Abstract
Diabetes mellitus (DM) is a metabolic disease that is rapidly increasing and has become a major public health problem. Type 2 DM (T2DM) is the most common type, accounting for up to 90-95% of the new diagnosed DM cases. The brain is very susceptible to glucose fluctuations and hyperglycemia-induced oxidative stress (OS). It is well known that DM and the risk of developing neurodegenerative diseases are associated. Tea, Camellia sinensis L., is one of the most consumed beverages. It contains several phytochemicals, such as polyphenols, methylxanthines (mainly caffeine) and L-theanine that are often reported to be responsible for tea’s health benefits, including in brain. Tea phytochemicals have been reported to be responsible for tea’s significant antidiabetic and neuroprotective properties and antioxidant potential. Epidemiological studies have shown that regular consumption of tea has positive effects on DM-caused complications and protects the brain against oxidative damage, contributing to an improvement of the cognitive function. Among the several reported benefits of tea consumption, those related with neurodegenerative diseases are of great interest. Herein, we discuss the potential beneficial effects of tea consumption and tea phytochemicals on DM and how their action can counteract the severe brain damage induced by this disease.
Keywords: Brain, caffeine, catechins, diabetes mellitus, L-theanine, tea
INTRODUCTION
Diabetes mellitus (DM) represents one of the greatest threats to modern global health and its incidence is rapidly increasing. The World Health Organization (WHO) estimated that 300 millions of people will develop DM in 2025 [1] and several factors, such as lifestyle, tend to aggravate these numbers. DM is described as a metabolic disorder of multiple etiologies, characterized by chronic hyperglycemia that can result from defects in insulin secretion and/or insulin action [2]. DM may be classified as Type 1 DM (T1DM) or Type 2 DM (T2DM). T1DM is only responsible for 5-10% of all diagnosed DM cases and results from the autoimmune destruction of the insulin-producing beta pancreatic cells, therefore there is a complete lack of insulin that leads to a increase of glucose levels in blood and urine [2]. In turn, T2DM is the most common type of DM and accounts for up to 90-95% of the newly diagnosed DM cases. Among other features, it is characterized by impaired insulin secretion and increased insulin resistance. As result, body glucose metabolism becomes compromised.
The brain uses glucose as a primary energy source, thus it is expectable that glucose metabolism dysfunction promoted by T2DM is responsible for brain damage in diabetic patients [3, 4]. In fact, hyperglycemia may produce several negative effects on cerebral function [5]. Moreover, the brain is quite vulnerable to oxidative stress (OS) due to its high consumption of oxygen, the abundance of easily oxidizable fatty acids and the relative low presence of antioxidant systems [6]. Increased OS plays an important role in the development of DM complications. The abnormal enhancement of free radicals and the decline of antioxidant defense mechanisms lead to damage of cellular organelles and enzymes, increase of lipids peroxidation and insulin resistance [7]. It has been reported that DM-related hyperglycemia and glycemia fluctuations can amplify OS [8, 9] by increasing the production of free radicals and/or by impairing the antioxidant defenses [10]. T1DM and T2DM are associated with pathological alterations in the Central Nervous System (CNS), leading to cognitive deficits and to an increased risk of brain vascular complications [11]. High blood glucose levels, obesity, increased blood triacylglycerols concentration and insulin resistance, are some risk factors that, individually or collectively, increase the probability of neurodegeneration or even neuronal death, which can result in neurodegenerative diseases [12].
Since ancient times, medicinal plants have been used to prevent and treat a wide range of diseases. Conventionally, DM is treated with exogenous insulin or with synthetic oral hypoglycemic agents, according to the type of DM. However, these drugs are not completely safe and may have adverse effects. Natural compounds are considered to be less toxic and relatively cheaper than synthetic ones and large amounts can be consumed in everyday diet [13]. In fact, many edible plants and natural compounds have demonstrated to be beneficial to health [14-17]. Tea, Camellia sinensis (L.), is one of the most consumed beverages in the world, surpassed only by water [18]. Each type of tea has a different composition, which depends on the type of processing, plant maturation, botanical variety, and geographical origin [19]. According to processing and collection, tea can be classified into black tea (BT), oolong tea (OT), green tea (GT) and white tea (WT). It is composed of several bioactive compounds with several health benefits [20-24]. Among those, antidiabetic [25] and neuroprotective [26, 27] properties have been attributed to tea’s high content of phenolic compounds, particularly catechins and other flavan-3-ols, and their antioxidant activity [28]. Moreover, tea supplementation has been reported to interact with several metabolic pathways, and is known to suppress insulin resistance and improve insulin sensitivity [29]. However, the mechanisms underlying the beneficial effects of tea are poorly understood. Herein, we discuss the potential beneficial effects of tea and tea phytochemicals on DM and how tea consumption can counteract the brain oxidative damage induced by this disease. Can tea be essential in the diet of diabetic patients?
GENERAL EFFECTS OF DM ON THE BRAIN
Blood glucose concentrations alter the function of several organs and tissues. As discussed, DM is a complex metabolic disorder and hyperglycemia is a hallmark of this disease. A main consequence of the disease is that glucose is not efficiently transported and metabolized in the target organs. This promotes a chronic hyperglycemia that is known to induce long-term injury and dysfunction of several organs, including a slow progressive brain damage [5]. The brain is highly dependent on glucose supply from the blood. Under diabetic conditions, the supply of glucose to the brain is affected and has an important impact on brain metabolism and function. Thus, a tight regulation of glucose metabolism is critical for the normal metabolic functioning of this organ. The brain depends on glucose as its obligatory fuel due the blood-brain barrier (BBB) and its selective permeability for this metabolite [4]. It is not consensual whether glucose transport into the brain is affected by the diabetic state. Some studies show that DM induces an increased permeability of the BBB [30] whereas others show that the structure of the BBB is maintained [31]. Glucose crosses BBB through specific glucose transporters (GLUTs). Several GLUTs have been identified in the brain, namely GLUT1, GLUT2, GLUT3, GLUT4, GLUT6, GLUT8 and GLUT10 [32]. The rate of glucose entry into the cell is limited by the number of GLUTs on the cell surface and the affinity of the glucose transporters. Notably, DM has been shown to induce a depletion of GLUTs [33] illustrating that diabetic individuals have inability to transport glucose, suggesting an association of this disease with brain damage. Changes in GLUTs function and expression have also been observed [32]. Therefore, there is compelling evidence that DM alters GLUTs expression and function, and consequently glucose transport to the brain, which induces dramatic effects in brain glucose homeostasis and function.
Insulin is responsible for the regulation of glucose homeostasis. In the brain, insulin signaling has been highlighted as a main regulator of neurodegenerative diseases. A recent study showed that insulin treatment in cerebral cortex of diabetic rats was able to normalize the alterations induced by T1DM [34]. However, this hormone affects some cerebral functions, such as cognition, memory and synaptic plasticity through complex insulin/insulin receptor signaling pathways [35]. Thus, it is possible that perturbation of insulin signaling is in the pathogenesis of neurological diseases and may lead to neurodegeneration [36].
The brain appears to use compensatory mechanisms to ensure adequate supply of glucose. For example, brain glycogen metabolism may be a contributing partner for these compensatory mechanisms [37]. Glycogen is an intracellular glucose reservoir and has the advantage of being rapidly metabolized when needed. Thus, it may play an important role in DM for the maintenance of a proper brain function. Besides, glycogen is important for maintaining neuronal activity during energy deprivation [38]. Hypoglycemic studies on rats have also shown that neuronal function was preserved longer when an elevated amount of glycogen was present in the brain at the onset of hypoglycemia [38]. Thus, these studies highlight that hypoglycemia should also be considered a major risk for the brain health of diabetic individuals. They also present compelling evidence that glycemia fluctuations and metabolic changes have a very negative impact in the brain.
CONNECTION BETWEEN HYPERGLYCEMIA AND OXIDATIVE STRESS-INDUCED NEURO-DEGENERATION
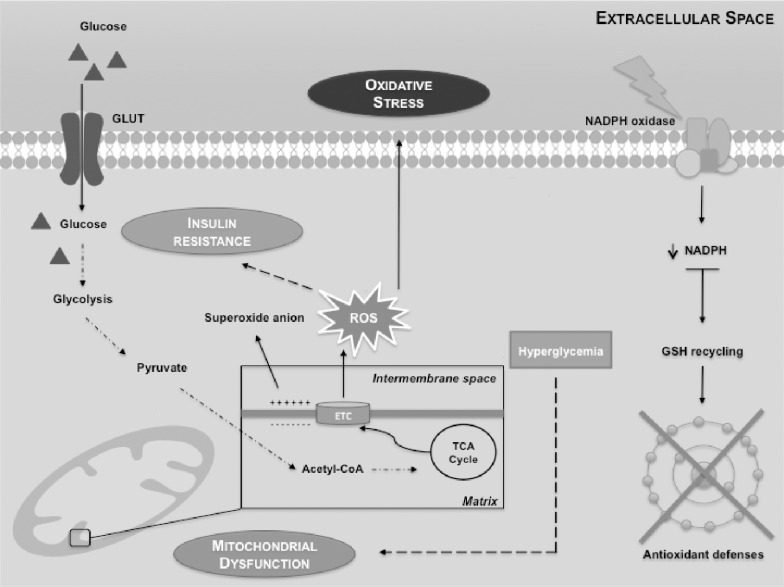
Hyperglycemia is a hallmark of DM and results from impaired insulin synthesis and/or insulin resistance. It is associated with long-term injury and dysfunction in the brain [5]. Any disturbance in glucose metabolism compromises the normal functioning of the brain [5]. Animal studies have reported that there is an overall reduced glucose metabolism and regional changes in brain’s glucose metabolism of animals with poorly controlled DM providing evidence that hyperglycemia alters brain glucose metabolism and physiology [39]. In fact, DM increases the vulnerability of specific brain areas to neuronal damage being the cerebral cortex particularly sensitive [40]. Indeed, it has been reported that cortical neurons and astrocytes are more vulnerable to deregulation of glucose metabolism than cells from striatum or hippocampus [41]. DM also increases the risk of cognitive impairments and dementia [11]. It has been reported an association between T1DM and several brain dysfunctions, such as cognitive decline [42] and atrophy [43]. These evidences indicate that DM induces structural, morphological and functional brain alterations. Furthermore, it is becoming evident that diabetic individuals have a higher risk for developing neurodegenerative diseases [44, 45], such as Alzheimer’s disease (AD). Data on the association between T1DM and AD are scarce. Noteworthy, T2DM has been clearly associated with AD and abnormalities in insulin metabolism are among the major factors proposed to mechanistically influence the onset of AD [44]. The impairment of insulin signaling is directly involved in the hyperphosphorylation of tau protein and intracellular deposition of amyloid beta, which potentiate the formation of neurofibrillary tangles and senile plaques, the neuro-pathological hallmarks of AD [44, 45]. Thus, AD has been recognized as an “insulin-resistant brain state”. Notably, mitochondrial abnormalities and OS are relevant events between these two diseases and proposed as common molecular mechanisms responsible for the onset of the diseases [46]. Nevertheless, more studies are needed to unveil the link between mitochondria, OS, DM and AD. Cerebral metabolism of glucose requires transport through the BBB, glycolytic conversion to pyruvate, metabolism via the tricarboxylic acid cycle and ultimately oxidation to carbon dioxide and water for full provision of adenosine triphosphate (ATP) and its high-energy equivalents. On the other hand, OS is also involved in the development and progression of DM [47]. It has been reported that DM-related hyperglycemia and glycemia fluctuations amplify OS [8, 9] by increasing the production of free radicals and/or by declining antioxidant defenses mechanisms [7]. The hyperglycemia-related increase in reactive oxygen species (ROS) and reactive nitrogen species (RNS) can be due to several factors, such as mitochondrial respiratory system [48], nicotinamide adenine dinucleotide phosphate (NADPH) oxidase [49], formation of advanced glycation end products (AGEs) [9] and imbalance of glutathione redox status [50]. Under normal conditions, glucose crosses BBB through specific GLUTs [51, 52]. Then, the glucose is decomposed to pyruvate and transported to the mitochondrial matrix, oxidized and decarboxylated by the pyruvate dehydrogenase forming the two carbon intermediate acetyl coenzyme A (Acetyl-CoA) which can enter the tricarboxylic acid (TCA) cycle. However, mitochondria is the main sites for ROS production within the electron transport chain (ETC) (mainly in complex I and III) [53], leading to OS and insulin resistance (Fig. 1).
Fig. (1).
Mitochondrial dysfunction and sustained activation of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase lead to insulin resistance, reactive oxygen species (ROS) production and impaired antioxidant defenses. Mitochondria are the main generators of ROS within electron transport chain (ETC). In normal conditions, glucose breakdown starts by glycolysis, generating among other compounds, pyruvate. Pyruvate is then converted into acetyl coenzyme A (acetyl-CoA) that enters the tricarboxylic acid (TCA) cycle. The produced electrons are stored in molecules that are then injected into the ETC, to generate the electrochemical gradient. Mitochondrial superoxide production is a major cause of the oxidative damage. This is particularly important since ROS may contribute to insulin resistance. Sustained activation of NADPH oxidase leads to decreased intracellular levels of NADPH and therefore the recycling of reduced glutathione (GSH) is limited, impairing antioxidants defenses.
Hyperglycemia contributes to mitochondrial dysfunction. Sustained activation of NADPH oxidase leads to impaired antioxidant defenses [49]. Moreover, if the levels of NADPH decrease, the recycling of reduced glutathione (GSH) is limited. NADPH oxidase can control the antioxidant potential of cells by regenerating GSH from oxidized glutathione (GSSG) [54]. Particularly, increased NADPH oxidase activity contributes to a large number of pathologies such as DM, cardiovascular diseases and neurodegeneration. Indeed, it has been proposed that sustained activation of NADPH oxidase in DM leads to ROS overproduction and impaired antioxidant defense [49] (Fig 1).
The forkhead (FOX) proteins are functionally diverse transcription factors that regulate a large number of regulatory genes and may be involved in a variety of cellular processes. The FOX subgroups range from FOXA to FOXS, designated by a letter, and within each subgroup proteins are given a number [55]: all capital letters refer to human (e.g. FOXO); only the first letter capitalized for mouse (e.g. Foxa2); and the first letter and subgroup capitalized for all other chordates (e.g. FoxA1). The FOX system was recently characterized and plays important roles in the development and pathogenesis of some diseases, including DM [55].
FOX proteins are expressed in several organs, such as liver, pancreas, and brain, among others. As transcription factors, have the ability to bind to deoxyribonucleic acid (DNA) to activate or repress target gene expression. For example, Foxa2 regulates gene expression in the liver, pancreatic islets and adipocytes [56]. It was reported that chronic hyperinsulinemia in T2DM causes the inactivation of Foxa2 by nuclear exclusion, thereby deteriorating hepatic lipid accumulation and lead to insulin resistance through increased fatty acid biosynthesis and reduced beta-oxidation [57]. Thus, preventing the inactivation of this transcription factor can decrease the complications arising from DM. Notably, it was reported that activation of Foxa2 in the liver of insulin-resistant mice resulted in decreased hepatic triglyceride content, increased insulin sensitivity, normalized plasma glucose and significantly lowered plasma insulin [57]. On the other hand, FoxO subgroup is involved in the onset of DM and its complications it has received much attention because of its recently discovered roles in ROS detoxification [58] and glucose metabolism [59]. FoxO protein expression has been reported in several organs [60]. FoxO1 and FoxO3a are expressed in adipose tissue and liver, respectively [55], and can act as cellular sensors of stress and survival signals. Notably, FoxO6 expression appears to be restricted to the brain [55]. FoxO1, which is phosphorylated and inhibited by protein kinase B (Akt), plays an important role in insulin signaling [55]. Moreover, the FoxO1 control of energy homeostasis is particularly striking under conditions of metabolic dysfunction and/or insulin resistance. Under pathophysiological conditions, such as those verified in DM, FoxO1 expression may alter the expression of genes involved in the combat of OS preserving the cellular function [55]. On the other hand, phosphorylation of FoxO3a is increased in rat and mouse renal cortical tissues two weeks after the induction of DM [55]. Furthermore, glial cell line-derived neurotrophic factor can protect enteric neurons from hyperglycemia, may affecting Akt signaling and preventing FoxO3a activation. FoxO proteins are also linked to the prevention of diabetic complications, preservating cellular energy reserves and mitochondrial integrity [61]. FoxOs are activated by protein kinases, which are also involved in OS, DNA damage, cytokines and ischemia. Moreover, FoxO transcription factors have been implicated in neuro-degenerative diseases, though their exact role remains controversial [62, 63]. Further studies are needed to clarify whether FOX proteins activation is neuroprotective or detrimental in neurodegenerative diseases and also under diabetic conditions. Thus, it may be suggested that if FOX proteins prove to be either beneficial or detrimental in conditions of hyperglycemia, a pharmacological intervention in this FOX system can be an effective treatment for T2DM and to decrease the risk of progression to neurodegenerative diseases. The use of compounds from natural products, in this case, can be a strategy for treating DM and its complications.
New ways to reduce brain damage caused by DM may pass through alterations in lifestyle, particularly by diet changes. There is a large interest in finding an effective therapy and tea seems to be a good candidate, with antidiabetic [25], neuroprotective [26, 27] and antioxidant [28] properties that may be useful to counteract and/or reduce DM-related dysfunction.
TYPES OF TEA
Tea plant (Camellia sinensis (L.)) is an evergreen shrub of the Theaceae family, native to Southeast China and now cultivated in over 30 countries across the world [27], including S. Miguel Island (Azores Archipelago, Portugal). Tea is one of the most widely consumed beverages in the world, surpassed only by water [18], with a per capita consumption of approximately 120 mL/day [64]. The popularity of tea consumption is probably related with its sensorial properties, relatively low retail price, stimulating effects and potential health benefits [20, 23, 24]. In fact, tea has been used for centuries in traditional chinese medicine to prevent and treat several diseases, such as DM [20, 65].
According to processing and collection, tea can be classified into BT (completely fermented), OT (semi-fermented), GT and WT (not fermented). Oxidation (frequently followed by polymerization), commonly called “fermentation”, occurs with the exposure to air and is a reaction catalyzed by the enzyme polyphenol oxidase [64]. Due to the level of “fermentation”, all types of tea have different chemical compositions (phenolic profiles) and organoleptic properties (appearances and tastes). To produce GT, the leaves are rolled and steamed to minimize the oxidation by inactivation of the enzyme before drying [64]. OT is produced with a shorter oxidation period than BT and has a taste and color somewhere between GT and BT [66]. In BT, the leaves are rolled and cellular compartmentalization is disrupted bringing the phenolic compounds to contact with polyphenol oxidases and then they undergo oxidation for 90 to 120 minutes [67].
TEA CHEMICAL COMPOSITION
Tea is composed of a complex mixture of about 2000 chemical compounds, including proteins, polysaccharides, minerals and trace elements, organic acids, lignin, polyphenols, methylxanthines and amino acids [20, 68]. Several of these compounds are bioactive and are believed to have health benefits [20-22, 24, 69]. In this review we will give special focus to phenolic compounds, caffeine and L-theanine, since these phytochemicals present strong antioxidant, antidiabetic and/or neuroprotective properties.
Phenolic Compounds
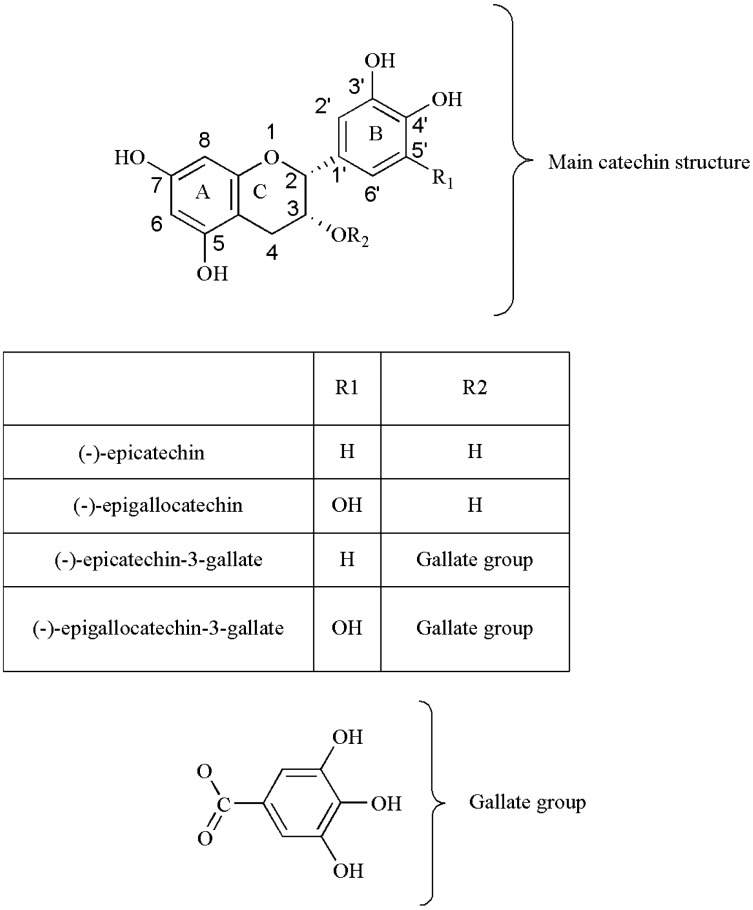
The polyphenols are secondary plant metabolites, widely distributed in nature. They can be pigments or products derived from defense reactions of plants against environmental aggressions. Catechins (also known as flavan-3-ols) and their derivatives are the main class of phenolic compounds present in tea leaves. The major catechins are (-)-epicatechin (EC), (-)-epigallocatechin (EGC), (-)-epicatechin-3-gallate (ECG), and (-)-epigallocatechin 3-gallate (EGCG) [20, 22, 23]. The health benefits attributed to catechins are mainly due to its chemical structure. The major catechins are composed of two aromatic rings (A and B) linked to a dihydropyran heterocyclic ring (C) and are characterized by the presence of several hydroxyl groups [70] (Fig. 2). Their chemical differences are due to the presence of different groups attached to those rings [20, 22, 70]. EC have an ortho-di-hydroxil group in the B ring (at carbons 3’ and 4’) and a hydroxyl group in the C ring (at carbon 3). EGC has a trihydroxyl group at carbons 3’, 4’, and 5’ on the B-ring, while ECG has a gallate moiety esterified at carbon 3 of the C-ring and EGCG has both a trihydroxyl group at carbons 3’, 4’, and 5’ on the B-ring and a gallate moiety esterified at carbon 3 on the C-ring. Tea composition is affected by the oxidation process, a reaction catalyzed by polyphenol oxidase [64]. Thus, the concentration of catechins is different for each type of tea. The tea types with higher levels of catechins are GT and WT. EGCG is the most abundant catechin in tea leaves and has been extensively studied [65, 68, 71, 72]. It represents 50–80% of total catechins, and is thought to contribute to the beneficial effects ascribed to tea [73, 74]. Theaflavins and thearubigins are formed during oxidation by polyphenol oxidase [75]. These polymers are responsible for BT bitter taste and dark color [65]. Theaflavins possess a basic chemical skeleton comprised of the bicyclic benzotropolone ring and are the result of main catechins dimerization [76]. Thearubigins are produced subsequently to a series of complex reactions that form its oligo-polymeric structures.
The redox properties of phenolic compounds are in the basis of tea antioxidant properties, which can be very useful if its consumption is adopted as a health practice [77]. Several reports have shown that tea catechins and other polyphenols are effective scavengers of ROS and RNS [78-80]. This is of extreme relevance since OS is known to induce neuronal death and to be involved in neuro-degenerative diseases [81, 82]. Thus, there is a growing interest in the possible neuronal benefits of tea consumption for DM patients.
Methylxanthines
Methylxanthines, such as caffeine, theophylline and theobromine, are purine bases derivatives present in tea, being the first predominant (2-4%) [83]. Caffeine is one of the most consumed substances in the world [84] and due to its chemical stability, the oxidation process does not affect its levels in tea [76]. However, some researchers found that BT and OT have greater caffeine content than GT [85] and that WT has also a higher content than GT [23, 86]. Some authors argue that the lowest caffeine content in GT contributes to its beneficial health properties [87]. Caffeine is absorbed by the stomach and small intestine within 45 minutes after intake and it reaches the maximum concentration in blood between 15-120 minutes [88]. It presents CNS stimulant properties and acts through stimulation of adenosine receptors and competitively inhibits the action of this nucleoside into cells, resulting in the release of norepinephrine, dopamine and serotonin [89]. Caffeine is also a likely candidate against memory loss [90] and has a great neuroprotective potential [91, 92]. Studies in rats have shown that this methylxanthine can interact with glucose transporters in adipocytes and act as an antagonist of adenosine receptors [93]. Interestingly, the consumption of caffeine-containing beverages, in particular tea, is associated with a lower risk of developing T2DM [25, 94]. Moreover, studies in humans have shown that caffeine decreases glucose uptake in the body [95] and increases insulin insensitivity [96]. Some authors have also reported that caffeine intake is inversely associated with body weight increase and satiety [97, 98]. Caffeine and theophylline are also involved in the stimulation of pancreatic beta cells [99]. In the brain it has been shown that increased levels of caffeine are associated with decreased risk of neuro-degenerative diseases [100].
L-theanine
L-theanine is a free amino acid which presents a structural similarity to glutamate, an important neuro-transmitter [101]. L-theanine constitutes between 1% and 3% of the dry weight of tea, but this percentage may vary according to growing location and method of cultivation, tea grade, variety, processing and collection time [102]. GT contains lower or similar levels of L-theanine as compared to BT and OT [103]. This amino acid is considered as a relaxing agent with antioxidant [104, 105] and neuroprotective effects [101]. However, its pharmacology is relatively unknown and human studies are inconclusive [106, 107]. Metabolically, it is easily absorbed from the gastrointestinal tract and peak plasma concentrations are detected 30 minutes after administration [101]. According to Yokogoshi and collaborators [108], L-theanine is partially transported to the brain via a leucine-preferring transporter system and can cross the BBB exercising protective and preventive effects on neuronal cell death. The benefits of L-theanine for health are reported to be associated with regulation of blood pressure, effective prophylaxis and treatment for neurodegenerative diseases, among others [109-111].
Antioxidant, Antidiabetic and Neuroprotective Properties of Tea and Tea Phytochemicals
New ways to reduce the brain damage caused by DM may arise by modifying lifestyles, particularly by changes in diet. There is a large interest in finding an effective therapy for DM-associated brain dysfunction. Many health benefits have been attributed to tea consumption [21-24, 112]. There are studies reporting that tea seems to be a good candidate, with interesting antioxidant [28], antidiabetic [25] and neuroprotective [27] properties. The CNS is particularly sensitive to oxidative damage. As discussed, oxidative damage has been linked to the development of DM and also to neurodegenerative diseases in general [113]. Therefore, antioxidants that scavenge ROS may be of great value in preventing the onset and/or the progression of oxidative-mediated diseases [114].
Most of the works focused on the health benefits of tea consumption are relatively recent and not very conclusive. The antioxidant power of tea is mainly due to its polyphenolic content [28]. Polyphenols present in the extracts of C. sinensis have been reported to possess anti-hyperglycemic activity, by enhancing insulin activity and possibly by preventing damage to pancreatic beta cells [115]. Although the exact mechanisms by which tea polyphenols ameliorate DM-related brain dysfunctions are not clear, there is compelling evidence that the high phenolic content of tea leaves have not only a lowering effect on OS but also an anti-hyperglycemic potential, by decreasing insulin resistance and improving insulin sensitivity [116]. Moreover, EGCG is the most effective tea catechin and can react against most of ROS. The antioxidant activity of phenolic compounds is mainly due to their redox properties, which allow them to act as reducing agents, singlet-oxygen quenchers and metallic-ion chelators [77]. As discussed above, tea components can help to fight several diseases. Although there is no consensus among researchers, tea and its individual phytochemical components are of great interest for its ability to counteract diseases such as DM and the possibility to avoid the development of neurodegenerative diseases. Nevertheless, reports focused on the effect of tea on DM-induced alterations in brain’s metabolism are very scarce. Previous studies have shown that tea polyphenols inhibit inflammatory response and have neuroprotective effects after ischemia reperfusion injury [87], and may be able to protect the BBB integrity [117]. Moreover, caffeine is one of the main tea phytochemicals and has the ability to cross BBB exerting pivotal effects on the brain and acting in the CNS. However, more studies must be carried out to evaluate the exact mechanism of action of tea and its phytocomponents in brain metabolism. In the last decades, the health benefits of tea have been evidenced by in vitro and in vivo studies, as well as by epidemiological studies. In addition to the antidiabetic and neuroprotective properties, the antioxidant capacity of tea has been very fashionable.
Protective Effects of Tea in Diabetes: in vitro Experiments and Animal Models
A study in streptozotocin (STZ)-induced diabetic rats with hepatic injury showed that rats treated with GT (prepared by using 1.5 g of GT tea leaves per 100 ml of boiling water) during 8 weeks, had a reduction of blood glucose level and revealed that daily treatment with GT extract markedly improved biochemical and histopathological status of these rats [118]. GSH levels were also reported to be increased by GT administration. GSH is a major non-protein thiol in living organisms, which plays a central role in the coordination of procedures for the body’s antioxidant defense [118]. These results illustrate that there is an improvement in OS and that daily treatment with GT extract markedly improves liver antioxidant status in rats with STZ-induced DM. In other experiments, normal and alloxan-induced diabetic rats were administered 50 and 100 mg/kg body weight GT extract [119]. The alloxan is a glucose analogue, such as STZ, which accumulates in pancreatic beta cells and selectively inhibits insulin secretion [120]. Alloxan generates ROS by a redox reaction in the presence of intracellular thiols, such as glutathione, in which the final product is dialuric acid. This acid to undergo auto-oxidation generates free radicals. Interestingly, the continuous administration of GT reversed these effects. In another study it was also demonstrated that EGCG, the major GT and WT component, has an hepatoprotective effect [121]. Ortsäter and collaborators [73] conducted an in vivo study in db/db mice that received diets supplemented with or without EGCG for 10 weeks. The db/db mice is a model of obesity, diabetes and dyslipidemia, where the mice are homozygous for a point mutation in the gene for the leptin receptor (leptin hormone regulates adipose-tissue) [122]. These mice were treated with EGCG and showed improved glucose tolerance, increased glucose-stimulated insulin secretion and preservation of islets of Langerhans structure. This study illustrates that dietary supplementation with EGCG can potentially be a nutritional strategy for the prevention and treatment of T2DM. The antidiabetic effects of tea were also demonstrated in rodent models of T2DM and H4IIE rat hepatoma cells [123]. The results showed that EGCG improves glucose and lipid metabolism in H4IIE cells and markedly enhances glucose tolerance in diabetic rodents.
The neuroprotective properties of tea are greatly associated with EGCG. In fact, EGCG can easily cross the BBB and reach the brain parenchyma [124]. Besides, long term administration was shown to improve spatial cognition and learning ability in rats [125] and to reduce cerebral amyloidosis in AD transgenic mice [126]. Moreover, the consumption of EGCG inhibits OS-induced neuronal degeneration and cell death in pre- and post-traumatic brain injury [127]. In an ischemic model, EGCG was also able to prevent free radical production after brain injury and, noteworthy, it also decreased brain edema [87, 128]. Lipid peroxidation and the levels of apoptotic markers were also found to be decreased after EGCG treatment in the brain of rats following ischemia-reperfusion [129]. Using a rat model of Parkinson Disease (PD), it was reported that EGCG inhibits the production of free radicals by mitochondria of hippocampal and glial cells, exerting a significant neuro-protective role [128]. A study by Rodrigues and collaborators [69] evaluated the effects of catechins in an extract of GT poor in EGCG. The authors showed that other catechins (EC, EGC and ECG) are effective protectors of proteins and lipids against oxidative changes related to aging. This study demonstrates that EGCG is not essential to some neuroprotective effects and that EC, EGC and ECG are able to improve behavioral performance and protect against oxidative damage. The potential neuroprotective effect of WT extract on hydrogen peroxide induced toxicity in PC12 cells has also been reported [27]. These cells were treated with various doses of WT (10–250 μg/ml) and cell survival was significantly higher in WT-treated cells compared to hydrogen peroxide-treated cells. Tea has demonstrated good antioxidant capacity and was able to reduce OS. Oral intake of tea catechins proved to be neuroprotective in animal models of neurotoxicity [130] and in animal models of aging, which are characterized by increased OS levels [131]. A study by Cho and collaborators [107] showed that L-theanine has antioxidant and neuroprotective effects against PD-related neurotoxicants and may be clinically useful for preventing PD symptoms. Another study proved the antioxidant power of this amino acid through the reduction of lipid peroxidation in the brain, illustrating that L-theanine can protect the brain against OS [104]. The brain is highly sensitive to OS due to its high concentration of readily oxidizable fatty acids and high oxygen consumption. Excessive activation of some pathways may lead to neuronal cell death [132]. Thus, inhibition of these pathways might be beneficial in the treatment of neurodegenerative diseases. L-theanine is able to inhibit some of these pathways preventing the neuronal death and thus loss of memory [133].
DM can trigger the onset of neurodegenerative diseases. The antidiabetic and neuroprotective effects of tea are often attributed to its polyphenols content [28, 134]. Several studies have reported that catechins exert protective effects in different models of neurotoxicity including glutamate, ischemia, OS and beta-amyloid peptides [27, 135, 136]. Moreover, the use of EGCG in the treatment and prevention of neurodegenerative diseases has been suggested. It has also been reported that EGCG inhibitory effect on malignant brain tumors is mediated through insulin-like growth factor-I (IGF-I) action [137]. Although the mechanisms remain unknown, this work illustrates that EGCG may also interact with insulin signaling. All studies reported herein (summarized in Table 1) show that tea consumption appears as an appealing and safe therapy against DM-induced neurodegeneration. However, most of the reported studies in animal models were performed using STZ to induce a DM state. This is not the only method for inducing a diabetic condition. However, its ability to damage the pancreatic beta cells turns it as the most advantageous. The alloxan is other method used to cause pancreatic injury and is responsible to produce free radicals. Most studies that relate tea and DM use STZ-induced diabetic animal models. Notably, tea consumption was able to decrease the damage caused by STZ as well by alloxan. Studies carried out in more physiologically relevant diabetes models are scarce and more studies are needed to further confirm this property of tea consumption.
Table 1.
Summary of the potential protective effects of tea and tea components, in vitro and in vivo, on DM and brain.
| Antioxidant Activity |
Oxidative Stress |
Anti-diabetic Activity | Insulin Activity | Hepato- protection |
Neuro- protection |
Cognitive Deficits | ||
|---|---|---|---|---|---|---|---|---|
| Studies in vitro | PC12 cells | ↑ [27] |
↓ [27] |
nd | nd | nd | ↑ [27] |
nd |
| H4IIE hepatoma cells | nd | nd | ↑ [123] |
nd | nd | nd | nd | |
| Hippocampal neuronal cells | ↑ [87] |
↓ [87] |
nd | nd | nd | ↑ [87] |
nd | |
| N18D3 cells | ↑ [129] |
nd | nd | nd | nd | ↑ [129] |
nd | |
| Dopaminergic cells | ↑ [107] |
nd | nd | nd | nd | ↑ [107] |
nd | |
| Studies in vivo | Wistar rats | ↑ [69, 118, 119, 121, 128] |
↓ [69, 118, 119, 121, 128] |
↑ [118, 119, 121] |
nd | ↑ [75, 118] |
↑ [27, 69, 87] |
↓ [125] |
| Animal models | ↑ [104, 131] |
↓ [104, 131] |
↑ [73, 123] |
↑ [73] |
↑ [73] |
nd | ↓ [133] |
|
| Sprague- Dawley rats | nd | nd | nd | nd | nd | ↑ [130] |
nd | |
| Tg APPsw mice | nd | nd | nd | nd | nd | ↑ [126] |
nd | |
Tg = Transgenic
Legend: ↑- increase; ↓- decrease; nd – non determined; numbers are references as indicated in references section.
Protective Effects of Tea in Human Intervention Studies
Tea extracts have been reported to act as hypoglycemic agents [138]. However, when we discuss human studies, the scenario becomes different. This field needs to be exploited to establish correlations between the results obtained using different models and draw accurate conclusions. Nevertheless, some studies have been performed. A prospective epidemiological study performed in Japan found that people reported to drink 6 or more cups of GT per day had one-third less incidence of T2DM over 5 years [139]. However, a randomized study by Mackenzie and collaborators [138] used capsules containing 150 mg of GT catechins (equivalent to the amount in 7 cups of GT), 75 mg of BT theaflavins (equivalent to the amount in 35 cups of BT) and 150 mg other tea polyphenols. The diabetic individuals consumed the capsules for 3 months. At the end of the study there were no significant effects of GT and BT extracts on glucose control in adults with T2DM. This could be due to several factors. The capsules used in this study did not contain caffeine nor L-theanine, which are main components of tea. On the other hand, it is possible that tea is more effective when continually consumed over the day, as is typically the case with tea drinking, as opposed to receiving it in a single or double bolus. The phyto-components used, the applied dose, the individuals in the study and the study design, among several other uncontrolled issues explain the differences in the results. WT and GT are less oxidized than BT, having relatively higher concentrations of catechins. Thus, the ingestion of these two types of tea should provide better results in a situation of DM. A study in men and women with 45-74 years reported a significant inverse association between BT consumption and risk of incident T2DM [140]. A study conducted by Haskell and collaborators [141] evaluated the neurocognitive effects of L-theanine, caffeine and their combination. The combination of these two compounds showed better results than each component alone. Moreover, epidemiological studies suggest that tea consumption can have beneficial effects by increasing the cognitive function, improving learning and memory, and protecting against neurodegenerative diseases [142]. A cross-sectional study in Japan evaluated the association between GT consumption and cognitive function in elderly patients. It was reported that the consumption of approximately 200 mL of GT lowered the prevalence of cognitive impairment [143]. This study demonstrated that the greater consumption of GT was associated with lower cognitive deficits.
A case-control study by Checkoway and collaborators [144] reported that individuals who consumed two or more cups of tea per day have a decreased risk of developing PD. In fact, GT consumption is inversely correlated with the incidence of dementias like AD and PD [145]. There is a lack of information about tea consumption not only by diabetic but also by non-diabetic individuals with neuro-degenerative diseases. It is known that tea phytochemicals reduce blood glucose levels, improve glucose tolerance and insulin sensitivity, and have hepatoprotective activity in diabetic conditions. Thus, and knowing that DM may lead to neurodegeneration, tea consumption may decrease the brain damage preventing neurodegenerative diseases. On the other hand, glycemic fluctuations can amplify brain OS inducing several alterations. Tea consumption may be beneficial due to its high antioxidant power, potentiated by its polyphenols content, protecting the brain against neurodegeneration.
A review by Hayat and collaborators [146] suggests that tea consumption has several health benefits but can also present some risks. However, the numerous benefits outweigh the reduced adverse effects. An adverse effect of tea (especially BT and GT) or its components occurs when they are consumed in excessive amounts. For example, caffeine increases blood pressure and high concentrations may be harmful to people with cardiovascular disease or cardiac problems. Pregnant and/or breastfeeding women should moderate their consumption of tea for the same reason. Some tea compounds may have deleterious effects in certain pharmacological concentrations, in vulnerable populations, in certain diseases or in a polypharmaceutical context. Besides, tea vitamins (e.g. vitamin K) may antagonize the effect of some drugs [147] and EGCG can induce hepatic and gastrointestinal toxicity [148, 149]. High proportions of tannins in BT may also contribute to decreased absorption of iron in organism [150]. Further human studies, particularly clinical trials, are needed to investigate the role of long-term tea consumption, and their innate bioactive compounds, in relation to T2DM risk. Several questions remain to be answered: What are the molecular mechanisms involved? In which doses should tea be consumed? How can we increase the bioavailability of these antioxidants in the brain? All these issues remain to be unraveled. It is unwise to recommend a direct increase in tea consumption until there is more thorough data from clinical trials related to the topic, with respect to the possible benefits but also the possible side effects or harm derived from excessive tea consumption. Very recent studies claim that EGCG has cytotoxic effects on certain types of cancers and cells, causing breaks in double-stranded DNA, apoptosis of normal cells and increased frequency of mutations [151]. Sayin and collaborators [152] claim that antioxidants in general can accelerate the growth of early tumors or precancerous lesions in high-risk populations.
The inverse association between tea intake, DM and neurodegenerative diseases in epidemiological studies (summarized in Table 2) may, or may not, be associated with antioxidants. Controlled interventional studies are needed to clarify the extent to which the antioxidant content and other biochemical features of tea may contribute to long-term health benefits. In addition, not only polyphenols may have protective effects in the brain. L-theanine and caffeine also provide clear cognitive benefits [153]. Several studies have reported that combination of L-theanine and caffeine improve the cognitive ability in healthy humans [110, 154]. Moreover, caffeine alone has also been reported to enhance the cognitive performance [155].
Table 2.
Summary of the potential protective effects of tea and tea components, observed in epidemiological studies, on DM and brain.
| Tea / Component Tested | Effects Observed | |
|---|---|---|
| Epidemiological studies | GT | ↓Risk of incident T2DM [139] Not found effects [138] ↓Cognitive deficits [143] ↑Cognitive performance [142] |
| BT | Not found effects [138] ↓Risk of incident T2DM [140] |
|
| Caffeine + L-Theanine | ↑Cognitive performance [141, 154] ↓Blood pressure [110] |
|
| Nonspecific | ↓Risk of PD incidence [110] | |
| Caffeine | ↑Cognitive performance [155] |
BT = Black tea; GT = Green tea; PD = Parkinson’s disease; T2DM = Type 2 diabetes mellitus; WT = White tea
Legend: ↑- increase; ↓
CONCLUSION
DM is a pandemic disease affecting an enormous number of people around the world. Although the progression of the disease results from several factors, OS is a key player. Thus, it is urgent to find new and effective preventive or therapeutic strategies to ameliorate DM-induced OS. Disturbances in glucose metabolism, insulin signaling and OS can cause severe brain damage and stimulate the occurrence of several neurodegenerative diseases. Most of the DM-related deleterious effects to the brain may be improved with glycemic control, but normoglycemia is virtually impossible to achieve 24-hours/day in diabetic individuals. C. sinensis is a medicinal plant that deserves special merit since tea is one of the most ancient and consumed beverages in the world and has been used to prevent and treat a wide variety of human diseases. In addition, in the last years, several studies have shown that the regular consumption of tea has important beneficial effects to the brain, namely when subjected to high OS environment as occurs in DM individuals. However, there is a lack of studies concerning the use of tea extracts and/or tea consumption by diabetic individuals and the possible beneficial effects to their brain. More studies are needed to unveil the mechanisms responsible for the action of phytocompounds present in tea. Moreover, in usual dietary practice, tea is generally prepared by using 1 g of tea leaves per 100 ml of boiling water. Nevertheless, the differences between the animal species subjected to research and humans may also hamper the correct interpretation, extrapolation and practical application. Thus, although tea consumption appears to be an effective and inexpensive choice as antidiabetic therapy, more studies are needed to unravel the role of tea and its phytochemicals in the protection against the injury caused by DM in the brain.
CONFLICT OF INTEREST
The author(s) confirm that this article content has no conflict of interest.
Figure 2.
Chemical structures of the main tea catechins. The figure illustrates two aromatic rings (A, B) and a dihydropyran heterocyclic ring (C), which is the basic structure of flavonoids. The (-)-epicatechin (EC) is constituted by an ortho-di-hydroxil group in the B ring (at carbons 3’ and 4’) and a hydroxyl group in the C ring (at carbon 3), and its ester derivative (-)-epicatechin 3-gallate (ECG) differs in this structure by possessing an additional gallate moiety esterified in the C ring, at carbon 3. On the other hand, (-)-epigallocatechin (EGC) contains a trihydroxil group on the B ring (at carbons 3’, 4’ and 5’) and its ester derivative (-)-epigallocatechin 3-gallate (EGCG) additionally possesses an esterified gallate at the carbon 3 of the C ring.
ACKNOWLEDGEMENTS
This work was supported by the “Fundaçãopara a Ciência e a Tecnologia”– FCT (PTDC/QUI-BIQ/121446/2010 and PEst-C/SAU/UI0709/2014) co-funded by Fundo Europeu de Desenvolvimento Regional - FEDER via Programa Operacional Factores de Competitividade - COMPETE/QREN. M.G. Alves (SFRH/BPD/80451/2011) was funded by FCT. P.F. Oliveira was funded by FCT through FSE and POPH funds (Programa Ciência 2008).
LIST OF ABBREVIATIONS
- Acetyl-CoA =
Acetylcoenzyme A
- AD =
Alzheimer’s disease
- AGEs =
Advanced glycation end products
- ATP =
Adenosine triphosphate
- BBB =
Blood brain-barrier
- BT =
Black tea
- CNS =
Central Nervous System
- DM =
Diabetes mellitus
- DNA =
Deoxyribonucleic acid
- EC =
(-)-epicatechin
- ECG =
(-)-epicatechin-3-gallate
- EGC =
(-)-epigallocatechin
- EGCG =
(-)-epigallocatechin 3-gallate
- ETC =
Electron transport chain
- FOX =
Forkhead
- GLUTs =
Glucose transporters
- GSH =
Reduced glutathione
- GSSG =
Oxidized glutathione
- GT =
Green tea
- IGF-I =
Insulin-like growth factor-I
- NADPH =
Nicotinamide adenine dinucleotide phosphate
- OS =
Oxidative stress
- OT =
Oolong tea
- PD =
Parkinson’s disease
- RNS =
Reactive nitrogen species
- ROS =
Reactive oxygen species
- T1DM =
Type 1 diabetes mellitus
- T2DM =
Type 2 diabetes mellitus
- TCA =
Tricarboxilic acid
- Tg =
Transgenic
- WHO =
World Health Organization
- WT =
White tea
REFERENCES
- 1.Agbaje I.M., Rogers D.A., McVicar C.M., McClure N., Atkinson A.B., Mallidis C., Lewis S.E. Insulin dependant diabetes mellitus: implications for male reproductive function. Hum. Reprod. 2007;22(7):1871–1877. doi: 10.1093/humrep/dem077. [DOI] [PubMed] [Google Scholar]
- 2.Association A.D., American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33(Suppl. 1):S62–S69. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McCall A.L. Cerebral glucose metabolism in diabetes mellitus. 2004. [DOI] [PubMed]
- 4.Alves M.G., Oliveira P.F., Socorro S., Moreira P.I. Impact of diabetes in blood-testis and blood-brain barriers: resemblances and differences. Curr. Diabetes Rev. 2012;8(6):401–412. doi: 10.2174/157339912803529896. [DOI] [PubMed] [Google Scholar]
- 5.Diaz-Parejo P., Ståhl N., Xu W., Reinstrup P., Ungerstedt U., Nordström C.H. Cerebral energy metabolism during transient hyperglycemia in patients with severe brain trauma. Intensive Care Med. 2003;29(4):544–550. doi: 10.1007/s00134-003-1669-3. [DOI] [PubMed] [Google Scholar]
- 6.Wang X., Michaelis E.K. Selective neuronal vulnerability to oxidative stress in the brain. Front. Aging Neurosci. 2010;2:12. doi: 10.3389/fnagi.2010.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maritim A.C., Sanders R.A., Watkins J.B., III Diabetes, oxidative stress, and antioxidants: a review. J. Biochem. Mol. Toxicol. 2003;17(1):24–38. doi: 10.1002/jbt.10058. [DOI] [PubMed] [Google Scholar]
- 8.Wen Y., Skidmore J.C., Porter-Turner M.M., Rea C.A., Khokher M.A., Singh B.M. Relationship of glycation, antioxidant status and oxidative stress to vascular endothelial damage in diabetes. Diabetes Obes. Metab. 2002;4(5):305–308. doi: 10.1046/j.1463-1326.2002.00212.x. [DOI] [PubMed] [Google Scholar]
- 9.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414(6865):813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 10.Kolluru G.K., Bir S.C., Kevil C.G. Endothelial Dysfunction and Diabetes: Effects on Angiogenesis, Vascular Remodeling, and Wound Healing. Int. J. Vasc. Med.2012(1-30), http://dx.doi. org/10.1155/2012/918267. 2012. [DOI] [PMC free article] [PubMed]
- 11.Biessels G.J., Gispen W.H. The impact of diabetes on cognition: what can be learned from rodent models? Neurobiol. Aging. 2005;26(1) Suppl. 1:36–41. doi: 10.1016/j.neurobiolaging.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 12.Duarte A.I., Candeias E., Correia S.C., Santos R.X., Carvalho C., Cardoso S., Plácido A., Santos M.S., Oliveira C.R., Moreira P.I. Crosstalk between diabetes and brain: glucagon-like peptide-1 mimetics as a promising therapy against neurodegeneration. Biochim. Biophys. Acta. 2013;1832(4):527–541. doi: 10.1016/j.bbadis.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 13.Saxena A., Vikram N.K. Role of selected Indian plants in management of type 2 diabetes: a review. J. Altern. Complement. Med. 2004;10(2):369–378. doi: 10.1089/107555304323062365. [DOI] [PubMed] [Google Scholar]
- 14.Palma H.E., Wolkmer P., Gallio M., Corrêa M.M., Schmatz R., Thomé G.R., Pereira L.B., Castro V.S., Pereira A.B., Bueno A., de Oliveira L.S., Rosolen D., Mann T.R., de Cecco B.S., Graça D.L., Lopes S.T., Mazzanti C.M. Oxidative stress parameters in blood, liver, and kidney of diabetic rats treated with curcumin and/or insulin. Mol. Cell. Biochem. 2014;386(1-2):199–210. doi: 10.1007/s11010-013-1858-5. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Z.C. Two new neuroprotective phenolic compounds from Gastrodia elata. J. Asian Nat. Prod. Res.DOI: 10.1080/10286020. 2013.791286. 2013;15(6):619–623. doi: 10.1080/10286020.2013.791286. [DOI] [PubMed] [Google Scholar]
- 16.Chang C.L.T., Lin Y., Bartolome A.P., Chen Y.C., Chiu S.C., Yang W.C. Herbal Therapies for Type 2 Diabetes Mellitus: Chemistry, Biology, and Potential Application of Selected Plants and Compounds. Evid. Based Compl. Alter. Med 378657. doi: 10.1155/2013/378657. 2012. [DOI] [PMC free article] [PubMed]
- 17.da Costa A.V., Calábria L.K., Furtado F.B., de Gouveia N.M., Oliveira R.J., de Oliveira V.N., Beletti M.E., Espindola F.S. Neuroprotective effects of Pouteria ramiflora (Mart.) Radlk (Sapotaceae) extract on the brains of rats with streptozotocin-induced diabetes. Metab. Brain Dis. 2013;28(3):411–419. doi: 10.1007/s11011-013-9390-6. [DOI] [PubMed] [Google Scholar]
- 18.Cheng T.O. All teas are not created equal: the Chinese green tea and cardiovascular health. Int. J. Cardiol. 2006;108(3):301–308. doi: 10.1016/j.ijcard.2005.05.038. [DOI] [PubMed] [Google Scholar]
- 19.de Mejia E.G., Ramirez-Mares M.V., Puangpraphant S. Bioactive components of tea: Cancer, inflammation and behavior. Brain Behav. Immun. doi: 10.1016/j.bbi.2009.02. 013. 2009;23(6):721–731. doi: 10.1016/j.bbi.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 20.Moderno P.M., Carvalho M., Silva B.M. Recent patents on Camellia sinensis: source of health promoting compounds. Recent Pat. Food Nutr. Agric. 2009;1(3):182–192. doi: 10.2174/2212798410901030182. [DOI] [PubMed] [Google Scholar]
- 21.Carvalho M., Jerónimo C., Valentão P., Andrade P.B., Silva B.M. Green tea: A promising anticancer agent for renal cell carcinoma. 2010. [DOI]
- 22.Dias T.R., Tomás G., Teixeira N.F., Alves M.G., Oliveira P.F., Silva B.M. White Tea (Camellia Sinensis (L.)): Antioxidant Properties and Beneficial Health Effects. Int. J. Food Sci. Nutr. Diet. 2013;11(2):1–15. [Google Scholar]
- 23.Dias T.R., Alves M.G., Tomás G.D., Socorro S., Silva B.M., Oliveira P.F. White tea as a promising antioxidant medium additive for sperm storage at room temperature: a comparative study with green tea. J. Agric. Food Chem. 2014;62(3):608–617. doi: 10.1021/jf4049462. [DOI] [PubMed] [Google Scholar]
- 24.Martins A.D., Alves M.G., Bernardino R.L., Dias T.R., Silva B.M., Oliveira P.F. Effect of white tea (Camellia sinensis (L.)) extract in the glycolytic profile of Sertoli cell. Eur. J. Nutr. 2014;53(6):1383–1391. doi: 10.1007/s00394-013-0640-5. [DOI] [PubMed] [Google Scholar]
- 25.van Dieren S., Uiterwaal C.S., van der Schouw Y.T., van der A D.L., Boer J.M., Spijkerman A., Grobbee D.E., Beulens J.W. Coffee and tea consumption and risk of type 2 diabetes. Diabetologia. 2009;52(12):2561–2569. doi: 10.1007/s00125-009-1516-3. [DOI] [PubMed] [Google Scholar]
- 26.Unno K., Takabayashi F., Yoshida H., Choba D., Fukutomi R., Kikunaga N., Kishido T., Oku N., Hoshino M. Daily consumption of green tea catechin delays memory regression in aged mice. Biogerontology. 2007;8(2):89–95. doi: 10.1007/s10522-006-9036-8. [DOI] [PubMed] [Google Scholar]
- 27.López V., Calvo M.I. White tea (Camellia sinensis Kuntze) exerts neuroprotection against hydrogen peroxide-induced toxicity in PC12 cells. Plant Foods Hum. Nutr. 2011;66(1):22–26. doi: 10.1007/s11130-010-0203-3. [DOI] [PubMed] [Google Scholar]
- 28.Almajano M.P., Carbó R., Jiménez J.A., Gordon M.H. Antioxidant and antimicrobial activities of tea infusions. Food Chem. 2008;108(1):55–63. doi: 10.1016/j.foodchem.2007.10.040. [DOI] [Google Scholar]
- 29.Hininger-Favier I., Benaraba R., Coves S., Anderson R.A., Roussel A.M. Green tea extract decreases oxidative stress and improves insulin sensitivity in an animal model of insulin resistance, the fructose-fed rat. J. Am. Coll. Nutr. 2009;28(4):355–361. doi: 10.1080/07315724.2009.10718097. [DOI] [PubMed] [Google Scholar]
- 30.Hawkins B.T., Lundeen T.F., Norwood K.M., Brooks H.L., Egleton R.D. Increased blood-brain barrier permeability and altered tight junctions in experimental diabetes in the rat: contribution of hyperglycaemia and matrix metalloproteinases. Diabetologia. 2007;50(1):202–211. doi: 10.1007/s00125-006-0485-z. [DOI] [PubMed] [Google Scholar]
- 31.Dai J., Vrensen G.F., Schlingemann R.O. Blood-brain barrier integrity is unaltered in human brain cortex with diabetes mellitus. Brain Res. 2002;954(2):311–316. doi: 10.1016/S0006-8993(02)03294-8. [DOI] [PubMed] [Google Scholar]
- 32.Shah K., Desilva S., Abbruscato T. The role of glucose transporters in brain disease: diabetes and Alzheimer’s Disease. Int. J. Mol. Sci. 2012;13(10):12629–12655. doi: 10.3390/ijms131012629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Handberg A., Vaag A., Damsbo P., Beck-Nielsen H., Vinten J. Expression of insulin regulatable glucose transporters in skeletal muscle from type 2 (non-insulin-dependent) diabetic patients. Diabetologia. http://dx.doi.org/10.1007/ bf00400207. 1990;33(10):625–627. doi: 10.1007/BF00400207. [DOI] [PubMed] [Google Scholar]
- 34.Santos R.X., Correia S.C., Alves M.G., Oliveira P.F., Cardoso S., Carvalho C., Duarte A.I., Santos M.S., Moreira P.I. Insulin therapy modulates mitochondrial dynamics and biogenesis, autophagy and tau protein phosphorylation in the brain of type 1 diabetic rats. Biochim. Biophys. Acta. 2014;1842(7):1154–1166. doi: 10.1016/j.bbadis.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 35.Zhao W.Q., Alkon D.L. Role of insulin and insulin receptor in learning and memory. Mol. Cell. Endocrinol. 2001;177(1-2):125–134. doi: 10.1016/S0303-7207(01)00455-5. [DOI] [PubMed] [Google Scholar]
- 36.Xu Q.G., Li X.Q., Kotecha S.A., Cheng C., Sun H.S., Zochodne D.W. Insulin as an in vivo growth factor. Exp. Neurol. 2004;188(1):43–51. doi: 10.1016/j.expneurol.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 37.Sickmann H.M., Waagepetersen H.S. Effects of diabetes on brain metabolism - is brain glycogen a significant player? Metab. Brain Dis. 2014 doi: 10.1007/s11011-014-9546-z. [DOI] [PubMed] [Google Scholar]
- 38.Suh S.W., Bergher J.P., Anderson C.M., Treadway J.L., Fosgerau K., Swanson R.A. Astrocyte glycogen sustains neuronal activity during hypoglycemia: studies with the glycogen phosphorylase inhibitor CP-316,819 ([R-R*,S*]-5-chloro-N-[2-hydroxy-3-(methoxymethylamino)-3-oxo-1-(phenylmethyl)propyl]-1H-indole-2-carboxamide). J. Pharmacol. Exp. Ther. 2007;321(1):45–50. doi: 10.1124/jpet.106.115550. [DOI] [PubMed] [Google Scholar]
- 39.Jacob R.J., Fan X., Evans M.L., Dziura J., Sherwin R.S. Brain glucose levels are elevated in chronically hyperglycemic diabetic rats: no evidence for protective adaptation by the blood brain barrier. Metabolism. 2002;51(12):1522–1524. doi: 10.1053/meta.2002.36347. [DOI] [PubMed] [Google Scholar]
- 40.Bree A.J., Puente E.C., Daphna-Iken D., Fisher S.J. Diabetes increases brain damage caused by severe hypoglycemia. Am. J. Physiol. Endocrinol. Metab. 2009;297(1):E194–E201. doi: 10.1152/ajpendo.91041.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu L., Sapolsky R.M., Giffard R.G. Differential sensitivity of murine astrocytes and neurons from different brain regions to injury. 2001. [DOI] [PubMed]
- 42.Ferguson S.C., Blane A., Perros P., McCrimmon R.J., Best J.J., Wardlaw J., Deary I.J., Frier B.M. Cognitive ability and brain structure in type 1 diabetes: relation to microangiopathy and preceding severe hypoglycemia. Diabetes. 2003;52(1):149–156. doi: 10.2337/diabetes.52.1.149. [DOI] [PubMed] [Google Scholar]
- 43.Lunetta M., Damanti A.R., Fabbri G., Lombardo M., Di Mauro M., Mughini L. Evidence by magnetic resonance imaging of cerebral alterations of atrophy type in young insulin-dependent diabetic patients. J. Endocrinol. Invest. 1994;17(4):241–245. doi: 10.1007/BF03348967. [DOI] [PubMed] [Google Scholar]
- 44.Moreira P.I. Alzheimer’s disease and diabetes: an integrative view of the role of mitochondria, oxidative stress, and insulin. J. Alzheimers Dis. 2012;30(Suppl. 2):S199–S215. doi: 10.3233/JAD-2011-111127. [DOI] [PubMed] [Google Scholar]
- 45.Correia S.C., Santos R.X., Perry G., Zhu X., Moreira P.I., Smith M.A. Insulin-resistant brain state: the culprit in sporadic Alzheimer’s disease? Ageing Res. Rev. 2011;10(2):264–273. doi: 10.1016/j.arr.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Santos R.X., Correia S.C., Alves M.G., Oliveira P.F., Cardoso S., Carvalho C., Seiça R., Santos M.S., Moreira P.I. Mitochondrial quality control systems sustain brain mitochondrial bioenergetics in early stages of type 2 diabetes. Mol. Cell. Biochem. 2014;394(1-2):13–22. doi: 10.1007/s11010-014-2076-5. [DOI] [PubMed] [Google Scholar]
- 47.Ceriello A. Oxidative stress and glycemic regulation. Metabolism. 2000;49(2) Suppl. 1:27–29. doi: 10.1016/S0026-0495(00)80082-7. [DOI] [PubMed] [Google Scholar]
- 48.Nishikawa T., Araki E. Impact of mitochondrial ROS production in the pathogenesis of diabetes mellitus and its complications. Antioxid. Redox Signal. 2007;9(3):343–353. doi: 10.1089/ars.2006.1458. [DOI] [PubMed] [Google Scholar]
- 49.Gao L., Mann G.E. Vascular NAD(P)H oxidase activation in diabetes: a double-edged sword in redox signalling. Cardiovasc. Res. 2009;82(1):9–20. doi: 10.1093/cvr/cvp031. [DOI] [PubMed] [Google Scholar]
- 50.Bravi M.C., Armiento A., Laurenti O., Cassone-Faldetta M., De Luca O., Moretti A., De Mattia G. Insulin decreases intracellular oxidative stress in patients with type 2 diabetes mellitus. 2006. [DOI] [PubMed]
- 51.Vannucci S.J., Maher F., Simpson I.A. Glucose transporter proteins in brain: delivery of glucose to neurons and glia. 1997. [DOI] [PubMed]
- 52.Vorbrodt A.W., Dobrogowska D.H., Tarnawski M. Immunogold study of interendothelial junction-associated and glucose transporter proteins during postnatal maturation of the mouse blood-brain barrier. J. Neurocytol. 2001;30(8):705–716. doi: 10.1023/A:1016581801188. [DOI] [PubMed] [Google Scholar]
- 53.Martin S.D., McGee S.L. The role of mitochondria in the aetiology of insulin resistance and type 2 diabetes. 2014. [DOI] [PubMed]
- 54.Afzal-Ahmed I., Mann G.E., Shennan A.H., Poston L., Naftalin R.J. Preeclampsia inactivates glucose-6-phosphate dehydrogenase and impairs the redox status of erythrocytes and fetal endothelial cells. Free Radic. Biol. Med. 2007;42(12):1781–1790. doi: 10.1016/j.freeradbiomed.2007.02.032. [DOI] [PubMed] [Google Scholar]
- 55.Yang X-F., Fang P., Meng S., Jan M., Xiong X., Yin Y., Wang H. The forkhead transcription factors are important in regulating vascular pathology, diabetes and regulatory T cells. Front. Biosci. 2009;1:420–436. doi: 10.2741/s35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang H., Wollheim C.B. Does chasing selected ‘Fox’ to the nucleus prevent diabetes? Trends Mol. Med. 2005;11(6):262–265. doi: 10.1016/j.molmed.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 57.Wolfrum C., Asilmaz E., Luca E., Friedman J.M., Stoffel M. Foxa2 regulates lipid metabolism and ketogenesis in the liver during fasting and in diabetes. Nature. 2004;432(7020):1027–1032. doi: 10.1038/nature03047. [DOI] [PubMed] [Google Scholar]
- 58.Stitt T.N., Drujan D., Clarke B.A., Panaro F., Timofeyva Y., Kline W.O., Gonzalez M., Yancopoulos G.D., Glass D.J. The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol. Cell. 2004;14(3):395–403. doi: 10.1016/S1097-2765(04)00211-4. [DOI] [PubMed] [Google Scholar]
- 59.Ni Y.G., Wang N., Cao D.J., Sachan N., Morris D.J., Gerard R.D., Kuro-O M., Rothermel B.A., Hill J.A. 2007. [DOI] [PMC free article] [PubMed]
- 60.Burgering B.M. A brief introduction to FOXOlogy. Oncogene. 2008;27(16):2258–2262. doi: 10.1038/onc.2008.29. [DOI] [PubMed] [Google Scholar]
- 61.Maiese K., Chong Z.Z., Shang Y.C. OutFOXOing disease and disability: the therapeutic potential of targeting FoxO proteins. Trends Mol. Med. 2008;14(5):219–227. doi: 10.1016/j.molmed.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pino E., Amamoto R., Zheng L., Cacquevel M., Sarria J.C., Knott G.W., Schneider B.L. FOXO3 determines the accumulation of α-synuclein and controls the fate of dopaminergic neurons in the substantia nigra. Hum. Mol. Genet. 2014;23(6):1435–1452. doi: 10.1093/hmg/ddt530. [DOI] [PubMed] [Google Scholar]
- 63.Kannike K., Sepp M., Zuccato C., Cattaneo E., Timmusk T. Forkhead transcription factor FOXO3a levels are increased in Huntington disease because of overactivated positive autofeedback loop. J. Biol. Chem. 2014;289(47):32845–32857. doi: 10.1074/jbc.M114.612424. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mckay D.L., Blumberg J.B. The role of tea in human health an update. J. Am. Coll. Nutr. 2002 doi: 10.1080/07315724.2002.10719187. [DOI] [PubMed] [Google Scholar]
- 65.Wheeler D., Wheeler W. The medicinal chemistry of tea. Drug Dev. Res. 2004;61(2):45–65. doi: 10.1002/ddr.10341. [DOI] [Google Scholar]
- 66.Del Rio D., Stewart A.J., Mullen W., Burns J., Lean M.E., Brighenti F., Crozier A. HPLC-MSn analysis of phenolic compounds and purine alkaloids in green and black tea. J. Agric. Food Chem. 2004;52(10):2807–2815. doi: 10.1021/jf0354848. [DOI] [PubMed] [Google Scholar]
- 67.Rusak G., Komes D., Likić S., Horžić D., Kovač M. Phenolic content and antioxidative capacity of green and white tea extracts depending on extraction conditions and the solvent used. 2008. [DOI] [PubMed]
- 68.Seeram N.P., Henning S.M., Niu Y., Lee R., Scheuller H.S., Heber D. Catechin and caffeine content of green tea dietary supplements and correlation with antioxidant capacity. J. Agric. Food Chem. 2006;54(5):1599–1603. doi: 10.1021/jf052857r. [DOI] [PubMed] [Google Scholar]
- 69.Rodrigues J., Assunção M., Lukoyanov N., Cardoso A., Carvalho F., Andrade J.P. Protective effects of a catechin-rich extract on the hippocampal formation and spatial memory in aging rats. Behav. Brain Res. 2013;246:94–102. doi: 10.1016/j.bbr.2013.02.040. [DOI] [PubMed] [Google Scholar]
- 70.Braicu C., Ladomeryc M.R., Chedead V.S., Irimief A., Berindan-Neagoea I. The relationship between the structure and biological actions of green tea catechins. 2013. [DOI] [PubMed]
- 71.Fresco P., Borges F., Diniz C., Marques M.P. New insights on the anticancer properties of dietary polyphenols. Med. Res. Rev. 2006;26(6):747–766. doi: 10.1002/med.20060. [DOI] [PubMed] [Google Scholar]
- 72.Yang C.S., Hong J., Hou Z., Sang S. Green tea polyphenols: antioxidative and prooxidative effects. J. Nutr. 2004;134(11):3181S. doi: 10.1093/jn/134.11.3181S. [DOI] [PubMed] [Google Scholar]
- 73.Ortsäter H., Grankvist N., Wolfram S., Kuehn N., Sjöholm A. Diet supplementation with green tea extract epigallocatechin gallate prevents progression to glucose intolerance in db/db mice. Nutr. Metab. (Lond) 2012;9(11):11. doi: 10.1186/1743-7075-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Khan N., Mukhatar H. Tea polyphenols for health promotion. 2007. [DOI] [PMC free article] [PubMed]
- 75.Song J., Xu H., Liu F., Feng L. Tea and cognitive health in late life: current evidence and future directions. J. Nutr. Health Aging. 2012;16(1):31–34. doi: 10.1007/s12603-011-0139-9. [DOI] [PubMed] [Google Scholar]
- 76.Li S., Lo C.Y., Pan M.H., Lai C.S., Ho C.T. Black tea: chemical analysis and stability. Food Funct. 2013;4(1):10–18. doi: 10.1039/C2FO30093A. [DOI] [PubMed] [Google Scholar]
- 77.Atoui A.K., Mansouri A., Boskou G., Kefalas P. Tea and herbal infusions: Their antioxidant activity and phenolic profile. Food Chem.http://dx.doi.org/10.1016/j.foodchem. 2004.01.075. 2005;89(1):27–36. [Google Scholar]
- 78.Paquay J.B., Haenen G.R., Stender G., Wiseman S.A., Tijburg L.B., Bast A. Protection against nitric oxide toxicity by tea. J. Agric. Food Chem. 2000;48(11):5768–5772. doi: 10.1021/jf981316h. [DOI] [PubMed] [Google Scholar]
- 79.Nakagawa T., Yokozawa T. Direct scavenging of nitric oxide and superoxide by green tea. Food Chem. Toxicol. 2002;40(12):1745–1750. doi: 10.1016/S0278-6915(02)00169-2. [DOI] [PubMed] [Google Scholar]
- 80.Guo Q., Zhao B., Shen S., Hou J., Hu J., Xin W. ESR study on the structure-antioxidant activity relationship of tea catechins and their epimers. Biochim. Biophys. Acta. 1999;1427(1):13–23. doi: 10.1016/S0304-4165(98)00168-8. [DOI] [PubMed] [Google Scholar]
- 81.Dumont M., Lin M.T., Beal M.F. Mitochondria and antioxidant targeted therapeutic strategies for Alzheimer’s disease. J. Alzheimers Dis. 2010;20(Suppl. 2):S633–S643. doi: 10.3233/JAD-2010-100507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Agostinho P., Cunha R.A., Oliveira C. Neuroinflammation, oxidative stress and the pathogenesis of Alzheimer’s disease. Curr. Pharm. Des. 2010;16(25):2766–2778. doi: 10.2174/138161210793176572. [DOI] [PubMed] [Google Scholar]
- 83.Hara Y., Luo S., Wickremasinghe R., Yamanishi T. Chemical composition of tea. Food Rev. Int. 1995;11(3):457–471. doi: 10.1080/87559129509541054. [DOI] [Google Scholar]
- 84.Hashimoto T., He Z., Ma W.Y., Schmid P.C., Bode A.M., Yang C.S., Dong Z. Caffeine inhibits cell proliferation by G0/G1 phase arrest in JB6 cells. Cancer Res. 2004;64(9):3344–3349. doi: 10.1158/0008-5472.CAN-03-3453. [DOI] [PubMed] [Google Scholar]
- 85.Lin Y.S., Tsai Y.J., Tsay J.S., Lin J.K. Factors affecting the levels of tea polyphenols and caffeine in tea leaves. J. Agric. Food Chem. 2003;51(7):1864–1873. doi: 10.1021/jf021066b. [DOI] [PubMed] [Google Scholar]
- 86.Unachukwu U.J., Ahmed S., Kavalier A., Lyles J.T., Kennelly E.J. White and Green Teas sinensis: Variation in Phenolic, Methylxanthine, and var. Camellia sinensis Antioxidant Profiles. J. Food Sci.http://dx.doi.org/10.1111/j. 1750-3841.2010.01705.x. 2010;75(6):C541–C548. doi: 10.1111/j.1750-3841.2010.01705.x. [DOI] [PubMed] [Google Scholar]
- 87.Lee H., Bae J.H., Lee S.R. Protective effect of green tea polyphenol EGCG against neuronal damage and brain edema after unilateral cerebral ischemia in gerbils. J. Neurosci. Res. 2004;77(6):892–900. doi: 10.1002/jnr.20193. [DOI] [PubMed] [Google Scholar]
- 88.Sinclair C.J., Geiger J.D. Caffeine use in sports. A pharmacological review. J. Sports Med. Phys. Fitness. 2000;40(1):71–79. [PubMed] [Google Scholar]
- 89.Nawrot P., Jordan S., Eastwood J., Rotstein J., Hugenholtz A., Feeley M. Effects of caffeine on human health. Food Addit. Contam. 2003;20(1):1–30. doi: 10.1080/0265203021000007840. [DOI] [PubMed] [Google Scholar]
- 90.Cunha R.A. [Caffeine, adenosine receptors, memory and Alzheimer disease]. Med. Clin. (Barc.) 2008;131(20):790–795. doi: 10.1016/S0025-7753(08)75506-4. [DOI] [PubMed] [Google Scholar]
- 91.Cunha R.A. Neuroprotection by adenosine in the brain: From A(1) receptor activation to A (2A) receptor blockade. Purinergic Signal. 2005;1(2):111–134. doi: 10.1007/s11302-005-0649-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Duarte J.M., Carvalho R.A., Cunha R.A., Gruetter R. Caffeine consumption attenuates neurochemical modifications in the hippocampus of streptozotocin-induced diabetic rats. 2009. [DOI] [PubMed]
- 93.Steinfelder H.J., Pethö-Schramm S. Methylxanthines inhibit glucose transport in rat adipocytes by two independent mechanisms. Biochem. Pharmacol. 1990;40(5):1154–1157. doi: 10.1016/0006-2952(90)90508-I. [DOI] [PubMed] [Google Scholar]
- 94.Sartorelli D.S., Fagherazzi G., Balkau B., Touillaud M.S., Boutron-Ruault M.C., de Lauzon-Guillain B., Clavel-Chapelon F. Differential effects of coffee on the risk of type 2 diabetes according to meal consumption in a French cohort of women: the E3N/EPIC cohort study. Am. J. Clin. Nutr. 2010;91(4):1002–1012. doi: 10.3945/ajcn.2009.28741. [DOI] [PubMed] [Google Scholar]
- 95.Greer F., Hudson R., Ross R., Graham T. Caffeine ingestion decreases glucose disposal during a hyperinsulinemic-euglycemic clamp in sedentary humans. Diabetes. 2001;50(10):2349–2354. doi: 10.2337/diabetes.50.10.2349. [DOI] [PubMed] [Google Scholar]
- 96.Keijzers G.B., De Galan B.E., Tack C.J., Smits P. Caffeine can decrease insulin sensitivity in humans. Diabetes Care. 2002;25(2):364–369. doi: 10.2337/diacare.25.2.364. [DOI] [PubMed] [Google Scholar]
- 97.Lopez-Garcia E., van Dam R.M., Rajpathak S., Willett W.C., Manson J.E., Hu F.B. Changes in caffeine intake and long-term weight change in men and women. Am. J. Clin. Nutr. 2006;83(3):674–680. doi: 10.1093/ajcn.83.3.674. [DOI] [PubMed] [Google Scholar]
- 98.Westerterp-Plantenga M.S., Lejeune M.P., Kovacs E.M. Body weight loss and weight maintenance in relation to habitual caffeine intake and green tea supplementation. Obes. Res. 2005;13(7):1195–1204. doi: 10.1038/oby.2005.142. [DOI] [PubMed] [Google Scholar]
- 99.Johnston K.L., Clifford M.N., Morgan L.M. Coffee acutely modifies gastrointestinal hormone secretion and glucose tolerance in humans: glycemic effects of chlorogenic acid and caffeine. Am. J. Clin. Nutr. 2003;78(4):728–733. doi: 10.1093/ajcn/78.4.728. [DOI] [PubMed] [Google Scholar]
- 100.Chen J.F., Xu K., Petzer J.P., Staal R., Xu Y.H., Beilstein M., Sonsalla P.K., Castagnoli K., Castagnoli N., Jr, Schwarzschild M.A. Neuroprotection by caffeine and A(2A) adenosine receptor inactivation in a model of Parkinson’s disease. J. Neurosci. 2001;21(10):RC143. doi: 10.1523/JNEUROSCI.21-10-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kakuda T. Neuroprotective effects of theanine and its preventive effects on cognitive dysfunction. Pharmacol. Res. 2011;64(2):162–168. doi: 10.1016/j.phrs.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 102.Vuong Q.V., Bowyer M.C., Roach P.D. L-Theanine: properties, synthesis and isolation from tea. J. Sci. Food Agric. 2011;91(11):1931–1939. doi: 10.1002/jsfa.4373. [DOI] [PubMed] [Google Scholar]
- 103.Ekborg-Ott K.H., Taylor A., Armstrong D.W. Varietal differences in the total and enantiomeric composition of theanine in tea. J. Agric. Food Chem. 1997;45(2):353–363. doi: 10.1021/jf960432m. [DOI] [Google Scholar]
- 104.Nishida K., Yasuda E., Nagasawa K., Fujimoto S. Altered levels of oxidation and phospholipase C isozyme expression in the brains of theanine-administered rats. Biol. Pharm. Bull. 2008;31(5):857–860. doi: 10.1248/bpb.31.857. [DOI] [PubMed] [Google Scholar]
- 105.Yokozawa T., Dong E. Influence of green tea and its three major components upon low-density lipoprotein oxidation. Exp. Toxicol. Pathol. 1997;49(5):329–335. doi: 10.1016/S0940-2993(97)80096-6. [DOI] [PubMed] [Google Scholar]
- 106.Egashira N., Hayakawa K., Osajima M., Mishima K., Iwasaki K., Oishi R., Fujiwara M. Involvement of GABA(A) receptors in the neuroprotective effect of theanine on focal cerebral ischemia in mice. J. Pharmacol. Sci. 2007;105(2):211–214. doi: 10.1254/jphs.SCZ070901. [DOI] [PubMed] [Google Scholar]
- 107.Cho H.S., Kim S., Lee S.Y., Park J.A., Kim S.J., Chun H.S. Protective effect of the green tea component, L-theanine on environmental toxins-induced neuronal cell death. Neurotoxicology. 2008;29(4):656–662. doi: 10.1016/j.neuro.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 108.Yokogoshi H., Kobayashi M., Mochizuki M., Terashima T. Effect of theanine, r-glutamylethylamide, on brain monoamines and striatal dopamine release in conscious rats. Neurochem. Res. 1998;23(5):667–673. doi: 10.1023/A:1022490806093. [DOI] [PubMed] [Google Scholar]
- 109.Yokogoshi H., Kobayashi M. Hypotensive effect of γ-glutamylmethylamide in spontaneously hypertensive rats. Life Sci. 1998;62(12):1065–1068. doi: 10.1016/S0024-3205(98)00029-0. [DOI] [PubMed] [Google Scholar]
- 110.Rogers P.J., Smith J.E., Heatherley S.V., Pleydell-Pearce C.W. Time for tea: mood, blood pressure and cognitive performance effects of caffeine and theanine administered alone and together. Psychopharmacology (Berl.) 2008;195(4):569–577. doi: 10.1007/s00213-007-0938-1. [DOI] [PubMed] [Google Scholar]
- 111.Di X., Yan J., Zhao Y., Zhang J., Shi Z., Chang Y., Zhao B. L-theanine protects the APP (Swedish mutation) transgenic SH-SY5Y cell against glutamate-induced excitotoxicity via inhibition of the NMDA receptor pathway. Neuroscience. 2010;168(3):778–786. doi: 10.1016/j.neuroscience.2010.04.019. [DOI] [PubMed] [Google Scholar]
- 112.Costa R.M., Magalhães A.S., Pereira J.A., Andrade P.B., Valentão P., Carvalho M., Silva B.M. Evaluation of free radical-scavenging and antihemolytic activities of quince (Cydonia oblonga) leaf: A comparative study with green tea (Camellia sinensis). Food Chem. Toxicol.http://dx.doi.org/10.1016/j.fct.2009. 01.019. 2009;47(4):860–865. doi: 10.1016/j.fct.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 113.Valko M., Leibfritz D., Moncol J., Cronin M.T., Mazur M., Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007;39(1):44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 114.Willett W.C. Diet and Health: What Should We Eat? Science.http://dx.doi.org/10.1126/science.8160011. 1994;264(51-58):532–537. doi: 10.1126/science.8160011. [DOI] [PubMed] [Google Scholar]
- 115.Chen C.H., Hsu H.J., Huang Y.J., Lin C.J. Interaction of flavonoids and intestinal facilitated glucose transporters. Planta Med. 2007;73(4):348–354. doi: 10.1055/s-2007-967172. [DOI] [PubMed] [Google Scholar]
- 116.Islam M. Effects of the aqueous extract of white tea (Camellia sinensis) in a streptozotocin-induced diabetes model of rats. Phytomedicine.http://dx.doi.org/10.1016/j. phymed.2011.06.025 . 2011;19(1):25–31. doi: 10.1016/j.phymed.2011.06.025. [DOI] [PubMed] [Google Scholar]
- 117.Xue R., Lv J., Gao J., Fu R., Li W., Lei X., Wu G., Xue L., Zhang Z. Protective effect of tea polyphenols on the blood-brain barrier. Transl. Neurosci. 2013;4(3):295–301. doi: 10.2478/s13380-013-0133-2. [DOI] [Google Scholar]
- 118.Abolfathi A.A., Mohajeri D., Rezaie A., Nazeri M. Protective effects of green tea extract against hepatic tissue injury in streptozotocin-induced diabetic rats. Evid. Based Compl. Alter. Med.2012, 740671. DOI: 10.1155/2012/740671. 2012. [DOI] [PMC free article] [PubMed]
- 119.Sabu M.C., Smitha K., Kuttan R. Anti-diabetic activity of green tea polyphenols and their role in reducing oxidative stress in experimental diabetes. J. Ethnopharmacol. 2002;83(1-2):109–116. doi: 10.1016/S0378-8741(02)00217-9. [DOI] [PubMed] [Google Scholar]
- 120.Lenzen S. The mechanisms of alloxan- and streptozotocin-induced diabetes. Diabetologia. 2008;51(2):216–226. doi: 10.1007/s00125-007-0886-7. [DOI] [PubMed] [Google Scholar]
- 121.Song E.K., Hur H., Han M.K. Epigallocatechin gallate prevents autoimmune diabetes induced by multiple low doses of streptozotocin in mice. Arch. Pharm. Res. 2003;26(7):559–563. doi: 10.1007/BF02976881. [DOI] [PubMed] [Google Scholar]
- 122.Sharma K., McCue P., Dunn S.R. Diabetic kidney disease in the db/db mouse. Am. J. Physiol. Renal Physiol. 2003;284(6):F1138–F1144. doi: 10.1152/ajprenal.00315.2002. [DOI] [PubMed] [Google Scholar]
- 123.Wolfram S., Raederstorff D., Preller M., Wang Y., Teixeira S.R., Riegger C., Weber P. Epigallocatechin gallate supplementation alleviates diabetes in rodents. J. Nutr. 2006;136(10):2512–2518. doi: 10.1093/jn/136.10.2512. [DOI] [PubMed] [Google Scholar]
- 124.Suganuma M., Okabe S., Oniyama M., Tada Y., Ito H., Fujiki H. Wide distribution of [3H](-)-epigallocatechin gallate, a cancer preventive tea polyphenol, in mouse tissue. Carcinogenesis. 1998;19(10):1771–1776. doi: 10.1093/carcin/19.10.1771. [DOI] [PubMed] [Google Scholar]
- 125.Haque A.M., Hashimoto M., Katakura M., Tanabe Y., Hara Y., Shido O. Long-term administration of green tea catechins improves spatial cognition learning ability in rats. J. Nutr. 2006;136(4):1043–1047. doi: 10.1093/jn/136.4.1043. [DOI] [PubMed] [Google Scholar]
- 126.Rezai-Zadeh K., Shytle D., Sun N., Mori T., Hou H., Jeanniton D., Ehrhart J., Townsend K., Zeng J., Morgan D., Hardy J., Town T., Tan J. Green tea epigallocatechin-3-gallate (EGCG) modulates amyloid precursor protein cleavage and reduces cerebral amyloidosis in Alzheimer transgenic mice. J. Neurosci. 2005;25(38):8807–8814. doi: 10.1523/JNEUROSCI.1521-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Itoh T., Imano M., Nishida S., Tsubaki M., Hashimoto S., Ito A., Satou T. (-)-Epigallocatechin-3-gallate protects against neuronal cell death and improves cerebral function after traumatic brain injury in rats. Neuromolecular Med. 2011;13(4):300–309. doi: 10.1007/s12017-011-8162-x. [DOI] [PubMed] [Google Scholar]
- 128.Jang S., Jeong H.S., Park J.S., Kim Y.S., Jin C.Y., Seol M.B., Kim B.C., Lee M.C. Neuroprotective effects of (-)-epigallocatechin-3-gallate against quinolinic acid-induced excitotoxicity via PI3K pathway and NO inhibition. Brain Res. 2010;1313:25–33. doi: 10.1016/j.brainres.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 129.Hong J.T., Ryu S.R., Kim H.J., Lee J.K., Lee S.H., Kim D.B., Yun Y.P., Ryu J.H., Lee B.M., Kim P.Y. Neuroprotective effect of green tea extract in experimental ischemia-reperfusion brain injury. Brain Res. Bull. 2000;53(6):743–749. doi: 10.1016/S0361-9230(00)00348-8. [DOI] [PubMed] [Google Scholar]
- 130.Kang K.S., Wen Y., Yamabe N., Fukui M., Bishop S.C., Zhu B.T. Dual beneficial effects of (-)-epigallocatechin-3-gallate on levodopa methylation and hippocampal neurodegeneration: in vitro and in vivo studies. PLoS One. 2010;5(8):e11951. doi: 10.1371/journal.pone.0011951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Li Q., Zhao H., Zhao M., Zhang Z., Li Y. Chronic green tea catechins administration prevents oxidative stress-related brain aging in C57BL/6J mice. Brain Res. 2010;1353:28–35. doi: 10.1016/j.brainres.2010.07.074. [DOI] [PubMed] [Google Scholar]
- 132.Bai Y., Li Q., Yang J., Zhou X., Yin X., Zhao D. p75(NTR) activation of NF-kappaB is involved in PrP106-126-induced apoptosis in mouse neuroblastoma cells. Neurosci. Res. 2008;62(1):9–14. doi: 10.1016/j.neures.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 133.Kim T.I., Lee Y.K., Park S.G., Choi I.S., Ban J.O., Park H.K., Nam S.Y., Yun Y.W., Han S.B., Oh K.W., Hong J.T. l-Theanine, an amino acid in green tea, attenuates beta-amyloid-induced cognitive dysfunction and neurotoxicity: reduction in oxidative damage and inactivation of ERK/p38 kinase and NF-kappaB pathways. Free Radic. Biol. Med. 2009;47(11):1601–1610. doi: 10.1016/j.freeradbiomed.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 134.Almajano M.P., Vila I., Gines S. Neuroprotective effects of white tea against oxidative stress-induced toxicity in striatal cells. Neurotox. Res. 2011;20(4):372–378. doi: 10.1007/s12640-011-9252-0. [DOI] [PubMed] [Google Scholar]
- 135.Choi Y.T., Jung C.H., Lee S.R., Bae J.H., Baek W.K. ; Choi Y.T., Jung C.H., Lee S.R., Bae J.H., Baek W.K., Suh M.H., Park J., Park C.W., Suh S.I. The green tea polyphenol (-)-epigallocatechin gallate attenuates beta-amyloid-induced neurotoxicity in cultured hippocampal neurons. Life Sci. 2001;70(5):603–614. doi: 10.1016/S0024-3205(01)01438-2. [DOI] [PubMed] [Google Scholar]
- 136.Lee J.H., Song D.K., Jung C.H., Shin D.H., Park J., Kwon T.K., Jang B.C., Mun K.C., Kim S.P., Suh S.I., Bae J.H. Epigallocatechin gallate attenuates glutamate-induced cytotoxicity via intracellular Ca modulation in PC12 cells. Clin. Exp. Pharmacol. Physiol. http://dx.doi.org/10. 1111/j.1440-1681.2004.04044.x. 2004;31(8):530–536. doi: 10.1111/j.1440-1681.2004.04044.x. [DOI] [PubMed] [Google Scholar]
- 137.Yokoyama S., Hirano H., Wakimaru N., Sarker K.P., Kuratsu J. Inhibitory effect of epigallocatechin-gallate on brain tumor cell lines in vitro. Neuro-oncol. 2001;3(1):22–28. doi: 10.1215/s1522851700000260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.MacKenzie T., Leary L., Brooks W.B. The effetc of an extract of green and black tea on glucose control in adults with type 2 diabetes mellitus: double-blind randomized study. Metabolism. http://dx.doi.org/10.1016/j.metabol. 2007.05.018. 2007;56(10):1340–1344. doi: 10.1016/j.metabol.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 139.Iso H., Date C., Wakai K., Fukui M., Tamakoshi A., JACC Study Group The relationship between green tea and total caffeine intake and risk for self-reported type 2 diabetes among Japanese adults. Ann. Intern. Med. 2006;144(8):554–562. doi: 10.7326/0003-4819-144-8-200604180-00005. [DOI] [PubMed] [Google Scholar]
- 140.Odegaard A.O., Pereira M.A., Koh W.P., Arakawa K., Lee H.P., Yu M.C. Coffee, tea, and incident type 2 diabetes: the Singapore Chinese Health Study. Am. J. Clin. Nutr. 2008;88(4):979–985. doi: 10.1093/ajcn/88.4.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Haskell C.F., Kennedy D.O., Milne A.L., Wesnes K.A., Scholey A.B. The effects of L-theanine, caffeine and their combination on cognition and mood. Biol. Psychol. 2008;77(2):113–122. doi: 10.1016/j.biopsycho.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 142.Borgwardt S., Hammann F., Scheffler K., Kreuter M., Drewe J., Beglinger C. Neural effects of green tea extract on dorsolateral prefrontal cortex. Eur. J. Clin. Nutr. 2012;66(11):1187–1192. doi: 10.1038/ejcn.2012.105. [DOI] [PubMed] [Google Scholar]
- 143.Kuriyama S., Hozawa A., Ohmori K., Shimazu T., Matsui T., Ebihara S., Awata S., Nagatomi R., Arai H., Tsuji I. Green tea consumption and cognitive function: a cross-sectional study from the Tsurugaya Project 1. Am. J. Clin. Nutr. 2006;83(2):355–361. doi: 10.1093/ajcn/83.2.355. [DOI] [PubMed] [Google Scholar]
- 144.Checkoway H., Powers K., Smith-Weller T., Franklin G.M., Longstreth W.T., Jr, Swanson P.D. Parkinson’s disease risks associated with cigarette smoking, alcohol consumption, and caffeine intake. Am. J. Epidemiol. 2002;155(8):732–738. doi: 10.1093/aje/155.8.732. [DOI] [PubMed] [Google Scholar]
- 145.Wang Y., Li M., Xu X., Song M., Tao H., Bai Y. Green tea epigallocatechin-3-gallate (EGCG) promotes neural progenitor cell proliferation and sonic hedgehog pathway activation during adult hippocampal neurogenesis. Mol. Nutr. Food Res. 2012;56(8):1292–1303. doi: 10.1002/mnfr.201200035. [DOI] [PubMed] [Google Scholar]
- 146.Hayat K., Iqbal H., Malik U., Bilal U., Mushtaq S. Tea and its consumption: benefits and risks. Crit. Rev. Food Sci. Nutr. 2013. [DOI] [PubMed]
- 147.Taylor J.R., Wilt V.M. Probable antagonism of warfarin by green tea. Ann. Pharmacother. 1999;33(4):426–428. doi: 10.1345/aph.18238. [DOI] [PubMed] [Google Scholar]
- 148.Lambert J.D., Sang S., Yang C.S. Possible controversy over dietary polyphenols: benefits vs risks. Chem. Res. Toxicol. 2007;20(4):583–585. doi: 10.1021/tx7000515. [DOI] [PubMed] [Google Scholar]
- 149.Molinari M., Watt K.D., Kruszyna T., Nelson R., Walsh M., Huang W.Y., Nashan B., Peltekian K. Acute liver failure induced by green tea extracts: case report and review of the literature. Liver Transpl. 2006;12(12):1892–1895. doi: 10.1002/lt.21021. [DOI] [PubMed] [Google Scholar]
- 150.Prystai E.A., Kies C.V., Driskell J.A. Calcium, Copper, Iron, Magnesium and Zinc Utilization of Humans as Affected by Consumption of Black, Decaffeinated Black and Green Teas. Nutr. Res. 1997;19(2):167–177. doi: 10.1016/S0271-5317(98)00181-X. [DOI] [Google Scholar]
- 151.Lu L.Y., Ou N., Lu Q.B. Antioxidant induces DNA damage, cell death and mutagenicity in human lung and skin normal cells. Sci. Rep. 2013;3:3169. doi: 10.1038/srep03169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sayin V.I., Ibrahim M.X., Larsson E., Nilsson J.A., Lindahl P., Bergo M.O. Antioxidants accelerate lung cancer progression in mice. Sci. Transl. Med. 2014;6(221):221ra15. doi: 10.1126/scitranslmed.3007653. [DOI] [PubMed] [Google Scholar]
- 153.Sun L., Tian X., Gou L., Ling X., Wang L., Feng Y., Yin X., Liu Y. Beneficial synergistic effects of concurrent treatment with theanine and caffeine against cerebral ischemia-reperfusion injury in rats. Can. J. Physiol. Pharmacol. 2013;91(7):562–569. doi: 10.1139/cjpp-2012-0309. [DOI] [PubMed] [Google Scholar]
- 154.Giesbrecht T., Rycroft J.A., Rowson M.J., De Bruin E.A. The combination of L-theanine and caffeine improves cognitive performance and increases subjective alertness. 2010. [DOI] [PubMed]
- 155.Smith A. Effects of caffeine on human behavior. Food Chem. Toxicol. 2002;40(9):1243–1255. doi: 10.1016/S0278-6915(02)00096-0. [DOI] [PubMed] [Google Scholar]