Abstract
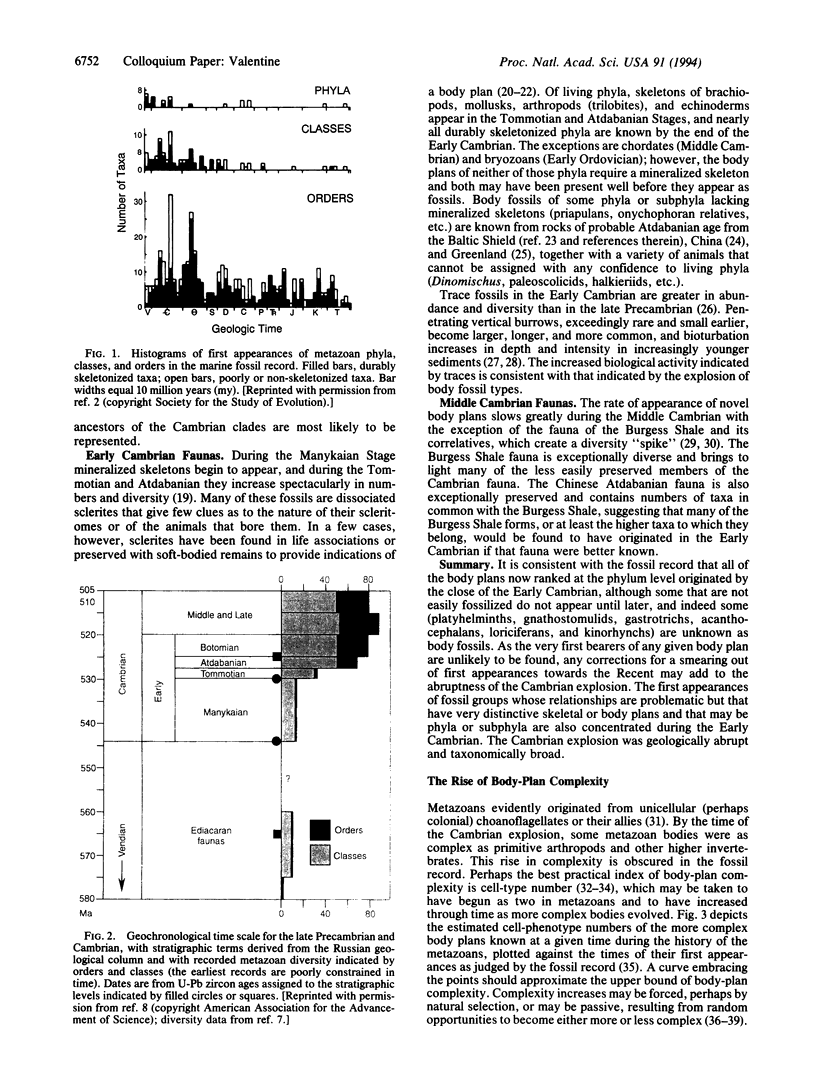
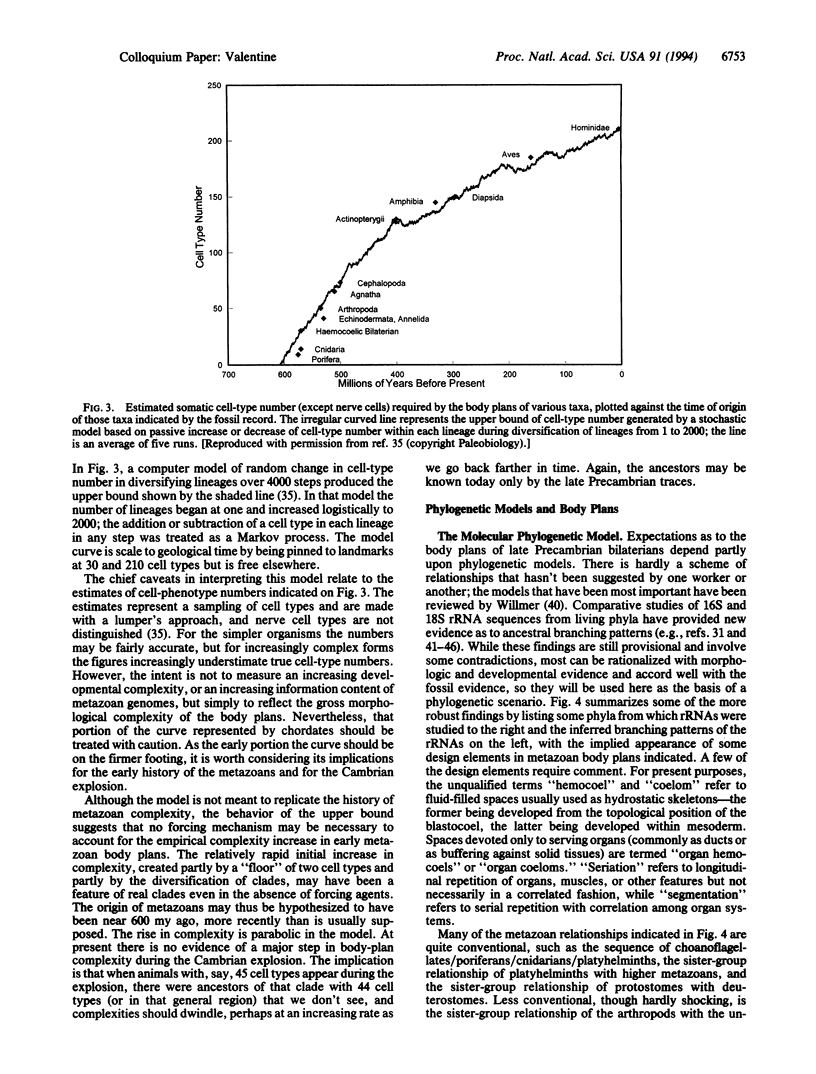
A broad variety of body plans and subplans appear during a period of perhaps 8 million years (my) within the Early Cambrian, an unequaled explosion of morphological novelty, the ancestral lineages represented chiefly or entirely by trace fossils. Evidence from the fossil record can be combined with that from molecular phylogenetic trees to suggest that the last common ancestor of (i) protostomes and deuterostomes was a roundish worm with a blood vascular system and (ii) of arthropods and annelids was similar, with a hydrostatic hemocoel; these forms are probably among trace makers of the late Precambrian. Cell-phenotype numbers in living phyla, and a model of cell-phenotype number increase, suggest an origin of metazoans near 600 my ago, followed by a passive rise in body-plan complexity. Living phyla appearing during the Cambrian explosion have a Hox/HOM gene cluster, implying its presence in the common ancestral trace makers. The explosion required a repatterning of gene expression that mediated the development of novel body plans but evidently did not require an important, abrupt increase in genomic or morphologic complexity.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akam M. The molecular basis for metameric pattern in the Drosophila embryo. Development. 1987 Sep;101(1):1–22. [PubMed] [Google Scholar]
- Ballard J. W., Olsen G. J., Faith D. P., Odgers W. A., Rowell D. M., Atkinson P. W. Evidence from 12S ribosomal RNA sequences that onychophorans are modified arthropods. Science. 1992 Nov 20;258(5086):1345–1348. doi: 10.1126/science.1455227. [DOI] [PubMed] [Google Scholar]
- Bartels J. L., Murtha M. T., Ruddle F. H. Multiple Hox/HOM-class homeoboxes in Platyhelminthes. Mol Phylogenet Evol. 1993 Jun;2(2):143–151. doi: 10.1006/mpev.1993.1014. [DOI] [PubMed] [Google Scholar]
- Bowring S. A., Grotzinger J. P., Isachsen C. E., Knoll A. H., Pelechaty S. M., Kolosov P. Calibrating rates of early Cambrian evolution. Science. 1993 Sep 3;261:1293–1298. doi: 10.1126/science.11539488. [DOI] [PubMed] [Google Scholar]
- Britten R. J., Davidson E. H. Repetitive and non-repetitive DNA sequences and a speculation on the origins of evolutionary novelty. Q Rev Biol. 1971 Jun;46(2):111–138. doi: 10.1086/406830. [DOI] [PubMed] [Google Scholar]
- Bürglin T. R., Ruvkun G. The Caenorhabditis elegans homeobox gene cluster. Curr Opin Genet Dev. 1993 Aug;3(4):615–620. doi: 10.1016/0959-437x(93)90097-9. [DOI] [PubMed] [Google Scholar]
- Cartwright P., Dick M., Buss L. W. HOM/Hox type homeoboxes in the chelicerate Limulus polyphemus. Mol Phylogenet Evol. 1993 Sep;2(3):185–192. doi: 10.1006/mpev.1993.1019. [DOI] [PubMed] [Google Scholar]
- Chalfie M. Homeobox genes in Caenorhabditis elegans. Curr Opin Genet Dev. 1993 Apr;3(2):275–277. doi: 10.1016/0959-437x(93)90034-m. [DOI] [PubMed] [Google Scholar]
- Davidson E. H. How embryos work: a comparative view of diverse modes of cell fate specification. Development. 1990 Mar;108(3):365–389. doi: 10.1242/dev.108.3.365. [DOI] [PubMed] [Google Scholar]
- Davidson E. H. Spatial mechanisms of gene regulation in metazoan embryos. Development. 1991 Sep;113(1):1–26. doi: 10.1242/dev.113.1.1. [DOI] [PubMed] [Google Scholar]
- Echelard Y., Epstein D. J., St-Jacques B., Shen L., Mohler J., McMahon J. A., McMahon A. P. Sonic hedgehog, a member of a family of putative signaling molecules, is implicated in the regulation of CNS polarity. Cell. 1993 Dec 31;75(7):1417–1430. doi: 10.1016/0092-8674(93)90627-3. [DOI] [PubMed] [Google Scholar]
- Erwin D. H., Valentine J. W., Sepkoski J. J., Jr A comparative study of diversification events: the early Paleozoic versus the Mesozoic. Evolution. 1987;41(6):1177–1186. [PubMed] [Google Scholar]
- Field K. G., Olsen G. J., Lane D. J., Giovannoni S. J., Ghiselin M. T., Raff E. C., Pace N. R., Raff R. A. Molecular phylogeny of the animal kingdom. Science. 1988 Feb 12;239(4841 Pt 1):748–753. doi: 10.1126/science.3277277. [DOI] [PubMed] [Google Scholar]
- Ingham P. W. The molecular genetics of embryonic pattern formation in Drosophila. Nature. 1988 Sep 1;335(6185):25–34. doi: 10.1038/335025a0. [DOI] [PubMed] [Google Scholar]
- Jacobs D. K. Selector genes and the Cambrian radiation of Bilateria. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4406–4410. doi: 10.1073/pnas.87.11.4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappen C., Schughart K., Ruddle F. H. Two steps in the evolution of Antennapedia-class vertebrate homeobox genes. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5459–5463. doi: 10.1073/pnas.86.14.5459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon C., Wang B. A cluster of Antennapedia-class homeobox genes in a nonsegmented animal. Science. 1991 Aug 2;253(5019):516–517. doi: 10.1126/science.1677487. [DOI] [PubMed] [Google Scholar]
- Krauss S., Concordet J. P., Ingham P. W. A functionally conserved homolog of the Drosophila segment polarity gene hh is expressed in tissues with polarizing activity in zebrafish embryos. Cell. 1993 Dec 31;75(7):1431–1444. doi: 10.1016/0092-8674(93)90628-4. [DOI] [PubMed] [Google Scholar]
- Lake J. A. Origin of the Metazoa. Proc Natl Acad Sci U S A. 1990 Jan;87(2):763–766. doi: 10.1073/pnas.87.2.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinnis W. Homeo box sequences of the Antennapedia class are conserved only in higher animal genomes. Cold Spring Harb Symp Quant Biol. 1985;50:263–270. doi: 10.1101/sqb.1985.050.01.033. [DOI] [PubMed] [Google Scholar]
- Oliver G., Vispo M., Mailhos A., Martínez C., Sosa-Pineda B., Fielitz W., Ehrlich R. Homeoboxes in flatworms. Gene. 1992 Nov 16;121(2):337–342. doi: 10.1016/0378-1119(92)90140-k. [DOI] [PubMed] [Google Scholar]
- Patel N. H., Martin-Blanco E., Coleman K. G., Poole S. J., Ellis M. C., Kornberg T. B., Goodman C. S. Expression of engrailed proteins in arthropods, annelids, and chordates. Cell. 1989 Sep 8;58(5):955–968. doi: 10.1016/0092-8674(89)90947-1. [DOI] [PubMed] [Google Scholar]
- Pendleton J. W., Nagai B. K., Murtha M. T., Ruddle F. H. Expansion of the Hox gene family and the evolution of chordates. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):6300–6304. doi: 10.1073/pnas.90.13.6300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle R. D., Johnson R. L., Laufer E., Tabin C. Sonic hedgehog mediates the polarizing activity of the ZPA. Cell. 1993 Dec 31;75(7):1401–1416. doi: 10.1016/0092-8674(93)90626-2. [DOI] [PubMed] [Google Scholar]
- Schierwater B., Murtha M., Dick M., Ruddle F. H., Buss L. W. Homeoboxes in cnidarians. J Exp Zool. 1991 Dec;260(3):413–416. doi: 10.1002/jez.1402600316. [DOI] [PubMed] [Google Scholar]
- Schummer M., Scheurlen I., Schaller C., Galliot B. HOM/HOX homeobox genes are present in hydra (Chlorohydra viridissima) and are differentially expressed during regeneration. EMBO J. 1992 May;11(5):1815–1823. doi: 10.1002/j.1460-2075.1992.tb05233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepkoski J. J., Jr Ten years in the library: new data confirm paleontological patterns. Paleobiology. 1993 Winter;19(1):43–51. doi: 10.1017/s0094837300012306. [DOI] [PubMed] [Google Scholar]
- Shashikant C. S., Utset M. F., Violette S. M., Wise T. L., Einat P., Einat M., Pendleton J. W., Schughart K., Ruddle F. H. Homeobox genes in mouse development. Crit Rev Eukaryot Gene Expr. 1991;1(3):207–245. [PubMed] [Google Scholar]
- Turbeville J. M., Field K. G., Raff R. A. Phylogenetic position of phylum Nemertini, inferred from 18S rRNA sequences: molecular data as a test of morphological character homology. Mol Biol Evol. 1992 Mar;9(2):235–249. doi: 10.1093/oxfordjournals.molbev.a040716. [DOI] [PubMed] [Google Scholar]
- Turbeville J. M., Pfeifer D. M., Field K. G., Raff R. A. The phylogenetic status of arthropods, as inferred from 18S rRNA sequences. Mol Biol Evol. 1991 Sep;8(5):669–686. doi: 10.1093/oxfordjournals.molbev.a040677. [DOI] [PubMed] [Google Scholar]
- Valentine J. W. Bilaterians of the Precambrian-Cambrian transition and the annelid-arthropod relationship. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2272–2275. doi: 10.1073/pnas.86.7.2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentine J. W., Campbell C. A. Genetic regulation and the fossil record. Am Sci. 1975 Nov-Dec;63(6):673–680. [PubMed] [Google Scholar]
- Valentine J. W., Tiffney B. H., Sepkoski J. J., Jr Evolutionary dynamics of plants and animals: a comparative approach. Palaios. 1991;6:81–88. [PubMed] [Google Scholar]
- Wainright P. O., Hinkle G., Sogin M. L., Stickel S. K. Monophyletic origins of the metazoa: an evolutionary link with fungi. Science. 1993 Apr 16;260(5106):340–342. doi: 10.1126/science.8469985. [DOI] [PubMed] [Google Scholar]
- Webster P. J., Mansour T. E. Conserved classes of homeodomains in Schistosoma mansoni, an early bilateral metazoan. Mech Dev. 1992 Jul;38(1):25–32. doi: 10.1016/0925-4773(92)90035-i. [DOI] [PubMed] [Google Scholar]




