Abstract
Immunopathology plays important roles in the development of different life-threatening diseases, such as atherosclerosis and its consequences (acute myocardial infarction and stroke), cancer, chronic inflammatory diseases. Effective modulation of the immune system may significantly increase the efficacy of prevention and therapy efforts. Currently there are no marketed drugs capable of normalizing immune system function in an intrinsic and comprehensive way. Here, we describe a test system designed for complex analysis of monocyte activity in individuals to diagnose immunopathology and monitor treatment efficacy. This cell-based test system may also be useful for screening compounds with an immune-correcting effects. Both diagnostic and screening systems are based on primary culture of human monocytes and/or monocyte-derived macrophages. This is the first step in creating a method for assessment of macrophage activity, which is required for further development of immune-correcting drugs. The existing preliminary data provide the basis for realization of this idea.
Keywords: Activation, atherosclerosis, chemokine, cytokine, diagnostics, immunopathology, macrophage, therapy.
1. INTRODUCTION
Immunopathology plays an important role in the development of or predisposition to such life-threatening conditions as atherosclerotic disease, cancer, and chronic inflammation [1-3]. Atherosclerosis per se is responsible for about one-half of all deaths in the industrial world, and malignant tumours cause at least one-third of deaths from causes other than atherosclerosis [4, 5]. It can therefore be assumed that all pathologic conditions associated with immunopathology are related to up to 80-90 percent of mortality in modern society. In this regard, comprehensive diagnostics of immunopathology and effective modulation of the immune system should significantly increase the efficacy of therapy and prevention, potentially leading to a substantial drop in morbidity and mortality. However, current methods in diagnosing immunopathology are imperfect, since they reveal only gross alterations of the immune state. This makes it difficult to interpret results of routine analysis. On the other hand, it is necessary to recognize that currently there are no drugs capable of normalizing functionality of the immune system in an adequate, intrinsic and comprehensive way. The existing challenge dictates the necessity of developing innovative methods for diagnosing immunopathology, as well as designing effective immune-correcting drugs. In particular, a universal, reliable and affordable test for assessment of functionality of the monocyte/macrophage system would provide an important advance in this direction [6].
2. DIAGNOSTICS
2.1. Macrophages in Immune Disorders
To solve the problem, it is necessary to define proper targets for research and development activity; one of the most promising is the innate immune system. Among the key regulators of innate immunity are monocytes and macrophages. Present in all tissues and organs of the human body, macrophages represent an important part of the immune system, providing the organism with first line defence and at the same time being responsible for everyday maintenance of homeostasis. Each pool of tissue macrophages stays relatively constant in a healthy organism; however, the macrophages are continuously replaced by new ones that originate from circulating monocytes. For several decades, it has been clear that various diseases involve modification of circulating and tissue monocyte function [7].
Studies of monocytogenesis have demonstrated that proliferative activity of promonocytes can be affected by relatively mild systemic inflammatory stimuli leading to an increased proliferation rate and an increase in the monocyte count in the blood [8-10]. A hypothesis has been proposed that changes in phagocytic capacity of macrophages are a result of a change in composition of the peripheral blood monocyte population [11]. It has been established that macrophages may adapt their phenotype to the changing microenvironment. According to their pro- and anti-inflammatory activity, macrophages can be classified as type 1 (M1) and type 2 (M2), respectively [12-14]. Various pro- and anti-inflammatory stimuli are responsible for high heterogeneity of macrophage phenotype in each class. Type 1 macrophages produce pro-inflammatory cytokines TNFα and IL-1β and express Fc-gamma receptors on the cell surface [14, 15]. Type 2 macrophages produce anti-inflammatory cytokines and chemokines IL-1 receptor antagonist (IL-1ra), IL-10, CCL18 and express various scavenger receptors on the surface [16-18]. The mechanism of macrophage activation is highly complex and includes several interacting signalling cascades, vesicular trafficking and interaction of these two processes. In cases of immune system dysfunction, blood monocytes that should be in an inactive state gain phenotypic and functional properties of activated macrophages. Therefore, the analysis of the balance between pro- and anti-inflammatory activities of primary human monocyte-derived macrophages is an attractive possibility for identification of immunopathology.
It is important to note that this balance may depend on macrophage inflammasome status, which controls processing and release of key pro-inflammatory cytokines IL-1β and IL-18 [19, 20]. In addition, inflammasome activation is accompanied by active caspase-1 release, which may further contribute to the death of the neighbouring cells [21-23]. Inflammasome activation has been described in a number of inflammatory diseases and evidence for the role of inflammasomes in different human pathologies is emerging [24]. In general, inflammasome is activated in response to numerous Danger- and Pathogen-Associated Molecular Patterns (DAMPs and PAMPs) [25, 26]. There are multiple studies showing that Chlamydia pneumonia, Helicobacter pylori, Porphyromonas ginigivalis and cytomegalovirus may play important roles in the development of atherosclerosis [27-29]. In addition to the impact of pathogens and PAMPs, DAMPs also contribute to inflammasome-dependent atherosclerosis. Inflammasome activation has been clearly shown in response to cholesterol deposition in arteries and to phagocytized intracellular cholesterol crystals [30, 31]. Uric acid crystals, known strong activators of the inflammasome, may further contribute to the inflammatory nature of atherosclerosis [32]. Impairment of autophagy and the inability to remove intracellular PAMPs and DAMPs may be another contributing factor in inflammasome activation and atherosclerosis progression [33]. Thus, activation of the inflammasome followed by macrophage pyroptosis and IL-1β, IL-18 and active caspase-1 release could add impact on the mechanism of disease progression.
Several decades ago, fast, easy and inexpensive blood tests for total cholesterol and different lipoproteins revolutionized diagnostic possibilities for determining which people are at risk for atherosclerosis and coronary heart diseases. Similarly, it is essential to develop a monocyte-based test system for diagnosis of human pathologies, including, but not limited to, atherosclerosis. Such a test systems may provide more diagnostic information and also be used to screen the effects of various immune-correcting drugs.
2.2. Methodology
There are several technical problems that hinder the use of monocyte-based functional tests for diagnostics. The primary problem is possible activation of monocytes during isolation. Traditional methodology includes an adhesion step and is widely criticized, since the adhesion event is one of the main factors leading to monocyte activation. A better alternative is provided by the use of fluorescence-activated cell sorting (FACS) or magnetic separation. However, these methods require antibodies for monocyte labelling which can also influence monocyte function not only upon binding to its target antigen, but also on binding to Fc receptors. In the case of magnetic separation, further monocyte activation may be induced by paramagnetic beads that may be phagocytosed. The only technology that seems to yield non-activated monocytes is elutriation [34, 35]; however, this method requires special laboratory equipment and cannot practically be adapted for use in routine diagnostics. All these technical difficulties stimulated the search for molecular markers that may substitute for functional tests in diagnostics of diseases. Development of flow cytometry and identification of a wide variety of surface markers and their functions revealed that circulating monocytes represent a heterogeneous cell population [8, 35, 36].
Analysis of monocyte functionality is mostly limited to the analysis of such basic monocyte functions as adhesion, migration, phagocytic activity, binding and endocytosis of low density lipoprotein [37-39]. In pathogenesis, however, monocytes encounter complex signals that include soluble factors affecting general monocyte function in circulation and local factors upon monocyte adhesion to the endothelium and migration into the tissue. The reaction of monocytes to these stimuli is defined by their priming or pre-activation by pathologic conditions in the circulation. Therefore, it can be hypothesized that the spectrum of monocyte response to certain endogenous stimuli has diagnostic potential. To date, two main directions of monocyte-to-macrophage differentiation are recognized: type 1 induced by inflammatory stimuli like IFN-gamma or LPS, and type 2 induced by IL-4, IL-13 and other anti-inflammatory cytokines [15, 18, 41]. Type 1 macrophages (M1) produce high amounts of reactive oxygen species and inflammatory cytokines like TNFα or IL-1β [14]. Type 2 macrophages show high expression of scavenger receptors, produce extracellular matrix components and remodelling enzymes, secrete anti-inflammatory cytokines IL-1ra, CCL18 and IL-10, and express typical surface markers: macrophage mannose receptor, CD163 and stabilin-1 [40-43]. We have previously found that atherogenic conditions enhance monocyte response to both type 1 and type 2 stimuli [8]. Considering this, it is important to develop functional assays and identify molecular markers reflecting altered response to the challenge of monocytes developed under pathologic conditions. Such an assay will provide a unique diagnostic possibility and may lead to discovery of novel targetable markers and molecular mechanisms.
In this test system, we propose monocyte isolation out of whole blood or buffy coats using magnetic separation, which guarantees a highly pure cell population [16, 40]. Complex functional analysis of macrophage activity should include measurement of concentrations of cytokines and chemokines produced by macrophages in response to endogenous bacterial products like lipopolysaccharide (LPS, ligand of TLR4), pro-inflammatory stimulus IFN-γ, and anti-inflammatory stimulus IL-4 [44]. TNFα and IL-1β should be measured as signatures of pro-inflammatory activity of macrophages, and CCL18 and IL-1ra as markers of anti-inflammatory activity of macrophages. To validate whether inflammasome assembly and function are involved in the process of monocyte activation, one can measure IL-1β expression at mRNA level and intracellular proIL-1β synthesis. Release of mature IL-1β, measured in cell culture media by ELISA provides evidence of inflammasome activation. Inflammasome activation results not only in mature IL-1β release but also in active caspase-1 release [21-22]. To complement the information about inflammasome activation, active caspase-1 release from stimulated monocytes should also be performed. As a control of the inflammasome activity, expression and release of the inflammasome-independent TNFα and IL-8 should be measured. Known cytokines that can serve as markers of macrophage activity may be tested: MMR, CD163, TGF-RII, CSFR1, TNFRI, CD16, CD32, CD64 and stabilin-1. TLR1, TLR2 and TLR4 at gene and surface expression levels may also be analysed. Changes in expression of these markers will be correlated with the type and intensity of stimulation.
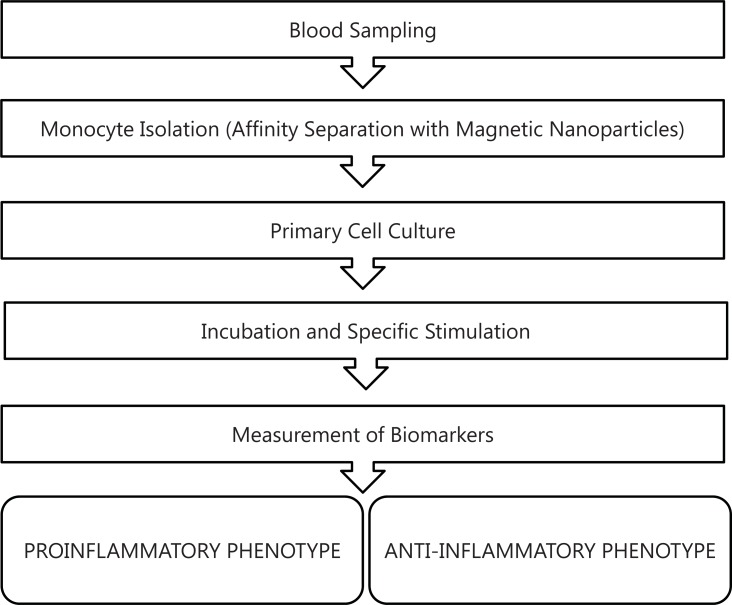
The resulting test system will include information about expression of major inflammation-related genes and soluble proteins, surface receptors and functional properties of macrophages (like inflammasome activation), sufficient for qualitative and quantitative analysis of monocyte activation state and for determination of individual predisposition for M1- or M2-skewed reaction to endogenous stimuli or pathogen. Figure 1 demonstrates the proposed assay design.
Fig. (1).
Disign of proposed assay for determanation of monocytes/ macrophages activation.
2.3. Preliminary Data
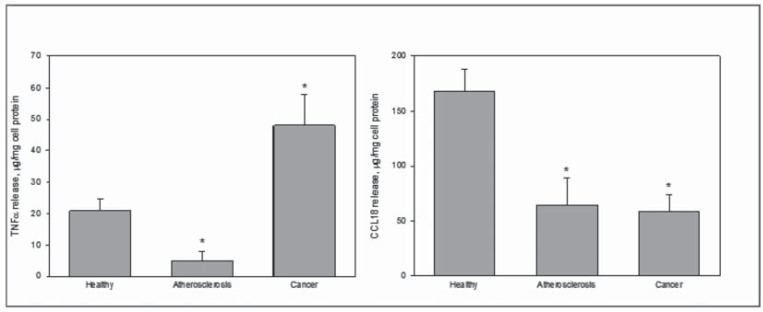
The method for assessment of functional analysis of macrophage activity based only on the measurements of TNFα and CCL18 production was established, and we obtained promising experimental data, adding support for the idea of a macrophage model for diagnostics (Fig. 2). The data obtained demonstrate the possibility of assigning profiles of cell activation specific to certain diseases, with respect to diagnostics of immunopathology.
Fig. (2).
The levels of stimulated activation of monocytes/macrophages from blood of healthy subjects (n = 19), atherosclerotic patients (n = 22), and breast cancer patients (n = 18).
Left panel, production of TNFα by monocytes/macrophages stimulated by combination of IFNg and LPS; right panel, production of CCL18 by monocytes/macrophages stimulated by IL-4.
Monocytes were isolated from the whole blood of patients with breast cancer at the baseline and after 2 weeks of chemotherapy by affinity magnetic separation on CD14-antibody-conjugated ferromagnetic nanobeads using MagCellect Human CD14+ Cell Isolation Kit (R&D Systems, USA), and seeded in primary cutlure. The cells were stimulated by interferon gamma (100 ng/mL) and LPS (10 ng/mL) or interleukin-4 (10 ng/mL), and after 72 h incubation the concentrations of TNFα or CCL 18 were measured in cultural medium by ELISA using Human TNF-α DuoSet or Human CCL18/PARC DuoSet (both, R&D Systems, USA), respectively.
As compared to apparently healthy subjects, stimulated production of TNFα was significantly lower in atherosclerotic patients, but significantly increased in breast cancer patients. As for IL-4 stimulated CCL 18 production, it was significantly lowered both in atherosclerotic and breast cancer patients.
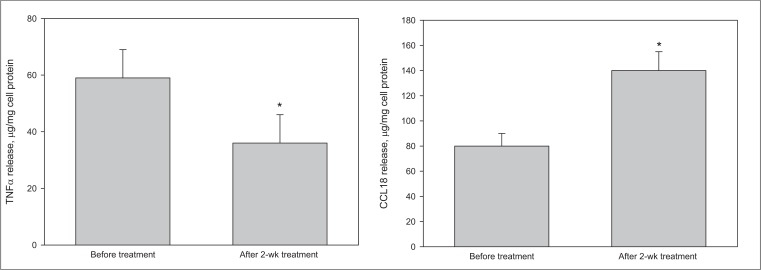
Chemotherapeutic intervention resulted in statistically significant trend to normalization of monocytes/macrophages activation profile, i.e., the decrease of stimulated TNFα production along with the increase of stimulated CCL 18 production.
Moreover, our data demonstrated the possibility of monitoring the effects of therapy on the profile of cell activation (Fig. 3).
Fig. (3).
The changes of levels of stimulated activation of monocytes/macrophages from the blood of breast cancer patients (n = 26) after initiation of chemotherapy.
Left panel, production of TNFα by monocytes/macrophages stimulated by combination of IFNγ and LPS; right panel, production of CCL18 by monocytes/macrophages stimulated by IL-4.
Monocytes were isolated from the whole blood of patients with breast cancer at the baseline and after 2 weeks of chemotherapy by affinity magnetic separation on CD14-antibody-conjugated ferromagnetic nanobeads using MagCellect Human CD14+ Cell Isolation Kit (R&D Systems, USA), and seeded in primary cutlure. The cells were stimulated by interferon gamma (100 ng/mL) and LPS (10 ng/mL) or interleukin-4 (10 ng/mL), and after 72 h incubation the concentrations of TNFα or CCL 18 were measured in cultural medium by ELISA using Human TNF-alpha DuoSet or Human CCL18/PARC DuoSet (both, R&D Systems, USA), respectively.
Preclinical (asymptomatic) atherosclerosis is an important problem because it can lead to sudden fatal outcomes. Diagnostics of preclinical atherosclerosis is not widely used because of the lack of reliable and efficient approaches. Since immunopathology is the most likely cause of preclinical atherosclerosis, we applied the macrophage test for integral evaluation of immunopathology in preclinical atherosclerosis.
Study participants were subdivided into three groups according to the results of ultrasonic evaluation of intima media layer thickness in the carotid arteries (CIMT). Group 1 (no atherosclerosis) included individuals belonging to the first and second quartiles of the age-adjusted CIMT distribution, without atherosclerotic plaques in any segment of the carotid arteries and identified as not predisposed to atherosclerosis. Group size was 21 persons (6 men and 15 women), mean age 60 ± 9 years. Group 2 (predisposition to atherosclerosis) included individuals belonging to the fourth quartile of the age-adjusted CIMT distribution, without atherosclerotic plaques in any segment of the carotid arteries. Group size was 21 persons (6 men and 15 women), mean age 63 ± 9 years. Group 3 (subclinical atherosclerosis) included individuals belonging to the third and fourth quartiles of the age-adjusted CIMT distribution, with atherosclerotic plaques (more than 10% of the arterial lumen) in at least one segment of the carotid arteries. Group size was 21 persons (6 men and 15 women), mean age 62 ± 7 years.
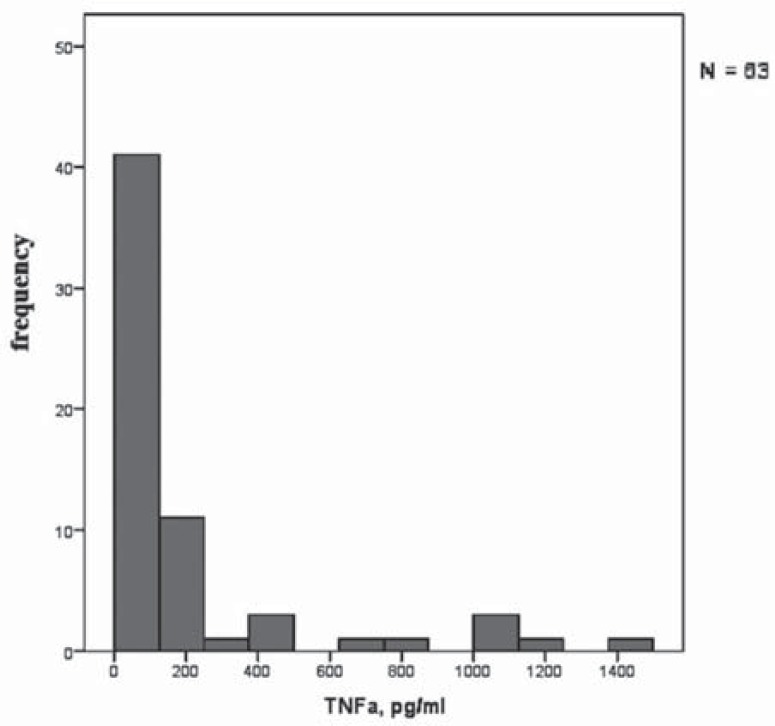
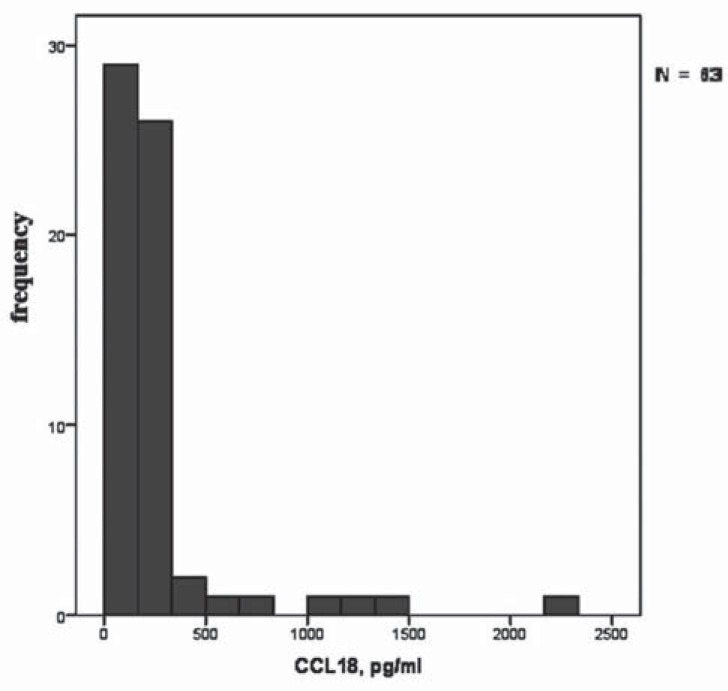
Figures 4 and 5 demonstrate the histograms of distributions of TNFα and CCL18 secretion by stimulated macrophages. The figures show that there are dramatic individual differences in the degree of macrophage activation.
Fig. (4).
Frequency distribution of TNFα secretion by macrophages from different subjects (n = 63).
Fig. (5).
Frequency distribution of CCL18 secretion by macrophages from different subjects (n = 63).
It was found that macrophage activation was very low in some subjects. Individuals with very low degree of macrophage activation belong mainly to group 3. Dramatic individual differences in the degree of macrophage activation are an important finding because these differences may determine individual predisposition to immunopathology.
Table 1shows the concentration of the pro-inflammatory cytokine TNFα and anti-inflammatory chemokine CCL18 in the culture medium of primary macrophage cultures obtained from individuals belonging to three study groups: healthy subjects, subjects predisposed to atherosclerosis, and patients with asymptomatic atherosclerosis. These data demonstrate that secretion of TNFα and CCL18 by stimulated macrophages is significantly lower in individuals predisposed to atherosclerosis and in patients with pre-clinical atherosclerosis. The obtained data indicate that the ability
Table 1.
TNF-α and CCL18 secretion by stimulated macrophages in primary culture.
| Group 1 no atherosclerosis |
Group 2 predisposition |
Group 3 atherosclerosis |
|
|---|---|---|---|
| TNF-α, pg/ml after IFNγ stimulation | 389 ± 100 | 161 ± 47 p (2 vs 1) = 0.05 |
101 ± 12 p (3 vs 1) = 0.01 p (3 vs 2) = 0.23 |
| CCL18, pg/ml after IL4 stimulation | 445 ± 126 | 179 ± 31 p (2 vs 1) = 0.05 |
201 ± 17 p (3 vs 1) = 0.06 p (3 vs 2) = 0.54 |
Monocytes were isolated from the blood of individuals belonging to three study groups (20 individuals in each group). In primary cultures, monocyte derived macrophages were stimulated by IFNγ (100 ng/mL) or IL4 (10 ng/mL). Secretion of TNFα and CCL18 was determined by ELISA. Data are presented as mean ± standard deviation.
The bars represent the number of cases per bin within the examined study participants (non-atherosclerotic subjects, persons predisposed to atherosclerosis, and patients with subclinical carotid atherosclerosis). These data demonstrate high variability and individual differences in stimulated TNFα secretion by macrophages. of macrophages to be activated to pro- and anti-inflammatory pathways significantly reduced with the development of atherosclerosis, suggesting that there is a tendency to depolarization of macrophages. However, these data do not indicate whether macrophage depolarization is the cause or the consequence of atherosclerosis. To clarify this more studies are needed.
3. DRUG DEVELOPMENT
3.1. Macrophages in Immune Disorders
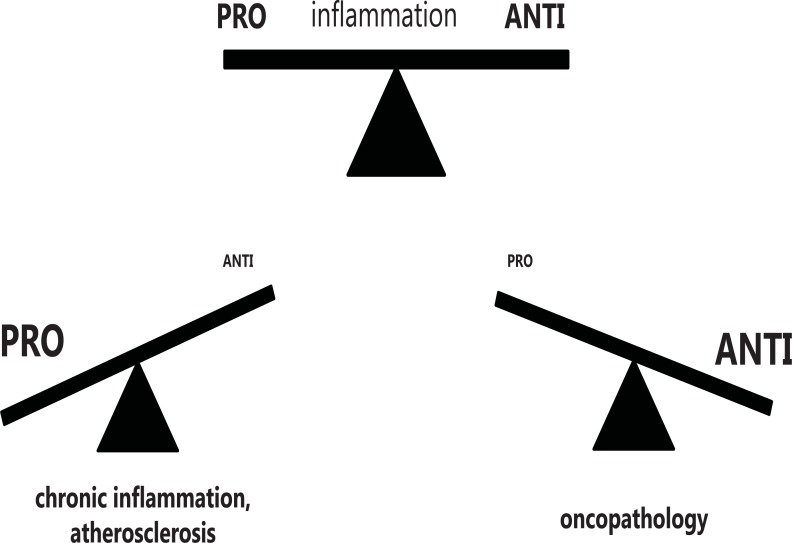
In contrast to obvious immunopathology in such diseases as lupus erythematosus, rheumatoid arthritis, scleroderma, ankylosing spondylitis, HIV infection, latent forms of immunopathology are occurring in atherosclerosis and oncopathology. Obvious immunopathology is specific for disease but has low prevalence in population. Latent immunopathology may be a trigger of the disease and accompanies its development. This form of immunopathology is non-specific for the disease but has extremely high prevalence, reaching up to 80% being associated with cardiovascular diseases and cancer. Playing an important if not crucial role in latent immunopathology is macrophage activation to pro- or anti-inflammatory phenotype. High level of activation leads to macrophage polarization and may cause chronic inflammation and atherosclerosis (pro-inflammatory phenotype) or cancer (anti-inflam-matory phenotype). Possible role of macrophage activation imbalance due to polarization is shown in scheme presented in (Fig. 6). It can be assumed that depolarization of macrophage might have a beneficial effect in the prevention and treatment of disease [45]. For example, it has been shown that macrophage depolarization from an M2 phenotype was associated with tumor regression [46].
Fig. (6).
Polarization of macrophages may lead to pathology.
Natural products widely used in herbal medicine may be the most suitable depolarizing agents, as they do not have serious side effects. This is a very important consideration because treatments aimed at prevention of macrophage polarization should be applied for very long periods of time.
3.2. Preliminary Data
To test this using in vitro assays, extracts were prepared from the following botanicals used in herbal medicine as anti-inflammatory agents: flowers of hawthorn (Crataegi flores), elderberry flowers (Sambuci nigrae flores), St. John's wort (Hyperici herba), calendula flowers (Calendula flores) and violets herbs (Violae herba). To study the effect of botanicals, macrophages were stimulated by IFNγ or IL4, with herbal extracts as indicated in Tables 2 and 3. After 6 days of culture, the culture medium was collected and used to determine the concentrations of cytokines TNFα and CCL18.
Table 2.
Modulation of IFNγ - induced TNFα secretion by botanicals’ extracts.
| Treatment | induced TNFα concentration |
|---|---|
| IFNγ | 520±135 pg/ml |
| IFNγ + hawthorn | 385±81 pg/ml |
| IFNγ + elder | 6590±2520 pg/ml |
| IFNγ + Saint John's wort | 11±4 pg/ml |
| IFNγ + calendula | 5460±2869 pg/ml |
| IFNγ + violet | 6900±2050 pg/ml |
Data are presented as mean ± standard deviation from 5 independent experiments.
Table 3.
Modulation of IL-4 – induced CCL18 secretion by botanicals’ extracts.
| Treatment | induced CCL18 concentration |
|---|---|
| IL-4 | 1604±321 pg/ml |
| IL-4 + hawthorn | 49±15 pg/ml |
| IL-4 + elder | 1408±488 pg/ml |
| IL-4 + Saint-John's-wort | 6±2 pg/ml |
| IL-4 + calendula | 1542±366 pg/ml |
| IFNγ + violet | 1471±558 pg/ml |
Data are presented as mean ± standard deviation from 5 independent experiments.
Table 2 demonstrates that the secretion of TNFα by macrophages stimulated with IFNγ in combination with three out of five studied extracts was over 10- 13-fold higher than by macrophages stimulated with IFNγ alone. Two extracts (hawthorn and St. John's wort) significantly inhibited the secretion of TNFα. Hawthorn extract inhibited TNFα secretion by 25%, whereas the St. John's wort extract almost completely suppressed TNFα secretion.
Analysis of CCL18 expression in response to stimulation with IL-4 demonstrated that extracts of hawthorn and Saint John's wort suppressed the secretion of CCL18 and the degree of suppression was highly significant (Table 3). In the case of the extract of hawthorn flowers, CCL18 secretion was suppressed by more than 30-fold, whereas the inhibitory effect of St. John's wort was even more pronounced: CCL18 secretion was inhibited by more than 250-fold.
Thus, St. John's wort and hawthorn extracts can be regarded as agents causing macrophage depolarization. Certainly, the study of these effects should be continued. However, the data confirmed the possibility of creating an immune-correcting agent causing macrophage depolarization. Such agents should find wide application in clinical practice as diseases associated with macrophage polarization are the most common and dangerous immune disorders.
3.3. Macrophages in Atherosclerosis-Related Models
As mentioned above, one of the diseases associated with immunopathology is atherosclerosis. Naturally, it is important to assess how the immune-correcting drugs can affect cellular manifestations of atherosclerosis. For such an assessment, macrophages could present a suitable cell model.
Current concepts of cellular and molecular mechanisms of atherogenesis are based on classical lipid theory of atherosclerosis implying a key role of cholesterol in the initiation of atherosclerotic lesions [47, 48]. The source of accumulating cholesterol is low-density lipoprotein (LDL) [49, 50]. However, native (intact) LDL does not cause cholesterol accumulation in the arterial cells [50]. On the other hand, chemically modified LDL is atherogenic as it is capable of causing the intracellular cholesterol accumulation.
Known atherogenic LDL modifications found in circulation are limited to three kinds: desialylation, the increase of the total negative surface charge, and the change of hydrated density of lipoprotein particles; all these forms may be accompanied by oxidation [50-52]. In all cases, there is the same type of multiple atherogenic modifications, but different methods are used for evaluation of each form [51]. The are also additional mechanisms enhancing LDL atherogenicity. The main one is the formation of large LDL-containing complexes [53], which form due to the ability of modified LDL to spontaneously self-associate [51]. Modified LDL possess antigenic properties, thus inducing the production of autoantibodies, which leads to the formation of LDL-containing circulating immune complexes [53]. These processes lead to the formation of large LDL-containing aggregates, the cellular metabolism of which at the cellular level is different from the classical receptor-dependent pathway [49]. The main pathway of internalization of such particles is uncontrolled phagocytosis. This leads to massive intracellular accumulation of cholesterol, mainly in the form of lipid droplets. These cholesterol-laden cells represent foam cells typical for atherosclerotic lesions.
One of approaches to prevention and treatment of atherosclerosis is the prevention of intracellular cholesterol accumulation. For screening of anti-atherosclerotic substances, a cell based model of a primary culture of human monocyte-derived macrophages has been developed [54-56] and used to analyze blood serum from patients after drug administration. We have earlier found that sera from atherosclerotic patients were pro-atherogenic, i.e. these sera induced cholesterol accumulation in cultured cells [57].
3.4. Preliminary Data
We evaluated the changes of atherogenic properties of serum samples from subjects treated with various preparations of botanical extracts. Blood was taken before and after single dose drug administration. Sera obtained from blood samples were added to the primary culture of monocyte-derived macrophages. An essential feature of this ex vivo model is the ability to assess anti-atherosclerotic potential of various substances and their active metabolites after digestion, distribution, and biotransformation in human organism. The study involved volunteers (groups of 4-8 people aged 45-60 years) treated with a single dose of investigated natural product and included. Monocytes were isolated from the blood of healthy donors by affinity magnetic separation on CD14-antibody-conjugated ferromagnetic nanobeads using MagCellect Human CD14+ Cell Isolation Kit (R&D Systems, USA), and seeded in primary cutlure. The tested serum samples taken from study participants were added to cultural medium at concentration of 10%. After 24 h incubation intracellular lipids were extracted by hexane-isopropanol mixture, and intracellular cholesterol level was measured as described elsewhere [54]. The baseline and follow-up levels of serum atherogenicity were quantitatively characterized as the ability of cultured cells to accumulate cholesterol during incubation with tested serum samples.
Using the macrophage test, the ability of St. John's wort and hawthorn to cause depolarization of macrophages has been demonstrated above. Next, these agents were tested on ex vivo cellular model to assess their effect on serum atherogenicity. After 2 hours of a single dose, hawthorn administration resulted in a decrease of serum atherogenicity by an average of 73% relative to baseline (Table 4). Four hours after administration there was a decrease of serum atherogenic potential by an average of 83%. Thus, hawthorn extract possesses may possess a pronounced anti-atherosclerotic effect that was observed after 2-4 hours after administration. Unlike hawthorn extract, St. John's wort did not display such effect, and reduction of serum atherogenicity was not statistically significant (Table 4).
Table 4.
Reduction of blood serum atherogenicity after a single dose of botanicals.
| time after administration, hours | serum atherogenicity, % from baseline | ||
|---|---|---|---|
| hawthorn | St. John's wort | ||
| 0 | 100 | 100 | |
| 2 | 26,7±12,1 * | 37,0±37,0 | |
| 4 | 16,7±16,7 * | 36,7±29,0 | |
| 8 | 35,3±24,3 | 56,0±29,5 | |
Significant reduction in blood serum atherogenicity, p <0.05.
Four patients were given water extract of 8 g hawthorn berries or 3 g St. John's wort herb. Before a single dose as well as 2, 4 and 8 hours after, the patient's blood was collected and blood serum was added to primary culture of subendothelial intimal cells from uninvolved human aorta as described [54-56]. In 24 hours cellular cholesterol was measured. Serum atherogenicity was determined as an increase of cholesterol content in cultured cells caused by serum sample.
It can be concluded that these two agents, St. John's wort and hawthorn, possess immuno-correcting (depolarizing) activity. St. John's wort exhibits pronounced depolarizing effect on macrophages but possesses unstable anti-atherosclerotic effect. The hawthorn extract is less effective as a depolarizing agent but has a pronounced anti-atherosclerotic effect. These findings suggest that the combination of St. John's wort and hawthorn may be the basis for the formulation of the anti-atherosclerotic immuno-correcting treatment.
4. CONCLUSION
Two innovative products have been developed. The first product is the test system designed for complex analysis of human monocyte activity in individuals, for diagnosing immunopathology and monitoring the efficacy of conducted treatment. It is based on primary culture of human monocytes isolated from patient’s blood. The second one is the universal cell-based test system for screening compounds with immuno-correcting effects; monocytes and monocyte-derived macrophages isolated from healthy donor’s blood are used. This test system will allow development of immuno-correcting drugs that will be used for prevention and treatment of human pathologies. The similarity of these two proposed innovative products is based on the idea of using primary culture of blood monocytes and/or derived macrophages.
The first product (diagnostic test-system) uses cells from blood of patients with established diagnosis; it will help to identify the set of cellular and molecular markers with the best diagnostic and/or prognostic potential, to estimate the presence and measure the severity of immunopathology (diagnostics), and to estimate the changes in pathologic profile of cellular activation in the course of therapy (monitoring of treatment efficacy in the given patient, as an approach to personalized medicine).
The second product (test-system for screening) uses cells from blood of healthy donors; this approach would allow researchers to reproduce a pathologic profile of cell activation under standardized conditions. This model allows testing of different compounds, both of synthetic and natural origin, to screen those capable of normalizing functionality of cells in intrinsic and comprehensive way. We illustrate applicability of this test system by analyzing the effects of natural extracts on macrophage polarization and atherogenesis. At this stage, it is not reasonable to use laboratory cell lines, as available cell lines generally cannot fully reproduce the properties of human cells. However, we understand that the use of cell lines should help standardizing the assay to the most achievable extent, and this should be the task for further developments.
AKNOWLEDGEMENTS
This work was supported by Russian Scientific Foundation (Grant # 14-15-00112).
CONFLICT OF INTEREST
The authors declare that there is no conflict of interests regarding the publication of this paper.
REFERENCES
- 1.Darveau R. Infection, inflammation, and cancer. Nat Biotechnol. 1999;1:19. doi: 10.1038/5188. [DOI] [PubMed] [Google Scholar]
- 2.Balkwill F, Mantovani A. Inflammation and cancer back to Virchow? Lancet. 2001;357:539–45. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 3.Balkwill FR. Re Possible role of ovarian epithelial inflammation in ovarian cancer. J Natl Cancer Inst. 2000;92:162–3. doi: 10.1093/jnci/92.2.162a. [DOI] [PubMed] [Google Scholar]
- 4.Fries JF. Strategies for reduction of morbidity. Am J Clin Nutr. 1992;55:1257S–62. doi: 10.1093/ajcn/55.6.1257S. [DOI] [PubMed] [Google Scholar]
- 5.Beaglehole R, Bonita R. Global public health a scorecard. Lancet. 2008;372:1988–96. doi: 10.1016/S0140-6736(08)61558-5. [DOI] [PubMed] [Google Scholar]
- 6.Gratchev A, Sobenin I, Orekhov A, Kzhyshkowska J. Monocytes as a diagnostic marker of cardiovascular diseases. Immunobiol. 2012;217:476–2. doi: 10.1016/j.imbio.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 7.Hurst NP, Nuki G. Evidence for defect of complement-mediated phagocytosis by monocytes from patients with rheumatoid arthritis and cutaneous vasculitis. BMJ. 1981;282:2081–3. doi: 10.1136/bmj.282.6282.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meuret G, Hoffmann G. Monocyte kinetic studies in normal and disease states. Br J Haematol. 1973;24:275–85. doi: 10.1111/j.1365-2141.1973.tb01652.x. [DOI] [PubMed] [Google Scholar]
- 9.Meuret G, Bammert J, Hoffmann G. Kinetics of human monocytopoiesis. Blood. 1974;44(6):801–16. [PubMed] [Google Scholar]
- 10.Averill LE, Meagher RC, Gerrity RG. Enhanced monocyte progenitor cell proliferation in bone marrow of hyperlipemic swine. Am J Pathol. 1989;135:369–77. [PMC free article] [PubMed] [Google Scholar]
- 11.Burke B, Lewis CE. 3rd ed. Oxford: Oxford University Press; 2008. The Macrophage. [Google Scholar]
- 12.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–64. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 13.Gordon S, Martinez FO. Alternative activation of macrophages mechanism and functions. Immunity. 2010;32:593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 14.Gratchev A, Schledzewski K, Guillot P, Goerdt S. Alternatively activated antigen-presenting cells molecular repertoire, immune regulation, and healing. Skin Pharmacol Appl Skin Physiol. 2001;14:272–9. doi: 10.1159/000056357. [DOI] [PubMed] [Google Scholar]
- 15.Gordon S. The macrophage. Bioessays. 1995;17:977–86. doi: 10.1002/bies.950171111. [DOI] [PubMed] [Google Scholar]
- 16.Gratchev A, Kzhyshkowska J, Duperrier K, Utikal J, Velten FW, Goerdt S. The receptor for interleukin-17E is induced by Th2 cytokines in antigen-presenting cells. Scand J Immunol. 2004;60:233–7. doi: 10.1111/j.0300-9475.2004.01443.x. [DOI] [PubMed] [Google Scholar]
- 17.Gratchev A, Kzhyshkowska J, Utikal J, Goerdt S. Interleukin-4 and dexamethasone counterregulate extracellular matrix remodelling and phagocytosis in type-2 macrophages. Scand J Immunol. 2005;61:10–17. doi: 10.1111/j.0300-9475.2005.01524.x. [DOI] [PubMed] [Google Scholar]
- 18.Kodelja V, Muller C, Politz O, Hakij N, Orfanos CE, Goerdt S. Alternative macrophage activation-associated CC-chemokine-1, a novel structural homologue of macrophage inflammatory protein-1 alpha with a Th2-associated expression pattern. J Immunol. 1998;160:1411–8. [PubMed] [Google Scholar]
- 19.Martinon F, Burns K, Tschopp J. The inflammasome a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417–26. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 20.Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011;145:341–55. doi: 10.1016/j.cell.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gavrilin MA, Mitra S, Seshadri S , et al. Pyrin critical to macrophage IL-1beta response to Francisella challenge. J Immunol. 2009;182:7982–9. doi: 10.4049/jimmunol.0803073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sarkar A, Mitra S, Mehta S, Raices R, Wewers MD. Monocyte derived microvesicles deliver a cell death message via encapsulated caspase-1. PLoS One. 2009;4:e7140. doi: 10.1371/journal.pone.0007140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gavrilin MA, Abdelaziz DH, Mostafa M, et al. Activation of the pyrin inflammasome by intracellular Burkholderia cenocepacia. J Immunol. 2012;188:3469–77. doi: 10.4049/jimmunol.1102272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Snalhoub J, Falck-Hansen MA, Davies AH. Innate immunity and monocyte-acrophages activation in atherosclerosis. J Inflamm. 2011;8:9. doi: 10.1186/1476-9255-8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martinon F, Mayor A, Tschopp J. The inflammasomes guardians of the body. Annu Rev Immunol. 2009;27:229–65. doi: 10.1146/annurev.immunol.021908.132715. [DOI] [PubMed] [Google Scholar]
- 26.Janeway CA Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 27.Valtonen VV. Infection as a risk factor for infarction and atherosclerosis. Annals of Medicine. 1991;23:539–43. doi: 10.3109/07853899109150515. [DOI] [PubMed] [Google Scholar]
- 28.Luque A, Turu MM, Rovira N, Juan-Babot JO, Slevin M, Krupinski J, editors. Early atherosclerotic plaques show evidence of infection by Chlamydia pneumoniae. Front Biosci . ((Elite Ed)) 2012;4:2423–32. doi: 10.2741/e554. [DOI] [PubMed] [Google Scholar]
- 29.Charakida M, Tousoulis D. Infections and atheromatous plaque current therapeutic implications. Curr Pharm Des. 2013;19:1638–50. [PubMed] [Google Scholar]
- 30.Duewell P, Kono H, Rayner KJ, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–61. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rajamäki K, Lappalainen J, Oörni K , et al. Cholesterol crystals activate the NLRP3 inflammasome in human macrophages a novel link between cholesterol metabolism and inflammation. PLoS One. 2010;5:e11765. doi: 10.1371/journal.pone.0011765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krishnan E. Inflammation, oxidative stress and lipids the risk triad for atherosclerosis in gout. Rheumatol. 2010;49:1229–38. doi: 10.1093/rheumatology/keq037. [DOI] [PubMed] [Google Scholar]
- 33.Razani B, Feng C, Coleman T, et al. Autophagy links inflam-masomes to atherosclerotic progression. Cell Metab. 2012;15:534–44. doi: 10.1016/j.cmet.2012.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Strasser EF, Eckstein R. Optimization of leukocyte collection and monocyte isolation for dendritic cell culture. Transfus Med Rev. 2010;24:130–9. doi: 10.1016/j.tmrv.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 35.Clarkson SB, Ory PA. Developmentally regulated IgG Fc receptors on cultured human monocytes. J Exp Med. 1988;167:408–20. doi: 10.1084/jem.167.2.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baumgartner I, Scheiner O, Holzinger C, et al. Expression of the VEP13 antigen (CD16):on native human alveolar macrophages and cultured blood monocytes. Immunobiol. 1988;177:317–26. doi: 10.1016/S0171-2985(88)80050-0. [DOI] [PubMed] [Google Scholar]
- 37.Krause S, Pohl A, Pohl C, et al. Ex vivo investigation of blood monocyte and platelet behaviour in pigs maintained on an atherogenic diet. Exp Pathol. 1992;44:144–6. doi: 10.1016/S0940-2993(11)80150-8. [DOI] [PubMed] [Google Scholar]
- 38.Mosig S, Rennert K, Krause S, et al. Different functions of monocyte subsets in familial hypercholesterolemia potential function of CD14+ CD16+ monocytes in detoxification of oxidized LDL. FASEB J. 2009;23:866–74. doi: 10.1096/fj.08-118240. [DOI] [PubMed] [Google Scholar]
- 39.Bell FP, Gerrity RG. Evidence for an altered lipid metabolic state in circulating blood monocytes under conditions of hyperlipemia in swine and its implications in arterial lipid metabolism. Arterioscler Thromb. 1992;12:155–62. doi: 10.1161/01.atv.12.2.155. [DOI] [PubMed] [Google Scholar]
- 40.Gratchev A, Guillot P, Hakiy N, et al. Alternatively activated macrophages differentially express fibronectin and its splice variants and the extracellular matrix protein betaIG-H3. Scand J Immunol. 2001;53:386–92. doi: 10.1046/j.1365-3083.2001.00885.x. [DOI] [PubMed] [Google Scholar]
- 41.Stein M, Keshav S, Harris N, Gordon S. Interleukin 4 potently enhances murine macrophage mannose receptor activity a marker of alternative immunologic macrophage activation. J Exp Med. 1992;176:287–92. doi: 10.1084/jem.176.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Politz O, Gratchev A, McCourt PA, et al. Stabilin-1 and -2 constitute a novel family of fasciclin-like hyaluronan receptor homologues. Biochem J. 2002;362:155–64. doi: 10.1042/0264-6021:3620155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schaer DJ, Boretti FS, Hongegger A, et al. Molecular cloning and characterization of the mouse CD163 homologue, a highly glucocorticoid-inducible member of the scavenger receptor cysteine-rich family. Immunogenetics. 2001;53:170–7. doi: 10.1007/s002510100304. [DOI] [PubMed] [Google Scholar]
- 44.Gratchev A, Kzhyshkowska J, Köthe K, et al. Mphi1 and Mphi2 can be re-polarized by Th2 or Th1 cytokines, respectively, and respond to exogenous danger signals. Immunobiol. 2006;211:473–86. doi: 10.1016/j.imbio.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 45.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–69. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pyonteck SM, Akkari L, Schuhmacher AJ, et al. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat Med. 2013;19:1264–72. doi: 10.1038/nm.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Andreeva ER, Pugach IM, Orekhov AN. Subendothelial smooth muscle cells of human aorta express macrophage antigen in situ and in vitro. Atherosclerosis. 1997;135:19–27. doi: 10.1016/s0021-9150(97)00136-6. [DOI] [PubMed] [Google Scholar]
- 48.Andreeva ER, Pugach IM, Orekhov AN. Collagen-synthesizing cells in initial and advanced atherosclerotic lesions of human aorta. Atherosclerosis. 1997;130:133–42. doi: 10.1016/s0021-9150(96)06056-x. [DOI] [PubMed] [Google Scholar]
- 49.Tertov VV, Orekhov AN. Metabolism of native and naturally occurring multiple modified low density lipoprotein in smooth muscle cells of human aortic intima. Exp Mol Pathol. 1997;64:127–45. doi: 10.1006/exmp.1997.2216. [DOI] [PubMed] [Google Scholar]
- 50.Tertov VV, Bittolo-Bon G, Sobenin IA, Cazzolato G, Orekhov AN, Avogaro P. Naturally occurring modified low density lipoproteins are similar if not identical more electronegative and desialylated lipoprotein subfractions. Exp Mol Pathol. 1995;62:166–72. doi: 10.1006/exmp.1995.1018. [DOI] [PubMed] [Google Scholar]
- 51.Jaakkola O, Solakivi T, Tertov VV, Orekhov AN, Miettinen TA, Nikkari T. Characteristics of low-density lipoprotein subfractions from patients with coronary artery disease. Coron Artery Dis. 1993;4:379–85. doi: 10.1097/00019501-199304000-00010. [DOI] [PubMed] [Google Scholar]
- 52.Tertov VV, Kaplun VV, Sobenin IA, Boytsova EY, Bovin NV, Orekhov AN. Human plasma trans-sialidase causes atherogenic modification of low density lipoprotein. Atherosclerosis. 2001;159:103–15. doi: 10.1016/s0021-9150(01)00498-1. [DOI] [PubMed] [Google Scholar]
- 53.Kacharava AG, Tertov VV, Orekhov AN. Autoantibodies against low-density lipoprotein and atherogenic potential of blood. Ann Med. 1993;25:551–5. [PubMed] [Google Scholar]
- 54.Orekhov AN, Tertov VV, Khashimov KhA, Kudryashov SA, Smirnov VN. Evidence of anti-atherosclerotic action of verapamil from direct effects on arterial cells. Am J Cardiol. 1987;59:495–6. doi: 10.1016/0002-9149(87)90971-4. [DOI] [PubMed] [Google Scholar]
- 55.Akopov SE, Orekhov AN, Tertov VV, Khashimov KA, Gabrielyan ES, Smirnov VN. Stable analogues of prostacyclin and thromboxane A2 display contradictory influences on atherosclerotic properties of cells cultured from human aorta. The effect of calcium antagonists. Atherosclerosis. 1988;72:245–8. doi: 10.1016/0021-9150(88)90087-1. [DOI] [PubMed] [Google Scholar]
- 56.Orekhov AN, Tertov VV, Khashimov KhA, Kudryashov SA, Smirnov VN. Antiatherosclerotic effects of verapamil in primary culture of human aortic intimal cells. J Hypertens. 1986;4:S153–5. doi: 10.1016/0021-9150(86)90002-x. [DOI] [PubMed] [Google Scholar]
- 57.Chazov EI, Tertov VV, Orekhov AN, et al. Atherogenicity of blood serum from patients with coronary heart disease. Lancet. 1986;2:595–8. doi: 10.1016/s0140-6736(86)92426-8. [DOI] [PubMed] [Google Scholar]