Abstract
The mortality rate among patients suffering acute respiratory distress syndrome (ARDS) remains high despite implementation at clinical centers of the lung protective ventilatory strategies recommended by the International Guidelines for Management of Severe Sepsis and Septic Shock, 2012. This suggests that such strategies are still sub-optimal for some ARDS patients. For these patients, tailored use of ventilator settings should be considered, including: further reduction of tidal volumes, administration of neuromuscular blocking agents if the patient’s spontaneous breathing is incompatible with mechanical ventilation, and adjusting positive end-expiratory pressure (PEEP) settings based on transpulmonary pressure levels.
Keywords: ARDS, transpulmonary pressure, lung protective strategy, strain
INTRODUCTION
Since the seminal report on “lung protective strategy” published by Amato et al. in the New England Journal of Medicine in 1998 [1], whether or not and in what ways the different strategies of mechanical ventilation affect the clinical outcome of ARDS patients remains a controversial topic among physicians and researchers. This concise review article focuses on the lessons that the Surviving Sepsis Campaign Guidelines (SSCG) 2012 report imparts regarding an optimized strategy for mechanically ventilating ARDS patients [2]. The SSCG 2012 report proposes a “lung protective strategy” for ARDS patients that includes the following 7 recommendations:
Tidal volume should be targeted to 6 mL/kg of predicted body weight (PBW)
Plateau pressures in a passively inflated lung should be limited to ≤30 cm H2O.
Positive end-expiratory pressure (PEEP) should be applied to avoid alveolar collapse at the end of expiration.
-
Higher levels of PEEP should be strategically used for patients with moderate or severe sepsis-induced ARDS
Even though it is difficult to specify the absolute values of higher levels of PEEP, we think the ARDSnet standard PEEP strategy is the reasonable choice for PEEP setting because the much higher PEEP strategy adopted in ALVEOLI trial’s higher PEEP group did not show significant improvement in survival [28]. Another choice may be the strategy adopted in EXPRESS trial [29]. They set PEEP as high as possible without increasing the maximum inspiratory plateau pressure > 28 – 30 cmH2O by keeping tidal volume of 6 mL/kg.
Recruitment maneuvers should be used in sepsis patients with severe refractory hypoxemia
Prone positioning should be used in sepsis-induced ARDS patients with a PaO2/FIO2 ratio < 100 mmHg in facilities that have experience with such practices.
A short-term course (< 48 h) of neuromuscular blocking agents (NMBAs), along with sedatives, should be prescribed for early, sepsis-induced ARDS and PaO2/FIO2< 150 mm Hg.
MORTALITY OF ARDS PATIENTS IN THE ERA OF LUNG PROTECTIVE STRATEGIES
A serious question that must be answered is whether strict implementation of the 7 recommendations proposed in the SSCG 2012 report will reduce the mortality rate in ARDS patients. Despite recent advances in medical treatment and technologies, the mortality rate among ARDS patients still remains as high as ~40% [3, 4]. One of the plausible explanations for this, despite the introduction of lung protective strategies, is that the patient populations included in the old [5, 6] and new [3, 4] studies might not be comparable, thereby introducing a misclassification bias [3]. As older studies [5, 6] tended to use relatively low levels of PEEP and FIO2, patients once classified as suffering ARDS might not meet current ARDS criteria. Therefore, older studies might have included less severe cases, potentially under-estimating the true mortality rate among ARDS-afflicted patients.
Another possible explanation could be a decrease of the incidence of ARDS [4]. In their population-based study Li et al. reported a lower incidence of ARDS despite an increase in patients’ severity of illness and comorbidities [4]. Although the ARDS mortality rate has not changed over time, the mortality rate of patients at risk of ARDS has been reduced. Fuller et al. reported in their systematic review that in mechanically ventilated patients who did not manifest ARDS at the time of endotracheal intubation, the use of lower tidal volume ventilation reduced their progression to ARDS [7]. Prophylactic use of lung protective strategies for those patients who are at risk of, but have not yet manifested, ARDS might help to avoid its progression. However, the mortality rate among patients who do suffer ARDS is still high.
HOW TO REDUCE MORTALITY OF ARDS PATIENTS?
What should we do to reduce mortality among patients afflicted with ARDS? The present authors propose the following. First, greater efforts to implement lung protective strategies should be undertaken. Needham et al. revealed in a prospective cohort study that lung protective strategies were used in only 41% of all eligible cases. And they confirmed that, compared with non-adherence to lung protective strategies, the estimated absolute mortality risk reduction over two years in a patient with half-adherence to such strategies was 4.0%, while perfect adherence resulted in a 7.8% risk reduction [8].
Second, limiting the tidal volume to 6 mL/kg PBW and the plateau pressure to 30 cmH2O may not be sufficient to minimize lung injury in certain severe ARDS patients. Terragni et al. showed that in patients with a large, dependent, non-aerated compartment, tidal volume of 6 mL/kgPBW resulted in an increasing number of hyper-inflated compartments and a decreasing number of normally aerated compartments. In fact, these patients exhibited increased levels of pulmonary inflammatory cytokines [9]. In such cases, the tidal volume should be further reduced to as low as 4 mL/kg PBW according to the ARDSnet protocol [10].
Third, as ARDS is a heterogeneous syndrome, optimization of ventilator settings for each individual would be required when mechanically ventilating ARDS patients. For example, among patients with recruitable lung, increasing PEEP may help to avoid the cyclic opening and closing of alveoli without increasing over-distention (alveolar “strain”). However, among patients with no- or little-recruitable lung, increasing PEEP may not prevent such cyclic opening and closing but also cause over-distention [11]. Therefore, while raising PEEP might cause harm in some patients it may benefit others. Alveolar recruitability may be assessed at bedside in the near future by computed tomography (CT) and electrical impedance tomography (EIT), thereby making it possible to individually optimize ventilator settings for ARDS patients.
Fourth, high-frequency oscillation (HFO) is now considered as an alternative to conventional ventilation (CV). Compared with CV, HFO was hypothesized to be a superior ventilatory strategy, as it could avoid cyclic collapse and hyperinflation of the alveoli. However, in two recent randomized controlled trials (RCTs) comparing HFO and CV in ARDS adult patients, while HFO prevented severe hypoxemia, it did not improve hospital mortality rates [12, 13]. In both trials, mean airway pressure was higher in the HFO group than in the CV group by more than 5 cmH2O. Higher airway pressure in HFO might cause more severe lung injury or hemodynamic compromise. Guervilly et al. demonstrated in a recent prospective study that using high-mean airway pressure in subjects under HFO worsened right ventricular function compared with CV in ARDS patients [14]. Sedative agents and NMBA were more frequently used in HFO groups, which may have negatively affected patients’ prognosis. Furthermore, many physicians have less clinical experience with HFO than with CV. As Malhorta et al. commented in their editorial, it was not HFO itself but the HFO protocols and management strategies used in these clinical trials that were less effective than the established lung protective strategy using CV [15]. In this context, there might be more room for HFO protocols to be better optimized for individual ARDS patients.
PLEURAL PRESSURE AND TRANSPULMONARY PRESSURE
In addition to CT and EIT, pleural pressure and transpulmonary pressure must be taken into account when optimizing ventilator settings for individual ARDS patients, as it is transpulmonary pressure (stress), but not airway pressure per se, that determines alveolar size (lung volume) during ventilation. Pleural pressure can be estimated from esophageal pressure measurements.
transpulmonary pressure
= alveolar pressure – pleural pressure
delta transpulmonary pressure / lung elastance
= delta lung volume
It has been reported that overstretch-induced lunginjury occurs when alveoli are stretched above a specific threshold level [16, 17]. In their animal study, Protti et al. demonstrated that lung damage develops at strain, the ratio of delta volume / functional residual capacity (FRC) > 2 [17].
strain (delta volume / FRC)
= delta transpulmonary pressure / (lung elastance x FRC)
= delta transpulmonary pressure / specific lung elastance.
specific lung elastance
= delta transpulmonary pressure / (delta lung volume / FRC)
= lung elastance x FRC (cmH2O)
Delta lung volume is the lung volume change from FRC.
Delta transpulmonary pressure is the transpulmonary pressure change from the atmosphere.
Elastance is a reciprocal number of compliance and compliance of ARDS is low.
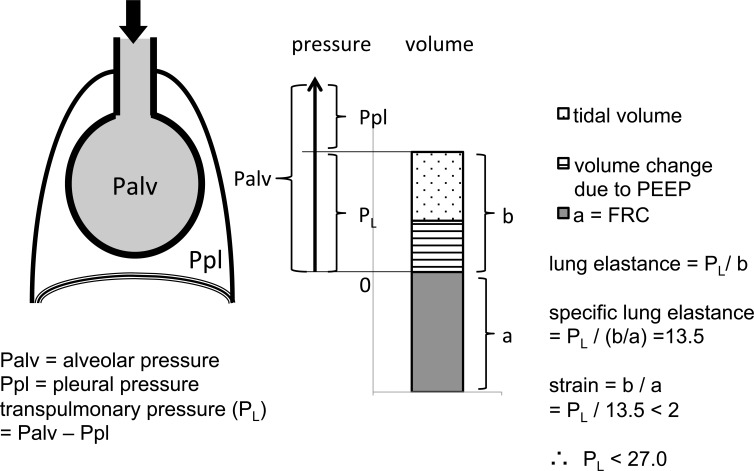
Specific lung elastance is a transpulmonary pressure that makes delta volume (b in the Fig. 1) equal to FRC (a in the Fig. 1).
Fig. (1).
Safe thresholds for transpulmonary (PL) and alveolar (Palv) pressures. This figure illustrates why the safe threshold of PL is 27 cmH2O while that of Palv may be more than 27 cmH2O. Strain must remain less than 2 to prevent lung injury [16, 17]. Because specific lung elastance (PL x FRC / b) is around 13.5 cmH2O [16], a PL divided by 13.5 must be less than 2.0. Therefore, PL must be less than 27 cmH2O. Pressure applied to the airway is utilized separately to inflate the thoracic cage (pleural pressure) and lung (transpulmonary pressure). Therefore, in some patients the safe threshold of Palv (PL + Ppl) may be higher than 30 -35 cmH2O.
Chiumello et al elucidated that the specific lung elastance was around 13.5 cmH2O in all the subgroups (surgical patients, medical patients, ALI patients and ARDS patients), and was not affected by the PEEP and tidal volume [16]. As the results, any transpulmonary pressure greater than 13.5 x 2 may represent the upper threshold at which overstretch-induced lung injury occurs (Fig. 1). This number of 27 cmH2O is close to the recommended upper transpulmonary pressure limit by Grasso et al who showed that overinflation starts from plateau transpulmonary pressure of around 25 cmH2O [30]. For example, if transpulmonary pressure was far less than 27 cmH2O and airway pressure was more than30 cmH2O, physicians should further increase PEEP to avoid cyclic alveolar collapse, and to permit proper ventilation of the lungs. Grasso et al. reported that in patients with influenza A (H1N1)-associated ARDS, a ventilatory strategy that raising PEEP (and plateau pressure) while maintaining transpulmonary pressure < 25 cmH2O lessened the possibility of respiratory failure such that the patients recovered without the use of extracorporeal membrane oxygenation (ECMO) [18]. On the other hand, all patients with a transpulmonary pressure > 25 cmH2O subsequently deteriorated and required ECMO support. In such cases, raising PEEP might have resulted in overstretch-induced lung injury.
One study proposed that when optimizing ventilator settings for individual ARDS patients, PEEP should be adjusted based on esophageal pressure measurements [19]. In those patients who exhibit excessively high pleural pressures and inadequately low PEEP, the calculated transpulmonary pressure at the end of expiration could be less than zero. For example, if a 10 cmH2O PEEP was applied to a patient with 15 cmH2O pleural pressure at the end of expiration, his/her transpulmonary pressure would be -5 cmH2O, a level incapable of sustaining the patency of the alveoli. Therefore, PEEP must be increased to a level that would result in positive transpulmonary pressure at the end of expiration. In an observational study, Talmor et al. showed that optimization of ventilator settings basedon transpulmonary pressure values significantly improve oxygenation and respiratory system compliance.
One must be aware that the onset of spontaneous breathing during mechanical ventilation can be harmful to some ARDS patients. Spontaneous breathing during lung protective mechanical ventilation in patients with ARDS could induce a large negative deflection in pleural pressure [20]. This large negative pleural pressure could, in turn, increase transpulmonary pressure, potentially inducing lung injury. Yoshida et al. demonstrated in their animal study that spontaneous breathing superimposed on CV could induce lung injury even when plateau pressure was kept below 30 cmH2O [21]. Large negative pleural pressure could also increase a transcapillary pressure gradient, thereby causing pulmonary edema [22]. Increased work of breathing due to a large increase in negative pleural pressure may be associated with elevated inflammatory cytokine levels [23].
Administration of NMBAs may be advisable to ease a patient’s inspiratory effort in this way thus reducing transpulmonary pressure. Papazian et al. have demonstrated in a randomized controlled trial (RCT) that short-term treatment with NMBAs (< 48 hours) reduced mortality rates in those patients with early, sepsis-induced severe ARDS [24]. However, NMBAs should not be routinely administered to all ARDS patients. Using an animal ARDS model, Yoshida et al. demonstrated that whereas spontaneous breathing could worsen severely injured lungs, it could alleviate mildly injured lungs, since lung recruitment was only possible in the latter [25]. It is worth noting that two identical tidal volume settings could result in different outcomes, depending on whether or not spontaneous breathing is permitted. Additionally, the distribution of a given tidal volume can occur differently. Yoshida et al. suggested that in cases of severe lung injury, strong spontaneous breathing efforts and high transpulmonary pressure can lead to increased rate of cyclic alveolar opening and collapse in those affected regions surrounding the diaphragm. However, the distributionof pleural pressure could be very inhomogeneous and unpredictable when expansion of lungs is inhomogenous [26]. In rabbits with positive pressure ventilation, Egan showed that the larger the amount of closed areas, the higher is the regional overdistension (the ratio of inflated volume to FRC) of the remaining open ones [31]. This study implies that if a considerable part of the lung suffers collapse, the transpulmonary pressure of the area adjacent to the collapsed tissue may become very high, because “vacuum effect” may be produced inside the chest wall by collapsed tissue. Therefore, we must be aware that imposing positive airway pressure to the patient with imhomogenous lung while a patient is paralyzed from NMBAs can cause much larger transpulmonary pressure than the pressure predicted in healthy lung.
Measuring pleural pressure at bedside is a challenging task. It is technically difficult to insert a catheter into the esophagus, and then properly position and calibrate it. When critically ill patients are in a supine position, measurements of esophageal pressure might result in artifacts associated with body position and pathologic conditions, thus rendering it less accurate and reliable. In a canine ARDS model Pelosi et al. found a vertical gradient of the pleural pressure in a supine position. The esophageal pressures closely matched the actual pleural pressures at the surface of the mid-lung when the animal was placed in a supine position [27]. However, the pleural pressures in the nondependentand dependent lung legions were 7 cmH2O lower and 4 cmH2O higher, respectively, than the esophageal pressures. However, when airway pressure was increased, the measured pleural pressure in all regions changed in similar fashion. This suggests that one could estimate, with some precision, variations in pleural pressure using the measured variation in esophageal pressure.
In summary, the authors believe that the protective ventilatory strategy recommended in the SSCG 2012 report is sub-optimal for some ARDS patients. For such patients, individual optimizations of ventilator settings should be performed. The authors would also propose to further reduce tidal volume, use NMBAs if necessary, and if possible, determine PEEP settings based on transpulmonary pressure for those ARDS patients who failed to improve using the SSCG 2012-recommended ventilatory protocol.
ACKNOWLEDGEMENTS
The authors thank Dr. Motomu Shimaoka (Mie University Medical School) for his valuable comments.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Amato MB, Barbas CS, Medeiros DM, Magaldi RB, Schettino GP, Lorenzi-Filho G, Kairalla RA, Deheinzelin D, Munoz C, Oliveira R, Takagaki TY, Carvalho CR. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N. Engl. J. Med. 1998;338:347–354. doi: 10.1056/NEJM199802053380602. [DOI] [PubMed] [Google Scholar]
- 2.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, Osborn TM, Nunnally ME, Townsend SR, Reinhart K, Kleinpell RM, Angus DC, Deutschman CS, Machado FR, Rubenfeld GD, Webb SA, Beale RJ, Vincent JL, Moreno R. Surviving Sepsis Campaign Guidelines Committee including the Pediatric Subgroup Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit. Care Med. 2013;41:580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 3.Villar J, Blanco J, Añón JM, Santos-Bouza A, Blanch L, Ambrós A, Gandía F, Carriedo D, Mosteiro F, Basaldúa S, Fernández RL, Kacmarek RM. ALIEN Network. The ALIEN study: incidence and outcome of acute respiratory distress syndrome in the era of lung protective ventilation. Intensive Care Med. 2011;37:1932–41. doi: 10.1007/s00134-011-2380-4. [DOI] [PubMed] [Google Scholar]
- 4.Li G, Malinchoc M, Cartin-Ceba R, Venkata CV, Kor DJ, Peters SG, Hubmayr RD, Gajic O. Eight-year trend of acute respiratory distress syndrome: a population-based study in Olmsted County, Minnesota. Am. J. Respir. Crit. Care Med. 2011;183:59–66. doi: 10.1164/rccm.201003-0436OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bersten AD, Edibam C, Hunt T, Moran J. Australian and New Zealand Intensive Care Society Clinical Trials Group.Incidence and mortality of acute lung injury and the acute respiratory distress syndrome in three Australian states. . Am. J. Respir. Crit. Care Med. 2002;165:443–448. doi: 10.1164/ajrccm.165.4.2101124. [DOI] [PubMed] [Google Scholar]
- 6.Luhr OR, Antonsen K, Karlsson M, Aardal S, Thorsteinsson A, Frostell CG, Bonde J. Incidence and mortality after acute respiratory failure and acute respiratory distress syndrome in Sweden, Denmark and Iceland. Am. J. Respir. Crit. Care Med. 1999;159:1849–1861. doi: 10.1164/ajrccm.159.6.9808136. [DOI] [PubMed] [Google Scholar]
- 7.Fuller BM, Mohr NM, Drewry AM, Carpenter CR. Lower tidal volume at initiation of mechanical ventilation may reduce progression to acute respiratory distress syndrome: a systematic review. Crit. Care. 2013;17:R11. doi: 10.1186/cc11936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Needham DM, Colantuoni E, Mendez-Tellez PA, Dinglas VD, Sevransky JE, Dennison Himmelfarb CR, Desai SV, Shanholtz C, Brower RG, Pronovost PJ. Lung protective mechanical ventilation and two year survival in patients with acute lung injury: prospective cohort study. BMJ. 2012;344:e2124. doi: 10.1136/bmj.e2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Terragni PP, Rosboch G, Tealdi A, Corno E, Menaldo E, Davini O, Gandini G, Herrmann P, Mascia L, Quintel M, Slutsky AS, Gattinoni L, Ranieri VM. Tidal hyperinflation during low tidal volume ventilation in acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2007;175:160–166. doi: 10.1164/rccm.200607-915OC. [DOI] [PubMed] [Google Scholar]
- 10.Acute Respiratory Distress Syndrome Network.Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N. Engl. J. Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 11.Caironi P, Cressoni M, Chiumello D, Ranieri M, Quintel M, Russo SG, Cornejo R, Bugedo G, Carlesso E, Russo R, Caspani L, Gattinoni L. Lung opening and closing during ventilation of acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2010;181:578–586. doi: 10.1164/rccm.200905-0787OC. [DOI] [PubMed] [Google Scholar]
- 12.Ferguson ND, Cook DJ, Guyatt GH, Mehta S, Hand L, Austin P, Zhou Q, Matte A, Walter SD, Lamontagne F, Granton JT, Arabi YM, Arroliga AC, Stewart TE, Slutsky AS, Meade MO. OSCILLATE Trial Investigators, Canadian Critical Care Trials Group.High-frequency oscillation in early acute respiratory distress syndrome. N. Engl. J. Med. 2013;368:795–805. doi: 10.1056/NEJMoa1215554. [DOI] [PubMed] [Google Scholar]
- 13.Young D, Lamb SE, Shah S, MacKenzie I, Tunnicliffe W, Lall R, Rowan K, Cuthbertson BH. OSCAR Study Group.High-frequency oscillation for acute respiratory distress syndrome. N. Engl. J. Med. 2013;368:806–13. doi: 10.1056/NEJMoa1215716. [DOI] [PubMed] [Google Scholar]
- 14.Guervilly C, Forel JM, Hraiech S, Demory D, Allardet-Servent J, Adda M, Barreau-Baumstark K, Castanier M, Papazian L, Roch A. Right ventricular function during high-frequency oscillatory ventilation in adults with acute respiratory distress syndrome. Crit. Care Med. 2012;40:1539–1545. doi: 10.1097/CCM.0b013e3182451b4a. [DOI] [PubMed] [Google Scholar]
- 15.Malhotra A, Drazen JM. High-frequency oscillatory ventilation on shaky ground. N. Engl. J. Med. 2013;368:863–865. doi: 10.1056/NEJMe1300103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chiumello D, Carlesso E, Cadringher P, Caironi P, Valenza F, Polli F, Tallarini F, Cozzi P, Cressoni M, Colombo A, Marini JJ, Gattinoni L. Lung stress and strain during mechanical ventilation for acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2008;178:346–355. doi: 10.1164/rccm.200710-1589OC. [DOI] [PubMed] [Google Scholar]
- 17.Protti A, Cressoni M, Santini A, Langer T, Mietto C, Febres D, Chierichetti M, Coppola S, Conte G, Gatti S, Leopardi O, Masson S, Lombardi L, Lazzerini M, Rampoldi E, Cadringher P, Gattinoni L. Lung stress and strain during mechanical ventilation: any safe threshold?. Am. J. Respir. Crit. Care Med. 2011;183:1354–1362. doi: 10.1164/rccm.201010-1757OC. [DOI] [PubMed] [Google Scholar]
- 18.Grasso S, Terragni P, Birocco A, Urbino R, Del Sorbo L, Filippini C, Mascia L, Pesenti A, Zangrillo A, Gattinoni L, Ranieri VM. ECMO criteria for influenza A (H1N1)-associated ARDS: role of transpulmonary pressure. Intensive Care Med. 2012;38:395–403. doi: 10.1007/s00134-012-2490-7. [DOI] [PubMed] [Google Scholar]
- 19.Talmor D, Sarge T, Malhotra A, O'Donnell CR, Ritz R, Lisbon A, Novack V, Loring SH. Mechanical ventilation guided by esophageal pressure in acute lung injury. N. Engl. J. Med. 2008;359:2095–2104. doi: 10.1056/NEJMoa0708638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kallet RH, Campbell AR, Dicker RA, Katz JA, Mackersie RC. Effects of tidal volume on work of breathing during lung-protective ventilation in patients with acute lung injury and acute respiratory distress syndrome. Crit. Care Med. 2006;34:8–14. doi: 10.1097/01.ccm.0000194538.32158.af. [DOI] [PubMed] [Google Scholar]
- 21.Yoshida T, Uchiyama A, Matsuura N, Mashimo T, Fujino Y. Spontaneous breathing during lung-protective ventilation in an experimental acute lung injury model: high transpulmonary pressure associated with strong spontaneous breathing effort may worsen lung injury. Crit. Care Med. 2012;40:1578–1585. doi: 10.1097/CCM.0b013e3182451c40. [DOI] [PubMed] [Google Scholar]
- 22.Kallet RH, Alonso JA, Luce JM, Matthay MA. Exacerbation of acute pulmonary edema during assisted mechanical ventilation using a low tidal-volume lung-protective strategy. Chest . 1999;116:1826–1832. doi: 10.1378/chest.116.6.1826. [DOI] [PubMed] [Google Scholar]
- 23.Toumpanakis D, Kastis GA, Zacharatos P, Sigala I, Michailidou T, Kouvela M, Glynos C, Divangahi M, Roussos C, Theocharis SE, Vassilakopoulos T. Inspiratory resistive breathing induces acute lung injury. Am. J. Respir. Crit. Care Med. 2011;182:1129–1136. doi: 10.1164/rccm.201001-0116OC. [DOI] [PubMed] [Google Scholar]
- 24.Papazian L, Forel JM, Gacouin A, Penot-Ragon C, Perrin G, Loundou A, Jaber S, Arnal JM, Perez D, Seghboyan JM, Constantin JM, Courant P, Lefrant JY, Guérin C, Prat G, Morange S, Roch A. ACURASYS Study Investigators Neuromuscular blockers in early acute respiratory distress syndrome. N. Engl. J. Med. 2010;363:1107–1116. doi: 10.1056/NEJMoa1005372. [DOI] [PubMed] [Google Scholar]
- 25.Yoshida T, Uchiyama A, Matsuura N, Mashimo T, Fujino Y. The comparison of spontaneous breathing and muscle paralysis in two different severities of experimental lung injury. Crit. Care Med. 2013;41:536–545. doi: 10.1097/CCM.0b013e3182711972. [DOI] [PubMed] [Google Scholar]
- 26.Marini JJ, Slutsky AS, Amato MBP, Marini JJ. Barotrauma, volutrauma, and the ventilation of acute lung injury Physiological basis of ventilatory support. New York Marcel Dekker. 1998:1187–1245. [Google Scholar]
- 27.Pelosi P, Goldner M, McKibben A, Adams A, Eccher G, Caironi P, Losappio S, Gattinoni L, Marini JJ. Recruitment and derecruitment during acute respiratory failure: an experimental study. Am. J. Respir. Crit. Care Med. 2001;164:122–130. doi: 10.1164/ajrccm.164.1.2007010. [DOI] [PubMed] [Google Scholar]
- 28.The National Heart, Lung, and Blood Institute ARDS Clinical Trials Network, Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N. Engl. J. Med. 2004;351:327–336. doi: 10.1056/NEJMoa032193. [DOI] [PubMed] [Google Scholar]
- 29.Mercat A, Richard JC, Vielle B, Jaber S, Osman D, Diehl JL, Lefrant JY, Prat G, Richecoeur J, Nieszkowska A, Gervais C, Baudot J, Bouadma L, Brochard L. Expiratory Pressure (Express) Study Group Positive end-expiratory pressure setting in adults with acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2008;299:646–655. doi: 10.1001/jama.299.6.646. [DOI] [PubMed] [Google Scholar]
- 30.Grasso S, Mascia L, Del Turco M, Malacarne P, Giunta F, Brochard L, Slutsky AS, Ranieri VM. Effects of recruiting maneuvers in patients with acute respiratory distress syndrome ventilated with protective ventilatory strategy. Anesthesiology. 2002;96:795–802. doi: 10.1097/00000542-200204000-00005. [DOI] [PubMed] [Google Scholar]
- 31.Egan EA. Lung inflation, lung solute permeability, and alveolar edema. J. Appl. Physiol. 1982;53:121–125. doi: 10.1152/jappl.1982.53.1.121. [DOI] [PubMed] [Google Scholar]