Abstract
Epilepsy is known as one of the most frequent neurological diseases, characterized by an enduring predisposition to generate epileptic seizures. Oxidative stress is believed to directly participate in pathways leading to neurodegeneration, which serves as the most important propagating factor, leading to the epileptic condition and cognitive decline. Moreover, there is also a growing body of evidence showing the disturbance of antioxidant system balance and consequently increased production of reactive species in patients with epilepsy. A meta-analysis, conducted in the present review confirms an association between epilepsy and increased lipid peroxidation. Furthermore, it was also shown that some of the antiepileptic drugs could potentially be responsible for additionally increased lipid peroxidation. Therefore, it is reasonable to propose that during the epileptic process neuroprotective treatment with antioxidants could lead to less sever structural damages, reduced epileptogenesis and milder cognitive deterioration. To evaluate this hypothesis studies investigating the neuroprotective therapeutic potential of various antioxidants in cells, animal seizure models and patients with epilepsy have been reviewed. Numerous beneficial effects of antioxidants on oxidative stress markers and in some cases also neuroprotective effects were observed in animal seizure models. However, despite these encouraging results, till now only a few antioxidants have been further applied to patients with epilepsy as an add-on therapy. Based on the several positive findings in animal models, a strong need for more carefully planned, randomized, double-blind, cross-over, placebo-controlled clinical trials for the evaluation of antioxidants efficacy in patients with epilepsy is warranted.
Keywords: Antiepileptic drugs, antioxidants, epileptogenesis, meta-analysis, neuroprotective, oxidative stress, reactive species
1. INTRODUCTION
Epilepsy is a neurological disease, characterized by an enduring predisposition to generate epileptic seizures and by enormous neurobiological, psychological, cognitive, and social consequences. Repeating seizures originate from hyperexcitation of certain group of neurons and lead to different transient clinical signs and laboratory findings. The crucial role in the disease development has the formation of the epileptogenic focus which has an uncontrolled hyperexcitability due to partial prolonged depolarization of cellular membranes [1]. Prolonged depolarization is the result of imbalance in neuronal system polarization in favour of hyperpolarizing influences as a consequence of enhanced excitation, decreased inhibition and/or diverse nervous system changes. This represents the underlying mechanism of the initiation and propagation of a seizure in a normal individual [2]. There is evidence that the formation of reactive species or the decreased activity of antioxidant systems may result in different forms of epilepsy as well as increased chances of repeating epileptic seizures [1].
Regardless, the abundance of antiepileptic drugs (AEDs) introduced after phenobarbitone (PB) in the beginning ofthe previous century, nowadays 20-30% of patients with epilepsy still continue having seizures [3]. These patients require a more aggressive approach, since monotherapy fails to control seizures. However, even polytherapy is not always effective. Furthermore, the incidence of adverse reactions, namely neurological disruptions, psychiatric and behavioural changes, as well as metabolic alterations is also increasingly important. Therefore, there is an urgent need for better tolerated and more efficient AEDs, especially in this group of patients [4].
Furthermore, during metabolic processes, numerous AEDs, especially from the older AEDs generation, including PB, phenytoin (PHT), carbamazepine (CBZ) and valproic acid (VPA), produce reactive metabolites which can covalently bind to different endogenous macromolecules or increase the formation of reactive oxygen species (ROS) that can induce oxidative damage and cause toxicity. To support this theory, numerous studies evaluating the influence of epilepsy and AEDs on the formation of free radicals, show that either of them could be connected to oxidative stress generation [1, 5-9].
Therefore, this review summarizes the rationale for use of antioxidants as add-on therapy for modulating free radical mediated oxidative stress in epilepsy and evaluates the existing studies investigating the efficacy of different antioxidant therapies.
2. OXIDATIVE STRESS AND ITS ROLE IN EPILEPTOGENESIS
Oxidative stress is a consequence of the increased oxidant burden which overwhelms the endogenous antioxidants and repair capacity or a consequence of diminished endogenous antioxidants and repair capacity which cannot encompass the normal oxidant burden [10]. Glutamate, which act as excitatory central nervous system (CNS) neurotransmitter and could potentially act excito-toxic, especially at higher concentrations, is believed to be one of the key factors involved in oxidative stress generation [11].
Increased formation of ROS results in elevated intracellular Ca2+concentration, which is seen in neuroplasticity changes, as well as seizure-induced neuronal death either through necrosis or apoptosis [12]. Increased intracellular Ca2+, which persist even through the chronic phase of epilepsy and is therefore crucial for the continuation of recurrent seizures, can influence on GABA A receptor recycling and thus alter neuronal excitability [13]. Moreover, increased intracellular Ca2+can change gene transcription, protein expression and turnover, neurogenesis, neuronal sprouting, and others cell physiological functions [14].
Epileptogenesis is defined as the process in which an initial CNS insult leads to the onset of the epileptic condition as well as to the propagation of events that occur after established epilepsy and can take years or even decades [15]. This supports the theory that sequence of changes after an initial insult is essential for the development of the epileptic condition [16, 17]. Although, the process of epileptogenesis is so far not clearly understood [18], the above mentioned neuronal cell death is believed to be one of the most important factor that could lead to the development of the epileptic condition [16].
Oxidative stress is known as a fundamental cytotoxic mechanism involved in pathogenesis of various neuro-degenerative diseases [19]. Its role in neurodegenerative diseases is especially important, because cells in the CNS, namely neurons, are particularly vulnerable to the destructive effects of reactive species [2]. ROS can cause deleterious effects on cells through acting on signalling pathways or through causing nonspecific oxidative damage to bio- macromolecules [10]. Generally, biological effects of ROS are successfully controlled by endogenous defence system of antioxidant enzymes (glutathione reductase -GR, glutathione peroxidase -GPx, superoxide dismutase -SOD, and catalase -CAT) and non-enzymatic antioxidants (namely glutathione - GSH, vitamin E, vitamin A, vitamin C and β-carotene) [20]. Antioxidant enzymes block the initiation of reactive species chain reactions, while the non-enzymatic antioxidants react directly with reactive species and thereby prevent the propagation of chain reactions [20]. A major source of ROS is the respiratory chain reaction, which takes place in cell mitochondria [21, 22].
Therefore, any changes in oxidative burden in favour of prooxidants can lead to initiation or propagation of already established epileptic seizures by proposed mechanisms that provoke neuronal cell death.
3. INFLUENCE OF OXIDATIVE STRESS ON BIOLOGICAL MACROMOLECULES
Free radicals, resulting from endogenous redox imbalance, are believed to play an important role in causing oxidative stress, cell death and consequently tissue damage. They are able of injuring different cell macromolecules, namely lipids, nucleic acids, proteins, and carbohydrates, finally leading to cell death and cognitive decline, as described in our previous work [2].
Since oxygen is lipophilic and therefore accumulates in higher amounts in biological membranes and since polyunsaturated fatty acids (PUFAs) in lipoproteins and phospholipids are especially vulnerable to oxidative injuries, the major consequence of increased oxidative stress reflects in lipid peroxidation. Lipid peroxidation disturbs biological membranes and is therefore particularly destructive to their structure and function. Moreover, several by-products are produced through lipid peroxidation, including unsaturated hydroperoxides, which can further break down to generate diverse reactive aldehydes. Through covalent binding to cellular proteins, reactive aldehydes can further alter their function and subsequently provoke cellular damage. One of the widely studied reactive aldehydes is malondyaldehyde (MDA). Moreover, peroxidation products, known as peroxyl radicals, are less reactive compared to ROS and have therefore longer time of action. Consequently, they are able to diffuse more distant, even through cells, where they can react with other cellular constituents and cause diffuse cellular damage. Due to greater stability, peroxidation end products are valuable laboratory markers. General laboratory markers of lipid peroxidation are MDA and F2-isoprostanes [23, 24].
Free radicals may further trigger disruption of nucleic acids. They can break deoxyribonucleic acid (DNA) strands or directly modify bases, which leads to deletions and other mutations. These modifications could result in aberrant gene expression and even cell death [25]. DNA damage activates the DNA repair enzyme poly-ADP-ribose polymerase-1, which over activation depletes its substrate, nicotinamide adenine dinucleotide, slowing the rate of glycolysis, electron transport, and ATP formation, eventually leading to functional impairment or cell death [26]. General laboratory marker of oxidative DNA damage is 8-hydroxy-2-deoxyguanosine (8-OHdG) [27].
The mitochondrial DNA (mtDNA), is even more vulnerable target for free radical damage because of diminished repair mechanisms and lack of histones and because it is situated much closer to the site of ROS generation. MtDNA disruption may lead to mitochondrial dysfunction that might result in disturbed cell function. Consequently disturbed electron transport chain and additional ROS production can result in a potentiation of oxidative stress [17]. After all, the most susceptible to oxidative damage is ribonucleic acid (RNA), since it is single stranded, not protected by hydrogen bonding, and less protected by proteins. RNA damages may result in errors in proteins or dysregulation of gene expression [28].
Moreover, free radicals can oxidize both the backbone and side chains of proteins [23]. These oxidative modifications may disturb the function of enzymes, receptors, neuro-transmitters, and structural proteins [29]. One of the most commonly used methods to quantify protein oxidation is by measuring the protein carbonyl level [23].
Finally, oxidation of monosaccharide sugars results in the formation of oxaldehydes, which can contribute to protein aggregation. The term advanced glycation end-product (AGE) describes either protein damage that results from adduction of reducing sugars and subsequent oxidative evolution, or adduction of more reactive sugar oxidation products, termed glycoxidation. Oxidation of carbohydrate polymers may cause depolymerization and disturbed function of the involved polymers. AGEs are known as useful markers of oxidative damage [30].
4. OXIDATIVE STRESS IN DRUG-NAIVE PATIENTS WITH EPILEPSY
Changes in antioxidant defence mechanisms, as a result of epileptic condition itself or the effects of antiepileptic therapy have been studied in humans, but the observed research findings are discordant [5-7, 31-47].
The majority of studies confirmed impaired oxidative status in untreated patients with epilepsy in terms of elevated lipid peroxidation reflected in elevated MDA levels [1, 33, 37, 42, 44, 46, 47] and significantly decreased serum total antioxidant capacity [7, 32, 44]. Studies further reported elevated [32, 35], decreased [43, 46] or unchanged [31, 37] SOD activity, elevated [31], unchanged [32, 37, 44] or decreased [36] GPx activity, unchanged [31] or decreased [37] GR activity and unchanged CAT activity [43]. Arhan et al. observed also significantly elevated nitric oxide (NO) levels in patients with epilepsy in contrast to healthy controls [45].
Although, MDA levels are elevated in most cases, unchanged [31, 43, 45] or even decreased [32] levels were also reported. In a study designed by Yis et al., it was shown that scavenger systems are activated in order to decrease lipid peroxidation, resulting in lower erythrocyte MDA levels and slightly, insignificantly higher SOD activities [32].
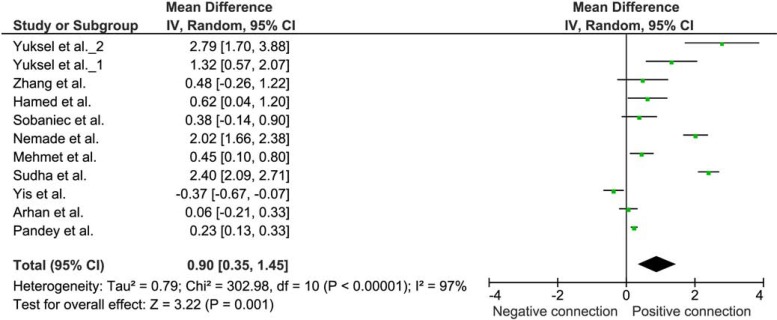
To evaluate the influence of epilepsy on oxidative status, we performed a meta-analysis on studies of lipid per-oxidation reflected in MDA levels. A selection criterion for inclusion of the study was the comparison of untreated patients with confirmed epilepsy with a control group, presented by healthy control matches. Eleven studies were found which met the inclusion criteria [1, 31-33, 37, 42-47].
Weighted mean difference (WMD) with 95% confidence interval (95% CI) was calculated from the extracted data, using Review Manager 5.2 (The Cochrane Collaboration, Oxford, UK). Due to heterogeneity of the results of various studies, random effect model was applied. WMD of the MDA levels was 0.90 µg/mL (95% CI 0.35 to 1.46) suggesting increased lipid peroxidation in epilepsy (Fig. 1).
Fig. (1).
Meta-analysis of the studies comparing the lipid peroxidation in untreated patients with epilepsy and healthy controls. The difference in plasma MDA concentration (μg/mL) in a group of untreated patients with epilepsy and a healthy control group.
5. OXIDATIVE STRESS IN PATIENTS WITH EPILEPSY TREATED WITH THE OLDER GENERATION OF AEDS
Oxidative stress is known to be present in epilepsy as a cause or/and a consequence of epileptogenesis process. Furthermore, it was also shown that long-term treatment with AEDs can cause elevated free radical production in neuronal cells and consequently increased oxidative damage, leading to neurodegeneration. Several conventional AEDs are known to be extensively metabolized in the body. Through their metabolic transformation, numerous reactive metabolites, which are capable of covalent binding to biological macromolecules, are formed. Thus, the AEDs may, besides their main inhibitory activity on the epileptic focuses, also provoke systemic toxicity, either through increased oxidative damage or covalent binding of their reactive metabolites to biological macromolecules [8, 9, 48, 49]. Hence, it is of particular importance to find out the impact of AEDs on oxidative stress.
5.1. Valproic Acid
Most studies have shown increased lipid peroxidation in children and adolescents [1, 31-33, 43, 45, 49] or adults [44] treated with VPA compared to untreated patients with epilepsy [1, 32, 33, 43-45] or healthy controls [1, 31, 33, 49]. Schulpis reported also decreased total antioxidant capacity levels [49]. Studies further showed decreased [50] or unchanged [5, 31, 51] SOD activity, elevated [44], unchanged [5, 31] or decreased [50, 52] GPx activity, elevated [52] or decreased [31] GR activity, unchanged [51] CAT activity and elevated [44] or decreased [52] serum Se levels. Furthermore, Schulpis in children and Varoglau, in adults found elevated 8-OHdG levels indicating increased DNA damage [49, 53]. On the other side, Peker et al. reported slightly higher NO concentrations in VPA group, however no significant differences in serum MDA levels were detected [51].
5.2. Carbamazepine
In the case of CBZ influence on lipid peroxidation inconsistent results were found. Some studies have shown increased oxidative stress expressed as lipid peroxidation [7, 33, 44, 53], some have shown ambiguous changes [1, 6] and the others decreased [31, 42] lipid peroxidation compared to untreated patients with epilepsy [1, 6, 31, 33, 42, 44] or healthy controls [7, 53]. Hammed et al. confirmed also decreased total antioxidant capacity [44]. Studies further reported elevated [6, 33], decreased [50] or unchanged [1, 5] SOD activity, elevated, unchanged [1, 5] or decreased [44, 50] GPx activity, unchanged or decreased GR activity, decreased CAT activity [50] and serum Se levels [44]. Moreover, Varoglau et al. found elevated 8-OHdG levels in adult patients with epilepsy, indicating increased DNA damage [53]. Niketić et al. further reported elevated levels of Hb ASSG, representing a glutathione adduct of haemoglobin (Hb). Occurrence of Hb ASSG serves as a marker of oxidative stress in erythrocytes [50]. A study, conducted by Yuksel et al., determined the modifications in the antioxidant system in children with epilepsy receiving long-term AEDs [1]. The results showed no significant differences in lipid per-oxidation, SOD and GPx activities in children with epilepsy on CBZ monotherapy in contrast to the healthy controls and untreated patients with epilepsy [1]. The levels were re-tested two years after the beginning of the treatment when increased lipid peroxidation and decreased SOD and GPx activities were noticed compared to healthy controls. SOD activity was lower even compared to untreated patients with epilepsy [33].
5.3. Phenobarbitone
Aycicek et al. investigated the effect of PB on different serum markers of oxidative stress. They examined the oxidative stress index, total antioxidant capacity, lipid hydro- peroxide, total peroxide, and concentrations of individual serum antioxidants such as albumin, bilirubin and uric acid. Apart from increased oxidative stress index and lipid hydro- peroxide in the group treated with PB in contrast to control group, no other significant changes were observed [7]. Furthermore, Niketić et al. observed decreased levels of SOD and GPx in adults treated with PB [50].
5.4. Polytherapy with Carbamazepine and Valproic Acid
In a large group of children and adolescents with epilepsy, receiving CBZ and VPA polytherapy, increased MDA concentrations and therefore lipid peroxidation were seen compared to healthy controls. Decreased SOD activities and significantly increased GPx activities were found in comparison to relevant levels in the healthy controls. Moreover, it was found that GR activity was slightly lower in polytherapy as well as in VPA monotherapy compared to healthy control. On the other hand, GR activity was higher in CBZ monotherapy group [31].
5.5. Meta-analysis
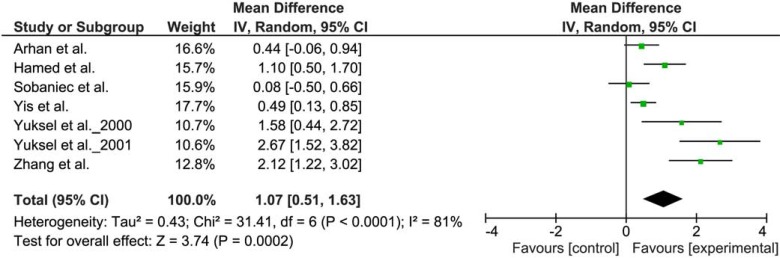
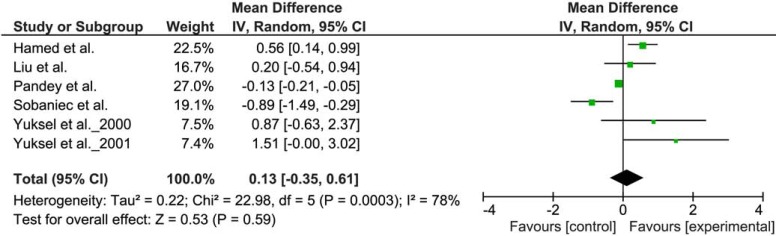
To explore the influence of AED therapy on oxidative status, a meta-analysis evaluating the influence of VPA and CBZ on MDA levels was designed. A selection criterion for inclusion of the study was the presence of a group of patients with epilepsy on therapy with VPA or CBZ and a control group of untreated patients with epilepsy. Seven [1, 31-33, 43-45] and six [1, 6, 31, 33, 42, 44] studies were found which met the inclusion criteria for VPA and CBZ, respectively. WMD with 95% confidence interval (95% CI) were calculated from the extracted data. Due to heterogeneity of the results of various studies, random effect model was applied. WMD of the MDA levels were 1.07 µg/mL (95% CI 0.51 to 1.63) in the case of VPA (Fig. 2) and 0.13 µg/mL (95% CI - 0.35 to 0.61) in the case of CBZ (Fig. 3).
Fig. (2).
Meta-analysis of the studies comparing the lipid peroxidation in patients with epilepsy, treated with VPA, and a control group of untreated patients with epilepsy. The difference in plasma MDA concentration (μg/mL).
Fig. (3).
Meta-analysis of the studies comparing the lipid peroxidation in epilepsy patients treated with CBZ and a control group of untreated patients with epilepsy. The difference in plasma MDA concentration (µg/mL).
Significantly increased MDA levels in VPA treated group were found, while in CBZ group the effect of treatment on MDA level was inconclusive. Increased MDA levels seen in VPA may be a consequence of afore mentioned conventional AEDs metabolism and subsequent elevated free radicals formation, causing numerous side effects [1, 48, 54]. Similarly, numerous studies have confirmed increased lipid peroxidation in older generation AEDs like PB [7], PHT [6] and already mentioned CBZ [44]. These drugs represent classical aromatic AEDs which have also been proven to provoke oxidative stress either alone or through their metabolites [55]. This free radical generation could be the reason for numerous side effects and even for the failure of seizure control. Unfortunately, there are sparse studies evaluating the effect of newer AEDs on oxidative status to perform reliable meta-analysis. However, there are few studies performed on newer AEDs generation which have shown better oxidative profile. Studies evaluating oxcarbazepine [34, 45], topiramate [56] and lamotrigine [57] have shown decreased lipid peroxidation compared to untreated patients with epilepsy or healthy controls.
These results confirm that in patients with epilepsy the oxidants-antioxidants balance is largely modified by epileptic condition as well as by antiepileptic therapy. Therefore, further research is needed, especially with the newer AEDs.
6. ANTIEPILEPTIC THERAPY AND OXIDATIVE STRESS
6.1. Medication Therapy
Despite better understanding of the pathophysiological processes related to seizure initiation and propagation in the brain, and despite the introduction of several newer, second and third generation AEDs, there are still 20-30% of patients with epilepsy that are inadequately treated with the recent frontline AEDs [58, 59]. Most of these patients havefocal epilepsies, which may be caused by brain trauma, brain tumors, complicated febrile convulsions, status epilepticus (SE) or ischemic lesions. As a consequence of such pathological processes a neural cell loss can be found in areas of epileptogenesis. Progressive neuronal cell loss is believed to have a major role in development of the resistant forms of epilepsy, such as temporal lobe seizures and further more even in development of the resistance to AED therapy at later stages of the disease [60].
Combination of two or more first- or second-generation AEDs or the application of novel third-generation AEDs are the most appropriate, but still often not sufficiently effective therapeutic options when surgical treatment cannot be offered [58, 61-63]. While oxidative stress is believed to have an important function in epilepsy, approaches covering neuroprotective as well as anticonvulsive actions are desirable. Use of AED zonisamide with independent neuro-protective and antiepileptic activity in drug resistant patients with epilepsy, has confirmed this theory [64, 65].
6.2. Add-on Therapy with Antioxidants
Currently, ROS generation is believed to represent one of the crucial processes, which underlies the mechanism of causing damaging effects on brain by epileptic activity. The resulting neuronal alterations in the brain circuitry represent the fundamental changes which could eventually lead to a condition of recurrent spontaneous seizures. Therefore, neuroprotective actions could slow or even prevent epileptogenesis, hence, decrease seizure severity and frequency, improve established AED therapy efficacy and reduce well known AED pharmacoresistance [66-69].
Since the neutralisation of the increased ROS formation during seizures depends on the ability of the antioxidant defence systems, the antiepileptogenic therapeutic potential of various substances possessing antioxidant activities, have been extensively studied [67-69]. This includes endogenously present antioxidants, such as α-lipoic acid [70-73], GSH [74], melatonin [75-85], ubiquinone (coenzyme Q10) [86, 87] and vitamin A [88], exogenous anticonvulsive and neuro-protective substances, such as α-tocopherol [53, 70-72], ascorbic acid [66, 89, 90], curcumin [91-100], N-acetylcisteine [101], omega-3 fatty acids [102-112] and resveratrol [113, 114], and novel synthetic, potent radical scavengers, like aspalatone [115], EPC-K1 [116], EUK-134 [117], MnTBAP [118-120], and tempol [121]. These compounds, generally defined as molecules with the ability to quench or reduce highly ROS, exerts neuroprotective effects and can therefore protect the brain against oxidative stress as seen in some experimental models of seizures [66, 70-74, 90-92, 96, 99, 122-125].
It is known that conventional AEDs fail to sufficiently control seizures. Furthermore, long-term use of these AEDs significantly increase oxidative stress and predispose to cognitive impairment [86]. The reason for seizures and cognitive deficit could lie in the inability of the current AEDs to establish the balance between oxidants and antioxidant defence mechanisms in the body [91]. Apart from epilepsy itself causing oxidative stress, increased oxidative stress triggered by AEDs is also suggested to contribute to the induction of seizures and cognitive impairment in patients with epilepsy [25, 37, 44, 91]. Therefore, the capability of antioxidants to inhibit seizure generation and decreased oxidative stress markers, further support their significant role as having supposed antiepileptic potential [126].
There is relatively sparse data regarding clinical activity of AEDs on cognitive impairment. Some AEDs, namely PB, VPA and CBZ have been shown to significantly disrupt the vital balance between oxidants and antioxidants [7, 43, 44]. CBZ and lamotrigine have also been shown to trigger an important decline in learning and memory tests [127]. To confirm these findings, significant cognitive decline after CBZ treatment has been reported in newly diagnosed patients with epilepsy [128]. Furthermore, Reeta et al. reported that PB and CBZ caused significant oxidative stress and therefore deterioration of learning and memory in rats after 21 days of treatment. PB caused more cognitive impairment compared to CBZ [91]. Comparable trends have been already reported in humans [129, 130]. Finally, it has been reported that PB, PHT and VPA can trigger deleterious effects on the immature brain, which can in some cases lead to severe deterioration of cognitive functions development [91].
According to mentioned above, the use of antioxidants as add-on therapy offers a potentially beneficial way of treatment in order to provide neuroprotective environmentin the injured or stressed brain. Potential benefits of antioxidants, used as add-on therapy, were extensively studied in different in vitro or in vivo animal models of seizures and epilepsy and in patients with epilepsy. The results and observed potential mechanisms of their anticonvulsive action are further described in details and summarized in Tables 1-3.
Table 1.
Observed anticonvulsive and neuroprotective actions of endogenous antioxidants.
| Substance | Cells/Animals/Humans | Seizure Model | References |
|---|---|---|---|
| Potential antioxidant actions | |||
| α-Lipoic acid | |||
| Anti-convulsive effects | Animal model (rats) | PIL | [79, 142] |
Inhibits seizure activity and oxidative damage
|
Animal model (rats) | PIL/Iron | [70, 72, 143] |
| Influence on neuronal excitability and further on development and propagation of certain seizure types, namely through its antioxidant activity |
In vitro & in vivo models |
- | [144] |
Strong antioxidant effects in vivo and in vitro
|
In vitro & in vivo models | - | [144] |
| Dihydrolipoic acid | |||
Strong antioxidant effects in vivo and in vitro
|
- | [144] | |
| Melatonin | |||
Attenuates seizure activity and neurodegeneration
|
Animal model (rats) | KA/PTZ/PIL | [76, 78, 139, 145] |
| Exerts antioxidant properties | Patients with epilepsy | Children | [146] |
Exerts anticonvulsive and neuroprotective properties
|
Animal model (rats) | KA/Amig. kindl | [142, 75] |
| Animal model (mice/rats) | KA/Iron | [185, 147] | |
| Animal model (mice) | KA | [76] | |
| Animal model (rats) | KA | [186, 149] | |
| Protects against seizures and decreased LPO | Animal model (rats) | Iron | [147, 150] |
Protects against oxidative stress
|
Animal model (rats) | Iron | [135] |
| Suppress epileptic activity by inhibiting peroxidation | Animal model (rats/mice) | Iron/KA | [147, 151] |
| Selen | |||
Provides protection against reactive oxygen species induced damage
|
Patients with epilepsy | Children | [36] |
| Ubiquinone | |||
Prevents cells from free radicals induced oxidative damage
|
Animal model (rats) | PIL | [86, 87] |
Potentiate the antiepileptic effects of PHT treatment
|
Animal model (rats) | PIL | [86] |
BBB – blood brain barrier, CAT – catalase, GPx – glutathione synthetase, GSH – glutathione, KA – kainic acid, LPO- lipid peroxidation, NOS - nitric oxide synthase, PIL – pilocarpin, PHT – phenytoin, PTZ – pentylenetetrazol, ROS – reactive oxygen species, RNS – reactive nitrogen species, SOD - superoxide dismutase
Table 3.
Observed anticonvulsive and neuroprotective actions of novel, potent antioxidants.
| Substance | Cells/Animals/Humans | Model | References |
|---|---|---|---|
| Potential antioxidant actions | |||
| Aspalatone | |||
| GPx mimetic [202] | |||
|
Animal modelIn vitro ESR study | KA - | [115] [115] |
| EUK-134 (new, potent SOD mimetic) | |||
Prevents oxidative stress and reduces neuronal damage [117]
|
In vitro model Animal model |
- KA |
[203] [127] |
|
Animal model | KA | [117, 118] |
| MnTBAP | |||
Inhibits mitochondrial oxidative stress and neuronal loss
|
Animal model (rats) | KA | [118-120] |
| Tempol | |||
Protects neuronal cells and exerts anticonvulsant effects via:
|
Animal models | KA | [121] |
| There were no effects exerted on seizure-like activity in hippocampus | Animal models | KA | [121] |
8-OHdG – 8-hydroxy-2-deoxyguanosine, AP-1 – activator protein, CAT – catalase, DNA – deoxyribonucleic acid, ESR - electron spin resonance, GPx – glutathione peroxidase, GSH – glutathione, MnTBAP – Mn(III)tetrakis (4-benzoic acid) porphyrin, KA – kainic acid, LPO- lipid peroxidation, NF – nuclear factor, SOD - superoxide dismutase.
6.2.1. Endogenous Antioxidants
α-Lipoic Acid
α-Lipoic acid (LA) is an important cofactor for mitochondrial enzymes and an essential natural antioxidant [70].
Pre-treatment with LA demonstrated decreased nitrite content and lipid peroxidation level, while increased SOD, CAT, and GPx activities in striatum were observed during the acute phase of seizures induced by pilocarpine in adult rats [70, 131, 132]. A reduction in free radical formation and an increase in the activity of antioxidant enzymes produced an important enhancement in the resistance to seizures. Animals exposed to LA treatment presented no differences in physical growth and brain development, suggesting that LA ameliorates metabolic parameters only in a pilocarpine model [133].
It was further demonstrated that pre-treatment with LA is able to reduce brain oxidative metabolism and consequently prevent pilocarpine-triggered seizures, SE and mortality of adult rats. These findings support an important function of free radicals in managing seizures development, maintenance and propagation [70].
After all, as seen above, LA could serve as neuro- protective treatment against seizures induced by pilocarpine.
Melatonin
Melatonin is a pineal hormone with its main function of regulating circadian rhythm [134]. It exhibits potent antioxidant activities by direct scavenging of hydroxyl and other free radicals, by stimulating GPx activity, and by inhibiting NO synthase [135].
Melatonin was reported to suppress generalized seizures in amygdala kindled rats [75]. It is also known that pineal- ectomy reduces the number of stimulations required to trigger amygdala kindling [136]. Similarly, in the model of pilocarpine induced seizures in rats, pinealectomy was also found to be associated with reduced time needed to first spontaneous seizures and further even increased number of spontaneous recurrent seizures during the chronic phase [79]. These findings together confirm the neuroprotective role of melatonin, both endogenous and exogenous [136].
Simultaneous admission of melatonin and kainic acid (KA) was further shown to completely inhibits KA-induced seizures and reduces mitochondrial DNA damage in the mouse brain cortex. These observed neuroprotective and anticonvulsant activity of melatonin could be mediated by scavenging of hydroxyl radicals [76, 77]. Besides, melatonin has been also reported to reduce iron-induced seizures in rats through inhibition of peroxidation [135].
Experiments conducted in mice showed that melatonin significantly raised the electroconvulsive threshold, and increased the CBZ and PB protective activity against electroshock. Since, melatonin was shown to increase the number of 3H GABA binding sites in animal hippocampus, it is reasonable to assume that these observed melatonin actions can be related to increased activity of GABA-ergic system [78].
Furthermore, supplementation with melatonin during the SE period in the pilocarpine model has been shown to decrease apoptosis in different limbic areas [79]. Anti- convulsive effects of melatonin were also observed in penicillin-induced epileptiform activity in rats. Melatonin administered intra-cerebroventricularly delayed the on-set of epileptic seizures confirmed by electrocorticogram.
This anticonvulsant activity can again be explained by increased activity of GABA-ergic system, induced by melatonin [80].
It was further seen that acutely administered melatonin considerably elevated the threshold for clonic convulsions triggered by pentylenetetrazol (PTZ) in mice [137]. In fact, melatonin prevented PTZ-induced glutamine and aspartate increases, while at higher doses, melatonin further decreases nitrite content in different brain areas, including the hippocampus [138]. Moreover, melatonin pre-treatment before PTZ administration in guinea pigs was reported to increase seizure latency, attenuate seizure severity, and lower the mortality rate [139].
Collectively, melatonin has shown numerous protective functions in various animal seizure models, therefore it seems a promising neuroprotective agent.
Ubiquinone
Ubiquinone is a potent antioxidant which reacts with ROS and therefore prevents cells free radicals induced oxidative damage, including mitochondrial membrane lipid peroxidation. Besides, ubiquinol, representing a reduced form of ubiquinone, is further capable of additional reduction in lipid peroxidation, since it has the ability of performing as a chain-breaking antioxidant and recycling of other antioxidants, namely α-tocopherol and lipoic acid [140, 141].
Recently, studies evaluating the neuroprotective effects of ubiquinone in rats’ pilocarpin-induced epileptic model were performed [86, 87]. In pilocarpine group, an important increase in hydroperoxide concentration and GPx activity was reported, while there were no changes observed in SOD and CAT activities. On the other hand, in rat hippocampusof ubiquinone group, markedly decreased hydroperoxide content and elevated SOD, CAT and GPx activities were observed [87]. Overall, ubiquinone has been reported to decrease the extent of oxidative stress and consequently the severity of pilocarpine-induced seizures [86].
Furthermore, ubiquinone was reported to potentiate the antiepileptic effects of PHT treatment by amelioration of oxidative stress and cognitive impairment caused by chronic PHT therapy in pilocarpine-induced seizures in rats. Therefore, ubiquinone can be used as a safe and effective add-on therapy to conventional epilepsy treatment, both to reduce seizure severity as well as to protect against seizure-induced oxidative damage [86].
These results suggest that ubiquinone may exert significant neuroprotective actions which might be helpful in the treatment of neurodegenerative disorders [86, 87].
6.2.2. Exogenous Antioxidants
Ascorbic Acid (Vitamin C)
Ascorbic acid represents a classical, potent water-soluble antioxidant, which reduces harmful oxidants and therefore protects biological macromolecules from oxidation [152]. Its main function is direct scavenging of superoxide and hydroxyl radical [66]. Furthermore, ascorbic acid is crucial for other antioxidants recycling, especially for vitamin E and lipoic acid. Moreover, it also enhances SOD and CAT enzymes activities [66, 90].
The use of ascorbic acid in animal studies demonstrated the reduction of neuronal damage, triggered by free radicals, which are particularly elevated in inflammation processes and neurodegenerative disorders [66].
Histopathological studies on animals, which were pre-treated with ascorbic acid, prior to pilocarpine induced seizure, have revealed a significant 60% reduction in the frequency of hippocampal brain damage induced by seizures and 5-fold decrease in the area of hippocampal damage [66]. The ascorbic acid pre-treatment has been further shown to increase hippocampal SOD and CAT activities, increase in the latency to first seizures, suppression of behavioural seizure episodes, and decrease in lipid peroxidation, nitrite content, brain damage, SE, severity of hippocampal lesions, and mortality of rats in pilocarpine induced seizures [66, 90, 153]. These findings are a consequence of ascorbic acidfree radicals scavenging abilities, which support its neuro- protective activity. Moreover, ascorbic acid could compensate for the reduction of GSH synthesis caused by nitrite and nitrate inhibition, and also for the loss of other endogenous antioxidants and antioxidant enzymes, including SOD and CAT [66].
Additionally, ascorbic acid was further reported to decrease or prevent epileptic seizures evoked by FeCl3 [116] or penicillin administration [154, 155].
Curcumin
Curcumin possess antioxidative and anti-apoptotic properties [92, 156]. It acts as an effective scavenger of ROS and RNS, which leads to decreased lipid peroxidation, oxidative DNA damage, mitochondrial dysfunction, and apoptotic cell death [93, 94]. Therefore, in experimental animal models, curcumin has been shown to exhibit protective effects against seizures, oxidative stress and cognitive deterioration in a dose-dependent manner [93, 96].
Curcumin pre-treatment was shown to prevent hippocampal neuronal cell death [92]. Precisely, curcumin, manganese complex of curcumin, and diacetylcurcumin treatment have been shown to attenuate hippocampal cell death induced by KA both, by inhibiting cell apoptosis as well as increasing neuroprotection accomplished through maintenance of intact blood brain barrier function [95, 96].
In PTZ animal models, curcumin expressed dose-dependent protection against seizures. It considerably extended latency phases presented before myoclonic, clonic and generalized tonic-clonic seizures occurred, and further decreased duration of generalized tonic-clonic seizures [96]. Furthermore, curcumin administration in rats inhibited brain MDA levels to increase, indicating a decreased lipid per- oxidation [157]. Additionally, a recent study of curcumin supplementation in PTZ kindled rats confirms antioxidant effect of curcumin through a decrease in MDA, and an increase in CAT and glutathione S-transferase levels observed in rat brain [100].
Curcumin pre-treatment in rats was further shown to poses protective effects against oxidative stress and cognitive deterioration induced by PHT [99] as well as against seizures in animal experimental models of epileptic seizures induced by iron administration [97] or electroshock [98].
Co-administration of curcumin with PHT, PB and CBZ showed a significant improvement in elevated plus maze test as well as in passive avoidance paradigm [91, 99, 158]. These two tests are often used as complementary to each other. The anxiety and memory in rodents can be estimated by elevated plus maze test [159]. In passive avoidance test, the animal teaches to stay away from a place or situation in which shock was experienced in the past. Consequently, both tests can measure animals’ capability to remember crucial environmental information [160]. In rats treated with curcumin, the improvement in both tests shows towards superior acquisition and retention of memory, and further increased capacity to learn [91]. Therefore, in rats treated with curcumin and the above mentioned AEDs, curcumin inhibited the progression of cognitive deterioration [91, 99]. Since curcumin administration in healthy rats was not associated with any changes in cognitive functions, it shows that curcumin alone does not improve memory in normal rats [91, 157].
Co-administration with curcumin in rat models of cognitive deterioration induced by PB and CBZ compared to controls shows a significant elevation and reduction in the brain reduced GSH and MDA levels, respectively. These could be at least partially responsible for the decrease of PB- and CBZ-induced cognitive deterioration [91].
The influence of curcumin on cognitive functions was further studied in rat PTZ-kindling induced seizure model [161, 162]. Curcumin group compared to controls showed a significant increase in retention latencies in the passive avoidance paradigm and a significant decrease in retention transfer latencies in the elevated plus maze test [161, 162]. Moreover, curcumin pre-treatment improved cognitive functions in PTZ-kindled rats that is at least partially a consequence of observed curcumin anti-seizure activity [157].
Curcumin antiepileptic actions have already been observed in former studies in which curcumin reduced aluminium chloride-induced, PHT-induced, and lead-induced memory deficit in rats [96]. Moreover, it was shown that curcumin raise the cellular GSH levels through promotion of glutamate cysteine ligase genes transcription [195].
Since the administration of curcumin in rats has been shown to be effective in preventing chemical induced seizures, oxidative damage and cognitive deterioration, it may have some potential as a possible neuroprotective agent. Therefore, curcumin could be used as an add-on therapy resulting in improved seizure control and cognitive functions.
Epigallocatechin (EGCG)
Pre-treatment with EGCG in PTZ-kindled rats has been shown to decrease the time of mean seizure phase and increase the duration of the latent period before myoclonic jerks and generalized tonic-clonic seizures occurred in a dose dependent manner compared to PTZ group. Furthermore, EGCG pre-treated group showed marked decrease in MDA and increased in GSH levels in brain tissue, caused by PTZ-induced seizures, compared to controls [196]. In addition, EGCG pre-treatment in lead-induced seizure models has been connected to significant decrease in MDA levels as well as an increase in GSH levels and SOD activity [197]. This is supported by the study where EGCG prevented iron-induced seizures [198].
On the other hand, pre-treatment with EGCG was further shown to significantly improve the declined learning and memory loss. These findings were confirmed by significantly improved results of passive avoidance paradigm and elevated plus maze tests in comparison to the PTZ group. While EGCG alone showed no effects on cognitive functions, this could confirm that EGCG attenuates the impaired cognition induced by PTZ. These observed neuro- protective effects of EGCG on cognitive decline induced by seizures can be at least partially a consequence of its anticonvulsant activity [196].
Therefore, it is suggested that pre-treatment with EGCG might decrease oxidative stress and improve cognitive decline after PTZ-induced seizures [196, 197, 199].
N-acetylcysteine - thiol Containing Compounds
The compounds in the thiol-containing group are similar to the major endogenous antioxidant glutathione. Their antioxidant activity comes from the reducing activity of the thiol group. This sulfhydryl provides an electron to ROS, causing ROS reduction and therefore decreased reactivity. N-acetylcysteine (NAC) is a simplified mimetic of GSH with a similar antioxidant mechanism utilizing the thiol group to reduce ROS [69].
NAC has demonstrated an ability to suppress epilepto-genesis in PTZ model, probably due to the direct antioxidant effect and increase in cellular GSH levels [69, 200].
Resveratrol
Resveratrol is a conjugated aromatic compound with a conjugated bridge between two aromatic rings. The antioxidant activity is due to the ability to delocalize an unpaired electron over an extended conjugated framework, thus stabilizing the radical [113]. Resveratrol was associated with reductions in severity of KA-induced seizures [114].
α-Tocopherol (Vitamin E)
α-Tocopherol is considered to be the main antioxidant substance in the human body, interfering with oxygen and the production of hydroxyl radical in cell membranes, thereby reducing lipid peroxidation [90]. Vitamin E type molecules are highly lipophilic. α-Tocopherol has been reported to prevent neurotoxicity and neurological symptoms in rat models of chemically-induced epilepsy [124]. Convulsive behaviour was attenuated in pentylenetetrazol-, methyl- malonate- and pilocarpine-induced seizures, where brain lipid peroxidation and nitrite content were lowered, while CAT and SOD activities were increased, with resulting lower hippocampal damage and increased survival [90, 125, 201]. In animals treated with α-tocopherol plus pilocarpine, the intensity of histopathological changes and mortality rate were lower in comparison to pilocarpine alone [90].
Diverse experimental models show that preliminary injections of α-tocopherol reduce seizure induced oxygen and nitrogen free radicals generation on a time scale of minutes-to-hours [124]. Short-term dietary α-tocopherol supplementation reduces brain lipid peroxidation evenfour days after KA-induced seizures. Together with a high consumption rate of brain α-tocopherol observed in supplemented rats after seizures, this finding indicates that conditions of oxidative stress initiated by SE persist long after the earliest post-ictal phases and that a-tocopherol can play a prolonged antioxidant effect after the triggering event. α-Tocopherol markedly reduces neuronal cell death after SE [124].
There is also considerable evidence of the prophylactic and inhibitory effects of α-tocopherol on the development of iron-induced epileptic seizures. α-Tocopherol has been shown to importantly delay the onset of epileptic seizures triggered by intra cerebral FeCl3 administration [135].
6.2.3.
Novel Synthetic, Potent Antioxidants
α-Tocopheryl-L-ascorbate-2-O-phosphatediester
α-Tocopheryl-L-ascorbate-2-O-phosphatediester (EPC-K1) represents a potent synthetic scavenger of hydroxyl radicals. EPC-K1 has been demonstrated to inhibit the formation of thiobarbituric acid reactive substances (TBARS) and protein carbonyl (P-Carb), induced by ferric ions in vitro in a dose dependant manner. Furthermore, pre-treatment or simultaneous treatment with EPC-K1 suppresses or delays the appearance of epileptic seizures induced by ferric ions [116].
As seen above, ROS have been implicated in seizure-induced neurodegeneration. These findings support the appropriateness of introducing the addition of antioxidants as an add-on to classical AEDs treatment. Most of the studies performed so far have confirmed that antioxidants as anadd-on therapy could potentially, at least to some extent, provide neuroprotective effects against seizure-induced neurotoxicity (Tables 1, 2, and 3).
Table 2.
Observed anticonvulsive and neuroprotective actions of exogenous antioxidants.
| Substance | Cells/Animals/Humans | Seizure Model | References |
|---|---|---|---|
| Potential Antioxidant Actions | |||
| Ascorbic acid (vitamin C) | |||
| Ameliorates convulsive behaviour and neuronal death Inhibits initial oxidative stress & maintains GSH homeostasis Directly scavenges free radicals & restores the endogenous antioxidant system Enhances CAT activity and decreased LPO |
Animal model (rats) Animal model (rats) Animal model (rats) Animal model (rats) |
PIL/KA/PTZ Trimethylin Stress PIL |
[89, 153, 163, 164] [165] [166] [89, 153] |
| β-catechin | |||
| Oral administration inhibits TBARS formation and increases the activity of SOD Pre-treatment results in a reduction in free radical formation |
Animal model (rats) Animal model (rats) |
Iron Iron |
[167] [168] |
| Curcumin | |||
| Neuroprotective effects produced by: | |||
|
Animal model (rats) | KA/- KA | [92, 169] [168] |
| Curcumin manganese complex | |||
| Possesses more powerful anticonvulsive and neuroprotective properties | Animal model (rats) | KA | [95, 170] |
Animal models show it:
|
Animal model (rats) | KA | [95, 170] |
| Ginkgo biloba | |||
Suppresses seizure generation and seizure induced ROS formation
|
Animal model (mice) | PTZ | [171] |
| *Neurotoxin (4’-O-methoxypridoxine) exerts pro-epileptic effects | Patients with epilepsy/Healthy subjects | / | [172] |
| Ginsenosides | |||
Attenuate seizure activity
|
Animal model (rats) Animal model (rats) Animal model (rats) Animal model (rats) |
KA PIL PTZ PTZ |
[173] [173] [174] [174] |
| Honeybee propolis | |||
Pre-treatment significantly attenuates oxidative stress, seizure activity and neuronal degenerations
|
Animal model (rats) | KA | [175] |
Anticonvulsive actions
|
Animal model (rats) | Trimethylin/ KA |
[165, 176] |
| Protects against seizures | Animal models (mice) | PTZ | [101] |
| Omega-3 fatty acids (PUFAs) | |||
Exert channel modulation, and anti-inflammatory action
|
Animal model (rats) Animal model (rats/mice) |
PIL KA |
[103, 104, 177] [171, 174] |
Observed anti-convulsive actions in animal studies
|
Mice/rats Mice/rats Animal model (rats) Animal model (mice) Animal model (rats) |
PTZ PTZ PTZ KA KA/GI |
[105, 178] [106, 107, 179] [180, 181] [108] [182] |
Anticonvulsant effects of n-3 PUFAs (EPA & DHA) in clinical studies [109]
|
Patients with epilepsy Patients with epilepsy |
CE Int./Ref. |
[110] [111, 183] |
| Plasma concentrations are elevated in children treated with KD | Children with epilepsy | KD | [184] |
| Resveratrol | |||
| Exerts anticonvulsive and neuroprotective properties, decreases LPO Delays the onset of seizures and decreases LPO |
Animal model (mice/rats) Animal model (rats) |
KA/AOMS Iron |
[171, 185] [186] |
| α-Tocopherol(vitamin E) | |||
Pre-treatment with α-tocopherol:
|
Animal model (rats) | PIL | [122, 125] |
| Prevents the development of epileptic seizures induced by iron administration Significantly delays the appearance of seizures triggered by intracerebral FeCl3 administration Decreases seizure activity and LPO Improvement in patients with complex partial seizures Exerts anticonvulsive and neuroprotective effects - reduced BBB disruption Fails to attenuate seizure activity |
Animal model (rats) Animal model (rats) Animal model (rats) Patients with epilepsy Animal model (rats) Animal model (rats) |
Iron Iron Iron - PTZ KA/Amig. kindl./BIC |
[135, 187, 188] [135, 187, 188] [189, 190] [191] [92-194] [115, 191] [191, 189] |
AOMS - artery occlusion model of stroke, CE – chronic epilepsy, DHA - docosahexaenoic acid, EPA - eicosapentaenoic acid, GI – global ischemia, GSH – glutathione, Int. – Intractable, KA – kainic acid, KD – ketogenic diet, LPO- lipid peroxidation, NMDA - N-methyl-D-aspartate, PIL – pilocarpin, PTZ – pentylenetetrazol, PUFA - polyunsaturated fatty acid, Ref. – refractory, SOD - superoxide dismutase, * Ginkgo biloba extracts can contain neurotoxin (4’-O-methoxypridoxine) which can exert pro-epileptic effects.
7. STUDIES OF ANTIOXIDANTS USAGE IN VARIOUS ANIMAL MODELS OF EPILEPSY
Numerous studies in animal models of seizures and epilepsy have been conducted in order to evolve the influence of an add-on antioxidant treatment on enzymatic antioxidant activity (SOD, CAT, GPx and GR), non-enzymatic endogenous antioxidant status (GSH), ROS markers (hydroperoxide), various markers of macromolecular oxidative stress damage (MDA, TBARS, 8-OHdG, mtDNA damage and P-carb), and nitrate/nitrite levels. Practically all antioxidants, including endogenously present α-lipoic acid 70, 73, 143, 204, coenzyme Q10 [87] and melatonin 135, 142, 145, 147, 148, exogenous anticonvulsive and neuro- protective substances, such as ascorbic acid [66, 89, 135, 153, 164, 165], curcumin [96, 99, 100, 205, 206], ginsenoside-Rd [207-209], propolis [175], α-tocopherol [122, 125, 135, 193, 201, 210], and naringin [211], and novel synthetic, potent radical scavengers, like aspalatone [115], EPC-K1 [135], and tempol [121], have shown neuroprotective effects against oxidative stress induced by different proconvulsive substances that are usually used in models of seizures and epilepsy, as summarized in the Table 4.
Table 4.
Effects of potential antioxidants on oxidative stress markers in different animal models of seizures and epilepsy.
| Antioxidant | Animal | Seizure Model | Observed Marker | Results | Investigated Material | References |
|---|---|---|---|---|---|---|
| α -Lipoic acid | Rats | Aging | SOD, CAT, GPx and GR | ↑ | Cortex, cerebellum, hippocampus, striatum and hypothalamus | [204] |
| MDA | ↓ | |||||
| Rats | PIL | SOD and CAT | ↑ | Striatum | [70] | |
| GPx | ↑ | Striatum/Hippocampus | [70, 143] | |||
| MDA and NO | ↓ | Striatum | [70] | |||
| GSH | ↑ | Hippocampus | [143] | |||
| Na+ and K+ ATP-ase | ↑ | Hippocampus | [143] | |||
| Rats | PIL | SOD, CAT and GPx | ↑ | Striatum/Hippocampus | [70, 673] | |
| MDA and NO | ↓ | Striatum/Hippocampus | [70, 673] | |||
| α -Tocopherol | Rats | PIL | SOD | ↑ | Hippocampus/Striatum | [153, 210] |
| CAT | ↑ | Hippocampus/Striatum | [125, 210] | |||
| LPO and nitrite | ↓ | Hippocampus/Striatum | [125, 210] | |||
| Rats | PTZ-kindled | TBARS and P-Carb | ↓ | Striatum | [201] | |
| LPO | ↓ | Whole brain | [193] | |||
| Rats | PIL | CAT | ↑ | Hippocampus | [122] | |
| Rats | Iron injection | H2O2 | ↓ | Whole Brain | [135] | |
| Ascorbic acid | Rats | PIL | SOD | ↑ | Hippocampus | [66] |
| CAT | ↑ | Hippocampus | [66, 89, 153] | |||
| MDA | ↓ | Hippocampus | [66, 89, 153] | |||
| NO | ↓ | Hippocampus | [66] | |||
| Rats | PTZ-kindled | P-carb | ↓ | Striatum | [164] | |
| Na+ and K+ ATP-ase | ↑ | Striatum | [164] | |||
| Rats | TMT | MDA | ↓ | Hippocampus | [165] | |
| P-carb and GSSG | ↓ | Hippocampus | [165] | |||
| GSH | ↑ | Hippocampus | [165] | |||
| Aspalatone | Rats | KA | P-carb | ↓ | Whole brain | [115] |
| MDA | ↓ | Whole brain | [115] | |||
| Curcumin | Rats | PTZ-kindled | GSH | ↑ | Whole brain | [96] |
| MDA | ↓ | Whole brain | [96] | |||
| Mice | PTZ-kindled | GSH | ↑ | Whole brain | [206] | |
| MDA | ↓ | Whole brain | [206 | |||
| Rats | PTZ-kindled | CAT | ↓ | Cerebrum, Cerebellum | [100] | |
| GSH and MDA | ↓ | Cerebrum, Cerebellum | [100] | |||
| Rats | PIL | NOS and LDH | ↓ | Hippocampus | [205] | |
| SOD and GSH | ↑ | Hippocampus | [205] | |||
| MDA | - | Hippocampus | [205] | |||
| Rats | PIL | CAT, MDA and NO | ↓ | Hippocampus | [212] | |
| GSH and Na+ and K+ ATP-ase | ↑ | Hippocampus | [212] | |||
| Rats | Phenytoin | GSH | ↑ | Whole brain | [99] | |
| MDA | ↓ | Whole brain | [99] | |||
| EPC-K1 | Rats | Iron injection | MDA | ↓ | Whole brain | [135] |
| P-carb | ↓ | Whole brain | [135] | |||
| Ginsenoside-Rd | Mice-SAM | Aging | SOD and CAT | - | Liver, serum | [207] |
| GPx and GR | ↑ | Liver, serum | [207] | |||
| MDA | ↓ | Liver, serum | [207] | |||
| GSH and GSH/GSSG | ↑ | Liver, serum | [207] | |||
| GSSG | ↓ | Liver, serum | [207] | |||
| Rats | Focal cerebral ischemia injury | SOD, CAT and GR | ↑ | Cerebral artery | [208] | |
| GSH/GSSG | ↑ | Cerebral artery | [208] | |||
| 8-OHdG, P-Carb, AGE, MDA | ↓ | Cerebral artery | [208] | |||
| Mice | Aging | SOD and GPx | ↑ | Serum | [209] | |
| MDA | ↓ | Serum | [209] | |||
| Melatonin | Mice | KA induced | mtDNA damage | ↓ | Whole brain | [145] |
| MDA | ↓ | Whole brain | [145] | |||
| Rats | KA induced | MDA | ↓ | Brain synaptosomes | [142] | |
| ROS generation | ↓ | Brain synaptosomes | [142] | |||
| Rats | KA induced | GPx and GR | ↑ | Forebrain | [148] | |
| GSH | ↑ | Hippocampus, amygdala | [148] | |||
| Rats | KA induced | GSH, GSH/GSSG | ↑ | Striatum and cortex | [149] | |
| Rats | Iron injection Iron injection |
GPx | ↑ | Whole brain | [135] | |
| NO formation | ↓ | Whole brain | [135] | |||
| Rats | Iron induced | TBARS | ↓ | Cortex | [147] | |
| Naringin | Rats | KA induced | GSH | ↑ | Whole brain | [211] |
| MDA | ↓ | Whole brain | [211] | |||
| Propolis | Rats | KA induced | MDA and P-carb | ↓ | Hippocampus | [175] |
| GSH/GSSG | ↑ | Hippocampus | [175] | |||
| Tempol | Rats | KA induced | SOD and DNA fragm. | ↓ | Hippocampus | [121] |
| Ubiquinone | Rats | PIL | SOD, CAT and GPx | ↑ | Hippocampus | [87] |
| H2O2 | ↓ | Hippocampus | [87] |
↓ - decreased, ↑ - increased, - no significant changes observed, 8-OHdG - 8-hydroxydeoxyguanosine, AGE - advanced glycation end-product, CAT – catalase, DNA – deoxyribonucleic acid, GPx - glutathione peroxidase, GR – glutathione reductase, GSH – glutathione, GSSG - glutathione disulfide, H2O2 – hydrogen peroxide, KA – kainic acid, LDH - lactate dehydrogenase, mtDNA – mitochondrial DNA, MDA – malondialdehyde, NO – nitric oxide, NOS - nitric oxide synthase, PIL – pilocarpin, PTZ – pentylenetetrazol, ROS – reactive oxygen species, SAM - senescence-accelerated mouse, SOD – superoxide dismutase, TBARS - thiobarbituric acid reactive substances, TMT –trimethylin
The majority of studies have confirmed neuroprotective effects of antioxidants, showing increased levels of endogenous antioxidant enzyme activities, increased endogenous non-enzymatic antioxidant levels, namely GSH and decreased markers of macromolecular oxidative stress damage in comparison to untreated animal models of seizures and epilepsy. However, there were few exceptions. Curcumin in seizure induced rats showed a decrease in CAT [100, 212] and GSH [100] levels and exerted no effect on MDA levels [205]. Ginsenoside-Rd in aging senescence-accelerated mouse (SAM) showed no effects on SOD and CAT activities [207]. Furthermore, tempol in KA-induced rats exerted decreased SOD activities [121]. A summary of findings from these studies is given in Table 4.
Oxidative stress occurring in the brain throughout substance-provoked seizures has been shown to play an important role in pathogenic consequences of seizures. As seen in Table 4 investigated potent antioxidants in animal models exert strong antioxidant effects. These findings therefore greatly support the idea of important neuro- protective and hence possible anticonvulsive role of using appropriate potent antioxidants as an add-on therapy in epilepsy.
8. STUDIES OF ANTIOXIDANTS USAGE IN PATIENTS WITH EPILEPSY
Based on the abundance of observed beneficial effects of antioxidants on markers of oxidative stress in vitro andin vivo in animal models of epileptic seizure, various antioxidants, namely vitamin E, melatonin and NAC,have also been used in patients with epilepsy as an add-on therapy [81, 83, 102, 104, 213-216]. On the other hand, unfortunately there is a lack of quality data obtained from straight clinical studies of antioxidants use in patients with epilepsy, and furthermore even the existing results are confusing.
For instance, Ogunmekan et al. reported that α-tocopherol as an add-on significantly reduced seizures in children [214], while Raju etal . observed no significant difference between α-tocopherol and placebo in adults [213]. Ogunmekan et al. published a randomized, double-blind, placebo-controlled clinical study in 24 children with medically refractory epilepsy. He investigated the effect of α-tocopherol supplementation on seizure control. It was noticed that the addition of D-α-tocopheryl acetate in a dosage of 400 mg/day produced a significant decrease in seizure occurrence in ten of twelve patients with epilepsy. Controls showed no changes in seizure incidence. Furthermore, since there were no changes in the plasma anticonvulsants concentrations, clinical benefits were attributed to increased serum E vitamin levels [214]. On the other hand, another randomized, double-blind, placebo-controlled clinical trial, evaluating the effect of the addition of D-α-tocopherol to the conventional AEDs treatment, carried out in 43 adults with refractory epilepsy, did not support the possible therapeutic effect of vitamin E [213].
Besides these two newer studies, there are also two older studies published which have evaluated the efficacy of α-tocopherol treatment in epilepsy. Kovalenko et al. have observed beneficial effects already one month after the α-tocopherol administration (600 mg once daily) in patients with confirmed pharmacoresistant epilepsy. Decreased lipid peroxidation, positive EEG alteration and reduced frequency of epileptic seizures were reported in the majority of patients [217]. Similarly, Tupeev et al. investigated the effect of600 mg of α-tocopherol applied daily as an add-on to conventional AEDs therapy. Only after one month of additional therapy, they reported greatly improved patients' general state, achieved namely through decreased epileptic seizure frequency, which was further supported by an observed increase in SOD activity and consequently significant improvement in EEG results. They concluded that using of α-tocopherol in the multiple-therapy approach to epilepsy treatment improved neuroprotective and antiepileptic effects [218].
Currently, only a few studies of using melatonin as add-on therapy in patients with epilepsy have shown potential beneficial effects on decreased epileptic seizures incidence. Assessing the effects of melatonin on the blood levels of antioxidant enzyme GPx and GR in children with epilepsy receiving CBZ monotherapy compared to the placebogroup, showed significantly and non-significantly increased
GR and GPx activities, respectively [83]. Similar findings were found in a study in children with epilepsy on VPA monotherapy [81]. Both of these studies demonstrate that melatonin exerts neuroprotective effects due to its antioxidant and antiexcitotoxic properties within the central nervous system [81, 83].
In a small clinical study of six children with intractable seizures, the addition of melatonin to the conventional AED treatment, demonstrated an improvement in seizure control in five cases. Moreover, after discontinuation of melatonin, all six patients have claimed their seizure activity returned to pre-treatment levels [84].
Another clinical study, performed in children with epilepsy investigated the effect of melatonin administration to the conventional AEDs on patient’s life quality. Since there were noticed an improvement in physical, cognitive and social functions, emotional well-being, and behaviour, it is suggested that melatonin may have beneficial neuro- protective effects when used as an add-on therapy in patients with epilepsy [82].
There is also a published study of using melatonin in children with sleep disturbances and therapy-resistant epilepsy. Six of ten participated children with epilepsy showed a significant decrease in epileptic seizure frequency, which could be a consequence of anticonvulsant effects as well as reduced sleep deprivation, caused by melatonin [85].
Moreover, in a patient with Unverricht-Lundborg disease, the influence of NAC treatment on serum GSH concentrations was studied. GSH concentrations increased during treatment, which corresponded to improved seizure control with exception in myoclonus and ataxia. There were also patients who showed a variable response [216]. Ben-Menachem et al. reported significantly reduced myoclonus and further decreased incidence of generalized seizures in numerous case reports of using NAC [215].
It is important to keep in mind that in patients with non-refractory epilepsy, even better responses to antioxidant as add-on therapy, could be expected. After all, for more reliable conclusions of potential beneficial effects of antioxidants, more carefully planned, randomized, double-blind, cross-over, placebo-controlled clinical trials including appropriate number of patients and a longer duration of oxidative stress biomarkers monitoring, are needed. Furthermore, to the best of our knowledge, till now no clinical studies have been performed, which would investigate the potential benefits of many other known antioxidants, such as ascorbate, flavonoids, melatonin, lipoic acid, exogenous novel potent mimetics of catalase or SOD, and coenzyme Q10, in the case of epilepsy in humans. Moreover, at this time even the assessment of beneficial effects of antioxidants as add-on treatment in the field of common genetic and acquired epilepsies can be considered as incomplete. Therefore, on the basis of the published studies and reports, it is very difficult to provide any relevant final conclusions of the potential benefits of using antioxidants as add-on neuroprotective therapy. To prevent potentially misleading conclusions, novel carefully designed and more comprehensive studies are needed.
9. POTENTIAL LIMITATIONS OF USING ANTI-OXIDANTS AS ADD-ON THERAPY IN EPILEPSY
The use of dietary supplements is very popular among the people around the world and patients with epilepsy are no exception. Despite the lack of evidence of their beneficial effects in epilepsy, studies confirmed that dietary supplements are consumed by 10 to 56% of patients with epilepsy [219-223]. Among reported dietary supplements many antioxidants can be found [219, 221]. In generally patients consider dietary supplements as safe medications with no adverse side effects. Although this is confirmed in some reports from clinical trials or case reports investigating dietary supplements use in patients with epilepsy, some studies also report that dietary supplements can affect central nervous system and potentially increase the risk of seizures [172, 219, 224, 225]. Moreover, the concomitant treatment of AEDs and dietary supplement can change effects of AEDs, since AEDs are highly prone to drug-drug and drug-dietary supplement interactions. The potential interactions between antioxidants and AEDs can be explained by pharmacokinetic (changes in absorption, distribution, metabolism or elimination) or pharmacodynamic (changesin drug effects or efficacy) mechanisms. The two most probable pharmacokinetic mechanisms by which antioxidants can interact with AEDs are via cytochrome P450 enzymes and transporter proteins. Ginkgo biloba induces CYP2C19 and can reduce the levels of phenytoin and valproate which are both substrates for this enzyme [172, 226]. Data about the effect of vitamin E on CYP are less evident. According to some reports Vitamin E may induce CYP3A4 [227, 228] and thus can reduce levels of carbamazepine, ethosuximide, felbamate, phenytoin phenobarbital, tiagabine, and zonisamide, which are all substrate for this enzyme [229]. Moreover, it was shown that resveratrol can inhibit CYP3A4 and increase levels of carbamazepine in rats [230]. We can also speculate that this interaction would probably occur with above mentioned AEDs which are substrates for CYP3A4. Resveratrol is also inhibitor of multidrug resistance-associated protein 2. This mechanism can also contribute to increased levels of CBZ in combination with resveratrol [230]. Additionally, AEDs are also substrates of P-glycoproteins and concomitant treatment with curcumin, catechins, and ginkgo biloba can probably affect their levels [172]. Therefore, concurrent use of antioxidants with AEDs, can lead to an increase or decrease in AED plasma concentrations. This can potentially results in an increase or decrease of drug efficacy, an increase of drug toxicity or in an increase in its adverse side effects. However, the potential clinical implication of interactions between concurrent use of antioxidants and AEDs is difficult to predict and assess. For this reason it is important to stress out that antioxidants should not be used concurrently with other drugs without proper medical supervision.
10. CONCLUSION
Oxidative stress has been revealed as one of the most important processes leading to neuronal cell death. Therefore, it is rational to anticipate that oxidative stress has a considerable role in epileptogenesis. There is an accumulation of free radicals and oxidative changes expressed during the acute phase after the initial insult or SE induced by various triggers and the latent phase in experimental models of epilepsy. This finding suggests that seizures, SE and cell death induced by different triggers, might possess a large participation in brain oxidative stress, which is closely related to the mechanism of propagation and/or maintenance of the epileptic focus. Furthermore, there is an accumulation of evidence that in patients with epilepsy the balance of antioxidant system is disturbed, and the production of free reactive radicals is increased.
Neuronal cells death resulting in neuronal loss appears to represent one of the most important neurobiological alterations in the epileptogenic and epileptic brain. Therefore, the use of antioxidants as add-on therapy should lead to less sever structural damages, reduced epileptogenesis and milder cognitive deterioration. Most of the conventional AEDs do not prevent neuronal damage resulting from prolonged or multiple seizures. Therefore, there is a strong need to develop newer antiepileptic treatments, including novel AEDs with broad spectrum of actions or conventional AEDs in combination with potent antioxidants, which would pose simultaneously neuroprotective and antiepileptogenic effects. The former effects seem to be very important, especially in the latent period, where targeted therapies could potentially prevent the progression of epileptic seizures, namely through epileptogenesis inhibition.
Only recently, the perception of epilepsy has extended from a condition almost entirely related to neurons to a condition further associated with the dysfunctions of glial cells. Astrocytes, representing the largest subgroup of glial cells, are known to play a crucial role in regulating and maintaining the extracellular chemical milieu of the central nervous system. Additionally, they interact with neurons, modulate neurons synaptic transmission, and participate in inflammation processes. It is therefore reasonable to propose a potentially important role of glial cells in epileptogenesis, as was indicated in few studies [231]. Moreover, we speculate that oxidative stress can also modulate the function of glial cells which can contributes to progression of epileptogenesis. However, new studies are needed to confirm this hypothesis.
In order to evaluate potentially positive effects of therapeutic interventions with antioxidant components, numerous studies of their use in animal models of epileptic seizures, were revised. However, even though numerous positive effects of antioxidants were observed in animal models of epileptic seizure, till now only few antioxidants have been further evaluated in patients with epilepsy as an add-on therapy and even these with only partial success. Based on the several positive findings in animal models, a strong need for more carefully planned, randomized, double-blind, cross-over, placebo-controlled clinical trials for the evaluation of antioxidants efficacy in patients with epilepsy is warranted.
Currently, upon enhanced investigation of possible causes and consequently improved understanding of underlying mechanisms of epileptogenesis, many new possibilities for epilepsy therapy have revealed. Future is currently reflected in designing newer AEDs, which will include anticonvulsant and neuroprotective activity. Therefore, it is of great importance to closely monitor various potent antioxidant systems and to study the possibilities of their inclusion to conventional AEDs treatment, which according to some studies can lead to improved life quality in patients with epilepsy.
ACKNOWLEDGEMENTS
This work was financially supported by the Slovenian Research Agency (ARRS Grant P1-0189).
ABBREVIATIONS
- 8-OHdG =
8-hydroxy-2-deoxyguanosine
- ADP =
Adenosine diphosphate
- AEDs =
Antiepileptic drugs
- AGE =
Advanced glycation end-product
- AOMS =
Artery occlusion model of stroke
- AP-1 =
Activator protein
- BBB =
Blood brain barrier
- CAT =
Catalase
- CBZ =
Carbamazepine
- CE =
Chronic epilepsy
- CI =
Confidence interval
- CNS =
Central nervous system
- DHA =
Docosahexaenoic acid
- DNA =
Deoxyribonucleic acid
- EEG =
Electroencephalogram
- EGCG =
Epigallocatechin
- EPA =
Eicosapentaenoic acid
- EPC-K1 =
α-Tocopheryl-L-ascorbate-2-O-phosphate diester
- ESR =
Electron spin resonance
- GABA =
γ-aminobutyricacid
- GI =
Global ishemia
- GPx =
Glutathione peroxidase
- GR =
Glutathione reductase
- GSH =
Glutathione
- GSSG =
Glutathione disuphide
- H2O2 =
Hydrogen peroxide
- Hb =
Haemoglobin
- Hb ASSG =
Glutathione adduct of haemoglobin
- KA =
Kainic acid
- KD =
Ketogenic diet
- LA =
Lipoic acid
- LDH =
Lactate dehydrogenase
- LPO =
Lipid peroxidation
- MDA =
Malondyaldehyde
- MnTBAP =
Mn(III)tetrakis (4-benzoic acid) porphyrin
- mtDNA =
Mitochondrial DNA
- NAC =
N-acetlcysteine
- NF =
Nuclear factor
- NMDA =
N-methyl-D-aspartate
- NO =
Nitric oxide
- NOS =
Nitric oxide synthase
- PB =
Phenobarbitone
- P-Carb =
Protein carbonyl
- PHT =
Phenitoin
- PIL =
Pilocarpin
- PTZ =
Pentylenetetrazol
- PUFA =
Polyunsaturated fatty acid
- RNA =
Ribonucleic acid
- RNS =
Reactive nitrogen species
- ROS =
Reactive oxygen species
- RR =
Relative risk
- SAM =
Senescence-accelerated mouse
- SE =
Status epilepticus
- SOD =
Superoxide dismutase
- TBARS =
Thiobarbituric acid reactive substances
- TMT =
Trimethylin
- VPA =
Valproic acid
- WMD =
Weighted mean difference
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Yüksel A., Cengiz M., Seven M., Ulutin T., et al. Erythrocyte glutathione, glutathione peroxidase, superoxide dismutase and serum lipid peroxidation in epileptic children with valproate and carbamazepine monotherapy. J. Basic Clin. Physiol. Pharmacol. 2000;11(1):73–81. doi: 10.1515/jbcpp.2000.11.1.73. [DOI] [PubMed] [Google Scholar]
- 2.Martinc B., Grabnar I., Vovk T. The role of reactive species in epileptogenesis and influence of antiepileptic drug therapy on oxidative stress. Curr. Neuropharmacol. 2012;10(4):328–343. doi: 10.2174/157015912804499447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brigo F., Del Felice A. Melatonin as add-on treatment for epilepsy. Cochrane Database Syst. Rev. 2012;6:CD006967. doi: 10.1002/14651858.CD006967.pub2. [DOI] [PubMed] [Google Scholar]
- 4.Deckers C.L., Hekster Y.A., Keyser A., van Lier H.J., Meinardi H., Renier W.O. Monotherapy versus polytherapy for epilepsy: a multicenter double-blind randomized study. 2001. [DOI] [PubMed]
- 5.Verrotti A., Basciani F., Trotta D., Pomilio M.P., Morgese G., Chiarelli F. Serum copper, zinc, selenium, glutathione peroxidase and superoxide dismutase levels in epileptic children before and after 1 year of sodium valproate and carbamazepine therapy. Epilepsy Res. 2002;48(1-2):71–75. doi: 10.1016/S0920-1211(01)00322-9. [DOI] [PubMed] [Google Scholar]
- 6.Liu C.S., Wu H.M., Kao S.H., Wei Y.H. Serum trace elements, glutathione, copper/zinc superoxide dismutase, and lipid peroxidation in epileptic patients with phenytoin or carbamazepine monotherapy. Clin. Neuropharmacol. 1998;21(1):62–64. [PubMed] [Google Scholar]
- 7.Aycicek A., Iscan A. The effects of carbamazepine, valproic acid and phenobarbital on the oxidative and antioxidative balance in epileptic children. Eur. Neurol. 2007;57(2):65–69. doi: 10.1159/000098053. [DOI] [PubMed] [Google Scholar]
- 8.Higuchi S., Yano A., Takai S., Tsuneyama K., Fukami T., Nakajima M., Yokoi T. Metabolic activation and inflammation reactions involved in carbamazepine-induced liver injury. Toxicol. Sci. 2012;130(1):4–16. doi: 10.1093/toxsci/kfs222. [DOI] [PubMed] [Google Scholar]
- 9.Lu W., Uetrecht J.P. Peroxidase-mediated bioactivation of hydroxylated metabolites of carbamazepine and phenytoin. Drug Metab. Dispos. 2008;36(8):1624–1636. doi: 10.1124/dmd.107.019554. [DOI] [PubMed] [Google Scholar]
- 10.Halliwell B., Gutteridge J. Free Radicals in Biology and Medicine 4th Ed., Chapter 4: Cellular responses to oxidative stress: adaptation, damage, repair, senscence and death. 2007. [Google Scholar]
- 11.Coyle J.T., Puttfarcken P. Oxidative stress, glutamate, and neurodegenerative disorders. Science. 1993;262(5134):689–695. doi: 10.1126/science.7901908. [DOI] [PubMed] [Google Scholar]
- 12.Waldbaum S., Patel M. Mitochondrial dysfunction and oxidative stress: a contributing link to acquired epilepsy? J. Bioenerg. Biomembr. 2010;42(6):449–455. doi: 10.1007/s10863-010-9320-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blair R.E., Sombati S., Lawrence D.C., McCay B.D., DeLorenzo R.J. Epileptogenesis causes acute and chronic increases in GABAA receptor endocytosis that contributes to the induction and maintenance of seizures in the hippocampal culture model of acquired epilepsy. J. Pharmacol. Exp. Ther. 2004;310(3):871–880. doi: 10.1124/jpet.104.068478. [DOI] [PubMed] [Google Scholar]
- 14.Morris T.A., Jafari N., Rice A.C., Vasconcelos O., DeLorenzo R.J. Persistent increased DNA-binding and expression of serum response factor occur with epilepsy-associated long-term plasticity changes. J. Neurosci. 1999;19(19):8234–8243. doi: 10.1523/JNEUROSCI.19-19-08234.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pitkänen A., Lukasiuk K. Mechanisms of epileptogenesis and potential treatment targets. Lancet Neurol. 2011;10(2):173–186. doi: 10.1016/S1474-4422(10)70310-0. [DOI] [PubMed] [Google Scholar]
- 16.Pitkänen A., Lukasiuk K. Molecular and cellular basis of epileptogenesis in symptomatic epilepsy. Epilepsy Behav. 2009;14(Suppl. 1):16–25. doi: 10.1016/j.yebeh.2008.09.023. [DOI] [PubMed] [Google Scholar]
- 17.Patel M. Mitochondrial dysfunction and oxidative stress: cause and consequence of epileptic seizures. 2004. [DOI] [PubMed]
- 18.Guerrini R., Casari G., Marini C. The genetic and molecular basis of epilepsy. Trends Mol. Med. 2003;9(7):300–306. doi: 10.1016/S1471-4914(03)00116-3. [DOI] [PubMed] [Google Scholar]
- 19.Valko M., Leibfritz D., Moncol J., Cronin M.T., Mazur M., Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007;39(1):44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 20.Barnham K.J., Masters C.L., Bush A.I. Neurodegenerative diseases and oxidative stress. Nat. Rev. Drug Discov. 2004;3(3):205–214. doi: 10.1038/nrd1330. [DOI] [PubMed] [Google Scholar]
- 21.Chuang Y.C. Mitochondrial dysfunction and oxidative stress in seizure-induced neuronal cell death. Acta Neurol. Taiwan. 2010;19(1):3–15. [PubMed] [Google Scholar]
- 22.Turrens J.F., Freeman B.A., Levitt J.G., Crapo J.D. The effect of hyperoxia on superoxide production by lung submitochondrial particles. Arch. Biochem. Biophys. 1982;217(2):401–410. doi: 10.1016/0003-9861(82)90518-5. [DOI] [PubMed] [Google Scholar]
- 23.Poon H.F., Calabrese V., Scapagnini G., Butterfield D.A. Free radicals and brain aging. Clin. Geriatr. Med. 2004;20(2):329–359. doi: 10.1016/j.cger.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 24.Devasagayam T.P., Boloor K.K., Ramasarma T. Methods for estimating lipid peroxidation: an analysis of merits and demerits. Indian J. Biochem. Biophys. 2003;40(5):300–308. [PubMed] [Google Scholar]
- 25.Choi B.H. Oxygen, antioxidants and brain dysfunction. Yonsei Med. J. 1993;34(1):1–10. doi: 10.3349/ymj.1993.34.1.1. [DOI] [PubMed] [Google Scholar]
- 26.Facchinetti F., Dawson V.L., Dawson T.M. Free radicals as mediators of neuronal injury. Cell. Mol. Neurobiol. 1998;18(6):667–682. doi: 10.1023/A:1020221919154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Valavanidis A., Vlachogianni T., Fiotakis C. 8-hydroxy-2' -deoxyguanosine (8-OHdG): A critical biomarker of oxidative stress and carcinogenesis. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2009;27(2):120–139. doi: 10.1080/10590500902885684. [DOI] [PubMed] [Google Scholar]
- 28.Nunomura A., Moreira P.I., Takeda A., Smith M.A., Perry G. Oxidative RNA damage and neurodegeneration. Curr. Med. Chem. 2007;14(28):2968–2975. doi: 10.2174/092986707782794078. [DOI] [PubMed] [Google Scholar]
- 29.Poon H.F., Calabrese V., Scapagnini G., Butterfield D.A. Free radicals: key to brain aging and heme oxygenase as a cellular response to oxidative stress. J. Gerontol. A Biol. Sci. Med. Sci. 2004;59(5):478–493. doi: 10.1093/gerona/59.5.M478. [DOI] [PubMed] [Google Scholar]
- 30.Sayre L.M., Moreira P.I., Smith M.A., Perry G. Metal ions and oxidative protein modification in neurological disease. Ann. Ist. Super. Sanita. 2005;41(2):143–164. [PubMed] [Google Scholar]
- 31.Sobaniec W., Solowiej E., Kulak W., Bockowski L., Smigielska-Kuzia J., Artemowicz B. Evaluation of the influence of antiepileptic therapy on antioxidant enzyme activity and lipid peroxidation in erythrocytes of children with epilepsy. J. Child Neurol. 2006;21(7):558–562. doi: 10.1177/08830738060210070301. [DOI] [PubMed] [Google Scholar]
- 32.Yiş U., Seçkin E., Kurul S.H., Kuralay F., Dirik E. Effects of epilepsy and valproic acid on oxidant status in children with idiopathic epilepsy. Epilepsy Res. 2009;84(2-3):232–237. doi: 10.1016/j.eplepsyres.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 33.Yüksel A., Cengiz M., Seven M., Ulutin T. Changes in the antioxidant system in epileptic children receiving antiepileptic drugs: two-year prospective studies. J. Child Neurol. 2001;16(8):603–606. doi: 10.1177/088307380101600814. [DOI] [PubMed] [Google Scholar]
- 34.Bolayir E., Celik K., Tas A., Topaktas S., Bakir S. The effects of oxcarbazepine on oxidative stress in epileptic patients. Methods Find. Exp. Clin. Pharmacol. 2004;26(5):345–348. doi: 10.1358/mf.2004.26.5.831325. [DOI] [PubMed] [Google Scholar]
- 35.Turkdogan D., Toplan S., Karakoc Y. Lipid peroxidation and antioxidative enzyme activities in childhood epilepsy. J. Child Neurol. 2002;17(9):673–676. doi: 10.1177/088307380201700904. [DOI] [PubMed] [Google Scholar]
- 36.Ashrafi M.R., Shams S., Nouri M., Mohseni M., Shabanian R., Yekaninejad M.S., Chegini N., Khodadad A., Safaralizadeh R. A probable causative factor for an old problem: selenium and glutathione peroxidase appear to play important roles in epilepsy pathogenesis. Epilepsia. 2007;48(9):1750–1755. doi: 10.1111/j.1528-1167.2007.01143.x. [DOI] [PubMed] [Google Scholar]
- 37.Sudha K., Rao A.V., Rao A. Oxidative stress and antioxidants in epilepsy. Clin. Chim. Acta. 2001;303(1-2):19–24. doi: 10.1016/S0009-8981(00)00337-5. [DOI] [PubMed] [Google Scholar]
- 38.Ono H., Sakamoto A., Sakura N. Plasma total glutathione concentrations in epileptic patients taking anticonvulsants. Clin. Chim. Acta. 2000;298(1-2):135–143. doi: 10.1016/S0009-8981(00)00286-2. [DOI] [PubMed] [Google Scholar]
- 39.Michoulas A., Tong V., Teng X.W., Chang T.K., Abbott F.S., Farrell K. Oxidative stress in children receiving valproic acid. J. Pediatr. 2006;149(5):692–696. doi: 10.1016/j.jpeds.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 40.Menon B., Ramalingam K., Kumar R.V. Oxidative stress in patients with epilepsy is independent of antiepileptic drugs. 2012. [DOI] [PubMed]
- 41.Sołowiej E., Sobaniec W. [The effect of antiepileptic drug therapy on antioxidant enzyme activity and serum lipid peroxidation in young patients with epilepsy]. Neurol. Neurochir. Pol. 2003;37(5):991–1003. [PubMed] [Google Scholar]
- 42.Pandey M.K., Mittra P., Maheshwari P.K. The Lipid Peroxidation Product as a Marker of Oxidative Stress in Epilepsy. 2012.
- 43.Zhang Y.J., Zhang M., Wang X.C., Yu Y.H., Jin P.J., Wang Y. [Effects of sodium valproate on neutrophils’ oxidative metabolism and oxidant status in children with idiopathic epilepsy]. Zhonghua Er Ke Za Zhi. 2011;49(10):776–781. [Effects of sodium valproate on neutrophils' oxidative metabolism and oxidant status in children with idiopathic epilepsy]. [PubMed] [Google Scholar]
- 44.Hamed S.A., Abdellah M.M., El-Melegy N. Blood levels of trace elements, electrolytes, and oxidative stress/antioxidant systems in epileptic patients. J. Pharmacol. Sci. 2004;96(4):465–473. doi: 10.1254/jphs.FPJ04032X. [DOI] [PubMed] [Google Scholar]
- 45.Arhan E., Serdaroglu A., Ozturk B., Ozturk H.S., Ozcelik A., Kurt N., Kutsal E., Sevinc N. Effects of epilepsy and antiepileptic drugs on nitric oxide, lipid peroxidation and xanthine oxidase system in children with idiopathic epilepsy. Seizure. 2011;20(2):138–142. doi: 10.1016/j.seizure.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 46.Nemade S.T., Melinkeri R.R. Oxidative and Antioxidative Status in Epilepsy. Pravara Med. Rev. 2010;2(4):8–10. [Google Scholar]
- 47.Mehmet UÇ, Sefer V, Yavuz Y, Eºref A, Tahsin Ç, Adalet A, Hatice Y, Mehmet UA. Serum paraoxonase-1 activities and malondialdehyde levels in patients with epilepsy. Dicel. Med. J. . 2012;39(4):557–560. [Google Scholar]
- 48.Hamed S.A., Abdellah M.M. Trace elements and electrolytes homeostasis and their relation to antioxidant enzyme activity in brain hyperexcitability of epileptic patients. J. Pharmacol. Sci. 2004;96(4):349–359. doi: 10.1254/jphs.CRJ04004X. [DOI] [PubMed] [Google Scholar]
- 49.Schulpis K.H., Lazaropoulou C., Regoutas S., Karikas G.A., Margeli A., Tsakiris S., Papassotiriou I. Valproic acid monotherapy induces DNA oxidative damage. Toxicology. 2006;217(2-3):228–232. doi: 10.1016/j.tox.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 50.Niketić V., Ristić S., Saicić Z.S., Spasić M., Buzadzić B., Stojković M. Activities of antioxidant enzymes and formation of the glutathione adduct of hemoglobin (Hb ASSG) in epileptic patients with long-term antiepileptic therapy. Farmaco. 1995;50(11):811–813. doi: 10.1016/0020-711x(92)90046-4. [DOI] [PubMed] [Google Scholar]
- 51.Peker E., Oktar S., Ari M., Kozan R., Doğan M., Cağan E., Söğüt S. Nitric oxide, lipid peroxidation, and antioxidant enzyme levels in epileptic children using valproic acid. Brain Res. 2009;1297:194–197. doi: 10.1016/j.brainres.2009.08.048. [DOI] [PubMed] [Google Scholar]
- 52.Graf W.D., Oleinik O.E., Glauser T.A., Maertens P., Eder D.N., Pippenger C.E. Altered antioxidant enzyme activities in children with a serious adverse experience related to valproic acid therapy. Neuropediatrics. 1998;29(4):195–201. doi: 10.1055/s-2007-973560. [DOI] [PubMed] [Google Scholar]
- 53.Varoglu A.O., Yildirim A., Aygul R., Gundogdu O.L., Sahin Y.N. Effects of valproate, carbamazepine, and levetiracetam on the antioxidant and oxidant systems in epileptic patients and their clinical importance. Clin. Neuropharmacol. 2010;33(3):155–157. doi: 10.1097/WNF.0b013e3181d1e133. [DOI] [PubMed] [Google Scholar]
- 54.Jurima-Romet M., Abbott F.S., Tang W., Huang H.S., Whitehouse L.W. Cytotoxicity of unsaturated metabolites of valproic acid and protection by vitamins C and E in glutathione-depleted rat hepatocytes. Toxicology. 1996;112(1):69–85. doi: 10.1016/0300-483X(96)03352-5. [DOI] [PubMed] [Google Scholar]
- 55.Santos N.A., Medina W.S., Martins N.M., Rodrigues M.A., Curti C., Santos A.C. 2008. Involvement of oxidative stress in the hepatotoxicity induced by aromatic antiepileptic drugs. [DOI] [PubMed] [Google Scholar]
- 56.Yürekli V.A., Nazıroğlu M. Selenium and topiramate attenuates blood oxidative toxicity in patients with epilepsy: a clinical pilot study. Biol. Trace Elem. Res. 2013;152(2):180–186. doi: 10.1007/s12011-013-9616-9. [DOI] [PubMed] [Google Scholar]
- 57.Lu W., Uetrecht J.P. Possible bioactivation pathways of lamotrigine. Drug Metab. Dispos. 2007;35(7):1050–1056. doi: 10.1124/dmd.107.015271. [DOI] [PubMed] [Google Scholar]
- 58.Bialer M. New antiepileptic drugs that are second generation to existing antiepileptic drugs. Expert Opin. Investig. Drugs. 2006;15(6):637–647. doi: 10.1517/13543784.15.6.637. [DOI] [PubMed] [Google Scholar]
- 59.Perucca E., French J., Bialer M. Development of new antiepileptic drugs: challenges, incentives, and recent advances. Lancet Neurol. 2007;6(9):793–804. doi: 10.1016/S1474-4422(07)70215-6. [DOI] [PubMed] [Google Scholar]
- 60.Margerison J.H., Corsellis J.A. Epilepsy and the temporal lobes. A clinical, electroencephalographic and neuropathological study of the brain in epilepsy, with particular reference to the temporal lobes. Brain. 1966;89(3):499–530. doi: 10.1093/brain/89.3.499. [DOI] [PubMed] [Google Scholar]
- 61.Bialer M, Johannessen SI, Kupferberg HJ, Levy RH, Perucca E, Tomson T. Progress report on new antiepileptic drugs. a summary of the Seventh Eilat Conference (EILAT VII). Epilepsy Res. 2004;61(1-3):1–48. doi: 10.1016/j.eplepsyres.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 62.Bialer M, Johannessen SI, Kupferberg HJ, Levy RH, Loiseau P, Perucca E. Progress report on new antiepileptic drugs. a summary of the Sixth Eilat Conference (EILAT VI). Epilepsy Res. 2002;51(1-2):31–71. doi: 10.1016/s0920-1211(02)00106-7. [DOI] [PubMed] [Google Scholar]
- 63.Kwan P., Brodie M.J. Early identification of refractory epilepsy. N. Engl. J. Med. 2000;342(5):314–319. doi: 10.1056/NEJM200002033420503. [DOI] [PubMed] [Google Scholar]
- 64.Kelemen A., Rásonyl G., Neuwirth M., Barcs G., Szucs A., Jakus R., Fabó D., Juhos V., Pálfy B., Halász P. Our clinical experience with zonisamide in resistant generalized epilepsy syndromes. Ideggyogy. Sz. 2011;64(5-6):187–192. [PubMed] [Google Scholar]
- 65.Biton V. Clinical pharmacology and mechanism of action of zonisamide. Clin. Neuropharmacol. 2007;30(4):230–240. doi: 10.1097/wnf.0b013e3180413d7d. [DOI] [PubMed] [Google Scholar]
- 66.Santos I.M., Tomé Ada.R., Saldanha G.B., Ferreira P.M., Militão G.C., Freitas R.M. Oxidative stress in the hippocampus during experimental seizures can be ameliorated with the antioxidant ascorbic acid. Oxid. Med. Cell. Longev. 2009;2(4):214–221. doi: 10.4161/oxim.2.4.8876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Devi P.U., Manocha A., Vohora D. Seizures, antiepileptics, antioxidants and oxidative stress: an insight for researchers. Expert Opin. Pharmacother. 2008;9(18):3169–3177. doi: 10.1517/14656560802568230. [DOI] [PubMed] [Google Scholar]
- 68.Costello D.J., Delanty N. Oxidative injury in epilepsy: potential for antioxidant therapy? Expert Rev. Neurother. 2004;4(3):541–553. doi: 10.1586/14737175.4.3.541. [DOI] [PubMed] [Google Scholar]
- 69.Rowles J., Olsen M. Perspectives on the development of antioxidant antiepileptogenic agents. Mini Rev. Med. Chem. 2012;12(10):1015–1027. doi: 10.2174/138955712802762266. [DOI] [PubMed] [Google Scholar]
- 70.Militão G.C., Ferreira P.M., de Freitas R.M. Effects of lipoic acid on oxidative stress in rat striatum after pilocarpine-induced seizures. Neurochem. Int. 2010;56(1):16–20. doi: 10.1016/j.neuint.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 71.Santos P.S., Campêlo L.M., Freitas R.L., Feitosa C.M., Saldanha G.B., Freitas R.M. Lipoic acid effects on glutamate and taurine concentrations in rat hippocampus after pilocarpine-induced seizures. Arq. Neuropsiquiatr. 2011;69(2B):360–364. doi: 10.1590/S0004-282X2011000300018. [DOI] [PubMed] [Google Scholar]
- 72.Meyerhoff J.L., Lee J.K., Rittase B.W., Tsang A.Y., Yourick D.L. Lipoic acid pretreatment attenuates ferric chloride-induced seizures in the rat. Brain Res. 2004;1016(2):139–144. doi: 10.1016/j.brainres.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 73.Freitas R.M. The evaluation of effects of lipoic acid on the lipid peroxidation, nitrite formation and antioxidant enzymes in the hippocampus of rats after pilocarpine-induced seizures. 2009. [DOI] [PubMed]
- 74.Pence S., Erkutlu I., Kurtul N., Bosnak M., Tan U. Total brain tissue sialic acid levels due to glutathione effect in experimental epilepsy. Int. J. Neurosci. 2007;117(11):1523–1535. doi: 10.1080/00207450601126384. [DOI] [PubMed] [Google Scholar]
- 75.Mevissen M., Ebert U. Anticonvulsant effects of melatonin in amygdala-kindled rats. Neurosci. Lett. 1998;257(1):13–16. doi: 10.1016/S0304-3940(98)00790-3. [DOI] [PubMed] [Google Scholar]
- 76.Mohanan P.V., Yamamoto H.A. Preventive effect of melatonin against brain mitochondria DNA damage, lipid peroxidation and seizures induced by kainic acid. 2002. [DOI] [PubMed]
- 77.Yamamoto H., Tang H.W. Preventive effect of melatonin against cyanide-induced seizures and lipid peroxidation in mice. 1996. [DOI] [PubMed]
- 78.Costa-Lotufo L.V., Fonteles M.M., Lima I.S., de Oliveira A.A., Nascimento V.S., de Bruin V.M., Viana G.S. Attenuating effects of melatonin on pilocarpine-induced seizures in rats. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2002;131(4):521–529. doi: 10.1016/s1532-0456(02)00037-6. [DOI] [PubMed] [Google Scholar]
- 79.de Lima E., Soares J.M., Jr, del Carmen Sanabria Garrido Y., Gomes Valente S., Priel M.R., Chada Baracat E., Abrão Cavalheiro E., da Graça Naffah-Mazzacoratti M., Amado D. Effects of pinealectomy and the treatment with melatonin on the temporal lobe epilepsy in rats. Brain Res. 2005;1043(1-2):24–31. doi: 10.1016/j.brainres.2005.02.027. [DOI] [PubMed] [Google Scholar]
- 80.Yildirim M., Marangoz C. Anticonvulsant effects of melatonin on penicillin-induced epileptiform activity in rats. Brain Res. 2006;1099(1):183–188. doi: 10.1016/j.brainres.2006.04.093. [DOI] [PubMed] [Google Scholar]
- 81.Gupta M., Gupta Y.K., Agarwal S., Aneja S., Kohli K. A randomized, double-blind, placebo controlled trial of melatonin add-on therapy in epileptic children on valproate monotherapy: effect on glutathione peroxidase and glutathione reductase enzymes. Br. J. Clin. Pharmacol. 2004;58(5):542–547. doi: 10.1111/j.1365-2125.2004.02210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gupta M., Aneja S., Kohli K. Add-on melatonin improves quality of life in epileptic children on valproate monotherapy: a randomized, double-blind, placebo-controlled trial. Epilepsy Behav. 2004;5(3):316–321. doi: 10.1016/j.yebeh.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 83.Gupta M., Gupta Y.K., Agarwal S., Aneja S., Kalaivani M., Kohli K. Effects of add-on melatonin administration on antioxidant enzymes in children with epilepsy taking carbamazepine monotherapy: a randomized, double-blind, placebo-controlled trial. 2004. [DOI] [PubMed]
- 84.Peled N., Shorer Z., Peled E., Pillar G. Melatonin effect on seizures in children with severe neurologic deficit disorders. Epilepsia. 2001;42(9):1208–1210. doi: 10.1046/j.1528-1157.2001.28100.x. [DOI] [PubMed] [Google Scholar]
- 85.Jones C, Huyton M, Hindley D. Melatonin and epilepsy. Arch. Dis. Child. 2005;90(11):1203. doi: 10.1136/adc.2005.077172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tawfik M.K. Coenzyme Q10 enhances the anticonvulsant effect of phenytoin in pilocarpine-induced seizures in rats and ameliorates phenytoin-induced cognitive impairment and oxidative stress. Epilepsy Behav. 2011;22(4):671–677. doi: 10.1016/j.yebeh.2011.09.018. [DOI] [PubMed] [Google Scholar]
- 87.Santos I.M., de Freitas R.L., da Silva E.P., Feitosa C.M., Saldanha G.B., Souza G.F., Tomé Ada.R., Feng D., de Freitas R.M. Effects of ubiquinone on hydroperoxide concentration and antioxidant enzymatic activities in the rat hippocampus during pilocarpine-induced seizures. Brain Res. 2010;1315:33–40. doi: 10.1016/j.brainres.2009.12.037. [DOI] [PubMed] [Google Scholar]
- 88.Sayyah M., Yousefi-Pour M., Narenjkar J. Anti-epileptogenic effect of beta-carotene and vitamin A in pentylenetetrazole-kindling model of epilepsy in mice. Epilepsy Res. 2005;63(1):11–16. doi: 10.1016/j.eplepsyres.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 89.Xavier S.M., Barbosa C.O., Barros D.O., Silva R.F., Oliveira A.A., Freitas R.M. Vitamin C antioxidant effects in hippocampus of adult Wistar rats after seizures and status epilepticus induced by pilocarpine. Neurosci. Lett. 2007;420(1):76–79. doi: 10.1016/j.neulet.2007.04.056. [DOI] [PubMed] [Google Scholar]
- 90.Tome Ada R, Ferreira PM, Freitas RM. Inhibitory action of antioxidants (ascorbic acid or alpha-tocopherol) on seizures and brain damage induced by pilocarpine in rats. Arq. Neuropsiquiatr. . 2010;68(3):355–361. doi: 10.1590/s0004-282x2010000300005. [DOI] [PubMed] [Google Scholar]
- 91.Reeta K.H., Mehla J., Gupta Y.K. Curcumin ameliorates cognitive dysfunction and oxidative damage in phenobarbitone and carbamazepine administered rats. Eur. J. Pharmacol. 2010;644(1-3):106–112. doi: 10.1016/j.ejphar.2010.07.022. [DOI] [PubMed] [Google Scholar]
- 92.Shin H.J., Lee J.Y., Son E., Lee D.H., Kim H.J., Kang S.S., Cho G.J., Choi W.S., Roh G.S. Curcumin attenuates the kainic acid-induced hippocampal cell death in the mice. 2007. [DOI] [PubMed]
- 93.Shukla P.K., Khanna V.K., Khan M.Y., Srimal R.C. Protective effect of curcumin against lead neurotoxicity in rat. Hum. Exp. Toxicol. 2003;22(12):653–658. doi: 10.1191/0960327103ht411oa. [DOI] [PubMed] [Google Scholar]
- 94.Wang Q., Sun A.Y., Simonyi A., Jensen M.D., Shelat P.B., Rottinghaus G.E., MacDonald R.S., Miller D.K., Lubahn D.E., Weisman G.A., Sun G.Y. Neuroprotective mechanisms of curcumin against cerebral ischemia-induced neuronal apoptosis and behavioral deficits. J. Neurosci. Res. 2005;82(1):138–148. doi: 10.1002/jnr.20610. [DOI] [PubMed] [Google Scholar]
- 95.Sumanont Y., Murakami Y., Tohda M., Vajragupta O., Watanabe H., Matsumoto K. Prevention of kainic acid-induced changes in nitric oxide level and neuronal cell damage in the rat hippocampus by manganese complexes of curcumin and diacetylcurcumin. Life Sci. 2006;78(16):1884–1891. doi: 10.1016/j.lfs.2005.08.028. [DOI] [PubMed] [Google Scholar]
- 96.Mehla J., Reeta K.H., Gupta P., Gupta Y.K. Protective effect of curcumin against seizures and cognitive impairment in a pentylenetetrazole-kindled epileptic rat model. Life Sci. 2010;87(19-22):596–603. doi: 10.1016/j.lfs.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 97.Jyoti A., Sethi P., Sharma D. Curcumin protects against electrobehavioral progression of seizures in the iron-induced experimental model of epileptogenesis. Epilepsy Behav. 2009;14(2):300–308. doi: 10.1016/j.yebeh.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 98.Bharal N., Sahaya K., Jain S., Mediratta P.K., Sharma K.K. Curcumin has anticonvulsant activity on increasing current electroshock seizures in mice. Phytother. Res. 2008;22(12):1660–1664. doi: 10.1002/ptr.2551. [DOI] [PubMed] [Google Scholar]
- 99.Reeta K.H., Mehla J., Gupta Y.K. Curcumin is protective against phenytoin-induced cognitive impairment and oxidative stress in rats. Brain Res. 2009;1301:52–60. doi: 10.1016/j.brainres.2009.09.027. [DOI] [PubMed] [Google Scholar]
- 100.Sharma V, Nehru B, Munshi A, Jyothy A. Antioxidant potential of curcumin against oxidative insult induced by pentylenetetrazol in epileptic rats. Methods Find Exp. Clin. Pharmacol. 2010;32(4):227–232. doi: 10.1358/mf.2010.32.4.1452090. [DOI] [PubMed] [Google Scholar]
- 101.Uma Devi P., Pillai K.K., Vohora D. Modulation of pentylenetetrazole-induced seizures and oxidative stress parameters by sodium valproate in the absence and presence of N-acetylcysteine. Fundam. Clin. Pharmacol. 2006;20(3):247–253. doi: 10.1111/j.1472-8206.2006.00401.x. [DOI] [PubMed] [Google Scholar]
- 102.Ferrari D., Cysneiros R.M., Scorza C.A., Arida R.M., Cavalheiro E.A., de Almeida A.C., Scorza F.A. Neuroprotective activity of omega-3 fatty acids against epilepsy-induced hippocampal damage: Quantification with immunohistochemical for calcium-binding proteins. Epilepsy Behav. 2008;13(1):36–42. doi: 10.1016/j.yebeh.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 103.Young C., Gean P.W., Chiou L.C., Shen Y.Z. Docosahexaenoic acid inhibits synaptic transmission and epileptiform activity in the rat hippocampus. Synapse. 2000;37(2):90–94. doi: 10.1002/1098-2396(200008)37:2<90::AID-SYN2>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 104.Xiao Y., Li X. Polyunsaturated fatty acids modify mouse hippocampal neuronal excitability during excitotoxic or convulsant stimulation. Brain Res. 1999;846(1):112–121. doi: 10.1016/S0006-8993(99)01997-6. [DOI] [PubMed] [Google Scholar]
- 105.Rabinovitz S., Mostofsky D.I., Yehuda S. Anticonvulsant efficiency, behavioral performance and cortisol levels: a comparison of carbamazepine (CBZ) and a fatty acid compound (SR-3). Psychoneuroendocrinology. 2004;29(2):113–124. doi: 10.1016/S0306-4530(02)00149-X. [DOI] [PubMed] [Google Scholar]
- 106.Taha A.Y., Filo E., Ma D.W., McIntyre Burnham W. Dose-dependent anticonvulsant effects of linoleic and alpha-linolenic polyunsaturated fatty acids on pentylenetetrazol induced seizures in rats. 2009. [DOI] [PubMed]
- 107.Taha A.Y., Jeffrey M.A., Taha N.M., Bala S., Burnham W.M. Acute administration of docosahexaenoic acid increases resistance to pentylenetetrazol-induced seizures in rats. 2010. [DOI] [PubMed]
- 108.Lauritzen I., Blondeau N., Heurteaux C., Widmann C., Romey G., Lazdunski M. Polyunsaturated fatty acids are potent neuroprotectors. EMBO J. 2000;19(8):1784–1793. doi: 10.1093/emboj/19.8.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Schlanger S., Shinitzky M., Yam D. Diet enriched with omega-3 fatty acids alleviates convulsion symptoms in epilepsy patients. Epilepsia. 2002;43(1):103–104. doi: 10.1046/j.1528-1157.2002.13601.x. [DOI] [PubMed] [Google Scholar]
- 110.Yuen A.W., Sander J.W., Fluegel D., Patsalos P.N., Bell G.S., Johnson T., Koepp M.J. Omega-3 fatty acid supplementation in patients with chronic epilepsy: a randomized trial. Epilepsy Behav. 2005;7(2):253–258. doi: 10.1016/j.yebeh.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 111.Bromfield E., Dworetzky B., Hurwitz S., Eluri Z., Lane L., Replansky S., Mostofsky D. A randomized trial of polyunsaturated fatty acids for refractory epilepsy. Epilepsy Behav. 2008;12(1):187–190. doi: 10.1016/j.yebeh.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 112.DeGiorgio C.M., Miller P. n-3 fatty acids (eicosapentanoic and docosahexanoic acids) in epilepsy and for the prevention of sudden unexpected death in epilepsy. Epilepsy Behav. 2008;13(4):712–713. doi: 10.1016/j.yebeh.2008.06.017. [DOI] [PubMed] [Google Scholar]
- 113.Murias M., Jäger W., Handler N., Erker T., Horvath Z., Szekeres T., Nohl H., Gille L. Antioxidant, prooxidant and cytotoxic activity of hydroxylated resveratrol analogues: structure-activity relationship. Biochem. Pharmacol. 2005;69(6):903–912. doi: 10.1016/j.bcp.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 114.Gupta Y.K., Chaudhary G., Srivastava A.K. Protective effect of resveratrol against pentylenetetrazole-induced seizures and its modulation by an adenosinergic system. Pharmacology. 2002;65(3):170–174. doi: 10.1159/000058044. [DOI] [PubMed] [Google Scholar]
- 115.Kim HC, Choi DY, Jhoo WK, Lee DW, Koo CH, Kim C. Aspalatone, a new antiplatelet agent, attenuates the neurotoxicity induced by kainic acid in the rat. Life Sci. 1997;61(24): PL 373–381. doi: 10.1016/s0024-3205(97)00963-6. [DOI] [PubMed] [Google Scholar]
- 116.Yamamoto N., Kabuto H., Matsumoto S., Ogawa N., Yokoi I. alpha-Tocopheryl-L-ascorbate-2-O-phosphate diester, a hydroxyl radical scavenger, prevents the occurrence of epileptic foci in a rat model of post-traumatic epilepsy. Pathophysiology. 2002;8(3):205–214. doi: 10.1016/S0928-4680(02)00009-3. [DOI] [PubMed] [Google Scholar]
- 117.Rong Y., Doctrow S.R., Tocco G., Baudry M. EUK-134, a synthetic superoxide dismutase and catalase mimetic, prevents oxidative stress and attenuates kainate-induced neuropathology. Proc. Natl. Acad. Sci. USA. 1999;96(17):9897–9902. doi: 10.1073/pnas.96.17.9897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Liang L.P., Ho Y.S., Patel M. Mitochondrial superoxide production in kainate-induced hippocampal damage. Neuroscience. 2000;101(3):563–570. doi: 10.1016/S0306-4522(00)00397-3. [DOI] [PubMed] [Google Scholar]
- 119.Patel M. Superoxide involvement in excitotoxicity: a SOD-mimetic holds promise as a novel neuroprotective agent. Mol. Psychiatry. 1996;1(5):362–363. [PubMed] [Google Scholar]
- 120.Patel M. Inhibition of neuronal apoptosis by a metalloporphyrin superoxide dismutase mimic. J. Neurochem. 1998;71(3):1068–1074. doi: 10.1046/j.1471-4159.1998.71031068.x. [DOI] [PubMed] [Google Scholar]
- 121.Chuang Y.C., Chen S.D., Liou C.W., Lin T.K., Chang W.N., Chan S.H., Chang A.Y. Contribution of nitric oxide, superoxide anion, and peroxynitrite to activation of mitochondrial apoptotic signaling in hippocampal CA3 subfield following experimental temporal lobe status epilepticus. Epilepsia. 2009;50(4):731–746. doi: 10.1111/j.1528-1167.2008.01778.x. [DOI] [PubMed] [Google Scholar]
- 122.Barros D.O., Xavier S.M., Barbosa C.O., Silva R.F., Freitas R.L., Maia F.D., Oliveira A.A., Freitas R.M., Takahashi R.N. Effects of the vitamin E in catalase activities in hippocampus after status epilepticus induced by pilocarpine in Wistar rats. Neurosci. Lett. 2007;416(3):227–230. doi: 10.1016/j.neulet.2007.01.057. [DOI] [PubMed] [Google Scholar]
- 123.Gaby A.R. Natural approaches to epilepsy. Altern. Med. Rev. 2007;12(1):9–24. [PubMed] [Google Scholar]
- 124.Betti M., Minelli A., Ambrogini P., Ciuffoli S., Viola V., Galli F., Canonico B., Lattanzi D., Colombo E., Sestili P., Cuppini R. Dietary supplementation with α-tocopherol reduces neuroinflammation and neuronal degeneration in the rat brain after kainic acid-induced status epilepticus. Free Radic. Res. 2011;45(10):1136–1142. doi: 10.3109/10715762.2011.597750. [DOI] [PubMed] [Google Scholar]
- 125.Tomé A.R., Feng D., Freitas R.M. The effects of alpha-tocopherol on hippocampal oxidative stress prior to in pilocarpine-induced seizures. Neurochem. Res. 2010;35(4):580–587. doi: 10.1007/s11064-009-0102-x. [DOI] [PubMed] [Google Scholar]
- 126.Martinc B., Grabnar I., Vovk T. The role of reactive species in epileptogenesis and influence of antiepileptic drug therapy on oxidative stress. Curr. Neuropharmacol. 2012;10(4):328–343. doi: 10.2174/157015912804499447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Arora T., Mehta A.K., Sharma K.K., Mediratta P.K., Banerjee B.D., Garg G.R., Sharma A.K. Effect of carbamazepine and lamotrigine on cognitive function and oxidative stress in brain during chemical epileptogenesis in rats. 2010. [DOI] [PubMed]
- 128.Wesnes K.A., Edgar C., Dean A.D., Wroe S.J. The cognitive and psychomotor effects of remacemide and carbamazepine in newly diagnosed epilepsy. Epilepsy Behav. 2009;14(3):522–528. doi: 10.1016/j.yebeh.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 129.Park S.P., Kwon S.H. Cognitive effects of antiepileptic drugs. J. Clin. Neurol. 2008;4(3):99–106. doi: 10.3988/jcn.2008.4.3.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tonekaboni S.H., Beyraghi N., Tahbaz H.S., Bahreynian S.A., Aghamohammadpoor M. Neurocognitive effects of phenobarbital discontinuation in epileptic children. Epilepsy Behav. 2006;8(1):145–148. doi: 10.1016/j.yebeh.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 131.Maczurek A., Hager K., Kenklies M., Sharman M., Martins R., Engel J., Carlson D.A., Münch G. Lipoic acid as an anti-inflammatory and neuroprotective treatment for Alzheimer’s disease. Adv. Drug Deliv. Rev. 2008;60(13-14):1463–1470. doi: 10.1016/j.addr.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 132.Bellissimo M.I., Amado D., Abdalla D.S., Ferreira E.C., Cavalheiro E.A., Naffah-Mazzacoratti M.G. Superoxide dismutase, glutathione peroxidase activities and the hydroperoxide concentration are modified in the hippocampus of epileptic rats. Epilepsy Res. 2001;46(2):121–128. doi: 10.1016/S0920-1211(01)00269-8. [DOI] [PubMed] [Google Scholar]
- 133.Mesulam M.M., Guillozet A., Shaw P., Levey A., Duysen E.G., Lockridge O. Acetylcholinesterase knockouts establish central cholinergic pathways and can use butyrylcholinesterase to hydrolyze acetylcholine. Neuroscience. 2002;110(4):627–639. doi: 10.1016/S0306-4522(01)00613-3. [DOI] [PubMed] [Google Scholar]
- 134.Gupta Y.K., Gupta M., Kohli K. Neuroprotective role of melatonin in oxidative stress vulnerable brain. Indian J. Physiol. Pharmacol. 2003;47(4):373–386. [PubMed] [Google Scholar]
- 135.Mori A., Yokoi I., Noda Y., Willmore L.J. Natural antioxidants may prevent posttraumatic epilepsy: a proposal based on experimental animal studies. Acta Med. Okayama. 2004;58(3):111–118. doi: 10.18926/AMO/32111. [DOI] [PubMed] [Google Scholar]
- 136.Janjoppi L., Silva de Lacerda A.F., Scorza F.A., Amado D., Cavalheiro E.A., Arida R.M. Influence of pinealectomy on the amygdala kindling development in rats. Neurosci. Lett. 2006;392(1-2):150–153. doi: 10.1016/j.neulet.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 137.Yalýn O., Arman F., Erdoğan F., Kula M. A comparison of the circadian rhythms and the levels of melatonin in patients with diurnal and nocturnal complex partial seizures. Epilepsy Behav. 2006;8(3):542–546. doi: 10.1016/j.yebeh.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 138.Bikjdaouene L., Escames G., León J., Ferrer J.M., Khaldy H., Vives F., Acuña-Castroviejo D. Changes in brain amino acids and nitric oxide after melatonin administration in rats with pentylenetetrazole-induced seizures. J. Pineal Res. 2003;35(1):54–60. doi: 10.1034/j.1600-079X.2003.00055.x. [DOI] [PubMed] [Google Scholar]
- 139.Solmaz I., Gürkanlar D., Gökçil Z., Göksoy C., Ozkan M., Erdoğan E. Antiepileptic activity of melatonin in guinea pigs with pentylenetetrazol-induced seizures. Neurol. Res. 2009;31(9):989–995. doi: 10.1179/174313209X385545. [DOI] [PubMed] [Google Scholar]
- 140.Geromel V., Rötig A., Munnich A., Rustin P. Coenzyme Q10 depletion is comparatively less detrimental to human cultured skin fibroblasts than respiratory chain complex deficiencies. Free Radic. Res. 2002;36(4):375–379. doi: 10.1080/10715760290021216. [DOI] [PubMed] [Google Scholar]
- 141.James A.M., Smith R.A., Murphy M.P. Antioxidant and prooxidant properties of mitochondrial Coenzyme Q. 2004. [DOI] [PubMed]
- 142.Giusti P., Franceschini D., Petrone M., Manev H., Floreani M. In vitro and in vivo protection against kainate-induced excitotoxicity by melatonin. J. Pineal Res. 1996;20(4):226–231. doi: 10.1111/j.1600-079X.1996.tb00263.x. [DOI] [PubMed] [Google Scholar]
- 143.de Freitas R.M. Lipoic acid alters delta-aminolevulinic dehydratase, glutathione peroxidase and Na+,K+-ATPase activities and glutathione-reduced levels in rat hippocampus after pilocarpine-induced seizures. Cell. Mol. Neurobiol. 2010;30(3):381–387. doi: 10.1007/s10571-009-9460-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Packer L., Witt E.H., Tritschler H.J. alpha-Lipoic acid as a biological antioxidant. Free Radic. Biol. Med. 1995;19(2):227–250. doi: 10.1016/0891-5849(95)00017-R. [DOI] [PubMed] [Google Scholar]
- 145.Yamamoto H.A., Mohanan P.V. Ganglioside GT1B and melatonin inhibit brain mitochondrial DNA damage and seizures induced by kainic acid in mice. Brain Res. 2003;964(1):100–106. doi: 10.1016/S0006-8993(02)04083-0. [DOI] [PubMed] [Google Scholar]
- 146.Ardura J., Andres J., Garmendia J.R., Ardura F. Melatonin in epilepsy and febrile seizures. J. Child Neurol. 2010;25(7):888–891. doi: 10.1177/0883073809351315. [DOI] [PubMed] [Google Scholar]
- 147.Kabuto H., Yokoi I., Ogawa N. Melatonin inhibits iron-induced epileptic discharges in rats by suppressing peroxidation. Epilepsia. 1998;39(3):237–243. doi: 10.1111/j.1528-1157.1998.tb01367.x. [DOI] [PubMed] [Google Scholar]
- 148.Floreani M., Skaper S.D., Facci L., Lipartiti M., Giusti P. Melatonin maintains glutathione homeostasis in kainic acid-exposed rat brain tissues. FASEB J. 1997;11(14):1309–1315. doi: 10.1096/fasebj.11.14.9409550. [DOI] [PubMed] [Google Scholar]
- 149.Chen S.T., Chuang J.I. The antioxidant melatonin reduces cortical neuronal death after intrastriatal injection of kainate in the rat. Exp. Brain Res. 1999;124(2):241–247. doi: 10.1007/s002210050619. [DOI] [PubMed] [Google Scholar]
- 150.Srivastava A.K., Gupta S.K., Jain S., Gupta Y.K. Effect of melatonin and phenytoin on an intracortical ferric chloride model of posttraumatic seizures in rats. 2002. [DOI] [PubMed]
- 151.Tan DX, Manchester LC, Reiter RJ, Qi W, Kim SJ, El- Sokkary GH. Melatonin protects hippocampal neurons in vivo against kainic acid-induced damage in mice. J. Neurosci. Res. . 1998;54(3):382–389. doi: 10.1002/(SICI)1097-4547(19981101)54:3<382::AID-JNR9>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 152.Padayatty S.J., Levine M. New insights into the physiology and pharmacology of vitamin C. 2001. [PMC free article] [PubMed]
- 153.Santos L.F., Freitas R.L., Xavier S.M., Saldanha G.B., Freitas R.M. Neuroprotective actions of vitamin C related to decreased lipid peroxidation and increased catalase activity in adult rats after pilocarpine-induced seizures. Pharmacol. Biochem. Behav. 2008;89(1):1–5. doi: 10.1016/j.pbb.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 154.Ayyildiz M., Yildirim M., Agar E. The effects of vitamin E on penicillin-induced epileptiform activity in rats. Exp. Brain Res. 2006;174(1):109–113. doi: 10.1007/s00221-006-0425-7. [DOI] [PubMed] [Google Scholar]
- 155.Ayyildiz M., Yildirim M., Agar E. The involvement of nitric oxide in the anticonvulsant effects of alpha-tocopherol on penicillin-induced epileptiform activity in rats. Epilepsy Res. 2007;73(2):166–172. doi: 10.1016/j.eplepsyres.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 156.Aggarwal B.B., Sundaram C., Malani N., Ichikawa H. Curcumin: the Indian solid gold. Adv. Exp. Med. Biol. 2007;595:1–75. doi: 10.1007/978-0-387-46401-5_1. [DOI] [PubMed] [Google Scholar]
- 157.Nabiuni M., Nazari Z., Abdolhamid Angaji S., Safayi Nejad Z. Neuroprotective Effects Of Curcumin. Australian J. Basic Appl. Sci. 2011;5(9):2224–2240. doi: 10.1007/978-0-387-46401-5_8. [DOI] [Google Scholar]
- 158.Reeta K.H., Mehla J., Pahuja M., Gupta Y.K. Pharmacokinetic and pharmacodynamic interactions of valproate, phenytoin, phenobarbitone and carbamazepine with curcumin in experimental models of epilepsy in rats. Pharmacol. Biochem. Behav. 2011;99(3):399–407. doi: 10.1016/j.pbb.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 159.Pereira L.O., da Cunha I.C., Neto J.M., Paschoalini M.A., Faria M.S. The gradient of luminosity between open/enclosed arms, and not the absolute level of Lux, predicts the behaviour of rats in the plus maze. Behav. Brain Res. 2005;159(1):55–61. doi: 10.1016/j.bbr.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 160.Rodriguiz R.M., Wetsel W.C. Assessments of Cognitive Deficits in Mutant Mice. 2006. [PubMed]
- 161.Keller J.N., Schmitt F.A., Scheff S.W., Ding Q., Chen Q., Butterfield D.A., Markesbery W.R. Evidence of increased oxidative damage in subjects with mild cognitive impairment. 2005. [DOI] [PubMed]
- 162.Fukui K., Omoi N.O., Hayasaka T., Shinnkai T., Suzuki S., Abe K., Urano S. Cognitive impairment of rats caused by oxidative stress and aging, and its prevention by vitamin E. 2002. [DOI] [PubMed]
- 163.MacGregor D.G., Higgins M.J., Jones P.A., Maxwell W.L., Watson M.W., Graham D.I., Stone T.W. Ascorbate attenuates the systemic kainate-induced neurotoxicity in the rat hippocampus. 1996. [DOI] [PubMed]
- 164.Schneider Oliveira M., Flávia Furian A., Freire Royes L.F., Rechia Fighera M., de Carvalho Myskiw J., Gindri Fiorenza N., Mello C.F. Ascorbate modulates pentylenetetrazol-induced convulsions biphasically. Neuroscience. 2004;128(4):721–728. doi: 10.1016/j.neuroscience.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 165.Shin E.J., Suh S.K., Lim Y.K., Jhoo W.K., Hjelle O.P., Ottersen O.P., Shin C.Y., Ko K.H., Kim W.K., Kim D.S., Chun W., Ali S., Kim H.C. Ascorbate attenuates trimethyltin-induced oxidative burden and neuronal degeneration in the rat hippocampus by maintaining glutathione homeostasis. Neuroscience. 2005;133(3):715–727. doi: 10.1016/j.neuroscience.2005.02.030. [DOI] [PubMed] [Google Scholar]
- 166.Zaidi S.M., Al-Qirim T.M., Banu N. Effects of antioxidant vitamins on glutathione depletion and lipid peroxidation induced by restraint stress in the rat liver. Drugs R D. 2005;6(3):157–165. doi: 10.2165/00126839-200506030-00004. [DOI] [PubMed] [Google Scholar]
- 167.Yoneda T., Hiramatsu M., Sakamoto M., Togasaki K., Komatsu M., Yamaguchi K. Antioxidant effects of “beta catechin”. Biochem. Mol. Biol. Int. 1995;35(5):995–1008. [PubMed] [Google Scholar]
- 168.Komatsu M., Hiramatsu M. The efficacy of an antioxidant cocktail on lipid peroxide level and superoxide dismutase activity in aged rat brain and DNA damage in iron-induced epileptogenic foci. Toxicology. 2000;148(2-3):143–148. doi: 10.1016/S0300-483X(00)00205-5. [DOI] [PubMed] [Google Scholar]
- 169.Scapagnini G., Foresti R., Calabrese V., Giuffrida Stella A.M., Green C.J., Motterlini R. Caffeic acid phenethyl ester and curcumin: a novel class of heme oxygenase-1 inducers. Mol. Pharmacol. 2002;61(3):554–561. doi: 10.1124/mol.61.3.554. [DOI] [PubMed] [Google Scholar]
- 170.Shin E.J., Jeong J.H., Kim A.Y., Koh Y.H., Nah S.Y., Kim W.K., Ko K.H., Kim H.J., Wie M.B., Kwon Y.S., Yoneda Y., Kim H.C. Protection against kainate neurotoxicity by ginsenosides: attenuation of convulsive behavior, mitochondrial dysfunction, and oxidative stress. J. Neurosci. Res. 2009;87(3):710–722. doi: 10.1002/jnr.21880. [DOI] [PubMed] [Google Scholar]
- 171.Sinha K., Chaudhary G., Gupta Y.K. Protective effect of resveratrol against oxidative stress in middle cerebral artery occlusion model of stroke in rats. Life Sci. 2002;71(6):655–665. doi: 10.1016/S0024-3205(02)01691-0. [DOI] [PubMed] [Google Scholar]
- 172.Samuels N., Finkelstein Y., Singer S.R., Oberbaum M. Herbal medicine and epilepsy: proconvulsive effects and interactions with antiepileptic drugs. Epilepsia. 2008;49(3):373–380. doi: 10.1111/j.1528-1167.2007.01379.x. [DOI] [PubMed] [Google Scholar]
- 173.Lian X.Y., Zhang Z., Stringer J.L. Anticonvulsant and neuroprotective effects of ginsenosides in rats. Epilepsy Res. 2006;70(2-3):244–256. doi: 10.1016/j.eplepsyres.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 174.Kim S., Rhim H. Ginsenosides inhibit NMDA receptor-mediated epileptic discharges in cultured hippocampal neurons. Arch. Pharm. Res. 2004;27(5):524–530. doi: 10.1007/BF02980126. [DOI] [PubMed] [Google Scholar]
- 175.Kwon Y.S., Park D.H., Shin E.J., Kwon M.S., Ko K.H., Kim W.K., Jhoo J.H., Jhoo W.K., Wie M.B., Jung B.D., Kim H.C. Antioxidant propolis attenuates kainate-induced neurotoxicity via adenosine A1 receptor modulation in the rat. Neurosci. Lett. 2004;355(3):231–235. doi: 10.1016/j.neulet.2003.10.075. [DOI] [PubMed] [Google Scholar]
- 176.Shin E.J., Ko K.H., Kim W.K., Chae J.S., Yen T.P., Kim H.J., Wie M.B., Kim H.C. Role of glutathione peroxidase in the ontogeny of hippocampal oxidative stress and kainate seizure sensitivity in the genetically epilepsy-prone rats. Neurochem. Int. 2008;52(6):1134–1147. doi: 10.1016/j.neuint.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 177.Xiao Y.F., Kang J.X., Morgan J.P., Leaf A. 1995.
- 178.Yehuda S, Carasso RL, Mostofsky DI. Essential fatty acid preparation (SR-3) raises the seizure threshold in rats. Eur. J. Pharmacol. 1994;254(1-2):193–198. doi: 10.1016/0014-2999(94)90387-5. [DOI] [PubMed] [Google Scholar]
- 179.Taha A.Y., Huot P.S., Reza-López S., Prayitno N.R., Kang J.X., Burnham W.M., Ma D.W. Seizure resistance in fat-1 transgenic mice endogenously synthesizing high levels of omega-3 polyunsaturated fatty acids. J. Neurochem. 2008;105(2):380–388. doi: 10.1111/j.1471-4159.2007.05144.x. [DOI] [PubMed] [Google Scholar]
- 180.Taha A.Y., Baghiu B.M., Lui R., Nylen K., Ma D.W., Burnham W.M. Lack of benefit of linoleic and alpha-linolenic polyunsaturated fatty acids on seizure latency, duration, severity or incidence in rats. 2006. [DOI] [PubMed]
- 181.Willis S., Samala R., Rosenberger T.A., Borges K. Eicosapentaenoic and docosahexaenoic acids are not anticonvulsant or neuroprotective in acute mouse seizure models. Epilepsia. 2009;50(1):138–142. doi: 10.1111/j.1528-1167.2008.01722.x. [DOI] [PubMed] [Google Scholar]
- 182.Blondeau N., Widmann C., Lazdunski M., Heurteaux C. Polyunsaturated fatty acids induce ischemic and epileptic tolerance. 2002. [DOI] [PubMed]
- 183.DeGiorgio CM, Miller P, Meymandi S, Gornbein JA. n-3 fatty acids (fish oil) for epilepsy cardiac risk factors, and risk of SUDEP clues from a pilot, double-blind, exploratory study. Epilepsy Behav. 2008;13(4):681–684. doi: 10.1016/j.yebeh.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 184.Fraser D.D., Whiting S., Andrew R.D., Macdonald E.A., Musa-Veloso K., Cunnane S.C. Elevated polyunsaturated fatty acids in blood serum obtained from children on the ketogenic diet. 2003. [DOI] [PubMed]
- 185.Gupta Y.K., Briyal S., Chaudhary G. Protective effect of trans-resveratrol against kainic acid-induced seizures and oxidative stress in rats. Pharmacol. Biochem. Behav. 2002;71(1-2):245–249. doi: 10.1016/S0091-3057(01)00663-3. [DOI] [PubMed] [Google Scholar]
- 186.Gupta Y.K., Chaudhary G., Sinha K., Srivastava A.K. Protective effect of resveratrol against intracortical FeCl3-induced model of posttraumatic seizures in rats. Methods Find. Exp. Clin. Pharmacol. 2001;23(5):241–244. doi: 10.1358/mf.2001.23.5.662120. [DOI] [PubMed] [Google Scholar]
- 187.Willmore L.J., Rubin J.J. Effects of antiperoxidants on FeCl2-induced lipid peroxidation and focal edema in rat brain. Exp. Neurol. 1984;83(1):62–70. doi: 10.1016/0014-4886(84)90046-3. [DOI] [PubMed] [Google Scholar]
- 188.Willmore L.J., Rubin J.J. Antiperoxidant pretreatment and iron-induced epileptiform discharges in the rat: EEG and histopathologic studies. Neurology. 1981;31(1):63–69. doi: 10.1212/WNL.31.1.63. [DOI] [PubMed] [Google Scholar]
- 189.Willmore L.J., Triggs W.J., Gray J.D. The role of iron-induced hippocampal peroxidation in acute epileptogenesis. Brain Res. 1986;382(2):422–426. doi: 10.1016/0006-8993(86)91356-9. [DOI] [PubMed] [Google Scholar]
- 190.Willmore L.J., Rubin J.J. The effect of tocopherol and dimethyl sulfoxide on focal edema and lipid peroxidation induced by isocortical injection of ferrous chloride. Brain Res. 1984;296(2):389–392. doi: 10.1016/0006-8993(84)90080-5. [DOI] [PubMed] [Google Scholar]
- 191.Levy S.L., Burnham W.M., Bishai A., Hwang P.A. The anticonvulsant effects of vitamin E: a further evaluation. Can. J. Neurol. Sci. 1992;19(2):201–203. [PubMed] [Google Scholar]
- 192.Oztaş B., Kiliç S., Dural E., Ispir T. Influence of antioxidants on the blood-brain barrier permeability during epileptic seizures. J. Neurosci. Res. 2001;66(4):674–678. doi: 10.1002/jnr.10023. [DOI] [PubMed] [Google Scholar]
- 193.Kaya M., Cimen V., Kalayci R., Kucuk M., Gurses C., Arican N., Elmas I. Catalase and alpha-tocopherol attenuate blood-brain barrier breakdown in pentylenetetrazole-induced epileptic seizures in acute hyperglycaemic rats. Pharmacol. Res. 2002;45(2):129–133. doi: 10.1006/phrs.2001.0915. [DOI] [PubMed] [Google Scholar]
- 194.Bashkatova V., Narkevich V., Vitskova G., Vanin A. The influence of anticonvulsant and antioxidant drugs on nitric oxide level and lipid peroxidation in the rat brain during penthylenetetrazole-induced epileptiform model seizures. 2003. [DOI] [PubMed]
- 195.Dickinson D.A., Iles K.E., Zhang H., Blank V., Forman H.J. Curcumin alters EpRE and AP-1 binding complexes and elevates glutamate-cysteine ligase gene expression. FASEB J. 2003;17(3):473–475. doi: 10.1096/fj.02-0566fje. [DOI] [PubMed] [Google Scholar]
- 196.Xie T., Wang W.P., Mao Z.F., Qu Z.Z., Luan S.Q., Jia L.J., Kan M.C. Effects of epigallocatechin-3-gallate on pentylenetetrazole-induced kindling, cognitive impairment and oxidative stress in rats. Neurosci. Lett. 2012;516(2):237–241. doi: 10.1016/j.neulet.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 197.Yin S.T., Tang M.L., Su L., Chen L., Hu P., Wang H.L., Wang M., Ruan D.Y. Effects of Epigallocatechin-3-gallate on lead-induced oxidative damage. Toxicology. 2008;249(1):45–54. doi: 10.1016/j.tox.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 198.Yokoi I., Kabuto H., Akiyama K., Mori A., Ozaki M. Tannins inhibit the occurrence of epileptic focus induced by FeCl3 injection in rats. Jpn. J. Psychiatry Neurol. 1989;43(3):552–553. doi: 10.1111/j.1440-1819.1989.tb02979.x. [DOI] [PubMed] [Google Scholar]
- 199.Baluchnejadmojarad T., Roghani M. Chronic epigallocatechin-3-gallate ameliorates learning and memory deficits in diabetic rats via modulation of nitric oxide and oxidative stress. Behav. Brain Res. 2011;224(2):305–310. doi: 10.1016/j.bbr.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 200.Silva L.F., Hoffmann M.S., Rambo L.M., Ribeiro L.R., Lima F.D., Furian A.F., Oliveira M.S., Fighera M.R., Royes L.F. The involvement of Na+, K+-ATPase activity and free radical generation in the susceptibility to pentylenetetrazol-induced seizures after experimental traumatic brain injury. J. Neurol. Sci. 2011;308(1-2):35–40. doi: 10.1016/j.jns.2011.06.030. [DOI] [PubMed] [Google Scholar]
- 201.Ribeiro M.C., de Avila D.S., Schneider C.Y., Hermes F.S., Furian A.F., Oliveira M.S., Rubin M.A., Lehmann M., Krieglstein J., Mello C.F. alpha-Tocopherol protects against pentylenetetrazol- and methylmalonate-induced convulsions. Epilepsy Res. 2005;66(1-3):185–194. doi: 10.1016/j.eplepsyres.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 202.Shin E.J., Jeong J.H., Chung Y.H., Kim W.K., Ko K.H., Bach J.H., Hong J.S., Yoneda Y., Kim H.C. Role of oxidative stress in epileptic seizures. 2011. [DOI] [PMC free article] [PubMed]
- 203.Liu W., Liu R., Schreiber S.S., Baudry M. Role of polyamine metabolism in kainic acid excitotoxicity in organotypic hippocampal slice cultures. J. Neurochem. 2001;79(5):976–984. doi: 10.1046/j.1471-4159.2001.00650.x. [DOI] [PubMed] [Google Scholar]
- 204.Arivazhagan P., Shila S., Kumaran S., Panneerselvam C. Effect of DL-alpha-lipoic acid on the status of lipid peroxidation and antioxidant enzymes in various brain regions of aged rats. Exp. Gerontol. 2002;37(6):803–811. doi: 10.1016/S0531-5565(02)00015-3. [DOI] [PubMed] [Google Scholar]
- 205.Du P., Tang H.Y., Li X., Lin H.J., Peng W.F., Ma Y., Fan W., Wang X. Anticonvulsive and antioxidant effects of curcumin on pilocarpine-induced seizures in rats. Chin. Med. J. (Engl.) 2012;125(11):1975–1979. [PubMed] [Google Scholar]
- 206.Agarwal N.B., Jain S., Agarwal N.K., Mediratta P.K., Sharma K.K. Modulation of pentylenetetrazole-induced kindling and oxidative stress by curcumin in mice. Phytomedicine. 2011;18(8-9):756–759. doi: 10.1016/j.phymed.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 207.Yokozawa T., Satoh A., Cho E.J. Ginsenoside-Rd attenuates oxidative damage related to aging in senescence-accelerated mice. J. Pharm. Pharmacol. 2004;56(1):107–113. doi: 10.1211/0022357022449. [DOI] [PubMed] [Google Scholar]
- 208.Ye R., Yang Q., Kong X., Han J., Zhang X., Zhang Y., Li P., Liu J., Shi M., Xiong L., Zhao G. Ginsenoside Rd attenuates early oxidative damage and sequential inflammatory response after transient focal ischemia in rats. Neurochem. Int. 2011;58(3):391–398. doi: 10.1016/j.neuint.2010.12.015. [DOI] [PubMed] [Google Scholar]
- 209.Zhao H., Li Q., Zhang Z., Pei X., Wang J., Li Y. Long-term ginsenoside consumption prevents memory loss in aged SAMP8 mice by decreasing oxidative stress and up-regulating the plasticity-related proteins in hippocampus. Brain Res. 2009;1256:111–122. doi: 10.1016/j.brainres.2008.12.031. [DOI] [PubMed] [Google Scholar]
- 210.dos Santos P.S., Costa J.P., Tome Ada R., Saldanha G.B., de Souza G.F., Feng D., de Freitas R.M. Oxidative stress in rat striatum after pilocarpine-induced seizures is diminished by alpha-tocopherol. 2011. [DOI] [PubMed]
- 211.Golechha M., Chaudhry U., Bhatia J., Saluja D., Arya D.S. Naringin protects against kainic acid-induced status epilepticus in rats: evidence for an antioxidant, anti-inflammatory and neuroprotective intervention. Biol. Pharm. Bull. 2011;34(3):360–365. doi: 10.1248/bpb.34.360. [DOI] [PubMed] [Google Scholar]
- 212.Ezz H.S., Khadrawy Y.A., Noor N.A. The neuroprotective effect of curcumin and Nigella sativa oil against oxidative stress in the pilocarpine model of epilepsy: a comparison with valproate. Neurochem. Res. 2011;36(11):2195–2204. doi: 10.1007/s11064-011-0544-9. [DOI] [PubMed] [Google Scholar]
- 213.Raju G.B., Behari M., Prasad K., Ahuja G.K. Randomized, double-blind, placebo-controlled, clinical trial of D-alpha-tocopherol (vitamin E) as add-on therapy in uncontrolled epilepsy. Epilepsia. 1994;35(2):368–372. doi: 10.1111/j.1528-1157.1994.tb02446.x. [DOI] [PubMed] [Google Scholar]
- 214.Ogunmekan A.O., Hwang P.A. A randomized, double-blind, placebo-controlled, clinical trial of D-alpha-tocopheryl acetate (vitamin E), as add-on therapy, for epilepsy in children. Epilepsia. 1989;30(1):84–89. doi: 10.1111/j.1528-1157.1989.tb05287.x. [DOI] [PubMed] [Google Scholar]
- 215.Ben-Menachem E., Kyllerman M., Marklund S. Superoxide dismutase and glutathione peroxidase function in progressive myoclonus epilepsies. 2000. [DOI] [PubMed]
- 216.Edwards M.J., Hargreaves I.P., Heales S.J., Jones S.J., Ramachandran V., Bhatia K.P., Sisodiya S. N-acetylcysteine and Unverricht-Lundborg disease: variable response and possible side effects. 2002. [DOI] [PubMed]
- 217.Kovalenko V.M., Kryzhanovskiĭ G.N., Kovalenko V.S., Pronina I.G., Nikushkin E.V. [Alpha-tocopherol in the complex treatment of several forms of epilepsy]. Zh. Nevropatol. Psikhiatr. Im. S S Korsakova. 1984;84(6):892–897. [PubMed] [Google Scholar]
- 218.Tupeev I.R., Kryzhanovskiĭ G.N., Nikushkin E.V., Bordiukov M.M., Iuzefova S.M. [The antioxidant system in the dynamic combined treatment of epilepsy patients with traditional anticonvulsant preparations and an antioxidant--alpha-tocopherol]. Biull. Eksp. Biol. Med. 1993;116(10):362–364. [The antioxidant system in the dynamic combined treatment of epilepsy patients with traditional anticonvulsant preparations and an antioxidant--alpha-tocopherol]. [PubMed] [Google Scholar]
- 219.Kaiboriboon K., Guevara M., Alldredge B.K. Understanding herb and dietary supplement use in patients with epilepsy. Epilepsia. 2009;50(8):1927–1932. doi: 10.1111/j.1528-1167.2009.02090.x. [DOI] [PubMed] [Google Scholar]
- 220.Eyal S., Rasaby S., Ekstein D. Concomitant therapy in people with epilepsy: potential drug-drug interactions and patient awareness. Epilepsy Behav. 2014;31:369–376. doi: 10.1016/j.yebeh.2013.09.041. [DOI] [PubMed] [Google Scholar]
- 221.Easterford K., Clough P., Comish S., Lawton L., Duncan S. The use of complementary medicines and alternative practitioners in a cohort of patients with epilepsy. Epilepsy Behav. 2005;6(1):59–62. doi: 10.1016/j.yebeh.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 222.Peebles C.T., McAuley J.W., Roach J., Moore J.L., Reeves A.L. Alternative medicine use by patients with epilepsy. Epilepsy Behav. 2000;1(1):74–77. doi: 10.1006/ebeh.2000.0031. [DOI] [PubMed] [Google Scholar]
- 223.Sirven JI, Drazkowski JF, Zimmerman RS, Bortz JJ, Shulman DL, Macleish M. Complementary/alternative medicine for epilepsy in Arizona. Neurology. 2003;61(4):576–577. doi: 10.1212/01.wnl.0000073541.08356.47. [DOI] [PubMed] [Google Scholar]
- 224.Lee S.W., Chung S.S. A review of the effects of vitamins and other dietary supplements on seizure activity. Epilepsy Behav. 2010;18(3):139–150. doi: 10.1016/j.yebeh.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 225.Haller C.A., Meier K.H., Olson K.R. Seizures reported in association with use of dietary supplements. Clin. Toxicol. (Phila.) 2005;43(1):23–30. doi: 10.1081/CLT-44771. [DOI] [PubMed] [Google Scholar]
- 226.Shi S., Klotz U. Drug interactions with herbal medicines. Clin. Pharmacokinet. 2012;51(2):77–104. doi: 10.2165/11597910-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 227.Clarke M.W., Burnett J.R., Croft K.D. Vitamin E in human health and disease. Crit. Rev. Clin. Lab. Sci. 2008;45(5):417–450. doi: 10.1080/10408360802118625. [DOI] [PubMed] [Google Scholar]
- 228.Murray M. Altered CYP expression and function in response to dietary factors: potential roles in disease pathogenesis. Curr. Drug Metab. 2006;7(1):67–81. doi: 10.2174/138920006774832569. [DOI] [PubMed] [Google Scholar]
- 229.Yap K.Y., Chui W.K., Chan A. Drug interactions between chemotherapeutic regimens and antiepileptics. Clin. Ther. 2008;30(8):1385–1407. doi: 10.1016/j.clinthera.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 230.Chi Y.C., Lin S.P., Hou Y.C. A new herb-drug interaction of Polygonum cuspidatum, a resveratrol-rich nutraceutical, with carbamazepine in rats. Toxicol. Appl. Pharmacol. 2012;263(3):315–322. doi: 10.1016/j.taap.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 231.Heuser K., Szokol K., Taubøll E. The role of glial cells in epilepsy. Tidsskr. Nor. Laegeforen. 2014;134(1):37–41. doi: 10.4045/tidsskr.12.1344. [DOI] [PubMed] [Google Scholar]