Abstract
Decreased nitric oxide (NO) bioavailability underlies a number of cardiovascular pathologies, including hypertension. The shear stress exerted by flowing blood is the main determinant of NO release. Rap1 promotes integrin- and cadherin-mediated signaling. Here, we show that Rap1 is a critical regulator of NO production and endothelial function. Rap1 deficiency in murine endothelium attenuates NO production and diminishes NO-dependent vasodilation, leading to endothelial dysfunction and hypertension, without deleterious effects on vessel integrity. Mechanistically, Rap1 is activated by shear stress, promotes the formation of the endothelial mechanosensing complex—comprised of PECAM-1, VE-cadherin and VEGFR2- and downstream signaling to NO production. Our study establishes a novel paradigm for Rap1 as a regulator of mechanotransduction.
Keywords: mechanotransduction, nitric oxide, shear stress, small GTPase Rap1, vasodilation
Introduction
NO is a key regulator of endothelial homeostasis. A defect in NO production leads to endothelial dysfunction, a pathological condition linked to inflammation and atherosclerosis, perturbed endothelial barrier, progression of diabetes, and, in particular, vasomotor dysfunction and hypertension 1, 2. Endothelial NO synthase (eNOS) produces NO in the vasculature 3, 4, and its activity is regulated by Ca2+/calmodulin 5, binding of regulatory cofactors and posttranslational modifications, including phosphorylation events which sensitize eNOS to Ca2+ and stimulate NO production 1. Increased shear stress resulting from increased vascular smooth muscle-mediated vessel contraction is a potent stimulus of NO release, together with other relaxing factors, from endothelium 6. Thus, smooth muscle contraction and endothelium-dependent vasodilation are two major mechanisms controlling vascular tone. Decreased NO, a hallmark of endothelial dysfunction, contributes to pathogenesis of hypertension 3, 4.
Shear-stress-induced NO release from endothelium is regulated by mechanisms involving several mechanosensing cell-surface receptors 7, 8, 9, 10. Among those, shear-induced, ligand-independent transactivation of VEGFR2, acting as a component of endothelial junctional mechanosensing complex, triggers signaling culminating at eNOS phosphorylation at Ser1177 that leads to enhanced NO release 11, 12. Conversely, inhibition of VEGFR2 activation reduces shear-induced vasodilation in vivo 8, 9. While much progress has been made 6, the exact mechanisms of mechanotransduction underlying endothelial response are not fully understood 13.
Rap1, a ubiquitously expressed small GTPase, integrates signals from multiple receptors to control cell adhesion, polarity, and migration 14, 15. Two Rap1 isoforms, Rap1a and Rap1b, share 95% identity, and deletion of either gene leads to developmental cardiovascular defects 16, 17, impaired adhesion of various blood cell populations 16, 18, 19 and altered angiogenesis 20, 21 in mice. In cultured endothelial cells (ECs), Rap1 has been implicated in promoting cell–cell junction formation and vascular barrier 22, 23, 24, also via interactions with KRIT-1 25, a gene product mutated in Cavernous Cerebral Malformations (CCM), a human neurological disorder leading to hemorrhage and stroke 26. Therefore, Rap1 has been linked with human CV disease 17.
In addition to the described in vivo defects, our observation of frequently increased neonatal lethality and sudden death in endothelium-restricted Rap1 knockout mice suggested additional underlying defects in cardiovascular function of these mice. In this manuscript, we report that endothelium-specific deletion of both Rap1 isoforms leads to a severe impairment of NO-dependent vasodilation and, significantly, is sufficient to induce hypertension in vivo. We show that, mechanistically, Rap1 activity is required for transmission of shear-induced signals from the endothelial mechanosensing complex leading to eNOS activation and normal NO release. Because shear stress from flowing blood is the main determinant of NO release, our novel finding positions Rap1 as a key regulator of endothelial function. Furthermore, to our knowledge, this is the first report of Rap1 involvement in mechanotransduction in vivo.
Results and Discussion
EC-restricted Rap1-knockout mice develop hypertension
Endothelial lineage-specific deletion of both Rap1 isoforms leads to complete embryonic lethality due to hemorrhage around mid-gestation 17, 27. Deletion of all but one Rap1a allele (Tie2-Cre+/0; Rap1af/+Rap1bf/f; Rap1-ECKO) reduces the level of total Rap1 to about 5% of that in WT ECs and has a milder defect with ∽30% of animals surviving to adulthood with increased spontaneous lethality and poor survival following anesthesia, particularly in male mice. Upon dissection, we discovered enlarged hearts in Rap1-ECKO mice (Fig1A), similar to what we have recently observed in total Rap1b-knockout mice 28. Such pathological hypertrophy often results from increased vascular load and in Rap1b-knockout mice is accompanied by hypertension and increased smooth muscle contractility 28. Because endothelium is an important regulator of vascular tone, we examined whether Rap1 deficiency in endothelium is sufficient for elevated blood pressure in mice and found that, indeed, Rap1-ECKO mice were hypertensive (Fig1B). Rap1 has been implicated in promoting EC cell–matrix and cell–cell adhesion 29, and therefore, its significant reduction in ECs might be expected to lead to defects in EC junctions and endothelial barrier. However, we found that basal vascular permeability in vivo was not elevated in Rap1-ECKO mice (Fig1C) and ex vivo, cell–cell junctions appeared normal (Fig1D and Supplementary Fig S1A). Further, neither EC turnover (Fig1D and Supplementary Fig S1A) nor apoptosis (Fig1E and Supplementary Fig S1B) were altered in aortae of these mice. Therefore, we concluded that basal EC defects are not responsible for the observed phenotypes. Instead, we considered decreased NO, a hallmark of endothelial dysfunction, as an underlying cause of the elevated blood pressure in endothelium-specific Rap1 knockout mice.
Figure 1. Endothelial-specific Rap1 deficiency induces hypertension and cardiac left-ventricular hypertrophy in Tie2-Cre+/0; Rap1af/+Rap1bf/f (Rap1-ECKO) mice but no basal endothelial cell defects.
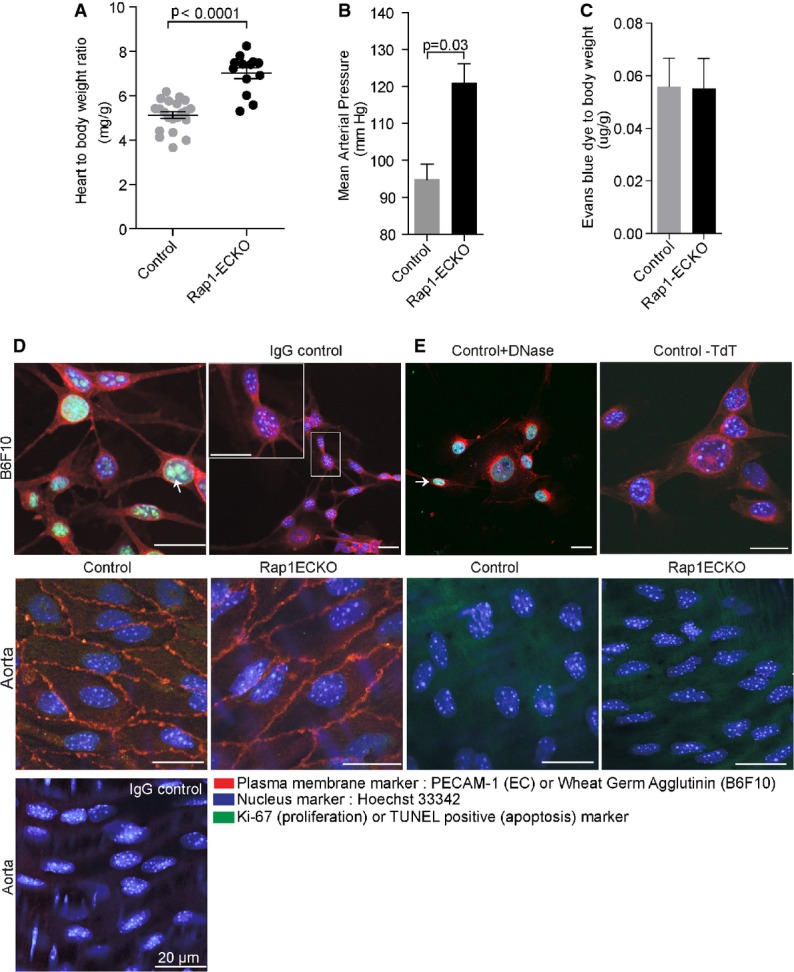
- A Quantification of heart-to-body weight ratios in controls and Rap1-ECKO mice (n ≥ 13).
- B Absolute values of telemetrically measured arterial blood pressure in Tie2-Cre-negative control and Rap1-ECKO mice (n = 4).
- C Unchanged basal permeability in Rap1-ECKO mice. Permeability of vessels was measured following injection of Evans blue/PBS into mice. Shown is the quantification of Evans blue dye remaining in lung tissue following perfusion with PBS (n = 4).
- D, E Lack of basal endothelial cell defects in Rap1-ECKO mice indicated by normal: PECAM-1 localization, Ki-67 staining (cell proliferation marker, D) and negative TUNEL reaction (apoptotic indicator, E) in Rap1ECKO aortic segments, stained en face. Top row: Representative images of B6F10 melanoma cells used as a positive control for proliferation and TUNEL reaction. Plasma membrane was stained with PECAM-1 or Wheat Germ Agglutinin (both shown in red). Nuclei are labeled with Hoechst 33342 (shown in blue). Goat and rabbit IgGs (D, top right) or cells without TdT labeling (E, top right) were used as negative controls. Representative images are shown (n = 3). Arrows indicate nuclei and nuclear bodies. Scale bars, 20 μm.
Source data are available online for this figure.
Endothelial dysfunction in EC-restricted Rap1-knockout mice
Because Tie2 promoter is expressed in the hematopoietic lineage 30 and Rap1 is excised in blood cells 27, it is possible that non-ECs may contribute to hypertension in Rap1-ECKO mice. Furthermore, vascular deficiency during development 17 may contribute to the vascular phenotype in adult mice. To avoid these complications, we generated inducible Rap1-knockout mice (Cadh5(PAC)-CreERT+/0;Rap1af/+Rap1bf/f; Rap1iΔEC), in which Rap1 is deleted in ECs with tamoxifen injections in neonates (Fig2A). We found that blood pressure was significantly increased in Rap1iΔEC mice (Fig2B), similar to that in Rap1-ECKO mice (Fig1B). Because endothelial dysfunction leads to hypertension 3, 4, we hypothesized that a defect in NO release underlies hypertension in endothelial Rap1-deficient mice. Therefore, we examined ex vivo NO-dependent vasodilation in aortic segments, where NO is the major vasodilating substance. We found that ACh-induced vessel dilation was significantly reduced in Rap1iΔEC aortic segments preconstricted with thromboxane analog U46619 (Fig2C and Supplementary Table S1). To distinguish whether Rap1 regulates NO release from ECs or NO sensitivity of SM, we blocked NO release with L-nitro-arginine methyl ester (L-NAME) and measured vasodilation in the presence of sodium nitroprusside (SNP). We found that the extent of SNP-induced vasodilation of aortic segments was not affected by Rap1-deficiency (Fig2D and Supplementary Table S2) compared to controls. Although SNP-induced vessel relaxation appeared decreased in Rap1iΔEC versus WT segments at the lowest dose of Ach, the difference was not statistically significant (Supplementary Fig S2). These results indicate that deficiency of both Rap1 isoforms leads to a defect in NO bioavailability in an EC-endogenous manner and suggest that Rap1 is required for optimal NO release and normal vascular tone. In contrast, endothelial deletion of Rap1b isoform alone leads to a more moderate defect in vasodilation and is not sufficient for blood pressure elevation 28. Cumulatively, these data show that genetic Rap1 deficiency in endothelium leads to endothelial dysfunction, which may be a primary mechanism explaining hypertension in these mice.
Figure 2. Hypertension and decreased NO-dependent vasodilation in Cadh5(PAC)-CreERT-Rap1 (Rap1iΔEC) mice.
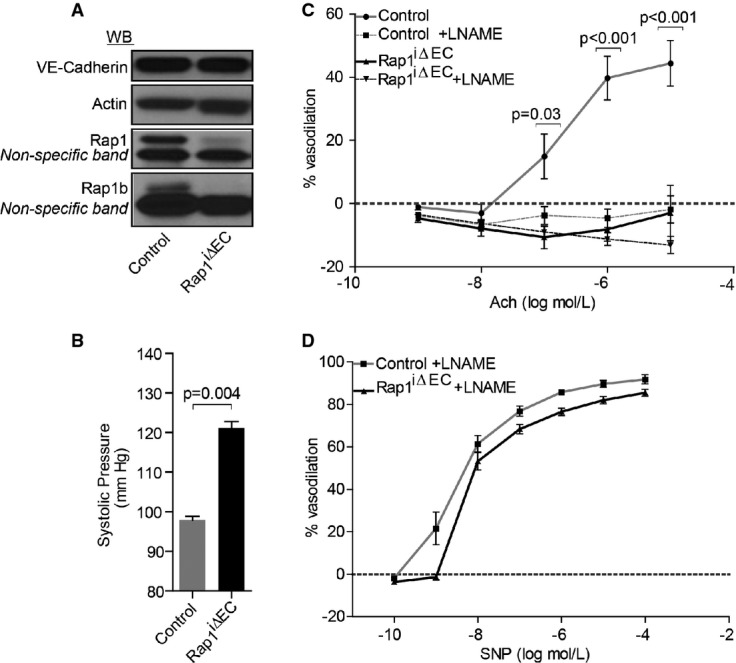
- A Representative immunoblot showing complete Rap1 excision in Rap1iΔEC mice, compared with Cre-negative controls. VE-cadherin is included as an EC marker (top blot).
- B Tail-cuff measurements of systolic blood pressure in Rap1iΔEC and control mice. Values are means ± s.e.m. (n = 3).
- C, D Decreased NO-dependent vasodilation of preconstricted aortae from Rap1iΔEC mice. Dose-dependent relaxation of control and Rap1iΔEC aortic segments preconstricted with U46619 in response to acetylcholine (ACh) in the absence or presence of L-NAME (C), and in response to sodium nitroprusside (SNP) in the presence of L-NAME (D) (n ≥ 3).
Source data are available online for this figure.
Rap1 is required for fluid shear-induced signaling leading to optimal NO release
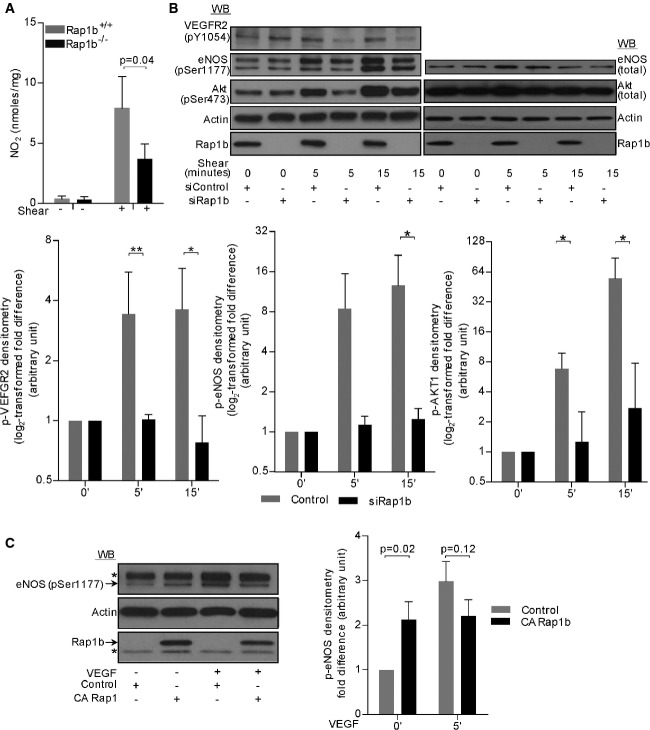
Blood flow-induced fluid shear stress is the main physiological stimulus regulating EC-dependent NO release in vivo 6. Therefore, we examined the effect of Rap1 deficiency on EC-dependent NO release in response to shear stress. Since Rap1b is the main Rap1 isoform in ECs 27, we used ECs isolated from Rap1b-KO mice as a model of Rap1-deficient ECs. Rap1b-KO ECs were subjected to shear stress at 10–15 dynes/cm2 using a cone viscometer, and NO release was quantified and compared to wild-type ECs treated in the same manner (Fig3A). In the absence of shear stress stimulus, both wild-type and Rap1b-KO ECs showed negligible NO production that was enhanced with shear stress several fold in wild-type but not in Rap1b-KO ECs.
Figure 3. Rap1 is required for shear-induced signaling leading to normal NO production.
- Shear-induced NO release from cultured, quiescent or sheared ECs isolated from Rap1b-deficient or WT mice measured by nitrite-dependent chemiluminescence using an NO Analyzer. Shown are mean values of concentrations calculated using authentic standards that were normalized to total protein in each sample (n = 3).
- Shear-induced VEGFR2 activation and signaling to NO production in cultured, HUVECs transfected with control, scrambled siRNA or Rap1b siRNA. Representative immunoblots of phosphorylated and total protein expression (top panel) and quantification of fold change in shear-induced phosphorylation of signaling molecules in Rap1b-deficient and control EC (bottom panel). Actin is shown as a loading control. Values are means ± s.e.m. (n = 3). *P < 0.05; **P < 0.01.
- Rap1 GOF experiment: overexpression of constitutively active (CA), Rap1b[G12V] mutant leads to elevated eNOS/Ser1177 phosphorylation in basal, but not VEGF-stimulated ECs. Representative immunoblot (top panel) and quantification of the phospho-eNOS band (bottom panel), normalized to actin. Exogenous expression of HA-tagged CA Rap1b was confirmed by Rap1b blot (top band) and HA immunoblot (data not shown). Values are means ± s.e.m. (n = 3). * indicates a non-specific band.
Source data are available online for this figure.
Sensed by a number of endothelial mechanoreceptors 6, including endothelial junctional mechanosensory complex comprising PECAM-1, VE-cadherin and VEGFR2, shear stress leads to Akt-dependent eNOS/Ser1177 phosphorylation 7, 8, 9, 10. Inhibition of VEGFR2 reduces shear-induced vasodilation in vivo 8, 9. Therefore, we examined shear-induced, ligand-independent VEGFR2 activation in a human EC model of Rap1 deficiency: HUVECs transfected with siRap1b 27. Time-dependent pVEGFR2 was evident in control but not in Rap1-deficient human ECs, which failed to increase phosphorylation of the receptor and Akt-S473 and eNOS-S1177 (Fig3B and C and Supplementary Fig S3A). Conversely, overexpression of a constitutively active mutant of Rap1b, Rap1b[G12V] led to an increased eNOS-S1177 phosphorylation in unstimulated HUVECs (Fig3C) to the level observed in VEGF-stimulated control cells, consistent with Rap1 and VEGFR2 acting in the same pathway leading to eNOS phosphorylation.
Consistent with decreased shear-induced VEGFR2 activation in Rap1-deficient ECs, association between VEGFR2 and p85 subunit of PI3K was decreased in these cells (Fig4A), which may explain the decreased phosphorylation of Akt-S473. In contrast, VE-cadherin association with p85 was constitutive and not affected by Rap1-deficiency (Fig4A). Together, these data show that Rap1 mediates fluid shear-induced recruitment of VEGFR2 to VE-cadherin-p85 complex and VEGFR2 activation promoting downstream signaling to phosphorylation-dependent eNOS activation and NO release from ECs.
Figure 4. Rap1 is required for shear-induced formation of the junctional mechanotransduction complex and eNOS activation.
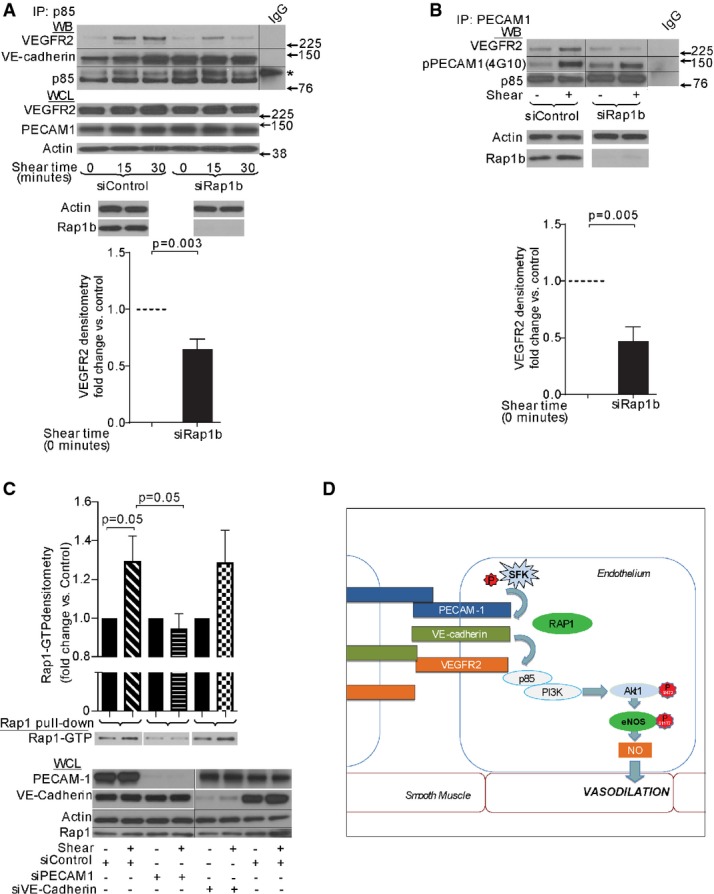
- A, B Interaction between VEGFR2 and p85-PI(3)K (A) or PECAM-1 (B) in HUVECs transfected with control, scrambled siRNA or Rap1b siRNA. Representative immunoblots (top panels) and quantification (bottom panels) of VEGFR2 in p85-PI(3)K (A), or PECAM-1 (B) immunoprecipitations from EC lysates. Basal and shear-induced (15-min treatment) levels are shown. Shear-induced PECAM-1 phosphorylation, detected by anti-phosphotyrosine mAb, 4G10, is not affected by siRap1b (B, middle row). p85 is shown as loading control. WCL: Immunoblots of EC lysates are shown to illustrate equal protein loading (actin, VEGFR2, PECAM-1 and VE-cadherin) or Rap1b knockdown efficiency and specificity. Values are means ± s.e.m. (n ≥ 4). * indicates a non-specific band. Black lines indicate where the PVDF membrane was cut to blot with specific antibodies, as indicated.
- C Shear-induced activation of Rap1 is blocked by siPECAM-1, but not siVE-cadherin. Quantification (top panel) and representative Rap1 immunoblot (middle panel) of RalGDS-bound Rap1-GTP from lysates of HUVECs transfected with control, PECAM-1 or VE-cadherin-specific siRNA and sheared for 1 min. Bottom panel, top row: representative PECAM-1 and VE-cadherin immunoblot demonstrating siRNA knockdown efficiency in transfected HUVECs. Values are means ± s.e.m. (n = 3). Black lines indicate where the PVDF membrane was cut to blot with specific antibodies, as indicated.
- D A model of Rap1 function in transducing shear stress signals leading to phosphorylation-dependent eNOS activation. Upon shear stress, activated downstream from Src-family kinase (SFK) and PECAM-1 52, Rap1 promotes increased association between PECAM-1 and VEGFR2, and ligand-independent VEGFR2 transactivation in the junctional mechanosensing complex 9. Such ligand-independent VEGFR2 activation promotes signaling to its downstream effectors, pAkt1 and eNOS, resulting in increased NO release and NO-dependent vasodilation 8.
Source data are available online for this figure.
Function of Rap1 in the mechanosensing complex
Our results suggest that under conditions of shear, Rap1 acts upstream from VEGFR2 promoting its activation. VEGFR2 activation in response to shear stress occurs as a result of association with PECAM-1, and, together with VE-cadherin, the three receptors form a mechanosensory complex sufficient to confer EC responsiveness to flow 9, 10. To determine the molecular basis of decreased shear-induced VEGFR2 activation in Rap1-deficient ECs, we analyzed association between PECAM-1 and VEGFR2 and found it was decreased in Rap1-deficient ECs compared with wild-type cells (Fig4B). Phosphorylation of tyrosines within the PECAM-1 cytoplasmic domain by Src-family tyrosine kinase Fyn modulates shear stress-induced activation of eNOS 10, 31; however, it was unchanged in Rap1-deficient cells under shear stress (Fig4B, top blot), suggesting that Rap1 acts downstream from PECAM-1.
To examine how Rap1 is involved in mechanosensing, we measured the effect of shear stress on Rap1 activity level. There is a considerable level of active Rap1 in quiescent ECs (Fig4C, Supplementary Fig S3A), which was transiently yet significantly increased in response to shear stress, with a peak within 30 s from initiation of flow (Fig4C). Such rapid activation of Rap1 has been reported to occur in hematopoietic cells in response to turbulence 32. In endothelium, this timeframe is consistent with flow-induced VEGFR2 transactivation 9. Further, to investigate where in the sequence of events mediating EC mechanosensing Rap1 becomes activated, we analyzed Rap1 activity upon siRNA knockdown of PECAM-1 or VE-cadherin. We found that shear-induced Rap1 activation was attenuated in PECAM-1-deficient but not in VE-cadherin-deficient ECs (Fig4C).
Based on our current report and previous findings in isolated cells, we propose the following model (Fig4D). In response to shear stress, Rap1 is rapidly and transiently activated downstream from PECAM-1, and through a GEF likely distinct from Epac, as treatment of ECs with 8-pCPT-2′-O-Me-cAMP (007), a cyclic adenosine monophosphate analog that directly activates Epac, failed to induce eNOS activation (Supplementary Fig S3B) 33. Active Rap1 promotes association of PECAM-1 with VEGFR2, which leads to ligand-independent VEGFR2 transactivation and triggers signaling for NO release to drive vasodilation. Rap1-dependent regulation of mechanotransduction pathways in the vasculature has not been previously considered. However, published data suggest that this novel paradigm of Rap1 involvement in transducing mechanical signals may also be applied to other cell types; Rap1 activation in response to turbulence has been reported in hematopoietic cells 32 and in response to stretching in permeabilized fibroblasts 34. It will be important, however, to establish in vivo relevance of Rap1 mechanotransduction in these cell types. While exact molecular mechanisms of Rap1 mechanotransduction in endothelium need to be elucidated, our report reveals a physiological significance of this function: release of NO in response to shear stress of flowing blood.
Novel paradigms of Rap1 signaling
Previous studies of Rap1 function in ECs have shown its importance in promoting integrin- and cadherin-mediated adhesion 29, functions important for angiogenesis and maintenance of vascular integrity 17, 35. In vivo analysis of endothelium-restricted Rap1-deficient mice demonstrates that Rap1 is particularly important for signaling by the adhesion molecules rather than their adhesive functions. Consistently, Rap1-deficient ECs are able to form grossly normal monolayers in vivo (Fig1D and E and Supplementary Fig S1) without a deleterious effect on vessel permeability (Fig1C) or gross vessel morphology. In contrast, other signaling functions of adhesion receptors are severely perturbed, as we demonstrate here in the context of shear stress sensing by the mechanotransduction complex; Rap1 acting downstream from PECAM-1 and upstream from VE-cadherin is critical for VEGFR2 transactivation and signaling to NO release. Interestingly, we have previously demonstrated that Rap1 acting upstream from ligand-dependent VEGFR2 activation promotes angiogenesis in a process dependent on integrin αvβ3 27. Therefore, Rap1-dependent-signaling converging on VEGFR2 activation is required for multiple EC responses. In addition, our novel discovery of Rap1's involvement in transducing mechanical signals may help explain other Rap1-related phenotypes; specifically, the role of Rap1 in hemodynamic regulation of vessel stability may be relevant for cranial hemorrhages observed in CCM and in a mouse model of Rap1 deficiency 17, 26. Furthermore, the implications of our findings may reach beyond the cardiovascular system as Rap1 is ubiquitously expressed and may be involved in transmitting mechanical forces in other tissues.
Functional significance of our findings for human health
The significance of our findings is underscored by the phenotype of Rap1-deficient mice, which includes endothelial dysfunction and hypertension. The extent of the defect: severely diminished vasodilation in aortic segments from Rap1iΔEC mice (Fig2C) and sudden lethality of ∽50% young male mice suggest that this may be physiologically the most critical Rap1 function in the cardiovascular system described so far 16, 18, 19, 20, 36, 37. Our studies with human ECs implicate Rap1 as an important regulator of NO signaling and suggest that perturbation of Rap1 activity may be linked with human disease. Interestingly, a genome-wide expression profiling study reported upregulated expression of a negative regulator of Rap1 activity, Rap1GAP, in lungs of patients with idiopathic pulmonary hypertension 38, a change that would be expected to lead to decreased Rap1 activity and increased airway resistance. Furthermore, endothelial dysfunction, in addition to hypertension, is linked with other endothelial pathologies, including inflammation, atherosclerosis and progression of diabetes. While additional studies exploring these functional consequences of endothelial dysfunction are required, and the exact Rap1 function in different vascular beds should be examined, our current study defines a novel framework for analysis of Rap1 function in the cardiovascular system and opens up the possibility for new therapeutic targets.
In conclusion, Rap1 critically regulates eNOS, NO release and endothelial function and plays a key role in maintenance of normal vascular tone. Granted the significance of NO for multiple cardiovascular functions, our findings suggest a broader impact of Rap1 signaling in the cardiovascular system. Further, by identifying mechano-effector functions of Rap1, our study also defines a novel conceptual framework for analysis of Rap1 biology.
Materials and Methods
Chemicals
Unless indicated, chemicals were purchased from Sigma-Aldrich. RNAiMAX was from Life Technologies. Antibodies used are described in Supplementary Methods.
Genetic mouse models
All mouse procedures were performed according to approved Medical College of Wisconsin Institutional Animal Use and Care Committee protocol 00001206. Generation of endothelial lineage-restricted Rap1 knockout mice (Tie2-Cre+/0;Rap1af/+Rap1bf/f; Rap1-ECKO) has been previously described 27. Inducible endothelial-restricted (Rap1iΔEC) mice were obtained by crossing mice containing floxed Rap1a and Rap1b alleles (rap1af/frap1bf/f mice) 39 with Cdh5(PAC)-CreERT2 mice and inducing Cre activity with tamoxifen injections, as previously described 40. Mice were euthanized by intraperitoneal injection of ketamine (100 mg/kg) and xylazine (10 mg/kg) followed by cervical dislocation. Because estrogen is known to have protective effects on regulation of blood pressure and, in particular release of NO 41, 3- to 6-month-old Rap1-ECKO and 2- to 4-month-old Rap1iΔEC male mice on mixed C57Bl6/129 Sv/Ev genetic background were used for blood pressure measurements and vasodilation studies.
Heart isolation
Isoflurane-anesthetized mice were weighed, and hearts were perfused with PBS. Dissected hearts were thoroughly rinsed in PBS, blotted quickly and weighed, and heart-to-body weight ratio was calculated 42.
Blood pressure measurements
Blood pressure and heart rate measurements were performed using telemetry and/or tail cuff, as described previously 28, 43, 44 and in Supplementary Methods. Tail-cuff measurement was performed because of the poor surgery survival of Rap1-deficient mice.
In vivo lung vessel permeability
Vascular permeability to Evans blue-labeled BSA was determined essentially as described earlier 45. Briefly, sterile 0.5% Evans blue/4% BSA in PBS was injected retro-orbitally into deeply anesthetized mice. After 1 h, mice were perfused with 5 mM EDTA/PBS, lungs were harvested and Evans blue dye was extracted with formamide at 60°C for 24 h. Concentration of the dye was determined by measuring absorbance at 620 nm, and the amount of Evans blue was calculated from the prepared standard curve.
Analysis of endothelial proliferation and apoptosis in isolated vessels
En face immunohistochemical and TUNEL staining was performed as described in Supplementary Methods 46.
Confocal microscopy
En face preparations and B6F10 cells were imaged using Laser Scanning Olympus FluoView1000 confocal microscope with 60×/1.2 water immersion objective lens. Images were recorded from five random fields within a sample, and at least 100 cells were analyzed using Olympus confocal software.
Endothelium-dependent relaxation of aortic rings in response to acetylcholine
Vasodilation was measured as previously described 28, 47. Briefly, the thoracic aorta from control and Rap1-mutant mice was dissected and placed in Krebs physiological saline aerated with 21%O2/5%CO2. 1.5-mm segments were mounted on parallel pins in conventional myographs and maintained at optimal tension in Krebs buffer for 60 min at 37°C. The rings were preconstricted with 10 nM U46619, exposed to increasing concentration of acetylcholine in the absence or presence of 100 μM L-NAME, and changes in vascular tension were recorded.
Cell culture, shear stress experiments and transfections
ECs isolated from neonatal Rap1b−/− mouse lungs 48 were used as cellular models of Rap1 deficiency in ECs, as Rap1b is the predominant Rap1 isoform in these cells 27. To demonstrate relevance for human biology, human umbilical vein ECs (HUVECs) were used, as an established model to study NO release and signaling in response to shear 9. HUVECs were isolated in-house from discarded umbilical cords obtained from local hospitals. Because the tissue was completely de-identified at the hospitals, our use does not constitute human subject research and therefore does not require approval of a local ethics review board. As the discard tissue came de-identified from multiple sources, we are unable to verify consent. Both mouse and human ECs were cultured in VascuLife EnGS Medium Complete (Lifeline Cell Technology). Mouse ECs were used for shear-induced NO release studies 49. For biochemical analysis, 30–40% confluent cultures HUVECs, passage 3 or fewer, were transfected with 50 nM Rap1b, PECAM-1 or VE-cadherin siGENOME siRNA pool (Dharmacon) or with scrambled siRNA using Opti-MEM medium and RNAiMAX reagent (Life Technologies) for 6 h and cultured for additional 48 h in Vasculife EnGS complete medium, until cells reached confluency. ECs were then serum-starved for 4 h in Vasculife EnGS basal medium, and NO release or mechanosignaling was analyzed as follows. For analysis of mechanotransduction, HUVECs were placed in Vasculife EnGS basal medium with 5% dextran and, using a cone viscometer, subjected to shear stress at 10–15 dynes/cm2 for indicated times. For a gain-of-function (GOF) experiment, HUVECs were transduced with lentiviral particles expressing constitutively active (CA) Rap1 (Rap1b[G12V] mutant) at five multiplicity of infection, cultured for 48 h including 6 h of starvation, as described above and lysed (no treatment) or stimulated with 40 ng/ml VEGF A, a potent inducer of eNOS phosphorylation 11 for 5 min. Experiments were terminated by cell lysis on ice followed by immunoprecipitations, Western blotting and Rap1 activity assay 50 as described in Supplementary Methods.
Measurement of nitric oxide concentration
NO release from sheared mouse lung ECs was analyzed by monitoring 4,5-diaminofluorescein-2 triazole (DAF-2T) formation from DAF-2 diacetate (DAF-2DA) by chemiluminescence methodology using an NO Analyzer 51. Detailed experimental procedure is described in Supplementary Methods.
Statistical analysis
Mean values and standard error of mean (s.e.m.) were obtained from three or more independent experiments, as indicated. Statistical significance of group differences was determined using two-sample Student's t-test (Figs1A–C and 2B), one-sample t-test (Figs3C and 4A and B) and repeated measures ANOVA (Figs2C and D and 4C), as appropriate for the experimental design. The Holm–Sidak adjustment for multiple comparisons was used within each plot. The assumptions of the tests were evaluated using tests of normality and equal variance; in cases where data did not follow Gaussian distribution, biologically meaningful Box–Cox family transformations were examined. Specifically, densitometry values were log2-transformed before statistical analysis. For analysis of shear-stress-induced signaling data, within each experiment, fold change in phosphorylation induction between Rap1-deficient and control values were calculated for each condition and such obtained values were statistically analyzed as described above. All tests were two-sided, and a 5% overall significance level was used.
Acknowledgments
We thank R. Adams (Cancer Research UK) for providing and T. Byzova (Cleveland Clinic) for breeding Cdh5-CreERT2 mice, P. Newman (Blood Research Institute) for anti-PECAM-1 antibodies, W. Campbell and J. Kelliher for assistance with blood pressure measurements, J. Idsvoog for animal care assistance and P. Joseph for proofreading the manuscript. This work was supported by American Heart Association grant (0950118G; to M.C.-W.) and the National Institutes of Health grants (HL111583 to M.C.-W. and HL096647 to D.X.Z.).
Author contributions
MC-W conceived of experiments. SL, MS, DXZ and MC-W designed the research. SL and MC-W analyzed the data. XZ, YN and DXZ performed and analyzed vasodilation experiments. SL and YN performed and analyzed blood pressure measurements. MS performed and analyzed in vivo permeability experiments. SL performed and analyzed endothelial cell experiments. SL and JV-V performed and analyzed nitric oxide measurement experiments. AS statistically analyzed data. MC-W wrote the manuscript. SL, AS, JV-V and DXZ critically read and edited the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Supporting Information
Supplementary Information
Source Data for Supplementary Figure S3
Review Process File
Source Data for Figure 1A–C
Source Data for Figure 2
Source Data for Figure 3
Source Data for Figure 4
References
- Forstermann U, Sessa WC. Nitric oxide synthases: regulation and function. Eur Heart J. 2012;33:829–837. doi: 10.1093/eurheartj/ehr304. , 837a–837d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widlansky ME, Gokce N, Keaney JF, Jr, Vita JA. The clinical implications of endothelial dysfunction. J Am Coll Cardiol. 2003;42:1149–1160. doi: 10.1016/s0735-1097(03)00994-x. [DOI] [PubMed] [Google Scholar]
- Huang PL, Huang Z, Mashimo H, Bloch KD, Moskowitz MA, Bevan JA, Fishman MC. Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature. 1995;377:239–242. doi: 10.1038/377239a0. [DOI] [PubMed] [Google Scholar]
- Shesely EG, Maeda N, Kim HS, Desai KM, Krege JH, Laubach VE, Sherman PA, Sessa WC, Smithies O. Elevated blood pressures in mice lacking endothelial nitric oxide synthase. Proc Natl Acad Sci USA. 1996;93:13176–13181. doi: 10.1073/pnas.93.23.13176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel T, Feron O. Nitric oxide synthases: which, where, how, and why? J Clin Invest. 1997;100:2146–2152. doi: 10.1172/JCI119750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn C, Schwartz MA. Mechanotransduction in vascular physiology and atherogenesis. Nat Rev Mol Cell Biol. 2009;10:53–62. doi: 10.1038/nrm2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K-D, Li Y-S, Kim M, Li S, Yuan S, Chien S, Shyy JY-J. Mechanotransduction in response to shear stress. J Biol Chem. 1999;274:18393–18400. doi: 10.1074/jbc.274.26.18393. [DOI] [PubMed] [Google Scholar]
- Jin Z-G, Ueba H, Tanimoto T, Lungu AO, Frame MD, Berk BC. Ligand-independent activation of vascular endothelial growth factor receptor 2 by fluid shear stress regulates activation of endothelial nitric oxide synthase. Circ Res. 2003;93:354–363. doi: 10.1161/01.RES.0000089257.94002.96. [DOI] [PubMed] [Google Scholar]
- Tzima E, Irani-Tehrani M, Kiosses WB, Dejana E, Schultz DA, Engelhardt B, Cao G, DeLisser H, Schwartz MA. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature. 2005;437:426–431. doi: 10.1038/nature03952. [DOI] [PubMed] [Google Scholar]
- Fleming I, Fisslthaler B, Dixit M, Busse R. Role of PECAM-1 in the shear-stress-induced activation of Akt and the endothelial nitric oxide synthase (eNOS) in endothelial cells. J Cell Sci. 2005;118:4103–4111. doi: 10.1242/jcs.02541. [DOI] [PubMed] [Google Scholar]
- Fulton D, Gratton JP, McCabe TJ, Fontana J, Fujio Y, Walsh K, Franke TF, Papapetropoulos A, Sessa WC. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature. 1999;399:597–601. doi: 10.1038/21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399:601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- Conway D, Schwartz MA. Lessons from the endothelial junctional mechanosensory complex. F1000 Biol Rep. 2012;4:1. doi: 10.3410/B4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frische EW, Zwartkruis FJT. Rap1, a mercenary among the Ras-like GTPases. Dev Biol. 2010;340:1–9. doi: 10.1016/j.ydbio.2009.12.043. [DOI] [PubMed] [Google Scholar]
- Gloerich M, Bos JL. Regulating Rap small G-proteins in time and space. Trends Cell Biol. 2011;21:615–623. doi: 10.1016/j.tcb.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Chrzanowska-Wodnicka M, Smyth SS, Schoenwaelder SM, Fischer TH, White GC. Rap1b is required for normal platelet function and hemostasis in mice. J Clin Invest. 2005;115:2296–2296. doi: 10.1172/JCI22973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrzanowska-Wodnicka M. Distinct functions for Rap1 signaling in vascular morphogenesis and dysfunction. Exp Cell Res. 2013;319:2350–2359. doi: 10.1016/j.yexcr.2013.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchniewicz M, Zemojtel T, Kolanczyk M, Grossmann S, Scheele JS, Zwartkruis FJT. Rap1A-deficient T and B cells show impaired integrin-mediated cell adhesion. Mol Cell Biol. 2006;26:643–653. doi: 10.1128/MCB.26.2.643-653.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Yan JL, De P, Chang HC, Yamauchi A, Christopherson KW, Paranavitana NC, Peng XD, Kim C, Munugulavadla V, et al. Rap1a null mice have altered myeloid cell functions suggesting distinct roles for the closely related Rap1a and 1b proteins. J Immunol. 2007;179:8322–8331. doi: 10.4049/jimmunol.179.12.8322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrzanowska-Wodnicka M, Kraus AE, Gale D, White GC, II, Vansluys J. Defective angiogenesis, endothelial migration, proliferation, and MAPK signaling in Rap1b-deficient mice. Blood. 2008;111:2647–2656. doi: 10.1182/blood-2007-08-109710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J, Li F, Ingram DA, Quilliam LA. Rap1a is a key regulator of fibroblast growth factor 2-induced angiogenesis and together with Rap1b controls human endothelial cell functions. Mol Cell Biol. 2008;28:5803–5810. doi: 10.1128/MCB.00393-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullere X, Shaw SK, Andersson L, Hirahashi J, Luscinskas FW, Mayadas TN. Regulation of vascular endothelial barrier function by Epac, a cAMP-activated exchange factor for Rap GTPase. Blood. 2005;105:1950–1955. doi: 10.1182/blood-2004-05-1987. [DOI] [PubMed] [Google Scholar]
- Fukuhara S, Sakurai A, Sano H, Yamagishi A, Somekawa S, Takakura N, Saito Y, Kangawa K, Mochizuki N. Cyclic AMP potentiates vascular endothelial cadherin-mediated cell-cell contact to enhance endothelial barrier function through an Epac-Rap1 signaling pathway. Mol Cell Biol. 2005;25:136–146. doi: 10.1128/MCB.25.1.136-146.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittchen ES, Worthylake RA, Kelly P, Casey PJ, Quilliam LA, Burridge K. Rap1 GTPase inhibits leukocyte transmigration by promoting endothelial barrier function. J Biol Chem. 2005;280:11675–11682. doi: 10.1074/jbc.M412595200. [DOI] [PubMed] [Google Scholar]
- Glading A, Han J, Stockton RA, Ginsberg MH. KRIT-1/CCM1 is a Rap1 effector that regulates endothelial cell–cell junctions. J Cell Biol. 2007;179:247–254. doi: 10.1083/jcb.200705175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revencu N, Vikkula M. Cerebral cavernous malformation: new molecular and clinical insights. J Med Genet. 2006;43:716–721. doi: 10.1136/jmg.2006.041079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakshmikanthan S, Sobczak M, Chun C, Henschel A, Dargatz J, Ramchandran R, Chrzanowska-Wodnicka M. Rap1 promotes VEGFR2 activation and angiogenesis by a mechanism involving integrin alphavbeta(3) Blood. 2011;118:2015–2026. doi: 10.1182/blood-2011-04-349282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakshmikanthan S, Zieba BJ, Ge ZD, Momotani K, Zheng X, Lund H, Artamonov MV, Maas JE, Szabo A, Zhang DX, et al. Rap1b in smooth muscle and endothelium is required for maintenance of vascular tone and normal blood pressure. Arterioscler Thromb Vasc Biol. 2014;34:1486–1494. doi: 10.1161/ATVBAHA.114.303678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boettner B, Van Aelst L. Control of cell adhesion dynamics by Rap1 signaling. Curr Opin Cell Biol. 2009;21:684–693. doi: 10.1016/j.ceb.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takakura N, Huang X-L, Naruse T, Hamaguchi I, Dumont DJ, Yancopoulos GD, Suda T. Critical role of the TIE2 endothelial cell receptor in the development of definitive hematopoiesis. Immunity. 1998;9:677–686. doi: 10.1016/s1074-7613(00)80665-2. [DOI] [PubMed] [Google Scholar]
- Chiu YJ, McBeath E, Fujiwara K. Mechanotransduction in an extracted cell model: Fyn drives stretch- and flow-elicited PECAM-1 phosphorylation. J Cell Biol. 2008;182:753–763. doi: 10.1083/jcb.200801062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bruyn KMT, Zwartkruis FJT, De Rooij J, Akkerman JWN, Bos JL. The small GTPase Rap1 is activated by turbulence and is involved in integrin αIIbβ3-mediated cell adhesion in human megakaryocytes. J Biol Chem. 2003;278:22412–22417. doi: 10.1074/jbc.M212036200. [DOI] [PubMed] [Google Scholar]
- Moon EY, Oh SY, Han GH, Lee CS, Park SK. Epac1-mediated Rap1 activation is not required for the production of nitric oxide in BV2, murine microglial cells. J Neurosci Res. 2005;81:38–44. doi: 10.1002/jnr.20535. [DOI] [PubMed] [Google Scholar]
- Tamada M, Sheetz MP, Sawada Y. Activation of a signaling cascade by cytoskeleton stretch. Dev Cell. 2004;7:709–718. doi: 10.1016/j.devcel.2004.08.021. [DOI] [PubMed] [Google Scholar]
- Chrzanowska-Wodnicka M. Regulation of angiogenesis by a small GTPase Rap1. Vascul Pharmacol. 2010;53:1–10. doi: 10.1016/j.vph.2010.03.003. [DOI] [PubMed] [Google Scholar]
- Chen YH, Yu M, Podd A, Wen R, Chrzanowska-Wodnicka M, White GC, Wang D. A critical role of Rap1b in B-cell trafficking and marginal zone B-cell development. Blood. 2008;111:4627–4636. doi: 10.1182/blood-2007-12-128140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu H, Awasthi A, White GC, II, Chrzanowska-Wodnicka M, Malarkannan S. Rap1b regulates B cell development, homing, and T cell-dependent humoral immunity. J Immunol. 2008;181:3373–3383. doi: 10.4049/jimmunol.181.5.3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkumar R, Konishi K, Richards TJ, Ishizawar DC, Wiechert AC, Kaminski N, Ahmad F. Genomewide RNA expression profiling in lung identifies distinct signatures in idiopathic pulmonary arterial hypertension and secondary pulmonary hypertension. Am J Physiol Heart Circ Physiol. 2010;298:H1235–H1248. doi: 10.1152/ajpheart.00254.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan BX, Vautier F, Ito W, Bolshakov VY, Morozov A. Enhanced cortico-amygdala efficacy and suppressed fear in absence of Rap1. J Neurosci. 2008;28:2089–2098. doi: 10.1523/JNEUROSCI.5156-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitulescu ME, Schmidt I, Benedito R, Adams RH. Inducible gene targeting in the neonatal vasculature and analysis of retinal angiogenesis in mice. Nat Prot. 2012;5:1518–1534. doi: 10.1038/nprot.2010.113. [DOI] [PubMed] [Google Scholar]
- Haynes MP, Sinha D, Russell KS, Collinge M, Fulton D, Morales-Ruiz M, Sessa WC, Bender JR. Membrane estrogen receptor engagement activates endothelial nitric oxide synthase via the PI3-kinase-Akt pathway in human endothelial cells. Circ Res. 2000;87:677–682. doi: 10.1161/01.res.87.8.677. [DOI] [PubMed] [Google Scholar]
- Nelson TJ, Ge ZD, Van Orman J, Barron M, Rudy-Reil D, Hacker TA, Misra R, Duncan SA, Auchampach JA, Lough JW. Improved cardiac function in infarcted mice after treatment with pluripotent embryonic stem cells. Anat Rec A Discov Mol Cell Evol Biol. 2006;288:1216–1224. doi: 10.1002/ar.a.20388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyama K, Wulff H, Chandy KG, Azam P, Raman G, Saito T, Fujiwara Y, Mattson DL, Das S, Melvin JE, et al. The intermediate-conductance calcium-activated potassium channel KCa3.1 contributes to atherogenesis in mice and humans. J Clin Invest. 2008;118:3025–3037. doi: 10.1172/JCI30836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriska T, Cepura C, Magier D, Siangjong L, Gauthier KM, Campbell WB. Mice lacking macrophage 12/15-lipoxygenase are resistant to experimental hypertension. Am J Physiol Heart Circ Physiol. 2012;302:H2428–H2438. doi: 10.1152/ajpheart.01120.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moitra J, Sammani S, Garcia JGN. Re-evaluation of Evans Blue dye as a marker of albumin clearance in murine models of acute lung injury. Transl Res. 2007;150:253–265. doi: 10.1016/j.trsl.2007.03.013. [DOI] [PubMed] [Google Scholar]
- Chen Z, Tzima E. PECAM-1 is necessary for flow-induced vascular remodeling. Arterioscler Thromb Vasc Biol. 2009;29:1067–1073. doi: 10.1161/ATVBAHA.109.186692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza SA, Fang J, Gutterman DD, Wilcox DA, Bubolz AH, Li R, Suzuki M, Zhang DX. TRPV4-mediated endothelial Ca2+ influx and vasodilation in response to shear stress. Am J Physiol Heart Circ Physiol. 2010;298:H466–H476. doi: 10.1152/ajpheart.00854.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobczak M, Dargatz J, Chrzanowska-Wodnicka M. Isolation and culture of pulmonary endothelial cells from neonatal mice. J Vis Exp. 2010;46 doi: 10.3791/2316. : doi: 10.3791/2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shikata Y, Rios A, Kawkitinarong K, DePaola N, Garcia JG, Birukov KG. Differential effects of shear stress and cyclic stretch on focal adhesion remodeling, site-specific FAK phosphorylation, and small GTPases in human lung endothelial cells. Exp Cell Res. 2005;304:40–49. doi: 10.1016/j.yexcr.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Franke B, van Triest M, de Bruijn KMT, van Willigen G, Nieuwenhuis HK, Negrier C, Akkerman J-WN, Bos JL. Sequential regulation of the small GTPase Rap1 in human platelets. Mol Cell Biol. 2000;20:779–785. doi: 10.1128/mcb.20.3.779-785.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan NS, Grisham MB. Methods to detect nitric oxide and its metabolites in biological samples. Free Radic Biol Med. 2007;43:645–657. doi: 10.1016/j.freeradbiomed.2007.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reedquist KA, Ross E, Koop EA, Wolthuis RM, Zwartkruis FJ, van Kooyk Y. The small GTPase, Rap1, mediates CD31-induced integrin adhesion. J Cell Biol. 2000;148:1151–1158. doi: 10.1083/jcb.148.6.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information
Source Data for Supplementary Figure S3
Review Process File
Source Data for Figure 1A–C
Source Data for Figure 2
Source Data for Figure 3
Source Data for Figure 4