Abstract
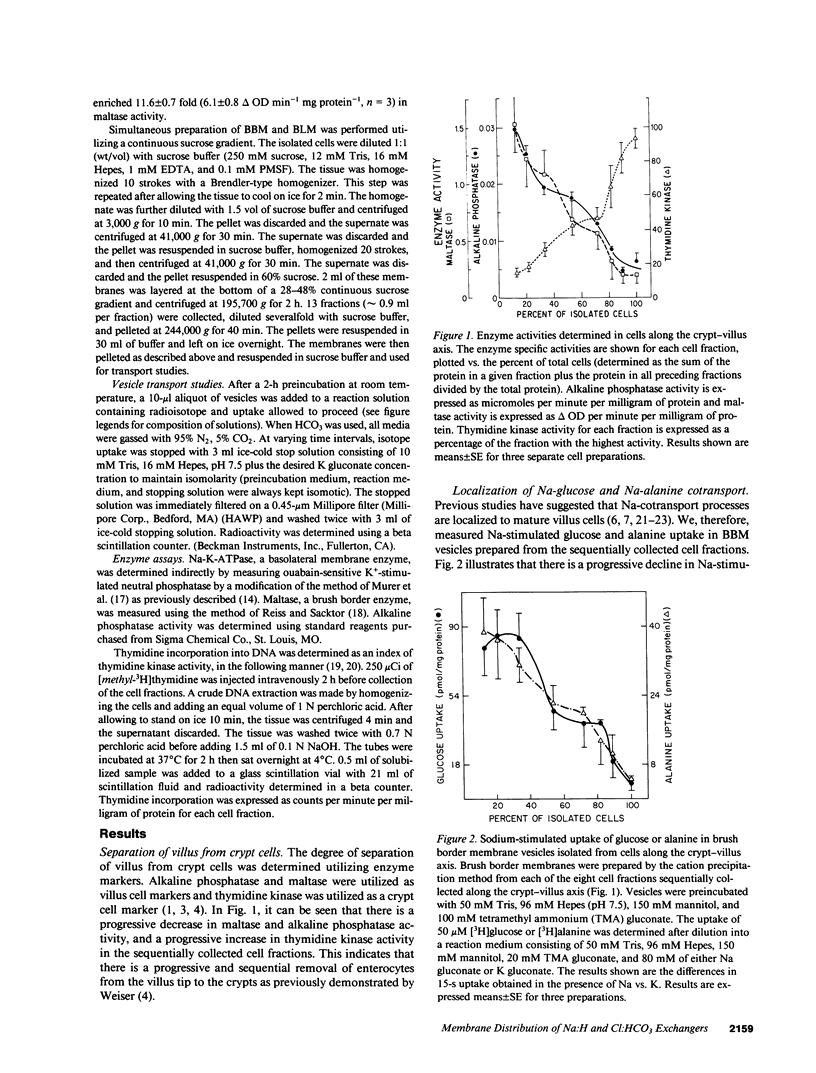
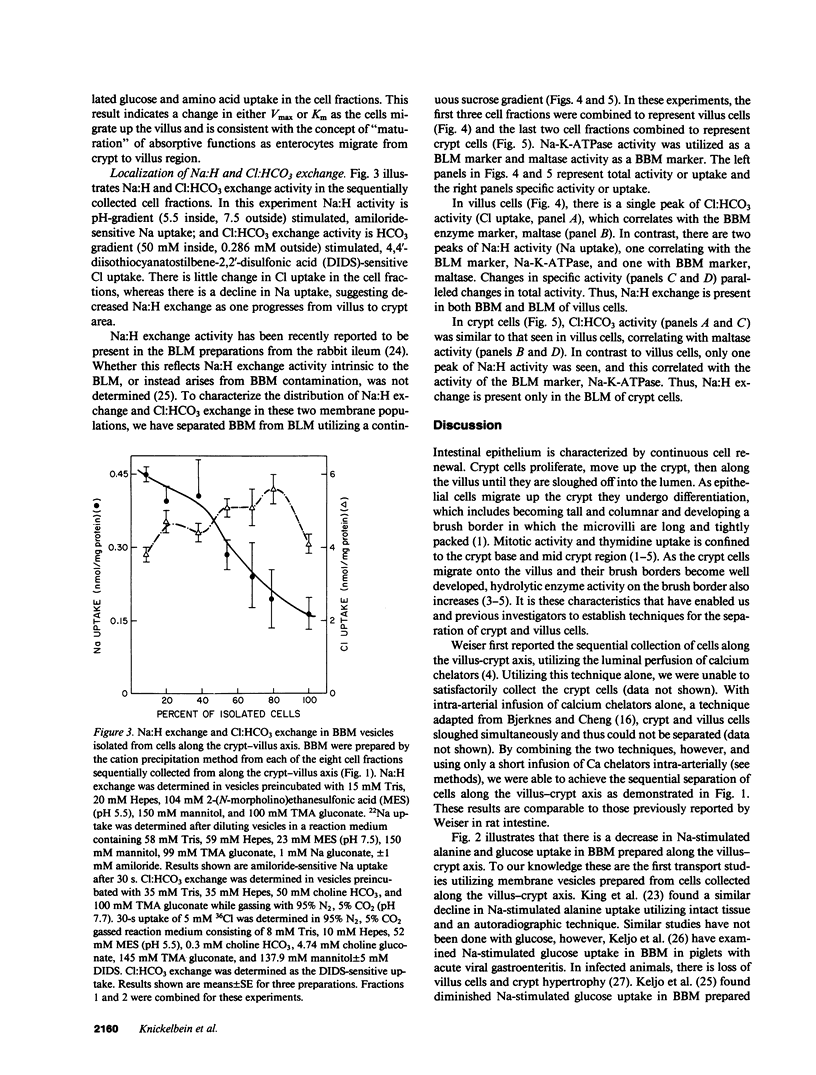
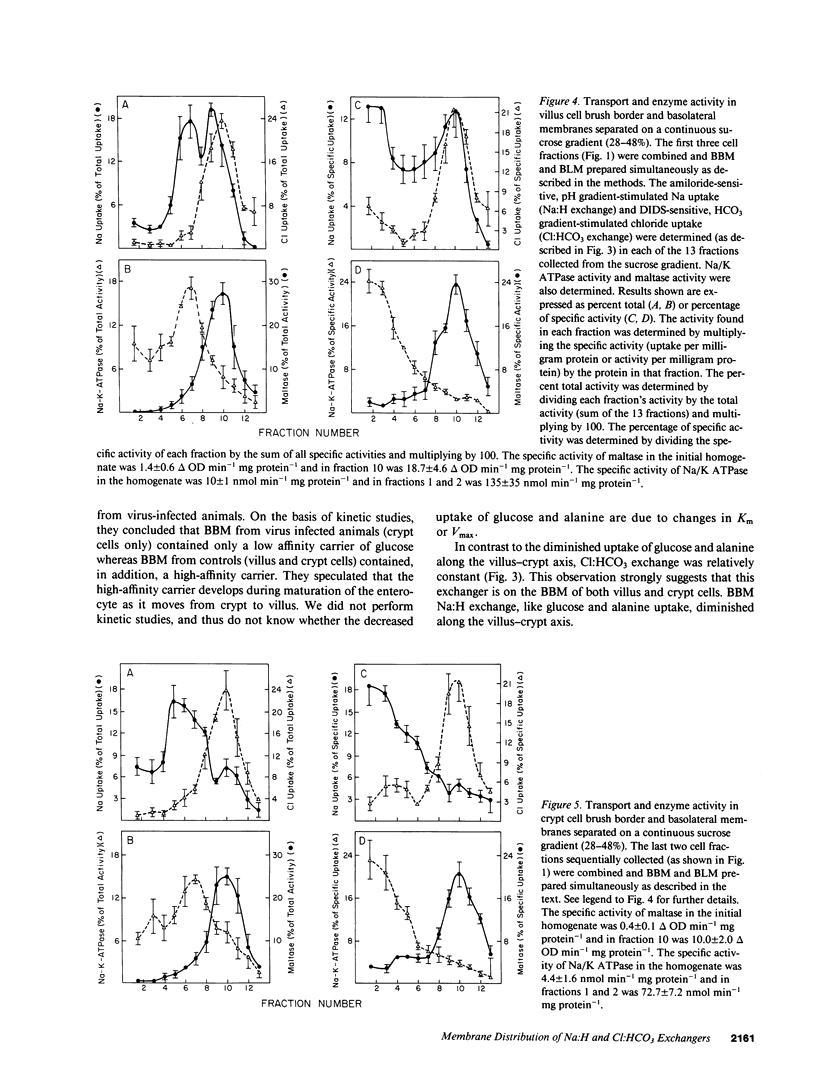
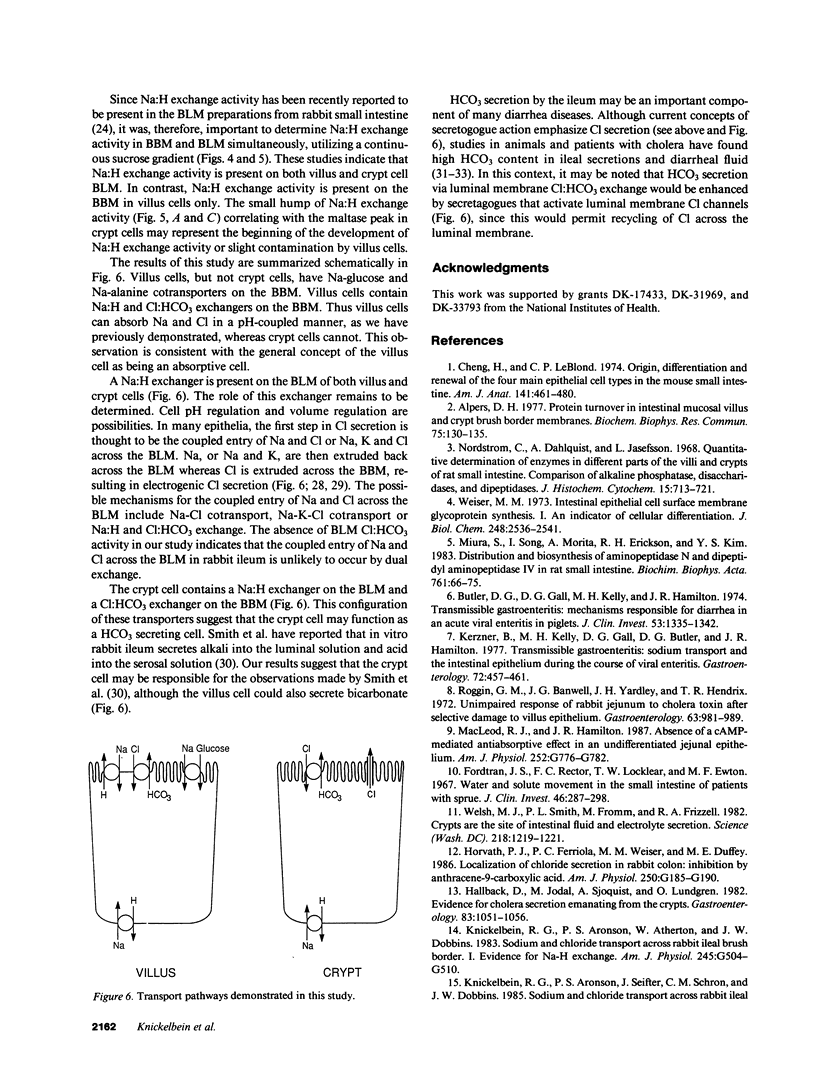
Present evidence suggests that in the small intestine, villus cells are primarily absorptive and crypt cells are primarily secretory. In order to further confirm that there are differences in transport properties between villus and crypt cells, we have separated villus from crypt cells, using calcium chelations techniques, and determined the distribution of Na:H and Cl:HCO3 exchange activity on brush border membrane and basolateral membrane preparations from these two cell populations. Separation of cells was determined utilizing alkaline phosphatase and maltase activity as a marker of villus cells and thymidine kinase activity as a marker of crypt cells. Utilizing these techniques, we were able to sequentially collect cells along the villus-crypt axis. Na-stimulated glucose and alanine uptake in brush border membrane vesicles diminished from the villus to the crypt region in the sequentially collected cells fractions, further suggesting separation of these cells. Brush border and basolateral membranes were then prepared from cells from the villus and crypt areas, utilizing a continuous sucrose gradient. In the villus cells, Na:H exchange activity was found associated with both the brush border and basolateral membrane, whereas, in crypt cells, Na:H exchange activity was only found on the basolateral membrane. Cl:HCO3 exchange activity was found only on the brush border membrane, in both villus and crypt cells. These studies suggest functional heterogeneity in ion transport between villus and crypt cells.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alpers D. H. Protein turnover in intestinal mucosal villus and crypt brush border membranes. Biochem Biophys Res Commun. 1977 Mar 7;75(1):130–135. doi: 10.1016/0006-291x(77)91299-2. [DOI] [PubMed] [Google Scholar]
- Banwell J. G., Pierce N. F., Mitra R. C., Brigham K. L., Caranasos G. J., Keimowitz R. I., Fedson D. S., Thomas J., Gorbach S. L., Sack R. B. Intestinal fluid and electrolyte transport in human cholera. J Clin Invest. 1970 Jan;49(1):183–195. doi: 10.1172/JCI106217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros F., Domínguez P., Velasco G., Lazo P. S. Na+/H+ exchange is present in basolateral membranes from rabbit small intestine. Biochem Biophys Res Commun. 1986 Jan 29;134(2):827–834. doi: 10.1016/s0006-291x(86)80495-8. [DOI] [PubMed] [Google Scholar]
- Bjerknes M., Cheng H. Methods for the isolation of intact epithelium from the mouse intestine. Anat Rec. 1981 Apr;199(4):565–574. doi: 10.1002/ar.1091990412. [DOI] [PubMed] [Google Scholar]
- Butler D. G., Gall D. G., Kelly M. H., Hamilton J. R. Transmissible gastroenteritis. Mechanisms responsible for diarrhea in an acute viral enteritis in piglets. J Clin Invest. 1974 May;53(5):1335–1342. doi: 10.1172/JCI107681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H., Leblond C. P. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. I. Columnar cell. Am J Anat. 1974 Dec;141(4):461–479. doi: 10.1002/aja.1001410403. [DOI] [PubMed] [Google Scholar]
- Fordtran J. S., Rector F. C., Locklear T. W., Ewton M. F. Water and solute movement in the small intestine of patients with sprue. J Clin Invest. 1967 Mar;46(3):287–298. doi: 10.1172/JCI105531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallbäck D. A., Jodal M., Sjöqvist A., Lundgren O. Evidence for cholera secretion emanating from the crypts. A study of villus tissue osmolality and fluid and electrolyte transport in the small intestine of the cat. Gastroenterology. 1982 Nov;83(5):1051–1056. [PubMed] [Google Scholar]
- Hartmann F., Owen R., Bissell D. M. Characterization of isolated epithelial cells from rat small intestine. Am J Physiol. 1982 Feb;242(2):G147–G155. doi: 10.1152/ajpgi.1982.242.2.G147. [DOI] [PubMed] [Google Scholar]
- Heintze K., Stewart C. P., Frizzell R. A. Sodium-dependent chloride secretion across rabbit descending colon. Am J Physiol. 1983 Apr;244(4):G357–G365. doi: 10.1152/ajpgi.1983.244.4.G357. [DOI] [PubMed] [Google Scholar]
- Hoffman A. G., Kuksis A. Improved isolation of villus and crypt cells from rat small intestinal mucosa. Can J Physiol Pharmacol. 1979 Aug;57(8):832–842. doi: 10.1139/y79-127. [DOI] [PubMed] [Google Scholar]
- Horvath P. J., Ferriola P. C., Weiser M. M., Duffey M. E. Localization of chloride secretion in rabbit colon: inhibition by anthracene-9-carboxylic acid. Am J Physiol. 1986 Feb;250(2 Pt 1):G185–G190. doi: 10.1152/ajpgi.1986.250.2.G185. [DOI] [PubMed] [Google Scholar]
- Hubel K. A. The mechanism of bicarbonate secretion in rabbit ileum exposed to choleragen. J Clin Invest. 1974 Apr;53(4):964–970. doi: 10.1172/JCI107662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keljo D. J., MacLeod R. J., Perdue M. H., Butler D. G., Hamilton J. R. D-Glucose transport in piglet jejunal brush-border membranes: insights from a disease model. Am J Physiol. 1985 Dec;249(6 Pt 1):G751–G760. doi: 10.1152/ajpgi.1985.249.6.G751. [DOI] [PubMed] [Google Scholar]
- Kerzner B., Kelly M. H., Gall D. G., Butler D. G., Hamilton J. R. Transmissible gastroenteritis: sodium transport and the intestinal epithelium during the course of viral enteritis. Gastroenterology. 1977 Mar;72(3):457–461. [PubMed] [Google Scholar]
- King I. S., Sepúlveda F. V., Smith M. W. Cellular distribution of neutral and basic amino acid transport systems in rabbit ileal mucosa. J Physiol. 1981;319:355–368. doi: 10.1113/jphysiol.1981.sp013913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knickelbein R., Aronson P. S., Atherton W., Dobbins J. W. Sodium and chloride transport across rabbit ileal brush border. I. Evidence for Na-H exchange. Am J Physiol. 1983 Oct;245(4):G504–G510. doi: 10.1152/ajpgi.1983.245.4.G504. [DOI] [PubMed] [Google Scholar]
- MacLeod R. J., Hamilton J. R. Absence of a cAMP-mediated antiabsorptive effect in an undifferentiated jejunal epithelium. Am J Physiol. 1987 Jun;252(6 Pt 1):G776–G782. doi: 10.1152/ajpgi.1987.252.6.G776. [DOI] [PubMed] [Google Scholar]
- Miura S., Song I. S., Morita A., Erickson R. H., Kim Y. S. Distribution and biosynthesis of aminopeptidase N and dipeptidyl aminopeptidase IV in rat small intestine. Biochim Biophys Acta. 1983 Nov 22;761(1):66–75. doi: 10.1016/0304-4165(83)90363-x. [DOI] [PubMed] [Google Scholar]
- Moore W. L., Jr, Bieberdorf F. A., Morawski S. G., Finkelstein R. A., Fordtran J. S. Ion transport during cholera-induced ileal secretion in the dog. J Clin Invest. 1971 Feb;50(2):312–318. doi: 10.1172/JCI106496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murer H., Ammann E., Biber J., Hopfer U. The surface membrane of the small intestinal epithelial cell. I. Localization of adenyl cyclase. Biochim Biophys Acta. 1976 May 21;433(3):509–519. doi: 10.1016/0005-2736(76)90277-7. [DOI] [PubMed] [Google Scholar]
- Nordström C., Dahlqvist A., Josefsson L. Quantitative determination of enzymes in different parts of the villi and crypts of rat small intestine. Comparison of alkaline phosphatase, disaccharidases and dipepeptidases. J Histochem Cytochem. 1967 Dec;15(12):713–721. doi: 10.1177/15.12.713. [DOI] [PubMed] [Google Scholar]
- Paterson J. Y., Sepúlveda F. V., Smith M. W. A sodium-indpendent low affinity transport system for neutral amino acids in rabbit ileal mucosa. J Physiol. 1980 Jan;298:333–346. doi: 10.1113/jphysiol.1980.sp013084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss U., Sacktor B. Kidney brush border membrane maltase: purification and properties. Arch Biochem Biophys. 1981 Jul;209(2):342–348. doi: 10.1016/0003-9861(81)90290-3. [DOI] [PubMed] [Google Scholar]
- Roggin G. M., Banwell J. G., Yardley J. H., Hendrix T. R. Unimpaired response of rabbit jejunum to cholera toxin after selective damage to villus epithelium. Gastroenterology. 1972 Dec;63(6):981–989. [PubMed] [Google Scholar]
- Schron C. M., Knickelbein R. G., Aronson P. S., Dobbins J. W. Evidence for carrier-mediated Cl-SO4 exchange in rabbit ileal basolateral membrane vesicles. Am J Physiol. 1987 Sep;253(3 Pt 1):G404–G410. doi: 10.1152/ajpgi.1987.253.3.G404. [DOI] [PubMed] [Google Scholar]
- Shepherd R. W., Butler D. G., Cutz E., Gall D. G., Hamilton J. R. The mucosal lesion in viral enteritis. Extent and dynamics of the epithelial response to virus invasion in transmissible gastroenteritis of piglets. Gastroenterology. 1979 Apr;76(4):770–777. doi: 10.1016/S0016-5085(79)80177-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. L., Cascairo M. A., Sullivan S. K. Sodium dependence of luminal alkalinization by rabbit ileal mucosa. Am J Physiol. 1985 Sep;249(3 Pt 1):G358–G368. doi: 10.1152/ajpgi.1985.249.3.G358. [DOI] [PubMed] [Google Scholar]
- Weiser M. M. Intestinal epithelial cell surface membrane glycoprotein synthesis. I. An indicator of cellular differentiation. J Biol Chem. 1973 Apr 10;248(7):2536–2541. [PubMed] [Google Scholar]
- Welsh M. J., Smith P. L., Fromm M., Frizzell R. A. Crypts are the site of intestinal fluid and electrolyte secretion. Science. 1982 Dec 17;218(4578):1219–1221. doi: 10.1126/science.6293054. [DOI] [PubMed] [Google Scholar]



