Abstract
In the vertebrate retina, visual signals are segregated into parallel ON and OFF pathways, which provide information for light increments and decrements. The segregation is first evident at the level of the ON and OFF bipolar cells and it apparently remains as signals propagate to higher brain visual centers. A fundamental question in visual neuroscience is how these two parallel pathways function: are they independent from each other or do they interact somehow? In the latter case, what kinds of mechanisms are involved and what are the consequences from this cross-talk? This review summarizes current knowledge about the types of interactions between the ON and OFF channels in nonmammalian and mammalian retina. Data concerning the ON-OFF interactions in distal retina revealed by recording of single bipolar cell activity and electroretinographic ON (b-wave) and OFF (d-wave) responses are presented. Special emphasis is put on the ON-OFF interactions in proximal retina and their dependence on the state of light adaptation in mammalian retina. The involvement of the GABAergic and glycinergic systems in the ON-OFF crosstalk is also discussed.
Keywords: Bipolar cells, electroretinogram, GABA, ganglion cells, glycine, ON-OFF interactions, retina
1. INTRODUCTION
In the vertebrate retina, visual information is processed into parallel ON and OFF pathways, which carry information for light increments and decrements, respectively [for reviews: [1-3]]. The ON–OFF segregation begins with the divergence of photoreceptor signals to two subclasses of bipolar cells (BCs) – ON and OFF types [4]. It has been shown that axon terminals of OFF BCs ramify in the distal portion of the inner plexiform layer (sublamina a), where they connect with dendrites of OFF ganglion cells (GCs); whereas axon terminals of ON BCs ramify in the proximal part of the inner plexiform layer (sublamina b), where they make contacts with ON GCs [5-11]. This segregation of ON and OFF channels is a fundamental principle of retinal organization. The ON and OFF signals generated in the retina appear to remain separate as they are transmitted to higher brain visual centres. One of the most intensively studied subjects lately is how do the ON and OFF pathways interact with each other? Evidence supporting interaction between the ON and OFF channels was first reported in studies of goldfish ganglion cells [12, 13]. Latter, McGuire et al. [14] argue, on anatomical grounds, that the centre response of each cat ganglion cell is mediated by both ON and OFF cone bipolar cells. This has been called the “push-pull” model. That is, a bipolar and ganglion cell of the same response polarity would communicate with a sign-conserving synapse (push), while a bipolar cell of the opposite response polarity would use a sign-inverting synapse (pull). A ganglion cell could receive sign-inverting synapse from an amacrine cell instead of bipolar cell as it has been demonstrated by recordings of amacrine–ganglion cell pairs in the carp [15]. Because the latter amacrine cells carry signals across the ON/OFF boundary of the inner plexiform layer, the inhibition they exert is referred as “crossover inhibition” [16]. Different types of inhibitory interactions between the ON and OFF channels have been described after the discovery that glutamate analog 2-amino-4-phosphonobutyric acid (APB or L-AP4) eliminates the responses of ON, but not OFF bipolar cells and thus can separate the activity of the two channels [17]. In addition to inhibitory interactions, a type of excitatory influences between the ON and OFF channels, which is often revealed after blockade of the GABAergic transmission, has also been reported. This review summarizes current knowledge about the types of interactions between the ON and OFF channels in distal and proximal retina in both nonmammalian and mammalian species and the involvement of the GABAergic and glycinergic systems in these interactions
2. ORIGIN OF RETINAL ON AND OFF CHANNELS
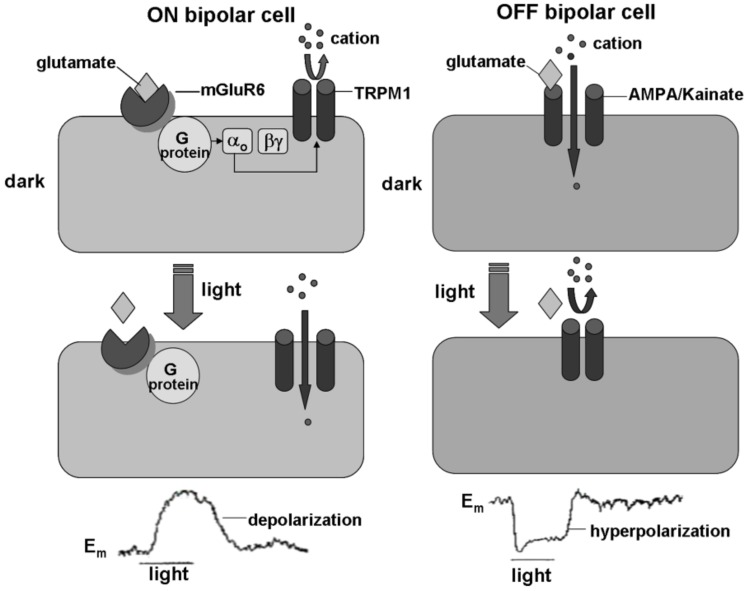
The ON-OFF segregation begins at the first synapse in the retina, where glutamate released from photoreceptors acts on different types of glutamate receptors on bipolar cells. The depolarizing (ON) bipolar cells express metabotropic glutamate receptors (mGluR6), while the hyperpolarizing (OFF) bipolar cells express ionotropic (AMPA/kainate) glutamate receptors [18-23]. In the dark, glutamate released from photoreceptors depolarizes OFF bipolar cells through activation of an ionotropic glutamate receptor, whereas glutamate hyperpolarizes ON bipolar cells through activation of mGluR6 with a decrease in cationic conductance [19, 24, 25] (Fig. 1). Metabotropic glutamate receptor mGluR6 is known as the APB or L-AP4 receptor, because it is selectively agonized by L-2-amino-4-phosphonobutyric acid (APB or L-AP4). The АРВ receptors have been found in the ON BCs of all vertebrate species investigated so far amphibians: [26, 27]; rodents: [20, 28, 29]; cats, monkeys: [30]. Binding of glutamate to these receptors activates the G protein Go [31-35] that leads to closure of a constitutively active nonselective cation channel, identified as transient receptor potential melastatin 1 (TRPM1) [36-39]. It has been shown that the ON bipolar cells do not response to light and there is no ERG b-wave in TRPM1-/- mice [37, 38, 40, 41]. Similar changes have been seen in mGluR6 -/- mice [19, 41-43], G(o-/- mice [33] and in wild type mice injected with APB [39]. These observations support the suggestion that mGluR6 receptors, Gαo and TRPM1 channels are required for the light responses of ON BCs and generation of the ERG b-wave. The proper binding of mGluR6 receptor and TRPM1 effector channel to ON bipolar cell distal dendrites is provided by nyctalopin [44, 45]. Cao et al. [44] suggest that the role of nyctalopin is “to coordinate the assembly of mGluR6-TRPM1 complex in the optimal configuration for signaling, which ultimately makes the fast signal transmission possible”. It has been shown that TRPM1 channels on rod bipolar cells are inactive and their expression at the distal dendrites is greatly reduced in mGluR6 -/- and nyctalopin-null mice [40, 43-45]. It is now known that mutations in TRPM1 [39, 46-48], mGluR6 [49, 50] and nyctalopin [51, 52] account for the majority of congenital night blindness cases in humans.
Fig. (1).
Glutamate transduction mechanisms in ON and OFF bipolar cells. In the dark, glutamate released from photoreceptors hyperpolarizes ON bipolar cell through activation of mGluR6 that in turn via G protein causes closure of TRPM1 channel and a decrease in cationic conductance (left, top). In the dark, glutamate depolarizes OFF bipolar cell through activation of an ionotropic glutamate AMPA/ kainite receptor that in turn causes an increase in cationic conductance (right, top). Light diminishes the glutamate release from photoreceptors, which causes depolarization of the ON bipolar cell (left, bottom) and hyperpolarization of the OFF bipolar cell (right bottom).
2.1. Effects of APB on the ON Channel Activity
It has been shown that exogenously applied APB saturates mGluR6 receptors on the ON bipolar cells and thus eliminates their responses to light-modulated glutamate release from the photoreceptors [17, 53, 54]. APB is proved to be a useful tool for eliminating the increment light responses throughout the visual pathway. It abolishes the light responses of ON bipolar cells [amphibians: [17, 55, 56, 57]; fish: [58]; rabbit: [59, 60]; review: [61]] and ERG b-wave in all vertebrate species [amphibians: [17, 62-64]; reptiles: [65]; fishes: [66, 67]; birds: [68]; rodents: [69-74]; rabbits: [75-79]; cats: [80]; monkeys: [77, 81-84]]. In fishes, however, two glutamate input mechanisms on the ON BCs exist: one mGluR6-dependent mechanism and one non-mGluR6 mechanism [85-87]. The latter mechanism is accomplished by excitatory amino acid transporter (EAAT) that in the dark transports glutamate and chloride from the synaptic cleft into the cell, resulting in hyperpolarization [86]. The ON BCs depolarize during illumination, because the EAAT becomes less activated by glutamate and less chloride enters the cells [86, 88, 89]. Connaughton and Nelson [89] have found that the majority of ON bipolar cells (60%) express a glutamate-gated chloride current, the minority of them (4 %) express APB responses only, while the remainder (14%) express a combination of the chloride and APB mechanisms. Wong et al. [87] reported that cone ON bipolar cells express only EAAT, while mixed rod-cone ON bipolar cells have both mGluR6 and EAAT on their dendrites. It has been proposed that mGluR6 mechanism mediates rod-driven light responses, while EAAT mediates cone-driven light responses of fish ON bipolar cells [85, 86, 88]. Wong et al. [87] have found, however, that APB receptors mediate a part of cone-driven responses of mixed rod-cone bipolar cells as well, although a majority of the cone input to these cells is mediated by EAAT. They presented data that mGluR6 inhibits the EAATs and propose that “this kind of interaction may have relevance to rod–cone suppression, a phenomenon that has been observed in many species”. Recently Tse et al. [90] have presented immunohistochemical data indicating the presence of postsynaptic EAAT5 on some cone ON BCs and some rod ON BCs in mouse retina. It remains to be determined if the ON BCs in other mammalian species express EAA5 transporters.
ERG studies in fishes show that APB abolishes the rod-driven b-wave and thus they confirm that mGluR6 mediates rod-driven light responses of ON bipolar cells [67, 91-93]. Contradictory results have been obtained, however, when the effects of APB on the cone-mediated b-wave were investigated in fishes. Some authors reported that APB eliminates nearly all of the b-wave [94-96], while other authors have found that a small part of cone-mediated b-wave persists even in the presence of APB, indicating that non-metabotropic mechanisms take part in its generation [91, 97-99]. This APB-resistant part is greater when the photoreceptor-to-bipolar cell synapse is isolated by picrotoxin + strychnine + tetrodotoxin [93]. Wong et al. [93] suggest that “L-AP4 activated group III mGluRs on amacrine cells, which suppressed ON bipolar cells by inhibitory synapses. Together, these 2 effects of L-AP4 led to a dramatic reduction of the photopic b-wave”. Saszik et al. [98] have found that in zebrafish the suppressing effect of L-AP4 on the photopic b-wave depends on stimulus wavelength. The effect is most apparent during blue and UV stimulation, indicating that metabotropic glutamate receptors mediate a great part of ON bipolar cell responses to ultraviolet and short-wavelength stimuli. Nelson and Singla [100] confirmed this observation and added that metabotropic glutamate receptors take part in responses of ON bipolar cell to input of all cone types.
The rod- and cone-mediated b-waves in mammalian retina may also show some differences with respect to their influence by APB. Green and Kapousta-Bruneau [101] have found that cone-mediated b-wave in rat ERG is more sensitive to APB that rod-mediated one. They concluded that “metabotropic receptors on depolarizing cone bipolar cells are affected by concentrations of APB (2 μM) that have minimal effects on rod bipolar cells”. The opposite results, however, have been reported recently in mouse retina [90]. Tse et al. [90] have found that the rod-mediated b-wave is more sensitive to depressing action of L-AP4 than the cone-mediated b-wave. In addition, the authors reported that the b-wave is fully suppressed (by L-AP4) only when measured with moderate mesopic stimuli, but not with lower or higher intensity stimuli. Tse et al. [90] have demonstrated that a great part of the residual L-AP4 insensitive b-waves, obtained in the photopic range, could be eliminated by adding of TBOA, which blocks EAAT5. TBOA by itself has effects similar to that of L-AP4 and these effects do not depend on the intact GABAergic and glycinergic retinal neurotransmission. The authors suggest that “EAAT5 plays a significant role in mediating cone-driven ON BC light responses, and perhaps a minor role in mediating rod-driven bipolar cell light responses”. Because there are multiple subtypes of BCs in mouse retina, Tse et al. [90] propose that “EAAT5 plays a role in mediating ON-light responses of some DBCs driven by cones. Other DBCs may either possess only the mGluR6 machinery, or possess both mGluR6 and EAAT5 machineries but have their light response dominated by the mGluR6 mechanism”. It is yet to be elucidated the role played by EAAT5 in mediating the ON BC light responses under different conditions of light stimulation in other mammalian species. However, it appears that mGluR6 and EAAT have additive action in mammalian ON BCs in contrast to their action in fish ON BCs where they suppress each other [87].
Many data indicate that АРВ eliminates the ON responses in third order retinal neurons [amphibians: [17, 55, 62]; reptiles: [65, 102]; rodents: [42, 103, 104]; rabbits: [75, 76, 105-108]; cats: [109, 110]; monkeys: [111]] and in neurons from higher brain visual centers [77, 110, 112]. Although the ON bipolar cells express also ionotropic glutamate receptor subunits [cat: [30]; mouse: [113]; rat: [114, 115]; fish: [116-118]] their function has not yet been described in the ON bipolar cells. Thus, APB can be used as a valuable tool for examining the impact that retinal ON channel blockade has on the activity in the OFF channel.
3. EFFECTS OF ON CHANNEL BLOCKADE ON THE DISTAL RETINAL OFF CHANNEL ACTIVITY: ROLE OF GLYCINE AND GABA
The effects of ON channel blockade on the OFF channel activity in distal retina have been investigated in two ways: 1) by recording the activity of individual OFF bipolar cells during retinal APB treatment and 2) by recording the electroretinographic (ERG) OFF response (d-wave) during the same treatment. It is known that the activity of large populations of ON and OFF bipolar cells is reflected in the b- and d-waves of the diffuse ERG [reviews: 119, 120]. The role of endogenous glycine in the retinal ON-OFF interactions is studied mainly by application of strychnine that is considered as a selective competitive antagonist of glycine gated Cl- channels [Review: 121]. Although strychnine, in the concentrations commonly used, is also a competitive antagonist of alpha7 nicotinic acetylcholine receptors [122], including that in proximal mammalian retina [123], in none of the studies summarized in the review a distinction has been made between the strychnine effects on glycine vs. nicotinic receptors. All strychnine effects have been attributed to glycine receptor blockade. The role of endogenous GABA in the retinal ON-OFF interactions is studied mostly by application of picrotoxin that blocks the chloride channel of both GABAA and GABAC receptors. In addition some selective GABAA (bicuculline, SR95531) and GABAC (TPMPA) receptor antagonists have also been used. It is well known that picrotoxin inhibits the glycine receptor currents in addition to the GABAergic ones [Review: 121]. Most of the authors have compared the effects of picrotoxin with that of strychnine and in some cases with the effects of selective GABA receptor antagonists in order to specify the receptors involved in picrotoxin action.
3.1. Effects on the OFF Bipolar Cell Activity
The effects of ON channel blockade by APB on the light responses of OFF bipolar cells have been investigated in a few studies. Some authors fail to obtain any effect of APB on the light responses of OFF bipolar cells [mudpuppy: [17]; tiger salamander: [57]; rabbit: [124]], while other authors reported that APB enhances the responses in some OFF bipolar cells without changing their resting membrane potential [55, 125]. Hare and Owen [56] reported that the effects of APB on the OFF BCs in tiger salamander retina depend on the photoreceptor input. APB increases the amplitudes of rod-driven responses and reduces the amplitudes of cone-driven responses. The authors suggest that APB reduces transmitter release from cones that should cause an increase in the impedance of the postsynaptic membrane with a concomitant increase in the gain between rods and OFF bipolar cells, but a net reduction in the voltage gain between cones and OFF bipolar cells. This suggestion is supported by data obtained in newt retina, where L-AP4 inhibits L-type calcium currents in cones, but not rods and thus suppresses synaptic transmission between cone and OFF BCs [126]. However, it has been shown that in tiger salamander retina the group II mGluRs act presynaptically to reduce glutamate release from both rods and cones, while group III mGluRs play a much lesser role [127, 128]. More studies are needed to fully understand the exact mechanism of APB action on the OFF BCs.
Molnar and Werblin [60] have found that APB eliminates the inhibition that occurs at the onset of a bright flash in rabbit OFF bipolar cells. This inhibition represents an interaction between the ON and OFF pathways and the authors call it reinforcing, because it acts to “reinforce the effects of the excitatory current”. Molnar and Werblin [60] have show that every OFF bipolar cell receives reinforcing ON inhibition, while no rod ON bipolar cells and only a half of cone ON bipolar cells receive reinforcing OFF inhibition. The latter in some cells is APB-resistant and thus originates in the OFF system, while in the other cells probably originates in the ON system, because it is eliminated by APB. The authors suggest that the reinforcing ON inhibition in OFF bipolar cells “must be carried by amacrine cells the processes of which receive excitatory input in the ON sublamina and deliver inhibition to the OFF sublamina”. Molnar and Werblin [60] reported that strychnine, but not picrotoxin blocks reinforcing ON inhibition in all OFF bipolar cells indicating that it is carried by glycine and not by GABA. On the other hand, strychnine has variable effect on the reinforcing OFF inhibition in cone ON bipolar cells. In some cells the inhibition was reduced but in other cells it was unaffected. Picrotoxin has no effect on the OFF reinforcing inhibition in ON cone bipolar cells. The authors proposed that “there are two pharmacologically distinct signals that appear as reinforcing OFF inhibition to ON cone bipolar cells: a strychnine-sensitive APB-insensitive signal that acts on more distal ON cells and a strychnine-insensitive APB-sensitive signal that acts on the more proximal ON cells”. Only the former is a type of ON-OFF interaction and it is more likely carried from the OFF sublamina by glycinergic amacrine cells. Molnar and Werblin [60] concluded that the “cross-lamina inhibition carried between the ON and OFF cone bipolar cells is predominantly glycinergic” and that “there is much less OFF-to-ON inhibition than ON-to-OFF inhibition, a significant asymmetry in signal flow between ON and OFF sublaminae”. Vight et al. [129] have found that axotomized terminals of mixed rod/cone ON BCs (Mb) in goldfish retina receive light-evoked cone mediated OFF inhibitory postsynaptic currents (IPSCs), which are resistant to L-AP4, indicating that they originate in the OFF pathway. These OFF IPSCs are mediated by GABA, acting on GABAC receptors, because they are partially blocked by TPMPA (GABAC antagonist) and fully eliminated by subsequent application of picrotoxin (GABAA and GABAC antagonist). Blocking of GABAA receptors by SR95531 markedly increases the OFF IPSCs, which is probably due to elimination of GABAA receptor mediated serial inhibition between amacrine cells. TPMPA reduces but does not completely block SR95531-elevated IPSPs. The authors proposed that disinhibited amacrine cell network (by SR95531) “released large amounts of GABA, and that the competitive antagonist TPMPA was unable to block all GABACRs” at bipolar cell terminals. On the other hand, glycinergic blockade by strychnine has no significant effect on the light-evoked OFF IPSCs. Thus, the OFF inhibition to the ON BCs in goldfish retina is mediated by GABA, but not glycine. It seems that different inhibitory neurotransmitters mediate the ON-OFF interactions at the level of bipolar cells in mammalian and nonmammalian retina. More studies are needed to evaluate the role played by GABA and glycinergic systems in crosstalk between retinal ON and OFF bipolar cells in different vertebrate species.
3.1. Effects on the ERG OFF Response
The effects of APB on the ERG OFF response have been investigated in a number of studies, but the results obtained are contradictory. Some authors have not seen any changes of the d-wave amplitude under the influence of APB [amphibians: [17]; fishes: [66]; rabbits: [76]; monkeys: [77]], while other authors reported for an increase of its amplitude caused by APB amphibians: [62, 63, 130-133]; reptiles: [65]; birds: [68]; monkeys: [81, 134]. Still other authors reported that APB reduces or fully eliminates the negative OFF response in rabbit ERG [77] and in monkey ERG in conditions favoring responses of rods or blue cones [77, 81]. One can suggest that the effect of APB upon the ERG OFF response is related to the type of the photoreceptor input. APB does not change or depresses the rod-dominated ERG OFF response [all rod retina of Raja erinacea: [66]; predominantly rod retina of rabbits: [76, 77], rod-mediated responses in monkeys: [77, 81]], but it increases the cone-dominated ERG OFF response [68, 134]. In our study on mixed (rod-cone) frog retina we have demonstrated, however, that APB increases the amplitude of the d-wave in nearly the same extent in the dark and under mesopic and photopic background illumination [63]. This indicates that the enhancing effect of APB on the ERG OFF response does not depend on the type of the photoreceptor input in frog retina. It remains to be determined if this is true for other mixed type retinas.
The enhancing effect of APB on the light responses of OFF bipolar cells and ERG d-wave can be attributed to disinhibition of OFF BCs from the canceling suppressive input coming from the ON bipolar cells via inhibitory (glycine and/or GABAergic) amacrine cells. Jardon et al. [131] argue that the glycinergic system takes part in these inhibitory interactions. They reported that strychnine injected intravitreally in frog eyes reduces the d-wave amplitude, which has previously been increased by APB. If APB blocks ON bipolar cells responses, the latter cells could not exert any light modulated inhibitory influences upon the OFF bipolar cells. Thus, the subsequent blockade of the glycinergic amacrine cells fed by ON bipolar cells would fail to influence the light responses of OFF bipolar cells. So, it is difficult to explain the decrease of the d-wave amplitude during strychnine application. In our study on frog retina we have shown that the blockade of the glycinergic synapses by strychnine as well as the blockade of the GABAergic synapses by picrotoxin does not alter significantly the enhancing effect of APB on the d-wave amplitude [133]. This is true for the responses mediated by red rods in dark adapted eyes as well as the responses mediated by green rods in chromatically adapted eyes. The enhancing effect of APB on the d-wave, however, was expressed to a smaller extent during the GABAergic blockade in chromatically-adapted eyes, where the responses were mediated by cones. Thus, it appears that the GABAergic system is involved in some cone-mediated inhibitory influences coming from the ON channel and directed towards the OFF channel in distal frog retina.
4. EFFECTS OF ON CHANNEL BLOCKADE ON THE PROXIMAL RETINAL OFF CHANNEL ACTIVITY: ROLE OF GLYCINE AND GABA
4.1. Nonmammalian Retina
The effects of ON channel blockade by APB on the OFF responses of third order retinal neurons have been investigated in a number of studies. Arkin and Miller [55] classified sustained OFF GCs in mudpuppy retina into three subtypes according to the effect of APB on them during intracellular recording. In the first group (disfacilitory cells) APB increases the sustained hyperpolarization caused by illumination, which is associated with resistance increase without altering the cells firing. These OFF GCs probably receive the excitatory input from OFF bipolar cells in the dark and the action of light is to reduce this excitatory drive (light-evoked disfacilitation). In the second group (inhibitory cells) APB causes a loss of sustained light-evoked hyperpolarization and an increase in transient potentials at light off. These cells probably receive a dominant ON bipolar cell input, providing sustained inhibition during illumination. In the third group (push-pull cells) APB eliminates part, but not all, of the sustained light-evoked hyperpolarization and incidentally caused an increase in the transient OFF postsynaptic potentials. These cells probably receive excitatory input from the OFF channel in the dark and inhibitory input from the ON channel during illumination. Arkin and Miller [55] reported that APB has no significant effect on the spiking of the OFF GCs and it either accentuates or has no effects on the OFF responses of ON-OFF GCs during extracellular recording. Awatramani and Slaughter [135] argue that the effect of L-AP4 on the OFF excitatory post synaptic currents (EPSCs) in OFF and ON-OFF GCs in tiger salamander depends on the stimulus intensity. The OFF EPSCs to the dimmer red stimuli (which preferentially stimulate cones) are suppressed, while those to the brighter red stimuli are slightly enhanced by L-AP4. These effects of L-AP4 are preserved in the presence of antagonists of GABA and glycine receptors (picrotoxin, imidazol-4-acetic acid, CGP35348 and strychnine), indicating that the effects of L-AP4 on GC OFF responses are independent of the inhibitory circuitry. The addition of mGluRs antagonist CPPG blocks the effect of L-AP4 on the OFF EPSCs to dim lights and the latter resembled the EPSCs registered in control conditions. On the other hand, CPPG reverses the effects of L-AP4 on the OFF EPSCs to bright-light stimuli. In 4 out of 6 cells, where the responses were enhanced by L-AP4, CPPG reduces the OFF EPSCs, indicating that “endogenous activation of mGluRs is only apparent with stronger stimulation”. Avatramani and Slaughter [135] propose that L-AP4 is acting on mGluRs at cone OFF bipolar cell terminals to reduce the transmitter release and this effect accounts for the suppression of OFF EPSCs in GCs at dim red stimuli (which activate only cones). According to the authors the enhancement of OFF EPSC by L-AP4 at brighter stimuli is “likely the result of augmented rod component that is only evident during strong light stimulation”. However, recently Sethuramanujam and Slaughter [136] presented data that do not support the hypothesis of Avatramani and Slaughter [135]. They have shown that L-AP4 greatly increases (instead of decreases) the cone-mediated light-evoked OFF EPSCs of transient ON-OFF GCs in tiger salamander retina. These results exclude the possibility that APB decreases the release of glutamate from cone OFF BCs. They have demonstrated that L-AP4 enhances the OFF NMDA receptor component during a 1-s stimulus, where this component is small, but L-AP4 produces little enhancement of the OFF NMDA receptor component during a 2-s stimulus, where this component is large. The authors concluded that short term cross talk from the ON pathway controls the level of activation of NMDA receptors in the OFF pathway. When this cross talk is blocked, the OFF response increases because of recruitment of NMDA receptor activation. Sethuramanujam and Slaughter [136] have demonstrated that the enhancing effect of L-AP4 on the light-evoked OFF EPSCs of ON-OFF GCs is occluded during simultaneous blockade of ionotropic glycine and GABA receptors. However, the authors do not investigate the relative contribution of each of the two inhibitory systems in the enhancing effect of L-AP4 on the OFF EPSCs. They concluded that the mechanism by which ON pathway regulates the light-evoked OFF EPSCs is yet to be deciphered.
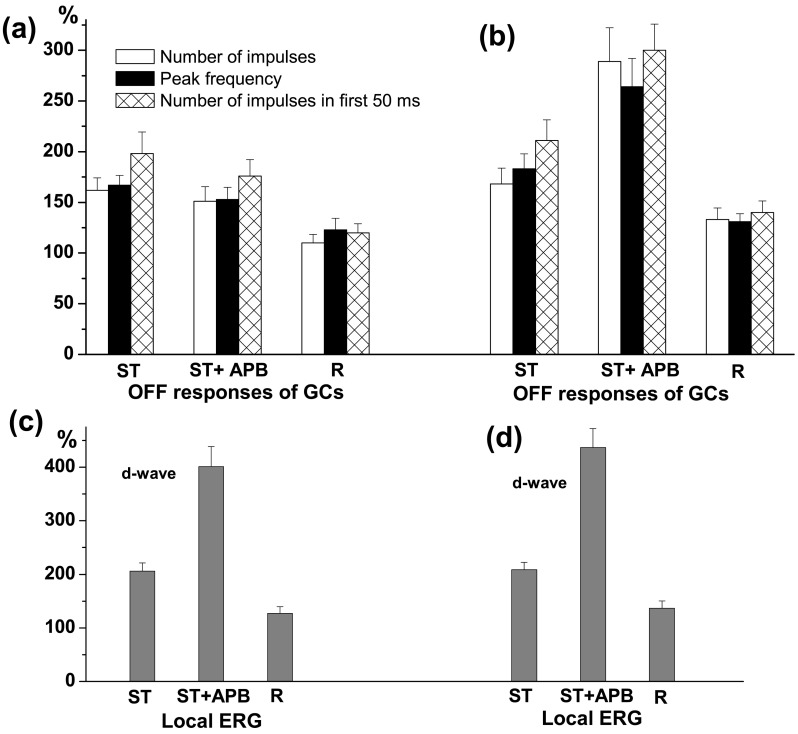
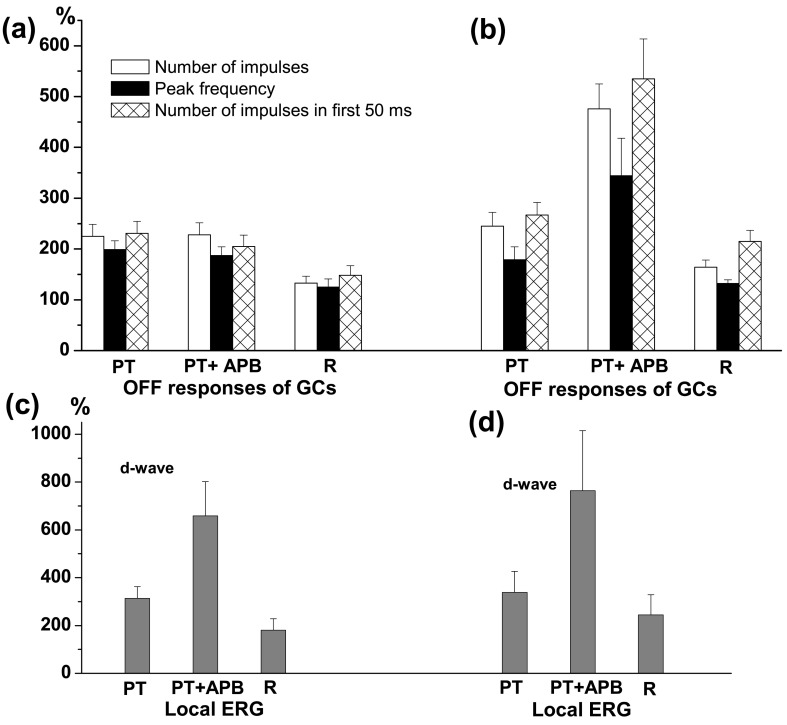
Many authors reported that APB causes an enhancement of the spiking OFF responses of retinal ganglion cells [amphibians: [57, 62, 137]; reptiles: [65, 102]]. АPB increases the absolute sensitivity of the OFF responses and eliminates the antagonistic effect of surround upon the ganglion cell centre response [102, 131]. Our results obtained in frog retina indicate that the effect of APB upon the OFF responses of ganglion cells depends on the type of the cell. APB has no effect on the light responses of tonic OFF GCs, but it increases the OFF responses in phasic OFF and ON-OFF GCs [138]. We have demonstrated that the latter effect of APB depends on the glycinergic and GABAergic neuro-transmission [138, 139]. Blocking of glycine receptors by strychnine prevents APB enhancing effect in 31 out of 69 GCs (Fig. 2a) and does not change it in the other cells (Fig. 2b). Blocking of ionotropic GABA receptors by picrotoxin eliminates APB enhancing effect in 24 out of 41 GCs (Fig. 3a) and does not alter it in the rest (Fig. 3b). On the other hand, neither strychnine nor picrotoxin eliminates the enhancing effect of APB on the d-wave amplitude of the local ERG, registered simultaneously with ganglion cell activity (Fig. 2c, 2d; Fig.3c, 3d). Thus, it appears that both glycinergic and GABAergic systems are involved in establishing the suppressive action that the ON channel exerts upon the OFF responses of frog phasic OFF and ON-OFF GCs. Jardon et al. [131] argue, however, that only the glycinergic system mediates the inhibitory influences of ON upon OFF channel in frog proximal retina. They have shown that strychnine decreases the OFF responses of ON-OFF GCs, which have been previously enhanced by APB. However, the diminution of the OFF responses caused by strychnine could not be explained by “push-pull” hypothesis as it has been pointed out for the diminution of the ERG d-wave amplitude. Granda et al. [102] have found that in turtle retina APB enhances the OFF responses of OFF and ON-OFF GCs in a wavelength dependent manner. In an OFF cell the enhancement is more to 640 nm than to 540 nm light, while in an ON-OFF cell the enhancement is more to 540 nm than to 640 nm light. The authors suggest that the enhancement of the OFF responses after APB derives from underlying ON inputs and “when ON responses to 640 nm light is greater in the pre-drug condition, the elimination of the ON responses releases opposing OFF responses, especially OFF responses to 540 nm light”.
Figure 2.
Effects of perfusion with strychnine (ST), ST+APB and Ringer solution in the recovery period (R) on the OFF responses of ganglion cells and d-wave in local ERG. (a) Changes of mean number of impulses (white columns), peak frequency (black columns) and number of impulses in the first 50 ms (hatched columns) of the OFF responses of ON-OFF and phasic OFF GCs expressed as % from their initial values, obtained in cells with blocked enhancing effect of APB on their OFF responses during the perfusion with ST+APB. The mean ± S.E.M. are represented. (b) Changes of the same parameters of the GCs’ OFF responses as (a), obtained in cells with preserved enhancing effect of APB on their OFF responses during the perfusion with ST+APB. (c) and (d): Amplitude of the d-wave of the local ERG (mean ± S.E.M.), expressed as % from its initial value during perfusion with ST, ST+APB and Ringer (during recovery period), recorded simultaneously with activity of GCs. It is seen that the enhancing effect of APB on the d-wave amplitude is preserved during the glycinergic blockade in all eyes irrespective of where the perfusion with ST+APB prevents (c) or does not change (d) the effect of APB on the ganglion cell OFF responses.
Figure 3.
Effects of perfusion with picrotoxin (PT), PT+APB and Ringer solution in the recovery period (R) on the OFF responses of ganglion cells and d-wave in local ERG. (a) Changes of mean number of impulses (white columns), peak frequency (black columns) and number of impulses in the first 50 ms (hatched columns) of the OFF responses of ON-OFF and phasic OFF GCs expressed as % from their initial values, obtained in cells with blocked enhancing effect of APB on their OFF responses during the perfusion with PT+APB. The mean ± S.E.M. are represented. (b) Changes of the same parameters of the GCs’ OFF responses as (a), obtained in cells with preserved enhancing effect of APB on their OFF responses during the perfusion with PT+APB. (c) and (d): Amplitude of the d-wave of the local ERG (mean ± S.E.M.), expressed as % from its initial value during perfusion with PT, PT+APB and Ringer (during recovery period), recorded simultaneously with activity of GCs. It is seen that the enhancing effect of APB on the d-wave amplitude is preserved during the GABAergic blockade in all eyes irrespective of where the perfusion with PT+APB prevents (c) or does not change (d) the effect of APB on the ganglion cell OFF responses.
DeMarco et al. [92] have shown that APB has a depressing effect upon the sensitivity of the OFF response recorded from the whole optic nerve in both dark and light adapted intact goldfishes. According to the authors “the decrease in sensitivity of optic nerve response would seem to reflect either a diminished number of OFF ganglion cells contributing to the response or a general decrease in sensitivity of the normal complement of cells”. Their study could not distinguish between these alternatives and could not be directly compared with the above cited results.
Summary. Most extracellular recordings from OFF and ON-OFF ganglion cells in nonmammalian species indicate that the ON channel inhibits the ganglion cell spiking at light stimulus offset. The inhibition occurs only in a part of the ganglion cells. Application of APB in these cells causes an enhancement of their OFF responses. What is the nature of this suppressive inhibition remains largely unknown, but it could include GABA and glycinergic mechanisms as well as NMDA receptor suppression. Intracellular recordings from OFF ganglion cells reveal that the ON channel provides a sustained inhibition, which occurs at the onset of a bright flash. This ON inhibition can account for all or a part of the hyperpolarization that is evident in OFF GCs during illumination. The underlying mechanism of the described inhibition has not been elucidated in nonmammalian retina.
4.2. Mammalian Retina
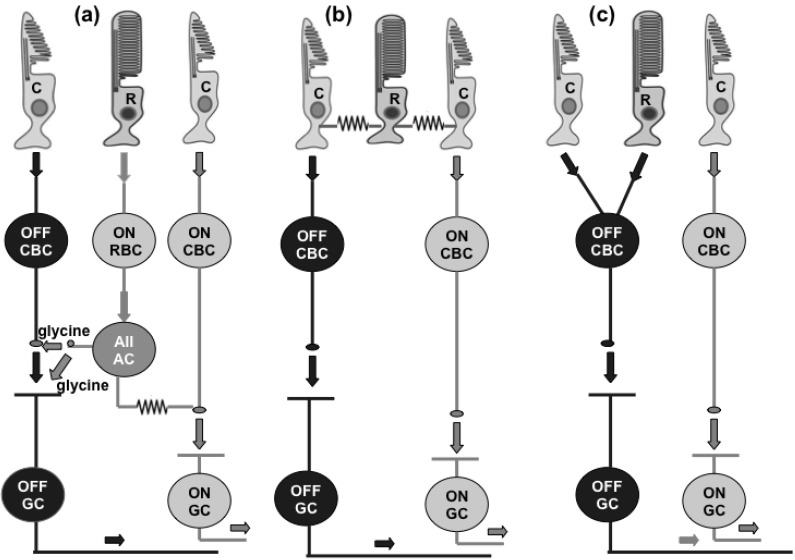
It is reasonable to expect that APB effects on the OFF responses of ganglion cells in mammalian retina will depend on the type of the photoreceptor input, because the rod and cone pathways differ in some aspects. Unlike the cold-blooded vertebrates, where rods and cones are connected to both types of bipolar cells (ON and OFF types), mammalian rods connect to a single type of bipolar cell, which depolarize in response to light. Rod bipolar cells make excitatory synapses with two postsynaptic neurons: AII and A17 amacrine cells [140-142]. The AII amacrine cells are coupled by gap junctions to each other and to the axon terminals of certain types of cone ON bipolar cells [review: 143] (Fig. 4a). The latter junctions serve to distribute the rod signals to cone ON bipolar pathway. The AII amacrine cells also make inhibitory glycinergic synapses onto the terminals of some cone OFF bipolar cells and onto the dendrites of some OFF ganglion cells review: 143 (Fig. 4a). Thus, rod signals can reach the cone OFF pathway as well. It has been proposed that rod signals can pass through gap junctions to cones and from there to the cone ON and OFF bipolar cells [144-146] (Fig. 4b). In addition to this “secondary rod pathway”, a “tertiary rod pathway” has been described, where rods make chemical synapses with cone OFF bipolar cells [mouse: [103, 147, 148]; rat: [149]; squirrel: [150, 151]; cat: [152]; rabbit: [153] (Fig. 4c). It is not known yet the number of pathways used by rod-driven OFF signals in different mammalian species.
Figure 4.
Effects of perfusion with picrotoxin (PT), PT+APB and Ringer solution in the recovery period (R) on the OFF responses of ganglion cells and d-wave in local ERG. (a) Changes of mean number of impulses (white columns), peak frequency (black columns) and number of impulses in the first 50 ms (hatched columns) of the OFF responses of ON-OFF and phasic OFF GCs expressed as % from their initial values, obtained in cells with blocked enhancing effect of APB on their OFF responses during the perfusion with PT+APB. The mean ± S.E.M. are represented. (b) Changes of the same parameters of the GCs’ OFF responses as (a), obtained in cells with preserved enhancing effect of APB on their OFF responses during the perfusion with PT+APB. (c) and (d): Amplitude of the d-wave of the local ERG (mean ± S.E.M.), expressed as % from its initial value during perfusion with PT, PT+APB and Ringer (during recovery period), recorded simultaneously with activity of GCs. It is seen that the enhancing effect of APB on the d-wave amplitude is preserved during the GABAergic blockade in all eyes irrespective of where the perfusion with PT+APB prevents (c) or does not change (d) the effect of APB on the ganglion cell OFF responses.
4.2.1. Rod-mediated Responses
One could expect that the responses of OFF GCs would be eliminated by APB in scotopic conditions, if the rod signals use the “primary rod pathway”. On the other hand, the scotopic responses of OFF GCs would survive during APB treatment if the signals travel through the other two rod pathways. Inhibiting effect of APB upon the OFF responses of all OFF and ON-OFF GC has been seen in dark adapted retina of some mammalian species [cat: [154]; rabbit: [107, 155]; rat: [156]]. Muller et al. [154] reported that in cats the sustained OFF GCs fire with a rather high maintained discharge rate, but the light modulation is entirely blocked during APB treatment. However, although the light modulation in transient OFF GCs is greatly reduced by APB, only in a few cells the light response is completely blocked. The authors suggest that the less pronounced effect of APB upon the transient OFF cells is due to their larger receptive field sizes. Protti et al. [156] have found that there is a species specific difference in APB action upon the rod-mediated ganglion cell OFF responses. In rat retina all the scotopic OFF responses are eliminated by APB; in the mouse retina a very high proportion of the OFF responses are eliminated by APB, while in the rabbit retina approximately one-half of the OFF responses in OFF and ON-OFF GCs are abolished by APB. In the other half of the cells the OFF responses are partially reduced in amplitude during APB treatment, but not fully eliminated. The authors suggest that APB-resistant OFF responses are mediated by direct contacts between rods and cone OFF bipolar cells, because they have found such contacts in rabbit retina. DeVries and Baylor [157] have found that the effects of APB on rabbit ganglion cells depend on the type of the cell. APB dramatically reduces the rod-mediated responses of all brisk OFF centre GCs, while leaving unaffected the responses of ON-OFF direction selective GCs and sluggish OFF GCs. The latter cells continued to respond to dim light in presence of strychnine indicating that transmission between AII amacrine cells and cone OFF bipolar cells does not take part in their generation. The authors suggest that these APB-resistant responses “are generated by the alternative pathway involving rod-cone junctions. This alternative APB-resistant pathway may carry the major rod input to OFF-centre sluggish and ON-OFF direction selective ganglion cells in rabbit retina”.
All authors working on dark adapted mouse retina reported that APB eliminates some, but not all of the rod-dominated GC OFF responses [103, 104, 158-160]. It has been shown that the effect of APB depends on the stimulus intensity. There is agreement among the authors that APB eliminates the OFF responses of OFF and ON-OFF GCs at lower stimulus intensities, indicating that the responses are mediated by “primary rod pathway” at lower intensities [103, 104, 159, 160]. However, contradictory results have been reported for the effects of APB on the OFF responses at higher stimulus intensities. Soucy et al. [103] have demonstrated that АРВ enhances the OFF responses of OFF and ON-OFF GCs at higher stimulus intensities in transgenic mice lacking cones. These OFF responses persist in strychnine, indicating that rod signals can reach OFF GCs by a pathway that excludes the rod ON bipolar cell and AII amacrine cell. The authors suggest that the “primary rod pathway” is responsible for response generation at lower stimulus intensities (≤ 1 Rh*/rod/s), but a direct excitatory input from rods to cone OFF bipolar cells mediated through ionotropic glutamate receptors (“tertiary rod pathway’) is involved in OFF response generation at higher stimulus intensities (> 10 Rh*/rod/s). The authors explain the enhanced OFF responses at higher intensities after APB treatment as being due to a reduction of the inhibitory glycinergic input from AII amacrine cells to cone OFF BCs. An enhancement of the APB-resistant OFF responses, obtained with high stimulus intensity (350 Rh*/rod/s) in conditions of dark adaptation has also been seen by Yang et al. [104]. The authors have found that strychnine partially blocks APB-induced increments of GC OFF responses, consistent with the notion that glycine mediates the inhibition from rod ON BCs to cone OFF BCs and OFF GCs. The authors suggest that APB-resistant OFF responses probably originate from the “secondary rod pathway”, because “in mouse retinas the tertiary pathway is rare”. Consistent with this suggestion are the results of Wang [158], who has found differences in the time characteristics of the OFF responses originating from APB-sensitive vs. APB-insensitive pathways. The OFF responses of the APB-insensitive pathway have significantly shorter latency and are capable of following substantially higher stimulus frequencies, which is a characteristic sign of cone responses. The author concluded that “APB sensitive and insensitive rod pathways can convey different types of information signaling light decrements in the dark-adapted retina”. In contrast to the above cited results [103, 104], other authors reported that APB decreases [159] or does not alter [160] the ganglion cell OFF responses at higher stimulus intensities in dark adapted mouse retina. Volgyi et al. [160] describe 3 physiological groups of rod-driven OFF GCs: high-sensitivity, intermediate-sensitivity and low-intermediate-sensitivity. APB eliminates the light responses only from the high-sensitivity OFF cells, while it has no effects on the responses from the other groups. The authors propose that the responses of high-sensitivity OFF GCs are mediated mainly by the “primary rod pathway”, the responses of intermediate-sensitivity OFF GCs originate mainly in “secondary rod pathway”, while the low-intermediate-sensitivity cells receive rod signals via “tertiary rod pathway”. The latter cells survive in the Cx36 KO mouse retina, where the gap junctions between neighbouring AII cells and between rods and cones are disrupted and thus both the “primary” and “secondary” rod pathways are eliminated. Volgyi et al. [160] have found that some OFF GCs receive mixed input from primary and secondary pathways, other cells receive mixed input from primary and tertiary pathways, but OFF cells never receive convergent inputs from all three pathways.
Summary. It appears that the scotopic OFF responses of mammalian ganglion cells are due entirely to input from the ON channel in the lowest intensity range (where they are mediated by “primary” rod pathway). However, the nature of interactions between the ON and OFF pathways at ganglion cell level remains largely unsolved in the higher scotopic range, where the responses are mediated by “secondary” and “tertiary” rod pathways. Some data indicate that the ON channel inhibits the activity of the OFF channel [103, 104], other data indicate that the activity of the OFF channel is not influenced by the ON channel [160], and still other data support the suggestion that the ON channel enhances the activity of the OFF channel [159].
4.2.2. Cone-mediated Responses
Four different types of influences of the ON channel upon the cone-mediated activity of the OFF channel have been described in proximal mammalian retina.
4.2.2.1. Reinforcing Inhibition at Light Onset
This type of inhibition is similar to that described at bipolar cell level, which occurs at the onset of a bright flash (ON inhibition). Symmetrically, the OFF pathway can exert reinforcing inhibition upon the ON pathway at the light offset. The convergence of ON inhibition with OFF excitation in OFF amacrine cells and OFF inhibition with ON excitation in ON amacrine cells has been reported in rabbit retina [161]. Hsueh et al. [161] have found that APB blocks the ON inhibition in almost half of OFF amacrine cells, indicating that this type of inhibition derives from the ON pathway. APB does not significantly affect the OFF inhibition that occurs in almost all ON amacrine cells, demonstrating that this inhibition likely originates from the OFF pathway. It is apparent that the crossover inhibition at the amacrine cell level is opposite to that at the bipolar cell level in rabbit retina: OFF crossover inhibition is more common than ON inhibition for the amacrine cells, while the reverse is true for the bipolar cells. Hsueh et al. [161] reported that strychnine, but not picrotoxin, eliminates the ON reinforcing inhibition in OFF amacrine cells and OFF reinforcing inhibition in ON amacrine cells, suggesting that this type of crossover inhibition among the amacrine cells is mediated primarily by glycine and not GABA.
Reinforcing crossover inhibition has been described for ganglion cells in many species [rabbit: [16, 162-164], cat: [165]; guinea pig: [166, 167]; mouse: [168]; monkey: [169]]. In monkeys this type of inhibition greatly diminishes at low stimulus contrasts, and does not contribute to their contrast sensitivity [169]. The inhibition in monkeys does not show ON-OFF asymmetry: both ON and OFF transient GCs receive crossover conductance, which is largely rectified. On the other hand, the reinforcing crossover inhibition shows a clear ON-OFF asymmetry in the other species. Molnar et al. [16] have shown that ON-OFF asymmetry of reinforcing inhibition in rabbit GCs is similar to that of bipolar cells and opposite to that of amacrine cells: almost all OFF GCs receive ON inhibition, while less than half of ON GCs receive OFF inhibition. Roska et al. [162] generate a “space-time map” of responses of GCs in light adapted rabbit retina and concluded that for many ganglion cells inhibition appears in regions complementary to excitation. For OFF GCs excitation occurs in regions driven by OFF bipolar cell input, whose activity survives during APB treatment, while inhibition occurs in regions driven by ON BCs, whose activity is blocked by APB. The opposite is true for the OFF GCs. The authors propose that “excitation and inhibition act in a complementary push-pull synergy” such that “excitatory and inhibitory currents combine and enhance, rather then offset each other”. Roska et al. [162] suggest that the active crossover inhibition of the GCs creates the antagonistic surround of their receptive field, because the antagonistic surround of bipolar cell receptive field is lost through rectification at the bipolar to ganglion cell synapse. The authors proposed that “this active, inhibitory surround antagonism in regions around the light stimulus at the ganglion cell level may spatially constrain the blurring of excitation across the ganglion cell dendrites”. Renteria et al. [42] argue, however, that crossover inhibition is not required for generation of GCs surrounds, because the receptive field surrounds of OFF GCs are normal in mGluR6 null mice, whose retina lack ON pathway signaling. The authors suggest that this same crossover inhibition may act to suppress spurious ON signals that otherwise appear in the OFF pathway. Chen et al. [163] examined the neurotransmitters involved in reinforcing crossover inhibition of rabbit ganglion cells and have found that they depend on the type of the cell. Sustained OFF GCs receive only glycinergic APB-sensitive ON inhibition, while transient OFF GCs receive both glycinergic and GABAergic ON inhibition. Sustained ON GCs receive both glycinergic and GABAergic APB-resistant OFF inhibition, while transient ON cells receive only GABAergic OFF inhibition. Buldyrev et al. [164] have found that the ON inhibition of brisk sustained OFF GCs in rabbits is blocked not only by L-AP4, but also during the blockade of kainate and AMPA glutamate receptors (with a combination of UPB 310 and GYKI 53655) as well as during the blockade of glycine receptors (by strychnine). The authors suggest that the ON inhibition in OFF GCs is due to direct input from a glycinergic amacrine cell “driven by conventional ionotropic glutamate receptor-mediated input and not via gap junction connections with cone ON BCs, as has been shown for the AII amacrine cell”. This glycinergic amacrine cell probably stratifies in both the ON and OFF sublaminae of the inner plexiform layer.
Some authors argue that only the OFF, but not the ON ganglion cells, receive reinforcing crossover inhibition. Zaghloul et al. [166] presented evidence that in guinea pig retina, hyperpolarizing response of ON GCs to dark depends on the high basal rate of glutamate release from the ON BCs and not to direct inhibition from the OFF pathway. On the other hand, hyperpolarizing response of OFF ganglion cells to light depends on direct inhibition. APB markedly decreases the amplitude of hyperpolarization of OFF GCs at light onset and changes it from direct inhibition to indirect inhibition. The authors conclude that “the direct inhibition during light increment in an OFF cell is driven by an ON amacrine cell” (crossover inhibition), while “the remaining hyperpolarization at light onset apparently depends on reducing the basal rate of glutamate release from the OFF bipolar cell”. The ON inhibition in guinea pig OFF GCs is observed under conditions driven by either rod or cone bipolar pathways [167]. Asymmetry of crossover inhibition similar to that described by Zaghloul et al. [166] has been demonstrated in cat retina. Cohen [165] reported that application of APB completely eliminates all light-evoked currents in sustained ON GCs, indicating that these cells receive no input from the OFF bipolar cells. On the other hand, APB causes a loss of the inhibitory current activated at light onset in the three sustained OFF GCs tested, indicating that it originates in the ON pathway. Thus, it appears that crossover inhibition does not exist in sustained ON GGs, but this type of inhibition exists in sustained OFF GCs in cat retina. Addition of a mixture of picrotoxin and strychnine blocks the inhibitory current at light onset in OFF GCs, indicating that it is mediated by GABA and glycine. The inhibitory current at light onset is largely blocked by ionotropic glutamate receptor antagonists (mixture of NBQX and D-AP5) in four OFF GCs, while it remained in another four OFF GCs. Cohen [165] suggests that the inhibition in former cells is mediated by amacrine cells driven by OFF BCs, while the inhibition in the latter cells is due to direct inhibition of ganglion cell dendrites by AII amacrine cells (fed by cone ON BCs). Cohen [165] does not comment why APB eliminates the inhibitory current activated at light onset in all OFF cells tested if some OFF GCs cells receive inhibition from the OFF BCs.
Many other functions of reinforcing crossover inhibition have been proposed in addition to the above mentioned. Molnar et al. [16] suggest that this type of inhibition “acts to suppress the distorting effects of synaptic rectification. The excitatory and inhibitory currents that impinge upon a given cell are rectified, but in opposing directions, so their recombination generates a membrane voltage representation of the visual signal that is more linear than either of its inputs”. Because ON bipolar cells and ON GCs show the least rectification in excitation, they also show the least rectified reinforcing crossover inhibition. Liang and Feed [170] argue, however, that crossover inhibition does not necessarily linearize the final output of the neuron and it may serve to rectify it. They have found that the excitatory currents elicited by white noise stimuli in guinea pig OFF GCs are clearly rectified and thus nonlinear. Blocking the ON channel with L-AP4 decreases rectification in OFF GCs, indicating that it is due to influences coming from the ON channel. This effect of L-AP4 is prevented by meclofenamic acid, which blocks the gap junctions. Blocking the glycinergic synapses by strychnine has effect similar to that of L-AP4. The authors propose that “the On cone bipolar cell sends current to the AII amacrine cell through gap junctions, depolarizing it, causing it to release glycine onto the Off cone bipolar cell. This inhibition hyperpolarizes the Off cone bipolar cell to near the threshold for glutamate release. Thus when dynamic stimulation begins, dimming depolarizes the Off bipolar cell and increases its release of glutamate, but brightening hyperpolarizes the Off bipolar cell below threshold and fails to decrease release. The Off bipolar cell’s inability to decrease release rectifies excitatory currents in the Off ganglion cell” and their responses are virtually restricted to negative contrasts. On the other hand, the excitatory currents in the ON GCs are less rectified and they are able to signal both positive and negative contrasts. Liang and Feed [170] propose that “asymmetrical rectification of ON and OFF cells may be an adaptation to natural scenes which have more contrast levels below the mean than above”. Werblin [171] argue that reinforcing crossover inhibition converts nonlinear Y-like responses of ganglion cells into linear X-like responses to an inverting grating. Without crossover inhibition, the currents elicited in bipolar cell at light ON and OFF are asymmetrical. When they add at the ganglion cell membrane, a net inward current is generated at both the onset of the light transition and the offset of the dark transition, leading to a response at each transition of the inverting grating. With reinforcing crossover inhibition, the excitatory currents under each stripe are combined with the inhibitory currents to generate symmetrical currents with each stripe inversion. According to Werblin [171] crossover inhibition serves also to reduce the net change in input conductance in the postsynaptic neuron. Because excitation and inhibition generate opposite conductance changes, their combination tends to reduce the net conductance change in the postsynaptic neuron. This is valuable because other inputs to the neuron will not be modified at different states of excitation or inhibition. Another valuable role of reinforcing crossover inhibition is its compensation for membrane potential offsets that are common to both excitation and inhibition in the retina. This decreases the distortions to the visual signal due to perturbations within the retina and the final output voltage resembles more closely the input signal.
Summary. Reinforcing crossover inhibition is widely distributed among mammalian ganglion cells under photopic conditions of illumination. It shows no ON-OFF asymmetry in primates, while in other species a clear ON-OFF asymmetry is evident. Almost all OFF GCs in rabbits, guinea pigs and cats receive ON inhibition, while less than half of rabbit ON GCs and none of guinea pig and cat ON GCs receive OFF inhibition. Both glycine and GABA appear to mediate crossover inhibition with their specific involvement in dependence on the ganglion cell type. Many functions of crossover inhibitions have been proposed. However, it is a matter of debate if this type of inhibition acts to suppress the distorting effects of synaptic rectification or it by itself serves to rectify the final output of the neurons.
4.2.2.2. Disinhibition at Light Offset
The OFF GCs receive disinhibitory input from the ON channel, which occurs at the offset of a bright flash. This type of cross talk enhances the OFF response because it now represents both excitation and disinhibition. Manookin et al. [167] using conductance analysis, have show that OFF GCs receive increased excitation in parallel with decreased inhibition (i.e., disinhibition) at all contrasts of decrement light stimuli. The authors have demonstrated that “at low contrasts, disinhibition plays a relatively large role, leading to an inward current at Vrest associated with a negative conductance. At high contrasts, disinhibition plays a smaller role, leading to an inward current at Vrest associated with a positive conductance”. APB significantly reduces the magnitude of the decreased inhibitory conductance at each contrast, but does not block the increased excitatory conductance. Manookin et al. [167] have shown that blocking of glycine receptors with strychnine in the presence of ionotropic glutamate receptor blockade (with CNQX and D-AP-5) completely eliminates disinhibition of OFF GCs, while blocking of GABAA receptors with bicuculline only slightly suppresses the response. Manookin et al. [167] suggest that “the disinhibition circuit is driven by the ON pathway through the following pathway: cone – cone ON bipolar cell - AII cell - OFF ganglion cell. Thus, to light decrement, AII cells, driven by electrical synapses with ON cone bipolar cells, would hyperpolarize and reduce glycine release”. This disinhibition of the OFF ganglion cell combines with conventional excitation from OFF bipolar cells to extend the operating range for encoding negative contrasts. Buldyrev et al. [164] have found that during the OFF phase, the decrease of the inhibitory input was small and variable compared with the magnitude of excitation in rabbit brisk sustained OFF GCs, indicating that these cells receive little tonic disinhibitory input. The authors reported that L-AP4 suppresses the peak in the excitatory conductance at the beginning of the OFF phase of the stimulus cycle, indicating that a part of it originates in the ON pathway. They have shown that a combination of selective kainate and AMPA receptor blockers (UPB 310 and GYKI 53655) that completely suppresses the responses of cone OFF BCs, does not completely eliminate the excitatory synaptic input to OFF GCs. A significant NMDA receptor-mediated component remains, which is blocked by L-AP4, indicating that it arises in the ON pathway. The same component is also blocked by strychnine, suggesting that a glycinergic amacrine cell drives the NMDA input through presynaptic inhibition at cone OFF BC terminals. The authors suggest that the AII glycinergic amacrine cell is involved in this disinhibitory circuit, while another type of glycinergic amacrine cell mediates reinforcing ON inhibition in OFF GCs. It is evident that the ON channel activity is needed for activation of NMDA component in rabbit OFF GCs, while the ON channel activity suppresses the same component of GC OFF responses in tiger salamander retina [136]. Thus, it appears that the ON pathway controls in an opposite manner the activation of NMDA component in cone-mediated OFF responses in nonmammalian and mammalian proximal retina. More studies are needed to understand the role of ON channel activity in modulating NMDA receptor activation in the OFF channel in both nonmammalian and mammalian species.
Chen and Linsenmeier [172, 173] propose that the combination of APB-sensitive and APB-resistant pathways increases the range of response amplitudes and temporal frequencies to which cat OFF GCs can respond. They have found that APB elevates the mean firing rate of OFF GCs, but suppresses their responsivity to photopic sinusoidal stimuli across all spatial frequencies and reduces all components of their cone-mediated light responses, except the transient increase in firing at light offset. The authors suggest that “the centre response mechanism of OFF GCs (X and Y subtypes) comprises APB-sensitive and APB-resistant components”. According to them “APB-sensitive component is more sustained and responds to both brightening and dimming stimuli, while the APB-resistant component is more transient and responds primarily to dimming stimuli”. Chen and Linsenmeier [172, 173] suggest that the APB-sensitive component is probably derived from ON bipolar cells via sign-reversing (inhibitory) synapse, while APB-resistant component is derived from OFF bipolar cells via sign-conserving synapse. Both the APB-sensitive and APB-resistant pathways could involve bipolar-to-amacrine-to ganglion cell input as well as direct bipolar-to-ganglion cell input. Recently Yang et al. [104] reported that APB decreases the OFF responses of mouse OFF and ON-OFF GCs under light adaptation conditions, but the authors proposed a new mechanism for this action. They have found that the blockade of dopamine D1 receptors (by SCH23390) or hyperpolarization-activated cyclic nucleotide-gated (HCN) channels (by ZD 7288) prevents the suppressing action of APB, while the blockade of GABAergic and glycinergic neurotransmission (by combination of strychnine, picrotoxin and TPMPA) has no effect on it. During treatment with SCH23390 or ZD 7288, APB, instead of decreasing, enhances the cone-mediated OFF responses of ganglion cells. The authors suggest that APB has two opposite functions on the OFF pathway in light adapted mouse retina. First, APB inhibits a subgroup of dopaminergic amacrine cells and consequently inhibits HCN channels in cone OFF bipolar cells, inducing a decrease in their glutamate release and subsequent reduction of light-evoked OFF responses of ganglion cells. Second, APB increases OFF responses of GCs via removal of inhibition from ON pathway to OFF pathway. Because the first function of APB is stronger than the second one, APB decreases OFF responses of ganglion cells in conditions of light adaptation. However, when the first function of APB is blocked (by SCH23390 or ZD 7288), the second function of APB becomes unmasked and APB increases the OFF responses. Whether the first, dopamine-dependent circuit exists in other mammalian species remains largely unknown.
Summary. The role played by the disinhibitory input that the OFF GCs receive from the ON channel at stimulus offset under photopic conditions of illumination remains largely unknown in most vertebrate species. It appears that disinhibition has a relatively large role at lower stimulus contrasts in guinea pig OFF GCs, but it is small and variable in rabbit sustained OFF GCs. In addition to disinhibition, the ON pathway may contribute to the excitatory conductance at light offset by NMDA receptor activation (in rabbit OFF GCs) or through network mechanism involving D1 receptors and HCN channels (in mouse OFF GCs). In both cases (disinhibition and excitation) the ON channel works together with the OFF channel to augment the OFF responses. That’s why blocking of the ON channel activity with APB causes a diminution of the ganglion cell OFF responses.
4.2.2.3. Suppression at Mean Luminance or Light Offset
The OFF ganglion cells receive suppression from the ON channel, which occurs at mean luminance or offset of light stimulus. Blocking this suppression with APB causes an enhancement of the maintained and light-evoked activity of OFF GCs [rodents: [166, 174]; rabbits: [75, 76, 106]; cats: [154, 165, 175]; monkeys: [111]]. Massey et al. [76] have seen that the OFF cells in rabbits are usually excited by APB, occasionally exhibiting high frequency firing with a typical bursting pattern. The excitatory effect of APB is not due to its direct action on OFF GCs, because it is prevented during a Mg2+ induced synaptic block. It has been shown that APB increases also the maintained discharges of cat OFF GCs in scotopic, mesopic and photopic range, indicating that these cells receive tonic inhibitory influences from the ON channel [109, 154, 175]. Bolz et al. [109] did not observe any effect of APB on light-modulated responses of OFF GCs, while Wassle et al. [175] and Muller et al. [154] have found that APB enhances the light-evoked spike activity in all OFF brisk GCs. It is seen from post-stimulus time histograms in their works, that APB increases the spike count both at light onset and light offset especially in sustained OFF GCs. The enhancement of the OFF GC activity under the influence of APB has been attributed to a reduction of ON inhibitory input mediated directly by ON bipolar cells or with amacrine cells interposed [154, 175]. The authors cited [154, 175] have shown that strychnine, but not bicuculline completely blocks the effects of APB on the OFF GCs, indicating that the glycinergic pathway is crucial for the described ON-OFF interaction. Wassle et al. [175] and Muller et al. [154] do not differentiate between APB effects during light onset and light offset. While the former is type of a reinforcing inhibition, the latter appears as a suppressive inhibition, which works to decrease the excitatory input from the OFF bipolar cells. Cohen [165] has shown that APB causes the cone-mediated excitatory inward currents at light offset to increase an average of 44% in cat sustained OFF GCs. The authors suggest that the excitation at light offset is primarily due to input from excitatory cone OFF BCs, but they do not offer any explanation why the light-evoked excitatory currents are augmented under the influence of APB.
The OFF GCs in rodents also receive suppressive input from the ON channel at mean luminance. Zaghloul et al. [166] have found that APB tonically depolarizes the transient OFF GCs in guinea pigs, which is associated with an increase in input resistance and noise in the membrane potential. APB increases also the spike rate in OFF GCs and as a consequence the cells could response to low contrasts. Zaghloul et al. [166] argue that “in addition to phasic inhibition at light onset, the ON pathway tonically inhibits the OFF GCs at mean luminance”. The authors suggest that the ON amacrine cells directly inhibit the OFF ganglion cell dendrites, but they could not determine how many amacrine cell types are involved in the two types of inhibition. Margolis and Detwiler [174] have shown that APB causes a depolarization and an increase of the maintained spiking rate of OFF GCs in mouse retina, indicating that these cells receive tonic inhibitory drive from the ON channel. The authors argue that “the synaptic input is not required for generation of the maintained activity in OFF GCs and that these cells are capable of intrinsically generated spontaneous activity”. The latter statement is based on the fact that the blockade of gap junctions (with carbenoxelone) and synaptic transmission (with antagonists of AMPA, NMDA, glycine, GABA and acetylchonine receptors) in addition to APB does not eliminate the maintained activity of sustained and transient OFF GCs. In contrast to OFF GCs, APB eliminates the maintained activity of ON GCs, indicating that it is due to tonic synaptic drive from ON bipolar cells.
Summary. Extracellular recordings from mammalian OFF GCs under photopic conditions of illumination indicate that many of them receive inhibitory input from the ON channel at mean luminance and stimulus offset. That’s why blocking of the ON channels with APB causes an enhancement of the maintained discharges and OFF responses of these ganglion cells. The inhibitory input is probably mediated by glycine in cat retina, but its network mechanism remains largely unknown. Intracellular recordings from OFF GCs indicate that the ON channel tonically hyperpolarizes these cells at mean luminance and also decreases the cone-mediated excitatory inward currents at light offset. The nature of these inhibitory influences is not yet elucidated.
4.2.2.4. Excitation at Light Onset
The OFF ganglion cells could receive an excitatory input from the ON channel at stimulus onset in addition to the inhibitory reinforcing input. The excitatory input could be revealed, however, only after blockade of retinal inhibitory transmission. Recently Farajian et al. [176] have found that the blockade of GABAergic transmission by picrotoxin reveals a robust centre-mediated ON response in every OFF α-ganglion cell studied under dark adapted conditions in both mouse and rabbit retinas. The ON response is sensitive to L-AP4, indicating that it originates in the ON pathway. The ON response is not unmasked during isolated GABAA receptor blockade (by SR95531), isolated GABAC receptor blockade (by TPMPA), isolated GABAB receptor blockade (by CGP-55845) and isolated glycine receptor blockade (by strychnine). However, when SR95531 and TPMPA are applied together, the robust ON response is reversibly unmasked in OFF GCs. Chloride channel blocker DNDS, which eliminates the effects of feedforward GABAA receptor activation is also unable to unmask an ON response in OFF GCs. This indicates that the inhibition responsible for masking of ON responses in OFF α-GCs is mediated by both GABAA and GABAC receptors on presynaptic bipolar and possibly amacrine cells. The ON response was abolished by gap junction blockers (18β-glycyrrhetinic acid, meclofenamic acid), suggesting that “the electrical synapses between OFF GCs and neighbouring multistratified amacrine cells form the pathway for excitatory crosstalk between the ON and OFF pathways”. The authors propose that “multistratified amacrine cells receive ON signals via chemical synapses with ON bipolar cell axon terminals in sublamina-b of the inner plexiform layer and then deliver these signals to OFF α-GC dendrites in sublamina-a”. They have found that the GABAergic blockade unmasks opposite polarity responses in over one-third of ON and OFF ganglion cells in rabbit and mouse retinas, indicating that “crossover excitation is a phenomenon expressed by significant number of mammalian ganglion cells”. The function of this phenomenon is yet unknown. The authors posit that “GABAergic inhibition is relieved under certain stimulus conditions allowing crossover excitation between the ON and OFF channels, which can enhance the efficiency and capacity of visual information flow to the brain”.
CONCLUSION
The parallel retinal ON and OFF pathways interact with each other at bipolar and ganglion cell levels. A common feature of these interactions is crossover reinforcing inhibition. This type of inhibition is evident in both nonmammalian and mammalian retina and it accounts for a part or all of the hyperpolarization that occurs at stimulus onset in OFF cells and at stimulus offset in ON cells. It shows clear ON-OFF asymmetry in some mammalian species, while in others is more symmetrical. The reinforcing inhibition is probably mediated by glycine and GABA, but their specific involvement depends on species and ganglion cell type. The second type of interactions between the ON and OFF channels is “disinhibition” that occurs in OFF GCs at stimulus offset. The “disinhibition” is derived from the ON channel and it works together with the input from the OFF channel to reinforce ganglion cell OFF responses. In some cases it represents a decreased inhibition at the light offset, while in other cases the ON channel contributes to the excitatory conductance at cessation of the light stimulus. The mechanisms involved in the described synergistic action between the ON and OFF channels are largely unknown. The third type of interaction between the ON and OFF channels is suppression of OFF ganglion cells at mean luminance or stimulus offset. The ON channel inhibits the maintained discharges and the light-evoked OFF responses of some ganglion cells in both nonmammalian and mammalian retina. These inhibitory influences are probably mediated by glycine and GABA, but their exact nature remains to be determined. More studies are needed to fully understand the complex interactions between the ON and OFF channels at the level of bipolar and ganglion cells in both nonmammalian and mammalian retina.
ACKNOWLEDGEMENTS
Declared none.
CONFLICT OF INTEREST
The author(s) confirm that this article content has no conflict of interest.
REFERENCES
- 1.Schiller P.H. The ON and OFF channels of the visual system. 1992. [DOI]
- 2.Schiller P.H. Parallel information processing channels created in the retina. 2010. [DOI] [PMC free article] [PubMed]
- 3.Westheimer G. The ON-OFF dichotomy in visual processing: from receptors to perception. Prog. Retin. Eye Res. 2007;26(6):636–648. doi: 10.1016/j.preteyeres.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 4.Werblin F.S., Dowling J.E. Organization of the retina of the mudpuppy, Necturus maculosus. II. Intracellular recording. J. Neurophysiol. 1969;32(3):339–355. doi: 10.1152/jn.1969.32.3.339. [DOI] [PubMed] [Google Scholar]
- 5.Famiglietti E.V., Jr, Kolb H. Structural basis for ON-and OFF-center responses in retinal ganglion cells. Science. 1976;194(4261):193–195. doi: 10.1126/science.959847. [DOI] [PubMed] [Google Scholar]
- 6.Famiglietti E.V., Jr, Kaneko A., Tachibana M. Neuronal architecture of on and off pathways to ganglion cells in carp retina. Science. 1977;198(4323):1267–1269. doi: 10.1126/science.73223. [DOI] [PubMed] [Google Scholar]
- 7.Stell W.K., Ishida A.T., Lightfoot D.O. Structural basis for on-and off-center responses in retinal bipolar cells. Science. 1977;198(4323):1269–1271. doi: 10.1126/science.201028. [DOI] [PubMed] [Google Scholar]
- 8.Nelson R., Famiglietti E.V., Jr, Kolb H. Intracellular staining reveals different levels of stratification for on- and off-center ganglion cells in cat retina. J. Neurophysiol. 1978;41(2):472–483. doi: 10.1152/jn.1978.41.2.472. [DOI] [PubMed] [Google Scholar]
- 9.Bloomfield S.A., Miller R.F. A functional organization of ON and OFF pathways in the rabbit retina. J. Neurosci. 1986;6(1):1–13. doi: 10.1523/JNEUROSCI.06-01-00001.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kolb H. The architecture of functional neural circuits in the vertebrate retina. The Proctor Lecture. Invest. Ophthalmol. Vis. Sci. 1994;35(5):2385–2404. [PubMed] [Google Scholar]
- 11.Wu S.M., Gao F., Maple B.R. Functional architecture of synapses in the inner retina: segregation of visual signals by stratification of bipolar cell axon terminals. J. Neurosci. 2000;20(12):4462–4470. doi: 10.1523/JNEUROSCI.20-12-04462.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levine M.W., Shefner J.M. Variability in ganglion cell firing patterns; implications for separate “on” and “off” processes. Vision Res. 1977;17(7):765–776. doi: 10.1016/0042-6989(77)90118-3. [DOI] [PubMed] [Google Scholar]
- 13.Shefner J.M., Levine M.W. An analysis of receptor inputs to and spatial distribution of ganglion cell on and off processes. Vision Res. 1979;19(6):647–653. doi: 10.1016/0042-6989(79)90240-2. [DOI] [PubMed] [Google Scholar]
- 14.McGuire B.A., Stevens J.K., Sterling P. Microcircuitry of beta ganglion cells in cat retina. J. Neurosci. 1986;6(4):907–918. doi: 10.1523/JNEUROSCI.06-04-00907.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Toyoda J., Shimbo K., Kondo H., Kujiraoka T. Push-pull modulation of ganglion cell responses of carp retina by amacrine cells. 1992. [DOI] [PubMed]
- 16.Molnar A., Hsueh H.A., Roska B., Werblin F.S. Crossover inhibition in the retina: circuitry that compensates for nonlinear rectifying synaptic transmission. J. Comput. Neurosci. 2009;27(3):569–590. doi: 10.1007/s10827-009-0170-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Slaughter M.M., Miller R.F. 2-amino-4-phosphonobutyric acid: a new pharmacological tool for retina research. Science. 1981;211(4478):182–185. doi: 10.1126/science.6255566. [DOI] [PubMed] [Google Scholar]
- 18.Nomura A., Shigemoto R., Nakamura Y., Okamoto N., Mizuno N., Nakanishi S. Developmentally regulated postsynaptic localization of a metabotropic glutamate receptor in rat rod bipolar cells. Cell. 1994;77(3):361–369. doi: 10.1016/0092-8674(94)90151-1. [DOI] [PubMed] [Google Scholar]
- 19.Masu M., Iwakabe H., Tagawa Y., Miyoshi T., Yamashita M., Fukuda Y., Sasaki H., Hiroi K., Nakamura Y., Shigemoto R., Takada M., Nakamura K., Nakao K., Katsuki M., Nakanishi S. Specific deficit of the ON response in visual transmission by targeted disruption of the mGluR6 gene. Cell. 1995;80(5):757–765. doi: 10.1016/0092-8674(95)90354-2. [DOI] [PubMed] [Google Scholar]
- 20.Vardi N., Morigiwa K. ON cone bipolar cells in rat express the metabotropic receptor mGluR6. Vis. Neurosci. 1997;14(4):789–794. doi: 10.1017/S0952523800012736. [DOI] [PubMed] [Google Scholar]
- 21.Morigiwa K., Vardi N. Differential expression of ionotropic glutamate receptor subunits in the outer retina. 1999. [DOI] [PubMed]
- 22.DeVries S.H. Bipolar cells use kainate and AMPA receptors to filter visual information into separate channels. Neuron. 2000;28(3):847–856. doi: 10.1016/S0896-6273(00)00158-6. [DOI] [PubMed] [Google Scholar]
- 23.Haverkamp S., Grünert U., Wässle H. Localization of kainate receptors at the cone pedicles of the primate retina. J. Comp. Neurol. 2001;436(4):471–486. doi: 10.1002/cne.1081. [DOI] [PubMed] [Google Scholar]
- 24.de la Villa P., Kurahashi T., Kaneko A. L-glutamate-induced responses and cGMP-activated channels in three subtypes of retinal bipolar cells dissociated from the cat. J. Neurosci. 1995;15(5 Pt 1):3571–3582. doi: 10.1523/JNEUROSCI.15-05-03571.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Euler T., Schneider H., Wässle H. Glutamate responses of bipolar cells in a slice preparation of the rat retina. J. Neurosci. 1996;16(9):2934–2944. doi: 10.1523/JNEUROSCI.16-09-02934.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nawy S., Jahr C.E. cGMP-gated conductance in retinal bipolar cells is suppressed by the photoreceptor transmitter. Neuron. 1991;7(4):677–683. doi: 10.1016/0896-6273(91)90380-I. [DOI] [PubMed] [Google Scholar]
- 27.Thoreson W.B., Miller R.F. Membrane currents evoked by excitatory amino acid agonists in ON bipolar cells of the mudpuppy retina. J. Neurophysiol. 1993;70(4):1326–1338. doi: 10.1152/jn.1993.70.4.1326. [DOI] [PubMed] [Google Scholar]
- 28.Karschin A., Wässle H. Voltage- and transmitter-gated currents in isolated rod bipolar cells of rat retina. J. Neurophysiol. 1990;63(4):860–876. doi: 10.1152/jn.1990.63.4.860. [DOI] [PubMed] [Google Scholar]
- 29.Yamashita M., Wässle H. Responses of rod bipolar cells isolated from the rat retina to the glutamate agonist 2-amino-4-phosphonobutyric acid (APB). J. Neurosci. 1991;11(8):2372–2382. doi: 10.1523/JNEUROSCI.11-08-02372.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vardi N., Morigiwa K., Wang T.L., Shi Y.J., Sterling P. Neurochemistry of the mammalian cone "synaptic complex". Vision Res. 1998;38:1359–1369. doi: 10.1016/s0042-6989(98)00007-8. [DOI] [PubMed] [Google Scholar]
- 31.Vardi N., Matesic D.F., Manning D.R., Liebman P.A., Sterling P. Identification of a G-protein in depolarizing rod bipolar cells. Vis. Neurosci. 1993;10(3):473–478. doi: 10.1017/S0952523800004697. [DOI] [PubMed] [Google Scholar]
- 32.Nawy S. The metabotropic receptor mGluR6 may signal through G(o), but not phosphodiesterase, in retinal bipolar cells. J. Neurosci. 1999;19(8):2938–2944. doi: 10.1523/JNEUROSCI.19-08-02938.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dhingra A., Lyubarsky A., Jiang M., Pugh E.N., Jr, Birnbaumer L., Sterling P., Vardi N. The light response of ON bipolar neurons requires G[alpha]o. J. Neurosci. 2000;20(24):9053–9058. doi: 10.1523/JNEUROSCI.20-24-09053.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dhingra A., Jiang M., Wang T.L., Lyubarsky A., Savchenko A., Bar-Yehuda T., Sterling P., Birnbaumer L., Vardi N. Light response of retinal ON bipolar cells requires a specific splice variant of Galpha(o). J. Neurosci. 2002;22(12):4878–4884. doi: 10.1523/JNEUROSCI.22-12-04878.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Okawa H., Pahlberg J., Rieke F., Birnbaumer L., Sampath A.P. Coordinated control of sensitivity by two splice variants of Gα(o) in retinal ON bipolar cells. J. Gen. Physiol. 2010;136(4):443–454. doi: 10.1085/jgp.201010477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morgans C.W., Zhang J., Jeffrey B.G., Nelson S.M., Burke N.S., Duvoisin R.M., Brown R.L. TRPM1 is required for the depolarizing light response in retinal ON-bipolar cells. Proc. Natl. Acad. Sci. USA. 2009;106(45):19174–19178. doi: 10.1073/pnas.0908711106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shen Y., Heimel J.A., Kamermans M., Peachey N.S., Gregg R.G., Nawy S. A transient receptor potential-like channel mediates synaptic transmission in rod bipolar cells. J. Neurosci. 2009;29(19):6088–6093. doi: 10.1523/JNEUROSCI.0132-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koike C., Numata T., Ueda H., Mori Y., Furukawa T. TRPM1: a vertebrate TRP channel responsible for retinal ON bipolar function. 2010. [DOI] [PubMed]
- 39.Nakamura M., Sanuki R., Yasuma T.R., Onishi A., Nishiguchi K.M., Koike C., Kadowaki M., Kondo M., Miyake Y., Furukawa T. TRPM1 mutations are associated with the complete form of congenital stationary night blindness. Mol. Vis. 2010;16:425–437. [PMC free article] [PubMed] [Google Scholar]
- 40.Koike C., Obara T., Uriu Y., Numata T., Sanuki R., Miyata K., Koyasu T., Ueno S., Funabiki K., Tani A., Ueda H., Kondo M., Mori Y., Tachibana M., Furukawa T. TRPM1 is a component of the retinal ON bipolar cell transduction channel in the mGluR6 cascade. Proc. Natl. Acad. Sci. USA. 2010;107(1):332–337. doi: 10.1073/pnas.0912730107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koyasu T., Kondo M., Miyata K., Ueno S., Miyata T., Nishizawa Y., Terasaki H. Photopic electroretinograms of mGluR6-deficient mice. Curr. Eye Res. 2008;33(1):91–99. doi: 10.1080/02713680701823232. [DOI] [PubMed] [Google Scholar]
- 42.Rentería R.C., Tian N., Cang J., Nakanishi S., Stryker M.P., Copenhagen D.R. Intrinsic ON responses of the retinal OFF pathway are suppressed by the ON pathway. J. Neurosci. 2006;26(46):11857–11869. doi: 10.1523/JNEUROSCI.1718-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu Y., Dhingra A., Fina M.E., Koike C., Furukawa T., Vardi N. mGluR6 deletion renders the TRPM1 channel in retina inactive. J. Neurophysiol. 2012;107(3):948–957. doi: 10.1152/jn.00933.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cao Y., Posokhova E., Martemyanov K.A. TRPM1 forms complexes with nyctalopin in vivo and accumulates in postsynaptic compartment of ON-bipolar neurons in mGluR6-dependent manner. J. Neurosci. 2011;31(32):11521–11526. doi: 10.1523/JNEUROSCI.1682-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pearring J.N., Bojang P., Jr, Shen Y., Koike C., Furukawa T., Nawy S., Gregg R.G. A role for nyctalopin, a small leucine-rich repeat protein, in localizing the TRP melastatin 1 channel to retinal depolarizing bipolar cell dendrites. J. Neurosci. 2011;31(27):10060–10066. doi: 10.1523/JNEUROSCI.1014-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Audo I., Kohl S., Leroy B.P., Munier F.L., Guillonneau X., Mohand-Saïd S., Bujakowska K., Nandrot E.F., Lorenz B., Preising M., Kellner U., Renner A.B., Bernd A., Antonio A., Moskova-Doumanova V., Lancelot M.E., Poloschek C.M., Drumare I., Defoort-Dhellemmes S., Wissinger B., Léveillard T., Hamel C.P., Schorderet D.F., De Baere E., Berger W., Jacobson S.G., Zrenner E., Sahel J.A., Bhattacharya S.S., Zeitz C. TRPM1 is mutated in patients with autosomal-recessive complete congenital stationary night blindness. Am. J. Hum. Genet. 2009;85(5):720–729. doi: 10.1016/j.ajhg.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van Genderen M.M., Bijveld M.M., Claassen Y.B., Florijn R.J., Pearring J.N., Meire F.M., McCall M.A., Riemslag F.C., Gregg R.G., Bergen A.A., Kamermans M. Kamermans, M. Mutations in TRPM1 are a common cause of complete congenital stationary night blindness. Am. J. Hum. Genet. 2009;85:730–736. doi: 10.1016/j.ajhg.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li Z., Sergouniotis P.I., Michaelides M., Mackay D.S., Wright G.A., Devery S., Moore A.T., Holder G.E., Robson A.G., Webster A.R. Recessive mutations of the gene TRPM1 abrogate ON bipolar cell function and cause complete congenital stationary night blindness in humans. Am. J. Hum. Genet. 2009;85(5):711–719. doi: 10.1016/j.ajhg.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zeitz C., van Genderen M., Neidhardt J., Luhmann U.F., Hoeben F., Forster U., Wycisk K., Mátyás G., Hoyng C.B., Riemslag F., Meire F., Cremers F.P., Berger W. Mutations in GRM6 cause autosomal recessive congenital stationary night blindness with a distinctive scotopic 15-Hz flicker electroretinogram. Invest. Ophthalmol. Vis. Sci. 2005;46(11):4328–4335. doi: 10.1167/iovs.05-0526. [DOI] [PubMed] [Google Scholar]
- 50.O’Connor E., Allen L.E., Bradshaw K., Boylan J., Moore A.T., Trump D. Congenital stationary night blindness associated with mutations in GRM6 encoding glutamate receptor MGluR6. Br. J. Ophthalmol. 2006;90(5):653–654. doi: 10.1136/bjo.2005.086678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bech-Hansen N.T., Naylor M.J., Maybaum T.A., Sparkes R.L., Koop B., Birch D.G., Bergen A.A., Prinsen C.F., Polomeno R.C., Gal A., Drack A.V., Musarella M.A., Jacobson S.G., Young R.S., Weleber R.G. Mutations in NYX, encoding the leucine-rich proteoglycan nyctalopin, cause X-linked complete congenital stationary night blindness. Nat. Genet. 2000;26(3):319–323. doi: 10.1038/81619. [DOI] [PubMed] [Google Scholar]
- 52.Leroy B.P., Budde B.S., Wittmer M., De Baere E., Berger W., Zeitz C. A common NYX mutation in Flemish patients with X linked CSNB. Br. J. Ophthalmol. 2009;93(5):692–696. doi: 10.1136/bjo.2008.143727. [DOI] [PubMed] [Google Scholar]
- 53.Peterson N.L., Thoreson W.B., Johnson R.L., Koerner J.F., Miller R.F. Characterization of retinal and hippocampal L-AP4 receptors using conformationally constrained AP4 analogues. Brain Res. 1991;568(1-2):15–23. doi: 10.1016/0006-8993(91)91374-A. [DOI] [PubMed] [Google Scholar]
- 54.Thoreson W.B., Ulphani J.S. Pharmacology of selective and non-selective metabotropic glutamate receptor agonists at L-AP4 receptors in retinal ON bipolar cells. Brain Res. 1995;676(1):93–102. doi: 10.1016/0006-8993(95)00093-6. [DOI] [PubMed] [Google Scholar]
- 55.Arkin M.S., Miller R.F. Subtle actions of 2-amino-4- phosphonobutyrate (APB) on the Off pathway in the mudpuppy retina. Brain Res. 1987;426:142–148. doi: 10.1016/0006-8993(87)90433-1. [DOI] [PubMed] [Google Scholar]
- 56.Hare W.A., Owen W.G. Effects of 2-amino-4-phosphonobutyric acid on cells in the distal layers of the tiger salamander retina. J. Physiol. Lond. 1992;445:741–757. doi: 10.1113/jphysiol.1992.sp018948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hensley S.H., Yang X.L., Wu S.M. Identification of glutamate receptor subtypes mediating inputs to bipolar cells and ganglion cells in the tiger salamander retina. J. Neurophysiol. 1993;69(6):2099–2107. doi: 10.1152/jn.1993.69.6.2099. [DOI] [PubMed] [Google Scholar]
- 58.Shiells R.A., Falk G., Naghshineh S. Action of glutamate and aspartate analogues on rod horizontal and bipolar cells. Nature. 1981;294(5841):592–594. doi: 10.1038/294592a0. [DOI] [PubMed] [Google Scholar]
- 59.Bloomfield S.A., Dowling J.E. Roles of aspartate and glutamate in synaptic transmission in rabbit retina. I. Outer plexiform layer. J. Neurophysiol. 1985;53(3):699–713. doi: 10.1152/jn.1985.53.3.699. [DOI] [PubMed] [Google Scholar]
- 60.Molnar A., Werblin F. Inhibitory feedback shapes bipolar cell responses in the rabbit retina. J. Neurophysiol. 2007;98(6):3423–3435. doi: 10.1152/jn.00838.2007. [DOI] [PubMed] [Google Scholar]
- 61.Miller R.F., Slaughter M.M. Excitatory amino acid receptors of the retina: diversity of subtypes and conductance mechanisms. 1986. [DOI]
- 62.Vitanova L., Kupenova P., Popova E., Mitova L., Belcheva S. Comparative investigation of retinal responses to brief light stimuli: 2-amino-4-phosphonobutyrate studies--I. Frog retina, Rana ridibunda. Comp. Biochem. Physiol. C. Comp. Pharmacol. Toxicol. 1993;104(2):289–297. doi: 10.1016/0742-8413(93)90037-L. [DOI] [PubMed] [Google Scholar]
- 63.Popova E., Kupenova P., Vitanova L., Mitova L. ERG OFF response in frog retina: light adaptation and effect of 2-amino-4-phosphonobutyrate. Acta Physiol. Scand. 1995;154(3):377–386. doi: 10.1111/j.1748-1716.1995.tb09921.x. [DOI] [PubMed] [Google Scholar]
- 64.Arnarsson A., Eysteinsson T. The role of GABA in modulating the Xenopus electroretinogram. Vis. Neurosci. 1997;14(6):1143–1152. doi: 10.1017/S0952523800011834. [DOI] [PubMed] [Google Scholar]
- 65.Vitanova L., Popova E., Kupenova P., Mitova L., Belcheva S. Comparative investigation of retinal responses to brief light stimuli: 2-amino-4-phosphonobutyrate studies--II. Turtle retina (Emys orbicularis). Comp. Biochem. Physiol. C. Comp. Pharmacol. Toxicol. 1993;104(2):299–305. doi: 10.1016/0742-8413(93)90038-M. [DOI] [PubMed] [Google Scholar]
- 66.Chappell R.L., Rosenstein F.J. Pharmacology of the skate electroretinogram indicates independent ON and OFF bipolar cell pathways. 1996. [DOI] [PMC free article] [PubMed]
- 67.Shiells R.A., Falk G. Contribution of rod, on-bipolar, and horizontal cell light responses to the ERG of dogfish retina. Vis. Neurosci. 1999;16(3):503–511. doi: 10.1017/S0952523899163119. [DOI] [PubMed] [Google Scholar]
- 68.Porciatti V., Bagnoli P., Alesci R. ON and OFF activity in the retinal and tectal response to focal stimulation with uniformed or patterned stimuli. Clin. Vis. Sci. 1987;2:93–102. [Google Scholar]
- 69.Hanitzsch R., Lichtenberger T., Mattig W.U. The influence of MgCl2 and APB on the light-induced potassium changes and the ERG b-wave of the isolated superfused rat retina. Vision Res. 1996;36(4):499–507. doi: 10.1016/0042-6989(95)00140-9. [DOI] [PubMed] [Google Scholar]
- 70.Green D.G., Kapousta-Bruneau N.V. A dissection of the electroretinogram from the isolated rat retina with microelectrodes and drugs. Vis. Neurosci. 1999;16(4):727–741. doi: 10.1017/S0952523899164125. [DOI] [PubMed] [Google Scholar]
- 71.Sharma S., Ball S.L., Peachey N.S. Pharmacological studies of the mouse cone electroretinogram. Vis. Neurosci. 2005;22(5):631–636. doi: 10.1017/S0952523805225129. [DOI] [PubMed] [Google Scholar]
- 72.Kang Derwent J.J., Saszik S.M., Maeda H., Little D.M., Pardue M.T., Frishman L.J., Pepperberg D.R. Test of the paired-flash electroretinographic method in mice lacking b-waves. Vis. Neurosci. 2007;24(2):141–149. doi: 10.1017/S0952523807070162. [DOI] [PubMed] [Google Scholar]
- 73.Shirato S., Maeda H., Miura G., Frishman L.J. Postreceptoral contributions to the light-adapted ERG of mice lacking b-waves. Exp. Eye Res. 2008;86(6):914–928. doi: 10.1016/j.exer.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Heikkinen H., Vinberg F., Pitkänen M., Kommonen B., Koskelainen A. Flash responses of mouse rod photoreceptors in the isolated retina and corneal electroretinogram: comparison of gain and kinetics. Invest. Ophthalmol. Vis. Sci. 2012;53(9):5653–5664. doi: 10.1167/iovs.12-9678. [DOI] [PubMed] [Google Scholar]
- 75.Neal M.J., Cunningham J.R., James T.A., Joseph M., Collins J.F. The effect of 2-amino-4-phosphonobutyrate (APB) on acetylcholine release from the rabbit retina: evidence for on-channel input to cholinergic amacrine cells. Neurosci. Lett. 1981;26(3):301–305. doi: 10.1016/0304-3940(81)90149-X. [DOI] [PubMed] [Google Scholar]
- 76.Massey S.C., Redburn D.A., Crawford M.L. The effects of 2-amino-4-phosphonobutyric acid (APB) on the ERG and ganglion cell discharge of rabbit retina. Vision Res. 1983;23(12):1607–1613. doi: 10.1016/0042-6989(83)90174-8. [DOI] [PubMed] [Google Scholar]
- 77.Knapp A.G., Schiller P.H. The contribution of on-bipolar cells to the electroretinogram of rabbits and monkeys. A study using 2-amino-4-phosphonobutyrate (APB). Vision Res. 1984;24(12):1841–1846. doi: 10.1016/0042-6989(84)90016-6. [DOI] [PubMed] [Google Scholar]
- 78.Lei B., Perlman I. The contributions of voltage- and time-dependent potassium conductances to the electroretinogram in rabbits. Vis. Neurosci. 1999;16(4):743–754. doi: 10.1017/S0952523899164137. [DOI] [PubMed] [Google Scholar]
- 79.Nishimura T., Machida S., Kondo M., Terasaki H., Yokoyama D., Kurosaka D. Enhancement of ON-bipolar cell responses of cone electroretinograms in rabbits with the Pro347Leu rhodopsin mutation. Invest. Ophthalmol. Vis. Sci. 2011;52(10):7610–7617. doi: 10.1167/iovs.11-7611. [DOI] [PubMed] [Google Scholar]
- 80.Gargini C., Demontis G.C., Cervetto L., Bisti S. Analysis of pharmacologically isolated components of the ERG. Vision Res. 1999;39(10):1759–1766. doi: 10.1016/S0042-6989(98)00281-8. [DOI] [PubMed] [Google Scholar]
- 81.Evers H.U., Gouras P. Three cone mechanisms in the primate electroretinogram: two with, one without off-center bipolar responses. Vision Res. 1986;26(2):245–254. doi: 10.1016/0042-6989(86)90019-2. [DOI] [PubMed] [Google Scholar]
- 82.Sieving P.A., Murayama K., Naarendorp F. Push-pull model of the primate photopic electroretinogram: a role for hyperpolarizing neurons in shaping the b-wave. Vis. Neurosci. 1994;11(3):519–532. doi: 10.1017/S0952523800002431. [DOI] [PubMed] [Google Scholar]
- 83.Kondo M., Sieving P.A. Primate photopic sine-wave flicker ERG: vector modeling analysis of component origins using glutamate analogs. Invest. Ophthalmol. Vis. Sci. 2001;42(1):305–312. [PubMed] [Google Scholar]
- 84.Khan N.W., Kondo M., Hiriyanna K.T., Jamison J.A., Bush R.A., Sieving P.A. Primate retinal signaling pathways: Suppressing ON-pathway activity in monkey with glutamate analogs mimics human CSNB1-NYX genetic night blindness. J. Neurophysiol. 2005;93(1):481–492. doi: 10.1152/jn.00365.2004. [DOI] [PubMed] [Google Scholar]
- 85.Nawy S., Copenhagen D.R. Multiple classes of glutamate receptor on depolarizing bipolar cells in retina. Nature. 1987;325(6099):56–58. doi: 10.1038/325056a0. [DOI] [PubMed] [Google Scholar]
- 86.Grant G.B., Dowling J.E. A glutamate-activated chloride current in cone-driven ON bipolar cells of the white perch retina. J. Neurosci. 1995;15(5 Pt 2):3852–3862. doi: 10.1523/JNEUROSCI.15-05-03852.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wong K.Y., Cohen E.D., Dowling J.E. Retinal bipolar cell input mechanisms in giant danio. II. Patch-clamp analysis of ON bipolar cells. J. Neurophysiol. 2005;93:94–107. doi: 10.1152/jn.00270.2004. [DOI] [PubMed] [Google Scholar]
- 88.Grant G.B., Dowling J.E. On bipolar cell responses in the teleost retina are generated by two distinct mechanisms. J. Neurophysiol. 1996;76(6):3842–3849. doi: 10.1152/jn.1996.76.6.3842. [DOI] [PubMed] [Google Scholar]
- 89.Connaughton V.P., Nelson R. Axonal stratification patterns and glutamate-gated conductance mechanisms in zebrafish retinal bipolar cells. J. Physiol. 2000;524(Pt 1):135–146. doi: 10.1111/j.1469-7793.2000.t01-1-00135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tse D.Y., Chung I., Wu S.M. Possible roles of glutamate transporter EAAT5 in mouse cone depolarizing bipolar cell light responses. 2014. [DOI] [PMC free article] [PubMed]
- 91.DeMarco P.J., Jr, Powers M.K. Sensitivity of ERG components from dark-adapted goldfish retinas treated with APB. Brain Res. 1989;482(2):317–323. doi: 10.1016/0006-8993(89)91194-3. [DOI] [PubMed] [Google Scholar]
- 92.DeMarco P.J., Jr, Bilotta J., Powers M.K. DL-2-amino-4-phosphonobutyric acid does not eliminate “ON” responses in the visual system of goldfish. Proc. Natl. Acad. Sci. USA. 1991;88(9):3787–3791. doi: 10.1073/pnas.88.9.3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wong K.Y., Adolph A.R., Dowling J.E. Retinal bipolar cell input mechanisms in giant danio. I. Electroretinographic analysis. J. Neurophysiol. 2005;93(1):84–93. doi: 10.1152/jn.00259.2004. [DOI] [PubMed] [Google Scholar]
- 94.DeMarco P.J., Jr, Nussdorf J.D., Brockman D.A., Powers M.K. APB selectively reduces visual responses in goldfish to high spatiotemporal frequencies. Vis. Neurosci. 1989;2(1):15–18. doi: 10.1017/S0952523800004272. [DOI] [PubMed] [Google Scholar]
- 95.Van Epps H.A., Yim C.M., Hurley J.B., Brockerhoff S.E. Investigations of photoreceptor synaptic transmission and light adaptation in the zebrafish visual mutant nrc. Invest. Ophthalmol. Vis. Sci. 2001;42(3):868–874. [PubMed] [Google Scholar]
- 96.Ren J.Q., Li L. Rod and cone signaling transmission in the retina of zebrafish: an erg study. Int. J. Neurosci. 2004;114(2):259–270. doi: 10.1080/00207450490269480. [DOI] [PubMed] [Google Scholar]
- 97.Demarco P.J., Jr, Powers M.K. APB alters photopic spectral sensitivity of the goldfish retina. Vision Res. 1994;34(1):1–9. doi: 10.1016/0042-6989(94)90251-8. [DOI] [PubMed] [Google Scholar]
- 98.Saszik S., Alexander A., Lawrence T., Bilotta J. APB differentially affects the cone contributions to the zebrafish ERG. Vis. Neurosci. 2002;19(4):521–529. doi: 10.1017/S0952523802194144. [DOI] [PubMed] [Google Scholar]
- 99.Wong K.Y., Gray J., Hayward C.J., Adolph A.R., Dowling J.E. Glutamatergic mechanisms in the outer retina of larval zebrafish: analysis of electroretinogram b- and d-waves using a novel preparation. Zebrafish. 2004;1:121–131. doi: 10.1089/zeb.2004.1.121. [DOI] [PubMed] [Google Scholar]
- 100.Nelson R.F., Singla N. A spectral model for signal elements isolated from zebrafish photopic electroretinogram. Vis. Neurosci. 2009;26(4):349–363. doi: 10.1017/S0952523809990113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Green D.G., Kapousta-Bruneau N.V. Evidence that L-AP5 and D,L-AP4 can preferentially block cone signals in the rat retina. Vis. Neurosci. 2007;24(1):9–15. doi: 10.1017/S0952523807230123. [DOI] [PubMed] [Google Scholar]
- 102.Granda A.M., Dearworth J.R., Jr, Subramaniam B. Balanced interactions in ganglion-cell receptive fields. Vis. Neurosci. 1999;16(2):319–332. doi: 10.1017/S0952523899162126. [DOI] [PubMed] [Google Scholar]
- 103.Soucy E., Wang Y., Nirenberg S., Nathans J., Meister M. A novel signaling pathway from rod photoreceptors to ganglion cells in mammalian retina. Neuron. 1998;21(3):481–493. doi: 10.1016/S0896-6273(00)80560-7. [DOI] [PubMed] [Google Scholar]
- 104.Yang J., Pahng J., Wang G-Y. Dopamine modulates the off pathway in light-adapted mouse retina. J. Neurosci. Res. 2013;91(1):138–150. doi: 10.1002/jnr.23137. [DOI] [PubMed] [Google Scholar]
- 105.Bloomfield S.A., Dowling J.E. Roles of aspartate and glutamate in synaptic transmission in rabbit retina. II. Inner plexiform layer. J. Neurophysiol. 1985;53(3):714–725. doi: 10.1152/jn.1985.53.3.714. [DOI] [PubMed] [Google Scholar]
- 106.Kittila C.A., Massey S.C. Effect of ON pathway blockade on directional selectivity in the rabbit retina. J. Neurophysiol. 1995;73(2):703–712. doi: 10.1152/jn.1995.73.2.703. [DOI] [PubMed] [Google Scholar]
- 107.Jin X.T., Brunken W.J. A differential effect of APB on ON- and OFF-center ganglion cells in the dark adapted rabbit retina. Brain Res. 1996;708(1-2):191–196. doi: 10.1016/0006-8993(95)01390-3. [DOI] [PubMed] [Google Scholar]
- 108.Bloomfield S.A., Xin D. Surround inhibition of mammalian AII amacrine cells is generated in the proximal retina. J. Physiol. 2000;523(Pt 3):771–783. doi: 10.1111/j.1469-7793.2000.t01-1-00771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bolz J., Wässle H., Thier P. Pharmacological modulation of on and off ganglion cells in the cat retina. Neuroscience. 1984;12(3):875–885. doi: 10.1016/0306-4522(84)90176-3. [DOI] [PubMed] [Google Scholar]
- 110.Horton J.C., Sherk H. Receptive field properties in the cat’s lateral geniculate nucleus in the absence of on-center retinal input. J. Neurosci. 1984;4(2):374–380. doi: 10.1523/JNEUROSCI.04-02-00374.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cohen E.D., Miller R.F. The role of NMDA and non-NMDA excitatory amino acid receptors in the functional organization of primate retinal ganglion cells. Vis. Neurosci. 1994;11(2):317–332. doi: 10.1017/S0952523800001668. [DOI] [PubMed] [Google Scholar]
- 112.Knapp A.G., Mistler L.A. Response properties of cells in rabbit’s lateral geniculate nucleus during reversible blockade of retinal on-center channel. J. Neurophysiol. 1983;50(5):1236–1245. doi: 10.1152/jn.1983.50.5.1236. [DOI] [PubMed] [Google Scholar]
- 113.Hughes T.E. Are there ionotropic glutamate receptors on the rod bipolar cell of the mouse retina? Vis. Neurosci. 1997;14(1):103–109. doi: 10.1017/S0952523800008804. [DOI] [PubMed] [Google Scholar]
- 114.Hughes T.E., Hermans-Borgmeyer I., Heinemann S. Differential expression of glutamate receptor genes (GluR1-5) in the rat retina. Vis. Neurosci. 1992;8(1):49–55. doi: 10.1017/S0952523800006489. [DOI] [PubMed] [Google Scholar]
- 115.Kamphuis W., Dijk F., O'Brien B.J. Gene expression of AMPA-type glutamate receptor subunits in rod-type ON bipolar cells of rat retina. 2003. [DOI] [PubMed]
- 116.Peng Y.W., Blackstone C.D., Huganir R.L., Yau K.W. Distribution of glutamate receptor subtypes in the vertebrate retina. Neuroscience. 1995;66(2):483–497. doi: 10.1016/0306-4522(94)00569-Q. [DOI] [PubMed] [Google Scholar]
- 117.Nelson R., Janis A.T., Behar T.N., Connaughton V.P. Physiological responses associated with kainate receptor immunoreactivity in dissociated zebrafish retinal neurons: a voltage probe study. Prog. Brain Res. 2001;131:255–265. doi: 10.1016/S0079-6123(01)31021-X. [DOI] [PubMed] [Google Scholar]
- 118.Klooster J., Yazulla S., Kamermans M. Ultrastructural analysis of the glutamatergic system in the outer plexiform layer of zebrafish retina. J. Chem. Neuroanat. 2009;37(4):254–265. doi: 10.1016/j.jchemneu.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 119.Perlman I. The Electroretinogram: ERG. In: Kolb H., Fernandez E., Nelsonm R., editors. Webvision: The Organization of the Retina and Visual System. Salt Lake City, UT: University of Utah Health Sciences Center; 1995. [Internet] [PubMed] [Google Scholar]
- 120.Frishman L.J. Origins of the electroretinogram. In: Heckenlively J.R., Arden G.B., editors. Principles and Practice of Clinical Electrophysiology of Vision. London: MIT Press; 2006. pp. 139–183. [Google Scholar]
- 121.Betz H., Laube B. Glycine receptors: recent insights into their structural organization and functional diversity. J. Neurochem. 2006;97(6):1600–1610. doi: 10.1111/j.1471-4159.2006.03908.x. [DOI] [PubMed] [Google Scholar]
- 122.Matsubayashi H., Alkondon M., Pereira E.F., Swanson K.L., Albuquerque E.X. Strychnine: a potent competitive antagonist of alpha-bungarotoxin-sensitive nicotinic acetylcholine receptors in rat hippocampal neurons. J. Pharmacol. Exp. Ther. 1998;284(3):904–913. [PubMed] [Google Scholar]
- 123.Renna J.M., Strang C.E., Amthor F.R., Keyser K.T. Strychnine, but not PMBA, inhibits neuronal nicotinic acetylcholine receptors expressed by rabbit retinal ganglion cells. Vis. Neurosci. 2007;24(4):503–511. doi: 10.1017/S0952523807070241. [DOI] [PubMed] [Google Scholar]
- 124.Bloomfield S.A., Dowling J.E. Roles of aspartate and glutamate in synaptic transmission in rabbit retina. I. Outer plexiform layer. J. Neurophysiol. 1985;53(3):699–713. doi: 10.1152/jn.1985.53.3.699. [DOI] [PubMed] [Google Scholar]
- 125.Slaughter M.M. APB receptors on OFF bipolar and horizontal cells. Invest. Ophthalmol. Vis. Sci. 1986;27(Suppl.):130. [Google Scholar]
- 126.Hosoi N., Arai I., Tachibana M. Group III metabotropic glutamate receptors and exocytosed protons inhibit L-type calcium currents in cones but not in rods. J. Neurosci. 2005;25(16):4062–4072. doi: 10.1523/JNEUROSCI.2735-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Higgs M.H., Lukasiewicz P.D. Activation of group II metabotropic glutamate receptors inhibits glutamate release from salamander retinal photoreceptors. Vis. Neurosci. 2002;19(3):275–281. doi: 10.1017/S0952523802192054. [DOI] [PubMed] [Google Scholar]
- 128.Higgs M.H., Romano C., Lukasiewicz P.D. Presynaptic effects of group III metabotropic glutamate receptors on excitatory synaptic transmission in the retina. Neuroscience. 2002;115(1):163–172. doi: 10.1016/S0306-4522(02)00381-0. [DOI] [PubMed] [Google Scholar]
- 129.Vigh J., Vickers E., von Gersdorff H. Light-evoked lateral GABAergic inhibition at single bipolar cell synaptic terminals is driven by distinct retinal microcircuits. J. Neurosci. 2011;31(44):15884–15893. doi: 10.1523/JNEUROSCI.2959-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zimmerman R.P., Corfman T.P. A comparison of the effects of isomers of alpha-aminoadipic acid and 2-amino-4-phosphonobutyric acid on the light response of the müller glial cell and the electroretinogram. Neuroscience. 1984;12(1):77–84. doi: 10.1016/0306-4522(84)90139-8. [DOI] [PubMed] [Google Scholar]
- 131.Jardon B., Yucel H., Bonaventure N. Glutamate separation of ON and OFF retinal channels: possible modulation by glycine and acetylcholine. Eur. J. Pharmacol. 1989;162:215–224. doi: 10.1016/0014-2999(89)90284-7. [DOI] [PubMed] [Google Scholar]
- 132.Stockton R.A., Slaughter M.M. B-wave of the electroretinogram. A reflection of ON bipolar cell activity. J. Gen. Physiol. 1989;93(1):101–122. doi: 10.1085/jgp.93.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Popova E., Kupenova P., Vitanova L., Mitova L. Effect of 2-amino-4-phosphonobutyrate on ERG OFF-response after glycinergic and GABAergic blockade. Vision Res. 1995;35(14):1945–1949. doi: 10.1016/0042-6989(94)00310-I. [DOI] [PubMed] [Google Scholar]
- 134.Bush R.A., Sieving P.A. A proximal retinal component in the primate photopic ERG a-wave. Invest. Ophthalmol. Vis. Sci. 1994;35(2):635–645. [PubMed] [Google Scholar]
- 135.Awatramani G.B., Slaughter M.M. Intensity-dependent, rapid activation of presynaptic metabotropic glutamate receptors at a central synapse. J. Neurosci. 2001;21(2):741–749. doi: 10.1523/JNEUROSCI.21-02-00741.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sethuramanujam S., Slaughter M.M. Disinhibitory recruitment of NMDA receptor pathways in retina. J. Neurophysiol. 2014;112(1):193–203. doi: 10.1152/jn.00817.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Jardon B., Bonaventure N., Scherrer E. Possible involvement of cholinergic and glycinergic amacrine cells in the inhibition exerted by the ON retinal channel on the OFF retinal channel. Eur. J. Pharmacol. 1992;210(2):201–207. doi: 10.1016/0014-2999(92)90672-Q. [DOI] [PubMed] [Google Scholar]
- 138.Popova E., Mitova L., Vitanova L., Kupenova P. Effect of 2-amino-4-phosphonobutyrate on the OFF responses of frog retinal ganglion cells and local ERG after glycinergic blockade. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2000;126(2):139–151. doi: 10.1016/S0742-8413(00)00107-9. [DOI] [PubMed] [Google Scholar]
- 139.Popova E., Mitova L., Vitanova L., Kupenova P. Participation of the GABAergic system in the action of 2-amino-4- phosphonobutyrate on the OFF responses of frog retinal ganglion cells. Vision Res. 2003;43:607–616. doi: 10.1016/s0042-6989(03)00008-7. [DOI] [PubMed] [Google Scholar]
- 140.Famiglietti E.V., Jr, Kolb H. A bistratified amacrine cell and synaptic cirucitry in the inner plexiform layer of the retina. Brain Res. 1975;84(2):293–300. doi: 10.1016/0006-8993(75)90983-X. [DOI] [PubMed] [Google Scholar]
- 141.Dacheux R.F., Raviola E. The rod pathway in the rabbit retina: a depolarizing bipolar and amacrine cell. J. Neurosci. 1986;6(2):331–345. doi: 10.1523/JNEUROSCI.06-02-00331.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Raviola E., Dacheux R.F. Excitatory dyad synapse in rabbit retina. Proc. Natl. Acad. Sci. USA. 1987;84(20):7324–7328. doi: 10.1073/pnas.84.20.7324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Demb J.B., Singer J.H. Intrinsic properties and functional circuitry of the AII amacrine cell. Vis. Neurosci. 2012;29(1):51–60. doi: 10.1017/S0952523811000368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Nelson R. Cat cones have rod input: a comparison of the response properties of cones and horizontal cell bodies in the retina of the cat. J. Comp. Neurol. 1977;172:109–135. doi: 10.1002/cne.901720106. [DOI] [PubMed] [Google Scholar]
- 145.Smith R.G., Freed M.A., Sterling P. Microcircuitry of the dark-adapted cat retina: functional architecture of the rod-cone network. J. Neurosci. 1986;6(12):3505–3517. doi: 10.1523/JNEUROSCI.06-12-03505.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Schneeweis D.M., Schnapf J.L. Photovoltage of rods and cones in the macaque retina. Science. 1995;268(5213):1053–1056. doi: 10.1126/science.7754386. [DOI] [PubMed] [Google Scholar]
- 147.Tsukamoto Y., Morigiwa K., Ueda M., Sterling P. Microcircuits for night vision in mouse retina. J. Neurosci. 2001;21(21):8616–8623. doi: 10.1523/JNEUROSCI.21-21-08616.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Pang J.J., Gao F., Lem J., Bramblett D.E., Paul D.L., Wu S.M. Direct rod input to cone BCs and direct cone input to rod BCs challenge the traditional view of mammalian BC circuitry. Proc. Natl. Acad. Sci. USA. 2010;107(1):395–400. doi: 10.1073/pnas.0907178107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hack I., Peichl L., Brandstätter J.H. An alternative pathway for rod signals in the rodent retina: rod photoreceptors, cone bipolar cells, and the localization of glutamate receptors. Proc. Natl. Acad. Sci. USA. 1999;96(24):14130–14135. doi: 10.1073/pnas.96.24.14130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.West R.W. Bipolar and horizontal cells of the gray squirrel retina: Golgi morphology and receptor connections. Vision Res. 1978;18(2):129–136. doi: 10.1016/0042-6989(78)90177-3. [DOI] [PubMed] [Google Scholar]
- 151.Li W., DeVries S.H. Synapse between rods and off cone bipolar cells in a mammalian retina. Invest. Ophthalmol. Vis. Sci. 2007.
- 152.Fyk-Kolodziej B., Qin P., Pourcho R.G. Identification of a cone bipolar cell in cat retina which has input from both rod and cone photoreceptors. J. Comp. Neurol. 2003;464(1):104–113. doi: 10.1002/cne.10784. [DOI] [PubMed] [Google Scholar]
- 153.Li W., Keung J.W., Massey S.C. Direct synaptic connections between rods and OFF cone bipolar cells in the rabbit retina. J. Comp. Neurol. 2004;474(1):1–12. doi: 10.1002/cne.20075. [DOI] [PubMed] [Google Scholar]
- 154.Müller F., Wässle H., Voigt T. Pharmacological modulation of the rod pathway in the cat retina. J. Neurophysiol. 1988;59(6):1657–1672. doi: 10.1152/jn.1988.59.6.1657. [DOI] [PubMed] [Google Scholar]
- 155.Jensen R.J. Involvement of glycinergic neurons in the diminished surround activity of ganglion cells in the dark-adapted rabbit retina. Vis. Neurosci. 1991;6(1):43–53. doi: 10.1017/S0952523800000894. [DOI] [PubMed] [Google Scholar]
- 156.Protti D.A., Flores-Herr N., Li W., Massey S.C., Wässle H. Light signaling in scotopic conditions in the rabbit, mouse and rat retina: a physiological and anatomical study. J. Neurophysiol. 2005;93(6):3479–3488. doi: 10.1152/jn.00839.2004. [DOI] [PubMed] [Google Scholar]
- 157.DeVries S.H., Baylor D.A. An alternative pathway for signal flow from rod photoreceptors to ganglion cells in mammalian retina. Proc. Natl. Acad. Sci. USA. 1995;92(23):10658–10662. doi: 10.1073/pnas.92.23.10658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wang G.Y. Unique functional properties of the APB sensitive and insensitive rod pathways signaling light decrements in mouse retinal ganglion cells. Vis. Neurosci. 2006;23(1):127–135. doi: 10.1017/S0952523806231110. [DOI] [PubMed] [Google Scholar]
- 159.Murphy G.J., Rieke F. Network variability limits stimulus-evoked spike timing precision in retinal ganglion cells. Neuron. 2006;52(3):511–524. doi: 10.1016/j.neuron.2006.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Völgyi B., Deans M.R., Paul DL., Bloomfield S.A. Convergence and segregation of the multiple rod pathways in mammalian retina. J. Neurosci. 2004;24:11182–11192. doi: 10.1523/JNEUROSCI.3096-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hsueh H.A., Molnar A., Werblin F.S. Amacrine-to-amacrine cell inhibition in the rabbit retina. J. Neurophysiol. 2008;100(4):2077–2088. doi: 10.1152/jn.90417.2008. [DOI] [PubMed] [Google Scholar]
- 162.Roska B., Molnar A., Werblin F.S. Parallel processing in retinal ganglion cells: how integration of space-time patterns of excitation and inhibition form the spiking output. J. Neurophysiol. 2006;95(6):3810–3822. doi: 10.1152/jn.00113.2006. [DOI] [PubMed] [Google Scholar]
- 163.Chen X., Hsueh H.A., Greenberg K., Werblin F.S. Three forms of spatial temporal feedforward inhibition are common to different ganglion cell types in rabbit retina. J. Neurophysiol. 2010;103(5):2618–2632. doi: 10.1152/jn.01109.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Buldyrev I., Puthussery T., Taylor W.R. Synaptic pathways that shape the excitatory drive in an OFF retinal ganglion cell. J. Neurophysiol. 2012;107(7):1795–1807. doi: 10.1152/jn.00924.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Cohen E.D. Interactions of inhibition and excitation in the light-evoked currents of X type retinal ganglion cells. J. Neurophysiol. 1998;80(6):2975–2990. doi: 10.1152/jn.1998.80.6.2975. [DOI] [PubMed] [Google Scholar]
- 166.Zaghloul K.A., Boahen K., Demb J.B. Different circuits for ON and OFF retinal ganglion cells cause different contrast sensitivities. J. Neurosci. 2003;23(7):2645–2654. doi: 10.1523/JNEUROSCI.23-07-02645.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Manookin M.B., Beaudoin D.L., Ernst Z.R., Flagel L.J., Demb J.B. Disinhibition combines with excitation to extend the operating range of the OFF visual pathway in daylight. J. Neurosci. 2008;28(16):4136–4150. doi: 10.1523/JNEUROSCI.4274-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Pang J.J., Gao F., Wu S.M. Light-evoked excitatory and inhibitory synaptic inputs to ON and OFF alpha ganglion cells in the mouse retina. J. Neurosci. 2003;23(14):6063–6073. doi: 10.1523/JNEUROSCI.23-14-06063.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Crook J.D., Packer O.S., Dacey D.M. A synaptic signature for ON- and OFF-center parasol ganglion cells of the primate retina. Vis. Neurosci. 2014;31(1):57–84. doi: 10.1017/S0952523813000461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Liang Z., Freed M.A. The ON pathway rectifies the OFF pathway of the mammalian retina. 2010. [DOI] [PMC free article] [PubMed]
- 171.Werblin F.S. Six different roles for crossover inhibition in the retina: correcting the nonlinearities of synaptic transmission. Vis. Neurosci. 2010;27(1-2):1–8. doi: 10.1017/S0952523810000076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Chen E.P., Linsenmeier R.A. Effects of 2-amino-4-phosphonobutyric acid on responsivity and spatial summation of X cells in the cat retina. J. Physiol. 1989;419:59–75. doi: 10.1113/jphysiol.1989.sp017861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Chen E.P., Linsenmeier R.A. Centre components of cone-driven retinal ganglion cells: differential sensitivity to 2-amino-4-phosphonobutyric acid. J. Physiol. 1989;419:77–93. doi: 10.1113/jphysiol.1989.sp017862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Margolis D.J., Detwiler P.B. Different mechanisms generate maintained activity in ON and OFF retinal ganglion cells. J. Neurosci. 2007;27(22):5994–6005. doi: 10.1523/JNEUROSCI.0130-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Wässle H., Schäfer-Trenkler I., Voigt T. Analysis of a glycinergic inhibitory pathway in the cat retina. J. Neurosci. 1986;6(2):594–604. doi: 10.1523/JNEUROSCI.06-02-00594.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Farajian R., Pan F., Akopian A., Völgyi B., Bloomfield S.A. Masked excitatory crosstalk between the ON and OFF visual pathways in the mammalian retina. J. Physiol. 2011;589(Pt 18):4473–4489. doi: 10.1113/jphysiol.2011.213371. [DOI] [PMC free article] [PubMed] [Google Scholar]