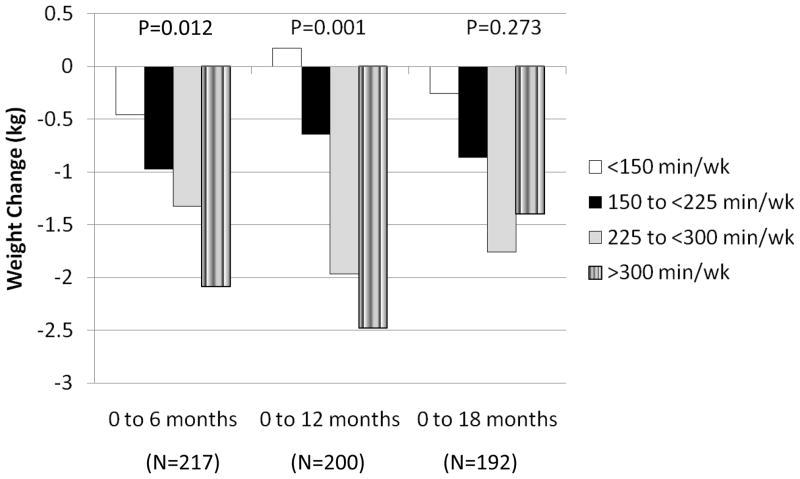
Weight change at 18 months was +5.4±2.6% (4.0±1.8 kg) for WT-GAIN, 0.0±1.3% (0.0±1.0 kg) for WT-STABLE, and −7.4±3.6% (−5.4±2.6 kg) for WT-LOSS (p<0.001) (Table 3 and Figure 2). Changes in waist circumference and percent body fat followed the same pattern as change in body weight. ANOVA was also performed with subjects retrospectively grouped according to level of physical activity at 6, 12, and 18 months. These retrospective groupings were defined as <150 min/wk, 150 to <225 min/wk, 225 to <300 min/wk, and ≥300 min/wk. Results are presented in Figure 4 and showed significant improvement in weight loss with a higher dose of PA at 6 (p=0.012) and 12 months (p=0.001), with weight loss not differing by PA category at 18 months (p=0.273).
Figure 2.
Percent weight change by 18 month weight change classification.
Figure 4.
Change in body weight by level of self-reported physical activity at 6, 12, and 18 months.
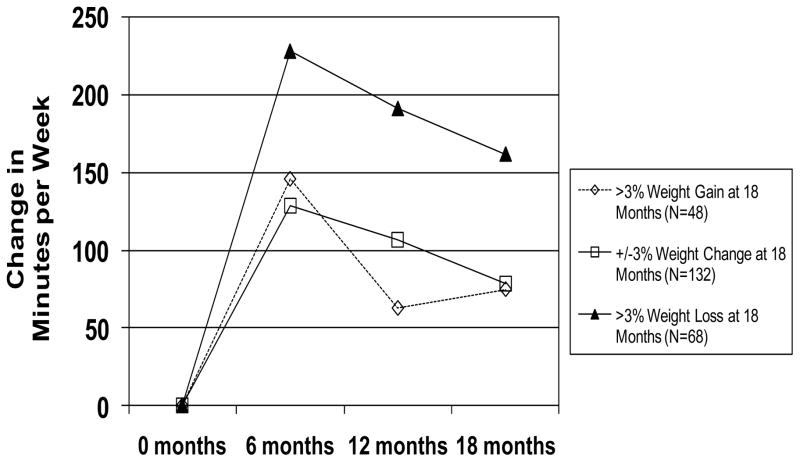
There was a significant Group X Time interaction for change in PA between WT-STABLE, WT-LOSS, and WT-GAIN (p<0.001). The increase in PA from baseline to 18 months was 78.2±162.6 min/wk for WT-STABLE, 161.9±252.6 min/wk for WT-LOSS, and 74.7±274.3 min/wk for WT-GAIN (Figure 3 and Table 3). There was a significant Group X Time (p<0.001) interaction for change in fitness between the groups with the increases in time to achieve 85% of age-predicted maximal heart rate being 4.7, 1.2, and 1.4 minutes for WT-LOSS, WT-STABLE, and WT-GAIN, respectively (Table 3).
Figure 3.
Change in physical activity by 18 month weight change classification.
There was a modest but significant overall decrease in dietary intake (1822.5±860.6 to 1621.5±782.6 kcal/d) and percent dietary fat intake (39.2±7.9% to 38.4±7.8%), an increase in percent carbohydrate intake (46.0±8.7% to 47.0±8.6%), with no change in percent protein intake (15.3±2.9% to 15.5±2.9%) from baseline to 18 months, with no significant difference between WT-STABLE, WT-LOSS, and WT-GAIN. However, there was a significant Group X Time interaction (p<0.001) for change in EBI score, with change from baseline to 18 month scores being 2.8±6.8, 10.0±9.9, and 4.9±10.4 for WT-STABLE, WT-LOSS, and WT-GAIN, respectively.
DISCUSSION
Two recent comprehensive reviews of the literature have reported that PA interventions result in a modest decrease in body weight. The literature review conducted for the Physical Activity Guidelines for Americans (5, 20) concluded that weight loss is typically 1–3% of initial body weight in response to ≥180 min/wk of PA, with PA <150 min/wk resulting in no reduction in body weight. The American College of Sports Medicine concluded that 150–250 min/wk of PA results in a similar magnitude of weight loss (3). Few of these studies conducted between 1998 and 2008 were long-term in duration, whereas the current study reports on the effects of an 18-month intervention. The results of the current study that included only overweight adults are consistent with these prior reviews of the literature that included studies of both overweight and obese adults. The current study demonstrated that a prescribed dose of 150 min/wk or 300 min/wk of PA resulted in <2% weight loss in overweight adults. These results are also consistent with earlier reviews of the literature conducted in the late 1990’s (2, 21). However, subjects in this study were not prescribed a reduction in energy intake, which most likely reduced the potential of a greater reduction in body weight in MOD-PA and HIGH-PA. We previous demonstrated that a prescribed reduction in energy intake combined with a sufficient dose of PA results in approximately an 8–10% reduction in body weight by the end of a 6 month intervention (8, 9, 19). Moreover, the progression of PA followed a reasonable clinical approach to minimize injury risk from overuse too early within the intervention, and is similar to a progression that we have used in a prior weight loss study (8, 9). However, this resulted in the MOD-PA group reaching their prescribed maximal PA dose much more rapidly than the HIGH-PA group, which may have influenced the weight loss observed when comparing these intervention conditions. However, despite this difference in progression to maximal PA dose, the HIGH-PA group was still prescribed a higher dose of PA during the final 15 months of the intervention compare to both MOD-PA and SELF, yet this still did not result in significant differences in weight loss.
Despite the findings from the primary analysis of the data that demonstrated a modest effect of PA on change in body weight in overweight adults, results from secondary analysis may provide an enhanced understanding of these data. When grouped based on whether there was weight gain (WT-GAIN), weight loss (WT-LOSS), or weight stability (WT-STABLE) it does appear that an increase in PA can result in significant weight loss. Overweight participants classified as WT-LOSS showed a weight loss of 7.4% (5.4 kg) by the completion of the 18 month intervention, and this was accompanied by an increase in PA by 228 min/wk at 6 months, and despite decreasing at 18 months still remained 161 min/wk above baseline. This may suggest that when compliant to an increase in the dose of PA that is 161 to 228 min/wk above baseline, weight loss may be significant in overweight adults. Of interest is that this dose of PA is similar to recent PA guidelines (5, 20) and recommended by the American College of Sports Medicine (3). However, it needs to be emphasized that these findings are based on secondary analysis with subjects retrospectively grouped by weight change to examine the dose of PA that may contribute to differences in these observed changes in weight.
Despite these findings, concluding that PA alone can result in the magnitude of weight loss observed in WT-LOSS within the retrospective secondary analysis may be misleading. There is some indication that participants classified as WT-LOSS, who lost 7.4% of their body weight compared to baseline (Table 4 and Figure 2) also made significant changes in their eating behavior as measured by the EBI questionnaire (Table 4), despite the lack of an intervention promoting a reduction in energy intake. In fact, these results are consistent with other studies with interventions that have prescribed both reductions in energy intake and increases in PA for weight loss (3, 22). Thus, it appears that changes in both behaviors are important to maximize weight loss in overweight adults. Moreover, as previously reported, weight loss behaviors such as PA and diet appear to cluster, and the results from this study support this initial observation (23). This may suggest that for some individuals an increase in PA may also indirectly affect eating behavior and potentially dietary intake that can result in weight loss, but this requires further study as few studies have been able to confirm this effect.
This study demonstrates that approximately 20% of overweight subjects gained significant weight, and this weight gain can be on the magnitude of approximately 4 kg over a period of 18 months (Figure 2 and Table 4). Weight gain was also accompanied by an increase in abdominal adiposity, which has been shown to increase risk of chronic health conditions such as cardiovascular disease and diabetes (24). Identifying characteristics of those individuals who gained weight may be important to identify for whom a behavioral intervention that focuses primarily on increasing PA is not effective for preventing weight gain.
The results of this study demonstrate the challenges of traditional behavioral intervention programs to maintain a sufficient dose of PA to reduce body weight. During the initial 6 months the MOD-PA group increased PA above baseline values by 131 min/wk and the HIGH-PA group increased PA by 245 min/d, which was 87.3% and 81.7% of the 150 min/wk and 300 min/wk goals for these groups, respectively. At 18-months the PA was 66 min/wk and 155 min/wk above baseline levels in MOD-PA and HIGH-PA, respectively, reflecting a 49.6% and 36.7% decrease compared to the peak increases observed at 6 months. This study did implement an intervention that included intensive contact and included theoretically based behavioral constructs to promote adherence. This suggests the need for additional research to implement effective interventions to achieve and maintain targeted PA goals in overweight adults. This was identified as a key research area by the Advisory Committee for the Physical Activity Guidelines for Americans (20).
The SELF group also showed increases in PA of 74.6 min/wk (442 kcal/wk) from baseline to 18 months (see Table 2). While this increase was only 49.7% of the consensus public health recommendation to increase PA by 150 min/wk, this increase was comparable to what was observed in MOD-PA. SELF was provided with a self-help manual that is evidence-based to improve physical activity, which may have contributed to the increase in PA observed in response to this intervention. However, in prior studies in which we have used a self-help manual, this type of intervention has demonstrated a minimal effect on behavior change resulting in PA increasing by 89 kcal/wk at 6 months and 122 kcal/wk at 24 months (18). Thus, future studies should examine the feasibility and effectiveness of these self-help interventions for improving physical activity that may affect body weight and related chronic disease risk factors.
This study is not without limitations. Dietary intake was assessed using a food frequency questionnaire, and this may explain why those individuals who gained weight (WT-GAIN) reported a decrease in total energy intake over the 18-month intervention (Table 4) based on retrospective secondary analysis. There was little change in percent dietary fat intake and the adoption of weight loss eating behaviors (EBI) for those who gained weight, whereas those who lost weight (WT-LOSS) showed decreases in dietary fat and increases in weight loss eating behaviors. This may demonstrate the difficulty of accurately assessing energy intake in a relatively large randomized intervention study, and future studies may need to use alternative assessment methods to more accurately assess changes in dietary intake in response to a behavioral intervention. This study also used a questionnaire to assess PA, with WT-LOSS reporting significantly greater increases in PA compared to WT-GAIN and WT-STABLE. However, the results of this study also demonstrated that WT-LOSS had significantly greater increases in objectively measured fitness (Table 4), which supports the finding that PA improved more in this group compared to the others. The pattern of change in PA also mirrored the pattern of change in fitness between SELF, MOD-PA, and HIGH-PA. However, PA was based on self-report and future studies should consider assessing PA using objective methods (e.g, accelerometers, multi-sensor devices) to define the dose of PA necessary for weight loss or weight maintenance in overweight adults. Moreover, this study used self-reported dietary intake, which has been shown to under-report dietart intake to objective methods such as doubly-labeled water, which may influence the interpretation of our results concerning the contribution to energy intake on observed changes in body weight. The body composition data are also based on bioelectrical impedance analysis and anthropometry, and future studies should consider the use of dual-energy x-ray absorptiometry (DXA), magnetic resonance imaging (MRI), and computed tomography (CT) to provide additional objective data with regard to the effects of this type of PA intervention on changes in body composition and fat distribution.
It is important to highlight that this study was performed in an outpatient setting, and this may explain the difference in findings related to weight change in this study compare to other studies that have used supervised PA. For example, 16-month results reported by Donnelly et al. (25) showed a 4.8 kg difference in weight between PA and control groups for men and a 5.2 kg in weight between the groups for women, with PA involving increasing energy expenditure by 371 kcal/d in men and 209 kcal/d in women. However, these doses of PA resulted in weight loss in men but prevention of weight gain in women compared to the control groups. Ross et al. (26) reported a 6.5 kg reduction in weight in response to an exercise dose of 3500 kcal/wk with no change in energy intake over a period of 14. Thus, the lower change in weight observed in this current study may reflect lower adherence to the prescribed dose of PA that may not be able to be detected using the self-report measures used in this study. However, Slentz et al. (27) showed that PA equivalent to 20 miles per week of walking or jogging, which would approximate 300 to 400 minutes per week assuming a 15 to 20 minute per mile walking pace, resulted in 2.9±2.8 kg of weight loss over a period of 8 months. Church et al. (28) reported on weight loss resulting from different doses of supervised PA with no change in dietary intake. A higher dose of PA (12 kcal/kg/week) resulted in 1.5 kg of weight loss, which was not greater than the 2.1 kg or 1.4 kg weight loss resulting from 8 kcal/kg/wk or 4 kcal/kg/wk, respectively. This may suggest that higher doses of PA may result in compensation of energy intake that affects energy balance, and this requires further investigation.
In summary, the mean change in body weight resulting from an intervention that promotes 150 to 300 min/wk of moderate-intensity PA with no reduction in energy intake is <2.0 kg. However, approximately 25% of subjects lost >3% of their initial body weight in this study, with these subjects categorized retrospectively as WT-LOSS for secondary analysis. Individuals categorized as WT-LOSS for these secondary analyses do appear to be more compliant with initially increasing PA by 245 min/wk and maintaining an increase of at least 161 min/wk at the conclusion of this 18-month study. These individuals may also self-select to change their eating behaviors, which in combination with PA, resulted in the significant decrease in weight. Health-care providers should promote modifications in these behaviors; however, strategies continue to be needed to facilitate the adoption and maintenance of these behaviors long-term.
Acknowledgments
This study was supported by funding provided by the National Heart, Lung and Blood Institute (R01 HL70257) to a grant award to Dr. Jakicic and by the University of Pittsburgh Obesity and Nutrition Research (P30 DK46204). The authors also recognize the significant contribution of the staff and graduate students in the Physical Activity and Weight Management Research Center at the University of Pittsburgh. Dr. Jakicic and Dr. Lang had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Trial Registration: ClinicalTrial.gov, NCT00177502
Disclosures
Dr. Jakicic is a consultant with UPMC Health Plan (Pittsburgh, PA), BodyMedia, Inc. (Pittsburgh PA), and Proctor & Gamble (Cincinnati, OH), and is on the Scientific Advisory Board for Free & Clear (Seattle, WA). Dr. Mohr is currently affiliated with Mohr Results, Inc. (Louisville, KY). Drs. Otto, Lang, Winters, Polzien, and Ms. Semler have no disclosures to report.
References
- 1.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–55. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 2.National Institutes of Health National Heart Lung and Blood Institute. Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults - The Evidence Report. Obes Res. 1998;6(suppl 2) [PubMed] [Google Scholar]
- 3.Donnelly JE, Jakicic J, Blair SN, Rankin J, Manore M. ACSM position stand on appropriate intervention strategies for weight loss and prevention of weigth regain for adults. Med Sci Sports Exerc. 2009;42:459–71. doi: 10.1249/MSS.0b013e3181949333. [DOI] [PubMed] [Google Scholar]
- 4.US Department of Health and Human Services and US Department of Agriculture. Dietary Guidelines for Americans. Washington, DC: 2005. [cited 2009 Dec 29]. Available from: www.healthierus.gov/dietaryguidelines. [Google Scholar]
- 5.US Department of Health and Human Services. 2008 Physical Activity Guidelines for Americans. Washington, DC: 2008. [cited 2009 Dec 29]. Available from: http://www.health.gov/paguidelines/guidelines/summary.aspx. [Google Scholar]
- 6.Cloud J. Why exercise won’t make you thin. Time. 2009;174(6) [PubMed] [Google Scholar]
- 7.Stevens J, Truesdale KP, McClain JE, Cai J. The definition of weight maintenance. International Journal of Obesity. 2006;30:391–9. doi: 10.1038/sj.ijo.0803175. [DOI] [PubMed] [Google Scholar]
- 8.Jakicic JM, Marcus BH, Gallagher KI, Napolitano M, Lang W. Effect of exercise duration and intensity on weight loss in overweight, sedentary women. A randomized trial. JAMA. 2003;290:1323–30. doi: 10.1001/jama.290.10.1323. [DOI] [PubMed] [Google Scholar]
- 9.Jakicic JM, Marcus BH, Lang W, Janney C. Effect of exercise on 24-month weight loss in overweight women. Arch Int Med. 2008;168:1550–9. doi: 10.1001/archinte.168.14.1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription. 8. Wolters Kluwer/Lippincott Williams & Wilkins; Philadelphia: 2009. p. 20. [Google Scholar]
- 11.Segal KR, Gutin B, Presta E, Wang J, Van Itallie TB. Estimation of human body composition by electrical impedance methods: a comparative study. J Appl Physiol. 1985;58:1565–71. doi: 10.1152/jappl.1985.58.5.1565. [DOI] [PubMed] [Google Scholar]
- 12.Paffenbarger RS, Hyde RT, Wing AL, Hsieh CC. Physical activity, all-cause mortality, and longevity of college alumni. N Engl J Med. 1986;314:605–13. doi: 10.1056/NEJM198603063141003. [DOI] [PubMed] [Google Scholar]
- 13.Ainsworth BE, Haskell WL, Leon AS, et al. Compendium of physical activities: classification of energy costs of human physical activities. Med Sci Sports Exerc. 1993;25:71–80. doi: 10.1249/00005768-199301000-00011. [DOI] [PubMed] [Google Scholar]
- 14.Block G, Hartman AM, Dresser CM, MDC, JG, LG A data-based approach to diet questionnaire design and testing. Am J Epidemiol. 1986;108:161–75. doi: 10.1093/oxfordjournals.aje.a114416. [DOI] [PubMed] [Google Scholar]
- 15.Block G, Woods M, Potosky A, Clifford C. Validation of a self-administered diet history questionnaire using multiple diet records. J Clin Epidemiol. 1990;43:1327–35. doi: 10.1016/0895-4356(90)90099-b. [DOI] [PubMed] [Google Scholar]
- 16.O’Neil PM, Currey HS, Hirsch AA, et al. Development and validation of the eating behavior inventory. Journal of Behavioral Assessment. 1979;1:123–32. [Google Scholar]
- 17.Blair SN, Dunn AL, Marcus BH, Carpenter RA, Jaret P. Active Living Every Day. Human Kinetics; Champaign, IL: 2001. [Google Scholar]
- 18.Wing RR, Venditti EM, Jakicic JM, Polley BA, Lang W. Lifestyle intervention in overweight individuals with a family history of diabetes. Diabetes Care. 1998;21:350–9. doi: 10.2337/diacare.21.3.350. [DOI] [PubMed] [Google Scholar]
- 19.Jakicic JM, Winters C, Lang W, Wing RR. Effects of intermittent exercise and use of home exercise equipment on adherence, weight loss, and fitness in overweight women: a randomized trial. Journal of the American Medical Association. 1999;282:1554–60. doi: 10.1001/jama.282.16.1554. [DOI] [PubMed] [Google Scholar]
- 20.US Department of Health and Human Services. Physical Activity Guidelines Advisory Committee Report 2008. US Department of Health and Human Services; Washington, DC: 2008. [Google Scholar]
- 21.Wing RR. Physical activity in the treatment of the adulthood overweight and obesity: current evidence and research issues. Med Sci Sports Exerc. 1999;31:S547–52. doi: 10.1097/00005768-199911001-00010. [DOI] [PubMed] [Google Scholar]
- 22.Seagle HM, Strain GW, Markis A, Reeves RS. Position of the American Dietetic Association: weight management. J Am Diet Assoc. 2009;109:330–46. doi: 10.1016/j.jada.2008.11.041. [DOI] [PubMed] [Google Scholar]
- 23.Jakicic JM, Wing RR, Winters-Hart C. Relationship of physical activity to eating behaviors and weight loss in women. Med Sci Sports Exerc. 2002;34:1653–9. doi: 10.1097/00005768-200210000-00018. [DOI] [PubMed] [Google Scholar]
- 24.Després JP. Intra-abdominal obesity: an untreated risk factor for type 2 diabetes and cardiovascular disease. J Endocrinol Invest. 2006;29:77–82. [PubMed] [Google Scholar]
- 25.Donnelly JE, Hill JO, Jacobsen DJ, et al. Effects of a 16-month randomized controlled exercise trial on body weight and composition in young, overweight men and women. Arch Int Med. 2003;163:1343–50. doi: 10.1001/archinte.163.11.1343. [DOI] [PubMed] [Google Scholar]
- 26.Ross R, janssen I, Dawson J, et al. Exercise-induced reduction in obesity and insulin resistance in women: a randomized controlled trial. Obesity Research. 2004;12:789–98. doi: 10.1038/oby.2004.95. [DOI] [PubMed] [Google Scholar]
- 27.Slentz CA, Duscha MS, Johnson JL, et al. Effects of the amount of exercise on body weight, body composition, and measures of central obesity. STRIDDE - a randomized controlled study. Arch Int Med. 2004;164:31–9. doi: 10.1001/archinte.164.1.31. [DOI] [PubMed] [Google Scholar]
- 28.Church TS, Earnest CP, Skinner JS, Blair SN. Effects of different doses of physical activity on cardiorespiratory fitness among sedentary, overweight or obese postmenopausal women with elevated blood pressure. JAMA. 2007;297:2081–91. doi: 10.1001/jama.297.19.2081. [DOI] [PubMed] [Google Scholar]