Abstract
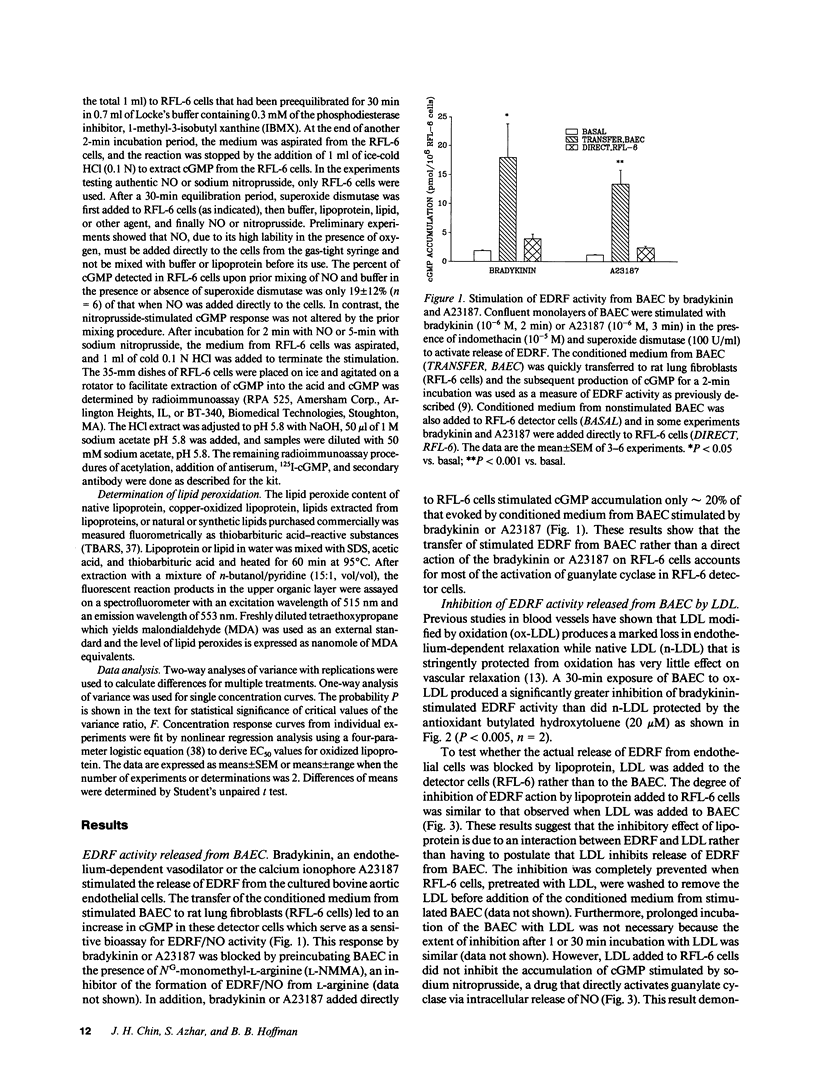
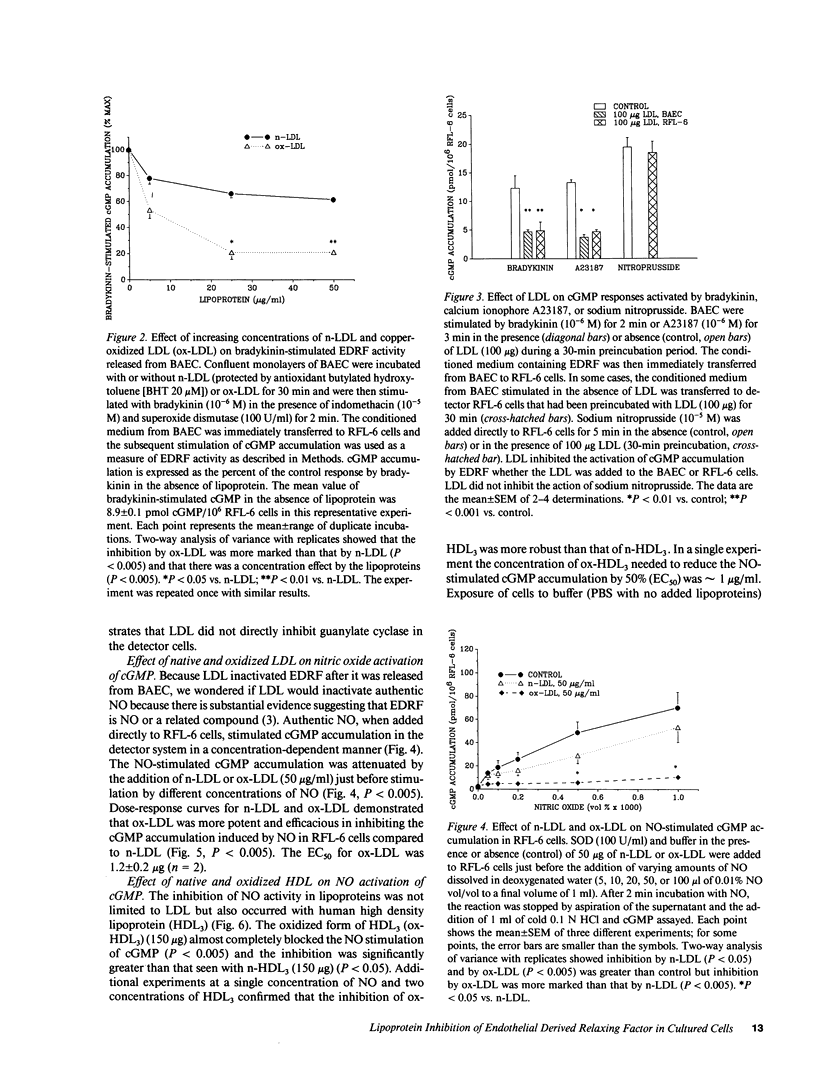
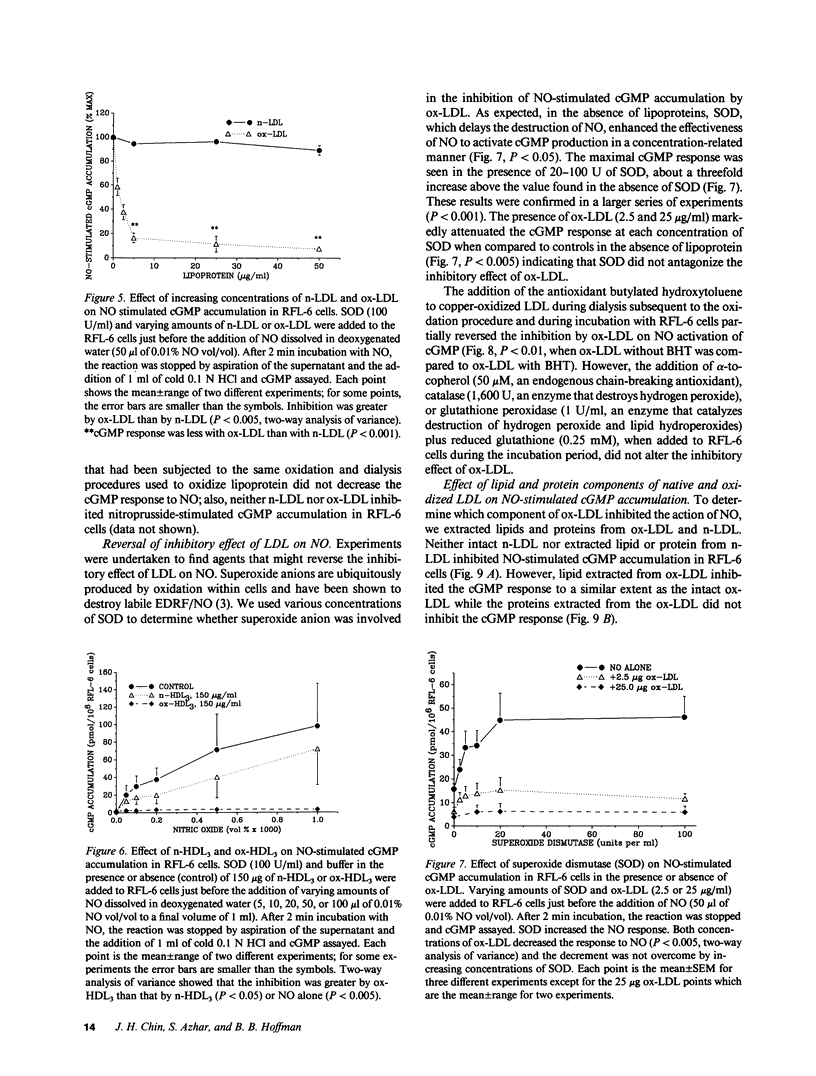
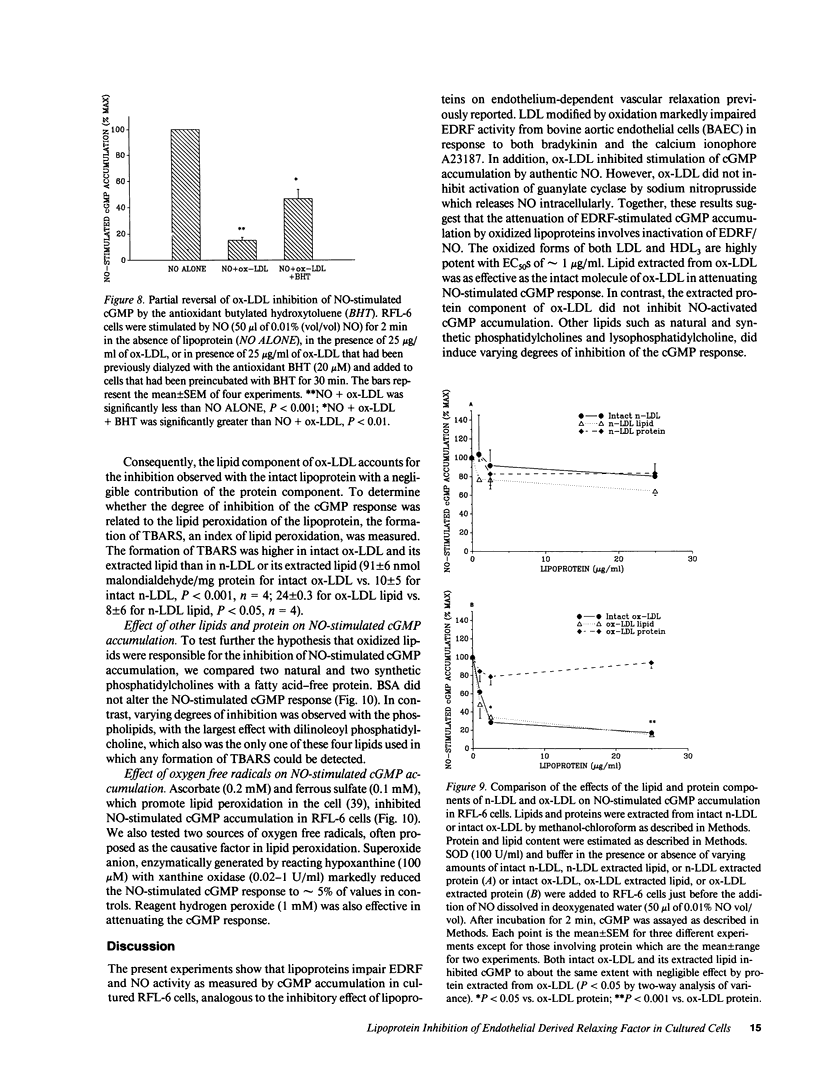
Endothelial cell derived relaxing factor (EDRF) mediated relaxation of blood vessels is impaired in vessels exposed to lipoproteins in vitro and in arteries of hyperlipidemic humans and animals. To investigate the mechanism by which lipoproteins impair the effects of EDRF, which is likely nitric oxide (NO) or a related molecule, we have bioassayed EDRF/NO activity by measuring its ability to increase cGMP accumulation in rat fetal lung cultured fibroblasts (RFL-6 cells). Low density lipoprotein modified by oxidation (ox-LDL) induced a concentration-dependent inhibition of EDRF activity that had been released from bovine aortic endothelial cells (BAEC) stimulated with bradykinin or the calcium ionophore A23187. In addition, lipoproteins directly impaired authentic NO-induced stimulation of cGMP accumulation in the detector cells; stimulation by sodium nitroprusside was unaffected. Ox-LDL or oxidized HDL3 were highly potent in blocking NO-stimulated cGMP accumulation with EC50's of approximately 1 microgram/ml. Lipid extracted from ox-LDL blocked NO-stimulated cGMP accumulation to about the same extent as intact ox-LDL, while the protein component of ox-LDL did not inhibit the cGMP response. These results suggest that the lipid component of oxidized lipoproteins inactivate EDRF after its release from endothelial cells.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews H. E., Bruckdorfer K. R., Dunn R. C., Jacobs M. Low-density lipoproteins inhibit endothelium-dependent relaxation in rabbit aorta. Nature. 1987 May 21;327(6119):237–239. doi: 10.1038/327237a0. [DOI] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Berliner J. A., Territo M. C., Sevanian A., Ramin S., Kim J. A., Bamshad B., Esterson M., Fogelman A. M. Minimally modified low density lipoprotein stimulates monocyte endothelial interactions. J Clin Invest. 1990 Apr;85(4):1260–1266. doi: 10.1172/JCI114562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossaller C., Habib G. B., Yamamoto H., Williams C., Wells S., Henry P. D. Impaired muscarinic endothelium-dependent relaxation and cyclic guanosine 5'-monophosphate formation in atherosclerotic human coronary artery and rabbit aorta. J Clin Invest. 1987 Jan;79(1):170–174. doi: 10.1172/JCI112779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulanger C., Hendrickson H., Lorenz R. R., Vanhoutte P. M. Release of different relaxing factors by cultured porcine endothelial cells. Circ Res. 1989 Jun;64(6):1070–1078. doi: 10.1161/01.res.64.6.1070. [DOI] [PubMed] [Google Scholar]
- Creager M. A., Cooke J. P., Mendelsohn M. E., Gallagher S. J., Coleman S. M., Loscalzo J., Dzau V. J. Impaired vasodilation of forearm resistance vessels in hypercholesterolemic humans. J Clin Invest. 1990 Jul;86(1):228–234. doi: 10.1172/JCI114688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLean A., Munson P. J., Rodbard D. Simultaneous analysis of families of sigmoidal curves: application to bioassay, radioligand assay, and physiological dose-response curves. Am J Physiol. 1978 Aug;235(2):E97–102. doi: 10.1152/ajpendo.1978.235.2.E97. [DOI] [PubMed] [Google Scholar]
- Esterbauer H., Jürgens G., Quehenberger O., Koller E. Autoxidation of human low density lipoprotein: loss of polyunsaturated fatty acids and vitamin E and generation of aldehydes. J Lipid Res. 1987 May;28(5):495–509. [PubMed] [Google Scholar]
- Furchgott R. F., Vanhoutte P. M. Endothelium-derived relaxing and contracting factors. FASEB J. 1989 Jul;3(9):2007–2018. [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Förstermann U., Mügge A., Alheid U., Haverich A., Frölich J. C. Selective attenuation of endothelium-mediated vasodilation in atherosclerotic human coronary arteries. Circ Res. 1988 Feb;62(2):185–190. doi: 10.1161/01.res.62.2.185. [DOI] [PubMed] [Google Scholar]
- Förstermann U., Mülsch A., Böhme E., Busse R. Stimulation of soluble guanylate cyclase by an acetylcholine-induced endothelium-derived factor from rabbit and canine arteries. Circ Res. 1986 Apr;58(4):531–538. doi: 10.1161/01.res.58.4.531. [DOI] [PubMed] [Google Scholar]
- Galle J., Bassenge E., Busse R. Oxidized low density lipoproteins potentiate vasoconstrictions to various agonists by direct interaction with vascular smooth muscle. Circ Res. 1990 May;66(5):1287–1293. doi: 10.1161/01.res.66.5.1287. [DOI] [PubMed] [Google Scholar]
- Guerra R., Jr, Brotherton A. F., Goodwin P. J., Clark C. R., Armstrong M. L., Harrison D. G. Mechanisms of abnormal endothelium-dependent vascular relaxation in atherosclerosis: implications for altered autocrine and paracrine functions of EDRF. Blood Vessels. 1989;26(5):300–314. doi: 10.1159/000158779. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J. Biosynthesis and metabolism of endothelium-derived nitric oxide. Annu Rev Pharmacol Toxicol. 1990;30:535–560. doi: 10.1146/annurev.pa.30.040190.002535. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J. Nitric oxide. A novel signal transduction mechanism for transcellular communication. Hypertension. 1990 Nov;16(5):477–483. doi: 10.1161/01.hyp.16.5.477. [DOI] [PubMed] [Google Scholar]
- Jacobs M., Plane F., Bruckdorfer K. R. Native and oxidized low-density lipoproteins have different inhibitory effects on endothelium-derived relaxing factor in the rabbit aorta. Br J Pharmacol. 1990 May;100(1):21–26. doi: 10.1111/j.1476-5381.1990.tb12045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kugiyama K., Kerns S. A., Morrisett J. D., Roberts R., Henry P. D. Impairment of endothelium-dependent arterial relaxation by lysolecithin in modified low-density lipoproteins. Nature. 1990 Mar 8;344(6262):160–162. doi: 10.1038/344160a0. [DOI] [PubMed] [Google Scholar]
- Leitman D. C., Agnost V. L., Tuan J. J., Andresen J. W., Murad F. Atrial natriuretic factor and sodium nitroprusside increase cyclic GMP in cultured rat lung fibroblasts by activating different forms of guanylate cyclase. Biochem J. 1987 May 15;244(1):69–74. doi: 10.1042/bj2440069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D. M., Aust S. D. Studies of ascorbate-dependent, iron-catalyzed lipid peroxidation. Arch Biochem Biophys. 1989 May 15;271(1):113–119. doi: 10.1016/0003-9861(89)90261-0. [DOI] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Moncada S., Radomski M. W., Palmer R. M. Endothelium-derived relaxing factor. Identification as nitric oxide and role in the control of vascular tone and platelet function. Biochem Pharmacol. 1988 Jul 1;37(13):2495–2501. doi: 10.1016/0006-2952(88)90236-5. [DOI] [PubMed] [Google Scholar]
- Morel D. W., Hessler J. R., Chisolm G. M. Low density lipoprotein cytotoxicity induced by free radical peroxidation of lipid. J Lipid Res. 1983 Aug;24(8):1070–1076. [PubMed] [Google Scholar]
- Ohkawa H., Ohishi N., Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979 Jun;95(2):351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ashton D. S., Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988 Jun 16;333(6174):664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Moncada S. A novel citrulline-forming enzyme implicated in the formation of nitric oxide by vascular endothelial cells. Biochem Biophys Res Commun. 1989 Jan 16;158(1):348–352. doi: 10.1016/s0006-291x(89)80219-0. [DOI] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Reaven E., Shi X. Y., Azhar S. Interaction of lipoproteins with isolated ovary plasma membranes. J Biol Chem. 1990 Nov 5;265(31):19100–19111. [PubMed] [Google Scholar]
- Rosenfeld M. E., Khoo J. C., Miller E., Parthasarathy S., Palinski W., Witztum J. L. Macrophage-derived foam cells freshly isolated from rabbit atherosclerotic lesions degrade modified lipoproteins, promote oxidation of low-density lipoproteins, and contain oxidation-specific lipid-protein adducts. J Clin Invest. 1991 Jan;87(1):90–99. doi: 10.1172/JCI115006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossitch E., Jr, Alexander E., 3rd, Black P. M., Cooke J. P. L-arginine normalizes endothelial function in cerebral vessels from hypercholesterolemic rabbits. J Clin Invest. 1991 Apr;87(4):1295–1299. doi: 10.1172/JCI115132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon B. C., Cunningham L. D., Cohen R. A. Oxidized low density lipoproteins cause contraction and inhibit endothelium-dependent relaxation in the pig coronary artery. J Clin Invest. 1990 Jul;86(1):75–79. doi: 10.1172/JCI114718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreeharan N., Jayakody R. L., Senaratne M. P., Thomson A. B., Kappagoda C. T. Endothelium-dependent relaxation and experimental atherosclerosis in the rabbit aorta. Can J Physiol Pharmacol. 1986 Nov;64(11):1451–1453. doi: 10.1139/y86-246. [DOI] [PubMed] [Google Scholar]
- Steinberg D., Parthasarathy S., Carew T. E., Khoo J. C., Witztum J. L. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med. 1989 Apr 6;320(14):915–924. doi: 10.1056/NEJM198904063201407. [DOI] [PubMed] [Google Scholar]
- Steinbrecher U. P., Lougheed M., Kwan W. C., Dirks M. Recognition of oxidized low density lipoprotein by the scavenger receptor of macrophages results from derivatization of apolipoprotein B by products of fatty acid peroxidation. J Biol Chem. 1989 Sep 15;264(26):15216–15223. [PubMed] [Google Scholar]
- Steinbrecher U. P., Zhang H. F., Lougheed M. Role of oxidatively modified LDL in atherosclerosis. Free Radic Biol Med. 1990;9(2):155–168. doi: 10.1016/0891-5849(90)90119-4. [DOI] [PubMed] [Google Scholar]
- Tanner F. C., Noll G., Boulanger C. M., Lüscher T. F. Oxidized low density lipoproteins inhibit relaxations of porcine coronary arteries. Role of scavenger receptor and endothelium-derived nitric oxide. Circulation. 1991 Jun;83(6):2012–2020. doi: 10.1161/01.cir.83.6.2012. [DOI] [PubMed] [Google Scholar]
- Tomita T., Ezaki M., Miwa M., Nakamura K., Inoue Y. Rapid and reversible inhibition by low density lipoprotein of the endothelium-dependent relaxation to hemostatic substances in porcine coronary arteries. Heat and acid labile factors in low density lipoprotein mediate the inhibition. Circ Res. 1990 Jan;66(1):18–27. doi: 10.1161/01.res.66.1.18. [DOI] [PubMed] [Google Scholar]
- Verbeuren T. J., Jordaens F. H., Van Hove C. E., Van Hoydonck A. E., Herman A. G. Release and vascular activity of endothelium-derived relaxing factor in atherosclerotic rabbit aorta. Eur J Pharmacol. 1990 Nov 27;191(2):173–184. doi: 10.1016/0014-2999(90)94145-n. [DOI] [PubMed] [Google Scholar]
- Verbeuren T. J., Jordaens F. H., Zonnekeyn L. L., Van Hove C. E., Coene M. C., Herman A. G. Effect of hypercholesterolemia on vascular reactivity in the rabbit. I. Endothelium-dependent and endothelium-independent contractions and relaxations in isolated arteries of control and hypercholesterolemic rabbits. Circ Res. 1986 Apr;58(4):552–564. doi: 10.1161/01.res.58.4.552. [DOI] [PubMed] [Google Scholar]
- Ylä-Herttuala S., Palinski W., Rosenfeld M. E., Parthasarathy S., Carew T. E., Butler S., Witztum J. L., Steinberg D. Evidence for the presence of oxidatively modified low density lipoprotein in atherosclerotic lesions of rabbit and man. J Clin Invest. 1989 Oct;84(4):1086–1095. doi: 10.1172/JCI114271. [DOI] [PMC free article] [PubMed] [Google Scholar]



