Abstract
Stroke is the third leading cause of human death. Endothelial dysfunction, thrombogenesis, inflammatory and oxidative stress damage, and angiogenesis play an important role in cerebral ischemic pathogenesis and represent a target for prevention and treatment. Statins have been found to improve endothelial function, modulate thrombogenesis, attenuate inflammatory and oxidative stress damage, and facilitate angiogenesis far beyond lowering cholesterol levels. Statins have also been proved to significantly decrease cardiovascular risk and to improve clinical outcome. Could statins be the new candidate agent for the prevention and therapy in ischemic stroke? In recent years, a vast expansion in the understanding of the pathophysiology of ischemic stroke and the pleiotropic effects of statins has occurred and clinical trials involving statins for the prevention and treatment of ischemic stroke have begun. These facts force us to revisit ischemic stroke and consider new strategies for prevention and treatment. Here, we survey the important developments in the non-lipid dependent pleiotropic effects and clinical effects of statins in ischemic stroke.
Keywords: Clinical effects, endothelial dysfunction, inflammation, ischemic stroke, oxidative stress, statins, thrombogenesis
INTRODUCTION
Despite considerable advances in the understanding of the pathophysiology of ischemic stroke, therapeutic options, particularly pharmacological agents for prevention and treatment are still limited. Stroke is still the third leading cause of death and the most frequent cause of permanent disability in adults worldwide [1]. Systemic and local processes of endothelial dysfunction, thrombogenesis, inflammatory and oxidative stress damage, and angiogenesis play an important role in cerebral ischemic pathogenesis and may represent strategic targets for prevention and treatment of ischemic stroke [2].
Statins lower serum cholesterol level by inhibiting hydroxymethylglutaryl-coenzymeA (HMG-CoA) reductase[3]. Statins have been found to improve endothelial function, modulate thrombogenesis, attenuate inflammatory and oxidative stress damage, and facilitate angiogenesis far beyond lowering cholesterol levels [4-7]. Statins have also been proved to significantly decrease cardiovascular risk and to improve clinical outcome [8]. Could statins be the new candidate agents for the prevention and treatment of ischemic stroke?
In recent years, a vast expansion in the understanding of the pathophysiology of ischemic stroke and the pleiotropic effects of statins has occurred. Clinical trials involving statins for prevention and treatment of ischemic stroke have begun. Treatment with statins either before, or early after cerebral arterial occlusion has been proved to associate with reduced infarct volume and improved neurological function in animal models [9-11]. In several large clinical trials, the effects of statins on stroke prevention and treatment have also been well established [12-14]. Statins may have surpassed other pharmacologic medicine in the reduction of the incidence of stroke and total mortality [15, 16]. These facts force us to revisit ischemic stroke and consider new strategies for prevention and treatment.
In this review, we survey recent important developments in the pleiotropic anti-inflammatory, antioxidative, antithrombotic and endothelial protective effects of statins (Fig. 1) and the data from the prospective and observational studies of statins focused on their preventive and therapeutic effects in ischemic stroke (Tables 1 and 2).
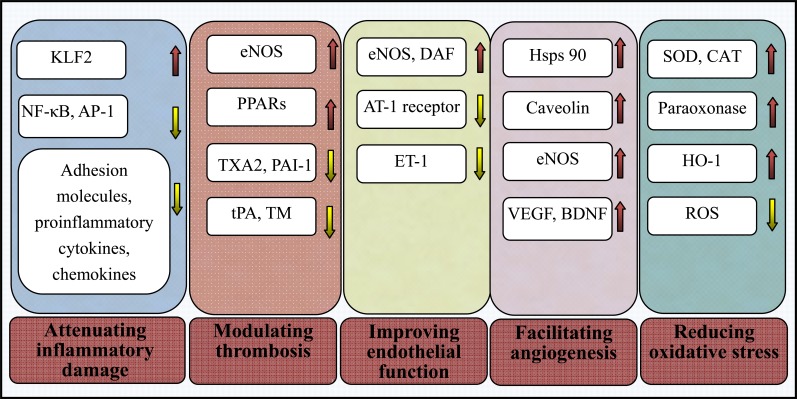
Fig. (1).
Many roles of statins beyond lowering cholesterol. The pleiotropic effects and the underlying associated mechanisms of statins beyond lowering cholesterol, including improving endothelial function, modulating thrombogenesis, attenuating inflammatory and oxidative stress damage, and facilitating angiogenesis. NF-ćB = nuclear factor-ćB; AP-1 = activatorprotein-1; PPARs = peroxisome proliferator-activated receptors; KLF2 = Kruppel-like factor-2; TXA2 = thromboxane A2; PAI-1 = plasminogen activator inhibitor-1; tPA = tissue plasminogen activator; TM = thrombomodulin; ET-1 = endothelin-1; AT-1 = angiotensin II type 1; eNOS = endothelial nitric oxide synthase; VEGF = vascular endothelial growth factor; BDNF = brain-derived neurotrophic factor; Hsp90 = heat-shock protein 90; ROS = reactive oxygen species
Table 1.
Summary of the statins clinical trials: effects on stroke prevention with or without ischemic heart disease.
| Trials [Reference] | Inclusion Criteria | Statins | Dose (mg/d) | RRR for Stroke | P value | Follow-up |
|---|---|---|---|---|---|---|
| 4S [16] | MI, UA | Simvastatin | 10 - 40 | 30% | 0.024 | 5.4 years |
| WOSCOP [66] | Hypercholesteremia, without IHD | Pravastatin | 40 | 11% | NS | 4.9 years |
| CARE [65] | MI | Pravastatin | 40 | 31% | 0.03 | 5.8 years |
| LIPID [12] | MI, UA, without hypercholesteremia | Pravastatin | 40 | 19% | 0.048 | 6.1 years |
| A-Z [76] | IHD | Simvastatin | 40/80 | 30% | NS | 6 - 24 months |
| PROSPER [13] | Vascular risk factors or MI, stroke | Pravastatin | 40 | 3% | NS | 3.2 years |
| HPS [75] | CHD, DM, stroke, other vascular diseases | Simvastatin | 40 | 25%, (2% for previous stroke) | 0.0001, (NS) | 5.0 years |
| SPARCL [73] | Stroke, TIA without IHD | Atorvastatin | 80 | 16% | 0.03 | 4.9 years |
| GREACE [70] | CHD | Atorvastatin | 10 - 80 | 47% | 0.0034 | 3.0 years |
| ASCOTLLA [69] | Hypertension with at least three other risk factors, without hypercholesterolemia and CHD | Atorvastatin | 10 | 27% | 0.024 | 5.0 years |
| ALLIANCE [71] | CHD | Atorvastatin | 10 - 80 | 13% | NS | 4.3 years |
| ALLHAT-LLT [67] | Hypertension | Pravastatin | 40 | 9% | NS | 4.8 years |
| MEGA [68] | Hypercholesterolemia | Pravastatin | 10 - 20 | 17% | NS | 5.3 years |
| ASPEN [72] | DM | Atorvastatin | 10 | 11% | NS | 4.0 years |
| JUPITER [77] | CRP > 2.0 mg/l | Rosuvastatin | 20 | 48% | 0.002 | 1.9 years |
Note: MI = myocardial infarction; CHD = coronary heart disease; DM = diabetes mellitus; HR = hazard ratio; IHD = ischemic heart disease; NS = not significant; RRR = relative risk reduction; TIA = transient ischemic attack; UA = unstable angina.
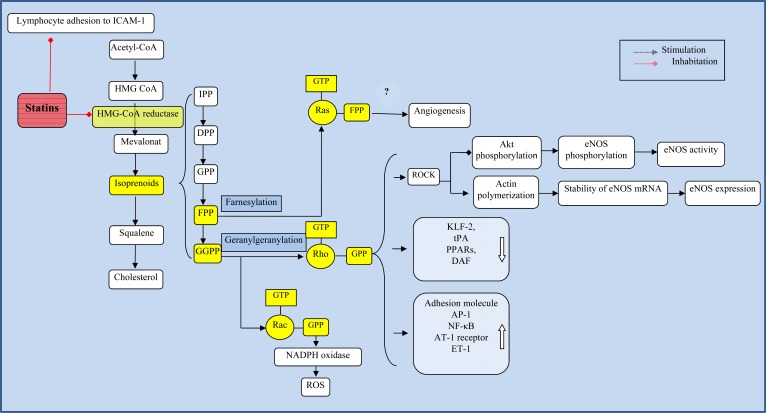
Fig. (2).
Isoprenoids-related mechanisms. Statins inhibit HMG-CoA reductase and decrease isoprenoids intermediates such as FPP and GGPP, which leads to an inhibition of isoprenylation of small GTPases such as Ras, Rho, and Rac. The many roles of statins in ischemic stroke may be due to the interruption of isoprenoid biosynthesis. IPP = isopentyl pyrophosphate; DPP = 3,3-dimethylallyl pyrophosphate, GPP = geranyl pyrophosphate, FPP = farnesyl pyrophosphate, GGPP = geranylgeranyl pyrophosphate, ROCK = Rho kinase, eNOS = endothelial nitric oxide synthase, NF-ćB = nuclear factor-ćB, KLF2 = Kruppel-like factor-2, tPA = tissue plasminogen activator, AT-1 = angiotensin II type 1, ET-1 = endothelin-1, DAF = decay-accelerating factor, AP-1 = activatorprotein-1, PPARs = peroxisome proliferator-activated receptors, ROS = reactive oxygen species, LFA-1 = lymphocyte function-associated antigen-1.
MAIN PLEIOTROPIC EFFECTS OF STATINS BEYOND LOWERING CHOLESTEROL
It has been known that statins exert lipid dependent effects on atherosclerosis by lowering the generation of serum low density lipoprotein (LDL), oxidized LDL and cholesterol [17]. Statins inhibit HMG-CoA reductase, ultimately leading to a reduction not only of cholesterol but also of a range of other intermediate metabolites, among which the formation of isoprenoids plays a key role in cellular signaling and control of cell functions such as proliferation, differentiation and migration [18]. It is not surprising that, other than reducing cholesterol, statins appear to lead to the non-lipid dependent, pleiotropic effects on ischemic stroke [19].
Inhibition of Isoprenoids Formation
There is growing evidence indicating that some of the lipid-independent effects are mediated by interruption of isoprenoids biosynthesis [19]. Isoprenoids, such as indicates isopentyl pyrophosphate (IPP); 3, 3-dimethylallyl pyrophosphate (DPP), geranyl pyrophosphate (GPP), farnesyl pyrophosphate (FPP), geranylgeranyl pyrophosphate (GGPP) are important intermediate metabolites in cholesterol biosynthesis pathway. Prenylation (isoprenylation), such as farnesylation and geranylgeranylation, is critical for the insertion and anchorage of proteins to cell membranes and for their full biological functionality [19]. The translocation of Ras and Ras-like proteins (Rho and Rac) to the membrane is dependent on farnesylation and geranylgeranylation, respectively [20]. Statins block the transformation of HMG-CoA into L-mevalonate, the formation of isoprenoids such as FPP and GGPP, and subsequent translocation of Ras and Ras-like proteins to the membrane (Fig. 2). Statins increase the production and bioavailability of endothelium-derived nitric oxide (NO) through reducing the Rho GTPase [21]. By inhibiting Rac prenylation, statins lead to a reduction in nicotinamide adenine dinucleotide phosphate oxidase (NOX) assembly and consequent generation of reactive oxygen species (ROS) [22]. Statins activate endothelial Ras which is associated with cellular proliferation and lead to proangio-genic effects [23].
Improvement of Endothelial Function and Vasomotor Reactivity
Endothelial dysfunction is one of the earliest manifestations of atherosclerosis and is strongly related to stroke occurrence [24]. Statins improve endothelial function through non-lipid dependent effect at least in part mediated by upregulating endothelial nitric oxide synthase (eNOS) [4]. Endothelium-derived NO may mediate vasodilation and decrease vascular smooth muscle cells (VSMCs) proliferation [25]. Parts of eNOS-induced effects on vascular wall are attributed to the inhibition of the Rho/Rho kinase (ROCK) pathway (Fig. 2). Inhibition of Rho/ROCK activates PI3K/Akt/eNOS pathway and increases the eNOS mRNA stability via changes in actin cytoskeleton and extension in eNOS mRNA half-life, which are reversed by GGPP [26, 27].
Statins protect vascular endothelium against complement-mediated injury through decay-accelerating factor (DAF) upregulation, which are mediated by inhibiting RhoA, independent of NO [28]. Statins reduce angiotensin II type 1 (AT-1) receptor gene expression with subsequent alleviation of vasoconstrictive angiotensin II (AT-II) effects through a Rho-dependent manner to promote vasorelaxation [29]. Statins also inhibit the expression of endothelin-1 (ET-1) in a Rho-dependent pathway, to limit vasoconstriction and VSMCs proliferation [30].
Giannopoulos et al. believed that statins pretreatment significantly improved cerebral vasomotor reactivity through the upregulation of eNOS in patients with severe small vessel disease [31]. Endres et al. thought that prophylactic treatment with statins augmented cerebral blood flow and reduced brain injury during cerebral ischemia by upregulating eNOS [32]. Combination of simvastatin and dipyridamole may have greater benefits in stroke protection than statin alone through NO- dependent vascular protection [33].
Modulation of Thrombogenesis
Thrombosis superimposed on atherosclerosis plays an important role during ischemic stroke. Statins reduce the production of thromboxane A2 (TXA2) in platelet and erythrocyte membranes, resulting in a decrease in thrombogenic potential of these cells [34]. Acute intravenous administration of lovastatin is associated with favorable alterations in platelet function including impaired aggregation, reduced dense granule release, and reduction in subsequent platelet-mediated thrombus formation in an animal study [5]. Statins reduce platelet activation and thrombus formation partly mediated by decreased Rho-GTPase prenylation and subsequent increased eNOS expression [35]. Peroxisome proliferator-activated receptor-α (PPAR-α) and peroxisome proliferator-activated receptor-γ (PPAR-γ) stimulation in platelets surface have been suggested as additional mechanisms to reduce platelet activation [36].
Statins increase Thrombomodulin (TM) expression and function through a NO-dependent mechanism in vitro [37]. Fu et al. provided the novel mechanisms for statins-induced TM upregulation that heat-shock factor-1 (HSF-1) dissociated from HSP-90 and activated Kruppel like factor (KLF)-2, subsequently both of the transcription factors translocating to the nucleus where they binded to promoter regions of TM involving heat shock element (HSE)-1 and -3 and Sp1/KLF [38]. Lovastatin increase tPA activity in rat aortic endothelial cells involving Rho proteins [39]. The signaling pathways by which statins induce downregulation of PAI-1 are still unclear.
Attenuation of Inflammatory Damage
Inflammatory processes have a key role in the pathophysiology of ischemic stroke [40]. Statins have been proved to inhibit inflammatory cell recruitment, adhesion and migration. Statins inhibit the expression of adhesion molecules such as vascular adhesion molecule-1 (VCAM-1), intercellular adhesion molecule-1 (ICAM-1) and E-selectin, thereby reducing inflammatory cell recruitment [6]. Statins reduce macrophage receptor-1 (MAC1) (CD11b/CD18) expression and CD11b dependent monocyte adhesion to endothelium [41], probably mediated by reducing isoprenylation of leukocyte G-proteins [42]. Statins also inhibit leukocyte adhesion by direct interactions with the leukocyte-function antigen-1 (LFA-1) (CD11a/CD18), rather than targeting HMG-CoA reductase [43]. (Fig. 2). Statins reduce expression of the chemokine such as monocyte chemoattractant protein-1 (MCP-1), interleukin (IL)-8, and regulated on activation normally T-cell expressed and secreted (RANTES) in vitro [44, 45].
Statins are associated with reductions in inflammatory biomarkers, referring to c-reactive protein (CRP), cytokines (IL-1, IL-6, IL-12, tumour necrosis factor-α (TNF-α), IFN-γ), lipoprotein-associated phospholipase A2 [45-47]. Statins inhibit the activation of inflammatory transcription factors activating protein-1 (AP-1) and NF-κB in human endothelial cells and VSMCs [48], probably mediated by Rho and Rac proteins [49, 50]. Further study shows that statins suppress the activation of NF-κB probably through inducing the expression of a novel transcriptional regulator Kruppel-like factor-2 (KLF2) [51], which can be abolished by GGPP, involving the Rho pathway [52].
Reduction of Oxidative Stress
The release and production of ROS is thought to be a key event in the pathogenesis of endothelial dysfunction and atherosclerosis [53]. Statins confer a reduction of AT-II-induced release of ROS by two important mechanisms involved in decreased geranylgeranyl-dependent activation of Rac1 GTPase and reduced AT-1 receptor expression mediated by destabilization of AT1 mRNA [7]. Activation of NOX is a major source of ROS during ischemic stroke [54]. NOX subunit nox1 and p22phox expression were found to decrease from an in-vitro and in-vivo study of atorvastatin [22].
Antioxidative defense systems are equally important for oxidative stress besides inhibiting ROS generation. The antioxidant effects of statins may attribute to increased heme oxygenase-1 (HO-1) expression and paraoxonase activity [55, 56]. Statins also produce broader antioxidant defenses by increasing radical-scavenging enzymes activity [57]. De Oliveira et al. proved that atorvastatin withdrawal led to oxidative/nitrosative damage in the rat cerebral cortex, and that mitochondrial superoxide dismutase (SOD) activities played a part in such harmful condition [58]. However, Wassmann et al. demonstrated that atorvastatin did not significantly alter the mRNA expression of SOD isoforms and glutathione peroxidase (GSH-Px), in addition to catalase [22].
Facilitation of Angiogenesis
The key role of angiogenesis is an unresolved issue in the understanding of recovery mechanisms after stroke. Atorvastatin promotes angiogenesis and enhances functional recovery after stroke through a mechanism involving an increase of vascular endothelial growth factor (VEGF), brain-derived neurotrophic factor (BDNF), cGMP, synaptic protein and synaptophysin in the rodent middle cerebral artery occlusion model [59, 60]. Statins increase bone marrow-derived endothelial progenitor cell (EPC) levels, which play a role in neovascularization after vascular injury, probably through NO-related mechanisms [61]. Phosphatidyl inositol 3-kinase (PI3K)/Akt/eNOS, caveolin/eNOS and Hsp90/Akt/eNOS are identified as key targets of statins to modulate NO-mediated angiogenesis [26, 62].
After initial reports on angiogenesis effects of statins, subsequent studies imply inhibition of angiogenesis by statins [63]. Disparities of angiogenesis may result from different statins concentrations. Low concentrations of atorvastatin or mevastatin activated endothelial Ras and promoted Akt and eNOS phosphorylation, leading to proangiogenic effects, while high concentrations resulted in anti-angiogenic effects through inhibiting Ras and RhoA without upregulating eNOS [23]. However, simvastatin of the same dose promoted angiogenesis in response to hypoxic conditions and inhibited angiogenesis during inflammation [64], which implies that the effect on angiogenesis also change according to the inner environment and underlying disease. More trials are needed to illuminate the complex relationship between statins and angiogenesis.
CLINICAL PREVENTIVE EFFECTS OF STATINS IN ISCHEMIC STROKE
Statins have emerged as guideline therapy for primary and secondary stroke prevention. The results from several large randomized, double-blind trials have firmly established that statins use in ischemic stroke is associated with reduced risk of incident and recurrent stroke (Table 1).
Pravastatin
The CARE study demonstrated that pravastatin (40 mg/day) significantly decreased incidence of stroke by 31% in patients with myocardial infarction (MI) (P = 0.03) [65]. The LIPID study demonstrated that pravastatin (40 mg/day) in patients with MI or unstable angina (UA) resulted in a 19% relative risk reduction (RRR) in stroke [12]. However, PROSPER, ALLHAT-LLT and WOSCOP study failed to demonstrate significant reduction in stroke incidence among patients taken pravastatin (40 mg/day) [13, 66, 67]. No significant difference was found in stroke RRR in MEGA study, in which people took pravastatin (10 to 20 mg/day) [68].
Atorvastatin
In the trial of ASCOTLLA, atorvastatin (10 mg/day) was associated with a 27% reduction in fatal or nonfatal stroke (P = 0.02) [69]. In the GREACE study, atorvastatin (10 to 80mg/day) was proved to reduce stroke incidence by 47% (P = 0.03) [70]. There was no significant difference of stroke RRR between atorvastatin group and control group in the trial of ALLIANCE and ASPEN [71, 72].
The SPARCL trial provided the best evidence to support the role of atorvastatin for the secondary stroke prevention. Atorvastatin 80 mg/day reduced the incidence of recurrent ischemic stroke versus placebo (RRR: 16%; P = 0.03) [73].
Simvastatin
The Scandinavian Simvastatin Survival Study (4S) showed that simvastatin 40 mg/day given in population with prior MI or UA reduced the incidence of stroke and TIA by 30% over the follow-up period of 5.4 years [16]. The Heart Protection Study (HPS) firmly established the efficacy of simvastatin in reducing stroke and other vascular events among 20, 536 adults with cerebrovascular and other occlusive arterial disease or diabetes. Simvastatin (40 mg/day) reduced the incidence of ischemic stroke by 25%, and statins therapy was beneficial for people with preexisting cerebrovascular disease, in consideration of the reduction by 20% (8% - 29%) in major vascular events (nonfatal myocardial infarction, stroke of any type, et al). However, there was no obvious reduction in stroke incidence and severity among those with previous cerebrovascular disease [74, 75]. In the phase Z of A to Z trial of patients with acute coronary syndrome, 2,232 patients received placebo for 4 months followed by simvastatin (20 mg/day) and 2,265 patients received simvastatin (40 mg/day) for 1 month followed by 80 mg thereafter, no significant differences were observed in stroke incidence [76].
Rosuvastatin
From the JUPITER trial, rosuvastatin was demonstrated to significantly reduce not only major cardiovascular events but also stroke risk (RRR: 48%; P = 0.002) in those apparently looked healthy individuals but with elevated high-sensitivity CRP levels. However, due to the low number of stroke events and the fact that JUPITER included individuals without a specific condition placing them at risk of stroke, the relevance for primary stroke prevention was limited [77].
Meta-analysis of the Preventive Effects
In consideration of above contradictory results, a large meta-analysis of 38 trials including 83,161 patients with a mean follow-up of 4.7 years showed that pre-stroke statins use was associated with a stroke RRR of 26% (P < 0.001) [78]. Another meta-analysis of 121,000 patients concluded that statins provided an obvious protection against all-cause mortality and non-hemorrhagic stroke [79]. Results from a meta-analysis of more than 170,000 participants showed a positive overall effect of statins treatment in all types of adults, even those with a relatively low risk for major vascular events [14].
CLINICAL THERAPEUTIC EFFECTS OF STATINS IN ISCHEMIC STROKE
In addition to the stroke risk reduction with pre-stroke statins therapy, evidences from clinical trials demonstrated that statins may also improve stroke prognosis (Table 2), even when administered after the event onset, but the effect of statins on stroke initial severity and subsequent functional outcomes is still controversial.
Table 2.
Summary of statins clinical trials in stroke severity and functional outcomes.
| Trials [Reference] | Inclusion Criteria | Statins | Dose (mg/d) | Control Group | Evaluation Criteria | Efficiency | Administration Time |
|---|---|---|---|---|---|---|---|
| MISTICS [83] | Cortical stroke | Simvastatin | 40 | Placebo | NIHSS | (46.4% vs. 17.9%, P = 0.022) by the third day |
3 - 12 h from symptom onset |
| North Dublin Study [84] |
Acute ischemic stroke |
Atorvastatin Pravastatin |
10 - 80 10 - 40 |
Statins-untreated | Decreased fatality | OR = 0.48; P = 0.05 at 1 year, OR = 0.23; P = 0.002 at 90 days, OR = 0.04; P = 0.003 at 7 days |
Pre-stroke |
| OR = 0.26; P < 0.001 at 1 year,
OR = 0.19; P < 0.001 at 90 days, OR = 0.12; P = 0.006 at 7 days |
Acute post-stroke (< 72 h) | ||||||
| Functional outcome (mRS 0 - 2) |
OR = 1.41; P = 0.37 at 1 year, OR = 2.21; P = 0.05 at 90 days, OR = 2.15; P = 0.07 at 7 days |
Pre-stroke | |||||
| OR = 1.69; P = 0.14 at 1 year, OR = 1.88; P = 0.09 at 90 days, OR = 2.06; P = 0.06 at 7 days |
Acute post-stroke (< 72 h) | ||||||
| Statins withdrawal for functional outcome [87] |
Hemispheric ischemic stroke within 24 h |
Statins withdrawal group | --- | Atorvastatin 20mg/d | mRS > 2 | OR = 4.66; P < 0.05 | Withdrawal for first 3 days after admission |
| END | OR = 8.7; P = 0.002 |
||||||
| Mean infarct volume | 63 ml (SE 10.01; P < 0.001) | ||||||
| Statins withdrawal for poststroke survival [88] | Acute ischemic stroke |
Statins prescription | --- | Statins use both before and during hospitalization | Poststroke survival | HR = 2.5; P < 0.001 | Withdrawal in hospital |
| No statins use before and during hospitalization | HR = 0.55; P < 0.001 | Initiation in the hospital | |||||
| No statins use before hospitalization | HR = 0.85; P < 0.001 | Before ischemic stroke | |||||
| No statins use before and during hospitalization | HR = 0.59; P < 0.001 | Before and during hospitalization | |||||
| Prestroke statins for initial severity [81] | Ischemic stroke | High dose (rosuvastatin; any other statins) |
40 80 |
No statins use | Mild stroke severity (NIHSS ≤ 5) |
OR = 3.297; 95% CI: 1.480 - 7.345 | Pre-stroke |
| Low to moderate dose | Stroke severity (NIHSS) |
Median [interquartile range]: 2 [4] P = 0.010 |
|||||
| Low to moderate dose (rosuvastatin; any other statins) | 0 - 40 0 - 80 |
No statins use | Mild stroke severity (NIHSS ≤ 5) | OR = 1.637; 95% CI: 1.156 - 2.319 | |||
| High dose | Stroke severity (NIHSS) | Median [interquartile range]: 4 [9] P = 0.010 |
|||||
| Prestroke statins on severity and outcome [82] | First-ever ischemic stroke |
Statins users | Non-statins users | Functional outcome (mRS) | OR = 0.76; P = 0.221 | Use before onset | |
| Initial severity (NIHSS) | Median[interquartile range]4 [7] versus 4 [9] P = 0.104 |
Note: HR = hazard ratio; ND = early neurological deterioration; mRS = modified Rankin Scale; NIHSS = national institute of health stroke scale; OR = odds ratio.
Improved Stroke Functional Outcome with Pre-stroke Therapy
Statins pretreatment improved clinical outcomes with a significant improvement in neurological deficit scores (NIHSS) over 1-month follow-up [80]. Patricia concluded that pretreatment with statins, at high (40 mg of rosuvastatin or 80 mg of any other statins) as well as at low to moderate (< 40 mg of rosuvastatin or < 80 mg of any other statins) doses, was associated with lower stroke severity (NIHSS ≤ 5) on admission among 969 ischemic stroke patients [81]. However, among 953 patients with first-ever ischemic stroke (127 with previous statins administration), prestroke statins therapy did not affect initial clinical severity and the association between prestroke statins treatment and better early functional outcomes after ischemic stroke was non-significant [82].
Improved Functional Outcome with Acute Post-stroke Therapy
In the MISTICS trial, simvastatin (40 mg/day) was given at 3-12 h from symptom onset. Patients treated with simvastatin had better functional outcomes at 3 days compared with placebo group (P = 0.02), but no neurological functional improvement was observed at 90 days [83]. Of the 448 ischemic stroke patients in North Dublin Study, modified Rankin Scale (mRS) score and fatality were assessed from 7 days to 1 year. Post-stroke statins therapy (within 72 h from stroke onset) was independently associated with improved survival and functional outcomes [84]. However, Insufficient data were available from randomized trials to indicate that statins were effective and safe in acute ischemic stroke and TIA, through analyzing eight randomized controlled trials - comparing statins of different type and dosage versus placebo or no treatment, administered within two weeks from stroke or TIA onset [85].
Meta-analysis of the Therapeutic Effects in Acute Stroke
Because the association between statins therapy and outcomes recovery after acute ischemic stroke from clinical studies is conflicting, the meta-analysis of observational and randomized trials by Danielle et al. investigated the relationship between statins therapy and outcome after acute ischemic stroke. They concluded that pre-stroke statins use was associated with improved functional outcomes (mRS score 0 to 2) at 90 days but not 1 year, and with reduced fatality at 90 days and 1 year among observational studies, In the single randomized controlled trial (SPARCL trial), statins treatment was associated with good 90-day functional outcomes. However, this association was not observed in thrombolysis-treated patients. Randomized controlled trials of acute post-stroke statins therapy in acute ischemic stroke were still needed [86].
Continuous Therapy in Acute Stroke
Although we still don’t have convincing data to support statins therapy for acute ischemic stroke, but it seems harmful to discontinue statins if the patient is already taking them before acute cerebral ischemic stroke. From 215 patients admitted within 24 hours of a hemispheric ischemic stroke, 89 patients with chronic statins treatment were randomly assigned within 24 hours of onset either to statins withdrawal for the first 3 days (n = 46) or to immediately receiving atorvastatin 20 mg/day (n = 43). This trial emphasized that statins treatment should be continued in the acute phase of ischemic stroke in consideration of increased brain damage and worsened functional outcomes with statins withdrawal during acute period [87]. Records from 12,689 patients admitted with ischemic stroke demonstrated that statins withdrawal even for a brief period was associated with worsened survival [88]. The guideline for the early management of patients with acute ischemic stroke from the American Heart Association/American Stroke Association (AHA/ASA) recommends that among patients already taking statins at the time of onset of ischemic stroke, continuation of statins therapy during the acute period is reasonable (Class IIa; Level of Evidence B) in 2013 [89].
DISCUSSION
In this review we have outlined the theoretical and clinical benefits of statins in ischemic stroke. The pleiotropic effects of statins offer new opportunity for the prevention and treatment of ischemic stroke. The guideline for the management of patients with acute ischemic stroke from the AHA/ASA recommended continuous statins therapy in the acute period of ischemic stroke among patients with pre-stroke statins therapy in 2013 [89]. Statins should be dubbed the most important medicines in ischemic stroke prevention and therapy since the introduction of aspirin.
Much effort has been taken to clarify the pleiotropic effect of statins, but the precise mechanisms responsible for these effects are still unclear. Further preclinical experimental data are required for better evaluation of statins. More experimental work is needed to illuminate the complex relationship between statins and angiogenesis in stroke recovery process, the mechanism of statins to facilitate EPC increment in acute stroke, and effective signaling pathways for antithrombotic effects of statins.
An increasing amount of clinical trials suggest that statins improve functional outcomes after ischemic stroke, but most are observational studies or sample sizes are limited. The potential bias from limited clinical trials is considered likely to reduce the estimated effect of statins. Therefore, we still need larger, randomized, placebo-controlled clinical trials to completely prove efficacy and safety of statins in acute ischemic stroke, especially the duration of pre-stroke and post-stroke statins therapy, as well as the effects of different doses and types on initial stroke severity and functional outcomes. Ongoing studies such as NeuSTART II, EUREKA may provide more valuable safety and efficacy information of statins therapy in acute ischemic stroke [90, 91]. However, we cannot neglect a fact that a large proportion of stroke patients survive stroke but die later of myocardial infarction rather than another stroke, or more stroke patients die from IHD than those from stroke. Consequently, it will be advisable to use statins therapy for stroke patients in consideration of these reasons.
In conclusion, new knowledge about statins has provided surprising insights into their pleiotropic beneficial effects in ischemic stroke, has offered new opportunities for prevention and treatment, and may lead to new candidate agents after aspirin for treatment of this life-threatening disease.
ACKNOWLEDGEMENTS
The authors thank Litao Li for his assistance in preparing this article.
Supported by grants from the National Natural Science Foundation of China (Grant no.81371287).
CONFLICT OF INTEREST
The authors declare that they have no conflict of interests.
References
- 1.Donnan G.A., Fisher M., Macleod M., Davis S.M. Stroke. Lancet. 2008;371(9624):1612–1623. doi: 10.1016/S0140-6736(08)60694-7. [DOI] [PubMed] [Google Scholar]
- 2.Dirnagl U., Iadecola C., Moskowitz M.A. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999;22(9):391–397. doi: 10.1016/S0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- 3.Endo A., Kuroda M., Tsujita Y. ML-236A, ML-236B, and ML-236C, new inhibitors of cholesterogenesis produced by Penicillium citrinium. J. Antibiot. 1976;29(12):1346–1348. doi: 10.7164/antibiotics.29.1346. [DOI] [PubMed] [Google Scholar]
- 4.Anderson T.J., Meredith I.T., Yeung A.C., Frei B., Selwyn A.P., Ganz P. The effect of cholesterol-lowering and antioxidant therapy on endothelium-dependent coronary vasomotion. N. Engl. J. Med. 1995;332(8):488–493. doi: 10.1056/NEJM199502233320802. [DOI] [PubMed] [Google Scholar]
- 5.Obi C., Wysokinski W., Karnicki K., Owen W.G., McBane R.D., II Inhibition of platelet-rich arterial thrombus in vivo: acute antithrombotic effect of intravenous HMG-CoA reductase therapy. Arterioscler. Thromb. Vasc. Biol. 2009;29(9):1271–1276. doi: 10.1161/ATVBAHA.109.190884. [DOI] [PubMed] [Google Scholar]
- 6.Rasmussen L.M., Hansen P.R., Nabipour M.T., Olesen P., Kristiansen M.T., Ledet T. Diverse effects of inhibition of 3-hydroxy-3-methylglutaryl-CoA reductase on the expression of VCAM-1 and E-selectin in endothelial cells. Biochem. J. 2001;360(Pt 2):363–370. doi: 10.1042/0264-6021:3600363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wagner A.H., Köhler T., Rückschloss U., Just I., Hecker M. Improvement of nitric oxide-dependent vasodilatation by HMG-CoA reductase inhibitors through attenuation of endothelial superoxide anion formation. Arterioscler. Thromb. Vasc. Biol. 2000;20(1):61–69. doi: 10.1161/01.ATV.20.1.61. [DOI] [PubMed] [Google Scholar]
- 8.Ward S., Lloyd J.M., Pandor A., Holmes M., Ara R., Ryan A., Yeo W., Payne A. A. A systematic review and economic evaluation of statins for the prevention of coronary events. Health Technol. Assess. 2007;11(1):160–iii-iv. doi: 10.3310/hta11140. [DOI] [PubMed] [Google Scholar]
- 9.Kawashima S., Yamashita T., Miwa Y., Ozaki M., Namiki M., Hirase T., Inoue N., Hirata K., Yokoyama M. HMG-CoA reductase inhibitor has protective effects against stroke events in stroke-prone spontaneously hypertensive rats. Stroke. 2003;34(1):157–163. doi: 10.1161/01.STR.0000048213.18751.52. [DOI] [PubMed] [Google Scholar]
- 10.Prinz V., Laufs U., Gertz K., Kronenberg G., Balkaya M., Leithner C., Lindauer U., Endres M. Intravenous rosuvastatin for acute stroke treatment: an animal study. Stroke. 2008;39(2):433–438. doi: 10.1161/STROKEAHA.107.492470. [DOI] [PubMed] [Google Scholar]
- 11.Sironi L., Cimino M., Guerrini U., Calvio A.M., Lodetti B., Asdente M., Balduini W., Paoletti R., Tremoli E. Treatment with statins after induction of focal ischemia in rats reduces the extent of brain damage. Arterioscler. Thromb. Vasc. Biol. 2003;23(2):322–327. doi: 10.1161/01.ATV.0000044458.23905.3B. [DOI] [PubMed] [Google Scholar]
- 12.White H.D., Simes R.J., Anderson N.E., Hankey G.J., Watson J.D., Hunt D., Colquhoun D.M., Glasziou P., MacMahon S., Kirby A.C., West M.J., Tonkin A.M. Pravastatin therapy and the risk of stroke. N. Engl. J. Med. 2000;343(5):317–326. doi: 10.1056/NEJM200008033430502. [DOI] [PubMed] [Google Scholar]
- 13.Shepherd J., Blauw G.J., Murphy M.B., Bollen E.L., Buckley B.M., Cobbe S.M., Ford I., Gaw A., Hyland M., Jukema J.W., Kamper A.M., Macfarlane P.W., Meinders A.E., Norrie J., Packard C.J., Perry I.J., Stott D.J., Sweeney B.J., Twomey C., Westendorp R.G., PROSPER study group. PROspective Study of Pravastatin in the Elderly at Risk Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet. 2002;360(9346):1623–1630. doi: 10.1016/S0140-6736(02)11600-X. [DOI] [PubMed] [Google Scholar]
- 14.Mihaylova B., Emberson J., Blackwell L., Keech A., Simes J., Barnes E.H., Voysey M., Gray A., Collins R., Baigent C., Cholesterol Treatment Trialists’ (CTT) Collaborators The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet. 2012;380(9841):581–590. doi: 10.1016/S0140-6736(12)60367-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Topol E.J. Intensive statin therapy--a sea change in cardiovascular prevention. N. Engl. J. Med. 2004;350(15):1562–1564. doi: 10.1056/NEJMe048061. [DOI] [PubMed] [Google Scholar]
- 16.Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet. 1994;344(8934):1383–1389. doi: 10.1016/S0140-6736(94)90566-5. [DOI] [PubMed] [Google Scholar]
- 17.Chen H., Ikeda U., Shimpo M., Shimada K. Direct effects of statins on cells primarily involved in atherosclerosis. Hypertens. Res. 2000;23(2):187–192. doi: 10.1291/hypres.23.187. [DOI] [PubMed] [Google Scholar]
- 18.Comparato C., Altana C., Bellosta S., Baetta R., Paoletti R., Corsini A. Clinically relevant pleiotropic effects of statins: drug properties or effects of profound cholesterol reduction? Nutr. Metab. Cardiovasc. Dis. 2001;11(5):328–343. [PubMed] [Google Scholar]
- 19.Liao J.K. Isoprenoids as mediators of the biological effects of statins. J. Clin. Invest. 2002;110(3):285–288. doi: 10.1172/JCI0216421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Aelst L, D’Souza-Schorey C. Rho GTPases and signaling networks. Genes Dev. 1997;, 11:2295–2322. doi: 10.1101/gad.11.18.2295. [DOI] [PubMed] [Google Scholar]
- 21.Laufs U., La Fata V., Plutzky J., Liao J.K. Upregulation of endothelial nitric oxide synthase by HMG CoA reductase inhibitors. 1998. [DOI] [PubMed]
- 22.Wassmann S, Laufs U, Müller K, Konkol C, Ahlbory K, Bäumer AT, Linz W, Böhm M, Nickenig G. Cellular antioxidant effects of atorvastatin in vitro and in vivo. Arterioscler. Thromb. Vasc. Biol. 2002;22:300–305. doi: 10.1161/hq0202.104081. [DOI] [PubMed] [Google Scholar]
- 23.Urbich C., Dernbach E., Zeiher A.M., Dimmeler S. Double-edged role of statins in angiogenesis signaling. Circ. Res. 2002;90(6):737–744. doi: 10.1161/01.RES.0000014081.30867.F8. [DOI] [PubMed] [Google Scholar]
- 24.Werns S.W., Walton J.A., Hsia H.H., Nabel E.G., Sanz M.L., Pitt B. Evidence of endothelial dysfunction in angiographically normal coronary arteries of patients with coronary artery disease. Circulation. 1989;79(2):287–291. doi: 10.1161/01.CIR.79.2.287. [DOI] [PubMed] [Google Scholar]
- 25.Janssens S., Flaherty D., Nong Z., Varenne O., van Pelt N., Haustermans C., Zoldhelyi P., Gerard R., Collen D. Human endothelial nitric oxide synthase gene transfer inhibits vascular smooth muscle cell proliferation and neointima formation after balloon injury in rats. Circulation. 1998;97(13):1274–1281. doi: 10.1161/01.CIR.97.13.1274. [DOI] [PubMed] [Google Scholar]
- 26.Kureishi Y., Luo Z., Shiojima I., Bialik A., Fulton D., Lefer D.J., Sessa W.C., Walsh K. The HMG-CoA reductase inhibitor simvastatin activates the protein kinase Akt and promotes angiogenesis in normocholesterolemic animals. Nat. Med. 2000;6(9):1004–1010. doi: 10.1038/79510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laufs U., Liao J.K. Post-transcriptional regulation of endothelial nitric oxide synthase mRNA stability by Rho GTPase. J. Biol. Chem. 1998;273(37):24266–24271. doi: 10.1074/jbc.273.37.24266. [DOI] [PubMed] [Google Scholar]
- 28.Mason J.C., Ahmed Z., Mankoff R., Lidington E.A., Ahmad S., Bhatia V., Kinderlerer A., Randi A.M., Haskard D.O. Statin-induced expression of decay-accelerating factor protects vascular endothelium against complement-mediated injury. Circ. Res. 2002;91(8):696–703. doi: 10.1161/01.RES.0000038151.57577.19. [DOI] [PubMed] [Google Scholar]
- 29.Ichiki T., Takeda K., Tokunou T., Iino N., Egashira K., Shimokawa H., Hirano K., Kanaide H., Takeshita A. Downregulation of angiotensin II type 1 receptor by hydrophobic 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors in vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 2001;21(12):1896–1901. doi: 10.1161/hq1201.099430. [DOI] [PubMed] [Google Scholar]
- 30.Hernández-Perera O., Pérez-Sala D., Soria E., Lamas S. Involvement of Rho GTPases in the transcriptional inhibition of preproendothelin-1 gene expression by simvastatin in vascular endothelial cells. 2000. [DOI] [PubMed]
- 31.Giannopoulos S., Katsanos A.H., Tsivgoulis G., Marshall R.S. Statins and cerebral hemodynamics. J. Cereb. Blood Flow Metab. 2012;32(11):1973–1976. doi: 10.1038/jcbfm.2012.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Endres M., Laufs U., Huang Z., Nakamura T., Huang P., Moskowitz M.A., Liao J.K. Stroke protection by 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase inhibitors mediated by endothelial nitric oxide synthase. Proc. Natl. Acad. Sci. USA. 1998;95(15):8880–8885. doi: 10.1073/pnas.95.15.8880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim H.H., Sawada N., Soydan G., Lee H.S., Zhou Z., Hwang S.K., Waeber C., Moskowitz M.A., Liao J.K. Additive effects of statin and dipyridamole on cerebral blood flow and stroke protection. J. Cereb. Blood Flow Metab. 2008;28(7):1285–1293. doi: 10.1038/jcbfm.2008.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Le Quan Sang K.H., Levenson J., Megnien J.L., Simon A., Devynck M.A. Platelet cytosolic Ca2+ and membrane dynamics in patients with primary hypercholesterolemia. Effects of pravastatin. Arterioscler. Thromb. Vasc. Biol. 1995;15(6):759–764. doi: 10.1161/01.ATV.15.6.759. [DOI] [PubMed] [Google Scholar]
- 35.Gaddam V, Li DY, Mehta JL. Anti-thrombotic effects of atorvastatin - an effect unrelated to lipid lowering. J. Cardiovasc. Pharmacol. Ther. 2002;7:247–253. doi: 10.1177/107424840200700408. [DOI] [PubMed] [Google Scholar]
- 36.Phipps R.P., Blumberg N. Statin islands and PPAR ligands in platelets. Arterioscler. Thromb. Vasc. Biol. 2009;29(5):620–621. doi: 10.1161/ATVBAHA.109.184648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shi J., Wang J., Zheng H., Ling W., Joseph J., Li D., Mehta J.L., Ponnappan U., Lin P., Fink L.M., Hauer-Jensen M. Statins increase thrombomodulin expression and function in human endothelial cells by a nitric oxide-dependent mechanism and counteract tumor necrosis factor alpha-induced thrombomodulin downregulation. Blood Coagul. Fibrinolysis. 2003;14(6):575–585. doi: 10.1097/00001721-200309000-00010. [DOI] [PubMed] [Google Scholar]
- 38.Fu Q., Wang J., Boerma M., Berbée M., Qiu X., Fink L.M., Hauer-Jensen M. Involvement of heat shock factor 1 in statin-induced transcriptional upregulation of endothelial thrombomodulin. Circ. Res. 2008;103(4):369–377. doi: 10.1161/CIRCRESAHA.108.174607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Essig M., Nguyen G., Prié D., Escoubet B., Sraer J.D., Friedlander G. 3-Hydroxy-3-methylglutaryl coenzyme A reductase inhibitors increase fibrinolytic activity in rat aortic endothelial cells. Role of geranylgeranylation and Rho proteins. Circ. Res. 1998;83(7):683–690. doi: 10.1161/01.RES.83.7.683. [DOI] [PubMed] [Google Scholar]
- 40.Liesz A., Suri-Payer E., Veltkamp C., Doerr H., Sommer C., Rivest S., Giese T., Veltkamp R. Regulatory T cells are key cerebroprotective immunomodulators in acute experimental stroke. Nat. Med. 2009;15(2):192–199. doi: 10.1038/nm.1927. [DOI] [PubMed] [Google Scholar]
- 41.Weber C., Erl W., Weber K.S., Weber P.C. HMG-CoA reductase inhibitors decrease CD11b expression and CD11b-dependent adhesion of monocytes to endothelium and reduce increased adhesiveness of monocytes isolated from patients with hypercholesterolemia. J. Am. Coll. Cardiol. 1997;30(5):1212–1217. doi: 10.1016/S0735-1097(97)00324-0. [DOI] [PubMed] [Google Scholar]
- 42.Chiloeches A., Usera F., Lasa M., Ropero S., Montes A., Toro M.J. Effect of mevalonate availability on the association of G-protein alpha-subunits with the plasma membrane in GH4C1 cells. FEBS Lett. 1997;401(1):68–72. doi: 10.1016/S0014-5793(96)01434-2. [DOI] [PubMed] [Google Scholar]
- 43.Weitz-Schmidt G., Welzenbach K., Brinkmann V., Kamata T., Kallen J., Bruns C., Cottens S., Takada Y., Hommel U. Statins selectively inhibit leukocyte function antigen-1 by binding to a novel regulatory integrin site. 2001. [DOI] [PubMed]
- 44.Diomede L., Albani D., Sottocorno M., Donati M.B., Bianchi M., Fruscella P., Salmona M. In vivo anti-inflammatory effect of statins is mediated by nonsterol mevalonate products. Arterioscler. Thromb. Vasc. Biol. 2001;21(8):1327–1332. doi: 10.1161/hq0801.094222. [DOI] [PubMed] [Google Scholar]
- 45.Rezaie-Majd A., Maca T., Bucek R.A., Valent P., Müller M.R., Husslein P., Kashanipour A., Minar E., Baghestanian M. 2002. [DOI] [PubMed]
- 46.Elkind M.S. Inflammation, atherosclerosis, and stroke. 2006. [DOI] [PubMed]
- 47.Albert M.A., Danielson E., Rifai N., Ridker P.M., PRINCE Investigators Effect of statin therapy on C-reactive protein levels: the pravastatin inflammation/CRP evaluation (PRINCE): a randomized trial and cohort study. JAMA. 2001;286(1):64–70. doi: 10.1001/jama.286.1.64. [DOI] [PubMed] [Google Scholar]
- 48.Dichtl W., Dulak J., Frick M., Alber H.F., Schwarzacher S.P., Ares M.P., Nilsson J., Pachinger O., Weidinger F. HMG-CoA reductase inhibitors regulate inflammatory transcription factors in human endothelial and vascular smooth muscle cells. 2003. [DOI] [PubMed]
- 49.Perona R., Montaner S., Saniger L., Sánchez-Pérez I., Bravo R., Lacal J.C. Activation of the nuclear factor-kappaB by Rho, CDC42, and Rac-1 proteins. Genes Dev. 1997;11(4):463–475. doi: 10.1101/gad.11.4.463. [DOI] [PubMed] [Google Scholar]
- 50.Chang J.H., Pratt J.C., Sawasdikosol S., Kapeller R., Burakoff S.J. The small GTP-binding protein Rho potentiates AP-1 transcription in T cells. Mol. Cell. Biol. 1998;18(9):4986–4993. doi: 10.1128/mcb.18.9.4986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Parmar K.M., Nambudiri V., Dai G., Larman H.B., Gimbrone M.A., Jr, García-Cardeña G. Statins exert endothelial atheroprotective effects via the KLF2 transcription factor. J. Biol. Chem. 2005;280(29):26714–26719. doi: 10.1074/jbc.C500144200. [DOI] [PubMed] [Google Scholar]
- 52.Sen-Banerjee S., Mir S., Lin Z., Hamik A., Atkins G.B., Das H., Banerjee P., Kumar A., Jain M.K. Kruppel-like factor 2 as a novel mediator of statin effects in endothelial cells. Circulation. 2005;112(5):720–726. doi: 10.1161/CIRCULATIONAHA.104.525774. [DOI] [PubMed] [Google Scholar]
- 53.Harrison D.G. Endothelial function and oxidant stress. Clin. Cardiol. 1997;20(11) Suppl. 2:II-11–II-17. [PubMed] [Google Scholar]
- 54.Wang Q., Tang X.N., Yenari M.A. The inflammatory response in stroke. 2007. [DOI] [PMC free article] [PubMed]
- 55.Lee T.S., Chang C.C., Zhu Y., Shyy J.Y. Simvastatin induces heme oxygenase-1: a novel mechanism of vessel protection. 2004. [DOI] [PubMed]
- 56.Bełtowski J., Wójcicka G., Jamroz A. Effect of 3-hydroxy-3-methylglutarylcoenzyme A reductase inhibitors (statins) on tissue paraoxonase 1 and plasma platelet activating factor acetylhydrolase activities. J. Cardiovasc. Pharmacol. 2004;43(1):121–127. doi: 10.1097/00005344-200401000-00018. [DOI] [PubMed] [Google Scholar]
- 57.Chen L., Haught W.H., Yang B., Saldeen T.G., Parathasarathy S., Mehta J.L. Preservation of endogenous antioxidant activity and inhibition of lipid peroxidation as common mechanisms of antiatherosclerotic effects of vitamin E, lovastatin and amlodipine. J. Am. Coll. Cardiol. 1997;30(2):569–575. doi: 10.1016/S0735-1097(97)00158-7. [DOI] [PubMed] [Google Scholar]
- 58.de Oliveira C.V., Funck V.R., Pereira L.M., Grigoletto J., Rambo L.M., Ribeiro L.R., Royes L.F., Furian A.F., Oliveira M.S. Atorvastatin withdrawal elicits oxidative/nitrosative damage in the rat cerebral cortex. Pharmacol. Res. 2013;71:1–8. doi: 10.1016/j.phrs.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 59.Chen J., Zhang C., Jiang H., Li Y., Zhang L., Robin A., Katakowski M., Lu M., Chopp M. 2005.
- 60.Chen J., Zhang Z.G., Li Y., Wang Y., Wang L., Jiang H., Zhang C., Lu M., Katakowski M., Feldkamp C.S., Chopp M. Statins induce angiogenesis, neurogenesis, and synaptogenesis after stroke. Ann. Neurol. 2003;53(6):743–751. doi: 10.1002/ana.10555. [DOI] [PubMed] [Google Scholar]
- 61.Sobrino T., Blanco M., Pérez-Mato M., Rodríguez-Yáñez M., Castillo J. Increased levels of circulating endothelial progenitor cells in patients with ischaemic stroke treated with statins during acute phase. 2012. [DOI] [PubMed]
- 62.Brouet A., Sonveaux P., Dessy C., Moniotte S., Balligand J.L., Feron O. Hsp90 and caveolin are key targets for the proangiogenic nitric oxide-mediated effects of statins. Circ. Res. 2001;89(10):866–873. doi: 10.1161/hh2201.100319. [DOI] [PubMed] [Google Scholar]
- 63.Vincent L., Soria C., Mirshahi F., Opolon P., Mishal Z., Vannier J.P., Soria J., Hong L. Cerivastatin, an inhibitor of 3-hydroxy-3-methylglutaryl coenzyme a reductase, inhibits endothelial cell proliferation induced by angiogenic factors in vitro and angiogenesis in in vivo models. Arterioscler. Thromb. Vasc. Biol. 2002;22(4):623–629. doi: 10.1161/01.ATV.0000012283.15789.67. [DOI] [PubMed] [Google Scholar]
- 64.Zhu X.Y., Daghini E., Chade A.R., Lavi R., Napoli C., Lerman A., Lerman L.O. Disparate effects of simvastatin on angiogenesis during hypoxia and inflammation. Life Sci. 2008;83(23-24):801–809. doi: 10.1016/j.lfs.2008.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sacks F.M., Pfeffer M.A., Moye L.A., Rouleau J.L., Rutherford J.D., Cole T.G., Brown L., Warnica J.W., Arnold J.M., Wun C.C., Davis B.R., Braunwald E. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. 1996. [DOI] [PubMed]
- 66.Shepherd J., Cobbe S.M., Ford I., Isles C.G., Lorimer A.R., MacFarlane P.W., McKillop J.H., Packard C.J. 1995. [DOI] [PubMed]
- 67.ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Major outcomes in moderately hypercholesterolemic, hypertensive patients randomized to pravastatin versus usual care: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT-LLT). JAMA. 2002;288:2998–3007. doi: 10.1001/jama.288.23.2998. [DOI] [PubMed] [Google Scholar]
- 68.Nakamura H., Arakawa K., Itakura H., Kitabatake A., Goto Y., Toyota T., Nakaya N., Nishimoto S., Muranaka M., Yamamoto A., Mizuno K., Ohashi Y., MEGA Study Group Primary prevention of cardiovascular disease with pravastatin in Japan (MEGA Study): a prospective randomised controlled trial. Lancet. 2006;368(9542):1155–1163. doi: 10.1016/S0140-6736(06)69472-5. [DOI] [PubMed] [Google Scholar]
- 69.Sever P.S., Dahlof B., Poulter N.R., Wedel H., Beevers G., Caulfield M., Collins R., Kjeldsen S.E., Kristinsson A. McInnes. G.T., Mehlsen, J., Nieminen, M., O'Brien, E., Ostergren, J., ASCOT investigators. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than average cholesterol concentrations, in the angloscandinavian cardiac outcomes trial-lipid lowering arm (ASCOTLLA): a multicentre randomised controlled tria. Lancet. 2003;361:1149–1158. doi: 10.1016/S0140-6736(03)12948-0. [DOI] [PubMed] [Google Scholar]
- 70.Athyros V.G., Papageorgiou A.A., Mercouris B.R., Athyrou V.V., Symeonidis A.N., Basayannis E.O., Demitriadis D.S., Kontopoulos A.G. Treatment with atorvastatin to the National Cholesterol Educational Program goal versus ‘usual’ care in secondary coronary heart disease prevention. The GREek Atorvastatin and Coronary-heart-disease Evaluation (GREACE) study. Curr. Med. Res. Opin. 2002;18(4):220–228. doi: 10.1185/030079902125000787. [DOI] [PubMed] [Google Scholar]
- 71.Koren M.J., Hunninghake D.B., ALLIANCE Investigators Clinical outcomes in managed-care patients with coronary heart disease treated aggressively in lipid-lowering disease management clinics: the alliance study. J. Am. Coll. Cardiol. 2004;44(9):1772–1779. doi: 10.1016/j.accreview.2004.12.117. [DOI] [PubMed] [Google Scholar]
- 72.Knopp R.H., d’Emden M., Smilde J.G., Pocock S.J. Efficacy and safety of atorvastatin in the prevention of cardiovascular end points in subjects with type 2 diabetes: the Atorvastatin Study for Prevention of Coronary Heart Disease Endpoints in non-insulin-dependent diabetes mellitus (ASPEN). Diabetes Care. 2006;29(7):1478–1485. doi: 10.2337/dc05-2415. [DOI] [PubMed] [Google Scholar]
- 73.Amarenco P., Bogousslavsky J., Callahan A., III, Goldstein L.B., Hennerici M., Rudolph A.E., Sillesen H., Simunovic L., Szarek M., Welch K.M., Zivin J.A., Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) Investigators High-dose atorvastatin after stroke or transient ischemic attack. N. Engl. J. Med. 2006;355(6):549–559. doi: 10.1056/NEJMoa061894. [DOI] [PubMed] [Google Scholar]
- 74.Heart Protection Study Collaborative Group MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360(9326):7–22. doi: 10.1016/S0140-6736(02)09327-3. [DOI] [PubMed] [Google Scholar]
- 75.Collins R., Armitage J., Parish S., Sleight P., Peto R., Heart Protection Study Collaborative Group Effects of cholesterol-lowering with simvastatin on stroke and other major vascular events in 20536 people with cerebrovascular disease or other high-risk conditions. Lancet. 2004;363(9411):757–767. doi: 10.1016/S0140-6736(04)15690-0. [DOI] [PubMed] [Google Scholar]
- 76.de Lemos J.A., Blazing M.A., Wiviott S.D., Lewis E.F., Fox K.A., White H.D., Rouleau J.L., Pedersen T.R., Gardner L.H., Mukherjee R., Ramsey K.E., Palmisano J., Bilheimer D.W., Pfeffer M.A., Califf R.M., Braunwald E., Investigators Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes: phase Z of the A to Z trial. JAMA. 2004;292(11):1307–1316. doi: 10.1001/jama.292.11.1307. [DOI] [PubMed] [Google Scholar]
- 77.Ridker P.M., Danielson E., Fonseca F.A., Genest J., Gotto A.M., Jr, Kastelein J.J., Koenig W., Libby P., Lorenzatti A.J., MacFadyen J.G., Nordestgaard B.G., Shepherd J., Willerson J.T., Glynn R.J., JUPITER Study Group Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. 2008. [DOI] [PubMed]
- 78.Corvol J.C., Bouzamondo A., Sirol M., Hulot J.S., Sanchez P., Lechat P. Differential effects of lipid-lowering therapies on stroke prevention: a meta-analysis of randomized trials. Arch. Intern. Med. 2003;163(6):669–676. doi: 10.1001/archinte.163.6.669. [DOI] [PubMed] [Google Scholar]
- 79.O’Regan C., Wu P., Arora P., Perri D., Mills E.J. Statin therapy in stroke prevention: a meta-analysis involving 121,000 patients. Am. J. Med. 2008;121(1):24–33. doi: 10.1016/j.amjmed.2007.06.033. [DOI] [PubMed] [Google Scholar]
- 80.Ford A.L., An H., D’Angelo G., Ponisio R., Bushard P., Vo K.D., Powers W.J., Lin W., Lee J.M. Preexisting statins use is associated with greater reperfusion in hyperacute ischemic stroke. 2011. [DOI] [PMC free article] [PubMed]
- 81.Martínez-Sánchez P., Fuentes B., Martínez-Martínez M., Ruiz-Ares G., Fernández-Travieso J., Sanz-Cuesta B.E., Cuéllar-Gamboa L., Díaz-Domínguez E., Díez-Tejedor E. Treatment with statin and ischemic stroke severity. 2013. [DOI] [PubMed]
- 82.Aboa-Eboulé C., Binquet C., Jacquin A., Hervieu M., Bonithon-Kopp C., Durier J., Giroud M., Béjot Y. Effect of previous statin therapy on severity and outcome in ischemic stroke patients: a population-based study. J. Neurol. 2013;260(1):30–37. doi: 10.1007/s00415-012-6580-9. [DOI] [PubMed] [Google Scholar]
- 83.Montaner J., Chacón P., Krupinski J., Rubio F., Millán M., Molina C.A., Hereu P., Quintana M., Alvarez-Sabín J. Simvastatin in the acute phase of ischemic stroke: a safety and efficacy pilot trial. Eur. J. Neurol. 2008;15(1):82–90. doi: 10.1111/j.1468-1331.2007.02015.x. [DOI] [PubMed] [Google Scholar]
- 84.Ní Chróinín D., Callaly E.L., Duggan J., Merwick Á., Hannon N., Sheehan Ó., Marnane M., Horgan G., Williams E.B., Harris D., Kyne L., McCormack P.M., Moroney J., Grant T., Williams D., Daly L., Kelly P.J. Association between acute statin therapy, survival, and improved functional outcome after ischemic stroke: the North Dublin Population Stroke Study. Stroke. 2011;42(4):1021–1029. doi: 10.1161/STROKEAHA.110.596734. [DOI] [PubMed] [Google Scholar]
- 85.Squizzato A., Romualdi E., Dentali F., Ageno W. Statins for acute ischemic stroke. Cochrane Database Syst. Rev. 2011;10(8):CD007551. doi: 10.1161/STROKEAHA.111.638940. [DOI] [PubMed] [Google Scholar]
- 86.Ní Chróinín D., Asplund K., Åsberg S., Callaly E., Cuadrado-Godia E., Díez-Tejedor E., Di Napoli M., Engelter S.T., Furie K.L., Giannopoulos S., Gotto A.M., Jr, Hannon N., Jonsson F., Kapral M.K., Martí-Fàbregas J., Martínez-Sánchez P., Milionis H.J., Montaner J., Muscari A., Pikija S., Probstfield J., Rost N.S., Thrift A.G., Vemmos K., Kelly P.J. Statin therapy and outcome after ischemic stroke: systematic review and meta-analysis of observational studies and randomized trials. Stroke. 2013;44(2):448–456. doi: 10.1161/STROKEAHA.112.668277. [DOI] [PubMed] [Google Scholar]
- 87.Blanco M., Nombela F., Castellanos M., Rodriguez-Yáñez M., García-Gil M., Leira R., Lizasoain I., Serena J., Vivancos J., Moro M.A., Dávalos A., Castillo J. Statin treatment withdrawal in ischemic stroke: a controlled randomized study. Neurology. 2007;69(9):904–910. doi: 10.1212/01.wnl.0000269789.09277.47. [DOI] [PubMed] [Google Scholar]
- 88.Flint A.C., Kamel H., Navi B.B., Rao V.A., Faigeles B.S., Conell C., Klingman J.G., Sidney S., Hills N.K., Sorel M., Cullen S.P., Johnston S.C. Statins use during ischemic stroke hospitalization is strongly associated with improved poststroke survival. 2012. [DOI] [PubMed]
- 89.Jauch E.C., Saver J.L., Adams H.P., Jr, Bruno A., Connors J.J., Demaerschalk B.M., Khatri P., McMullan P.W., Jr, Qureshi A.I., Rosenfield K., Scott P.A., Summers D.R., Wang D.Z., Wintermark M., Yonas H., American Heart Association Stroke Council. Council on Cardiovascular Nursing. Council on Peripheral Vascular Disease. Council on Clinical Cardiology Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44(3):870–947. doi: 10.1161/STR.0b013e318284056a. [DOI] [PubMed] [Google Scholar]
- 90.Elkind M.S. (Principal Investigator). NeuSTART II. Neuro-protection with Statin Therapy for Acute Recovery Trial (Neu START): A Phase 2 safety and pilot efficacy study of lovastatin for the treatment of acute ischemic stroke . from the Stroke Center Clinical Trials Registry: http://www.strokecenter.org/trials/clinicalstudies/neuroprotection-with-statin-therapy-for-acute-recovery-trial-neu-starta-phase-2-safety-and-pilot-efficacy-study-of-lovastatin-for-the-treatment-of-acute-ischemic-stroke-spotrias . [Accessed 24.08.12].
- 91.Hoe Heo J. (Principal Investigator). The Effects of Very Early Use of Rosuvastatin in Preventing Recurrence of Ischemic Stroke: EUREKA. NCT01364220 ClinicalTrials . from the Stroke Center Clinical Trials Registry: http://www.strokecenter.org/trials/clini-calstudies/the-effects-of-very-early-use-of-rosuvastatin-in-preventing-recurrence-of-ischemic-stroke . [Accessed 24.08.12 ].