Abstract
Brain-computer interfaces (BCIs) might restore communication to people severely disabled by amyotrophic lateral sclerosis (ALS) or other disorders. We sought to: 1) define a protocol for determining whether a person with ALS can use a visual P300-based BCI; 2) determine what proportion of this population can use the BCI; and 3) identify factors affecting BCI performance. Twenty-five individuals with ALS completed an evaluation protocol using a standard 6 × 6 matrix and parameters selected by stepwise linear discrimination. With an 8-channel EEG montage, the subjects fell into two groups in BCI accuracy (chance accuracy 3%). Seventeen averaged 92 (± 3)% (range 71–100%), which is adequate for communication (G70 group). Eight averaged 12 (± 6)% (range 0–36%), inadequate for communication (L40 subject group). Performance did not correlate with disability: 11/17 (65%) of G70 subjects were severely disabled (i.e. ALSFRS-R < 5). All L40 subjects had visual impairments (e.g. nystagmus, diplopia, ptosis). P300 was larger and more anterior in G70 subjects. A 16-channel montage did not significantly improve accuracy. In conclusion, most people severely disabled by ALS could use a visual P300-based BCI for communication. In those who could not, visual impairment was the principal obstacle. For these individuals, auditory P300-based BCIs might be effective.
Keywords: Amyotrophic lateral sclerosis (ALS), brain-computer interface (BCI), augmentative and alternative communication (AAC), event-related potentials (ERP), rehabilitation, P300
Introduction
As amyotrophic lateral sclerosis (ALS) progresses, communication becomes increasingly difficult; and consequently a person may progress through a variety of assistive devices (e.g. speech output keyboards, single switch scanning, eye-gaze) (1). All assistive communication technologies require some form of muscle control. In the late stages of the disease, communication that does not depend on muscle control may become the only viable option. Brain-computer interfaces (BCIs) translate brain signals into new outputs that replace, restore, enhance, supplement, or improve central nervous system (CNS) function (2). Thus, they might be used to restore simple communication to people severely disabled by ALS.
To date, most people with ALS have not elected to accept mechanical ventilation as the disease progresses, and thus the numbers who might benefit from BCI-based communication have been small. However, the new availability and effectiveness of mechanical ventilation (3–5), the growing evidence that people with late-stage ALS can enjoy a reasonable quality of life (6,7), and the high impact of communication on quality of life (8) have increased the need for and the value of BCIs that can restore communication ability. People who accept ventilation usually have longer life spans and thus need communication systems longer. BCI technology might provide a longer-term solution for these individuals when other devices (i.e. eye-gaze systems) are no longer reliable (9). BCI could be introduced at any time and could thus help to ensure communication and control throughout the course of disease.
Over the past two decades, both non-invasive and invasive BCI systems have been explored as possible means to provide communication and control to severely disabled people (10). For example, electroencephalographic (EEG) activity recorded from the scalp can measure P300 evoked potentials (11), sensorimotor rhythms (SMR) (12), and slow cortical potentials (SCP) (13). Other non-invasive BCIs use magnetoencephalography (MEG) (14) and functional magneto-resonance imaging (fMRI) (15). Invasive methods include electrocorticography (ECoG) in which signals are recorded from electrode arrays on the cortical surface (16), and recording of local field potentials (LFPs) or single-neuron activity by microelectrode arrays inserted into the cortex (17). At the same time, very few studies have explored BCI capability in the population that needs this technology, people with severe neuromuscular disabilities such as late-stage ALS (18).
Use of the P300 evoked potential (also called the oddball response) as the basis for a BCI was initially demonstrated by Farwell and Donchin (11). Since this original description, P300-based BCIs have been explored in the laboratory by many different research groups (reviewed in (19)). For example, in the P300 BCI speller, a 6 × 6 matrix containing 36 characters is displayed on a screen and the user attends to a desired character (the target) while the characters flash randomly. About 300 ms after the desired character flashes, a positive deflection occurs in the EEG. This is the P300 event-related potential (ERP). By flashing each character a number of times, and averaging the EEG following each one, the BCI can usually identify the desired character (i.e. the character that evokes a P300 ERP). A P300 BCI requires minimal user training, and can provide a capacity for slow but reliable communication to most people.
P300-based BCI usage has been explored in small numbers of people with ALS or other severe neuromuscular disabilities (9,20–24). These initial studies indicate that P300 BCIs might be useful to people with ALS for communication and control. In order to help to realize this potential benefit, the present study sought: 1) to define an effective and efficient standard protocol for determining whether a person severely disabled by ALS can use a P300-based BCI for basic communication; 2) to determine what proportion of this population can use this BCI for communication; and 3) to identify the factors that may impair or prevent effective BCI use. The results validate a useful standard protocol, indicate that most people severely disabled by ALS can use the BCI, and identify factors that prevent use. Thus, they contribute significantly to the clinical dissemination of BCIs to serve people with severe neuromuscular disabilities.
Methods
Subjects
The 25 subjects (19 males and 6 females) were referred for an initial evaluation with the Wadsworth (P300-based) BCI-Home system by the Center for Rehabilitation Technology (CRT) at Helen Hayes Rehabilitation Hospital (West Haverstraw, New York) or were self-referred. The study was reviewed and approved by the Institutional Review Board of Helen Hayes Hospital. Evaluation took place in the subject’s home (n = 23) or the CRT (n = 2). The inclusion criteria were those of Vaughan et al. (10): diagnosis of ALS (referred by medical professionals with a probable or definite diagnosis); little or no remaining useful motor control; dissatisfaction with current communication device; stable physical and social environment; caregiver and/or family support for participation; desire to live (e.g. on mechanical ventilation or planning to adopt it when needed) and to retain useful communication; medically stable; an interest and willingness to use BCI technology; ability to provide reliable “yes” and “no” responses. Informed consent was obtained from each subject in the following manner: the investigator learned from the caregiver or a family member the individual’s method of providing a yes/no response; the consent form was either read by or to the subject; and the subject was asked five questions verifying that she/he understood the consent, had had all questions answered, and agreed to participate. The mean age of the subjects was 55.8 (± 8.6 SD, range 41–72) years; their mean ALS Functional Rating Scale-Revised (ALSFRS-R) (25) score was 6.2 (± 8.1 SD, range 0–25). Eighteen (72%) were 100% ventilator dependent (scores < 10); five had scores of 13–18; and two had scores in the 20s. The inclusion of many people who were extremely disabled is a distinctive feature of this study.
Evaluation protocol
All aspects of BCI operation and data collection were controlled by the BCI2000 software platform (26) running on a Lenovo T61 laptop (Intel Core2 Duo CPU, 2 Ghz, 1.9 GB of RAM, Windows XP SP3). The user viewed a separate 50-cm Dell monitor at a distance of about 1 m.
Subject task
The subject sat in his/her own wheelchair or bed or in a comfortable chair facing the monitor. The monitor was either on a rolling stand or an over-the-bed table; and its position was adjusted appropriately for each user. The evaluation consisted of nine runs. Each run represented one word (i.e. The/quick/brown/fox/jumps/over/the/lazy/dog), for a total of 35 characters (trials) that included every character in the English alphabet. For each trial, the subject was asked to pay attention to the target character and to count the number of times it flashed. These instructions were carefully explained and illustrated; and the first run did not begin until the subject indicated full understanding of the task. After each run, each subject was asked if she/he wished to continue. The entire evaluation, including consenting process, electrode cap application, task instructions, nine runs, and cap removal occupied 60–90 min.
Stimuli presentation
The user’s monitor (Figure 1) displayed 36 items (i.e. English characters and numbers) arranged in a 6 × 6 matrix. The items were light gray and the background was black. In the evaluation protocol, the ‘text-to-spell’ bar above the matrix displayed the word to be spelled. At the beginning, the words ‘Waiting to start’ were displayed over the matrix and the first target (i.e. the first letter of the word to be spelled) was shown in parenthesis at the end of the word. For example, in Figure 1 the target is the letter ‘B’. After 4 s, items began to flash in groups of 4–6 items (i.e. either a scattered set of items with no two items adjacent (checkerboard (CB) format; 21 subjects) or a single row or column (RC format; 4 subjects)) (11,22). For each letter selection (i.e. referred to as a single trial), the groups flashed in a random order (at a rate of 5.3 Hz for the RC format and 4 Hz for the CB format) until each target had flashed 10–30 times (depending on the subject). Each trial was followed by a 4-s pause during which the matrix items did not flash and the next letter in the word to be spelled was displayed in parenthesis in the ‘text-to-spell’ bar. When each letter of the word had served as the target item, the phrase ‘Time Out’ was displayed and the run was over. After several minutes, the next run began.
Figure 1.
A subject wearing an electrode cap (Electro-Cap International, Inc.) attends to the 6 × 6 matrix of items (i.e. letters and numbers) displayed on a 20” monitor at his bedside. Lower left inset: the 6 × 6 matrix and the gray ‘text-to-spell’ bar showing the word to be spelled (‘BROWN’) and the target letter for the first trial, the letter ‘B’, in parentheses at the end of the word. In this example, a group of four items is flashing according the checkerboard (CB) format (22). To encourage attention to the target item, the subject is asked to count the number of times it flashed. Lower right inset: the 16-channel electrode montage and the standard 8-channel subset (marked with ‘X’s).
Electroencephalography (EEG)
A 16-channel electrode cap (Electro-Cap International, Inc.) with tin electrodes at locations F3, Fz, F4, T7, C3, Cz, C4, T8, CP3, CP4, P3, Pz, P4, PO7, PO8 and Oz (Expanded 10–20 System (27) (Figure 1, right-lower inset)) was used for data collection. EEG was referenced to the right mastoid and grounded to the left mastoid. Signals were amplified using a Guger Technologies 16-channel g. USBamp biosignal amplifier. Signals were sampled at a rate of 256 Hz, high-pass filtered at 0.5 Hz, and low-pass filtered at 30 Hz. Within the practical limitations of working in the home environment with extremely disabled, often bedridden patients, every effort was made to keep electrode impedances as low as possible, generally below 20 kΩ. (Kappenman and Luck found minimal impedance-related attenuation of P300 with a 0.5-Hz high-pass filter. (28)) The noisy home environment and the close proximity of life support equipment often necessitated use of the 58–62-Hz notch filter to reduce 60-Hz line noise. To evaluate the impact of the recording conditions, later offline assessment by an investigator blind to the evaluation results gave the EEG traces of each subject a Record Quality Score (RQS) of 1–4 (1: Poor (i.e. frequent large physiological and/or mechanical artifacts (e.g. 60-Hz noise, sudden voltage shifts, EKG activity)); 2: Acceptable (i.e. frequent minor mechanical and/or physiological artifacts); 3: Good (i.e. few artifacts); 4: Excellent (i.e. no artifacts)) (based on (29)).
Data analysis
A stepwise linear discriminant function (SWLDA) (30) was used to select and weight the EEG features (i.e. voltages at specific EEG electrode locations in specific time-periods in the 800 ms after the matrix flashed) that were used to classify the subject’s response to each item and to thereby determine which item was the target (i.e. the desired selection) (see (30) for full description of analysis). SWLDA was used to derive features from the entire 16-electrode montage and from the 8-electrode subset shown in Figure 1. The results for the 8-electrode and 16-electrode montages were compared.
Using the data of the first five runs (21 characters), we developed SWLDA classifier weights with the 8-channel subset. We then applied them online during the last four runs of data collection (14 characters) to determine online accuracy for each subject. We used the data from all nine runs (i.e. 35 trials) to compute for each electrode of each subject the average response to the target item. Typically, the response was dominated by a positive peak at 250–500 ms (P300) and a later negative peak (LN) at 420–680 ms.
We examined the impact of the severity of the subject’s disability (assessed by ALSFRS-R score) on BCI accuracy and on P300 and LN amplitudes and latencies. We also examined the impact on BCI accuracy of: subject age; EEG montage (8- or 16-channel); EEG record quality score (RQS); number of flashes for each target item; and the number of trials used to calculate the SWLDA parameters (i.e. EEG features and their weights). Finally, we compared subjects with high BCI accuracy (> 70%) to those with low BCI accuracy (< 40%) in regard to P300 and LN amplitudes, latencies, and scalp topographies. Most measures were not normally distributed (31), and thus non-parametric statistical methods were used (32). A t-test was used for measures that were normally distributed.
Results
BCI performance
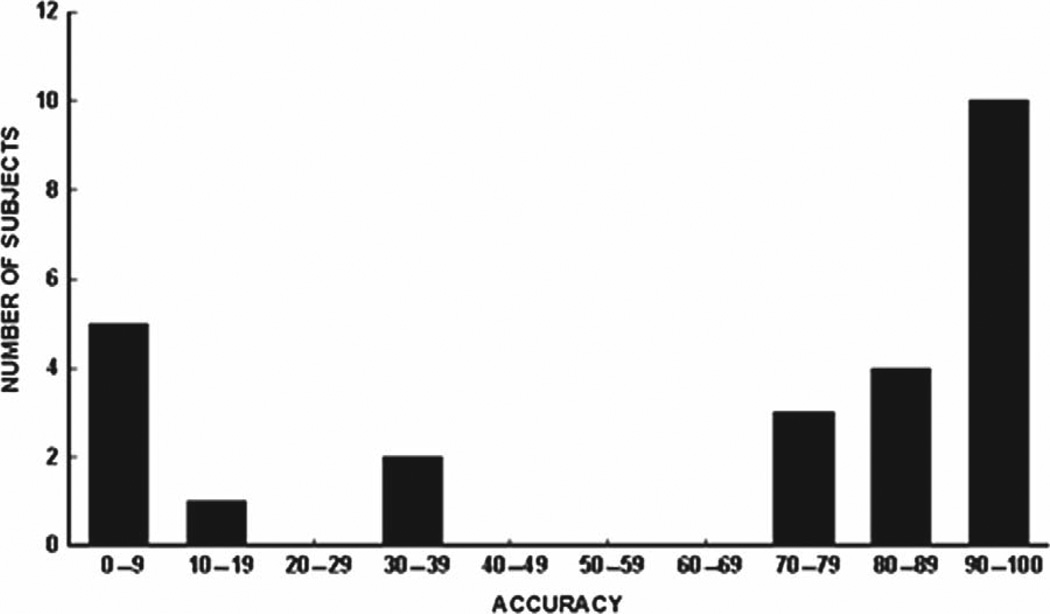
Figure 2 shows the BCI accuracies for all 25 subjects. Chance performance is 2.8% (i.e. 1/36). Subject accuracies fall into two distinct groups. Seventeen subjects had accuracies above 70% (average 92.1 ± 2.5% SE, range 71–100%). The other eight subjects had accuracies below 40% (average 12.3 ± 6.2% SE, range 0–36%). The accuracies of the first group (labeled the G70 (i.e. > 70%) group) were high enough to support successful communication, while the accuracies of the second group (labeled the L40 (i.e. < 40%) group) were not (33).
Figure 2.
BCI accuracy for all subjects (chance accuracy 2.8%). Two groups are apparent: those with accuracy >70% (the G70 group) and those with accuracy < 40% (the L40 group).
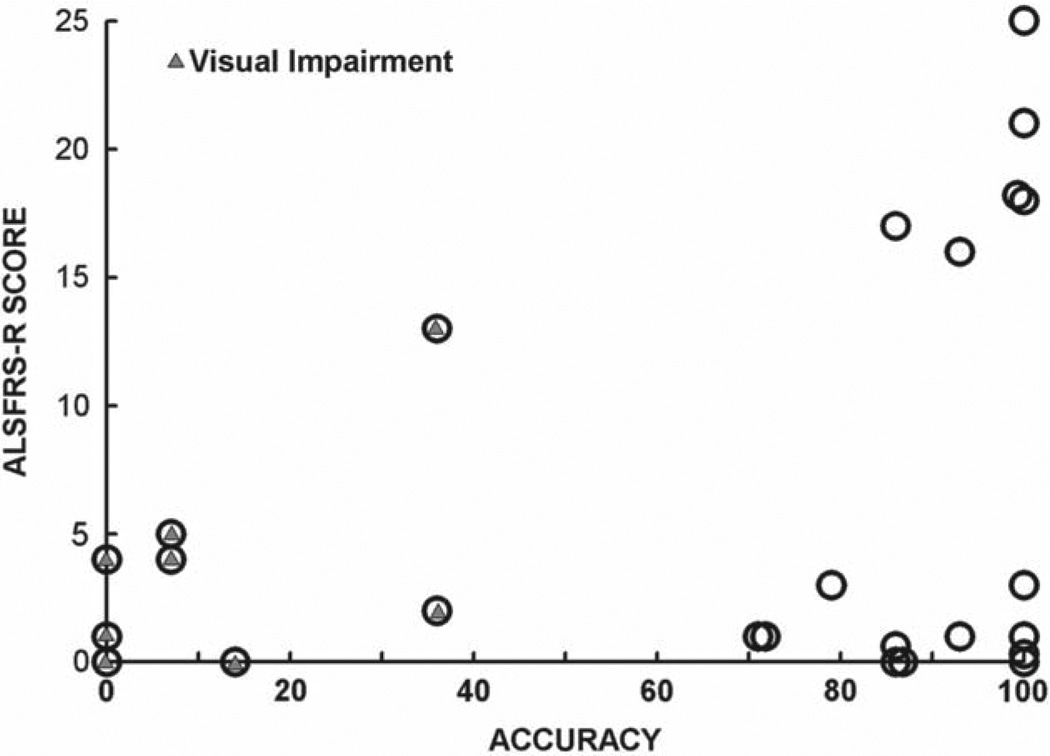
Figure 3 displays subject accuracy vs. ALSFRS-R score. BCI accuracy is not correlated with ALS-FRS-R score (r = 0.12, p = 0.57). Furthermore, and most notably, many people who were very severely disabled (score 0–5), had high BCI accuracies, indicating that severe disability (including complete dependence on mechanical ventilation) does not in itself preclude effective use of the BCI-Home system. This finding confirms earlier results with smaller numbers of subjects with ALS (9,20–22).
Figure 3.
ALSFRS-R score versus BCI accuracy for all subjects. A triangle within the circle indicates the presence of visual impairment. All those with ALSFRS-R ≤ 5 were ventilator-dependent. It is clear that many who were severely disabled had high BCI accuracy, and that all those with low BCI accuracy had visual impairments.
The G70 and L40 subject groups did not differ significantly in age, ALSFRS-R score, or EEG record quality. The distinctive characteristic of the subjects in the L40 group was the presence in all eight of a visual impairment (e.g. diplopia, nystagmus, ptosis) that apparently prevented them from seeing the flashing items well enough to achieve high accuracy.
Characteristics of the target responses
The G70 and L40 groups differed significantly in their target ERPs (Table I). P300 amplitude averaged 3.7 µV (± 0.5 SE) in the G70 group and 1.8 µV (± 0.4 SE) in the L40 group (p = 0.008 by Mann-Whitney rank sum); and LN amplitude averaged −3.4 µV (± 0.6 SE) in the G70 group and −1.4 µV (± 0.4 SE) in the L40 group (p = 0.002). BCI accuracy was significantly correlated with the amplitude of each peak (r = 0.55 and −0.70 for P300 and LN, respectively). The two peak amplitudes were highly correlated with each other (r = − 0.73, p < 0.001). In addition, P300 amplitude and latency were negatively correlated (r = −0.41, p = 0.04), indicating that shorter latency was associated with higher amplitude. The shorter P300 latency in the G70 group than in the L40 group neared significance (p = 0.06). P300 and LN amplitudes and latencies did not correlate with ALSFRS-R score.
Table I.
Average amplitudes and latencies for the positive peak at 250–500 ms (P300) and the later negative peak (LN) at 420–680 ms for the G70 and L40 subject groups.
| P300 amplitude (µV) * |
P300 latency (ms) |
LN amplitude (µV) * |
LN latency (ms) |
|
|---|---|---|---|---|
| G70 group | 3.7 ± 0.5 SE | 288 ± 21 SE | − 3.4 ± 0.6 SE | 624 ± 23 SE |
| L40 group | 1.8 ± 0.4 SE | 364 ± 34 SE | − 1.4 ± 0.4 SE | 648 ± 36 SE |
(* indicates a significant difference (p < 0.01 by Mann-Whitney rank sum) between the G70 and L40 groups.).
The two groups also differed in the location of the maximum P300 amplitude. In the G70 group, P300 was largest at frontal or central locations in 14 of 17 subjects and at occipital or parietal locations in only three, whereas in the L40 group it was largest at frontal or central locations in only two of eight subjects and at occipital or parietal locations in six (p = 0.03 by Mann-Whitney rank sum).
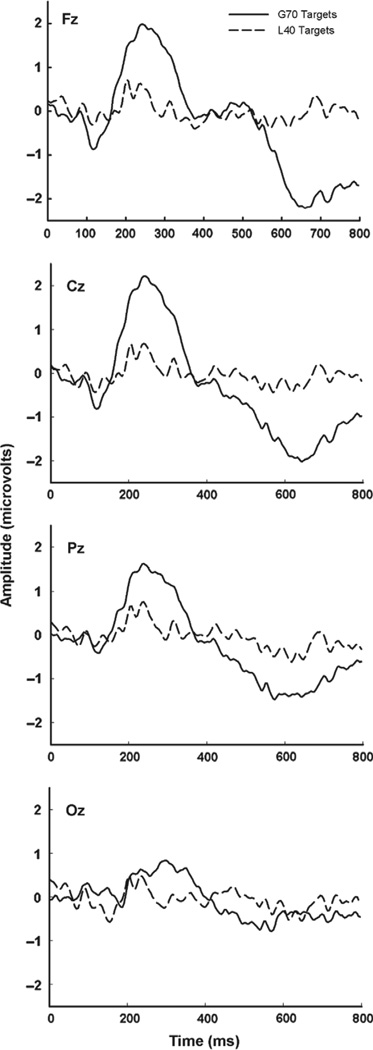
Figure 4 shows the average target responses at Fz, Cz, Pz, and Oz for the G70 and L40 groups. The greater peak amplitudes and shorter latencies of the G70 group are evident. The difference in maximum peak locations (i.e. more anterior in the G70 group and more posterior in the L40 group) is also apparent.
Figure 4.
Average ERPs to the target items at Fz, Cz, Pz, and Oz for the G70 group (solid line, n = 17) and the L40 Group (dashed line, n = 8). The G70 ERPs are larger and are focused more anteriorly than the L40 ERPs.
Alternative protocol parameters
In offline analyses, we investigated the effects of using: 1) the full 16-channel electrode montage rather than the standard 8-channel montage; 2) fewer numbers of trials to determine the SWLDA parameters; and 3) fewer flashes.
The 16-channel and 8-channel montage provided comparable accuracies. For the G70 group, 16-channel accuracy averaged 92.9 (± 2.7 SE)% (range 64–100%) and 8-channel accuracy averaged 92.1 (± 2.5 SE)% (range 71–100%). For the L40 group, 16-channel accuracy averaged 7.0 (± 3.0 SE)% (range 0–21%) and 8-channel accuracy averaged 12.3 (± 6.2 SE)% (range 0–36%). The difference was not significant for either group.
The accuracies presented so far were achieved using SWLDA parameters calculated from five runs comprising a total of 21 trials. In further offline analyses, we determined the accuracies provided by SWLDA parameters calculated from 4, 3, 2, or 1 runs (comprising 16, 13, 8, or 3 trials, respectively). Table II shows the average accuracies for the G70 group and the number of subjects versus the number of trials used to calculate those parameters. Accuracy declines as the number of trials used declines. Most importantly, when the number of trials is reduced from 21 to 16, two of the 17 subjects who previously achieved accuracies >70% no longer do so; and when the number of trials is reduced to eight, five subjects no longer reach 70%. Thus, it appears that 21 trials were necessary. On the other hand, the relatively modest reductions in accuracy produced by reducing the number of trials used, and the fact that the other eight subjects had accuracies below 40%, suggests that using more than 21 trials would not have enabled any of the L40 subjects to achieve accuracies that would support BCI use. The five runs (21 trials) used appear to be both necessary and sufficient.
Table II.
BCI accuracy and the number of subjects in the G70 group versus the number of trials used to parameterize the SWLDA algorithm for the 8-channel montage.
| Trials | 21 | 16 | 13 | 8 | 3 |
|---|---|---|---|---|---|
| Average accuracy | 94.2% | 92.5% | 89.5% | 92.8% | 74.1% |
| # Subjects > 70% | 17 | 15 | 15 | 12 | 12 |
Finally, offline analyses determined for each subject and each montage the minimum number of flashes to provide maximum accuracy. The notable aspect of the results was that 16 of the 17 G70 subjects (8-channel montage) and all of the G70 subjects (16-channel montage) achieved their maximum accuracy with fewer flashes than actually presented (Table III). This reduction could markedly reduce trial time and thus substantially increase the communication rate for people using this system.
Table III.
Online accuracies (8-channel) and maximum accuracy at the least number of flashes for the G70 group determined in offline analysis of the 8- and 16-channel montages.
| Subject Number |
Online 8-channel accuracy |
Offline 16-channel accuracy |
Online Flashes |
Offline Maximum Accuracies and Optimal Flashes | |||
|---|---|---|---|---|---|---|---|
| 8-channel accuracy |
8-channel optimal flashes |
16-channel accuracy |
16-channel optimal flashes |
||||
| G70 Group | |||||||
| 1 | 100 | 100 | 14 | 100 | 6 | 100 | 6 |
| 2 | 100 | 100 | 20 | 100 | 8 | 100 | 8 |
| 3 | 100 | 100 | 20 | 100 | 6 | 100 | 4 |
| 4 | 100 | 100 | 24 | 100 | 12 | 100 | 12 |
| 5 | 100 | 100 | 14 | 100 | 12 | 100 | 10 |
| 6 | 100 | 100 | 20 | 100 | 8 | 100 | 6 |
| 7 | 100 | 100 | 20 | 100 | 8 | 100 | 8 |
| 8 | 100 | 100 | 14 | 100 | 8 | 100 | 10 |
| 9 | 86 | 93 | 14 | 86 | 12 | 93 | 10 |
| 10 | 93 | 100 | 20 | 93 | 10 | 100 | 12 |
| 11 | 86 | 93 | 20 | 86 | 20 | 93 | 18 |
| 12 | 93 | 100 | 30 | 100 | 20 | 100 | 10 |
| 13 | 86 | 86 | 20 | 100 | 6 | 100 | 8 |
| 14 | 86 | 93 | 24 | 93 | 12 | 93 | 12 |
| 15 | 79 | 79 | 24 | 79 | 14 | 93 | 22 |
| 16 | 71 | 64 | 24 | 79 | 22 | 64 | 22 |
| 17 | 71 | 71 | 30 | 86 | 16 | 86 | 18 |
| Mean (SE) | 92% (± 2) | 93% (± 3) | 21 (± 1) | 94% (± 2) | 12 (± 1) | 95% (± 2) | 11 (± 1) |
Discussion
People with ALS are living longer and with better quality of life due in large part to earlier and improved respiratory support. The P300-based BCI described here could provide communication for individuals with ALS and other motor disorders when conventional devices are no longer suitable. Several studies have explored P300-based BCI communication in small numbers of people with ALS and have demonstrated stable performance over time (21,34). In this study, we sought to determine: 1) what proportion of people with advanced ALS (mean ALSFRS-R = 6) can use the P300-based Wadsworth BCI-Home system; 2) what factors correlate with BCI performance; and 3) the key requirements for an efficient and reliable effective protocol with which to evaluate these individuals for BCI use.
We evaluated the ability of 25 people severely disabled by ALS to use the BCI. Seventy-two percent were already completely ventilator dependent and others were sufficiently disabled to be considering ventilator use. This is the largest such group that has been systematically evaluated for BCI use to date. The preponderance of males in this group (76%) is consistent with the male preponderance among those with ALS who are ventilator-dependent (35).
The results indicate that the majority (68%) of the subjects could use the BCI with accuracy sufficient for basic communication (i.e. the G70 group). Visual impairments (i.e. ptosis, diplopia, nystagmus) were present in those for whom BCI performance was not adequate (i.e. the L40 group) and appeared to explain the poor performance. This strong correlation between poor BCI performance and visual impairments in people with advanced ALS is an important finding, especially since oculomotor function is generally believed to be preserved in these individuals (36,37). Whether continued disease progression and longer periods of ventilator dependence impair BCI performance in more people is unclear. Tracking these metrics would aid in determining the potential value of auditory or tactile BCIs (38,39) and in deciding when they should be provided. To address these questions, future studies should examine the impact on visual function and BCI performance of the length of time since diagnosis and/or since ventilator dependency.
All the subjects were able to provide informed consent and to participate appropriately in the BCI protocol, indicating that their cognition was not severely impaired. Nevertheless, recent studies strongly suggest that some may have had cognitive deficits (40). Future studies should assess the impact of such deficits on BCI performance.
In this extremely disabled population, the amplitudes of the target ERPs were positively correlated with BCI accuracy. In a less disabled group of ALS subjects (mean ALSFRS-R = 32), Silvoni et al. (41) found that their relatively small P300 amplitudes (at Pz) (6.3 µV vs. 10.5 µV in healthy control subjects) did not affect performance in a cursor movement task. In the present study, P300 amplitude (measured from zero-baseline) was much smaller, averaging only 3.7 µV in the G70 group and 1.8 µV in the L40 group. These small amplitudes may in part reflect disease progression, but the results indicate that the decrease does not necessarily degrade BCI performance. The significant difference in the amplitudes of both the negative and positive peaks between the G70 and L40 groups most likely resulted from the latter’s inability to see the flashing items clearly enough to generate a classifiable response. While this suggests that P300 amplitude might be used as a tool to evaluate an individual’s ability to use the BCI, seven of the 17 subjects in the G70 group had P300 amplitudes < 2.5 µV. Thus, small P300 amplitude is not in itself a bar to BCI use. The scalp location of the maximum P300 amplitude might be a more reliable indicator: the maximum was at frontal-central locations for almost all the G70 subjects and at parietal-occipital locations for almost all the L40 subjects. In addition, P300 latency was < 300 ms in 12 of 17 in the G70 group and > 320 ms in six of eight in the L40 group.
Offline analyses of the present results indicate that the 16-channel montage was not necessary; the 8-channel montage provided essentially the same results both overall and in terms of the individual subjects. The two montages yielded the same G70 and L40 groups, and thus the simpler one could be used in the evaluation protocol. On the other hand, analysis also indicated that the number of trials used for parameterizing the SWLDA algorithm could not safely be reduced below the 21 used here. Such reductions would have removed several subjects from the G70 group. Although using more than 21 trials for parameterization was not evaluated, the results also strongly suggested that more trials would not have enabled any of the L40 subjects to move into the G70 group.
For the immediate future at least, a second evaluation session would be worthwhile. In addition to enabling assessment of the effect of having more trials for parameterization, a second session would compensate to some degree for the often unpredictable and uncontrollable day-to-day variations in subject state, home environment, and other factors. In addition, the adoption of a simple pre-evaluation questionnaire concerning the subject’s visual and auditory capabilities would be useful.
For the subjects unable to use the BCI due to visual impairments (i.e. the L40 group), simple measures such as ptosis-glasses (42), or an eye-patch (for diplopia) may be effective. BCIs that use non-visual (i.e. auditory or somatosensory) stimuli are obviously a more general solution. Several promising auditory BCI paradigms, and an eyes-closed steady-state visual evoked potential (SSVEP) BCI, have been described (43,44). With further development, they may become clinically useful. The cost of the BCI system described here is similar to currently available eye-gaze devices (45).
This study indicates that most people extremely disabled by ALS can still use a P300-based BCI system. Several subjects could no longer use their eye-gaze devices, and many wanted to be tested with the BCI to determine if it was a possible means of communication for them in the future. Some individuals with ALS who can no longer use an eye-gaze device have already benefited from BCI technology (9); and others, such as those with spinal muscle atrophy, severe cerebral palsy, or high-level spinal cord injury, may also find BCI applications valuable (46).
Small P300 amplitudes did not preclude adequate BCI performance. Adequate performance was associated with frontal-central P300 locations. An 8-channel EEG montage and the 60–90 min protocol described here appears sufficient for reliable evaluation of BCI performance. At the same time, the addition of a second similar session on a subsequent day is probably worthwhile to control for variations in subject state or environmental factors. For those with visual impairments, BCIs that use non-visual (e.g. auditory) stimuli may provide a solution.
Acknowledgments
We are extremely grateful to the subjects and their caregivers for their time and effort. We also thank Laura Tenteromano and Rafi Kupferman of Helen Hayes Hospital for their contributions.
Funding was provided by the NIH, NIBIB & NINDS and the James S. McDonnell, Altran, ALS Hope, and NEC Foundations. None had any role in this study or in this article.
Footnotes
Declaration of interest: At the time of data collection, data analysis, and manuscript preparation, none of the authors had any relationship that could potentially bias the results presented here. The authors alone are responsible for the content and writing of the paper.
References
- 1.Doyle M, Phillips B. Trends in Augmentative and Alternative Communication Use by Individuals with Amyotrophic Lateral Sclerosis. Augmentative and Alternative Communication. 2001;17:167–178. [Google Scholar]
- 2.Wolpaw JR, Wolpaw EW. Brain-Computer Interfaces: Something New Under the Sun. New York, NY: Oxford University Press; 2012. [Google Scholar]
- 3.Heinman-Patterson T, Aboussouan LS. Respiratory Care in Amyotrophic Lateral Sclerosis. In: Mitsumoto H, Przedborski S, Gordon PH, editors. Amyotrophic Lateral Sclerosis. New York, NY: Taylor & Francis Group; 2006. pp. 737–755. [Google Scholar]
- 4.Rabkin JG, Albert SM, Tider T, Del Bene ML, O’Sullivan I, Rowland LP, et al. Predictors and course of elective long-term mechanical ventilation: a prospective study of ALS patients. Amyotroph Lateral Scler. 2006;7:86–95. doi: 10.1080/14660820500515021. [DOI] [PubMed] [Google Scholar]
- 5.Miller RG, Anderson F, Brooks BR, Mitsumoto H, Bradley WG, Ringel SP, et al. Outcomes research in amyotrophic lateral sclerosis: lessons learned from the amyotrophic lateral sclerosis clinical assessment, research, and education database. Annals of Neurology. 2009;65(Suppl 1):S24–S28. doi: 10.1002/ana.21556. [DOI] [PubMed] [Google Scholar]
- 6.Lule D, Pauli S, Altintas E, Singer U, Merk T, Uttner I, et al. Emotional adjustment in amyotrophic lateral sclerosis (ALS) Journal of Neurology. 2012;259:334–341. doi: 10.1007/s00415-011-6191-x. [DOI] [PubMed] [Google Scholar]
- 7.Simmons Z. Rehabilitation of motor neuron disease. Handbook of Clinical Neurology. 2013;110:483–498. doi: 10.1016/B978-0-444-52901-5.00041-1. [DOI] [PubMed] [Google Scholar]
- 8.Korner S, Sieniawski M, Kollewe K, Rath KJ, Krampfl K, Zapf A, et al. Speech therapy and communication device: impact on quality of life and mood in patients with amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener. 2013;14:20–25. doi: 10.3109/17482968.2012.692382. [DOI] [PubMed] [Google Scholar]
- 9.Sellers EW, Vaughan TM, Wolpaw JR. A brain-computer interface for long-term independent home use. Amyotrophic lateral sclerosis. 2010;11:449–455. doi: 10.3109/17482961003777470. [DOI] [PubMed] [Google Scholar]
- 10.Vaughan TM, McFarland DJ, Schalk G, Sarnacki WA, Krusienski DJ, Sellers EW, et al. The Wadsworth BCI Research and Development Program: at home with BCI. IEEE transactions on neural systems and rehabilitation engineering. 2006;14:229–233. doi: 10.1109/TNSRE.2006.875577. [DOI] [PubMed] [Google Scholar]
- 11.Farwell LA, Donchin E. Talking off the top of your head: toward a mental prosthesis utilizing event-related brain potentials. Electroencephalography and Clinical Neurophysiology. 1988;70:510–523. doi: 10.1016/0013-4694(88)90149-6. [DOI] [PubMed] [Google Scholar]
- 12.Pfurtscheller G, McFarland DJ. BCIs That Use Sensorimotor Rhythms. In: Wolpaw JR, Wolpaw EW, editors. Brain-computer interfaces: principles and practice. New York, NY: Oxford University Press; 2012. pp. 227–240. [Google Scholar]
- 13.Birbaumer N, Ghanayim N, Hinterberger T, Iversen I, Kotchoubey B, Kubler A, et al. A spelling device for the paralysed. Nature. 1999;398:297–298. doi: 10.1038/18581. [DOI] [PubMed] [Google Scholar]
- 14.Sudre G, Parkkonen L, Bock E, Baillet S, Wang W, Weber DJ. rtMEG: a real-time software interface for magnetoencephalography. Computational Intelligence and Neuroscience. 2011;2011:327953. doi: 10.1155/2011/327953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sitaram R, Lee S, Birbaumer N. BCIs that use Brain Metabolic Signals. In: Wolpaw JR, Wolpaw EW, editors. Brain-computer interfaces: principles and practice. New York, NY: Oxford University Press; 2012. pp. 301–323. [Google Scholar]
- 16.Schalk G. BCIs that use Electrocortiographic Activity. In: Wolpaw JR, Wolpaw EW, editors. Brain-computer interfaces: principles and practice. New York, NY: Oxford University Press; 2012. pp. 251–264. [Google Scholar]
- 17.Donoghue JP. BCIs that use Brain Signals Recorded in the Motor Cortex. In: Wolpaw JR, Wolpaw EW, editors. Brain-computer interfaces: principles and practice. New York, NY: Oxford University Press; 2012. pp. 265–288. [Google Scholar]
- 18.Mak JN, Arbel Y, Minett JW, McCane LM, Yuksel B, Ryan D, et al. Optimizing the P300-based brain-computer interface: current status, limitations and future directions. Journal of Neural Engineering. 2011;8:025003. doi: 10.1088/1741-2560/8/2/025003. [DOI] [PubMed] [Google Scholar]
- 19.Sellers EW, Arbel Y, Donchin E. BCIs that use P300 Event-Realted Potentials. In: Wolpaw JR, Wolpaw EW, editors. Brain-computer interfaces: principles and practice. New York, NY: Oxford University Press; 2012. pp. 215–226. [Google Scholar]
- 20.Nijboer F, Sellers EW, Mellinger J, Jordan MA, Matuz T, Furdea A, et al. A P300-based brain-computer interface for people with amyotrophic lateral sclerosis. Clinical neurophysiology: official journal of the International Federation of Clinical Neurophysiology. 2008;119:1909–1916. doi: 10.1016/j.clinph.2008.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sellers EW, Donchin E. A P300-based brain-computer interface: initial tests by ALS patients. Clinical Neurophysiology. 2006;117:538–548. doi: 10.1016/j.clinph.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 22.Townsend G, LaPallo BK, Boulay CB, Krusienski DJ, Frye GE, Hauser CK, et al. A novel P300-based brain-computer interface stimulus presentation paradigm: moving beyond rows and columns. Clinical Neurophysiology. 2010;121:1109–1120. doi: 10.1016/j.clinph.2010.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Piccione F, Giorgi F, Tonin P, Priftis K, Giove S, Silvoni S, et al. P300-based brain computer interface: reliability and performance in healthy and paralysed participants. Clinical Neurophysiology. 2006;117:531–537. doi: 10.1016/j.clinph.2005.07.024. [DOI] [PubMed] [Google Scholar]
- 24.Hoffmann U, Vesin JM, Ebrahimi T, Diserens K. An efficient P300-based brain-computer interface for disabled subjects. Journal of Neuroscience Methods. 2008;167:115–125. doi: 10.1016/j.jneumeth.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 25.Cedarbaum JM, Stambler N, Malta E, Fuller C, Hilt D, Thurmond B, et al. The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS Study Group (Phase III) Journal of the Neurological Sciences. 1999;169:13–21. doi: 10.1016/s0022-510x(99)00210-5. [DOI] [PubMed] [Google Scholar]
- 26.Schalk G, McFarland DJ, Hinterberger T, Birbaumer N, Wolpaw JR. BCI2000: a general purpose brain-computer interface (BCI) system. IEEE Transactions on Bio-medical Engineering. 2004;51:1034–1043. doi: 10.1109/TBME.2004.827072. [DOI] [PubMed] [Google Scholar]
- 27.Sharbrough F, Chatrian GE, Lesser RP, Luders H, Nuwer M, Picton TW. AEEGS Guidelines for Standard Electrode Position Nomenclature. Journal of Clinical Neurophysiology. 1991;8:200–202. [PubMed] [Google Scholar]
- 28.Kappenman ES, Luck SJ. The effects of electrode impedance on data quality and statistical significance in ERP recordings. Psychophysiology. 2010;47:888–904. doi: 10.1111/j.1469-8986.2010.01009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Portier F, Portmann A, Czernichow P, Vascaut L, Devin E, Benhamou D, et al. Evaluation of home versus laboratory polysomnography in the diagnosis of sleep apnea syndrome. American Journal of Respiratory and Critical Care Medicine. 2000;162:814–818. doi: 10.1164/ajrccm.162.3.9908002. [DOI] [PubMed] [Google Scholar]
- 30.Krusienski DJ, Sellers EW, McFarland DJ, Vaughan TM, Wolpaw JR. Toward enhanced P300 speller performance. Journal of Neuroscience Methods. 2008;167:15–21. doi: 10.1016/j.jneumeth.2007.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Razali N, Wah Y. Power Comparisons of Shapiro-Wilk, Kolmogorov-Smirnov, Lilliefors and Anderson-Darling tests. Journal of Statistical Modeling and Analytics. 2011;2:21–33. [Google Scholar]
- 32.Siegel S. Non-parametric Statistics. The American Statistician. 1957;11:13–19. [Google Scholar]
- 33.Sellers EW, Krusienski DJ, McFarland DJ, Vaughan TM, Wolpaw JR. A P300 event-related potential brain-computer interface (BCI): the effects of matrix size and inter-stimulus interval on performance. Biological Psychology. 2006;73:242–252. doi: 10.1016/j.biopsycho.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 34.Silvoni S, Cavinato M, Volpato C, Ruf CA, Birbaumer N, Piccione F. Amyotrophic lateral sclerosis progression and stability of brain-computer interface communication. Amyotroph Lateral Scler Frontotemporal Degener. 2013;14:390–396. doi: 10.3109/21678421.2013.770029. [DOI] [PubMed] [Google Scholar]
- 35.Tollefsen E, Midgren B, Bakke P, Fondenes O. Amyotrophic lateral sclerosis: gender differences in the use of mechanical ventilation. European Journal of Neurology. 2010;17:1352–1357. doi: 10.1111/j.1468-1331.2010.03036.x. [DOI] [PubMed] [Google Scholar]
- 36.Brockington A, Ning K, Heath PR, Wood E, Kirby J, Fusi N, et al. Unravelling the enigma of selective vulnerability in neurodegeneration: motor neurons resistant to degeneration in ALS show distinct gene expression characteristics and decreased susceptibility to excitotoxicity. Acta Neuropathologica. 2013;125:95–109. doi: 10.1007/s00401-012-1058-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hayashi H, Oppenheimer EA. ALS patients on TPPV: totally locked-in state, neurologic findings and ethical implications. Neurology. 2003;61:135–137. doi: 10.1212/01.wnl.0000069925.02052.1f. [DOI] [PubMed] [Google Scholar]
- 38.Kaufmann T, Holz EM, Kubler A. Comparison of tactile, auditory, and visual modality for brain-computer interface use: a case study with a patient in the locked-in state. Frontiers in Neuroscience. 2013;7:129. doi: 10.3389/fnins.2013.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hill NJ, Ricci E, Haider L, McCane LM, Heckman SM, Wolpaw JR, et al. A practical, intuitive brain-computer interface for communicating a “yes” or “no” by listening. Journal of Neural Engineering. 2013 doi: 10.1088/1741-2560/11/3/035003. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Raaphorst J, de Visser M, Linssen WH, de Haan RJ, Schmand B. The cognitive profile of amyotrophic lateral sclerosis: a meta-analysis. Amyotroph Lateral Scler. 2010;11:27–37. doi: 10.3109/17482960802645008. [DOI] [PubMed] [Google Scholar]
- 41.Silvoni S, Volpato C, Cavinato M, Marchetti M, Priftis K, Merico A, et al. P300-based Brain-Computer Interface Communication: Evaluation and Follow-up in Amyotrophic Lateral Sclerosis. Frontiers in Neuroscience. 2009;3:60. doi: 10.3389/neuro.20.001.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Walsh G, Rafferty PR, Lapin J. A simple new method for the construction of a ptosis crutch. Ophthalmic & Physiological Optics: the Journal of the British College of Ophthalmic Opticians. 2006;26:404–407. doi: 10.1111/j.1475-1313.2006.00388.x. [DOI] [PubMed] [Google Scholar]
- 43.Lim JH, Hwang HJ, Han CH, Im CH. Classification of binary intentions for individuals with impaired oculomotor funtion: ‘eyes-closed’ SSVEP based brain-computer interface (BCI) Journal of Neural Engineering. 2013;10:049501. doi: 10.1088/1741-2560/10/2/026021. [DOI] [PubMed] [Google Scholar]
- 44.Kubler A, Furdea A, Halder S, Hammer EM, Nijboer F, Kotchoubey B. A brain-computer interface controlled auditory event-related potential (p300) spelling system for locked-in patients. Annals of the New York Academy of Sciences. 2009;1157:90–100. doi: 10.1111/j.1749-6632.2008.04122.x. [DOI] [PubMed] [Google Scholar]
- 45.Augmentative Communication [Website] ALS Association Website: ALS Association; 2013. [cited 2013 September]; Available from: http://www.alsa.org. [Google Scholar]
- 46.Munssinger JI, Halder S, Kleih SC, Furdea A, Raco V, Hosle A, et al. Brain Painting: First Evaluation of a New Brain-Computer Interface Application with ALS Patients and Healthy Volunteers. Frontiers in Neuroscience. 2010;4:182. doi: 10.3389/fnins.2010.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]