Abstract
Objective
The E26 transformation-specific domain transcription factor Etv2/Etsrp/ER71 is a master regulator of vascular endothelial differentiation during vasculogenesis, although its later role in sprouting angiogenesis remains unknown. Here, we investigated in the zebrafish model a role for Etv2 and related E26 transformation-specific factors, Fli1a and Fli1b in developmental angiogenesis.
Approach and Results
Zebrafish fli1a and fli1b mutants were obtained using transposon-mediated gene trap approach. Individual fli1a and fli1b homozygous mutant embryos display normal vascular patterning, yet the angiogenic recovery observed in older etv2 mutant embryos does not occur in embryos lacking both etv2 and fli1b. Etv2 and fli1b double-deficient embryos fail to form any angiogenic sprouts and show greatly increased apoptosis throughout the axial vasculature. In contrast, fli1a mutation did not affect the recovery of etv2 mutant phenotype. Overexpression analyses indicate that both etv2 and fli1b, but not fli1a, induce the expression of multiple vascular markers and of each other. Temporal inhibition of Etv2 function using photoactivatable morpholinos indicates that the function of Etv2 and Fli1b during angiogenesis is independent from the early requirement of Etv2 during vasculogenesis. RNA-Seq analysis and chromatin immunoprecipitation suggest that Etv2 and Fli1b share the same transcriptional targets and bind to the same E26 transformation-specific sites.
Conclusions
Our data argue that there are 2 phases of early vascular development with distinct requirements of E26 transformation-specific transcription factors. Etv2 alone is required for early vasculogenesis, whereas Etv2 and Fli1b function redundantly during late vasculogenesis and early embryonic angiogenesis.
Keywords: angiogenesis, ETS transcription factor, vasculogenesis, zebrafish
The vertebrate vasculature forms early in development to support the metabolic needs of the developing embryo. The first blood vessels arise de novo from mesodermally derived endothelial precursors called angioblasts through vasculogenesis.1,2 Growth, expansion, and remodeling of the primary vasculature into a mature vascular network occur through the sprouting or longitudinal division of pre-existing vessels, termed angiogenesis.3,4 Angiogenesis remains important throughout adulthood during tissue repair,5,6 estrus and pregnancy,7,8 and during muscle growth with exercise.9,10 Effective therapeutic control of angiogenic potential remains a critical unmet goal in the treatment of pathologies of hyper-vascularization (eg, cancer and macular degeneration)11-14 and in situations requiring increased blood flow to ischemic tissues (eg, coronary heart disease, peripheral arterial disease, and wound healing disorders). 15-18 A key difficulty associated with antiangiogenic strategy design is that although many of the individual molecules required for the vertebrate vascular development and maintenance have been identified, the signaling cascades underlying these phenomena remain poorly understood because of a high degree of functional redundancy and overlapping target specificities. The functional overlap known to exist among the E26 transformation-specific (ETS) family of transcription factors is of particular interest because several vascular-specific ETS factors sit atop transcriptional hierarchies and thus represent potential therapeutic targets. 19-21
The ETS domain transcription factor Etv2/Etsrp has been identified as a critical regulator of embryonic vasculogenesis, with functional orthologs identified in multiple vertebrates, including zebrafish, Xenopus, mice (ER71/ETV2), and humans (ETV2).22-27 Like other ETS factors, Etv2/Etsrp contains a conserved 85-amino acid ETS domain that binds to a core GGA(A/T) motif to transcriptionally activate downstream target genes. Murine Etv2 binds synergistically with FoxC2 to activate the expression of a variety of endothelial genes (eg, VegfR2/flkl, tie2, and cdh5).28 In zebrafish and in mice, etv2 is expressed during early somitogenesis in the lateral plate mesoderm (LPM) and later restricted to the vascular endothelium. Etv2 null mice and zebrafish lack a formed vasculature, do not achieve circulation, and die during embryogenesis.22,25,26,29
A related vascular endothelial–specific ETS factor, FLI1, has received significant research focus because translocations between the ETS domain of FLI1 and the transactivation domain of exchange web services generate an aberrant transcriptional activator leading to Ewing sarcoma.30,31 In Xenopus embryos, loss of Fli1 results in a substantial reduction in the number of hemangioblasts,32 suggesting that Fli1 is required for early hemangioblast specification and the subsequent formation of the blood and endothelial lineages. Mutant mice similarly display a deficit in the number of both blood and endothelial cells, and although the vasculature initially seems normal in these mutants, they hemorrhage from the dorsal aorta and ventricles of the brain and die by E12.5 because of endothelial cell apoptosis.33,34 Fli1 is induced by Etv2 during early mouse embryogenesis and subsequently maintains its own expression and the expression of other vascular markers through a positive autoregulatory feedback mechanism.34 However, the role of Fli1 during vasculogenesis and the initial stages of angiogenesis have not been established.
The zebrafish (Danio rerio) has emerged as an important vertebrate system for studying vasculogenesis and angiogenesis because of the optical transparency, rapid external embryonic development, and high fecundity. Zebrafish have 2 closely related FLI1 paralogs, fli1a and fli1b. Fli1a and Fli1b share 55% identity and thus exhibit greater sequence homology with each other than either does with Etv2 (which shares 14% identity with Fli1b and 16% with Fli1a; http://uswest.ensembl.org). Both are initially expressed in the LPM and later restricted to the vascular endothelium.35-37Overexpression of wild-type (WT) zebrafish Fli1a did not induce ectopic vascular marker expression, whereas a constitutively active VP16-fused form of Fli1a was shown to induce several hemangioblast markers, including etv2 and kdrl.32 Far less is known about the role played by fli1b, although it contains a predicted ETS-binding domain and thus potentially regulates a similar subset of ETS-target genes. Fli1b is located ≈4.2 kb downstream of etv2 on chromosome 16 and is transcribed in the opposite direction, mirroring the arrangement of ets1 and fli1a on zebrafish chromosome 18 and of Ets1 and Fli1 in mice and humans.22,37 Interestingly, related fish species including stickleback and medaka also share similar arrangement of etv2 and fli1b genes, whereas in most other vertebrates, a single Fli1 homolog is located adjacent to Ets1 gene and not Etv2.23,38 Previously, combined morpholino (MO) knockdown of all 4 of these ETS factors (fli1a, fli1b, etv2, and ets1) in zebrafish was shown to result in a complete absence of angiogenic sprouting.29 By contrast, knockdown of fli1a or fli1b individually resulted in minor sprouting defects, suggesting that ≥1 of these ETS factors have redundant function. However, only limited phenotypic analysis of vascular defects has been performed, and the specific role of each ETS factor remains unclear. Furthermore, specificity of the observed phenotypes (except for etv2 MO) has not been demonstrated by RNA rescue or mutant confirmation. Recent studies have called into question many MO-based phenotypes, including Ets1 knockdown, which were not recapitulated in genetic mutants.39
In this study, we isolated genetic fli1a and fli1b zebrafish mutants that were homozygous viable and displayed no apparent angiogenic defects, suggesting that at least some of the previously reported defects29 were caused by MO off-target effects. We demonstrate that Etv2 and Fli1b function together as regulators of vasculogenesis and angiogenesis. Combined loss of Etv2 and Fli1b, but not Etv2 and Fli1a, blocks the angiogenic recovery otherwise observed in etv2 mutants and results in increased endothelial cell apoptosis throughout the LPM. Overexpression analyses indicate that both etv2 and fli1b induce the expression of multiple vascular endothelial markers and of each other. Finally, RNA-Seq, chromatin immunoprecipitation, and fluorescent reporter assays indicate that Fli1b, similar to Etv2, is capable of regulating the expression of multiple vascular endothelial markers and that Fli1a is a potential direct transcriptional target of Fli1b. Taken together, our data indicate that fli1b is induced by Etv2 during early vasculogenesis and later acts redundantly with Etv2 to support late vasculogenesis and early developmental angiogenesis. Furthermore, our data provide critical mechanistic insight into the combinatorial roles played by ETS factors in vascularization of the developing embryo.
Materials and Methods
Materials and Methods are available in the online-only Data Supplement.
Results
Etv2/etsrp Mutant and Morphant Embryos Exhibit Late-Stage, Aberrant Sprouting Angiogenesis
Although etsrp/etv2y11 null mutants (henceforth referred to as etv2-/- and morphants exhibit strong defects in vasculogenesis before 24 hours post fertilization (hpf),22,29 they undergo a partial recovery of the intersegmental vessels (ISVs) by sprouting angiogenesis at later stages. To understand what promotes this recovery, we analyzed vascular development in etv2-/- embryos ranging in age from the 9-somite stage (13.5 hpf) through 72 hpf (Figure 1). Etv2-/- embryos lacked detectable fli1a:GFP expression in the anterior or posterior LPM at the 9-somite stage (Figure 1A and 1B), consistent with previous reports, indicating that etv2 is both necessary and sufficient for initiating early vascular endothelial marker expression (eg, fli1a).22 The first indication of vascular recovery in etv2-/- embryos came at the 15-somite stage (16 hpf) with the appearance of a posterior pool of fli1a:GFP+ cells (Figure 1D, arrow). Fli1a:GFP+ vascular progenitor cells remained disorganized, and vasculogenesis was impaired in 24 hpf etv2-/- mutants relative to WT embryos, resulting in a complete absence of lumenized axial vessels, ISV sprouts, or blood circulation (Figure 1F). At 36 hpf, arterial and venous boundaries were poorly defined and lumenized segments were largely absent in etv2-/- embryos (Figure 1H), yet intermittent ISV sprouts were observed extending from the dorsal boundary of the loosely organized fli1a:GFP+ pool of vascular endothelial progenitors. By 48 hpf, small segments of the dorsal aorta and posterior cardinal vein in etv2-/- embryos appeared lumenized, particularly in the tail, although mutant embryos never achieved circulation (Figure 1J). At 72 hpf (Figure 1L), the mutant vascular bed contained several full-length ISVs and a discontinuous dorsal longitudinal anastomotic vessel, although the majority of ISVs remained mispatterned. A similar recovery was observed in etv2 morphant embryos (Figure 3A-3D). The etv2 morpholino has been validated in multiple previous studies22,27,40 and results in the same vascular phenotype as etv2-/- null mutants (Figures 1 and 3A-3D).
Figure 1.
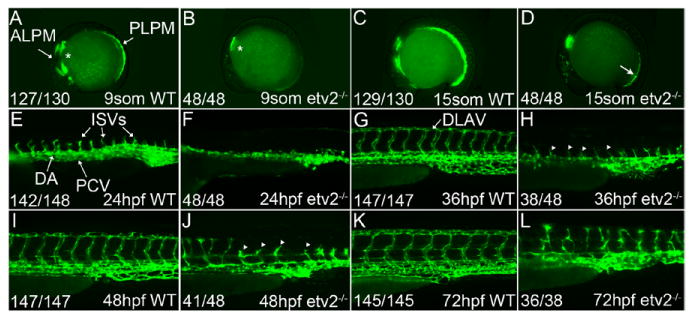
Etv2-/- mutants undergo a partial recovery of vasculogenesis and aberrant sprouting angiogenesis by 72 hpf. A-L, Lateral views of wild-type (WT) and etv2-/- mutant embryos in the Tg(fli1a:GFP) vascular reporter line. Fli1a:GFP expression was evident at the 9-somite stage (13.5 hpf) in WT embryos (A) and localized to the bilateral angioblasts of the anterior and posterior lateral plate mesoderm (ALPM and PLPM, respectively). Endothelial fli1a:GFP was not observed in etv2-/- mutant embryos at this stage (B), but an additional nonendothelial population of fli1a+ cells located in the presumptive pharyngeal endoderm was retained in etv2-/- mutant embryos (asterisks, A and B). The first sign of vascular recovery in etv2-/- mutant embryos was observed at 15-somites (17 hpf, arrow, D). Uniform pairs of intersegmental vessels (ISVs) were observed sprouting from fully lumenized dorsal aorta (DA) vessels at 24 hpf in WT embryos (E), whereas vasculogenesis remained aberrant and ISVs absent in age-matched etv2-/- mutants (F). Fully formed ISVs could be seen extending from the DA and posterior cardinal vein (PCV) and branching at their dorsal terminus to form the dorsal longitudinal anastomotic vessel (DLAV) by 36 hpf in WT embryos (G), whereas ISV sprouts in age-matched etv2-/- mutants were just starting to form (arrowheads, H). The regularly patterned ISVs observed in 48-hpf WT embryos (I) were disrupted in etv2-/- mutants, with a majority mispatterned (arrowheads, J). Continued recovery was observed at 72 hpf (L). Fractions indicate the number of embryos with the observed pattern of green fluorescent protein (GFP) expression (numerator) and the total evaluated (denominator).
Figure 3.
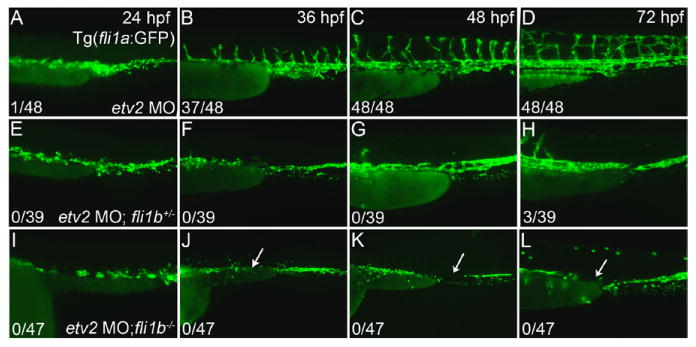
Fli1b functions redundantly with etv2 to support vascular development. A-D, Vascular recovery in etv2 morphant Tg(fli1a:GFP) embryos from 24 to 72 hpf (lateral views, anterior to the left). C and D, Etv2 morpholino knockdown delayed angiogenic sprouting (compare A with Figure 1E) and caused a large fraction of mispatterned intersegmental vessels (ISVs) in wild-type fli1a:GFP embryos. E-H, Limited vascular recovery in fli1b heterozygous, etv2 morphant embryos visualized using enhanced green fluorescent protein fluorescence from the gene trap vector. The etv2 morphant phenotype was exacerbated by the loss of 1 fli1b allele with embryos exhibiting impaired vasculogenesis from 24 to 36 hpf (E and F), and a limited recovery of angiogenic sprouting by 72 hpf (H). I-L, No vascular recovery observed in fli1b homozygous mutant, etv2 morphant embryos. Loss of both fli1b copies caused a significant impairment in vasculogenesis (I-K) and completely blocked angiogenesis through 72 hpf (L). Note the significant reduction in the number of vascular endothelial cells and large gaps within the axial vasculature (bottom, arrows). Fractions indicate the number of embryos with >15 ISVs (full or partial, numerator) and the total evaluated (denominator).
Angiogenic Recovery in etv2-Deficient Embryos Is Mediated by Existing Vascular Endothelial Cells Rather Than a Latent Population of Progenitor Cells
To properly interpret the basis of the recovery of etv2-deficient embryos, the source of cells contributing to this recovery needed to be determined. We used the photoconvertible TgBAC(etv2:Kaede) line that specifically labels angioblasts and their daughter (ie, differentiated) vascular endothelial cells (VECs) to identify populations of VECs arising before and after key stages of the observed recovery. Using this approach, VECs present before photoconversion exhibit both red and green fluorescence, whereas those arising later seem green only. Etv2 morpholino MO1 inhibits translation of Etv2 protein but is designed to not affect etv2:Kaede reporter expression, which allows observation of vascular endothelial progenitors in etv2 morphant embryos. Etv2:Kaede embryos injected with etv2 morpholino exhibited a partial impairment of the axial vasculature at 24 hpf and absence of ISVs and a lack of blood circulation (not shown), indicating that etv2 morpholino knockdown was successful. Embryos were photoconverted at the 20-somite stage (19 hpf) to enable the assessment of the endothelial contribution to ISVs during the recovery period (Figure 2). Photoconversion at the 20-somite stage in control embryos resulted in 97% (n=91 of 95) of trunk ISVs exhibiting both red and green fluorescence (Figure 2A), indicating that a majority of cells contributing to the ISVs in WT embryos were specified before the 20-somite stage, as we have previously reported.41 Morphants showed a partial recovery of axial and intersegmental vasculature by 36 hpf, but the average number of fluorescent ISVs per fish was significantly reduced relative to WT controls (2.1±0.3 in morphants versus 7.3±0.3 in controls, Figure 2B). Because vascular expression in etv2:Kaede embryos was mosaic, only a fraction of the total number of ISVs exhibited fluorescence. Ninety percent of the ISVs in the morphant embryos were both red and green (n=40 of 46), arguing that the angiogenic recovery in control and morphant embryos was also largely mediated by VECs present before the photoconversion period. Occasionally, green only cells were detected in the vascular plexus of both WT and morphant embryos at 36 hpf, providing confirmation that new VECs had formed, but these new cells did not significantly contribute to the observed recovery. It is likely that red fluorescence was not apparent in some of the green cells because of faint fluorescence and turnover of the photoconverted Kaede protein. Photoconversions performed before the 20-somite stage were uninformative because converted Kaede expression was not detectable through the target angiogenic recovery period because of weak etv2:Kaede expression during early stages (data not shown).
Figure 2.
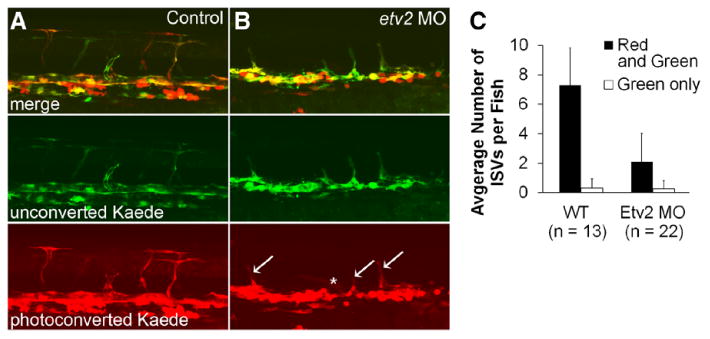
Vascular recovery in etv2 morpholino (MO)-injected etv2:Kaede embryos is mediated by vascular endothelial cells specified before the 20-somite stage. A and B, etv2:Kaede expression in the trunk of 36-hpf embryos after photoconversion at the 20-somite stage (19 hpf). A, Control embryos developed intersegmental vessels (ISVs) which were almost exclusively both red and green indicating that cells contributing to ISVs were specified before the 20-somite stage in normally developing embryos. B, Etv2 morpholino knockdown in etv2:Kaede embryos reduced angiogenic sprouting, as expected. A majority of ISVs in etv2 morphant embryos were red+green (arrows), with a small number of green only ISVs (asterisk) observed. C, Etv2 knockdown did not cause a significant change in the number of green only ISVs. Error bars, +1SD. WT indicates wild-type.
Loss of fli1b Exacerbates the etv2 Vascular Phenotype
We hypothesized that other ETS transcription factors function redundantly with Etv2 during the later stages of vascular development to mediate the vascular recovery observed in etv2 mutants and morphants. Three related ETS factors Ets1, Fli1a and Fli1b have been previously implicated as having a redundant function with Etv2.29 However, the previous study used a month-only approach without stringent controls, such as RNA rescue. Furthermore, the individual roles of each of these transcription factors have not been determined, and only limited phenotypic analysis has been previously performed. We focused our analysis on the 2 Fli1 homologs, Fli1a and Fli1b. As previously reported, strong expression of fli1a and fli1b continues in vascular endothelium past 24 hpf, whereas etv2 expression becomes greatly reduced29,36 (Figure IA through IK in the online-only Data Supplement). Furthermore, fli1b expression was significantly reduced but detectable by whole-mount in situ hybridization in etv2 mutants from 24 hpf through 36 hpf (Figure IL and IM in the online-only Data Supplement), suggesting that fli1b expression occurs in an etv2-independent manner throughout the recovery period of etv2-deficient embryos.
Fli1a and fli1b mutant lines were generated in Tol2-mediated insertional mutagenesis screens. The fli1ais10Gt mutant line contains a gene trap vector insertion in the third intron of fli1a gene with a splice acceptor site followed by enhanced green fluorescent protein (GFP) and polyA sequence (Figure IIA in the online-only Data Supplement). The insertion causes a truncation of the sterile alpha motif/pointed protein-binding domain, and the resulting Fli1a protein is predicted to lack the C-terminal ETS DNA-binding domain (Figure IIB in the online-only Data Supplement). The insertion resulted in 21.3-fold reduction in expression of full-length message as determined by reverse transcription polymerase chain reaction (Figure IIL in the online-only Data Supplement) and whole-mount in situ hybridization using an antisense probe against the 3′ end of the message containing the ETS-binding domain (Figure III and IIJ in the online-only Data Supplement). Fluorescence of the truncated fli1ais10Gt-GFP fusion protein was evident in the vascular endothelium, detectable by the 10-somite stage (14 hpf), and vascular-restricted expression was observed throughout embryogenesis (Figure UK in the online-only Data Supplement and data not shown). A gene-trap fli1btpl50Gt mutant line was generated in a Tol2-mediated insertional mutagenesis screen using GBT-B4 gene trap vector (C. Seiler et al, unpublished data, 2015) similar to the recently published GBT-B1.42-44 The gene trap transposon (Figure IIE in the online-only Data Supplement) integrated into the first intron and near the start of the coding sequence resulting in a Gal4-VP16 fusion protein lacking the ETS DNA-binding domain (Figure IIF through IIH in the online-only Data Supplement); therefore, the mutation is expected to be null or severe hypomorph.43 Consistent with this view, the gene-trap disruption resulted in an 18.5-fold (5.4±2.0%) reduction in full-length fli1b message as determined by reverse transcription polymerase chain reaction and whole-mount in situ hybridization (Figure IIM, IIN, and IIP in the online-only Data Supplement). RNA-Seq also confirmed significant reduction of terminal exon 9 reads of fli1b (2.4±2.6%, n=2 samples of 20 embryos, age 24 hpf, read at a depth of 20 million reads per sample). Gene trap embryos exhibited high levels of vascular endothelial GFP reporter expression beginning at the 12-somite stage (15 hpf), vascular restricted at 48 hpf (Figure IIO in the online-only Data Supplement), and maintained into adulthood (data not shown). In stark contrast to the previous study using morpholinos,29 fli1ais10Gt or fli1btpl50Gt mutant embryos had no apparent defects in vascular development (Figure IIK and IIO in the online-only Data Supplement) or vascular marker expression (Figure III in the online-only DataSupplement). Homozygous Fli1ais10Gt and Fli1btpl50Gt mutants (referred to as Fli1a-/- and fli1b-/-) developed normally into adulthood with no overt effect on growth or fecundity.
To assess the potential interaction between etv2 and the fli1a and fli1b ETS factors, we used etv2 morpholino knockdown in the corresponding mutant lines. Fli1a mutant embryos were injected with etv2 MO at the 1-cell stage (0.2 hpf) and imaged at stages from 24 to 72 hpf. Double fli1a-/-; etv2 MO embryos were indistinguishable from etv2 morphant embryos (Figure IV in the online-only Data Supplement), thus suggesting that the loss of Fli1a did not significantly enhance on the etv2 morphant phenotype. In contrast, the vascular phenotype associated with etv2 morpholino knockdown alone (Figure 3A-3D) was clearly exacerbated in fli1b+/- embryos (Figure 3E-3H) and further impaired in fli1b-/- embryos (Figure 3I-3L). Flilb+/- and fli1b-/- embryos with etv2 morpholino knockdown were easily distinguishable from etv2 morphant embryos by 24 hpf because of a reduction in the number of GFP-positive VECs when compared with etv2 morphant embryos (Figure 3E and 3I). Although endothelial cells in etv2 morphants coalesced to form the axial vasculature by 24 hpf, angioblasts in etv2 MO;fli1b-deficient embryos remained dispersed. At 36 hpf, both etv2 MO;fli1b+/- and etv2 MO;fli1b-/- embryos completely lacked intersegmental sprouts and showed a reduced number of GFP-positive cells relative to etv2 morphants. At 48 hpf, a few intersegmental sprouts were observed in the anterior trunk of etv2 MO;fli1b+/- (Figure 3G) but not in etv2 MO;fli1b-/- embryos (Figure 3K), which we interpret as a dose-effect resulting from the loss of functional fli1b protein. By 72 hpf, etv2 morphants exhibited a significant recovery in both the axial and ISVs (Figure 3D), whereas embryos also heterozygous for the fli1b gene trap displayed only a moderate recovery of the axial vessels and sporadic ISVs in the anterior trunk region (Figure 3). Finally, etv2 MO;fli1b-/- embryos showed a persistent gap in the axial vessels (Figure 3J–3L, arrow), indicating further impaired vasculogenesis. These embryos did not undergo angiogenic sprouting (Figure 3L), and their vasculature remained largely unchanged until they died at 5 dpf (data not shown). Because of the close proximity of etv2 and fli1b genes within the zebrafish genome, it was not possible to obtain the double etv2;fli1b-/- mutants using a simple cross because of the expected low frequency of recombination between the 2 genes. Nevertheless, etv2 MO has been validated in multiple previous studies and results in the same vascular phenotype as etv2-/- null mutants (Figures 1 and 3). We also tested whether double heterozygous etv2+/-;fli1b+/- mutants showed any vascular defects. Etv2+/-;fli1b+/- double heterozygous embryos were indistinguishable from WT embryos and did not display any defects in vascular patterning (Figure VA in the online-only Data Supplement).
We and others have previously reported a strong loss of multiple vascular endothelial marker expression observed in etv2 morphant and mutant embryos at 24 hpf.22,29 By 36 hpf, a partial recovery of the endothelial marker expression was observed in etv2 mutant and morphant embryos (Figure 4), consistent with the partial recovery of ISV sprouting observed in etv2 morphants. However, no significant vascular marker recovery was observed in embryos deficient in both etv2 and fli1b, and expression of the cdh5, fli1a, flt4, and kdrl vascular markers was largely undetectable through 36 hpf. Interestingly, a bilateral population of etv2-positive cells located in the trunk was retained in double-knockdown embryos, potentially representing a population of nondifferentiated etv2-positive vascular endothelial progenitors. Taken together, these data argue that Fli1b is capable of supporting vascular development in the absence of etv2 and that it participates in the angiogenic recovery of etv2-deficient embryos.
Figure 4.
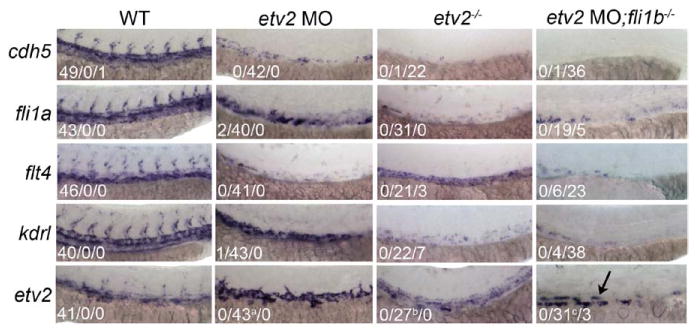
Expression of vascular markers is reduced in etv2 morphants and further reduced in embryos deficient in both fli1b and etv2. Whole-mount in situ hybridization (WISH) analysis performed on 36-hpf wild-type (WT) embryos shows strong expression of cdh5, fli1a, flt4, kdrl, and etv2 in the trunk vasculature. All vascular markers tested excluding etv2 itself were reduced in both etv2 morphants and etv2 mutants, and further reduced in double-knockdown embryos. A population of etv2-positive presumptive endothelial progenitor cells was retained in the double-knockdown embryos (arrow). Fractions indicate the number of embryos with the strong/low/no expression, excluding etv2 WISH panels for which the middle number indicates astrong but disorganized, breduced and disorganized or cstrong expression in a few remaining cells.
We also tested whether double fli1a-/-;fli1b-/- mutants showed any vascular defects. However, vascular patterning in fli1a-/-;fli1b-/- mutant embryos was normal (Figure VB in the online-only Data Supplement). fli1a-/-;fli1b-/- embryos exhibited impaired circulation at 32 hpf (not shown) and pericardial edema at 48 hpf, and the vessels appeared to fail to lumenize. Although the primary cause of this phenotype needs furtfromWTher investigation, these results show that the initial vasculogenesis and angiogenesis proceed normally in the absence of fli1a and fli1b functions.
Etv2 and fli1b Function in a Redundant Manner to Support Angiogenesis
The complete lack of angiogenic recovery observed in etv2 MO;fli1b-/- mutant embryos could be attributed to a direct requirement for etv2 and fli1b in angiogenesis or an indirect consequence of the failed vasculogenesis observed in etv2 MO;fli1b-/- mutant embryos. To determine whether etv2 and fli1b contribute combinatorially to sprouting angiogenesis, we used a previously validated photoactivatable (ie, caged) etv2 morpholino41 to block endogenous etv2 function at selected stages. Early photoactivation at 50% epiboly (5.3 hpf) confirmed effective uncaging and morpholino function as evidenced by a complete absence of angiogenesis at 24 hpf (data not shown). Fli1a:GFP expression at 42 hpf was robust in control embryos (Figure 5A), whereas embryos which were injected with the caged MO and uncaged at the 50% epiboly stage exhibited a partial recovery of the ISVs (Figure 5B), consistent with standard morpholino results (Figure 3B). Etv2-caged MO–injected embryos uncaged at the 18-somite stage (18 hpf) appeared grossly normal (Figure 5C) and were indistinguishable from control embryos that were injected with caged etv2 MO and never uncaged (Figure 5D). GFP fluorescence in 42 hpf fli1b-/- embryos (Figure 5E) mirrored the vascular-specific expression observed in the fli1a:GFP line (Figure 5A). Fli1b homozygous mutant embryos that were injected with the caged MO and uncaged at the 50% epiboly stage exhibited a more pronounced vascular phenotype relative to uncaged etv2 morphants in a WT background (Figure 5F compared with Figure 5B), as expected. Fli1b mutant embryos uncaged at the 18-somite stage showed a clear impairment of sprouting angiogenesis at 42 hpf (Figure 5G) compared with their never uncaged controls (Figure 5H). In these embryos, the axial vessels formed normally, but no complete ISVs and only partial ISVs were observed in the imaged portion of the trunk (average of 14±4 partial ISVs), compared with 24±0 fully formed ISVs in the corresponding caged etv2 morphants (Figure 5C). These results indicate that both etv2 and fli1b are redundantly required for angiogenesis independent from the etv2 earlier role in vasculogenesis.
Figure 5.
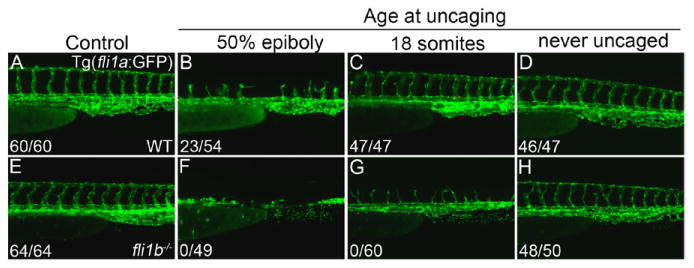
Etv2 and fli1b are required for angiogenesis independently from Etv2 early requirement in vasculogenesis. A-D, Caged etv2 morpholino (MO) analyses in 42-hpf wild-type (WT) embryos in a Tg(fli1a:GFP) background (lateral views, anterior to the left). A, Vascular patterning was normal and all intersegmental vessels (ISVs) were complete in control (uninjected) WT embryos that underwent the 30-minute UV exposure. B, All ISVs were incomplete in embryos injected with the caged etv2 MO and uncaged at 50% epiboly (5.3 hpf). Embryos uncaged at this stage showed truncated ISVs, typical for similarly staged morphants (compare with Figure 3B), thus confirming efficient morpholino uncaging. WT embryos uncaged at the 18-somite stage (C) were indistinguishable from the never uncaged embryos (D) indicating etv2 knockdown alone at 18-somites (18 hpf) did not block angiogenic sprouting. E-H, Caged etv2 MO analyses in 42-hpf fli1b-/- embryos visualized using the green fluorescent protein (GFP) expressed in the gene trap line. E, Fli1b mutants had a normally appearing vasculature. Uncaging at 50% epiboly in the fli1b mutant background again resulted in a more significant impairment of both vasculogenesis and angiogenesis (F) than observed in WT background (B). G, Uncaging of the etv2 MO at 18-somites in a fli1b mutant background resulted in truncated ISV sprouts (with no full-length sprouts observed). D and H, Embryos which were injected with caged MO and never uncaged had normally patterned ISVs (ie, <2 mispatterned ISVs), thus confirming effective caging. Fractions indicate the number of embryos with the >15 full ISVs (numerator) and the total evaluated (denominator).
Embryos Deficient in Both Etv2 and Fli1b Have an Expanded Zone of Apoptosis During Vasculogenesis
Because embryos deficient in both Etv2 and Fli1b exhibited a dramatic reduction in the number of VECs, particularly in the axial vasculature, we sought to determine whether this deficiency was because of increased apoptosis. Minimal caspase 3 staining was observed in WT embryos (Figure 6A), whereas etv2 morphant embryos showed increased apoptosis all along the axial vasculature (Figure 6B), similar to previous reports.29 Apoptotic staining in fli1b-/- embryos was not significantly different from WT embryos (Figure 6C). Staining was significantly expanded in etv2 MO;fli1b-/- embryos into the trunk axial vasculature (Figure 6D), suggesting that the observed reduction in VEC number was because of apoptotic cell death, either as a direct result of combined Etv2 and Fli1b loss or as an indirect consequence of impaired VEC differentiation. Cyclopamine induction of apoptosis was used to confirm caspase 3 staining, and a no antibody control was used to confirm a lack of nonspecific staining (data not shown). However, little overlap between endothelial GFP and caspase staining was detected. To rule out the possibility that the caspase-positive cells were primitive erythrocytes that originate in the adjacent LPM, we performed heme staining (data not shown) and whole-mount in situ analysis for hematopoietic markers hbbe3 and gata1 that were not changed in etv2 MO;fli1b-/- embryos (Figure 6A′-D′ and A″-D″), thus indicating that primitive erythropoiesis is normal in etv2 MO;fli1b-/- embryos. We repeated caspase 3 staining at earlier 10-somite (14 hpf) and 20-somite (19 hpf) stages. In both cases, caspase 3 staining was observed in the LPM, but no costaining with fli1a:GFP was observed (data not shown). We thus conclude that the vascular endothelial progenitors undergo apoptosis before they can initiate fli1a:GFP expression.
Figure 6.
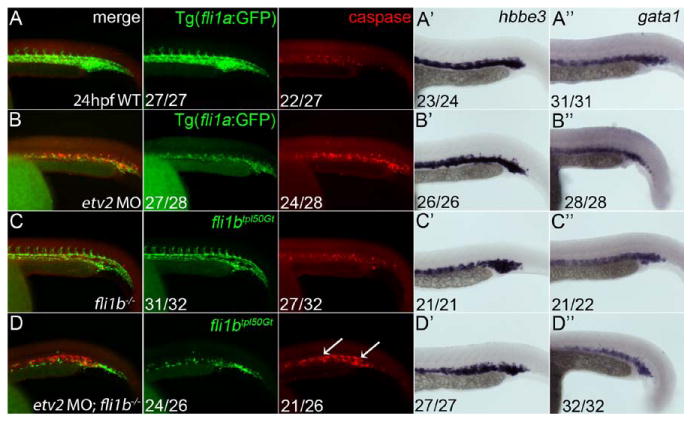
Apoptosis of vascular endothelial cells is expanded in double Etv2;Fli1b-deficient embryos. A-D, Whole-mount immunohistochemical staining of 48-hpf embryos for caspase 3 and green fluorescent protein (GFP) expression. Lateral views shown with anterior to the left. No distinct zones of apoptosis were noted in wild-type (A) and fli1b-/- embryos (C). By contrast, etv2 morphant embryos showed a narrow zone of apoptosis in the vascular plexus (B). D, This zone of apoptosis was expanded in embryos deficient in both fli1b and etv2 (arrows). Hbbe3 (A′-D′) and gata1 (A″-D″) levels determined by whole-mount in situ hybridization show normal erythrocyte staining in all groups indicating the observed apoptosis was not because of a loss of blood cells. Fractions indicate the number of embryos with the observed pattern of marker expression (numerator) and the total evaluated (denominator).
Etv2 and fli1b Can Independently Induce Vascular Endothelial Marker Expression
Previous studies have shown that etv2 overexpression is sufficient for the specification of endothelial and myeloid but not the erythroid lineages.22,23,27 To determine whether these functional roles also hold true for fli1a and fli1b, sense mRNA was injected into WT embryos. Overexpression of fli1a had no effect on the expression of vascular endothelial markers kdrl, fli1b, or etv2, hemangioblast marker scl, or erythroid marker gata1 expression (Figure VI in the online-only Data Supplement). In contrast, overexpression of fli1b mRNA induced ectopic expression of the vascular endothelial kdrl:GFP reporter and fli1a marker (Figure 7B and 7D). Also consistent with the published function of etv2,22 fli1b overexpression induced the early hemangioblast marker scl (Figure 7F) but had no effect on the erythroid marker gata1 (Figure 7H), thus arguing that fli1b overexpression is sufficient to induce vascular endothelial differentiation, similar to Etv2. To determine the relationship between etv2 and fli1b, a series of epistatic interaction analyses were performed. Overexpression of etv2 caused ectopic expression of fli1b (Figure 7J), and conversely, overexpression of fli1b was sufficient to induce expression of etv2 (Figure 7L), suggesting that a positive feedback relationship potentially exists between the 2 transcription factors.
Figure 7.
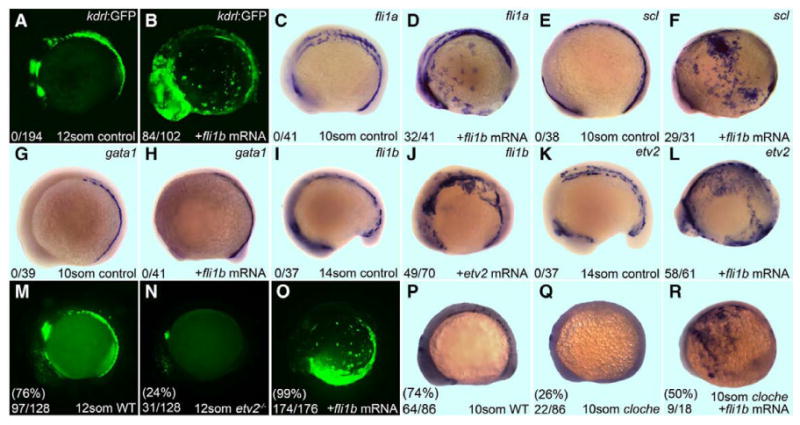
Fli1b overexpression is sufficient to induce expression of vascular endothelial markers in wild-type (WT), etv2-/- and cloche mutant embryos. A, C, E, G, I and K, Uninjected controls. Whole-mount in situ analysis of embryos is shown in lateral view with anterior of embryo oriented left. A-H, Effect of fli1b overexpression on vascular and hematopoietic markers during the indicated stages. Fli1b induces kdrl:GFP (B), fli1a (D), and scl (F), but not gata1 (H). I-L, Lateral views of WT embryos injected with fli1b and etv2 mRNA. J, Fli1b overexpression induces ectopic etv2 in WT embryos. L, Conversely, fli1b overexpression induces ectopic etv2. The number of embryos with ectopic expression (numerator) and the total number evaluated (denominator) lower left panel, the age and injected mRNA in the bottom left, and gene probed by in situ in the upper right. M-O, Effect of fli1b overexpression in etv2-/- mutant embryos. An in-cross of etv2+/- Tg(fli1a:GFP) adults yielded the expected Mendelian ratio of phenotypically WT (M) and etv2-/- embryos (N) as determined by fli1a:GFP expression. O, 99% embryos injected with fli1b mRNA exhibited ectopic expression of fli1a, indicating that fli1b is capable of inducing vascular fli1a in the absence of etv2. P-R, Fli1b mRNA induces ectopic kdrl expression in cloche mutant embryos. An in-cross of cloche heterozygous adults yielded the expected Mendelian ratio of 10-somite stage (14 hpf) WT (P) and mutant (Q) embryos, based on the presence or absence of kdrl expression. Injection of fli1b mRNA induced ectopic expression of kdrl (R) indicating that Fli1b acts downstream of cloche.
To determine whether fli1b was capable of inducing vascular marker expression in the absence of functional etv2, fli1b sense mRNA was injected into embryos from an etv2+/- Tg(fli1a:GFP) carrier in-cross. Robust expression of the downstream vascular marker fli1a was observed in the endothelial progenitor cell pools of WT embryos (Figure 7M) but was restricted to nonendothelial group of cells in the presumptive pharyngeal endoderm region in 12-somite (15 hpf) etv2 homozygous mutant embryos (Figure 7N). Embryos obtained from the etv2+/- carrier in-cross and injected with fli1b mRNA showed strong ectopic expression of fli1a:GFP (Figure 7O), thereby arguing that fli1b is capable of inducing the vascular marker fli1a expression independent of etv2 function.
Cloche mutant zebrafish are deficient in both endothelial and hematopoietic lineages,45 and etv2 is reduced in cloche mutants,46 suggesting that cloche is required for the differentiation of the hemangioblasts and functions upstream of etv2. Etv2 overexpression in cloche mutants is sufficient to induce expression of vascular markers, such as kdrl/flk1/VegfR2.22 We found that overexpression of fli1b in cloche mutant embryos also caused ectopic expression of kdrl (Figure 7P through 7R), suggesting that fli1b is also downstream of cloche and is sufficient to initiate vascular endothelial differentiation. Taken together, our data show that etv2 and fli1b, but not fli1a, are capable of inducing a similar set of vascular endothelial markers, which suggests that etv2 and fli1b may have an overlapping function.
Etv2 and Fli1b Have a Combinatorial Effect on Multiple Vascular Endothelial Markers
RNA-Seq analysis was performed on 24- and 48-hpf embryos to profile gene expression changes in etv2 morphant, fli1b-/-, and etv2 MO;fli1b-/- embryos. As expected, the expression of multiple vascular endothelial markers was significantly reduced in etv2 morphant embryos at 24 hpf and partially recovered at 48 hpf, consistent with the partial angiogenic recovery observed in morphant embryos (Table). Vascular markers were largely normal in fli1b mutant embryos, as expected, because these embryos develop a normally appearing vasculature. Vascular markers were further reduced in double-deficient embryos (Table), consistent with the severe vascular deficit in these embryos. Interestingly, most of the endothelial markers partially recovered by 48 hpf in the double-deficient embryos (as observed in the etv2 morphant embryos), despite the lack of an angiogenic recovery in these embryos. Of note, etv2 was significantly increased at 24 hpf in etv2 morphant embryos and double-deficient embryos (confirming in situ analysis shown in Figure 4). Etv2 levels were further increased in morphants during the angiogenic recovery occurring at 48 hpf, but no further increase was observed in double-deficient embryos, consistent with the failed angiogenic recovery observed in these embryos.
Table. Fold Changes in Vascular Gene Expression in etv2 Morphants, fli1b-/- Mutants, and Double etv2 MO;fli1b-/- Mutant Embryos When Compared With Uninjected Wild-Type Control Embryos Measured by RNA-Seq.
| Gene Symbol | 24hpf etv2 MO |
24-hpf fli1b-/- mutants |
24-hpf etv2 MO;li1b-/- |
48-hpf etv2 MO |
48-hpf fli1b-/- mutants |
48-hpf etv2 MO;fli1b-/- |
|---|---|---|---|---|---|---|
| aqp8a.1 | −29.4* | 1.6 | −4.4* | −7.3* | −1.0 | −14.2*† |
| mrc1a | −21.2* | 2.2* | −11.6* | −6.3* | −1.3 | −9.5*† |
| Fli1b | −7.5* | −3.9*† | −9.7*† | −3.3* | −3.2*† | −3.8* |
| gpr182 | −8.5* | 1.1 | −10.2*† | −2.9* | −1.2 | −8.7*† |
| tie1 | −4.3* | 1.1 | −17.6*† | −3.0* | −1.1 | −11.8*† |
| stab2 | −3.9* | 1.0 | −1.7 | −1.8* | 1.1 | −2.5*† |
| stab1l | −3.8* | −1.3 | −4.1*† | −2.4* | −1.4 | −2.8*† |
| flt4 | −3.5* | −1.1 | −3.3* | −1.6* | 1.1 | −1.6*† |
| micall2a | −3.4* | −1.9 | −11.1*† | −1.5* | −2.5 | −3.5*† |
| flt1 | −3.3* | −1.3 | −17.1*† | −1.9* | 1.1 | −1.5* |
| clec14a | −3.0* | 1.4 | −8.3*† | −1.5* | 1.2 | −3.0*† |
| dusp5 | −3.0* | 1.3 | −2.8* | 1.3* | −1.0 | −2.7*† |
| she | −2.7* | −1.0 | −5.9*† | −1.6* | 1.1 | −2.4*† |
| cldn5b | −2.6* | 1.1 | −10.0*† | −2.0* | 1.4 | −2.2*† |
| vsg1 | −2.5* | −2.1 | −3.1*† | −1.6* | −1.7 | −3.9*† |
| dab2 | −2.4* | −1.4 | −4.5*† | −1.2 | −1.2 | −1.1† |
| esama | −2.2* | −1.3 | −6.0*† | −1.5* | −1.5 | −3.2*† |
| sox7 | −1.9* | −1.4 | −2.9*† | −1.3 | 1.3 | −1.0 |
| cdh5 | −1.8* | 1.4 | −14.0*† | −1.9* | −1.2 | −4.5*† |
| kdrl | −1.7* | −1.0 | −3.7*† | −1.8* | 1.0 | −1.8*† |
| Fli1a | −1.3* | 1.1 | −2.5*† | −1.4* | −1.1 | −1.6† |
| ETV2 | 6.8* | −1.3 | 6.6*† | 21.5* | −1.1 | 8.6* |
All genes listed were altered >1.5-fold in 24-hpf morphants relative to 24-hpf wild-type controls (P<0.1; with the exclusion of fli1a which has been included for reference purposes). Note that etv2 expression was significantly increased in morphant embryos likely because of compensatory feedback in the absence of functional protein. Most of the vascular genes (18/20) showed less reduction in 48-hpf etv2 morphants than in 24-hpf etv2 morphants. Vascular genes were largely unaffected in fli1b mutants consistent with the lack of a vascular phenotype in mutant embryos. Vascular marker expression in double-deficient embryos was reduced beyond that of the age-matched morphant embryos in 15/20 (75%) instances in 24-hpf embryos and in 17/20 instances (85%) in 48-hpf embryos, consistent with the exacerbated phenotype in double-knockdown embryos.
Significant expression changes (P<0.1).
Fold changes which are enhanced in age-matched double-deficient embryos.
Further analysis of our RNA-Seq data (Table) provided additional insight into the role played by fli1b relative to etv2 and fli1a. Fli1a message was reduced 1.3-fold in etv2 morphants and further reduced (down 2.45-fold) in etv2 MO;fli1b-/- embryos at 24 hpf, suggesting that Etv2 and Fli1b are involved in regulating fli1a expression.
Fli1b Directly Induces Fli1a Expression
Etv2 binds together with FoxC homologs to the FOX-ETS motif present within the promoters of multiple vascular endothelial–specific genes.28 Evolutionarily conserved ETS sites have been identified within the proximal mouse Fli1 and zebrafish Fli1 a promoters with direct binding of Etv2 to these sites shown.34,47 Putative ETS-binding sites corresponding to the functional murine ETS sites were identified in the zebrafish fli1a promoter (Figure VIIA in the online-only Data Supplement). In addition, a conserved FOX-ETS motif with several adjacent ETS motifs was identified within the first intron of fli1a gene (Figure VII in the online-only Data Supplement). All of these identified sites are present within the fli1a[ep] enhancer–promoter region, which has been previously demonstrated to be necessary and sufficient for vascular endothelial reporter expression.47 To determine whether Fli1b directly binds to the DNA fragments containing these consensus binding sites, we performed chromatin immunoprecipitation analysis on zebrafish embryos injected with FLAG-fli1b RNA. Overexpression of FLAG-tagged fli1b, similar to the overexpression of untagged fli1b, resulted in specific induction of vascular endothelial markers, including fli1a:GFP. Embryos were fixed at the tailbud stage and subjected to chromatin immunoprecipitation using FLAG antibody. Specific enrichment for 2 fragments, containing the previously described ETS sites within the proximal fli1a promoter (2.27±0.80 fold enrichment) and a consensus FOX-ETS site within the first intron of fli1a (4.62±1.64 fold enrichment), was observed. Multiple other genomic regions tested showed no enrichment in the pull down fraction (data not shown), which argues for the specificity of the observed binding.
Functional activity of the predicted ETS-binding sites was verified by cloning the fli1a[ep] enhancer–promoter region upstream of an enhanced GFP reporter and evaluating the ability of fli1b mRNA to induce reporter expression (Figure VIIIA in the online-only Data Supplement). All predicted ETS-binding sites within the fli1a[ep] promoter–enhancer were mutated to test their functional requirement (Figure IX in the online-only Data Supplement). Control injections of the reporter fli1a[ep]:GFP construct with etv2 mRNA yielded ectopic GFP expression at the 8-somite stage (13 hpf) in 44% of embryos (n=192). Mutation of the ETS-binding sites in the fli1a[ep]:GFP reporter construct blocked its induction by etv2, thus indicating that etv2 induced expression in an ETS-binding site–dependent manner. Coinjection of the reporter construct with fli1b mRNA induced GFP reporter expression in 61% of embryos (n=184). Because we had already demonstrated that Fli1b injection induces etv2 expression, we used etv2 MO to inhibit Etv2 function and to test fli1b activity directly. When the fli1a[ep]:GFP construct was coinjected with etv2 MO and fli1b mRNA, strong ectopic GFP expression was observed in 64% (n=170) of embryos, indicating that Fli1b is capable of activating fli1a[ep] promoter in the absence of etv2 (Figure VIIIB and VIIIC in the online-only Data Supplement). Injections made with the enhanced GFP reporter construct lacking predicted ETS-binding sites yielded no fluorescent embryos (n=146), indicating that ETS sites are required for induction of fli1a[ep]:GFP by Fli1b. In summary, these results argue that Fli1b directly induces expression of fli1a by binding to the ETS-binding sites within the fli1a promoter–enhancer.
Discussion
In this study, we describe a novel critical function for 2 related ETS transcription factors Etv2 and Fli1b in initiating angiogenesis and completing vasculogenesis during early embryonic development. The data presented herein are consistent with a model of embryonic vascularization in which etv2 initiates the vascular endothelial program (including expression of fli1a, fli1b, and related markers) during early vasculogenesis, and later etv2 and fli1b are redundantly required during late vasculogenesis and early sprouting angiogenesis (Figure 8). During early stages of vascular development (1–15 somite stages), zebrafish etv2 single mutants display essentially complete lack of VECs and have little if any vascular endothelial marker expression; therefore, Etv2 seems to be the single major master regulator of early vasculogenesis. The lack of a vascular phenotype in fli1a and fli1b double-mutant embryos suggests that both Fli1a and Fli1b are largely dispensable for early vasculogenesis in WT embryos. However, etv2 mutants do form vascular endothelial progenitors at later stages and initiate angiogenesis after 36 hpf. Only double-deficient etv2;fli1b embryos show persistent loss of vascular endothelial progenitors within the axial vasculature and complete absence of angiogenesis during 24 to 72 hpf. This argues for the functional redundancy between Etv2 and Fli1b during these stages of late vasculogenesis and early angiogenesis (Figure 8).
Figure 8.
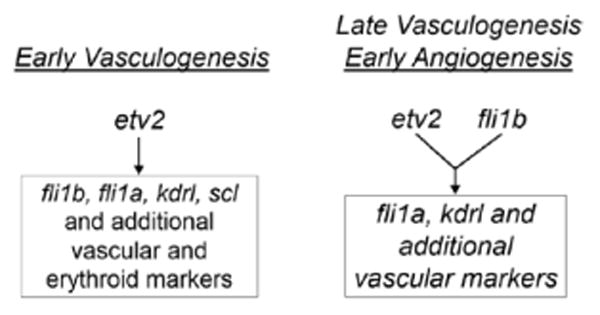
Proposed Etv2 and Fli1b roles in vasculogenesis and angiogenesis. During early vasculogenesis, Etv2 induces the expression of multiple vascular endothelial and hemangioblast genes including fli1a, fli1b, and others. During late vasculogenesis and early angiogenesis, Etv2 and Fli1b both function redundantly to upregulate expression of fli1a and other vascular target genes. WT indicates wild-type.
In support of this model, the reduced endothelial cell numbers and gaps in the axial vasculature at later stages are only observed in double etv2;fli1b knockdown embryos, which argues that Etv2 and Fli1b function redundantly and that Fli1b responsible for mediating the angiogenic recovery observed in etv2 mutants and morphants. The compensatory supporting function of fli1b in etv2-deficient embryos is further supported by the induction of multiple vascular markers by Fli1b overexpression, including direct induction of fli1a by binding to ETS sites fli1a promoter–enhancer region.
Whole-mount in situ hybridization and reverse transcription polymerase chain reaction data presented here and elsewhere indicate that fli1b is expressed at low levels beginning at the 8-somite stage (13 hpf) and then increases to a sustained plateau of high-level expression at around the 20-somite stage (19 hpf) when etv2 expression is waning.29,48 Because fli1b expression exceeds that of etv2 during the angiogenic recovery of both etv2 morphants and mutants (ie, after 24 hpf) and because Fli1b is capable of inducing the same vascular target genes as Etv2, it is likely that the angiogenic recovery observed in etv2-deficient embryos occurs through the compensatory action of fli1b. In this scenario, loss of Fli1b in etv2 mutants would result in a failure of angiogenic sprouting altogether, as we observed. Our data do not exclude potential contribution from other vascular ETS factors (eg, Erg or Ets-1), as suggested previously,29 but the normal profile of vascular markers in fli1a mutants combined with the lack of vascular marker induction in fli1a injected embryos suggests that fli1a is not mediating the observed angiogenic recovery and thus serves a functional role, which is distinct from fli1b. It is possible that such a functional redundancy has evolved as a means of protecting the embryo from vascular development defects and that additional ETS factors have the capacity to support vascularization in the absence of the normally dominant etv2.
It has been previously reported that fli1a-/- and fli1b-/- single-MO knockdown embryos exhibit defects in ISV sprouting.29 Despite the profound loss of target gene expression in the fli1a and fli1b gene trap lines, we did not observe any apparent vascular patterning defects in either mutant line. Therefore, it is likely that some of the previously reported defects were caused by MO off-target effects. However, we cannot rule out the possibility that small amounts of WT transcript that remained in fli1a-/- and fli1b-/- lines were sufficient to allow normal development. An increased incidence of hemorrhages attributed to a loss of vessel integrity has been previously reported in fli1a MO knockdown zebrafish embryos,37 and in Fli1 mutant mice,33 but hemorrhages were not observed in fli1a-/- or fli1b-/- embryos.
Our observation that the failed angiogenic recovery in embryos deficient in Fli1b and Etv2 correlated with an elevated level of apoptosis and a reduction in the number of VECs (relative to Etv2-deficiency alone) is consistent with other studies which have implicated ETS factors as modulators of apoptosis. Overexpression of human Fli1 and Erg inhibit apoptosis in vitro, thus providing critical insight into the progression of Ewing sarcoma.49 Further, exogenous expression of both Ets-2 and PU.1 increase Bcl-x activation to inhibit apoptosis in macrophages.50 While it is possible that Etv2 and Fli1b directly serve to inhibit apoptosis in vascular progenitor cells, our data cannot rule out an indirect initiation of apoptosis as a mechanism to remove undifferentiated vascular progenitor cells which fail to differentiate in the double Etv2;Fli1b knockdown embryos.
Previous studies have demonstrated that Etv2 directly binds to multiple endothelial enhancers and acts as a potent transcriptional regulator.28 We and others have also demonstrated that Etv2 overexpression results in strong upregulation of multiple vascular endothelial–specific genes.22,40,41. In this study, we report that Fli1b overexpression upregulates a subset of vascular endothelial and early hematopoietic markers in the same manner as Etv2. Furthermore, Fli1b is sufficient to induce vascular endothelial marker expression in Etv2 null and cloche mutant embryos. These results suggest that Fli1b and Etv2 bind to a similar set of transcriptional targets. RNA-Seq analysis seems to support this contention because double-knockdown embryos show a marked reduction in vascular markers compared with etv2 MO alone. Our analysis also indicates that fli1b directly induces fli1a expression in an ETS-binding site–dependent manner by binding to known Etv2-target ETS sites28,34 within the fli1a promoter and first intron. Further testing is required to identify additional direct transcriptional targets of Fli1b and to determine the extent of transcriptional target overlap with Etv2 in both vasculogenesis and angiogenesis.
Previous reports have shown that etv2 is significantly post-transcriptionally repressed by the let-7 family of micro-RNAs during the formation of the peripheral vasculature and that protein levels are drastically reduced shortly thereafter, as determined by immunostaining in WT embryos.48 By contrast, Fli1a and Fli1b are not predicted targets of the let-7 family, which could explain the persistent expression of both in later development. Despite the reduced expression of etv2 during angiogenesis, our data suggest that etv2 functions beyond its generally recognized role in vasculogenesis and acts in combination with fli1b in these later stages of angiogenesis (ie, 24–72 hpf). This later role is supported by caged morpholino knockdown of etv2 in fli1b mutant background, which caused impaired angiogenic sprouting in embryos with intact vasculogenesis (Figure 5). It is important to point out that limited ISV sprouting was observed in fli1b mutant embryos injected with caged etv2 MO and photoactivated at the 18-somite (18 hpf) stage, whereas earlier inhibition of Etv2 function in fli1b-/- mutant background resulted in the complete absence of ISVs. The partial sprouting observed in caged etv2 MO;fli1b-/- embryos after photoactivation at the 18-somite stage could be explained by the contribution of other vascular ETS factors that may also serve a compensatory role and support angiogenesis in the absence of etv2 function.
Data published previously34 and several lines of data presented herein suggest that Etv2 is the primary driver of early expression of the downstream target fli1a, including (1) reduced fli1a:GFP transgenic expression in etv2 morphants and mutants, (2) reduced fli1a expression in etv2-deficient embryos by in situ analyses, (3) failure of fli1a mutant embryos to recapitulate (or even enhance) the etv2 mutant phenotype, and (4) direct evidence of Etv2 binding to fli1a promoter–enhancer in fluorescent reporter assays. However, the observation that fli1a:GFP transgene expression and fli1a message partially recovered by 48 hpf suggests that etv2 is not solely required for inducing fli1a in vivo. Rather, because fli1a is significantly reduced only in etv2 MO;fli1b-/- embryos and ectopic Fli1b induced expression of the fli1a[ep]:GFP reporter construct, it is possible that Etv2 and Fli1b act cooperatively to induce fli1a expression or that both bind to ETS sites within the fli1a[ep] sequence and that the contribution of each ETS factor is based on the temporal concentrations of each. Although beyond the scope of this study, it would be interesting, therefore, to see whether etv2 MO knockdown further exacerbates the fli1a-/-;fli1b-/- mutant phenotype. Also, it is possible that additional transcription factors or cofactors not part of this study are required for sustained expression of fli1a. Interestingly, a recent study demonstrated that in mouse embryos, Etv2 initiates Fli1 expression, which is further maintained by Fli1 autoregulation.34 Similarly, our results show that zebrafish Etv2 initiates fli1a and fli1b expression, and then Fli1b is further involved in maintaining fli1a and possibly its own expression. Our results do not exclude the possibility that Fli1a also participates in maintaining its own expression. Thus, it seems that transcriptional regulation of Etv2 and Fli1 expression is evolutionarily conserved.
Taken together, our data indicate that Etv2 and Fli1b have redundant roles during late vasculogenesis and early embryonic angiogenesis. It is also intriguing to consider the possibility that they are involved in the remarkably similar processes of adult neoangiogenesis or in pathological hypervascularization. Our findings demonstrate a novel role for ETS transcription factors in angiogenesis and eventually may lead to the design of improved therapeutics to enhance wound healing or block tumor-induced vascularization.
Supplementary Material
Significance.
The E26 transformation-specific transcription factor Etv2/Etsrp has been previously shown to function as a master regulator during early embryonic vasculogenesis. Here, we demonstrate that Etv2 has a previously unrecognized role in angiogenesis and late embryonic vascuologenesis and that this novel function is supported by the redundant activity of a related E26 transformation-specific factor, Fli1b. We demonstrate for the first time that knockdown of 2 E26 transformation-specific factors results in a complete absence of angiogenesis and nearly complete absence of vasculogenesis. We show that Fli1b, similar to Etv2, directly initiates transcription of multiple endothelial–specific genes and that Etv2 and Fli1b induce the expression of each other. Taken together, our findings argue that fli1b and etv2 function together as critical regulators of endothelial differentiation. We expect that our results will be highly significant in elucidating molecular mechanisms of vascular development.
Acknowledgments
We thank Matthew Weirauch for help with identifying candidate ETS-binding sites.
Sources of Funding: This work was supported by the National Institutes of Health (NIH) 5T32HL007752-18 (Dr Whitsett), NIH R01 HD061749 (Dr Balciunas), DK100325 (Dr Park), DA14546 and GM63904 (Dr Ekker), HL107369 (Dr Sumanas) and pilot awards from Cancer Free Kids and Ohio Cancer Research Associates (Dr Sumanas).
Nonstandard Abbreviations and Acronyms
- ETS
E26 transformation-specific
- GFP
green fluorescent protein
- ISV
intersegmental vessel
- MO
morpholino
- VEC
vascular endothelial cell
- LPM
lateral plate mesoderm
- WT
wild-type
Footnotes
Disclosures: None.
The online-only Data Supplement is available with this article at http://atvb.ahajournals.org/lookup/suppl/doi:10.1161/ATVBAHA.114.304768/-/DC1.
Contributor Information
Michael P. Craig, Department of Molecular and Cellular Physiology, University of Cincinnati College of Medicine, OH; Division of Developmental Biology, Cincinnati Children's Hospital Medical Center, OH
Viktorija Grajevskaja, Department of Biology, Temple University, Philadelphia, PA; Department of Zoology, Faculty of Natural Sciences, Vilnius University, Vilnius, Lithuania.
Hsin-Kai Liao, Department of Genetics, Development and Cell Biology, Iowa State University, Ames.
Jorune Balciuniene, Department of Biology, Temple University, Philadelphia, PA.
Stephen C. Ekker, Department of Biochemistry and Molecular Biology, Mayo Clinic, Rochester, MN
Joo-Seop Park, Division of Developmental Biology, Department of Pediatric Urology, Cincinnati Children's Hospital Medical Center, OH.
Jeffrey J. Essner, Department of Genetics, Development and Cell Biology, Iowa State University, Ames
Darius Balciunas, Department of Biology, Temple University, Philadelphia, PA.
Saulius Sumanas, Department of Pediatrics, Cincinnati Children's Hospital Medical Center, OH.
References
- 1.Schmidt A, Brixius K, Bloch W. Endothelial precursor cell migration during vasculogenesis. Circ Res. 2007;101:125–136. doi: 10.1161/CIRCRESAHA.107.148932. [DOI] [PubMed] [Google Scholar]
- 2.Risau W, Flamme I. Vasculogenesis. Annu Rev Cell Dev Biol. 1995;11:73–91. doi: 10.1146/annurev.cb.11.110195.000445. [DOI] [PubMed] [Google Scholar]
- 3.Semenza GL. Vasculogenesis, angiogenesis, and arteriogenesis: mechanisms of blood vessel formation and remodeling. J Cell Biochem. 2007;102:840–847. doi: 10.1002/jcb.21523. [DOI] [PubMed] [Google Scholar]
- 4.Kobayashi D, Williams DA, Cook AL. Mixed-type total anomalous pulmonary venous connection. Pediatr Cardiol. 2010;31:929–930. doi: 10.1007/s00246-010-9728-3. [DOI] [PubMed] [Google Scholar]
- 5.Montesinos MC, Desai A, Chen JF, Yee H, Schwarzschild MA, Fink JS, Cronstein BN. Adenosine promotes wound healing and mediates angiogenesis in response to tissue injury via occupancy of A(2A) receptors. Am J Pathol. 2002;160:2009–2018. doi: 10.1016/S0002-9440(10)61151-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morgan C, Nigam Y. Naturally derived factors and their role in the promotion of angiogenesis for the healing of chronic wounds. Angiogenesis. 2013;16:493–502. doi: 10.1007/s10456-013-9341-1. [DOI] [PubMed] [Google Scholar]
- 7.Bołzan E, Andronowska A, Bodek G, Morawska-Pucińska E, Krawczyński K, Dabrowski A, Ziecik AJ. The novel effect of hCG administration on luteal function maintenance during the estrous cycle/pregnancy and early embryo development in the pig. Pol J Vet Sci. 2013;16:323–332. doi: 10.2478/pjvs-2013-0044. [DOI] [PubMed] [Google Scholar]
- 8.Plendl J, Neumtiller C, Sinowatz F. Differences of microvascular endothelium in the bovine corpus luteum of pregnancy and the corpus luteum of the estrous cycle. Biol Cell. 1996;87:179–188. [PubMed] [Google Scholar]
- 9.Gustafsson T, Kraus WE. Exercise-induced angiogenesis-related growth and transcription factors in skeletal muscle, and their modification in muscle pathology. Front Biosci. 2001;6:D75–D89. doi: 10.2741/gustafss. [DOI] [PubMed] [Google Scholar]
- 10.Gavin TP, Drew JL, Kubik CJ, Pofahl WE, Hickner RC. Acute resistance exercise increases skeletal muscle angiogenic growth factor expression. Acta Physiol (Oxf) 2007;191:139–146. doi: 10.1111/j.1748-1716.2007.01723.x. [DOI] [PubMed] [Google Scholar]
- 11.Sweeney K. Angiogenesis inhibitors: an upcoming therapy for cancer and wet age-related macular degeneration. Drug Discov Today. 2005;10:1346–1348. doi: 10.1016/S1359-6446(05)03607-X. [DOI] [PubMed] [Google Scholar]
- 12.Kumaran G, Clamp AR, Jayson GC. Angiogenesis as a therapeutic target in cancer. Clin Med. 2008;8:455–458. doi: 10.7861/clinmedicine.8-4-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prager GW, Poettler M. Angiogenesis in cancer. Basic mechanisms and therapeutic advances. Hamostaseologie. 2012;32:105–114. doi: 10.5482/ha-1163. [DOI] [PubMed] [Google Scholar]
- 14.Luo L, Zhang X, Hirano Y, et al. Targeted intraceptor nanoparticle therapy reduces angiogenesis and fibrosis in primate and murine macular degeneration. ACS Nano. 2013;7:3264–3275. doi: 10.1021/nn305958y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seiler C, Stoller M, Pitt B, Meier P. The human coronary collateral circulation: development and clinical importance. Eur Heart J. 2013;34:2674–2682. doi: 10.1093/eurheartj/ehtl95. [DOI] [PubMed] [Google Scholar]
- 16.Hammer A, Steiner S. Gene therapy for therapeutic angiogenesis in peripheral arterial disease - a systematic review and meta-analysis of randomized, controlled trials. Vasa. 2013;42:331–339. doi: 10.1024/0301-1526/a000298. [DOI] [PubMed] [Google Scholar]
- 17.Lara-Hernández R, Lozano-Vilardell P, Cordobés-Gual J. Novel therapies of non-revascularizing peripheral arterial occlusive disease: therapeutic angiogenesis. Med Clin (Barc) 2008;131:665–669. doi: 10.1157/13128727. [DOI] [PubMed] [Google Scholar]
- 18.Rubanyi GM. Therapeutic Angiogenesis and Wound Healing–IBC's International Conference: 15–16 November 2004. Cambridge, MA, USA. IDrugs. 2005;82:94–96. [PubMed] [Google Scholar]
- 19.Feng FY, Brenner JC, Hussain M, Chinnaiyan AM. Molecular pathways: Targeting ETS gene fusions in cancer. Clin Cancer Res. 2014;20:4442–4448. doi: 10.1158/1078-0432.CCR-13-0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kar A, Gutierrez-Hartmann A. Molecular mechanisms of ETS transcription factor-mediated tumorigenesis. Crit Rev Biochem Mol Biol. 2013;48:522–543. doi: 10.3109/10409238.2013.838202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oettgen P. The role of ets factors in tumor angiogenesis. J Oncol. 2010;2010 doi: 10.1155/2010/767384. 767384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sumanas S, Lin S. Ets1-related protein is a key regulator of vasculogenesis in zebrafish. PLoS Biol. 2006;4:e10. doi: 10.1371/journal.pbio.0040010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sumanas S, Gomez G, Zhao Y, Park C, Choi K, Lin S. Interplay among Etsrp/ER71, Scl, and Alk8 signaling controls endothelial and myeloid cell formation. Blood. 2008;111:4500–4510. doi: 10.1182/blood-2007-09-110569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Haro L, Janknecht R. Cloning of the murine ER71 gene (Etsrp71) and initial characterization of its promoter. Genomics. 2005;85:493–502. doi: 10.1016/j.ygeno.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 25.Ferdous A, Caprioli A, Iacovino M, Martin CM, Morris J, Richardson JA, Latif S, Hammer RE, Harvey RP, Olson EN, Kyba M, Garry DJ. Nkx2-5 transactivates the Ets-related protein 71 gene and specifies an endothelial/endocardial fate in the developing embryo. Proc Natl Acad Sci U S A. 2009;106:814–819. doi: 10.1073/pnas.0807583106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee D, Park C, Lee H, Lugus JJ, Kim SH, Arentson E, Chung YS, Gomez G, Kyba M, Lin S, Janknecht R, Lim DS, Choi K. ER71 acts downstream of BMP, Notch, and Wnt signaling in blood and vessel progenitor specification. Cell Stem Cell. 2008;2:497–507. doi: 10.1016/j.stem.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salanga MC, Meadows SM, Myers CT, Krieg PA. ETS family protein ETV2 is required for initiation of the endothelial lineage but not the hematopoietic lineage in the Xenopus embryo. Dev Dyn. 2010;239:1178–1187. doi: 10.1002/dvdy.22277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Val S, Chi NC, Meadows SM, et al. Combinatorial regulation of endothelial gene expression by ets and forkhead transcription factors. Cell. 2008;1356:1053–1064. doi: 10.1016/j.cell.2008.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pham VN, Lawson ND, Mugford JW, Dye L, Castranova D, Lo B, Weinstein BM. Combinatorial function of ETS transcription factors in the developing vasculature. Dev Biol. 2007;303:772–783. doi: 10.1016/j.ydbio.2006.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang L, Lemarchandel V, Romeo PH, Ben-David Y, Greer P, Bernstein A. The Fli-1 proto-oncogene, involved in erythroleukemia and Ewing sarcoma, encodes a transcriptional activator with DNA-binding specificities distinct from other Ets family members. Oncogene. 1993;8:1621–1630. [PubMed] [Google Scholar]
- 31.Zucman J, Melot T, Desmaze C, Ghysdael J, Plougastel B, Peter M, Zucker JM, Triche TJ, Sheer D, Turc-Carel C. Combinatorial generation of variable fusion proteins in the Ewing family of tumours. EMBO J. 1993;12:4481–4487. doi: 10.1002/j.1460-2075.1993.tb06137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu F, Walmsley M, Rodaway A, Patient R. Fli1 acts at the top of the transcriptional network driving blood and endothelial development. Curr Biol. 2008;18:1234–1240. doi: 10.1016/j.cub.2008.07.048. [DOI] [PubMed] [Google Scholar]
- 33.Spyropoulos DD, Pharr PN, Lavenburg KR, Jackers P, Papas TS, Ogawa M, Watson DK. Hemorrhage, impaired hematopoiesis, and lethality in mouse embryos carrying a targeted disruption of the Fli1 transcription factor. Mol Cell Biol. 2000;20:5643–5652. doi: 10.1128/mcb.20.15.5643-5652.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abedin MJ, Nguyen A, Jiang N, Perry CE, Shelton JM, Watson DK, Ferdous A. Fli1 acts downstream of Etv2 to govern cell survival and vascular homeostasis via positive autoregulation. Circ Res. 2014;114:1690–1699. doi: 10.1161/CIRCRESAHA.1134303145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patterson LJ, Gering M, Patient R. Scl is required for dorsal aorta as well as blood formation in zebrafish embryos. Blood. 2005;105:3502–3511. doi: 10.1182/blood-2004-09-3547. [DOI] [PubMed] [Google Scholar]
- 36.Thompson MA, Ransom DG, Pratt SJ, et al. The cloche and spadetail genes differentially affect hematopoiesis and vasculogenesis. Dev Biol. 1998;197:248–269. doi: 10.1006/dbio.1998.8887. [DOI] [PubMed] [Google Scholar]
- 37.Liu F, Patient R. Genome-wide analysis of the zebrafish ETS family identifies three genes required for hemangioblast differentiation or angiogenesis. Circ Res. 2008;103:1147–1154. doi: 10.1161/CIRCRESAHA.108.179713. [DOI] [PubMed] [Google Scholar]
- 38.Meadows SM, Myers CT, Krieg PA. Regulation of endothelial cell development by ETS transcription factors. Semin Cell Dev Biol. 2011;22:976–984. doi: 10.1016/j.semcdb.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kok FO, Shin M, Ni CW, et al. Reverse genetic screening reveals poor correlation between morpholino-induced and mutant phenotypes in zebrafish. Dev Cell. 2015;32:97–108. doi: 10.1016/j.devcel.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wong KS, Proulx K, Rost MS, Sumanas S. Identification of vasculature-specific genes by microarray analysis of Etsrp/Etv2 overexpressing zebrafish embryos. Dev Dyn. 2009;238:1836–1850. doi: 10.1002/dvdy.21990. [DOI] [PubMed] [Google Scholar]
- 41.Kohli V, Schumacher JA, Desai SP, Rehn K, Sumanas S. Arterial and venous progenitors of the major axial vessels originate at distinct locations. Dev Cell. 2013;25:196–206. doi: 10.1016/j.devcel.2013.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rehn K, Wong KS, Balciunas D, Sumanas S. Zebrafish enhancer trap line recapitulates embryonic aquaporin 1a expression pattern in vascular endothelial cells. Int J Dev Biol. 2011;55:613–618. doi: 10.1387/ijdb.103249kp. [DOI] [PubMed] [Google Scholar]
- 43.Balciuniene J, Nagelberg D, Walsh KT, Camerota D, Georlette D, Biemar F, Bellipanni G, Balciunas D. Efficient disruption of zebrafish genes using a Gal4-containing gene trap. BMC Genomics. 2013;14:619. doi: 10.1186/1471-2164-14-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Balciuniene J, Balciunas D. Gene trapping using gal4 in zebrafish. J Vis Exp. 2013;(79):e50113. doi: 10.3791/50113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stainier DY, Weinstein BM, Detrich HW, 3rd, Zon LI, Fishman MC. Cloche, an early acting zebrafish gene, is required by both the endothelial and hematopoietic lineages. Development. 1995;121:3141–3150. doi: 10.1242/dev.121.10.3141. [DOI] [PubMed] [Google Scholar]
- 46.Sumanas S, Jorniak T, Lin S. Identification of novel vascular endothelial-specific genes by the microarray analysis of the zebrafish cloche mutants. Blood. 2005;106:534–541. doi: 10.1182/blood-2004-12-4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Villefranc JA, Amigo J, Lawson ND. Gateway compatible vectors for analysis of gene function in the zebrafish. Dev Dyn. 2007;236:3077–3087. doi: 10.1002/dvdy.21354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moore JC, Sheppard-Tindell S, Shestopalov IA, Yamazoe S, Chen JK, Lawson ND. Post-transcriptional mechanisms contribute to Etv2 repression during vascular development. Dev Biol. 2013;384:128–140. doi: 10.1016/j.ydbio.2013.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yi H, Fujimura Y, Ouchida M, Prasad DD, Rao VN, Reddy ES. Inhibition of apoptosis by normal and aberrant Fli-1 and erg proteins involved in human solid tumors and leukemias. Oncogene. 1997;14:1259–1268. doi: 10.1038/sj.onc.1201099. [DOI] [PubMed] [Google Scholar]
- 50.Sevilla L, Zaldumbide A, Carlotti F, Dayem MA, Pognonec P, Boulukos KE. Bcl-XL expression correlates with primary macrophage differentiation, activation of functional competence, and survival and results from synergistic transcriptional activation by Ets2 and PU. 1. J Biol Chem. 2001;276:17800–17807. doi: 10.1074/jbc.M008270200. [DOI] [PubMed] [Google Scholar]
- 51.Gomez G, Lee JH, Veldman MB, Lu J, Xiao X, Lin S. Identification of vascular and hematopoietic genes downstream of etsrp by deep sequencing in zebrafish. PLoS One. 2012;7:e31658. doi: 10.1371/journal.pone.0031658. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


