Abstract
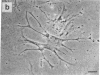
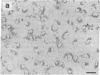
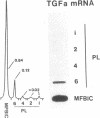
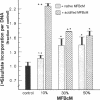
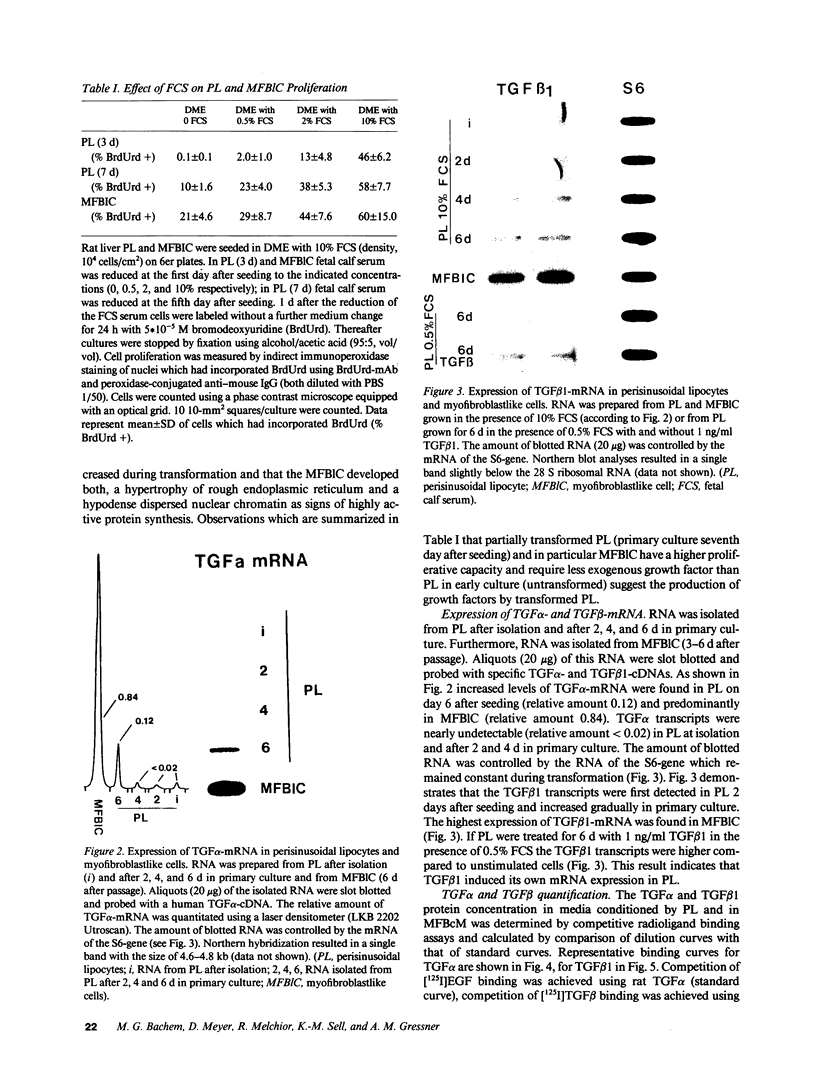
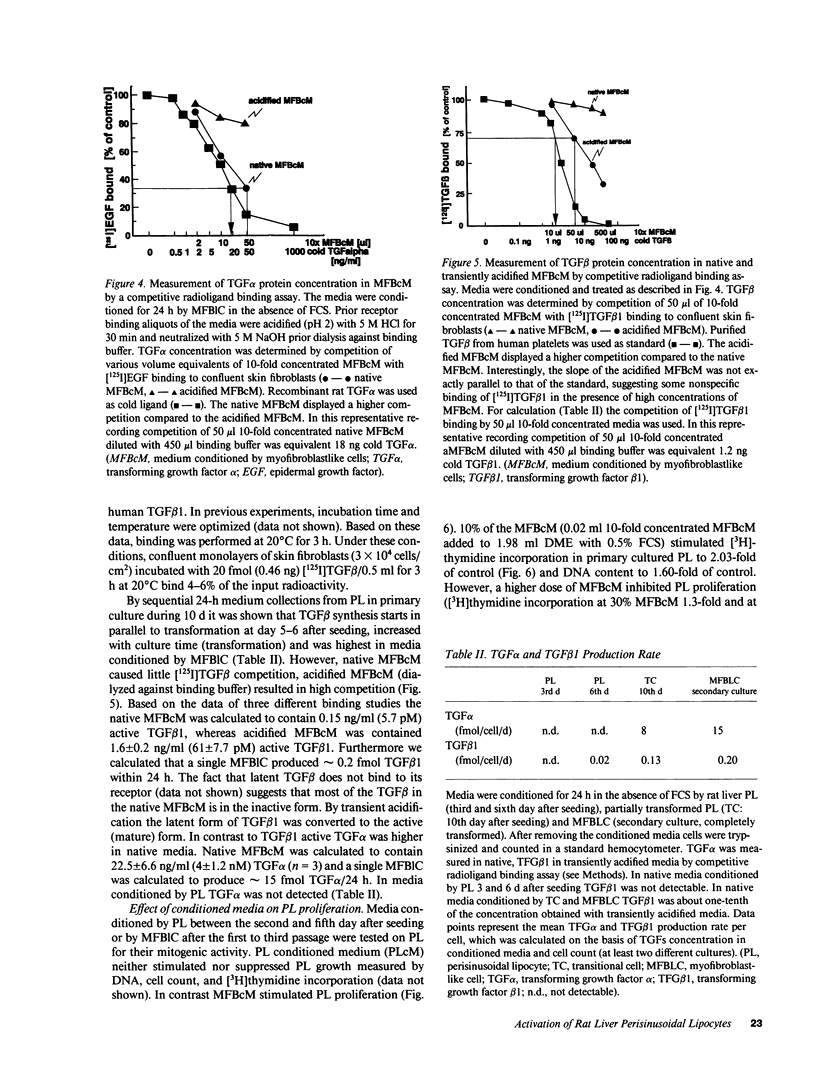
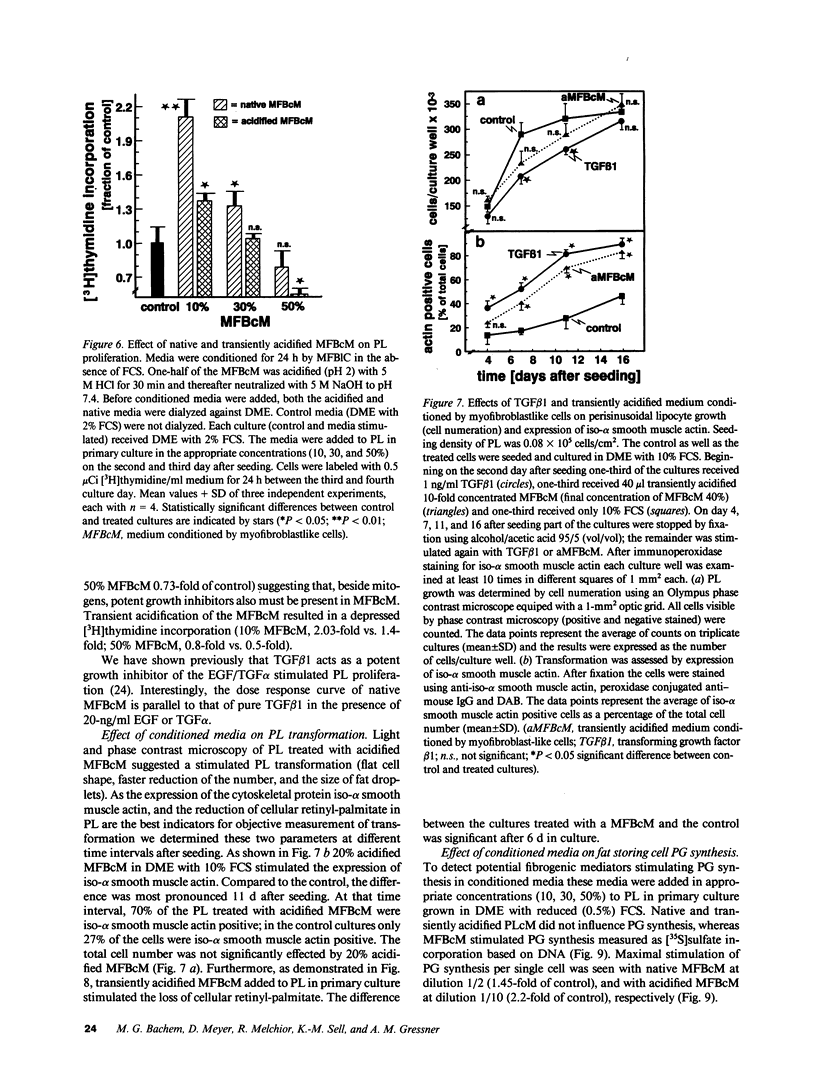
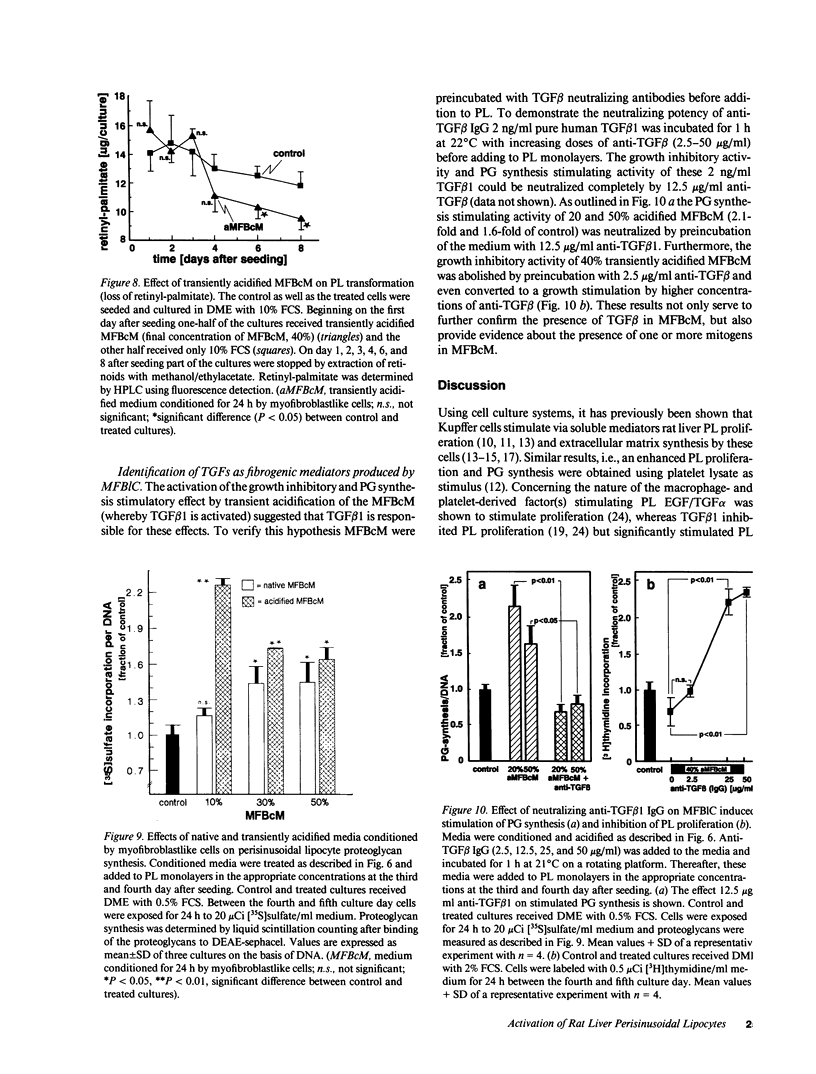
Rat liver perisinusoidal lipocytes (PL) cultured on uncoated plastic transform spontaneously within 6-10 d to myofibroblastlike cells (MFBlC). Parallel to the transformation the TGF alpha- and TGF beta 1-mRNA expression increased and was highest in MFBlC. Competitive radioligand binding assays demonstrated that in contrast to untransformed PL the MFBlC synthesize and secrete transforming growth factor (TGF)-alpha (15 fmol/cell per 24 h) and predominantly the latent form of TGF beta 1 (0.2 fmol/cell per 24 h). Medium conditioned by MFBlC (MFBcM) significantly stimulated PL proliferation with little effect on PL proteoglycan synthesis. By transient acidification of the MFBcM, known to activate the latent form of TGF beta 1, the stimulatory effect on PL proteoglycan synthesis was enhanced and furthermore PL transformation (measured by expression of iso-alpha smooth muscle actin and loss of retinylpalmitate) was accelerated. Preincubation of this medium with neutralizing antibodies to TGF beta resulted in (a) the growth inhibitory effect was converted to a growth stimulation and (b) the stimulatory effect on proteoglycan synthesis was abolished. In summary our data indicate that progressive activation of PL on plastic (transformation to MFBlC) leads to an enhanced expression of the TGF alpha- and TGF beta 1-mRNAs and secretion of the corresponding proteins. Medium conditioned by MFBIC stimulates proliferation, transformation, and PG synthesis of untransformed PL. These mechanisms are suggested to be relevant in self perpetuation of liver fibrogenesis.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andus T., Ramadori G., Heinrich P. C., Knittel T., Meyer zum Büschenfelde K. H. Cultured Ito cells of rat liver express the alpha 2-macroglobulin gene. Eur J Biochem. 1987 Nov 2;168(3):641–646. doi: 10.1111/j.1432-1033.1987.tb13464.x. [DOI] [PubMed] [Google Scholar]
- Bachem M. G., Melchior R., Gressner A. M. The role of thrombocytes in liver fibrogenesis: effects of platelet lysate and thrombocyte-derived growth factors on the mitogenic activity and glycosaminoglycan synthesis of cultured rat liver fat storing cells. J Clin Chem Clin Biochem. 1989 Sep;27(9):555–565. doi: 10.1515/cclm.1989.27.9.555. [DOI] [PubMed] [Google Scholar]
- Bachem M. G., Riess U., Gressner A. M. Liver fat storing cell proliferation is stimulated by epidermal growth factor/transforming growth factor alpha and inhibited by transforming growth factor beta. Biochem Biophys Res Commun. 1989 Jul 31;162(2):708–714. doi: 10.1016/0006-291x(89)92368-1. [DOI] [PubMed] [Google Scholar]
- Bachem M. G., Riess U., Melchior R., Sell K. M., Gressner A. M. Transforming growth factors (TGF alpha and TGF beta 1) stimulate chondroitin sulfate and hyaluronate synthesis in cultured rat liver fat storing cells. FEBS Lett. 1989 Oct 23;257(1):134–137. doi: 10.1016/0014-5793(89)81804-6. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Davis B. H. Transforming growth factor beta responsiveness is modulated by the extracellular collagen matrix during hepatic ito cell culture. J Cell Physiol. 1988 Sep;136(3):547–553. doi: 10.1002/jcp.1041360323. [DOI] [PubMed] [Google Scholar]
- Friedman S. L., Arthur M. J. Activation of cultured rat hepatic lipocytes by Kupffer cell conditioned medium. Direct enhancement of matrix synthesis and stimulation of cell proliferation via induction of platelet-derived growth factor receptors. J Clin Invest. 1989 Dec;84(6):1780–1785. doi: 10.1172/JCI114362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman S. L. Cellular sources of collagen and regulation of collagen production in liver. Semin Liver Dis. 1990 Feb;10(1):20–29. doi: 10.1055/s-2008-1040454. [DOI] [PubMed] [Google Scholar]
- Friedman S. L., Roll F. J., Boyles J., Arenson D. M., Bissell D. M. Maintenance of differentiated phenotype of cultured rat hepatic lipocytes by basement membrane matrix. J Biol Chem. 1989 Jun 25;264(18):10756–10762. [PubMed] [Google Scholar]
- Geerts A., Vrijsen R., Rauterberg J., Burt A., Schellinck P., Wisse E. In vitro differentiation of fat-storing cells parallels marked increase of collagen synthesis and secretion. J Hepatol. 1989 Jul;9(1):59–68. doi: 10.1016/0168-8278(89)90076-7. [DOI] [PubMed] [Google Scholar]
- Gressner A. M., Bachem M. G. Cellular sources of noncollagenous matrix proteins: role of fat-storing cells in fibrogenesis. Semin Liver Dis. 1990 Feb;10(1):30–46. doi: 10.1055/s-2008-1040455. [DOI] [PubMed] [Google Scholar]
- Gressner A. M., Haarmann R. Regulation of hyaluronate synthesis in rat liver fat storing cell cultures by Kupffer cells. J Hepatol. 1988 Dec;7(3):310–318. doi: 10.1016/s0168-8278(88)80003-5. [DOI] [PubMed] [Google Scholar]
- Gressner A. M., Zerbe O. Kupffer cell-mediated induction of synthesis and secretion of proteoglycans by rat liver fat-storing cells in culture. J Hepatol. 1987 Dec;5(3):299–310. doi: 10.1016/s0168-8278(87)80036-3. [DOI] [PubMed] [Google Scholar]
- Huang S. S., O'Grady P., Huang J. S. Human transforming growth factor beta.alpha 2-macroglobulin complex is a latent form of transforming growth factor beta. J Biol Chem. 1988 Jan 25;263(3):1535–1541. [PubMed] [Google Scholar]
- Kondaiah P., Van Obberghen-Schilling E., Ludwig R. L., Dhar R., Sporn M. B., Roberts A. B. cDNA cloning of porcine transforming growth factor-beta 1 mRNAs. Evidence for alternate splicing and polyadenylation. J Biol Chem. 1988 Dec 5;263(34):18313–18317. [PubMed] [Google Scholar]
- Labarca C., Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem. 1980 Mar 1;102(2):344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- Lyons R. M., Keski-Oja J., Moses H. L. Proteolytic activation of latent transforming growth factor-beta from fibroblast-conditioned medium. J Cell Biol. 1988 May;106(5):1659–1665. doi: 10.1083/jcb.106.5.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak K. M., Leo M. A., Lieber C. S. Alcoholic liver injury in baboons: transformation of lipocytes to transitional cells. Gastroenterology. 1984 Jul;87(1):188–200. [PubMed] [Google Scholar]
- Matsuoka M., Pham N. T., Tsukamoto H. Differential effects of interleukin-1 alpha, tumor necrosis factor alpha, and transforming growth factor beta 1 on cell proliferation and collagen formation by cultured fat-storing cells. Liver. 1989 Apr;9(2):71–78. doi: 10.1111/j.1600-0676.1989.tb00382.x. [DOI] [PubMed] [Google Scholar]
- Matsuoka M., Tsukamoto H. Stimulation of hepatic lipocyte collagen production by Kupffer cell-derived transforming growth factor beta: implication for a pathogenetic role in alcoholic liver fibrogenesis. Hepatology. 1990 Apr;11(4):599–605. doi: 10.1002/hep.1840110412. [DOI] [PubMed] [Google Scholar]
- McCaffrey T. A., Falcone D. J., Brayton C. F., Agarwal L. A., Welt F. G., Weksler B. B. Transforming growth factor-beta activity is potentiated by heparin via dissociation of the transforming growth factor-beta/alpha 2-macroglobulin inactive complex. J Cell Biol. 1989 Jul;109(1):441–448. doi: 10.1083/jcb.109.1.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee J. O., Patrick R. S. The role of perisinusoidal cells in hepatic fibrogenesis. An electron microscopic study of acute carbon tetrachloride liver injury. Lab Invest. 1972 Apr;26(4):429–440. [PubMed] [Google Scholar]
- Mendoza-Figueroa T., Argüello C., Kuri-Harcuch W. Culture of proliferating and differentiating fat-storing cells in 3T3-conditioned medium. Biol Cell. 1988;64(1):29–38. doi: 10.1016/0248-4900(88)90090-1. [DOI] [PubMed] [Google Scholar]
- Meyer D. H., Bachem M. G., Gressner A. M. Modulation of hepatic lipocyte proteoglycan synthesis and proliferation by Kupffer cell-derived transforming growth factors type beta 1 and type alpha. Biochem Biophys Res Commun. 1990 Sep 28;171(3):1122–1129. doi: 10.1016/0006-291x(90)90801-s. [DOI] [PubMed] [Google Scholar]
- Milani S., Herbst H., Schuppan D., Hahn E. G., Stein H. In situ hybridization for procollagen types I, III and IV mRNA in normal and fibrotic rat liver: evidence for predominant expression in nonparenchymal liver cells. Hepatology. 1989 Jul;10(1):84–92. doi: 10.1002/hep.1840100117. [DOI] [PubMed] [Google Scholar]
- Miyazono K., Heldin C. H. Role for carbohydrate structures in TGF-beta 1 latency. Nature. 1989 Mar 9;338(6211):158–160. doi: 10.1038/338158a0. [DOI] [PubMed] [Google Scholar]
- Miyazono K., Hellman U., Wernstedt C., Heldin C. H. Latent high molecular weight complex of transforming growth factor beta 1. Purification from human platelets and structural characterization. J Biol Chem. 1988 May 5;263(13):6407–6415. [PubMed] [Google Scholar]
- Mooradian D. L., Lucas R. C., Weatherbee J. A., Furcht L. T. Transforming growth factor-beta 1 binds to immobilized fibronectin. J Cell Biochem. 1989 Dec;41(4):189–200. doi: 10.1002/jcb.240410404. [DOI] [PubMed] [Google Scholar]
- Nakatsukasa H., Evarts R. P., Hsia C. C., Thorgeirsson S. S. Transforming growth factor-beta 1 and type I procollagen transcripts during regeneration and early fibrosis of rat liver. Lab Invest. 1990 Aug;63(2):171–180. [PubMed] [Google Scholar]
- O'Connor-McCourt M. D., Wakefield L. M. Latent transforming growth factor-beta in serum. A specific complex with alpha 2-macroglobulin. J Biol Chem. 1987 Oct 15;262(29):14090–14099. [PubMed] [Google Scholar]
- Ogawa K., Suzuki J., Narasaki M., Mori M. Healing of focal injury in the rat liver. Am J Pathol. 1985 Apr;119(1):158–167. [PMC free article] [PubMed] [Google Scholar]
- Prinz R., Klein U., Sudhakaran P. R., Sinn W., Ullrich K., von Figura K. Metabolism of sulfated glycosaminoglycans in rat hepatocytes. Synthesis of heparan sulfate and distribution into cellular and extracellular pools. Biochim Biophys Acta. 1980 Jul 3;630(3):402–413. doi: 10.1016/0304-4165(80)90289-5. [DOI] [PubMed] [Google Scholar]
- Schäfer S., Zerbe O., Gressner A. M. The synthesis of proteoglycans in fat-storing cells of rat liver. Hepatology. 1987 Jul-Aug;7(4):680–687. doi: 10.1002/hep.1840070411. [DOI] [PubMed] [Google Scholar]
- Shiratori Y., Geerts A., Ichida T., Kawase T., Wisse E. Kupffer cells from CCl4-induced fibrotic livers stimulate proliferation of fat-storing cells. J Hepatol. 1986;3(3):294–303. doi: 10.1016/s0168-8278(86)80481-0. [DOI] [PubMed] [Google Scholar]
- Wakefield L. M., Smith D. M., Flanders K. C., Sporn M. B. Latent transforming growth factor-beta from human platelets. A high molecular weight complex containing precursor sequences. J Biol Chem. 1988 Jun 5;263(16):7646–7654. [PubMed] [Google Scholar]
- Zerbe O., Gressner A. M. Proliferation of fat-storing cells is stimulated by secretions of Kupffer cells from normal and injured liver. Exp Mol Pathol. 1988 Aug;49(1):87–101. doi: 10.1016/0014-4800(88)90023-8. [DOI] [PubMed] [Google Scholar]