Abstract
Objectives:
With aging, the oral mucosa becomes more susceptible to external stimuli. The aims of this study were to obtain baseline data on the prevalence of oral mucosal lesions (OMLs) in a population of elderly Yemeni patients and to investigate differences in the presentation of these findings in relation to age, gender, education level, and the wearing of dentures.
Patients and Methods:
The prevalence of OMLs was assessed by clinical examination of a sample of 310 elderly Yemeni patients aged 60 years and older. A single examiner performed detailed oral examinations of the oral cavity according to international criteria and the World Health Organization codes.
Results:
The overall prevalence of OMLs was 77.1%, with a significant difference (P < 0.05) between men (80.3%) and women (69.6%). The prevalence rate of OMLs indicated a significant decrease with advancing age. The most frequently observed lesions were fissured tongue (34.2%), benign tumors (17.1%), hairy tongue (16.5%), and qat-induced white lesions (12.6%). Hairy tongue, qat-induced white lesions, and shammah keratosis were associated with men (P < 0.01, P < 0.05, and P < 0.05, respectively), whereas geographic tongue was associated with women (P < 0.05). The presence of one or more lesions was significantly associated with low education level (P < 0.05). Certain OMLs showed a significant association with smoking and qat chewing (P < 0.05). No association was found between the occurrence of OMLs and denture wearing (P > 0.05).
Conclusions:
The present study has shown a high prevalence of oral lesions among Yemeni elders.
Keywords: Elderly, oral lesions, prevalence, Yemen
INTRODUCTION
Worldwide, the elderly person demographic is growing faster than that of any other age group.[1] Approximately 600 million people are aged 60 years and older, and this number will double by 2025. By 2050, this number will reach 2 billion, with 80% living in developing countries.[2] As in other developing countries, the number of older people in Yemen is also increasing dramatically.
Elderly populations are mainly affected by non-transmissible diseases, which are quickly becoming the leading causes of disability and mortality, and many of these diseases share common risk factors with different oral diseases.[3,4] In addition to dental caries and periodontal disease, oral mucosal disease is another significant problem found in elderly populations. The oral mucosa performs essential protective functions that significantly affect the general health of an individual. A decline in the protective functions of the oral mucosa could expose the aging individual to a variety of pathogens and chemicals that enter the oral cavity.[5] As human beings age, the oral mucosa becomes more permeable to toxic substances and more vulnerable to external carcinogens.[4,5] The oral epithelium has been reported to become thinner with age, and collagen synthesis by connective tissue decreases. As a result, decreased tissue regeneration and disease resistance would be expected.[5,6]
Globally, poor oral health of elders is evidenced by high levels of tooth loss, dental caries, and periodontal disease, accompanied by other conditions such as xerostomia, mucosal lesions, pre-malignant lesions, and oral cancer.[3,4,5,6,7] The prevalence of lesions affecting the oral mucosa is an important parameter in evaluating the oral health of elders,[5] and its assessment is important for government decisions regarding health programs. Nevertheless, the information currently available about the oral health of elders is scarce, especially in Yemen and other Arab countries, where no research has been done on the subject. Therefore, the aims of this study were to obtain baseline information on the prevalence of oral mucosal lesions (OMLs) in a population of elderly Yemeni dental patients and to investigate differences in the presentation of these findings in relation to age, gender, education level, and the wearing of dentures.
PATIENTS AND METHODS
This cross-sectional study, conducted between January and June 2014, included a sample of 310 elderly dental patients (aged 60 years and older) attending the outpatient dental clinics in Sana’a University, Yemen. The Faculty of Dentistry of Sana’a University is the only public dental school in Sana’a, and it offers free dental services to the general public.
The minimum sample size for such a study was calculated to be 210 at 95% confidence level with an absolute precision of 5% and the expected prevalence rate of 83.6%.[5]
The study was approved by the Research and Ethics Committee of the Faculty of Medicine and Health Sciences, Sana’a University, and informed consent was obtained from the study participants.
Before clinical examination, demographic characteristics and clinical information including age, gender, education level, oral risk habits (smoking and qat chewing), oral hygiene practices, systemic health, and past and current use of medications were obtained from all participants through interviews. Participants were categorized into two groups according to their education status: Illiterate (those with no school education) and literate (those with any kind of formal education).
Extra- and intra-oral examination of the mouth was carried out by an oral pathologist from the Department of Oral Medicine and Pathology (Al-Jamaei AA) using an artificial light, dental mirror, tweezers, gauze, and a wooden tongue depressor. Cotton swabs were used for removing debris and examining whether white lesions could be wiped off. Any abnormality of the oral mucosa was diagnosed according to the diagnostic criteria described in the World Health Organization (WHO) guide to epidemiology and diagnosis of oral mucosal diseases.[8] Recurrent herpetic lesions and aphthous stomatitis were recorded only if observed at the time of the examination. No biopsies or laboratory tests were done in the present study. The number of natural teeth and the presence of dentures, either partial or complete, were also recorded. After the oral examination, participants who presented with lesions were referred for appropriate treatment.
The collected data were analyzed by the SPSS 20.0 program. Chi-square and Fisher's exact tests were used to compare between groups. P values lower than 0.05 were considered statistically significant.
RESULTS
Table 1 presents the demographics and characteristics of the study participants. Of the 310 participants studied, 218 (70.3%) were men and 92 (29.7%) were women. The mean age of the participants was 64.77 ± 6.38 years (range 60–86), with 72.3% in the 60–69-year-old age group. One-quarter of the participants had at least one systemic disease, and 19.7% reported consuming some type of drugs. The vast majority of the participants (72.6%) were illiterate, and only 85 (27.4%) had received a formal education. A total of 68.4% of the participants were qat (or khat) chewers and 38.4% were smokers.
Table 1.
Demographic characteristics of the study subjects
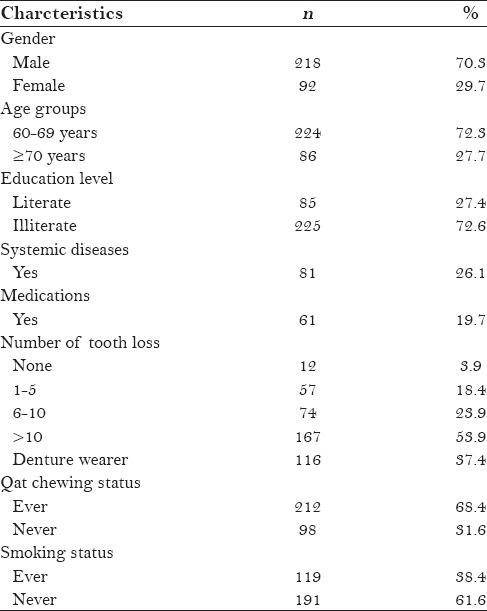
The mean score of tooth loss was 13.91 ± 9.15 (range 0–28), with 77.8% of the participants having lost at least six teeth. In the sample, 12 participants (3.9%) were fully dentate, 255 (82.2%) were partially edentulous, and 43 (13.9%) were edentulous. More than one-third (37.4%) of the participants wore dentures.
A total of 239 participants (77.1%) were diagnosed with at least one OML. The prevalence rate was significantly higher in men than in women (80.3% vs. 69.6%; P < 0.05). The diagnosed OMLs were categorized into seven main groups: Tongue lesions, white lesions, denture-related lesions, ulcerative lesions, exophytic lesions, pigmentation lesions, and miscellaneous. The most prevalent lesions were fissured tongue (34.2%), benign tumors (17.1%), hairy tongue (16.5%), and qat-induced white lesions (12.6%) [Table 2].
Table 2.
The frequency of OMLs according to gender and age groups, n (%)
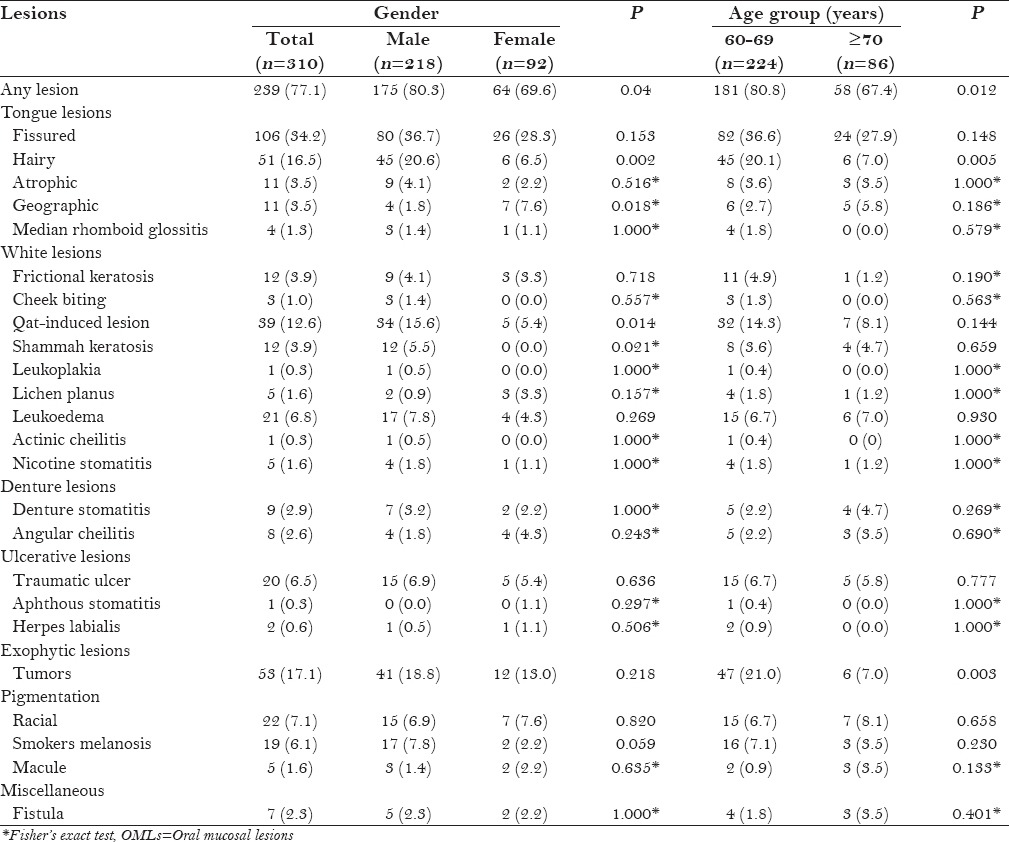
Table 2 shows the distribution of various OMLs in relation to gender and age groups. There were some differences in the distribution of OMLs between men and women. Hairy tongue, qat-induced white lesions, and shammah keratosis were associated with men (P < 0.01, P < 0.05, and P < 0.05, respectively), whereas geographic tongue was associated with women (P < 0.05). Smoker's melanosis showed a weak correlation with gender (P = 0.059).
Overall, the prevalence of OMLs was significantly higher in the 60–69-year-old group than in the 70 years and older group (80.8% vs. 67.4%; P < 0.05). The frequencies of benign tumors and hairy tongue were significantly higher in the 60–69-year-old group (P < 0.01). On the other hand, denture stomatitis was observed in 4.7% of the participants in the 70 years and older group compared with 2.2% in the 65–69-year-old group; however, the difference was not significant (P > 0.05).
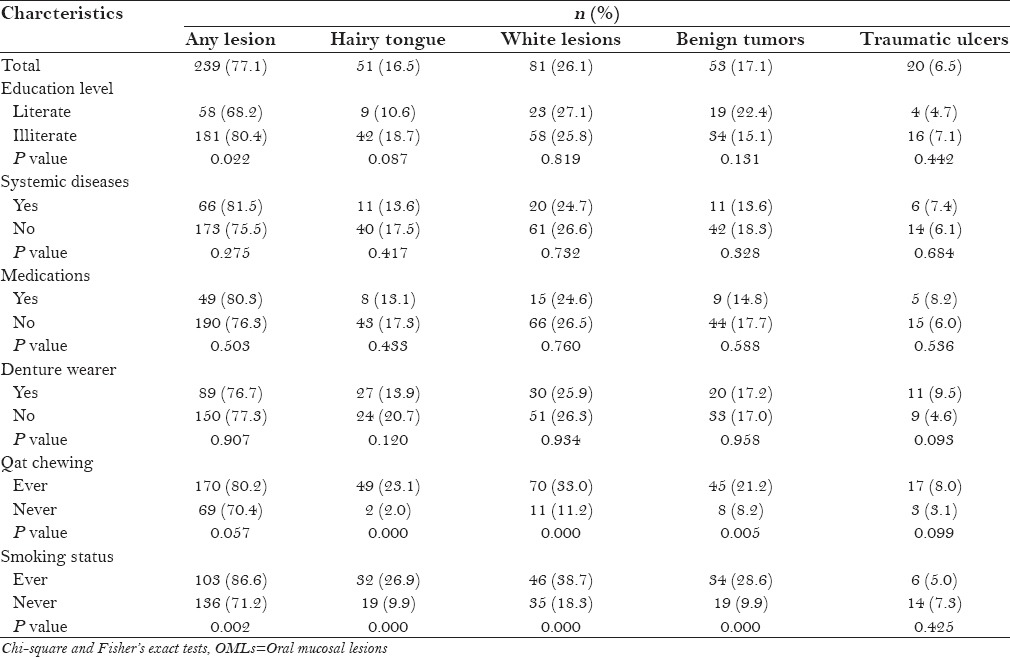
Table 3 illustrates the distribution of the most frequent OMLs in relation to various clinical and demographic variables. The presence of one or more lesions was significantly associated with low education level (P < 0.05). White lesions, hairy tongue, and benign tumors were significantly associated with qat chewing (P < 0.001, P < 0.001, and P < 0.01, respectively). Likewise, the presence of one or more lesions, white lesions, hairy tongue, and benign tumors was significantly associated with smoking (P < 0.05). There was no significant association between the occurrence of OMLs and denture wearing, systemic diseases, or medication consumption (P > 0.05).
Table 3.
Distribution of the most frequent OMLs according to various variables
DISCUSSION
Disease prevalence is usually determined by cross-sectional studies. To the best of our knowledge, this is the first study to provide data on the prevalence and characteristics of OMLs among an elderly population in Yemen.
In our study, the prevalence of OMLs was 77.1%, which is slightly lower than that reported among elderly patients in Thailand[5] (83.6%) and Iran[9] (86.1%). However, this rate is much higher than the results of other epidemiological studies, where investigators recorded a prevalence of 15.5–67%.[4,6,10,11,12,13] It is important to mention that it is difficult to compare results with those of other studies because of the methodological variability and sociocultural and demographic differences present in the studied populations.
The present authors observed that OMLs were more common among men than women, which is in accordance with several studies.[13,14,15,16,17,18,19] This finding is possibly because men are more exposed to risk habits than are women. Also, women in our community are usually more meticulous than men regarding their oral hygiene. Thus, a higher prevalence of certain lesions among men was not a surprise. The present authors found a decrease in the incidence of OMLs with advancing age, which is a finding consistent with other studies.[11,20] However, this result differs from the ones reported by Jainkittivong et al.[5] and Ferreira et al.[7] in which the prevalence increases with age, although we must consider that the population of the latter study consisted of institutionalized elders. In the present study, the highest prevalence of OMLs was in the 60–69-year-old group (80.8%) as compared with the older group (67.4%). Decrease in the incidence of OMLs with advancing age can be attributed to the fact that older people usually, due to certain medical reasons, quit oral risk habits such as smoking and habitual qat chewing with advancing age. Consequently, the incidence of such lesions will decrease significantly.
Several previous studies have shown that tongue lesions constitute a considerable proportion of OMLs, and their prevalence varies in different parts of the world.[5,9,10,21,22,23] Our study supports these findings; fissured tongue and hairy tongue were among the most prevalent lesions that we identified.
The prevalence of fissured tongue (34.2%) was higher than that reported among the elderly populations in Thailand[5] (28%), Brazil[7] (6.6%), and Germany[24] (19.0%), whereas it was lower than that described by Mozafari et al.[25] regarding institutionalized elderly people in Northeast Iran (66.5%). Tongue fissuring is suggested to be genetically determined, and this could be the reason for such prevalent variability worldwide.[26]
Hairy tongue was observed in 16.5% of our study population, which is much higher than that reported in previous studies in Germany[24] (1.8%) and Thailand[5] (6%). Similar to most published studies, our study also found hairy tongue to be more frequent in men and in tobacco users.[5,10,27,28,29,30] In addition to tobacco use, hairy tongue has also been associated with poor oral hygiene. Therefore, dentists should identify and eliminate the predisposing factors and advise regular brushing of the tongue and maintaining proper oral hygiene. The prevalence of geographic tongue in the present study was 3.5%, a percentage similar to the 3.0% prevalence reported by Banoczy et al.[31] in a Hungarian population. However, this rate is much higher than that reported by many other studies.[4,5,24]
White lesions were the second most common lesions observed in our study. The most frequent white lesions were qat-induced white lesions (12.6%), leukoedema (6.8%), and shammah keratosis (3.9%).
Qat-induced lesions are keratotic white lesions associated with the habit of qat chewing (a widespread social habit in Yemen) and are strictly confined to the site of chewing.[32,33,34] These lesions are confined primarily to the buccal mucosa and, to a lesser extent, to the vestibular and mucobuccal fold mucosa. Gorsky et al.[33] suggested that such lesions are related to the combination of mucosal dryness and exposure to chemical and mechanical irritation. Our findings are in accordance with previous studies, which reported these lesions as a common finding in populations where qat chewing is a popular habit.[32,33,34,35] Also, similar to previous researchers, the present authors found these lesions to be more frequent in men than in women, probably because of the higher practice of qat chewing among Yemeni men.
Although not significant, leukoedema was found to be more common among men than women in our study population. This higher prevalence among men supports previous findings by Reichart,[24] Jainkittivong et al.,[5] and Vieira-Andrade et al.[36]
Regarding ulcerative lesions, traumatic ulcers were the most common (6.5%), followed by herpes labialis (0.6%). This finding is in line with those of earlier studies which reported traumatic ulcers as the most common type of ulcerative lesions.[4,5,11,25] The traumatic ulcers in this study population were most commonly located on the buccal mucosa and lateral surface of the tongue, and were mainly caused by trauma from ill-fitting dentures, fractured restorations, and sharp edges of broken teeth.
In the present study, traumatic fibroma was found to be the most common type of exophytic lesions, which is in agreement with earlier reports.[3,6,25,30] The major cause of irritation fibroma is mechanical irritation from dentures, lip biting, calculus deposition, sharp margins of teeth, and long-standing cheek biting and tongue thrusting. The most common location for traumatic fibromas in our study population was the tongue dorsum, followed by the buccal mucosa and lips.
Denture-related lesions were not a common finding in our study population, as only 2.9% of the participants had denture stomatitis. This low rate contradicts the findings from most of the previous studies in which denture stomatitis was reported to be one of the most common lesions in elderly populations.[4,5,9,11,24,25] This is possibly due to non-usage or usage limited to eating and on social occasions among our study population. Moreover, it is of interest to note that the percentage of denture wearers in the present study population was 37.4%, which was very much lower as compared with the data from the aforementioned studies, where 50–94.3% of the elders used dentures.[4,5,9,11,24,25] Another possible reason could be the non-inclusion of ulcers caused by dentures under this category of lesions in the present study.
Melanin pigmentation was found to be more prevalent in this study than in the study among the Malaysian elderly.[21] It is expected to be mainly ethnical in predisposition in this part of the world, although there may be a small possibility for pigmentation to be due to smoking, which has been indicated as an important etiologic factor.
Premalignant lesions were not a common finding, and the incidence was similar to other reports. The 0.3% prevalence of leukoplakia in our study population was lower than that reported among German[24] (1.2%), Thai[5] (4.8%), and Chilean[6] (1.7%) elders. According to epidemiological data from different countries over the past 30 years, the prevalence of leukoplakia varies from 1 to 13%, with a mean value of 3%.[37] Lichen planus was observed in 1.6% of our study population, a finding similar to the ones reported by Reichart[24] and Shet et al.[13] The present authors observed lichen planus more frequently in women than in men. This finding has been confirmed by other authors.[5,13,38] No oral cancer cases were identified in this study, indicating rarity of this lesion in the oral cavity. Nevertheless, dental practitioners should remain alert for any suspicious lesions encountered in the oral cavity.
Also, no difference was found in the prevalence of OMLs among denture wearers as compared with non-denture wearers (P > 0.05) [Table 3]. This finding is different from those of other authors, where a higher prevalence of OMLs was correlated with denture wearing.[3,5,6,7,10,12]
Our results showed a significant association between tobacco habits and the presence of one or more lesions, white lesions, hairy tongue, tumors, and tumor-like lesions. These findings confirm previously published reports.[5,12,39] Also, the results showed a significant association between qat chewing and the occurrence of certain oral lesions: White lesions (P < 0.001), hairy tongue (P < 0.001), and oral tumors (P < 0.05). The role of qat chewing in causing white lesions has been extensively studied and well established in the literature.[32,33,34,35] Regarding hairy tongue, the exact mechanism by which qat chewing may affect the tongue is still unclear. There are no available data in the literature about the subject, which indicates a need for further investigations. However, a high prevalence of hairy tongue among qat chewers might be attributed to xerostomia induced by qat, which is one of the contributory factors for causing tongue conditions.[40]
In our study, the presence of one or more lesions was associated with low education; the lower the education level, the higher prevalence of one or more oral lesions. This result is in agreement with the findings reported by Hashibe et al.[41] and Hand and Whitehill.[42]
The reduction in salivary flow is common in elderly people and can be related to systemic diseases, sometimes because of medicine use, causing oral side effects such as mucosal burning, pain, or burning mouth sensation, interfering in the deglutition, speaking, and mastication, as well as in the taste, prosthesis adherence, and also contributing for the emergence of oral lesions.[12] In the present study, however, the occurrence of OMLs was not associated either with systemic diseases or with medication use [Table 3].
Although the most common OMLs seen in this study were benign, several participants had premalignant lesions. Thus, a periodic oral checkup for the detection of precancerous and cancerous lesions is mandatory, especially in elderly populations.
LIMITATIONS AND CONCLUSIONS
This study has several limitations. The main limitation of the study is the fact that data regarding oral hygiene practices, duration of prosthesis, night use of dentures, and the socio-economic status of the participants were not obtained. Such data might have revealed some correlation between the study findings and the socio-demographic data of the subjects. Another limitation is the lack of blood and/or salivary analyses to investigate the etiology of oral lesions among this population. These investigations could have objectively explained the results and might have shed some light on the etiopathogenesis of these lesions among this elderly population.
In conclusion, this study demonstrated a high prevalence of OMLs among elders in Yemen. Also, the current results support those of other investigators who have shown that the presence of OMLs in elders is related to age, gender, low education level, and practicing oral habits. However, no association was found between the occurrence of oral lesions and denture wearing.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Atashrazm P, Sadri D. Prevalence of oral mucosal lesions in a group of Iranian dependent elderly complete denture wearers. J Contemp Dent Pract. 2013;14:174–8. doi: 10.5005/jp-journals-10024-1295. [DOI] [PubMed] [Google Scholar]
- 2.Geneva, Switzerland: World Health Organization (WHO); 2002. World Health Organization. Active Ageing: A Policy Framework. [PubMed] [Google Scholar]
- 3.Petersen PE, Yamamoto T. Improving the oral health of older people: The approach of the WHO Global Oral Health Programme. Community Dent Oral Epidemiol. 2005;33:81–92. doi: 10.1111/j.1600-0528.2004.00219.x. [DOI] [PubMed] [Google Scholar]
- 4.Cueto A, Martínez R, Niklander S, Deichler J, Barraza A, Esguep A. Prevalence of oral mucosal lesions in an elderly population in the city of Valparaiso, Chile. Gerodontology. 2013;30:201–6. doi: 10.1111/j.1741-2358.2012.00663.x. [DOI] [PubMed] [Google Scholar]
- 5.Jainkittivong A, Aneksuk V, Langlais RP. Oral mucosal conditions in elderly dental patients. Oral Dis. 2002;8:218–23. doi: 10.1034/j.1601-0825.2002.01789.x. [DOI] [PubMed] [Google Scholar]
- 6.Espinoza I, Rojas R, Aranda W, Gamonal J. Prevalence of oral mucosal lesions in elderly people in Santiago, Chile. J Oral Pathol Med. 2003;32:571–5. doi: 10.1034/j.1600-0714.2003.00031.x. [DOI] [PubMed] [Google Scholar]
- 7.Ferreira RC, Magalhães CS, Moreira AN. Oral mucosal alterations among the institutionalized elderly in Brazil. Braz Oral Res. 2010;24:296–302. doi: 10.1590/s1806-83242010000300007. [DOI] [PubMed] [Google Scholar]
- 8.Kramer IR, Pindborg JJ, Bezroukov V, Infirri JS. Guide to epidemiology and diagnosis of oral mucosal diseases and conditions. World Health Organization. Community Dent Oral Epidemiol. 1980;8:1–26. doi: 10.1111/j.1600-0528.1980.tb01249.x. [DOI] [PubMed] [Google Scholar]
- 9.Rabiei M, Kasemnezhad E, Masoudi rad H, Shakiba M, Pourkay H. Prevalence of oral and dental disorders in institutionalised elderly people in Rasht, Iran. Gerodontology. 2010;27:174–7. doi: 10.1111/j.1741-2358.2009.00313.x. [DOI] [PubMed] [Google Scholar]
- 10.Mumcu G, Cimilli H, Sur H, Hayran O, Atalay T. Prevalence and distribution of oral lesions: A cross-sectional study in Turkey. Oral Dis. 2005;11:81–7. doi: 10.1111/j.1601-0825.2004.01062.x. [DOI] [PubMed] [Google Scholar]
- 11.Mujica V, Rivera H, Carrero M. Prevalence of oral soft tissue lesions in an elderly venezuelan population. Med Oral Patol Oral Cir Bucal. 2008;13:E270–4. [PubMed] [Google Scholar]
- 12.Saintrain MV, Almeida CB, Naruse TM, Gonçalves VP. Oral lesions in elderly patients of a community in Brazilian Northeast. Gerodontology. 2013;30:283–7. doi: 10.1111/j.1741-2358.2012.00680.x. [DOI] [PubMed] [Google Scholar]
- 13.Shet RG, Shetty SR, Kalavathi M, Naveen Kumar M, Yadav RD, Soumya S. A study to evaluate the frequency and association of various mucosal conditions among geriatric patients. J Contemp Dent Pract. 2013;14:904–10. doi: 10.5005/jp-journals-10024-1424. [DOI] [PubMed] [Google Scholar]
- 14.Van Wyk CW, Staz J, Farman AG. The chewing lesion of the cheeks and lips: Its features and prevalence among a selected group of adolescents. J Dent. 1977;5:193–9. doi: 10.1016/0300-5712(77)90003-3. [DOI] [PubMed] [Google Scholar]
- 15.Lin HC, Corbet EF, Lo EC. Oral mucosal lesions in adult Chinese. J Dent Res. 2001;80:1486–90. doi: 10.1177/00220345010800052001. [DOI] [PubMed] [Google Scholar]
- 16.Castellanos JL, Díaz-Guzmán L. Lesions of the oral mucosa: An epidemiological study of 23785 Mexican patients. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105:79–85. doi: 10.1016/j.tripleo.2007.01.037. [DOI] [PubMed] [Google Scholar]
- 17.Pentenero M, Broccoletti R, Carbone M, Conrotto D, Gandolfo S. The prevalence of oral mucosal lesions in adults from the Turin area. Oral Dis. 2008;14:356–66. doi: 10.1111/j.1601-0825.2007.01391.x. [DOI] [PubMed] [Google Scholar]
- 18.Jahanbani J, Sandvik L, Lyberg T, Ahlfors E. Evaluation of oral mucosal lesions in 598 referred Iranian patients. Open Dent J. 2009;3:42–7. doi: 10.2174/1874210600903010042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhatnagar P, Rai S, Bhatnagar G, Kaur M, Goel S, Prabhat M. Prevalence study of oral mucosal lesions, mucosal variants, and treatment required for patients reporting to a dental school in North India: In accordance with WHO guidelines. J Family Community Med. 2013;20:41–8. doi: 10.4103/2230-8229.108183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saintrain MV, Holanda TG, Bezerra TM, de Almeida PC. Prevalence of soft tissue oral lesion in elderly and its relations with deleterious habits. Gerodontology. 2012;29:130–4. doi: 10.1111/j.1741-2358.2011.00618.x. [DOI] [PubMed] [Google Scholar]
- 21.Taiyeb Ali TB, Razak IA, Raja Latifah RJ, Zain RB. An epidemiological survey of oral mucosal lesions among elderly Malaysians. Gerodontology. 1995;12:37–40. doi: 10.1111/j.1741-2358.1995.tb00128.x. [DOI] [PubMed] [Google Scholar]
- 22.Patil S, Kaswan S, Rahman F, Doni B. Prevalence of tongue lesions in the Indian population. J Clin Exp Dent. 2013;5:e128–32. doi: 10.4317/jced.51102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Al-Maweri SA, Al-Soneidar WA, Halboub ES. Oral lesions and dental status among institutionalized orphans in Yemen: A matched case-control study. Contemp Clin Dent. 2014;5:81–4. doi: 10.4103/0976-237X.128673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reichart PA. Oral mucosal lesions in a representative cross-sectional study of aging Germans. Community Dent Oral Epidemiol. 2000;28:390–8. doi: 10.1034/j.1600-0528.2000.028005390.x. [DOI] [PubMed] [Google Scholar]
- 25.Mozafari PM, Dalirsani Z, Delavarian Z, Amirchaghmaghi M, Shakeri MT, Esfandyari A, et al. Prevalence of oral mucosal lesions in institutionalized elderly people in Mashhad, Northeast Iran. Gerodontology. 2012;29:e930–4. doi: 10.1111/j.1741-2358.2011.00588.x. [DOI] [PubMed] [Google Scholar]
- 26.Kullaa-Mikkonen A, Sorvari T, Kotilainen R. Morphological variations on the dorsal surface of the human tongue. Proc Finn Dent Soc. 1985;81:104–10. [PubMed] [Google Scholar]
- 27.Campisi G, Margiotta V. Oral mucosal lesions and risk habits among men in an Italian study population. J Oral Pathol Med. 2001;30:22–8. doi: 10.1034/j.1600-0714.2001.300104.x. [DOI] [PubMed] [Google Scholar]
- 28.Martínez Díaz-Canel AI, García-Pola Vallejo MJ. Epidemiological study of oral mucosa pathology in patients of the Oviedo School of Stomatology. Med Oral. 2002;7:4–16. [PubMed] [Google Scholar]
- 29.Al-Mobeeriek A, AlDosari AM. Prevalence of oral lesions among Saudi dental patients. Ann Saudi Med. 2009;29:365–8. doi: 10.4103/0256-4947.55166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ali M, Joseph B, Sundaram D. Prevalence of oral mucosal lesions in patients of the Kuwait University Dental Center. Saudi Dent J. 2013;25:111–8. doi: 10.1016/j.sdentj.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bánóczy J, Rigó O, Albrecht M. Prevalence study of tongue lesions in a Hungarian population. Community Dent Oral Epidemiol. 1993;21:224–6. doi: 10.1111/j.1600-0528.1993.tb00761.x. [DOI] [PubMed] [Google Scholar]
- 32.Ali AA, Al-Sharabi AK, Aguirre JM, Nahas R. A study of 342 oral keratotic white lesions induced by qat chewing among 2500 Yemeni. J Oral Pathol Med. 2004;33:368–72. doi: 10.1111/j.1600-0714.2004.00145.x. [DOI] [PubMed] [Google Scholar]
- 33.Gorsky M, Epstein JB, Levi H, Yarom N. Oral white lesions associated with chewing khat. Tob Induc Dis. 2004;2:145–50. doi: 10.1186/1617-9625-2-3-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Halboub E, Dhaifullah E, Abdulhuq M. Khat chewing and smoking effect on oral mucosa: A clinical study. Acta Medica (Hradec Kralove) 2009;52:155–8. doi: 10.14712/18059694.2016.122. [DOI] [PubMed] [Google Scholar]
- 35.Schmidt-Westhausen AM, Al Sanabani J, Al-Sharabi AK. Prevalence of oral white lesions due to qat chewing among women in Yemen. Oral Dis. 2014;20:675–81. doi: 10.1111/odi.12188. [DOI] [PubMed] [Google Scholar]
- 36.Vieira-Andrade RG, Zuquim Guimarães Fde F, Vieira Cda S, Freire ST, Ramos-Jorge ML, Fernandes AM. Oral mucosa alterations in a socioeconomically deprived region: Prevalence and associated factors. Braz Oral Res. 2011;25:393–400. doi: 10.1590/s1806-83242011000500004. [DOI] [PubMed] [Google Scholar]
- 37.Dombi C, Vörös-Balog T, Czeglédy A, Hermann P, Vincze N, Bánóczy J. Risk group assessment of oral precancer attached to X-ray lung-screening examinations. Community Dent Oral Epidemiol. 2001;29:9–13. [PubMed] [Google Scholar]
- 38.Corrêa L, Frigerio ML, Sousa SC, Novelli MD. Oral lesions in elderly population: A biopsy survey using 2250 histopathological records. Gerodontology. 2006;23:48–54. doi: 10.1111/j.1741-2358.2006.00090.x. [DOI] [PubMed] [Google Scholar]
- 39.Sujatha D, Hebbar PB, Pai A. Prevalence and correlation of oral lesions among tobacco smokers, tobacco chewers, areca nut and alcohol users. Asian Pac J Cancer Prev. 2012;13:1633–7. doi: 10.7314/apjcp.2012.13.4.1633. [DOI] [PubMed] [Google Scholar]
- 40.Darwazeh AM, Almelaih AA. Tongue lesions in a Jordanian population. Prevalence, symptoms, subject's knowledge and treatment provided. Med Oral Patol Oral Cir Bucal. 2011;16:e745–9. doi: 10.4317/medoral.17098. [DOI] [PubMed] [Google Scholar]
- 41.Hashibe M, Jacob BJ, Thomas G, Ramadas K, Mathew B, Sankaranarayanan R, et al. Socioeconomic status, lifestyle factors and oral premalignant lesions. Oral Oncol. 2003;39:664–71. doi: 10.1016/s1368-8375(03)00074-5. [DOI] [PubMed] [Google Scholar]
- 42.Hand JS, Whitehill JM. The prevalence of oral mucosal lesions in an elderly population. J Am Dent Assoc. 1986;112:73–6. doi: 10.14219/jada.archive.1986.0009. [DOI] [PubMed] [Google Scholar]


