Abstract
Background
The European Randomized study of Screening for Prostate Cancer (ERSPC) is a randomized multi-center trial with a predefined centralized database, analysis plan and core age group (55–69 years) evaluating prostate-specific antigen (PSA) testing in eight European countries.
Methods
The present results are based on prostate cancer (PCa) incidence and mortality truncated at 9, 11, and 13 years of follow-up in the intervention arm (offered PSA testing) relative to the control arm. A secondary analysis corrected for selection bias due to non-participation was performed. Because of incomplete follow-up, only incidence and no mortality data at 9 years follow-up are reported for the French centers.
Findings
The rate ratio (RR) of PCa incidence between the intervention and control arms was 1.91 after 9 years (1.64 including France), 1.66 after 11 years and 1.57 after 13 years. The RR of PCa mortality was 0.85, 0.78 and 0.79 at 9, 11 and 13 years respectively (95% confidence interval 13-year 0.69–0.91, p = 0.001). This corresponds to a relative risk reduction of 21% and an absolute risk reduction of death from PCa at 13 years of 0.11 per 1,000 person-years or 1.28 per 1,000 men randomized, which is equivalent to one PCa death averted per 781 men invited for screening or one per 27 additional PCa detected. PCa mortality reduction in screened men after adjustment for non–participation was 27%.
Interpretation
This update of ERSPC confirms a substantial PCa mortality reduction due to PSA testing, with a substantially increased absolute effect at 13 years compared to findings after 9 and 11 years.
Funding
All sources of funding per center are indicated in the “Web extra material” section.
Trial identification
This trial is registered under Current Controlled Trials number: ISRCTN49127736.
Keywords: Prostate cancer, prostate specific antigen (PSA), randomised controlled trial, mortality, mass screening
Introduction
The European Randomized study of Screening for Prostate Cancer (ERSPC) has demonstrated significant reductions in prostate cancer (PCa) mortality after 9 and 11 years of follow-up1, 2. In spite of this, screening for prostate cancer remains controversial because of adverse effects such as overdiagnosis, which is estimated to comprise 40–50% of screen-detected cases and often results in overtreatment with subsequent side effects3–5. However a modeling study, partly based on ERSPC data, showed with a 4-year screening interval a gain of 52 life-years and a gain of 41 quality of life adjusted life years (QALY’s) despite some reduction in quality of life owing to overdiagnosis and long-term side-effects of treatment5.
The present report gives updated PCa mortality results with follow-up through 2010, with analyses truncated at 9, 11 and 13 years of follow-up. For the first time, we include France in the analysis of PCa incidence at 9 years of follow-up, but not of PCa mortality because of incomplete follow-up to the end of 2010.
Methods
Study design
The ERSPC is a multi-center, randomized screening trial with the main goal to compare PCa mortality between an intervention arm invited to screening and a control arm with no intervention offered. The trial was initiated in 1993 in the Netherlands and in Belgium6, 7. Five other centers (Sweden, Finland, Italy, Spain and Switzerland) joined the study between 1994 and 1998. Two French centers started in 2000 and 2003.
Randomization and Masking
The ERSPC trial protocol has been published previously1, 2, 8, 9. In short, eligible subjects (men aged 50–74 years of age at time of randomization) were identified from population registers and randomization was performed individually based on random numbers (with 1:1 allocation, except in Finland where an intervention/control ratio of approximately 1:1.5 was used). Due to different legal regulations, randomization after informed consent was used in some and randomization before consent in other countries8, 9. Allocation of participants to the trial arms was concealed to the investigators.
Recruitment of participants
Recruitment was completed by the end of 2003, except in France with recruitment up to 2005. The screening interval of four years (two years in Sweden) was chosen on the basis of lead time estimated as >8 years at the time of trial initiation10, 11. Prostate-specific antigen (PSA) determination in serum with a cut-off of ≥3.0 ng/ml was the main screening test and indication for biopsy (an ancillary test was used for men with PSA 3.0–3.9 ng/ml in Finland and Italy). Sextant biopsies were initially recommended for screen-positive men, in line with practice recommendations during the initiation of ERSPC. Screening was discontinued after three screening rounds in Belgium, Finland and Spain and after two rounds in France, but continued up to five rounds in the Netherlands and ten in Sweden. During 1994 and 1995, performance criteria were established as indicators of successful conduct of the trial. These criteria included: a pilot study, randomization with concealed allocation, adherence to the common trial protocol, participation in quality control assessments and continuous conduct of the study (recruitment, screening and data collection)8. An independent quality control committee was in charge of the supervision of compliance with the performance criteria. Full access to the ERSPC data, including disease-specific mortality outcome, was provided by the protocol after the first end-point publication1.
Primary end-points
The primary endpoint of the study is PCa mortality12. Overall mortality was assessed mainly to ensure comparability between the trial arms, as no reduction in overall mortality was anticipated from the intervention (given the small fraction of all deaths caused by PCa). Data on overall mortality were obtained by linkage to national registries. PCa deaths were ascertained by local, independent, causes of death committees evaluating all deaths in men diagnosed with PCa and/or PCa as a cause of death in the death certificate, blinded to trial arm and following the same algorithm in all centers13. If consensus was not reached, the international causes of death committee was consulted. Of the evaluated deaths, those classified as ‘definitely PCa’ and ‘probably PCa’ and intervention related deaths were used as the outcome events in the analysis. Death certificates were used in Finland after a very high concordance with committee assignments was demonstrated (κ>0.9)14.
Safety assessments were conducted by the independent Data Monitoring Committee. Stopping rules covered an excess of overall or PCa mortality in the screening arm relative the control arm15.
Statistical analysis
The primary analysis evaluated PCa mortality and addressed the upfront agreed core age group 55–69 years, with follow-up through 2010 truncated at 9, 11 and 13 years. All results were calculated with the control group for Finland weighted by approximately 1:1.5. The analysis was carried out on the basis of the intention-to-treat (or intention-to-screen, ITS) principle, comparing groups formed by randomization regardless of compliance with the assignment. Rate ratios (RR) were calculated using Poisson regression. Reported p-values are two-sided. In addition, an analysis of mortality in men screened, corrected for selection bias due to non-participation, was performed16. France was excluded from all analyses of PCa mortality because of incomplete follow-up (median follow-up for the two French centers was only 6.4 and 7.5 years). France was included in a secondary analysis of PCa incidence using the follow-up period 1–9 years. An analysis considering all available ages is included as part of the appendix tables 1–3 and appendix figures 1, 2. A further secondary analysis shows the results per center for the core age group excluding France (Appendix table 4). No adjustment of significance for alpha-spending in sequential analyses was applied because the present analysis is protocol-based and not driven by statistical significance17, 18. Cumulative PCa mortality by arm was calculated using the Nelson-Aalen method17. Number needed to invite (NNI) to avert one PCa death was calculated as the inverse of the absolute risk reduction and number needed to detect (NND) as the NNI multiplied by the excess PCa incidence in the intervention group.
Role of funding sources
Most funding was obtained from national cancer research funding agencies, European funding in the form of Framework programs, some private sponsors and an unconditional grant of the former Beckman/Hybritech company. All details are given in the “Web extra material”.
Results
Screening results
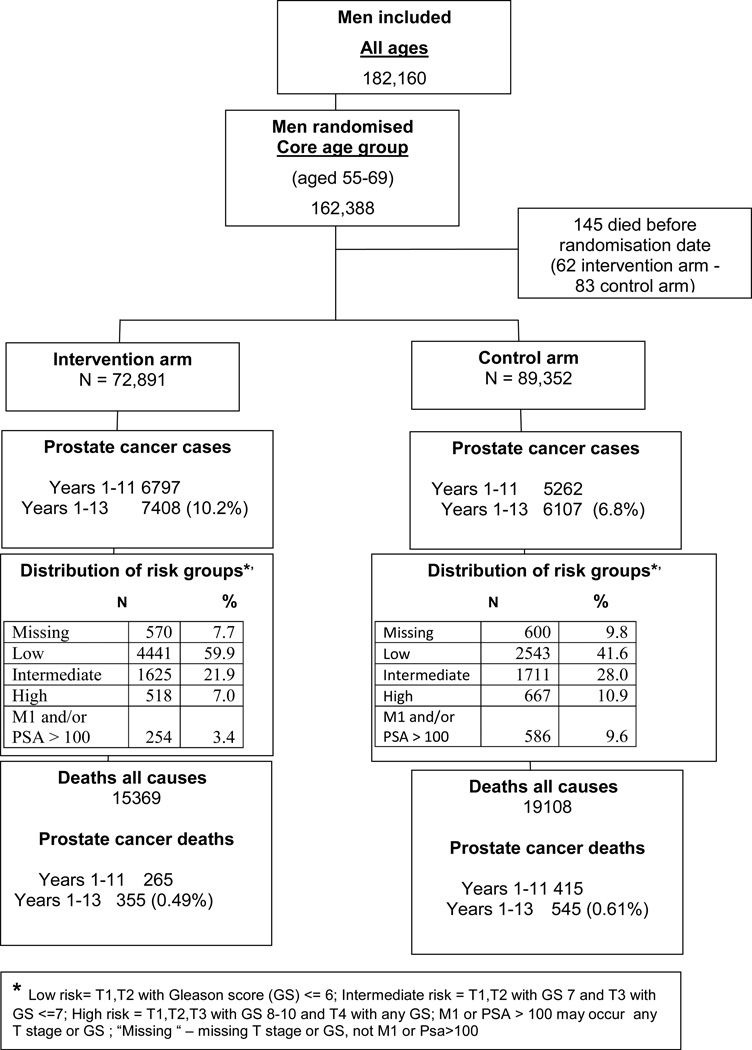
In the core age group of men aged 55–69 years, excluding France, 162,388 were randomized, of whom 145 died between randomization and screening. With data truncated at 13 years of follow-up, 7,408 PCa cases were diagnosed in the intervention arm and 6,107 cases in the control arm (Figure 1).
Figure 1.
Flow diagram of the ERSPC trial; core age group, excluding France.
The median age at randomization was 60.2 years. The overall compliance with biopsies was 85.6% of 23,574 screen-positive tests. On average, men in the intervention group were screened 2.3 times (ranging from 1.6 times in Belgium with a 7-year interval to 3.5 times in Sweden with a 2-year interval). Of the screen-positive men who underwent a biopsy, 24.2% were diagnosed with PCa within 12 months after testing (Table 1).
Table 1.
Randomization, participants and results of screening all centres (core age group, cut-off date December 31, 2010, data truncated at 13 years of follow-up).
| ERSPC – Randomization, participants and results of screening per centre (core ages, cut-off date December 31, 2010) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Netherlands | Belgium | Sweden | Finland | Italy | Spain | Switzerland | Total Excluding France |
France Herault June 2003 – Mar 2005 |
France Tarn Dec 2000 – June 2004 |
Total | |
| Period of randomization | Nov 1993 – March 2000 |
June 1991- Dec 2003 |
31-Dec-1994 (all randomized on same day) |
Jan 1996- Jan 1999 |
Oct 1996 – Oct 2000 |
Feb 1996 – June 1999 |
Sep 1998 -Aug-03 |
||||
| Median age at randomisation (years) | 61.7 | 63.0 | 59.7 | 58.7 | 61.8 | 60.4 | 61.1 | 60.2 | 62.0 | 62.0 | 61.1 |
| Randomized – N | 34,833 | 8,562 | 11,852 | 80,379 | 14,517 | 2,197 | 9,903 | 162,243 | 57,662 | 21,349 | 241,254 |
| - screening | 17,443 | 4,307 | 5,901 | 31,970 | 7,266 | 1,056 | 4,948 | 72,891 | 28,793 | 10,879 | 112,563 |
| 50.1% | 50.3% | 49.8% | 39.8% | 50.1% | 48.1% | 50.0% | 44.9% | 49.9% | 51.0% | 46.7% | |
| - control | 17,390 | 4,255 | 5,951 | 48,409 | 7,251 | 1,141 | 4,955 | 89,35 | 28,869 | 10,470 | 128,691 |
| 49.9% | 49.7% | 50.2% | 60.2% | 49.9% | 51.9% | 50.0% | 55.1% | 50.1% | 49.0% | 53.3% | |
| Screened at least once N | 16502 | 3908 | 4478 | 23771 | 5730 | 1056 | 4810 | 60255 | 7164 | 4143 | 71,562 |
| (%) | (94.6) | (90.7) | (75.9) | (74.4) | (78.9) | (100.0) | (97.2) | (82.7) | (24.9) | (38.1) | (63.6) |
| Screen tests done – N | 39345 | 6446 | 15468 | 52142 | 12731 | 1846 | 12062 | 140,040 | 7164 | 5358 | 152,562 |
| Mean no of screens / man screened | 2.4 | 1.6 | 3.5 | 2.2 | 2.2 | 1.7 | 2.5 | 2.3 | 1.0 | 1.3 | 2.1 |
| Positive tests – N | 9301 | 1058 | 2897 | 5925 | 1443 | 354 | 2596 | 23574 | 1091 | 821 | 25,486 |
| (%) | (23.6) | (16.4) | (18.7) | (11.4) | (11.3) | (19.2) | (21.5) | (16.8) | (15.2) | (15.3) | (16.7) |
| Biopsies – N | 8332 | 752 | 2510 | 5404 | 902 | 263 | 2025 | 20188 | 315 | 418 | 20921 |
| (% of screen positive) | (89.6) | (71.1) | (86.6) | (91.2) | (62.5) | (74.3) | (78.0) | (85.6) | (28.9) | (50.9) | (82.1) |
| Prostatecancers | |||||||||||
| Screening cohort total – N | 2180 | 417 | 738 | 3018 | 396 | 87 | 572 | 7408 | 1196 | 559 | 9163 |
| Screen detected N | 1809 | 187 | 570 | 1631 | 197 | 60 | 429 | 4883 | 163 | 121 | 5167 |
| Interval and Non attender N | 371 | 230 | 168 | 1387 | 199 | 27 | 143 | 2525 | 1033 | 438 | 3996 |
| PPV (S det cancers/biopsy, %) | (21.7) | (24.9) | (22.7) | (30.2) | (21.8 | (22.8) | (21.2) | (24.2) | (51.7) | (28.9) | (24.7) |
| Cumulative incidence (total cancers / all rand. To S arm, %) | (12.5) | (9.7) | (12.5) | (9.4) | ) (5.5) | (8.2) | (11.6) | (10.2) | (4.2) | (5.1) | (8.1) |
| Prostatecancer | |||||||||||
| Control group – N | 1070 | 321 | 469 | 3609 | 289 | 52 | 297 | 6107 | 1094 | 506 | 7707 |
| Cumulativeincidence (%) | (6.2) | (7.5) | (7.9) | (7.5) | (4.0) | (4.6) | (6.0) | (6.8) | (3.8) | (4.8) | (6.0) |
| Mean follow up (SD) (years) | 11.5 (2.8) | 10.8 (3.1) | 11.7 (3.0) | 11.4 (3.0) | 11.3 (2.7) | 11.9 (2.3) | 9.8 (2.0) | 11.3 (2.9) | 6.2 (1.1) | 7.3 (1.5) | 9.7 (3.4) |
| Median follow-up (IQR) (years) | 13.0 (1.6) | 13.0 (4.0) | 13.0 (0.0) | 13.0 (1.0) | 12.6 (2.3) | 12.7 (0.7) | 10.2 (2.5) | 13.0 (1.8) | 6.4 (0.6) | 7.5 (0) | 11.3 (6.4) |
Prostate cancer incidence and mortality
With follow-up truncated at 13 years, PCa incidence was 9.55 per 1,000 person-years in the intervention and 6.23 in the control arm, corresponding to a RR of 1.57 (95% CI 1.51–1.62) (Table 2a).
Tables 2a and 2b.
Prostate cancer incidence and mortality in the intervention and control arms during 3 time periods truncated – All centers, core age group, France excluded except for years 1–9
| a) Prostate cancer incidence | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Rate Ratio1 (95% CI) |
Rate difference per 1000 persons year1 (95% CI) |
Rate difference per 1000 men1 |
|||||
| Prostate Cancer N |
Person years | Rate per 1000 person years |
Prostate Cancer N |
Person years | Rate per 1000 person years |
||||
| Year 1–9 inc France | 7902 | 835353 | 9.46 | 5726 | 984993 | 5.81 | 1.64 (1.58–1.69) | 3.69 (3.42–3.95) | 26.5 |
| Years 1–9 | 6147 | 585627 | 10.50 | 4127 | 736688 | 5.60 | 1.91 (1.83–1.99) | 5.00 (4.68–5.32) | 39.0 |
| Years 1–11 | 6797 | 692186 | 9.82 | 5262 | 873415 | 6.02 | 1.66 (1.60 –1.73) | 3.90 (3.61 –4.20) | 35.5 |
| Years 1–13 | 7408 | 775527 | 9.55 | 6107 | 980474 | 6.23 | 1.57 (1.51 – 1.62) | 3.44 (3.16 – 3.72) | 34.8 |
| b: Prostate cancer mortality | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Rate Ratio1 (95% CI) |
Rate difference1 per 1000 persons year (95% CI) |
Rate difference per 1000 men1 |
Adjusted rate ratio in attenders1 |
|||||
| Prostate Cancer deaths N |
Person years | Rate per 1000 person years |
Prostate Cancer deaths N |
Person years | Rate per 1000 person years |
|||||
| Years 1–9 | 193 | 614590 | 0.31 | 278 | 751777 | 0.37 | 0.85 (0.70–1.03) p=0.10 | −0.06 (−0.12 – +0.01) | −0.46 | |
| Years 1–11 | 265 | 732133 | 0.35 | 415 | 896367 | 0.46 | 0.78 (0.66 – 0.91) P=0.002 | −0.10(−0.17 – −0.04) | −1.02 | 0.71 (0.58 −0.88), p= 0.001 |
| Years 1–13 | 355 | 825018 | 0.43 | 545 | 1011192 | 0.54 | 0.79 (0.69–0.91) p = 0.001 | −0.11 (−0.18 – −0.05) | −1.28 | 0.73 (0.61–0.88), p < 0.001 |
Control group for Finland weighted by 1:1,5
Adjusted by centre and for the randomization ratio 1:1.5 intervention versus control group in Finland
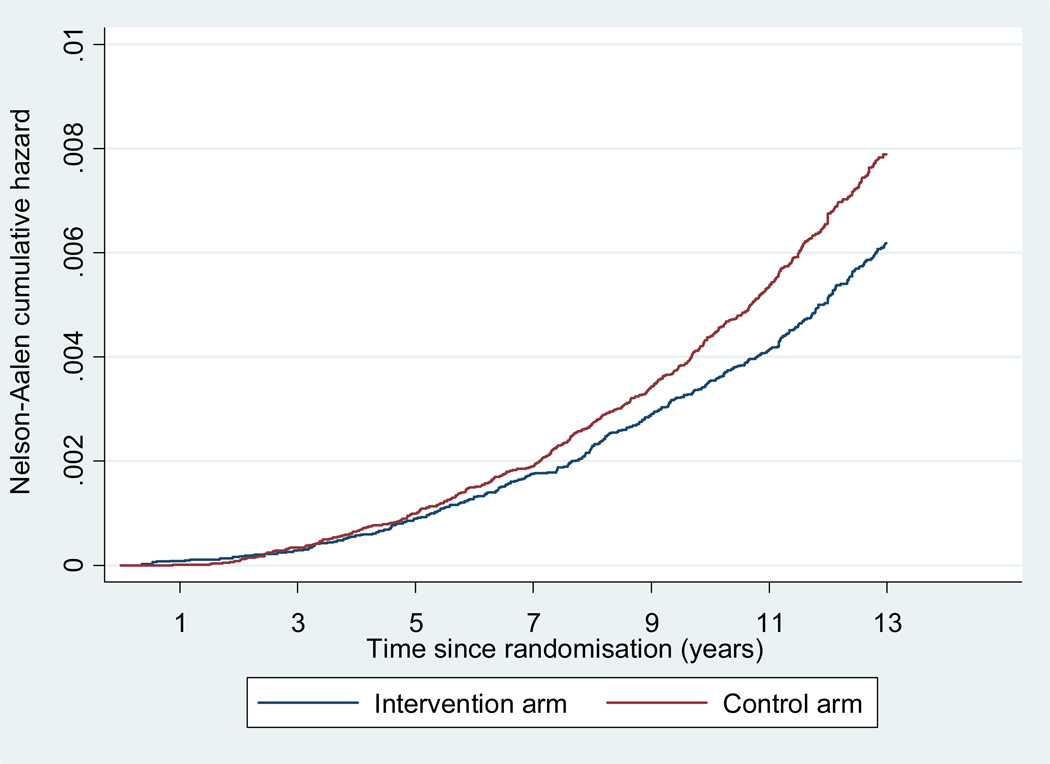
With follow-up truncated at 13 years, PCa mortality was 0.43 per 1,000 person-years in the intervention arm and 0.54 per 1,000 person-years in the control arm translating into a RR of 0.79 (95% CI 0.69–0.91, p=0.001), or a relative risk reduction of 21% in men randomized to screening (Table 2b, Figure 2). A similar RR of 0.78 (95% CI 0.66–0.91, p=0.002) was seen after 11 years. After adjustment for non-participation, RR’s of 0.71 and 0.73 were seen after 11 and 13 years, relative risk reductions of 29 and 27% (p=0.001 and p<0.001 respectively).
Figure 2.
Nelson Aalen Estimates of cumulative PCa mortality (All centres excluding France).
The absolute risk reduction in PCa mortality at 13 years of FU, in the intervention compared to the control arm after adjustment for the randomization ratio 1:1.5 in Finland, was 0.11 PCa deaths per 1,000 person-years or 1.28 PCa deaths per 1,000 men, which yielded a number needed to invite (NNI) of 781 (95% CI 490–1929) and a number needed to detect (NND) of 27 (95% CI 17–66) (Table 3). The NNI and NND are substantially decreased from follow-up to 9 (NNI 1410, NND 48) and 11 years (NNI 979, NND 35)1, 2.
Table 3.
Numbers needed to be invited (NNI) and numbers needed to be diagnosed (NND) per centre and follow-up period: core age group
| 11 years of follow-up | 13 years of follow-up | |||
|---|---|---|---|---|
| NNI (95% CI) | NND (95% CI) | NNI (95% CI) | NND (95% CI) | |
| Excl. France | 979 (594 – 2770) | 35 (21–96) | 781 (490 – 1929) | 27 (17–66) |
As shown in table 4, all-cause mortality did not differ between the two trial arms (18.6 and 18.9 per 1,000 person-years in the core age group, RR 1.00 (95%CI 0.98–1.02, p=0.82)).
Table 4.
All cause and prostate cancer mortality by age at randomization, France excluded.
| Intervention arm | Control arm | Rate Ratios | 95% CI | P-value | |||||
|---|---|---|---|---|---|---|---|---|---|
| All cause mortality | |||||||||
| Deaths (N) | Person years | Rate per 1000 personyears |
Deaths (N) | Person years | Rate per 1000 personyears |
||||
| Core age group | 15369 | 825018 | 18.6 | 19108 | 1011192 | 18.9 | 1.00 | 0.98–1.02 | 0.82 |
| All ages | 18251 | 935185 | 19.5 | 21992 | 1120432 | 19.6 | 1.00 | 0.98–1.02 | 0.98 |
| Prostate cancer mortality | |||||||||
| Age groups (yrs) | |||||||||
| <=54 | 6 | 64265 | 0.09 | 7 | 62312 | 0.11 | 0.84 | 0.28–2.49 | 0.75 |
| 55–59 | 114 | 411834 | 0.28 | 174 | 524314 | 0.33 | 0.81 | 0.93–1.03 | 0.09 |
| 60–64 | 121 | 240895 | 0.50 | 159 | 280404 | 0.57 | 0.90 | 0.71–1.15 | 0.41 |
| 65–69 | 120 | 172289 | 0.70 | 212 | 2064774 | 1.03 | 0.69 | 0.55–0.87 | 0.002 |
| 70+ | 66 | 45903 | 1.44 | 58 | 46928 | 1.24 | 1.17 | 0.82–1.66 | 0.40 |
| Core age group | 355 | 825018 | 0.43 | 545 | 1011192 | 0.54 | 0.79 | 0.69–0.91 | 0.001 |
| All ages | 427 | 935185 | 0.46 | 610 | 1120432 | 0.54 | 0.83 | 0.73–0.94 | 0.004 |
Test for heterogeneity: (PC mortality) All ages χ24 = 6.26 p= 0.18 Core age group χ22 = 2.31 p= 0.32
Correction for selection bias due to non-participation resulted in adjusted RRs for PCa mortality of 0.71 (95% CI 0.58–0.88) at 11 years and 0.73 (0.61–0.88) at 13 years, corresponding to relative risk reductions 29% and 27%, respectively (Table 2b).
In addition to the core age group, a significant reduction in PCa mortality was found for all 181,999 men aged 50–74 years at entry (excluding France), with a rate ratio 0.83 (95% CI 0.73–0.94, p=0.004) (Table 4). The screening effect did not differ significantly across five-year bands in the core age group or over the entire age range, but, most likely by chance, a significant PCa mortality reduction was found in the age group 65–69 years and a non-significantly increased PC mortality was seen in the screening arm in the age group 70+. However, the latter men were screened only once and this may explain the lack of an effect of starting screening late in life.
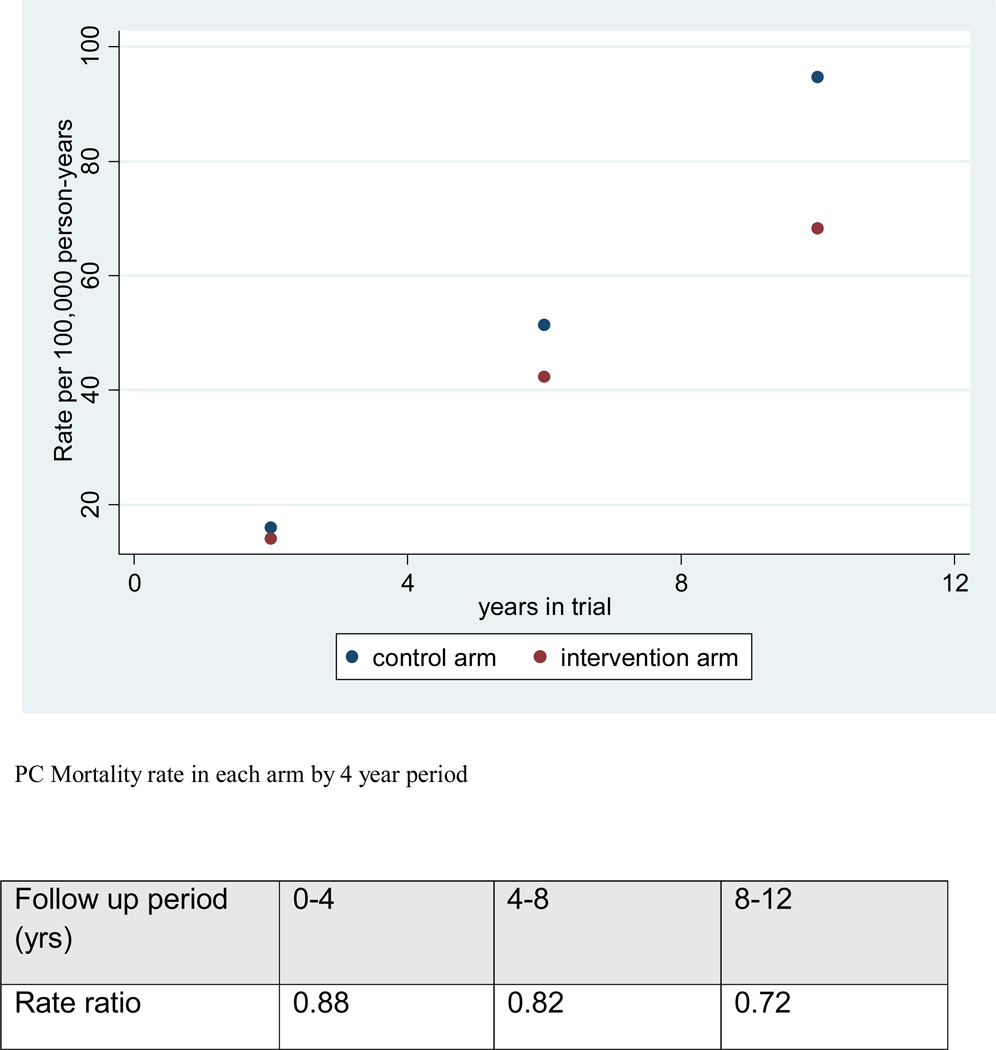
Figure 3 shows the PCa mortality rate by trial arm in four year intervals from date of randomization. The RRs decreased from 0.88 to 0.82 and 0.72 during years 0–4, 4–8 and 8–12 (relative risk reductions of 12%, 18% and 28%).
Figure 3.
Nelson Aalen estimates of cumulative PCa mortality in each arm by 4 year period (all centers, France excluded).
An analysis of PCa mortality in the intervention and control arms in the core age group of individual centers shows significant RR’s only for Sweden (RR 0.62) and the Netherlands (RR 0.67) (appendix table 4). A more extensive comparison including adjustments to non-compliance is pending.
Discussion
The results of our primary analysis based on extended follow-up up to 13 years indicate no further increase in the relative effect of screening on PCa mortality with an RR of 0.79, similar to 11 years2, but an enhanced absolute mortality reduction of 0.11 per 1,000 person-years of 1.28 per 1,000 men randomized. In line with ERSPC rules of participation and reporting (8) France is included in the analysis of incidence, but not in that of mortality because of incomplete follow-up to the end of 2010. The absolute effect i.e. absolute risk reduction is a key indicator of the effectiveness of screening and it should guide decision-making at both policy and patients levels. At 13 years of follow-up, one death from PCa was averted per 781 men invited to screening, which is reduced from 979 at 11 years and from 1,410 at 9 years. At 13 years of FU men in the intervention arm were screened on average 2.3 times. For comparison, the corresponding figures of NNI estimated for breast cancer screening trials are 1339–2000 based on 13 year follow-up19. The NND, which expresses the mortality reduction in relation to excess incidence, was estimated as 27 at 13 years, 35 at 11 years and 48 at 9 years.
In terms of relative effect, most of the screening impact was achieved during the follow-up years 1–11 with little further divergence occurring during the years 11–13. The secondary analysis correcting for non-attendance showed a RR of 0.73, a relative risk reduction of 27% for screened men, at 13 years follow-up (Table 2b).
Differences between age groups and centers
PCa mortality was significantly lower in the screened arm in the core age group and for all ages.
Our previous reports1, 2 did not include France because of short follow-up. French data are shown here for the first time in an analysis of incidence up to 9 years of follow-up. The French centers have mean follow-up periods of only 6.2 and 7.3 years, the lowest compliance with biopsy indications (28.9 and 50.9%), contributed with only 1–2 rounds of screening and their incidence data are suggestive of a very high contamination rate (PCa incidence RR 1.1 for the screening arm, Table 1). Inclusion of these centers in the analysis of data truncated at 9 years gave a RR of PCa incidence of 1.64 (1.58–1.69) compared with 1.91 (1.83–1.99) without these centers (Table 2a).
Differences in the screening effect were seen between centers but none of these were significant (Appendix table 4, France excluded). PCa mortality reduction was significant in the Swedish and Dutch centers, but not in the others. Finland, the largest component, still does not show a significant mortality reduction. Differences between centers are most likely due to differences in length of follow-up, underlying incidence and mortality, as well as contamination in the control arm, but possibly also to performance of screening and to the duration of the intervention.
Possible mechanisms which may explain the lack of further increase of the relative effect by screening in the 1–11 versus 1–13 year periods may include non-compliance in the intervention arm and contamination in the control arm by screening, as well as a decreasing difference in the frequency of screening between the intervention and control arm, reflected in approaching PCa incidence rates (rate differences of incidence in the intervention versus control arms at years 1–9 versus 1–13 are 4.90 versus 3.32 per 1,000 person-years respectively). In addition, latent advanced PCa at the time of randomization (influence of advanced, incurable cases detected in the first screen on PCa mortality)20 may approach the end of their treated natural course. In addition, biopsy compliance or variations in treatment may have an impact. A complete adjustment for contamination and non-participation according to16 is not possible at present because of unavailability of opportunistic PSA-testing data in the control arm in some centers. The change of the occurrence of T1c disease in the control arm over time might serve as a surrogate. An increase of the T1c detection rate per 1,000 person-years within the control arm of the core age group from 0.85 during year 1 to 3.58 during year 12 was seen (appendix table 5, excluding France). It is also possible that the follow-up is still too short to see the full effect of PSA screening, given the long natural history of screen detected PCa. Although the follow-up from randomization is 13 years, the median follow-up from diagnosis of PCa is only 6.4 and 4.3 years in the intervention and control arms (data not shown), and previous studies have shown that the natural course of early PCa usually is in the range of 15–25 years21, 22. Differences in treatment for PCa with similar tumor characteristics between the two arms of the trial could, in theory, explain apparent differences assigned to screening. A previous analysis, however, showed that this is unlikely23. This analysis shows only one major difference in treatment between arms, a higher rate of radiotherapy combined with endocrine treatment in favor of the control group. An update of the evaluation of treatments per arm and center is in preparation. In addition, an alternative analysis applying the excess mortality methodology was conducted and reported24. This analysis takes into account the differences in deaths which may be related to treatment. The results of this analysis does not differ from the data reported in the present report.
As previously, no difference in all-cause mortality was seen. As in other cancer screening trials (except lung cancer and regionally cervix cancer), all-cause mortality is not an endpoint, but similar death rates confirm the comparability of the trial arms.
Harmful effects of screening
Overdiagnosis occurs in approximately 40% of the screen-detected cases3, 4 resulting in a high risk of overtreatment with unavoidable adverse effects, which is a major adverse consequence of prostate cancer screening. Our current results show a 1.57-fold higher incidence in the screening arm (absolute excess 3.44 per 1,000 person-years), which is consistent with earlier assessments. Yet, our recent modeling study showed a favorable balance of benefits (mortality reduction) and harms (positive net impact despite a smaller gain in Quality of Life Adjusted Life Years (QALYs) than life-years overall)5. The model estimate of over diagnosis is 41%. Assuming no over diagnosis increases QALY’S gained per 1000 men screened annually from 56 to 79. To avoid over diagnosis, preferably by avoiding unnecessary biopsies, and to decrease the very large number of men who must be screened, biopsied, and treated to help a few is a top current research priority.
Limitations
Our study has limitations including heterogeneity between centers which is not excluded by the analysis of homogeneity in terms of screening protocol and performance, contamination in the control arm (reported in the range of 23–40%) and the short follow-up (more than 70% of all participants of the study population are still alive).
Despite evidence of the effectiveness of PSA-screening in reducing PCa mortality from our trial, the uncertain balance between benefits and harms needs to be considered in decisions about population screening. Informed decision-making, using well-designed decision aids, is necessary for individuals who consider PSA-based screening for PCa25, 26. Another issue which requires consideration is the different outcome of the ERSPC and prostate arm of the Prostate, Lung, Colon and Prostate Cancer screening trial (PLCO) of which a recent update again reports no effect on PCa mortality27 in spite of the diagnosis of more PCa in the screen arm. The comparability of the two trials is subject to heavy debate28, 29. Complications of diagnostic procedures have recently been reported in two other publications30, 31.
Panel: research in context
Summary of previous research findings
The ERSPC study has been published previously in 2009 and 20121, 2. Results have changed significantly, mainly concerning the absolute effect of screening on prostate cancer mortality. The number needed to invite changed from 1,410 to 1,055 and the number needed to detect from 48 to 37. The relative difference in mortality between the screening and control arm improved from 20% to 21% but the level of significance increased from p=0.04 to p=0.001 with 9 versus 11 years of follow-up. A systematic review was not conducted by the ERSPC; the recent Cochrane analysis of all screening trials is subject to heavy debate, mainly concerning the comparability of ERSPC with other screening trials29.
Interpretation
Our data show a significant relative reduction of prostate cancer mortality comparing the screening and control group of 21% and 27% in those men who actually participated. The main downside of screening is a high rate of overdiagnosis and overtreatment which are discussed in our report and which has been subject to a previous publication5. This leads the authors to the concluding statement that the time for population based screening has not arrived.
What clinicians and healthcare providers need to know
The fact that the time of population based screening has not come should not withhold clinicians and other healthcare providers to consider the application of PSA driven testing to men who wish to undergo such study. In the present situation extensive, well-balanced information should be given and discussed preferably on the basis of validated decision aids25. Instruments to decrease the proportion of unnecessary biopsies and the risk of overdiagnosis in the form of risk calculators are freely available on the internet (www.prostatecancer-riskcalculator.com). Our hope lies in the further development of multi-parametric MRI imaging technology of the prostate.
Conclusions
With data truncated at 13-years of follow-up, our study continues to demonstrate a significant 21% relative PCa mortality reduction in favor of screening, with one PCa death averted per 781 men invited and 27 excess cases detected. The relative risk reduction in men actually screened was 27% after adjustment for selection effects. In spite of these findings further quantification of harms and their reduction are still considered as pre-requirements for the introduction of population based screening.
Supplementary Material
Acknowledgments
Role of funding sources
All details of the of the international coordination and per participating country/center are given in the “Web extra material” section. Financial contributors, agencies and the only contributing company had no influence on study design, collection, analysis or interpretation of data. Sponsors were not involved in the writing or decision to submit this report.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
All authors have, among others, contributed by including substantial numbers of participants, as indicated in table 1 of the web extra Material, by providing data to the independent data center twice a year, and by critically revising the manuscript for important intellectual content.
Conflicts of interest
Dr. Lilja reports grants from National Cancer Institute grants R01CA160816; P50-CA92629 to MSKCC, New York, NY, USA, grants from David H. Koch trough the Prostate Cancer Foundation funding to the The Sidney Kimmel centre for prostate and Urological cancers at MSKCC, New York, NY, USA, grants from Swedish Cancer Society grant nr 11-0624 to Lund University, Malmö, Sweden, grants from Fundacion Federico SA grant to Lund University, Malmö, Sweden, grants from FiDiPro grant from TEKES, Finland to IBT, University of Tampere, Tampere, Finland, grants from the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre Program, University of Oxford, Oxford, UK, during the conduct of the study; other from Arctic Partners, outside the submitted work; In addition, Dr. Lilja has a patent free PSA, intact PSA and hK2 assays with royalties paid to OPKO.Dr. Lilja reports grants from National Cancer Institute (R01CA160816; P50-CA92629) to MSKCC, New York, NY, USA, grants from David H. Koch trough the Prostate Cancer Foundation to the The Sidney Kimmel Center for Prostate and Urological cancers at MSKCC, New York, NY, USA, grants from Swedish Cancer Society (nr 11-0624) to Lund University, Malmö, Sweden, grants from Fundacion Federico SA grant to Lund University, Malmö, Sweden, grants from FiDiPro grant from TEKES, Finland to IBT, University of Tampere, Tampere, Finland , grants from the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre Program, University of Oxford, Oxford, UK, during the conduct of the study; In addition, Dr. Lilja has a patent free PSA, intact PSA and hK2 assays with royalties paid to Arctic Partners, and a patent application for a statistical method to detect prostate cancer licensed to Arctic Partners. Dr. Moss reports grants from Rotterdam Prostate Cancer Research Foundation (SWOP) during the conduct of the study. Dr. TAMMELA reports personal fees from Astellas, personal fees from Janssen, personal fees from Orion Pharma, personal fees from AMGEN, outside the submitted work. Dr. Stenman has a patent Determination of free PSA with royalties paid to Perkin-Elmar Wallac. All other authors declare that they have no conflicts of interest.
References
- 1.Schröder FH, Hugosson J, Roobol MJ, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360(13):1320–1328. doi: 10.1056/NEJMoa0810084. [DOI] [PubMed] [Google Scholar]
- 2.Schröder FH, Hugosson J, Roobol MJ, et al. Prostate-cancer mortality at 11 years of follow-up. N Engl J Med. 2012;366:981–990. doi: 10.1056/NEJMoa1113135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Draisma G, Boer R, Otto SJ, et al. Lead times and overdetection due to prostate-specific antigen screening: estimates from the European Randomized study of Screening for Prostate Cancer. J Natl Cancer Inst. 2003;95(12):868–878. doi: 10.1093/jnci/95.12.868. [DOI] [PubMed] [Google Scholar]
- 4.Cooperberg MR, Lubeck DP, Meng MV, Mehta SS, Carroll PR. The changing face of low-risk prostate cancer: trends in clinical presentation and primary management. J Clin Oncol. 2004;22(11):2141–2149. doi: 10.1200/JCO.2004.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heijnsdijk EAM, Wever EM, Auvinen A, et al. Quality-of-life effects of Prostate-Specific Antigen screening. N Engl J Med. 2012;367(7):595–605. doi: 10.1056/NEJMoa1201637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schröder FH, Damhuis RAM, Kirkels WJ, et al. European randomized study of screening for prostate cancer –The Rotterdam pilot studies. Int J Cancer. 1996;65:145–151. doi: 10.1002/(SICI)1097-0215(19960117)65:2<145::AID-IJC4>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 7.Schröder FH, Denis LJ, Kirkels WJ, de Koning HJ, Standaert B. European randomized study of screening for prostate cancer: Progress report of Antwerp and Rotterdam pilot studies. Cancer. 1995;76:129–134. doi: 10.1002/1097-0142(19950701)76:1<129::aid-cncr2820760120>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 8.European Randomized study of Screening for Prostate Cancer. publications section – www.erspc.org/publist.php.
- 9.Roobol MJ, Schröder FH. European Randomized study of Screening for Prostate Cancer: achievements and presentation. BJU Int. 2003;92(Suppl.2):117–122. doi: 10.1111/j.1464-410x.2003.4698x.x. [DOI] [PubMed] [Google Scholar]
- 10.Stenman UH, Hakama M, Knekt P, Aromaa A, Teppo L, Leinonen J. Serum concentrations of prostate specific antigen and its complex with alpha 1-antichymotrypsin before diagnosis of prostate cancer. Lancet. 1994;344(8937):1594–1598. doi: 10.1016/s0140-6736(94)90405-7. [DOI] [PubMed] [Google Scholar]
- 11.Gann PH, Hennekens CH, Stampfer MJ. A prospective evaluation of plasma prostate-specific antigen for detection of prostatic cancer. JAMA. 1995;273(4):289–294. [PubMed] [Google Scholar]
- 12.De Koning HJ, Liem MK, Baan CA, Boer R, Schröder FH, Alexander FE ERSPC. Prostate cancer mortality reduction by screening: power and time frame with complete enrolment in the European Randomised Screening for Prostate Cancer (ERSPC) trial. Int J Cancer. 2002;98(2):268–273. doi: 10.1002/ijc.10188. [DOI] [PubMed] [Google Scholar]
- 13.De Koning HJ, Blom J, Merkelbach JW, et al. Determining the cause of death in randomized screening trial(s) for prostate cancer. BJU Int. 2003;92(Suppl 2):71–78. doi: 10.1111/j.1465-5101.2003.04402.x. [DOI] [PubMed] [Google Scholar]
- 14.Mäkinen T, Karhunen P, Aro J, Lahtela J, Määttänen L, Auvinen A. Assessment of causes of death in a prostate cancer screening trial. Int J Cancer. 2008;122(2):413–417. doi: 10.1002/ijc.23126. [DOI] [PubMed] [Google Scholar]
- 15.De Koning HJ, Hakulinen T, Moss SM, Adolfsson J, Smith PH, Alexander FE ERSPC. BJU Int. 2003;92(Suppl 2):112–114. doi: 10.1111/j.1464-410x.2003.4410x.x. [DOI] [PubMed] [Google Scholar]
- 16.Cuzick J, Edwards R, Segnan N. Adjusting for non-compliance and contamination in randomized clinical trials. Stat Med. 1997;16(9):1017–1029. doi: 10.1002/(sici)1097-0258(19970515)16:9<1017::aid-sim508>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 17.Aalen OO. Nonparametric inference for a family of counting processes. Ann Stat. 1978;6:701–727. [Google Scholar]
- 18.DeMets DL, Lan KK. Interim analysis: the alpha spending function approach. Stat Med. 1994;13:1341–1352. doi: 10.1002/sim.4780131308. [DOI] [PubMed] [Google Scholar]
- 19.Independent UK Panel on Breast Cancer Screening. The benefits and harms of breast cancer screening: an independent review. Lancet. 2012;380(9855):1778–1786. doi: 10.1016/S0140-6736(12)61611-0. [DOI] [PubMed] [Google Scholar]
- 20.Schröder FH, Hugosson J, Carlsson S, Tammela T, Määttänen L, Auvinen A, Kwiatkowski M, Recker F, Roobol M. Screening for prostate cancer decreases the risk of developing metastatic disease: findings from the European Randomised study of Screening for Prostate Cancer (ERSPC) Eur Urol. 2012;62(5):745–752. doi: 10.1016/j.eururo.2012.05.068. [DOI] [PubMed] [Google Scholar]
- 21.Popiolek M, Rider JR, Andren O, Andersson SO, Holmberg L, Adami HO, Johansson JE. Natural history of early, localized prostate cancer: a final report from three decades of follow-up. Eur Urol. 2013;63(3):428–435. doi: 10.1016/j.eururo.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 22.Vickers AJ, Ulmert D, Sjoberg DD, Bennette CJ, Björk T, Gerdtsson A, Manjer J, Nilsson PM, Dahlin A, Bjartell A, Scardino PT, Lilja H. Strategy for detection of prostate cancer based on relation between prostate specific antigen at age 40-55 and long term risk of metastasis: case-control study. BMJ. 2013;346:2023. doi: 10.1136/bmj.f2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wolters T, Roobol MJ, Steyerberg EW, van den Bergh RC, Bangma CH, Hugosson J, Ciatto S, Kwiatkowski M, Villers A, Lujan M, Nelen V, Tammela TL, Schröder FH. The effect of study arm on prostate cancer treatment in the large screening trial ERSPC. Int J Cancer. 2010;126(10):2387–2393. doi: 10.1002/ijc.24870. [DOI] [PubMed] [Google Scholar]
- 24.Zappa M, Puliti D, Hugosson J, et al. A different method of evaluation of the ERSPC trials confirms that prostate-specific antigen testing has a significant impact on prostate cancer mortality. Eur Urol. 2014 Jan 7; doi: 10.1016/j.eururo.2013.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Société International d’Urologie. www.siu-urology.org.
- 26.Movember. www.movember.com.
- 27.Andriole GL, Crawford ED, Grubb R, 3rd, et al. PLCO Project Team. Prostate cancer screening in the randomized Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial: mortality results after 13 years of follow-up. J Natl Cancer Inst. 2012 Jan 18;104(2):125–132. doi: 10.1093/jnci/djr500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schröder FH. ERSPC, PLCO Studies and Critique of Cochrane review 2013. Recent Results Cancer Res. 2014;202:59–63. doi: 10.1007/978-3-642-45195-9_7. [DOI] [PubMed] [Google Scholar]
- 29.Ilic D. Screening for prostate cancer: reflecting on the quality of evidence from the ERSPC and PLCO studies. Recent Results Cancer Res. 2014;202:65–71. doi: 10.1007/978-3-642-45195-9_8. [DOI] [PubMed] [Google Scholar]
- 30.Loeb S, Vellekoop A, Ahmed HU, Catto J, Emberton M, Nam R, Rosario DJ, Scattoni V, Lotan Y. Systematic review of complications of prostate biopsy. Eur Urol. 2013 Dec;64(6):876–892. doi: 10.1016/j.eururo.2013.05.049. [DOI] [PubMed] [Google Scholar]
- 31.Loeb S, Carter HB, Berndt SI, Ricker W, Schaeffer EM. Is repeat prostate biopsy associated with a greater risk of hospitalization? Data from SER-Medicare. J Urol. 2013 Mar;189(3):867–870. doi: 10.1016/j.juro.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.