Abstract
Background
Long-term exposure to particulate matter less than 2.5 μm in diameter (PM2.5) has been consistently associated with risk of all-cause mortality. The methods used to assess exposure, such as area averages, nearest monitor values, land use regressions, and spatio-temporal models in these studies are subject to measurement error. However, to date, no study has attempted to incorporate adjustment for measurement error into a long-term study of the effects of air pollution on mortality.
Methods
We followed 108,767 members of the Nurses’ Health Study (NHS) 2000–2006 and identified all deaths. Biennial mailed questionnaires provided a detailed residential address history and updated information on potential confounders. Time-varying average PM2.5 in the previous 12-months was assigned based on residential address and was predicted from either spatio-temporal prediction models or as concentrations measured at the nearest USEPA monitor. Information on the relationships of personal exposure to PM2.5 of ambient origin with spatio-temporal predicted and nearest monitor PM2.5 was available from five previous validation studies. Time-varying Cox proportional hazards models were used to estimate hazard ratios (HRs) and 95 percent confidence intervals (95%CI) for each 10 μg/m3 increase in PM2.5. Risk-set regression calibration was used to adjust estimates for measurement error.
Results
Increasing exposure to PM2.5 was associated with an increased risk of mortality, and results were similar regardless of the method chosen for exposure assessment. Specifically, the multivariable adjusted HRs for each 10 μg/m3 increase in 12-month average PM2.5 from spatio-temporal prediction models were 1.13 (95%CI:1.05, 1.22) and 1.12 (95%CI:1.05, 1.21) for concentrations at the nearest EPA monitoring location. Adjustment for measurement error increased the magnitude of the HRs 4-10% and led to wider CIs (HR = 1.18; 95%CI: 1.02, 1.36 for each 10 μg/m3 increase in PM2.5 from the spatio-temporal models and HR = 1.22; 95%CI: 1.02, 1.45 from the nearest monitor estimates).
Conclusions
These findings support the large body of literature on the adverse effects of PM2.5, and suggest that adjustment for measurement error be considered in future studies where possible.
Keywords: PM2.5, Measurement error, Mortality, Air pollution
Background
Long-term exposures to ambient particulate air pollution have been associated with an increased risk of all-cause mortality in a number of studies [1-14]. In a recent meta-analysis of cohort studies [6], each 10 μg/m3 increase in particulate matter 2.5 μm or less in aerodynamic diameter (PM2.5) was associated with a 6.2% (95% confidence interval 4.1-8.4%) increased risk of all-cause mortality. As noted in that review, the assessment of exposure has varied across studies from city level measures to central ambient monitoring locations to complex land-use regression or other spatial exposure models, all of which have the potential to induce substantial measurement error. These measurement errors have been shown to be both classical, leading to attenuation of the exposure-response association, as well as Berkson, leading to increases in the width of the confidence intervals, resulting in overall biased results [15-19]. Each exposure modeling approach is likely subject to a different blend of classical and Berkson errors, however, to date, no studies have incorporated measurement error corrections into a study of the effects of air pollution on mortality.
In occupational and nutritional epidemiology, regression calibration is a widely used method to adjust for exposure measurement error [20,21]. This calibration is usually applied when surrogate exposure data have been collected in the majority of the participants, and measurements of the “true” exposure of interest (which themselves are also collected with error) have only been collected in a limited subset or are only available from an external study. Previous methods to incorporate calibration factors required that the exposures be time invariant; however, a more recent risk set regression calibration approach (RRC) now allows for the calibration of time-varying exposures in Cox proportional hazards models [22]. This new method makes it possible to utilize regression calibration in long-term studies of chronic exposure to air pollution.
In a previous analysis in a subset of members of the prospective Nurses’ Health Study living in the Northeastern and Midwestern US between 1992 and 2002, we observed increases in all-cause mortality, a multivariable adjusted hazard ratio (HR) of 1.26 (95% confidence interval: 1.02-1.54) for each 10 μg/m3 increase in 12-month average PM2.5 [2]. Our current objectives are to expand these analyses spatially to include the full nationwide cohort, incorporate additional follow-up, examine the impact of different methods of obtaining exposure estimates, and to apply RRC to adjust the obtained effect estimates for measurement error. We anticipated that PM2.5 exposures would be positively associated with all-cause mortality risk, that there would be differences in the magnitude and precision of the effect estimates from the two exposure modeling approaches, and that the magnitude of the effects would increase and the precision of the estimates would decrease after adjustment for exposure measurement error. To the best of our knowledge, this will be the first analyses to be able to adjust long-term PM2.5 and all-cause mortality effect estimates for measurement error, and will provide a framework for this method of adjustment for future studies.
Methods
Study population and assessment of outcome
The Nurses’ Health Study (NHS) is a long-term prospective cohort study of US female nurses. The cohort was initiated in 1976 when 121,700 married female US registered nurses, 30 to 55 years old, completed a mailed questionnaire and provided informed consent. At the study inception the nurses resided in eleven states; however, there is now at least one cohort member in all fifty states. Follow-up questionnaires, with response rates above 90%, are mailed every two years to update information on risk factors and the occurrence of major illnesses. The mailing lists for each questionnaire also provide updated information on residential address. Women were included in the current study if they were still alive in June of 2000 and had at least one geocoded address within the contiguous US between 2000 and 2006 A total of 108,767 women were available for analysis. We assessed incident cases of non-accidental mortality June 2000 through May 2006. Deaths were identified from state vital statistics records and the National Death Index or were reported by the families and the postal system and subsequently confirmed by death certificate.
Exposure assessment
To ascertain each participant’s exposure to air pollution at each geocoded questionnaire mailing address, nationwide expansions of our spatio-temporal models of PM2.5 [23,24] were developed to estimate monthly PM2.5 exposures. These models and their previous use in assessing chronic PM exposures among the NHS cohort are described in detail elsewhere [3,25-29]. Briefly, a PM2.3 model was developed using monitor data from the US Environmental Protection Agency’s (USEPA) Air Quality System (AQS), the IMPROVE network, and Harvard research studies. The model also included meteorological and Geographic Information System (GIS)-derived covariates, such as: urban land use within 1 km, elevation, tract- and county-level population density, distance to nearest road for road classes A1-A3, and point-source emission density within 7.5 km. The model was evaluated using a cross-validation approach, where a sub-selection of monitors were held out to compare predicted to observed values [25-27] and were shown to exhibit little bias and high precision. For comparison to other studies that have used estimates from the nearest exposure monitoring location, we also calculated the monthly average PM2.5 from the nearest USEPA AQS monitoring location for all addresses 2000–2006.
Exposure validation study
The details of the validation study and information on the populations included have been described previously [30-43]. Briefly, personal and ambient measurements of PM2.5 were available from a number of short-term panel exposure studies performed in nine US cities between 1999 and 2002. Personal exposures to PM2.5 of ambient origin (as the “true” exposures of interest) were estimated using the personal to ambient sulfate ratio, with ambient sulfate serving as a tracer for PM2.5 of ambient origin [44,45], or as the weighted average of indoor PM2.5 of ambient origin and ambient PM2.5, using home infiltration efficiencies and the proportion each subject spent indoors and outdoors [46]. Personal PM2.5 of ambient origin could only be calculated in five cities (Atlanta, GA, Baltimore, MD, Boston, MA, Seattle, WA and Steubenville, OH). Using the spatio-temporal model described above and data from the nearest EPA AQS monitor, we predicted monthly PM2.5 at the home addresses of the participants of each validation study for the time of the personal and ambient sampling. The pooled dataset of paired information on the surrogate exposures (spatio-temporal model prediction or nearest monitor) and the “true” exposure (personal PM2.5 of ambient origin) from the 5 cities was used to for the risk set calibrations.
Potential confounders
Information on potential confounders is available every two years (every four years for diet information) from the follow-up questionnaires. Therefore, when appropriate, each woman was assigned updated covariate values for each questionnaire cycle. We examined possible confounding by numerous risk factors for all-cause mortality including: age (in months), race, physical activity, body mass index (BMI), hypercholesterolemia, and family history of MI. To control for smoking, we used lifetime smoking history to calculate pack-years (number of packs/day multiplied by number of years of cigarette smoking) and current smoking status (current/former/never). Diet was controlled for using a summary score based on the Alternate Healthy Eating Index (AHEI) [47]. As previously used in this cohort, the score included eight components of the AHEI: higher intakes of vegetables, fruit, nuts, soy and cereal fiber, alcohol consumption, high ratios of chicken plus fish to red meat and polyunsaturated to saturated fat, low intake of trans fat and multivitamin use of ≥5 years. To control for individual level socioeconomic status, we included several variables including nurses’ educational level, the occupation of both of the nurses’ parents when she was 16, marital status, and if applicable, husband’s education. To control for area-level socioeconomic status, we included area level information from the 2000 Census on tract level median income and house value for each residence. To control for long-term, regional, and seasonal patterns in mortality and pollution, we also adjusted all models for calendar year, season, and Census region.
Statistical methods
Time-varying Cox proportional hazards models were used to assess the association of exposure to PM2.5 in the previous 12-months from either the spatio-temporal models or the nearest monitor with the incidence of all-cause mortality. Person-months of follow-up were calculated from June 2000 until the earliest of end of follow-up (May 2006), death, or loss to follow-up. All models were stratified by age in months and calendar month and year and were used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs). Multivariate models were additionally adjusted a priori for the potential confounders listed above.
Risk set regression calibration (RRC) for time-varying exposures [22] was used to correct for bias due to exposure measurement error in the hazard ratios of all-cause mortality, utilizing the data from the external multi-city validation study. The goal of the measurement error correction was to quantify the difference in effect estimates induced by using ambient, as opposed to personal, measures of exposure to PM2.5 and to be able to apply these corrections in the setting of time-varying exposures. The “true” exposure of interest was assumed to be long-term personal PM2.5 of ambient origin, parameterized by the 12-month moving average. In brief, as an improvement over previous methods that applied the same calibration factor to all participants and time periods, the RRC method recalibrates the measurement error model for monthly PM2.5 exposure for each risk set observed in the main study by its counterpart in the validation study, and the 12-month average personal PM2.5 of ambient origin is then constructed from the monthly PM2.5 exposures estimated by the risk set-specific exposure measurement error models. A sandwich variance estimator is then used to calculate Wald-type asymptotic confidence intervals and p-values. Although adjusted for in the Cox model for all-cause mortality, calendar year was not included in the measurement error model because the validation studies were conducted over a 3 year calendar period. To account for the seasonal heterogeneity observed in the measurement error model for the spatio-temporal exposure predictions [30], we included an interaction term of season and PM2.5 to these measurement error models. Because the average number of people per household in a Census tract accounted for the between-city heterogeneity observed in the risk set calibration factors for the nearest EPA AQS monitor in the validation study [30], we included interaction terms of the number of people per household and PM2.5 in the measurement error models for this exposure. Non-linearity of all exposure response relationships was investigated through stepwise restricted cubic splines [48,49]. The analyses were performed in SAS version 9.2 and Fortran 90. User-friendly publicly available software to implement the RRC methodology is available for download [50]. To quantify the impact of measurement error adjustment, we calculated the percent difference in the HRs [((HR – HR measurement error)/HR)*100], as well as the percent increase in width in the confidence intervals [((UCL–LCL)-(UCL measurement error –LCL measurement error))/(UCL–LCL)]*100], after adjustment.
Results
Selected characteristics of the population over follow-up are presented in Table 1. The average age was 69.0 (SD 7.3), with a mean BMI of 25.8 (SD 7.4). Most (94%) of the women were Caucasian and 44% were never smokers. The average PM2.5 in the previous 12 months from the spatial temporal model was 12.0 μg/m3 (SD 2.8 μg/m3) and from the nearest monitoring location was 12.7 μg/m3 (SD 3.1 μg/m3).
Table 1.
Selected age-standardized characteristics of the Nurses’ Health Study participants throughout follow-up 6/2000-5/2006
| Characteristic | Mean (SD) or % |
|---|---|
| Na | 108,767 |
| Person-yearsa | 628,186 |
| Age (in years)a | 69.0 (7.3) |
| Caucasian race | 94 |
| BMI (kg/m2) | 25.8 (7.4) |
| Average PM2.5 over the previous 12 months | |
| Spatio-temporal model | 12.0 (2.8) |
| Nearest USEPA monitor | 12.7 (3.1) |
| Smoking status | |
| Never | 44 |
| Former | 45 |
| Current | 10 |
| Pack-yearsb | 24.3 (21.8) |
| Physical activity (MET hr/week) | |
| <3 | 23 |
| 3 to <9 | 21 |
| 9 to <18 | 18 |
| 18 to <27 | 11 |
| > = 27 MET | 19 |
| Alternative Healthy Eating Index | 175 (95) |
| Hypertension | 53 |
| Hypercholesterolemia | 61 |
| Diabetes | 11 |
| Individual level SES | |
| RN degree | 73 |
| Housewife mother at age 16 | 64 |
| Professional or manager father at age 16 | 26 |
| Married | 64 |
| Husband’s education | |
| less than high school | 4 |
| high school | 26 |
| greater than high school | 35 |
| Census tract SES | |
| Median home value ($1,000) | 170.2 (124.9) |
| Median income ($1,000) | 63.4 (24.4) |
aValue not age adjusted.
bAmong ever smokers only.
Over 628,186 person-years of follow-up, there were a total of 8,617 non-accidental deaths. The associations for a 10 μg/m3 increase in spatio-temporal model predicted or nearest USEPA AQS monitor ambient average PM2.5 in the previous 12 months with all-cause mortality are shown in Table 2 with and without adjustment for measurement error. The age, calendar time, region and season adjusted HR was 1.20 (95%CI 1.11,1.29) for models using the spatio-temporal model predictions and 1.14 (95%CI: 1.06, 1.22) for models using the nearest USEPA monitor values. Both HRs remained elevated but became more comparable after adjustment for measurement error (1.27 (95%CI: 1.08, 1.48) and 1.26 (1.07, 1.48)), reflecting an increase of 5.8% for the spatio-temporal estimates, and 10.5% for the nearest monitor estimates. The effect estimates both remained statistically significant even with >100% widening of confidence intervals after accounting for the uncertainty due to exposure measurement error and the adjustment procedure.
Table 2.
Associations of 12-month average PM 2.5 (per 10 μg/m 3 increase) from spatio-temporal model predictions or the nearest USEPA monitoring location values with all-cause mortality, with and without adjustment for exposure measurement error
| Spatio-temporal model | Nearest USEPA monitor | |
|---|---|---|
| Cases | 8,617 | 8,617 |
| Person-years | 628,186 | 628,186 |
| Basic HR (95%CI)1 | 1.20 (1.11, 1.29) | 1.14 (1.06, 1.22) |
| Basic Measurement Error Adjusted HR (95%CI)1,2 | 1.27 (1.08, 1.48) | 1.26 (1.07, 1.48) |
| % increase in HR3 | 5.8% | 10.5% |
| % increase in 95% CIs4 | 122.2% | 156.3% |
| Multivariable HR (95%CI)5 | 1.13 (1.05, 1.22) | 1.12 (1.05, 1.21) |
| Multivariable Measurement Error Adjusted HR (95%CI)2,5 | 1.18 (1.02, 1.36) | 1.22 (1.02, 1.45) |
| % increase in HR3 | 4.4% | 8.9% |
| % increase in 95% CIs4 | 100.0% | 168.8% |
1Basic model: models stratified by age in months, adjusted for race, region, year and season.
2Additionally adjusted for exposure measurement error.
3[(HR – HR measurement error)/HR]*100.
4[((UCL–LCL)-(UCL measurement error –LCL measurement error))/ (UCL–LCL)]*100.
5Multivariable: models stratified by age in months, adjusted for race. region, year, season, smoking status, pack-yrs, family history of MI, BMI, hypercholesterolemia, median family income in census tract of residence, median house value in census tract of residence, physical activity, race, Alternate Healthy Eating Index (AHEI), individual level socioeconomic status (nurses’ education level, occupation of both parents, marital status, and husband’s education).
Similar patterns were observed in multivariable models. Models unadjusted for measurement error were more comparable for the two exposure assignment methods (1.13 (95%CI: 1.05-1.22) for the spatio-temporal model predictions and 1.12 (95%CI: 1.05-1.21) for the nearest monitor estimates), and the magnitude of HR increases and increases in the width of the 95% confidence intervals were comparable to those from the basic models.
As shown in Figure 1 (spatio-temporal model) and Figure 2 (nearest monitor), the multivariable adjusted predicted mortality rates for a given 12-month average PM2.5 level were higher when using the measurement error adjusted estimates, compared to estimates ignoring measurement error.
Figure 1.
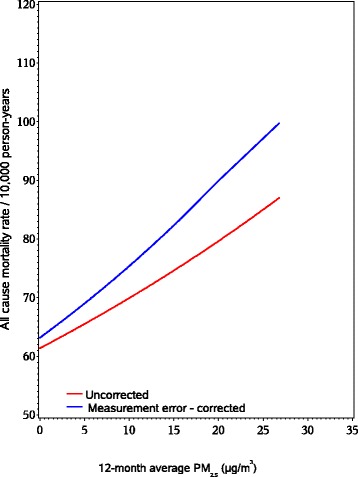
Measurement error corrected and uncorrected rates of all-cause mortality by 12-month spatio-temporal predicted average PM2.5. Multivariable models adjusted for age in months, adjusted for region, year, season, smoking status, pack-yrs, family history of MI, BMI, hypercholesterolemia, diabetes, hypertension, median family income in census tract of residence, median house value in census tract of residence, physical activity, race, Alternate Healthy Eating Index (AHEI), individual level socioeconomic status (nurses’ education level, occupation of both parents, marital status, and husband’s education).
Figure 2.
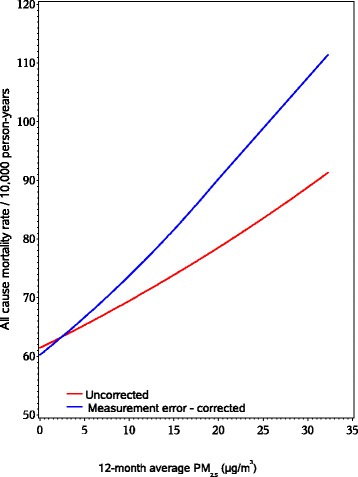
Measurement error corrected and uncorrected rates of all-cause mortality by 12-month nearest USEPA monitor average PM2.5. Multivariable models adjusted for age in months, adjusted for region, year, season, smoking status, pack-yrs, family history of MI, BMI, hypercholesterolemia, diabetes, hypertension, median family income in census tract of residence, median house value in census tract of residence, physical activity, race, Alternate Healthy Eating Index (AHEI), individual level socioeconomic status (nurses’ education level, occupation of both parents, marital status, and husband’s education).
Discussion
In analyses of this nationwide cohort of middle-aged and older women, increasing exposure to PM2.5 was associated with an increased risk of mortality. Contrary to our a priori hypothesis of elevated and more precise results using the spatio-temporal model predictions, the results were similar regardless of the method chosen for exposure prediction. Specifically, the HRs for 2000–2006 were 1.14 (95%CI: 1.05-1.23) and 1.13 (95%CI: 1.05-1.21) for predictions from the spatio-temporal prediction models and nearest USEPA monitoring location, respectively. Adjustment for measurement error increased the magnitude of the HRs by 10-15%, and also widened the confidence intervals, suggesting the presence of both classical and Berkson errors when using ambient, as opposed to personal exposures of ambient origin, as the exposures of interest [17].
Our estimated effects are lower than our previous findings in this cohort. Among women living in a selection of Northeastern and Midwestern states of the US between 1992–2002, each 10 μg/m3 increase in PM2.5 was associated with an HR = 1.26 (95%CI: 1.02-1.54) [51]. These differences are likely due to a combination of factors including different follow-up periods, the expansion to all contiguous states, differences in inclusion and exclusion criteria, and differences in parameterizations of the statistical models. However, our current results for the whole country 2000–2006 are similar to the equivalent meta-estimate for a 10 μg/m3 increase in PM2.5 from 19 cohorts participating in the European Study of Cohorts for Air Pollution Effects (ESCAPE) project (HR = 1.14; 95%CI: 1.04-1.26) [52].
Our measurement error correction method was designed to correct for the most likely source of error in long-term pollution studies, namely differences between ambient and personal levels of PM2.5 of ambient origin. Importantly, we were able to use these methods to correct for measurement error using both concentrations at the nearest monitoring location and predictions at the home address from spatio-temporal models. This allowed us to examine the impact of both methods of exposure prediction and the impact of measurement error on our findings. Notably, although additional information was needed to estimate the impact of measurement error from the nearest monitor, our results suggest similar levels of risk from the two prediction methods. An important point is that the measurements from the validation studies are also subject to measurement error. As long as these errors are uncorrelated with the errors in nearest monitor and spatio-temporal predictions, which we believe is likely to be true, the measurement error-corrected results will give valid point estimates and confidence intervals [60,61].
A number of methods to assess and quantify exposure measurement error have recently been proposed. In a study based in the Netherlands Cohort Study on Diet and Cancer, we recently used related non-time varying measurement error correction methods to assess the impact of measurement error on associations of air pollution and traffic parameters on lung cancer risk. Adjustment for measurement error to account for the differences between personal and ambient exposures led to modest increases in the HRs (0–3.3%) for exposures to black smoke and PM2.5 (9.7-37.2%), accompanied by substantial widening of the 95%CIs (10.2-216.8%) [53]. Other methods, including the use imputation based on random effects meta-analysis of correlations between personal and ambient exposures reported in the literature [54,55], have also been used to demonstrate increases in effect estimates and widening of 95%CIs in a study of heart rate variability [56]. Another group of methods have been developed to address errors induced by spatial modeling of exposures or spatial autocorrelation and have shown promise in simulation studies [17,57-59]. Overall, these methods suggest that air pollution studies are subject to complex measurement error structures, and that it is likely a variety of methods are needed to appropriately adjust the large number of study types.
There are a number of limitations to our study to note. First, although we were able to obtain information from a number of exposure panel studies, only a limited number (n = 5) of validation studies had information available to determine levels of PM2.5 of ambient origin. It is possible that differences in time-activity patterns between the participants in these validation studies and those in our study population could have been different, even with our approach of matching risk sets based on current age. There was also limited geographic coverage of the validation studies, which prevented us from applying region-specific adjustments. These differences in activity patterns and possible regional differences may cause our measurement error correction to incorporate error either away from or towards the null. Since most of the covariates adjusted for in the multivariable Cox models shown in Table 2 were not available in the validation study, we had to assume they are not the confounders in the measurement error model. Additionally, individuals in the validation studies wore personal sampling devices for short periods of time (median duration: 7 days), which were then used to calculate monthly “gold standard” exposures. The methods used here assume that the relationship between the personal measurements and the surrogate exposures observed on these days was representative of that would have been observed over an entire month. This is reasonable, given that the days within a month were chosen at random and we have shown in our validation study that the number of days each person participated had little impact on the size of the calibration factors [30]. We are also assuming that the month-specific relationships between the short-term personal and surrogate exposures collected in 1999–2002 can be used to correct 12-month average exposures between 2000 and 2006. The interclass correlation of 1-month exposures from the spatio-temporal model for all participants was 0.41 and the correlation between the 1-month and 12-month averages was 0.68; therefore, this is a reasonable assumption.
This study also has several major strengths. Our long follow-up period and consideration of residential address history allowed us to estimate time-varying exposures to PM2.5 over almost two decades. The availability of a wealth of follow-up data also allowed us to tightly control for a number of potential confounders in a time-varying manner, lessening concerns about residual confounding. The large number of cases allowed us to examine differences in regional patterns in risk and to examine risks after the availability of PM2.5 monitoring in the US. Lastly, our quantification of the potential bias due to measurement error induced by use of ambient levels of PM2.5 instead of personal exposures of ambient origin provides a sense of the potential level of underestimation in previous studies that may exist if similar associations between personal PM2.5 of ambient origin and spatio-temporal or nearest monitor predictions can be assumed.
Conclusions
In this large nationwide cohort of middle-aged and elderly women, exposures to PM2.5 were associated with an increased risk of all-cause mortality using two complementary approaches to exposure assessment. There was evidence that this risk varied by region of the county even after adjustment for a number of lifestyle and demographic factors. Therefore, our study provides evidence that 12-month average PM2.5 exposures below the current USEPA standard are associated with an increased risk of all-cause mortality and that measurement error corrections should be implemented in studies whenever possible.
Acknowledgements
Sources of funding: NIH grants UM1 CA186107, R01 ES009411, and R01 ES017017, EPA STAR FP-9172890 and NIH T32 ES007069 (Kioumourtzoglou). The funding agencies had no role in the design, collection, analysis, or interpretation of analyses, and had no role on the decision to submit the manuscript for publication.
Abbreviations
- AHEI
Alternate healthy eating index
- AQS
Air quality system
- CI
Confidence interval
- ESCAPE
European Study of Cohorts for Air Pollution Effects
- GIS
Geographic Information System
- HR
Hazard ratio
- NHS
Nurses’ Health Study
- PM2.5
Particulate matter less than 2.5 microns in aerodynamic diameter
- PM10
Particulate matter less than 10 microns in aerodynamic diameter
- RRC
Risk set regression calibration
- USEPA
United States Environmental Protection Agency
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
JEH was involved in the study conception, design, data acquisition, data analysis, interpretation of results, and drafted the manuscript. She agrees to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. XL was involved in study design, data analysis, interpretation of results, and has revised the manuscript critically. BH was involved in data analysis and has revised the manuscript critically. RCP was involved in study design, interpretation of results, and has revised the manuscript critically. JDY was involved in study design, data acquisition, interpretation of results, and has revised the manuscript critically. HS was involved in study conception and design, interpretation of results, and has revised the manuscript critically. MAK was involved in study design, data acquisition, interpretation of results, and has revised the manuscript critically. DS was involved in study conception and design, data analysis, interpretation of results, and has revised the manuscript critically. FL was involved in study conception and design, data acquisition, interpretation of results, and has revised the manuscript critically. All authors read and approved the final manuscript.
Contributor Information
Jaime E Hart, Email: Jaime.hart@channing.harvard.edu.
Xiaomei Liao, Email: stxia@channing.harvard.edu.
Biling Hong, Email: stbho@channing.harvard.edu.
Robin C Puett, Email: rpuett@umd.edu.
Jeff D Yanosky, Email: JYanosky@phs.psu.edu.
Helen Suh, Email: h.suh@neu.edu.
Marianthi-Anna Kioumourtzoglou, Email: marianthi.anna@mail.harvard.edu.
Donna Spiegelman, Email: stdls@hsph.harvard.edu.
Francine Laden, Email: Francine.laden@channing.harvard.edu.
References
- 1.Pope CA, 3rd, Dockery DW. Health effects of fine particulate air pollution: lines that connect. J Air Waste Manag Assoc. 2006;56(6):709–42. doi: 10.1080/10473289.2006.10464485. [DOI] [PubMed] [Google Scholar]
- 2.Puett RC, Hart JE, Yanosky JD, Paciorek CJ, Schwartz J, Suh H, et al. Chronic fine and coarse particulate exposure, mortality and coronary heart disease in the nurses’ health study. Environ Health Perspect. 2009;117(11):1697–701. doi: 10.1289/ehp.0900572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Puett RC, Schwartz J, Hart JE, Yanosky JD, Speizer FE, Suh H, et al. Chronic particulate exposure, mortality, and coronary heart disease in the nurses’ health study. Am J Epidemiol. 2008;168(10):1161–8. doi: 10.1093/aje/kwn232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brunekreef B. Health effects of air pollution observed in cohort studies in Europe. J Expo Sci Environ Epidemiol. 2007;17(Suppl 2):S61–5. doi: 10.1038/sj.jes.7500628. [DOI] [PubMed] [Google Scholar]
- 5.Hart JE, Garshick E, Dockery DW, Smith TJ, Ryan LM, Laden F. Long-term ambient multipollutant exposures and mortality. Am J of Respir Crit Care Med. 2011;183(1):73–8. doi: 10.1164/rccm.200912-1903OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoek G, Krishnan RM, Beelen R, Peters A, Ostro B, Brunekreef B, et al. Long-term air pollution exposure and cardio- respiratory mortality: a review. Environ Health. 2013;12(1):43. doi: 10.1186/1476-069X-12-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laden F, Schwartz J, Speizer FE, Dockery DW. Reduction in fine particulate air pollution and mortality: extended follow-up of the Harvard Six Cities study. Am J Respir Crit Care Med. 2006;173(6):667–72. doi: 10.1164/rccm.200503-443OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lepeule J, Laden F, Dockery D, Schwartz J. Chronic exposure to fine particles and mortality: an extended follow-up of the Harvard Six Cities study from 1974 to 2009. Environ Health Perspect. 2012;120(7):965–70. doi: 10.1289/ehp.1104660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pope CA, 3rd, Thun MJ, Namboodiri MM, Dockery DW, Evans JS, Speizer FE, et al. Particulate air pollution as a predictor of mortality in a prospective study of U.S. adults. Am J Respir Crit Care Med. 1995;151(3 Pt 1):669–74. doi: 10.1164/ajrccm/151.3_Pt_1.669. [DOI] [PubMed] [Google Scholar]
- 10.Pope CA, 3rd, Burnett RT, Thun MJ, Calle EE, Krewski D, Ito K, et al. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. Jama. 2002;287(9):1132–41. doi: 10.1001/jama.287.9.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heinrich J, Thiering E, Rzehak P, Kramer U, Hochadel M, Rauchfuss KM, et al. Long-term exposure to NO2 and PM10 and all-cause and cause-specific mortality in a prospective cohort of women. Occup Environ Med. 2013;70(3):179–86. doi: 10.1136/oemed-2012-100876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beelen R, Hoek G, van den Brandt PA, Goldbohm RA, Fischer P, Schouten LJ, et al. Long-term effects of traffic-related air pollution on mortality in a Dutch cohort (NLCS-AIR study) Environ Health Perspect. 2008;116(2):196–202. doi: 10.1289/ehp.10767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zeger SL, Dominici F, McDermott A, Samet JM. Mortality in the medicare population and chronic exposure to fine particulate air pollution in urban centers (2000–2005) Environ Health Perspect. 2008;116(12):1614–9. doi: 10.1289/ehp.11449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ostro B, Lipsett M, Reynolds P, Goldberg D, Hertz A, Garcia C, et al. Long-term exposure to constituents of fine particulate air pollution and mortality: results from the California teachers study. Environ Health Perspect. 2010;118(3):363–9. doi: 10.1289/ehp.0901181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bateson TF, Coull BA, Hubbell B, Ito K, Jerrett M, Lumley T, et al. Panel discussion review: session three–issues involved in interpretation of epidemiologic analyses–statistical modeling. J Expo Sci Environ Epidemiol. 2007;17(Suppl 2):S90–6. doi: 10.1038/sj.jes.7500631. [DOI] [PubMed] [Google Scholar]
- 16.Setton E, Marshall JD, Brauer M, Lundquist KR, Hystad P, Keller P, et al. The impact of daily mobility on exposure to traffic-related air pollution and health effect estimates. J Expo Sci Environ Epidemiol. 2011;21(1):42–8. doi: 10.1038/jes.2010.14. [DOI] [PubMed] [Google Scholar]
- 17.Szpiro AA, Sheppard L, Lumley T. Efficient measurement error correction with spatially misaligned data. Biostatistics. 2011;12(4):610–23. doi: 10.1093/biostatistics/kxq083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thomas D, Stram D, Dwyer J. Exposure measurement error: influence on exposure-disease. relationships and methods of correction. Annu Rev Public Health. 1993;14:69–93. doi: 10.1146/annurev.pu.14.050193.000441. [DOI] [PubMed] [Google Scholar]
- 19.Zeger SL, Thomas D, Dominici F, Samet JM, Schwartz J, Dockery D, et al. Exposure measurement error in time-series studies of air pollution: concepts and consequences. Environ Health Perspect. 2000;108(5):419–26. doi: 10.1289/ehp.00108419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Armstrong BG. The effects of measurement errors on relative risk regressions. Am J Epidemiol. 1990;132(6):1176–84. doi: 10.1093/oxfordjournals.aje.a115761. [DOI] [PubMed] [Google Scholar]
- 21.Carroll RJ, Ruppert D, LStefanski LA, Crainiceanu C. Measurement error in nonlinear models: A modern perspective: Chapman & hall. 2006. [Google Scholar]
- 22.Liao X, Zucker DM, Li Y, Spiegelman D. Survival analysis with error-prone time-varying covariates: a risk set calibration approach. Biometrics. 2011;67(1):50–8. doi: 10.1111/j.1541-0420.2010.01423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yanosky J, Paciorek C, Suh H. Predicting chronic fine and coarse particulate exposures using spatio-temporal models for the Northeastern and Midwestern United States. Environ Health Perspect. 2009;117(4):522–9. doi: 10.1289/ehp.11692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yanosky JD, Paciorek CJ, Schwartz J, Laden F, Puett R, Suh HH. Spatio-temporal modeling of chronic PM10 exposure for the nurses’ health study. Atmos Environ. 2008;42(18):4047–62. doi: 10.1016/j.atmosenv.2008.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paciorek CJ, Yanosky JD, Puett RC, Laden F, Suh H. Practical large-scale spatio-temporal modeling of particulate matter concentrations. Ann Appl Stat. 2009;3:369–96. doi: 10.1214/08-AOAS204. [DOI] [Google Scholar]
- 26.Yanosky JD, Paciorek CJ, Schwartz J, Laden F, Puett RC, Suh H. Spatio-temporal modeling of chronic PM10 exposure for the nurses’ health study. Atmos Environ. 2008;42(18):4047–62. doi: 10.1016/j.atmosenv.2008.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yanosky JD, Paciorek CJ, Laden F, Hart JE, Puett RC, Liao D, et al. Spatio-temporal modeling of particulate air pollution in the conterminous United States using geographic and meteorological predictors. Environ Health. 2014;13(1):63. doi: 10.1186/1476-069X-13-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Puett RC, Hart JE, Yanosky JD, Paciorek C, Schwartz J, Suh H, et al. Chronic fine and coarse particulate exposure, mortality, and coronary heart disease in the nurses’ health study. Environ Health Perspect. 2009;117(11):1697–701. doi: 10.1289/ehp.0900572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weuve J, Puett RC, Schwartz J, Yanosky JD, Laden F, Grodstein F. Exposure to particulate air pollution and cognitive decline in older women. Arch Intern Med. 2012;172(3):219–27. doi: 10.1001/archinternmed.2011.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kioumourtzoglou MA, Spiegelman D, Szpiro AA, Sheppard L, Kaufman JD, Yanosky JD, et al. Exposure measurement error in PM2.5 health effects studies: A pooled analysis of eight personal exposure validation studies. Environ Health. 2014;13(1):2. doi: 10.1186/1476-069X-13-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sarnat SE, Coull BA, Schwartz J, Gold DR, Suh HH. Factors affecting the association between ambient concentrations and personal exposures to particles and gases. Environ Health Perspect. 2006;114(5):649–54. doi: 10.1289/ehp.8422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koutrakis P, Suh HH, Sarnat JA, Brown KW, Coull BA, Schwartz J. Characterization of particulate and gas exposures of sensitive subpopulations living in Baltimore and Boston. Res Rep Health Eff Inst. 2005;131:1–65. [PubMed] [Google Scholar]
- 33.Liu LJ, Box M, Kalman D, Kaufman J, Koenig J, Larson T, et al. Exposure assessment of particulate matter for susceptible populations in Seattle. Environ Health Perspect. 2003;111(7):909–18. doi: 10.1289/ehp.6011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meng QY, Turpin BJ, Korn L, Weisel CP, Morandi M, Colome S, et al. Influence of ambient (outdoor) sources on residential indoor and personal PM2.5 concentrations: analyses of RIOPA data. J Expo Anal Environ Epidemiol. 2005;15(1):17–28. doi: 10.1038/sj.jea.7500378. [DOI] [PubMed] [Google Scholar]
- 35.Sarnat JA, Koutrakis P, Suh HH. Assessing the relationship between personal particulate and gaseous exposures of senior citizens living in Baltimore. MD J Air Waste Manag Assoc. 2000;50(7):1184–98. doi: 10.1080/10473289.2000.10464165. [DOI] [PubMed] [Google Scholar]
- 36.Suh HH, Zanobetti A. Exposure error masks the relationship between traffic-related air pollution and heart rate variability. J Occup Environ Med. 2010;52(7):685–92. doi: 10.1097/JOM.0b013e3181e8071f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suh HH, Koutrakis P, Chang LT. Characteristics of the composition of personal, indoor, and outdoor particulate exposures. CA, California Air Resources Board: In Sacramento; 2003. [Google Scholar]
- 38.Suh H, Koutrakis P, Ebelt S. Detailed Characterization of Indoor and Personal Particulate Matter Concentrations. CA, Calfornia Air Resources Board: In Sacramento; 2004. [Google Scholar]
- 39.Brown KW, Sarnat JA, Suh HH, Coull BA, Koutrakis P. Factors influencing relationships between personal and ambient concentrations of gaseous and particulate pollutants. Sci Total Environ. 2009;407(12):3754–65. doi: 10.1016/j.scitotenv.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 40.Brown KW, Sarnat JA, Suh HH, Coull BA, Spengler JD, Koutrakis P. Ambient site, home outdoor and home indoor particulate concentrations as proxies of personal exposures. J Environ Monit. 2008;10(9):1041–51. doi: 10.1039/b805991h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weisel CP, Zhang J, Turpin BJ, Morandi MT, Colome S, Stock TH, et al. Relationships of Indoor, Outdoor, and Personal Air (RIOPA). Part I. Collection methods and descriptive analyses. Res Rep Health Eff Inst 2005(130 Pt 1):1–107; discussion 109–127. [PubMed]
- 42.Williams R, Suggs J, Rea A, Leovic K, Vette A, Croghan C. The Research triangle park particulate matter panel study: PM mass concentration relationships. Atmos Environ. 2003;37:5349–63. doi: 10.1016/j.atmosenv.2003.09.019. [DOI] [Google Scholar]
- 43.Williams R, Suggs J, Rea A, Sheldon L, Rhodes C, Thornburg J. The Research Triangle Park Particulate Matter Panel Study: Modeling Ambient Source Contribution to Personal and Residential PM Mass Concentrations. Atmos Environ. 2003;37:5365–5378. doi: 10.1016/j.atmosenv.2003.09.010. [DOI] [Google Scholar]
- 44.Sarnat JA, Long CM, Koutrakis P, Coull B, Schwartz J, Suh H. Using sulfur as a tracer of outdoor fine particles. Environ Sci Technol. 2002;36(24):5305–14. doi: 10.1021/es025796b. [DOI] [PubMed] [Google Scholar]
- 45.Wilson WE, Mage DT, Grant LD. Estimating separately personal exposure to ambient and nonambient particulate matter for epidemiology and risk assessment: why and how. J Air Waste Manag Assoc. 2000;50(7):1167–83. doi: 10.1080/10473289.2000.10464164. [DOI] [PubMed] [Google Scholar]
- 46.Koenig JQ, Mar TF, Allen RW, Jansen K, Lumley T, Sullivan JH, et al. Pulmonary effects of indoor- and outdoor-generated particles in children with asthma. Environ Health Perspect. 2005;113(4):499–503. doi: 10.1289/ehp.7511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chiuve SE, Rexrode KM, Spiegelman D, Logroscino G, Manson JE, Rimm EB. Primary prevention of stroke by healthy lifestyle. Circulation. 2008;118(9):947–54. doi: 10.1161/CIRCULATIONAHA.108.781062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Govindarajulu U, Spiegelman D, Thurston S, Eisen E. Comparing smoothing techniques for modeling exposure-response curves in Cox models. Stat Med. 2007;26:3735–52. doi: 10.1002/sim.2848. [DOI] [PubMed] [Google Scholar]
- 49.Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8(5):551–61. doi: 10.1002/sim.4780080504. [DOI] [PubMed] [Google Scholar]
- 50.%rcc Macro [http://www.hsph.harvard.edu/donna-spiegelman/software/rrc-macro/]
- 51.Puett RC, Hart JE, Yanosky JD, Paciorek C, Schwartz J, Suh H, et al. Chronic fine and coarse particulate exposure, mortality, and coronary heart disease in the nurses’ health study. Environ Health Perspect. 2009;117(11):1697–701. doi: 10.1289/ehp.0900572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Beelen R, Raaschou-Nielsen O, Stafoggia M, Andersen ZJ, Weinmayr G, Hoffmann B, et al. Effects of long-term exposure to air pollution on natural-cause mortality: an analysis of 22 European cohorts within the multicentre ESCAPE project. Lancet. 2013;383(9919):785–95. doi: 10.1016/S0140-6736(13)62158-3. [DOI] [PubMed] [Google Scholar]
- 53.Hart JE, Spiegelman D, Beelen R, Hoek G, Brunekreef B,Schouten LJ, van den Brandt P. Long-Term Ambient Residential Traffic–Related Exposures and Measurement Error–Adjusted Risk of Incident Lung Cancer in the Netherlands Cohort Study on Diet and Cancer. Environ Health 2 Perspect; http://dx.doi.org/10.1289/ehp.1408762. [DOI] [PMC free article] [PubMed]
- 54.Avery CL, Mills KT, Williams R, McGraw KA, Poole C, Smith RL, et al. Estimating error in using ambient PM2.5 concentrations as proxies for personal exposures: a review. Epidemiology. 2010;21((2):215–23. doi: 10.1097/EDE.0b013e3181cb41f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Avery CL, Mills KT, Williams R, McGraw KA, Poole C, Smith RL, et al. Estimating error in using residential outdoor PM2.5 concentrations as proxies for personal exposures: a meta-analysis. Environmental Health Perspectives. 2010;118(5):673–8. doi: 10.1289/ehp.0901158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Holliday KM, Avery CL, Poole C, McGraw K, Williams R, Liao D, et al. Estimating personal exposures from ambient air pollution measures: using meta-analysis to assess measurement error. Epidemiology. 2014;25(1):35–43. doi: 10.1097/EDE.0000000000000006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Szpiro AA, Paciorek CJ. Measurement error in two-stage analyses, with application to air pollution epidemiology. Environmetrics. 2013;24(8):501–17. doi: 10.1002/env.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Szpiro AA, Paciorek CJ, Sheppard L. Does more accurate exposure prediction necessarily improve health effect estimates? Epidemiology. 2011;22(5):680–5. doi: 10.1097/EDE.0b013e3182254cc6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dionisio KL, Baxter LK, Chang HH. An empirical assessment of exposure measurement error and effect attenuation in bipollutant epidemiologic models. Environ Health Perspect. 2014;122(11):1216–24. doi: 10.1289/ehp.1307772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Spiegelman D, Schneeweiss S, McDermott A. Measurement error correction for logistic regression models with an “alloyed gold standard”. Am. J. Epidemiol. 1997;145(2):184–196. doi: 10.1093/oxfordjournals.aje.a009089. [DOI] [PubMed] [Google Scholar]
- 61.Spiegelman D, Zhao B, Kim J. Correlated errors in biased surrogates: study designs and methods for measurement error correction. Stat. Med. 2005;24(11):1657–1682. doi: 10.1002/sim.2055. [DOI] [PubMed] [Google Scholar]


