Abstract
Background:
Patients with type 2 diabetes have an increased prevalence of periodontitis and, in turn, periodontitis adversely affects the diabetic status. Oxidative stress plays a key role in affecting the pathophysiology of both the diseases and adjunctive systemic antioxidant therapy may have beneficial effect on the treatment outcome. This study was planned to compare the efficacy of systemic antioxidant therapy with lycopene as an adjunct to scaling and root planing versus scaling and root planing alone in chronic periodontitis patients with type 2 diabetes mellitus.
Materials and Methods:
40 diabetic subjects with periodontitis, attending the OP wing of the Department of Periodontics of a tertiary referral care hospital were randomized and equally divided into group A and group B. Diabetes status was recorded as per ADA guidelines and the periodontitis status as per American Academy of Periodontology (AAP) guidelines. Group A patients underwent scaling and root planing with administration of lycopene 8 mg and group B patients were treated with scaling and root planing alone. Clinical parameters like gingival index (GI), probing depth (PD), and clinical attachment level (CAL) were recorded. Serum markers, i.e. malondialdehyde (MDA) (TBARS assay) and C reactive protein (CRP) (ELISA), and glycated hemoglobin (HbA1c) levels were assessed at baseline and at 2 months and 6 months post-therapy.
Results:
Inter-group comparison showed group A giving statistically significant results in reducing mean serum MDA levels at 2 months and 6 months, and in reducing mean PD (mm) and mean HbA1c (%) levels at 2 months (P < 0.005).
Conclusion:
Lycopene as an adjunctive treatment was effective in reducing oxidative stress and restoring altered glycemic levels. Further longitudinal studies with a larger sample size are required to establish the role of lycopene in the management of chronic periodontitis.
Keywords: Antioxidant, diabetes, oxidative stress, periodontitis
INTRODUCTION
Periodontitis has been identified as the sixth complication of diabetes[1] and its prevalence in type 2 diabetic patients is more than twice that in non-diabetic patients.[2,3,4,5,6] Diabetic patients display an increased severity of disease, with the severity being related to diabetic control[7] but is unrelated to diabetic duration.[8] Periodontitis appears to have a reciprocating negative impact on diabetic status.[9,10]
Oxidative stress is an imbalance between the production of a reactive oxygen species and the antioxidant defense, leading to tissue damage. The produced reactive oxygen species, such as superoxide anion, hydroxyl radical, and peroxyl radical, result in damage to many biological molecules (including DNA, lipids, and protein), and the prolonged existence of these reactive oxygen species promotes severe tissue damage and cell death.[11,12] It has been proposed that there is a causal relationship between insulin resistance, oxidative stress, and periodontitis, and that hyperglycemia is a major factor responsible for the activation of oxidative stress.[11,12]
Various antioxidants have been tried locally and systemically in periodontal diseases and have shown beneficial outcome. Lycopene is a hydrocarbon carotenoid that has received a lot of attention. It has a potential role in preventing prostate cancer,[13,14] cardiovascular disease,[15] and long-term diabetic complications in humans,[16] and has shown promising results in preeclampsia,[17,18] leukoplakia,[19] oral submucous fibrosis,[20] gingivitis[21] and chronic periodontitis.[22,23,24]
The present study was undertaken to evaluate the efficacy of scaling and root planing (SRP) with adjunctive systemic lycopene versus SRP alone based on the assessment of clinical parameters, lipid peroxidation serum marker malondialdehyde (MDA), and systemic inflammatory marker serum C reactive protein (CRP), in patients with both the chronic diseases, i.e., type 2 diabetes and chronic periodontitis.
MATERIALS AND METHODS
Screening for patients commenced from January 2013. This was a randomized, controlled, double-blinded clinical trial which was carried out between March and August 2013 in the Department of Periodontics of a tertiary referral care hospital in Hyderabad. The protocol was approved by the institutional ethical committee.
Study population
Fifty-two subjects were screened for the study, out of which 12 subjects were excluded (8 did not meet the inclusion criteria and 4 refused to participate). Study sample consisted of 40 subjects (22 males and 18 females) aged between 35 and 50 years. Investigator KRR enrolled the participants and assigned them into two groups [CONSORT 2010 Flow Diagram]. Subjects with type 2 diabetes and chronic periodontal disease were recruited in the study. Patients who were known diabetics at least from the past 2 years were included.
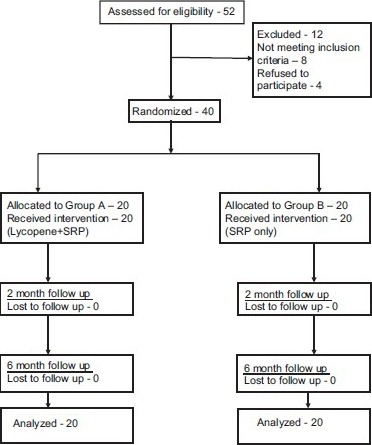
CONSORT 2010 Flow Diagram
Inclusion criteria
Type 2 diabetes as per American Dental Association (ADA) criteria, i.e. a fasting plasma glucose of >126 mg/dl
Patients on oral hypoglycemic drugs with no modification in therapy from the past 12 months
Chronic periodontitis as per American Academy of Periodontology (AAP) guidelines
Minimum of 15 natural teeth
At least four teeth with one or more sites with probing depth (PD) ≥5 mm, clinical attachment level (CAL) ≥4 mm, and bleeding on probing (BOP).
Exclusion criteria
History of antibiotic therapy within the previous 6 months
History of anti-inflammatory drugs within the previous 3 months
Pregnancy and lactation
Use of contraceptives or any other form of hormone
Usage of tobacco and tobacco-related products
Periodontal treatment within 12 months.
Forty subjects were randomly allocated into two groups, group A and group B, by the investigator KRR and the treatment was performed by investigator AM who was blinded to the randomization process.
Group A comprised 20 subjects supplemented with systemic lycopene (Lycored soft gels; Jagsonpal Pharma, New Delhi, India) 8 mg daily for a period of 8 weeks along with SRP.
Group B was the control group consisting of 20 subjects who underwent SRP alone.
Assessment of clinical parameters
Modified gingival index[25] (MGI), probing pocket depth (PPD), and clinical attachment level (CAL) were assessed for full mouth at six sites per tooth. Measurements were done with periodontal probe (Williams periodontal probe; Hu-Friedy, Chicago, USA).
Assessment of serum markers
Blood samples were collected by venipuncture of antecubital vein. Two milliliters of blood was collected, with 1 ml in each test tube. One milliliter of whole blood was used for glycosylated hemoglobin (HbA1c) estimation. Ten minutes after collection, 1 ml blood was subjected to centrifugation at 3000 rpm for 10 min; the supernatant straw-colored fluid (serum) was separated and collected in two storage vials for serum MDA and serum CRP estimation.
Assessment of serum MDA
MDA was assessed by spectrophotometric estimation of serum thiobarbituric acid substances[26] (TBARS). The TBARS assay[27] measures MDA,[27] a reactive compound formed from lipid peroxides that are generated under conditions of oxidative stress. Oxidative modification of lipids occurs with aging and various diseases, and increased oxidative stress is associated with diabetes and its complications. MDA forms an adduct with thiobarbituric acid (TBA). Results are calculated from a standard curve constructed with authentic MDA.
Assessment of serum CRP
CRP assessment was done by ELISA kit [Alpha Diagnostic International (ADI), San Antonio, Texas, USA] method.
Principle of the test
Human CRP ELISA kit is based on simultaneous binding of human CRP from samples to two antibodies, one immobilized on the microtiter well plates and the other conjugated to the enzyme horseradish peroxidase. After a washing step, chromogenic substrate is added and color is developed. The enzymatic reaction (color) is directly proportional to the amount of CRP present in the sample. Adding stopping solution terminates the reaction. Absorbance is then measured on a microtiter well ELISA reader at 450 nm and the concentration of CRP in samples and control is read off the standard curve.
Measurement of HbA1c
Measurement of HbA1c (glycated hemoglobin) was done by spectrophotometric analysis using commercial kit (Bio Systems, Barcelona, Spain).
All these clinical, biochemical and inflammatory parameters were measured at baseline, 2 and 6 months post-therapy.
Statistical analysis
The observations recorded were subjected to independent sample t-test for statistical analysis for inter-group comparison.
RESULTS
Drug adherence/safety
No adverse effects were reported in this study with lycopene supplementation.
Other results
The mean values for PD in group A (lycopene + SRP) and group B (SRP only) were 5.46 ± 0.47 and 5.39 ± 0.44 mm, respectively, at baseline [Table 1]. At 2 months follow-up [Table 2], the PPD reduced to 2.12 ± 0.45 and 2.63 ± 0.38 mm, respectively. From baseline to 2 months, the PD reduction was 3.34 mm in group A and only 1.76 mm in group B, which showed statistically significant (P < 0.001) difference in group A [Figure 1]. At 6 months follow-up [Table 3], the PD was 2.67 ± 0.39 and 3.17 ± 1.41 mm, respectively, which was not statistically significant between the groups.
Table 1.
Baseline parameters
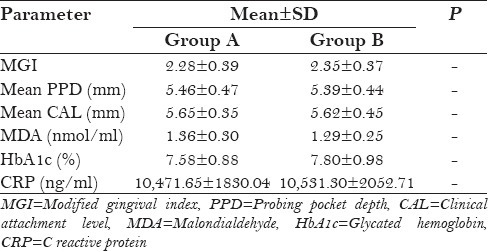
Table 2.
Inter-group evaluation (post-treatment at 2 months)
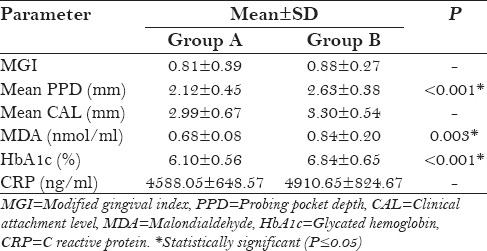
Figure 1.
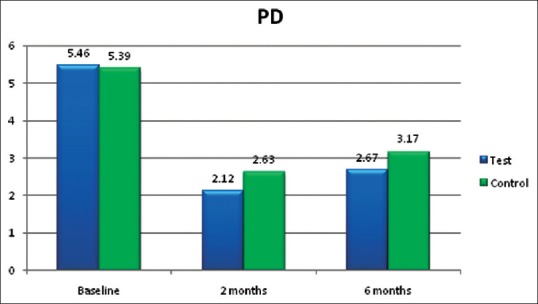
Probing depth (in mm)
Table 3.
Inter-group evaluation (post-treatment at 6 months)
The mean values of serum MDA in group A (lycopene + SRP) and group B (SRP only) were 1.36 ± 0.30 and 1.29 ± 0.25 nmol/ml, respectively, at baseline [Table 1]. At 2 months follow-up, the MDA values reduced to 0.68 ± 0.08 and 0.84 ± 0.20 nmol/ml, respectively, and at 6 months follow-up, the values were 1.17 ± 0.22 and 1.38 ± 0.26 nmol/ml, respectively. Both at 2 months follow-up (P = 0.003) and 6 months follow-up (P = 0.01), these reductions were statistically significant in group A (lycopene + SRP) compared to group B [Tables 2 and 3, Figure 2].
Figure 2.
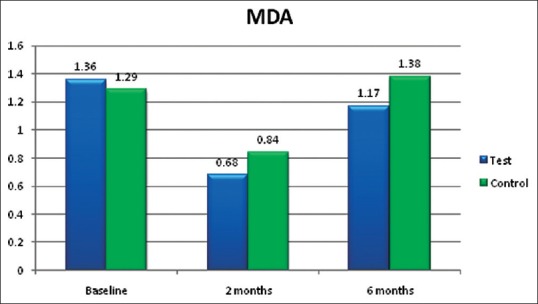
Serum malondialdehyde (MDA) in nmol/ml
The mean values of HbA1c in group A (lycopene + SRP) and group B (SRP only) were 7.58 ± 0.88 and 7.80 ± 0.98%, respectively, at baseline [Table 1]. At 2 months follow-up [Table 2], the HbA1c values reduced to 6.10 ± 0.56 and 6.84 ± 0.65%, respectively, and at 6 months follow-up [Table 3], the values were 6.82 ± 0.61 and 7.12 ± 0.41%, respectively. From baseline to 2 months, these reductions were statistically significant (P < 0.001) in group A (lycopene + SRP) when compared to group B, but there was no difference between the groups at 6 months [Figure 3].
Figure 3.
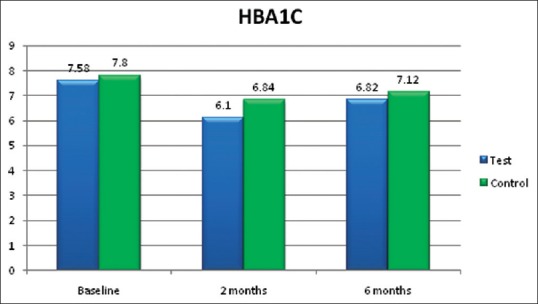
Glycated hemoglobin (HbA1c) in %
DISCUSSION
One of the common pathological mechanisms linking periodontitis and diabetes is oxidative stress; others are activation of innate immunity and inflammation.
Oxidative stress is an imbalance between the production of reactive oxygen species and antioxidant defense, leading to tissue damage. With regard to diabetes, advanced glycation endproducts (AGEs) present in diabetic gingiva may be associated with a state of enhanced oxidative stress, a potential mechanism for accelerated tissue injury.[28] The reactive oxygen species produced, such as superoxide anion, hydroxyl radical, and peroxyl radical, result in damage to many biological molecules including DNA, lipids, and protein, and prolonged existence of these reactive oxygen species promotes severe tissue damage and cell death.[28] Therefore, treating this aspect of oxidative stress by means of systemic antioxidant therapy improves the symptoms of both the diseases.
In this study, systemic lycopene in physiological doses, i.e. 8 mg daily for 2 months, was used as an adjunct to SRP and compared with SRP alone.
Lycopene is a hydrocarbon carotenoid that has recently received a lot of attention. Although similar in structure to the much studied β-carotene, lycopene does not have provitamin A activity. Di Mascio et al.[29] showed that lycopene had the strongest singlet oxygen-quenching capacity of several carotenoids, with α-carotene, β-carotene, and lutein ranking next in capacity.
In the present study, the oxidative stress marker assessed was serum MDA, a marker of lipid peroxidation. A recent study by Trivedi et al.[30] found that serum MDA levels were high in both chronic periodontitis patients and chronic periodontitis patients with diabetes.
Neyestani et al.[16] treated type 2 diabetic patients with 10 mg lycopene daily for 2 months, which resulted in increased total antioxidant capacity (TAOC) to MDA and enhanced serum immunoglobulin M (IgM) levels at 2 months follow-up. This is in accordance with the present study results wherein lycopene + SRP treated group A (0.68 and 1.17 nmol/ml) showed statistically significant reduction of serum MDA at 2 months and 6 months follow-up (P value 0.003 and 0.01, respectively) [Figure 2] compared to SRP alone group B (0.84 and 1.38 nmol/ml, respectively) [Tables 2 and 3].
A meta-analysis by Valero et al.[31] analyzed the possible role of lycopene on type 2 diabetes mellitus. Results showed that lycopene decreased the MDA and lipid peroxidation and was inconclusive for glycemic control, but in the present study in addition to decrease in MDA, lycopene + SRP treated group A (6.10%) also showed statistically significant reduction (P < 0.001) in HbA1c levels at 2 months follow-up [Figure 3] compared to SRP alone group B (6.84%) [Table 2].
There is no published data regarding systemic lycopene associated glycemic control in chronic periodontitis patients with type 2 diabetes mellitus. This is the first study showing positive effect of lycopene on glycemic control in patients with both the diseases, i.e. chronic periodontitis and type 2 diabetes mellitus. This may be due to the reduction in oxidative stress and periodontal inflammation, which together may have controlled the insulin resistance resulting in glycemic control.
In this study, the immunological parameter CRP did not show any statistically significant difference between (lycopene + SRP) group A and (SRP alone) group B at 2 months and 6 months follow-up [Tables 2 and 3, Figure 4]. In a recent study by Arora.[24] it was observed that the immunological marker serum tumor necrosis factor-α (TNF-α) did not show statistically significant reduction in the test (lycopene) group compared to placebo group at 2 months follow-up. In both studies, systemic lycopene as an adjunct to SRP has not shown any additional effect on improvement of serum inflammatory markers.
Figure 4.

Serum C reactive protein (CRP) in ng/ml
Chandra et al.[21] and Arora et al.[24] concluded in their studies that systemic lycopene and oral prophylaxis showed statistically significant reduction in gingival index, compared to placebo oral prophylaxis group. In both the studies, patients were followed up for 2 weeks in gingivitis patients and 2 months in chronic periodontitis patients, respectively. In the present study also, gingival index [Figure 5] was reduced in (lycopene + SRP) group A compared to (SRP only) group B, but this was not statistically significant at 2 months and at 6 months [Tables 2 and 3].
Figure 5.
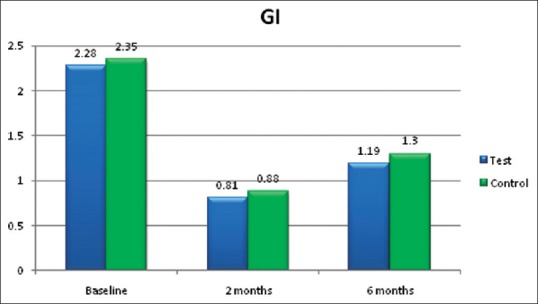
Gingival index (GI)
PD reduction [Figure 1] was statistically significant (P < 0.001) in (lycopene + SRP) group A (2.12 mm) than in (SRP alone) group B (2.63 mm) at 2 months [Table 2] in the present study. This finding was contradictory to the study finding of Nupur et al.[24] which showed no statistically significant reduction in the lycopene group.
CAL [Figure 6] did not show any difference between both the groups in the present study [Tables 2 and 3].
Figure 6.
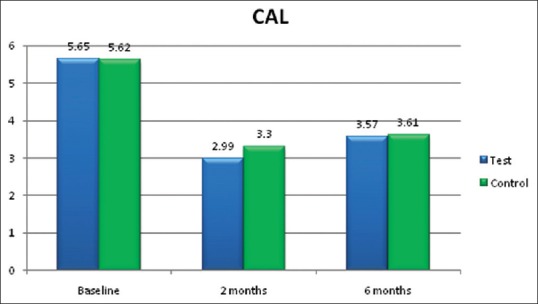
Clinical attachment level (CAL) in mm
The present study differs from the previous studies as it was done on patients with two chronic diseases, i.e., periodontitis and type 2 diabetes mellitus. These patients had been evaluated for 6 months. In addition to decrease in oxidative stress marker MDA with lycopene therapy, it was also found in the present study that systemic lycopene played a role in the glycemic control of diabetic patients.
Based on the results of the study, it can be emphatically said that systemic antioxidant therapy with lycopene has an effective role in reducing oxidative stress in chronic periodontitis patients with type 2 diabetes mellitus.
Limitations of the study
Although lycopene is generally recognized as safe (GRAS) drug, there are no studies with the drug supplemented beyond 2–3 months. So, in this study also, lycopene was supplemented for 2 months only.
As lycopene is available in natural food items like tomatoes and watermelon, lycopene diet washout period was not considered.
CONCLUSION
Oxidative stress has been observed to be a major pathological link between reactive oxygen species induced tissue destruction in patients with chronic periodontitis and altered glycemic control in diabetics. Lycopene has the strongest signet oxygen quenching activity among the antioxidants of β-carotene family. This short-term study shows that it has a beneficial role both in combating oxidative stress induced tissue destruction and restoring glycemic control in patients with chronic periodontitis and type 2 diabetes mellitus. The benefits of lycopene administration can be fully appreciated if it could be used on a long-term basis or as a stop gap monotherapy, for which studies evaluating the consequences of its prolonged administration should be conducted.
Inter-group comparison showed the following:
Lycopene along with nonsurgical periodontal therapy was effective in combating oxidative stress by significantly reducing serum MDA levels both at 2 months and 6 months
Lycopene along with nonsurgical periodontal therapy was effective in restoring altered glycemic control due to oxidative stress in diabetic patients
Lycopene along with nonsurgical periodontal therapy was also efficacious in reducing the PPD.
ACKNOWLEDGMENTS
We sincerely thank Mr. Shiva Prakash, Scientist D, Assistant Director and Madhavi Gurrapu, Research assistant, National Institute of Nutrition (NIN), Tarnaka, Hyderabad for helping us and giving consent to utilize the facilities at NIN for biochemical and immunological analysis. We extend our humble thanks to Dr. Kalyan Chakravathy for his statistical expertise that contributed immensely to the successful completion of this study. We would like to thank Jagsonpal Pharma for their support.
Footnotes
Source of Support: Panineeya Mahavidyalaya Institute of Dental Sciences
Conflict of Interest: None declared.
REFERENCES
- 1.Löe H. Periodontal Disease. The sixth complication of diabetes mellitus. Diabetes Care. 1993;16:329–34. [PubMed] [Google Scholar]
- 2.Cohen DW, Friedman LA, Shapiro J, Kyle JC, Franklin S. Diabetes mellitus and Periodontal Disease: Two year longitudinal observations. I. J Periodontol. 1970;41:709–12. doi: 10.1902/jop.1970.41.12.709. [DOI] [PubMed] [Google Scholar]
- 3.Nelson RG, Shlossman M, Budding LM, Pettitt DJ, Saad MF, Genco RJ, et al. Periodontal disease and NIDDM in Pima Indians. Diabetes Care. 1990;13:836–40. doi: 10.2337/diacare.13.8.836. [DOI] [PubMed] [Google Scholar]
- 4.Seppälä B, Seppälä M, Ainamo J. A longitudinal study on insulin-dependent diabetes mellitus and periodontal disease. J Clin Periodontol. 1993;20:161–5. doi: 10.1111/j.1600-051x.1993.tb00338.x. [DOI] [PubMed] [Google Scholar]
- 5.Firatli E. The relationship between clinical periodontal status and insulin-dependent diabetes mellitus. Results after 5 years. J Periodontol. 1997;68:136–40. doi: 10.1902/jop.1997.68.2.136. [DOI] [PubMed] [Google Scholar]
- 6.Taylor GW, Burt BA, Becker MP, Genco RJ, Shlossman M, Knowler WC, et al. Non-insulin dependent diabetes mellitus and alveolar bone loss progression over 2 years. J Periodontol. 1998;69:76–83. doi: 10.1902/jop.1998.69.1.76. [DOI] [PubMed] [Google Scholar]
- 7.Tsai C, Hayes C, Taylor GW. Glycemic control of type 2 diabetes and severe periodontal disease in the US adult population. Community Dent Oral Epidemiol. 2002;30:182–92. doi: 10.1034/j.1600-0528.2002.300304.x. [DOI] [PubMed] [Google Scholar]
- 8.Tervonen T, Oliver RC. Long-term control of diabetes and periodontitis. J Clin Periodontol. 1993;20:431–5. doi: 10.1111/j.1600-051x.1993.tb00384.x. [DOI] [PubMed] [Google Scholar]
- 9.Collin HL, Uusitupa M, Niskanen L, Kontturi-Närhi V, Markkanen H, Koivisto AM, et al. Periodontal findings in elderly patients with non-insulin dependent diabetes mellitus. J Periodontol. 1998;69:962–6. doi: 10.1902/jop.1998.69.9.962. [DOI] [PubMed] [Google Scholar]
- 10.Grossi SG, Skrepcinski FB, DeCaro T, Robertson DC, Ho AW, Dunford RG, et al. Treatment of periodontal disease in diabetics reduces glycated haemoglobin. J Periodontol. 1997;68:713–9. doi: 10.1902/jop.1997.68.8.713. [DOI] [PubMed] [Google Scholar]
- 11.Nassar H, Kantarci A, van Dyke TE. Diabetic periodontitis: A model for activated innate immunity and impaired resolution of inflammation. Periodontol 2000. 2007;43:233–44. doi: 10.1111/j.1600-0757.2006.00168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mahadev K, Wu X, Zilbering A, Zhu L, Lawrence JT, Goldstein BJ. Hydrogen peroxide generated during cellular insulin stimulation is integral to activation of the distal insulin signaling cascade in 3T3-L1 adipocytes. J Biol Chem. 2001;276:48662–9. doi: 10.1074/jbc.M105061200. [DOI] [PubMed] [Google Scholar]
- 13.Giovannucci E, Ascherio A, Rimm EB, Stampfer MJ, Colditz GA, Willett WC. Intake of carotenoids and retinol in relation to risk of prostate cancer. J Natl Cancer Inst. 1995;87:1767–76. doi: 10.1093/jnci/87.23.1767. [DOI] [PubMed] [Google Scholar]
- 14.Giovannucci E, Clinton SK. Tomatoes, lycopene, and prostate cancer. Proc Soc Exp Biol Med. 1998;218:129–39. doi: 10.3181/00379727-218-44277. [DOI] [PubMed] [Google Scholar]
- 15.Sesso HD, Buring JE, Norkus EP, Gaziano JM. Plasma lycopene, other carotenoids, and retinol and the risk of cardiovascular disease in men. Am J Clin Nutr. 2005;81:990–7. doi: 10.1093/ajcn/81.5.990. [DOI] [PubMed] [Google Scholar]
- 16.Neyestani TR, Shariathzadeh N, Gharavi A, Kalayi A, Khalaji N. Physiological dose of lycopene suppressed oxidative stress and enhanced serum levels of immunoglobulin M in patients with Type 2 diabetes mellitus: A possible role in the prevention of long-term complications. J Endocrinol Invest. 2007;30:833–8. doi: 10.1007/BF03349224. [DOI] [PubMed] [Google Scholar]
- 17.Sharma JB, Kumar A, Kumar A, Malhotra M, Arora R, Prasad S, et al. Effect of lycopene on pre-eclampsia and intra-uterine growth retardation in primigravidas. Int J Gynaecol Obstet. 2003;81:257–62. doi: 10.1016/s0020-7292(03)00040-7. [DOI] [PubMed] [Google Scholar]
- 18.Sibai BM. Prevention of preeclampsia: A big disapointment. Am J Obstet Gynecol. 1998;179:1275–8. doi: 10.1016/s0002-9378(98)70146-2. [DOI] [PubMed] [Google Scholar]
- 19.Singh M, Krishanappa R, Bagewadi A, Keluskar V. Efficacy of oral lycopene in the treatment of oral leukoplakia. Oral Oncol. 2004;40:591–6. doi: 10.1016/j.oraloncology.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 20.Kumar A, Bagewadi A, Keluskar V, Singh M. Efficacy of lycopene in the management of oral submucous fibrosis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:207–13. doi: 10.1016/j.tripleo.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 21.Chandra RV, Prabhuji ML, Roopa DA, Ravirajan S, Kishore HC. Efficacy of lycopene in the treatment of gingivitis: A randomized, placebo- controlled clinical trial. Oral Health Prev Dent. 2007;5:327–36. [PubMed] [Google Scholar]
- 22.Chandra RV, Sandhya YP, Nagarajan S, Reddy BH, Naveen A, Murthy KR. Efficacy of lycopene as a locally delivered gel in the treatment of chronic periodontitis: Smokers vs nonsmokers. Quintessence Int. 2012;43:401–11. [PubMed] [Google Scholar]
- 23.Chandra RV, Srinivas G, Reddy AA, Reddy BH, Reddy C, Nagarajan S, et al. Locally delivered antioxidant gel as an adjunct to nonsurgical therapy improves measures of oxidative stress and periodontal disease. J Periodontal Implant Sci. 2013;43:121–9. doi: 10.5051/jpis.2013.43.3.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arora N, Avula H, Avula JK. The adjunctive use of systemic antioxidant therapy (lycopene) in nonsurgical treatment of chronic periodontitis: A short-term evaluation. Quintessence Int. 2013;44:395–405. doi: 10.3290/j.qi.a29188. [DOI] [PubMed] [Google Scholar]
- 25.Lobene RR, Weatherford T, Ross NM, Lamm RA, Menaker L. A modified gingival index for use in clinical trials. Clin Prev Dent. 1986;8:3–6. [PubMed] [Google Scholar]
- 26.Pryor WA. The antioxidant nutrients and disease prevention-what do we know and what do we need to find out? Am J Clin Nulr. 1991;53(Suppl 1):391S–3. doi: 10.1093/ajcn/53.1.391S. [DOI] [PubMed] [Google Scholar]
- 27.Janero DR. Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radic Biol Med. 1990;9:515–40. doi: 10.1016/0891-5849(90)90131-2. [DOI] [PubMed] [Google Scholar]
- 28.Chapple IL, Matthews JB. The role of reactive oxygen and antioxidant species in periodontal tissue destruction. Periodontol 2000. 2007;43:160–232. doi: 10.1111/j.1600-0757.2006.00178.x. [DOI] [PubMed] [Google Scholar]
- 29.Di Mascio P, Kaiser S, Sies H. Lycopene as the most efficient biological carotenoid singlet oxygen quencher. Arch Biochem Biophys. 1989;274:532–8. doi: 10.1016/0003-9861(89)90467-0. [DOI] [PubMed] [Google Scholar]
- 30.Trivedi S, Lal N, Mahdi AA, Mittal M, Singh B, Pandey S. Evaluation of antioxidant enzymes activity and malondialdehyde levels in patients with chronic periodontitis and diabetes mellitus. J Periodontol. 2014;85:713–20. doi: 10.1902/jop.2013.130066. [DOI] [PubMed] [Google Scholar]
- 31.Valero MA, Vidal A, Burgos R, Calvo FL, Martínez C, Luengoy LM, et al. Meta-analysis on the role of lycopene in type 2 diabetes mellitus. Nutr Hosp. 2011;26:1236–41. doi: 10.1590/S0212-16112011000600007. [DOI] [PubMed] [Google Scholar]


