SUMMARY
MicroRNA-132 (miR-132) has been demonstrated to affect multiple neuronal functions and its dysregulation is linked to several neurological disorders. We previously showed that acute T. gondii infection induces miR-132 expression both in vitro and in vivo. To investigate the impact of chronic infection on miR-132, we infected mice with T. gondii PRU strain and performed assessment 5 months later in six brain regions (cortex, hypothalamus, striatum, cerebellum, olfactory bulb and hippocampus) by qPCR. We found that while acute infection of T. gondii increases the expression of miR-132, chronic infection has the opposite effect. The effect varied amongst different regions of the brain and presented in a sex-dependent manner, with females exhibiting more susceptibility than males. MiR-132 and brain derived neurotrophic factor (BDNF, an inducer of miR-132) were not co-varies in the brain areas of infected mice. T. gondii DNA/RNA was found in all tested brain regions and a selective tropism towards the hippocampus, based on bradyzoite density, was observed in both males and females. However, the expressions of miR-132 or BDNF were poorly reflected by the density of T. gondii in brain areas. Our findings highlight the importance of investigating the miR-132-mediated neuronal function in mice infected with T. gondii.
Keywords: Toxoplasma gondii, miR-132, BDNF, bradyzoite density, hippocampus, host sex
INTRODUCTION
Toxoplasma gondii (T. gondii) is a common protozoan that infects humans and other animals. Felines serve as the primary host in which T. gondii can undergo sexual reproduction and complete its life cycle. Humans, rodents, and other non-feline vertebrates can become infected with T. gondii and serve as intermediate hosts. It has been reported that T. gondii modifies the behavior of its intermediate hosts when the infection proceeds into its latent phase (Vyas et al. 2007), which is characterized by the presence of parasite cysts in the brain. This thereby raises the question of whether or not there is any tropism of T. gondii for a specific location in the brain. It is possible that preferential localization is the key to the behavioral manipulation.
T. gondii encystment has been found in most brain regions, yet tropism for specific brain regions remains controversial in the literature (Vyas et al. 2007; Hermes et al. 2008; Berenreiterová et al. 2011). Previous estimates of tropism were determined by tissue cyst density using the microscope-based method. However, tissue cysts can range from 5 to 100 μm in size containing just a few to thousands of encysted bradyzoites (Tomita et al. 2013). This fact makes direct comparisons based on cyst numbers less precise. As the number of bradyzoites is proportional to the size of the cyst, we assumed that the parasite’s preference for certain brain regions can be reflected in bradyzoite number, i.e. it is possible that bradyzoites divide differently in different regions due to preference or suitability, thereby forming cysts of varying sizes. This may provide an alternative way to examine the brain tropism of T. gondii and its influence on behavior. We thus explored T. gondii tropism for different brain regions in reference to bradyzoite number using a PCR-based approach.
MicroRNAs are a class of small, noncoding RNAs of 21–23 nucleotides that regulate gene expression at the post-transcriptional level by binding to the mRNA of protein coding genes. MicroRNA-132 (miR-132) is a neuron-enriched microRNA. Several targets for miR-132 have been described, including mediators of neurological development, synaptic transmission, inflammation and angiogenesis (Wanet et al. 2012). MiR-132 has thus been reported to play important roles in different physiological processes. Dysregulation of miR-132 is associated with several neurological disorders, such as schizophrenia, Alzheimer, Parkinson’s disease and tauopathies (Miller et al. 2012; Wanet et al. 2012), suggesting that this miRNA has a broader impact on different brain diseases. Brain derived neurotrophic factor (BDNF) is essential for a variety of neuronal aspects, including proliferation, differentiation, and survival in the CNS. Recent studies suggest that BDNF exerts beneficial effects on CNS neurons via upregulation of miR-132 (Remenyi et al. 2010). The BDNF-induced miR-132 transcription was dependent on the activation of ERK1/2 (Remenyi et al. 2010).
In a previous study, we demonstrated that acute infection with T. gondii induces the level of host miR-132 and this induction is associated with an altered dopamine pathway in infected mice by repressing the expression of relevant proteins (Xiao et al. 2014). However, the long-term effects of persistent brain infection with T. gondii on this microRNA are not known. Given the involvement of miR-132 in neurological disorders, it is possible that chronic T. gondii infection may also have an effect on the expression of miR-132. Hence the aim of the present study was to evaluate the expression of miR-132 in the latent phase of infection in multiple brain regions of a mouse model. We also examined several factors that may have the potential to modulate miR-132 expression within the brain, such as BDNF, T. gondii’s preferential localization, parasite load, and host sex.
MATERIALS AND METHODS
Animals
Male and female BALB/c mice (4.5 weeks old, The Jackson Laboratory, Bar Harbor, ME, USA) were used in this study. Animal protocols were reviewed and approved by the Animal Care and Use Committee of Johns Hopkins University (JHU), and all efforts were made to minimize the number of mice used and their suffering. Mice were housed 5 per cage in the JHU animal facility with 14.5/9.5 h of light/dark cycle and had free access to food and water.
T. gondii culture and infection
T. gondii Prugniaud strain (PRU, type II) was maintained by passage in human foreskin fibroblast (HFF) monolayers. Tachyzoites were released from cells using 18-, 23-, and 27-gauge needles in succession. Parasites were separated from cell debris by filter sterilization (Polycarbonate Membrane Filter, Whatman) and resuspended in Dulbecco’s phosphate buffered saline (DPBS). Mice of each sex were either mock-infected with sterile DPBS or infected with 400 tachyzoites (2 parasites/μl) intraperitoneally.
Brain Tissue Collection
The surviving mice (infected female: n = 10, infected male: n = 10, female control: n = 8, male control: n = 10) were sacrificed approximately 5 month post infection. The stage of the oestrus cycle was not monitored for females in this study. To collect tissue samples, mice were sacrificed following approved protocols. Their brains were dissected on wet ice to collect the following areas: hypothalamus (HTH), hippocampus (HC), cortex (CTX), striatum (STR), olfactory bulb (OB) and cerebellum (CRBL). These tissue samples were frozen immediately on dry ice and stored at −80 °C for subsequent RNA or DNA extraction.
Reverse transcription and quantitative PCR
Tissue material was divided in two: one half was used for RNA extraction and the other was used for DNA isolation. However, DNA from male cortex is not available in the current study. As our study is part of a larger ongoing project that evaluates the T. gondii hypothesis of schizophrenia in mouse models, the male cortex was used in many experiments that analyzed RNA and protein levels, thus not enough sample was left for DNA extraction. The total RNA was extracted from frozen tissue in 700 μL of QIAzol Lysis Reagent (Qiagen, Valencia, CA, USA) by tissue disruption and homogenization using a QIAshredder spin column (Qiagen) at 20,000g for 2mins. RNA was purified using the miRNeasy Mini kit (Qiagen). Reverse transcription was performed using either Multiscribe reverse transcriptase and random primers (Applied Biosystems, Foster City, CA, USA) to generate cDNA, or the MultiScribe miRNA Reverse Transcription Kit (Applied Biosystems) using miRNA-specific primers to produce miRNA. QPCR was performed using inventoried TaqMan miRNA and mRNA assays (Applied Biosystems) with standard ABI protocols and reagents. All samples were run in quadruplicate using snoRNA135 as endogenous miRNA controls and mouse β-actin as an endogenous mRNA control. Relative abundance was determined using the comparative ΔCt method. Levels of mature miR-132, miR-195 and BDNF of mice were determined. MiR-195 was used as a negative control for miR-132 quantification as its abundance would not be expected to differ between the control and infection groups (Xiao et al. 2014).
In vivo parasite load determination by qPCR
Parasite load was measured at both the DNA and RNA level by qPCR targeting T. gondii 5S rRNA and bradyzoite antigen1 (BAG1) genes (Ueno et al. 2009; Xiao et al. 2011), respectively, in samples of brain. BAG1 is the most abundant bradyzoite-specific gene. DNA was extracted using the DNeasy Blood & Tissue Kit (Qiagen). The expression levels of the target genes were normalized to mouse β-actin with the ΔCt method.
Statistical analysis
QPCR experiments were repeated at least three times with similar results, and values shown are means of three times. The results, after checking normal distribution, were first analyzed by multiple analysis of variance (MANOVA). Our data consists of two independent factors (infection and sex) and three dependent variables (miR-132, BDNF and miR-195). For each brain region, MANOVA was performed to determine which factors independently affected the dependent variables. Once the significant overall F-test values (Pillai’s Trace) were identified in each MANOVA, we used univariate analysis of variance (ANOVA) to determine which independent factors and which dependent variables were statistically significant. For comparison of parasite load among brain regions, data relating to parasite density from infected mice of the same sex were analyzed by one-way ANOVA. Further post hoc Bonferroni testing was conducted to explore any significant main effects resulting from the ANOVAs. The correlation of parasite density with gene expression was analyzed using scatter plots and correlation coefficients. Statistical analyses were conducted in both SPSS package (Version 21.0, SPSS, Chicago, IL, USA) and GraphPad Prism V5.02 (GraphPad Software Inc., La Jolla, CA, USA). P < 0.05 was used for the significance level.
RESULTS
We measured the expression of miR-132, BDNF and miR-195 in different brain regions of male and female mice 5 months later with or without T. gondii infection. BDNF was examined due to its potential to modulate neuronal expression of miR-132 (Remenyi et al. 2010). MiR-195 was employed as a negative control for miR-132 quantification as its abundance would not be expected to differ between the control and infection groups (Xiao et al. 2014).
Gene expression in the hippocampus
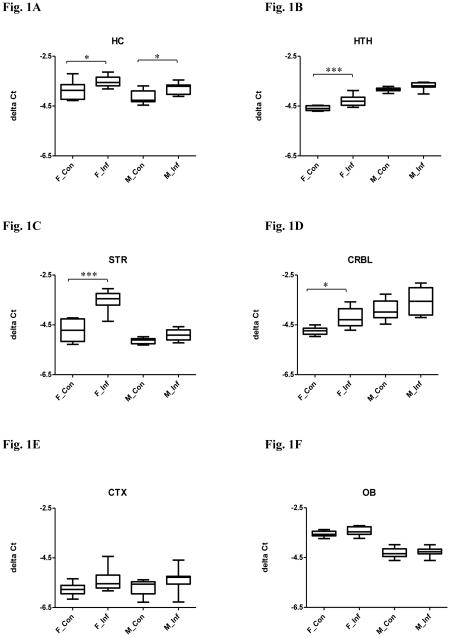
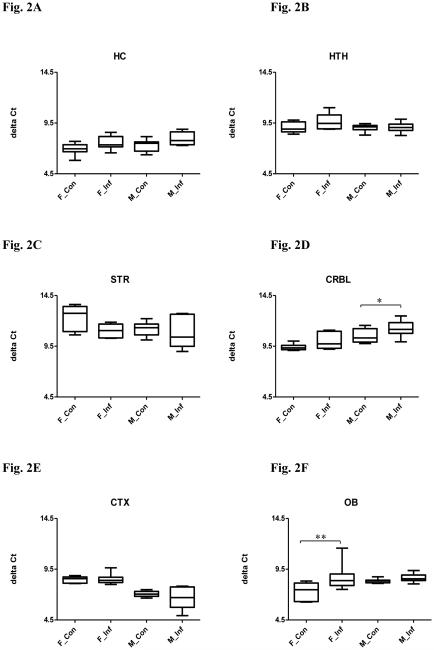
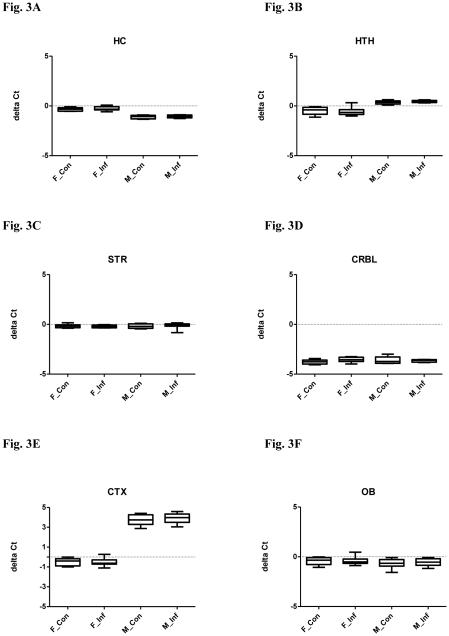
A MANOVA was performed with the independent facts being: infection and sex, and the dependent variables being: miR-132, BDNF and miR-195. The MANOVA revealed an overall significant difference in the expression of miR-132, BDNF and miR-195 between infected and uninfected mice (Pillai’s P = 0.011) and between males and females (P < 0.000), but there was no significant interaction between infection status and sex (P = 0.961). In the univariate analysis, miR-132 was downregulated in infected compared to uninfected mice (Fig. 1A, F(1,30) = 13.31, P = 0.001), and further post hoc Bonferroni tests confirmed the differences were in both males and females (Ps < 0.05). Univariate analysis for BDNF revealed a significant difference between infected and uninfected mice (Fig. 2A, F(1,29) = 8.499, P = 0.007), but further post hoc tests failed to find any difference between groups. No significant difference in levels of miR-195 was observed between infected and uninfected mice (Fig. 3A, F(1,32) = 1.265, P = 0.269). Univariate tests also indicated that the expression of miR-132 (Fig. 1A, F(1,30) = 8.578, P = 0.006) and miR-195 (Fig. 3A, F(1,32) = 174.5, P < 0.0001) vary between sex, with males displaying higher levels than females. The expression of BDNF was not significantly different between males and females (Fig. 2A, F(1,29) = 3.251, P = 0.082).
Figure 1.
Relative miR-132 expression in selected brain regions in both male and female mice with or without chronic T. gondii infection using qPCR. The box and whisker plot represents the delta Ct distribution: the bottom and top of the box are the first and third quartiles, and the band inside the box is the median. The ends of the whiskers represent the minimum and maximum values of the data. X axis represents the different experimental groups: F_Con (female control), F_Inf (female infection), M_Con (male control), and M_Inf (male infection). miR-132 expression was normalized to snoRNA135 and is given as delta Ct value, with lower values representing higher expression levels. Significant infection effects are shown. *P < 0.05, *** P < 0.001. (A) hippocampus (HC); (B) hypothalamus (HTH); (C) striatum (STR); (D) cerebellum (CRBL); (E) cortex (CTX) and (F) olfactory bulb (OB).
Figure 2.
Relative BDNF expression in selected brain regions in both male and female mice with or without chronic T. gondii infection using qPCR. The box and whisker plot represents the delta Ct distribution: the bottom and top of the box are the first and third quartiles, and the band inside the box is the median. The ends of the whiskers represent the minimum and maximum values of the data. X axis represents the different experimental groups: F_Con (female control), F_Inf (female infection), M_Con (male control), and M_Inf (male infection). BDNF expression was normalized to mouse β-actin and is given as delta Ct value, with higher values representing lower expression levels. Significant infection effects are shown. *P < 0.05, ** P < 0.01. (A) hippocampus (HC); (B) hypothalamus (HTH); (C) striatum (STR); (D) cerebellum (CRBL); (E) cortex (CTX) and (F) olfactory bulb (OB).
Figure 3.
Relative miR-195 expression in selected brain regions in both male and female mice with or without chronic T. gondii infection using qPCR. The box and whisker plot represents the delta Ct distribution: the bottom and top of the box are the first and third quartiles, and the band inside the box is the median. The ends of the whiskers represent the minimum and maximum values of the data. X axis represents the different experimental groups: F_Con (female control), F_Inf (female infection), M_Con (male control), and M_Inf (male infection). miR-195 expression was normalized to snoRNA135 and is given as delta Ct value, with lower values representing higher expression levels. (A) hippocampus (HC); (B) hypothalamus (HTH); (C) striatum (STR); (D) cerebellum (CRBL); (E) cortex (CTX) and (F) olfactory bulb (OB).
Gene expression in the hypothalamus
In MANOVA, an overall significant difference was noted in the expression of miR-132, BDNF and miR-195 between infected and uninfected mice (P < 0.000) and between males and females (P < 0.000). There was no significant interaction between infection status and sex (P = 0.114). In the univariate test, miR-132 expression was decreased in infected compared to uninfected mice (Fig. 1B, F(1,32) = 21.51, P < 0.0001), but further post hoc Bonferroni tests indicated a significant difference only in females (P < 0.001). No significant difference in levels of BDNF (Fig. 2B, F(1,34) = 2.988, P = 0.093) or miR-195 (Fig. 3B, F(1,32) = 0.0008, P = 0.978) was observed between infected and uninfected mice.
Univariate tests also revealed that the levels of miR-132 (Fig. 1B, F(1,32) = 203.1, P < 0.0001) and miR-195 (Fig. 3B, F(1,32) = 86.58, P < 0.0001) vary between sex, with males displaying lower expression than females. The expression of BDNF was not significantly different between males and females (Fig. 2B, F(1,34) = 2.719, P = 0.108).
Gene expression in the striatum
The MANOVA revealed an overall significant difference in miR-132, BDNF and miR-195 expression between infected and uninfected mice (P < 0.000) and between males and females (P < 0.000). There was also a significant interaction between infection status and sex (P < 0.000). In the univariate test, miR-132 expression was decreased in infected compared to uninfected mice (Fig. 1C, F(1,33) = 49.70, P < 0.0001), but further post hoc tests confirmed that the difference was in females only (P < 0.001). Univariate testing also revealed a significant difference in levels of BDNF between infected and uninfected mice (Fig. 2C, F(1,29) = 4.250, P = 0.048), but further post hoc tests failed to find any difference between groups. No significant difference was found for miR-195 (Fig. 3C, F(1,34) = 0.032, P = 0.859) between infected and uninfected mice.
Univariate testing also revealed that the levels of miR-132 (Fig. 1C, F(1,33) = 77.54, P < 0.0001) vary between sex, with males displaying higher expression than females. The expression of BDNF (Fig. 2C, F(1,29) = 2.539, P = 0.122) and miR-195 (Fig. 3C, F(1,34) = o.164, P = 0.688) was not significantly different between males and females. The relationship between infection and sex was proved significant for miR-132 (Fig. 1C, F(1,33) = 22.00, P < 0.0001).
Gene expression in the cerebellum
The MANOVA revealed an overall significant difference in miR-132, BDNF and miR-195 expression between infected and uninfected mice (P = 0.003) and between males and females (P < 0.000), but there was no independent effect of interaction (P = 0.272). In the univariate test, miR-132 expression was decreased in infected compared to uninfected mice (Fig. 1D, F(1,33) = 11.07, P = 0.002), but further post hoc Bonferroni tests indicated that the difference was in females only (P < 0.05). An analysis of univariate effect for BDNF revealed a significant difference between infected and uninfected mice (Fig. 2D, F(1,33) = 8.94, P = 0.005), but further post hoc tests confirmed the difference was in males only (P < 0.05). No difference in miR-195 levels was observed between infected and uninfected mice (Fig. 3D, F(1,26) = 0.882, P = 0.356).
Univariate tests also revealed that levels of miR-132 (Fig. 1D, F(1,33) = 32.20, P < 0.0001) and BDNF (Fig. 2D, F(1,33) = 28.43, P < 0.0001) vary between sex, with males displaying lower expression than females. The expression of miR-195 was not significantly different between males and females (Fig. 3D, F(1,26) = 0.061, P = 0.806).
Gene expression in the cortex
The MANOVA indicated that there was an overall significant difference in miR-132, BDNF and miR-195 expression between males and females (P < 0.000). There were no independent effects of infection (P = 0.095) and interaction (P = 0.272). In univariate testing, levels of BDNF (Fig. 2E, F(1,30) = 67.53, P < 0.0001) and miR-195 (Fig. 3E, F(1,33) = 828.2, P < 0.0001) vary between sex. The expression of BDNF was higher in males, while miR-195 expression was higher in females. The expression of miR-132 was not significantly different between males and females (Fig. 1E, F(1,32) = 0.183, P = 0.6715).
Gene expression in the olfactory bulb
The MANOVA revealed an overall significant difference in miR-132, BDNF and miR-195 expression between infected and uninfected mice (P = 0.028) and between males and females (P < 0.000), but there was no independent effect of interaction (P = 0.200). In the univariate test, BDNF was decreased in infected compared to uninfected mice (Fig. 2F, F(1,32) = 11.38, P = 0.002), but further post hoc tests proved the difference was in females only (P < 0.01). Univariate tests revealed no difference in levels of miR-132 (Fig. 1F, F(1,32) = 1.891, P = 0.179) and miR-195 (Fig. 3F, F(1,32) = 0.252, P = 0.619) between infection and control groups.
Univariate test also revealed that levels of miR-132 (Fig. 1F, F(1,32) = 185.0, P < 0.0001) vary between sex, with males displaying higher expression than females. The expression of BDNF (Fig. 2F, F(1,32) = 3.553, P = 0.069) and miR-195 (Fig. 3F, F(1,32) = 1.864, P = 0.182) was not significantly different between males and females.
The hippocampus exhibited a higher parasite density
We next used qPCR to investigate T. gondii distribution and density in infected mice at both DNA and RNA levels. The general pattern of parasite density in each brain region was consistent at both levels (Fig. 4), and indicated that parasite load in the hippocampus was significantly higher than most of the other brain areas in both sexes.
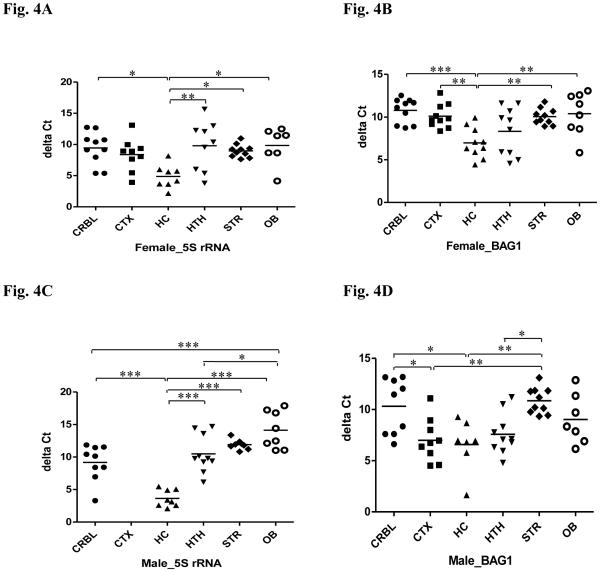
Figure 4.
Relative gene expression of T. gondii in selected brain regions in both male and female mice with chronic T. gondii infection using qPCR. (A) relative expression of T. gondii 5S rRNA in females; (B) relative expression of T. gondii BAG1 in females; (C) relative expression of T. gondii 5S rRNA in males; (D) relative expression of T. gondii BAG1 in males. Relative gene expression was normalized to mouse β-actin and is given as delta Ct value, with higher values representing lower expression levels. CRBL: cerebellum; CTX: cortex; HC: hippocampus; HTH: hypothalamus; STR: striatum; OB: olfactory bulb. *P < 0.05, **P < 0.01, *** P < 0.001.
Results from DNA measurement targeting T. gondii 5S rRNA sequence revealed that the hippocampus has a higher parasite density in both infected males and females, although DNA from the cortex in males is not available. For females (Fig. 4A), one-way ANOVA across the six brain regions revealed a significant difference in the parasite density (F(5,47) = 4.084, p = 0.0037). The post hoc Bonferroni tests showed significantly higher levels of 5S rRNA in the hippocampus than in the other regions except for cortex (P < 0.01 vs. hypothalamus; Ps < 0.05 vs. cerebellum, striatum and olfactory bulb). Likewise for males (Fig. 4C), ANOVA revealed a significant difference in the parasite density in the five brain regions (F(4,38) = 22.66, p < 0.0001), and a further post hoc test showed significantly higher levels of DNA in the hippocampus than all of the other regions (Ps < 0.001). Moreover, the parasite density was significantly lower in the olfactory bulb compared to both cerebellum (P < 0.001) and hypothalamus (P < 0.05).
Results from RNA measurements targeting T. gondii BAG1 gene indicated also that the hippocampus had a higher parasite density than other brain regions in both sexes. For females (Fig. 4B), one-way ANOVA revealed a significant difference in the parasite density (F(5,52) = 5.989, P = 0.0002). The post hoc Bonferroni test showed significantly higher levels of BAG1 in the hippocampus than in other brain regions (P < 0.001 vs. cerebellum; Ps < 0.01 vs. cortex, striatum and olfactory bulb). For males (Fig. 4D), ANOVA revealed a significant difference in the parasite density in the six brain regions (F(5,46) = 5.975, P = 0.0002). Notably, the post hoc test showed significantly higher levels of BAG1 in both the hippocampus and the cortex (Ps < 0.01 vs. striatum; Ps < 0.05 vs. cerebellum). Moreover, the parasite density in the hypothalamus was significantly higher compared to striatum (P < 0.05).
Correlation between parasite density and gene expression
We examined whether or not the changes in gene expression were due to the amount of parasite present in different brain regions. Because of the lack of T. gondii 5S rRNA data in the cortex of males, we applied data from BAG1 gene expression for the correlation analysis. The general pattern was that levels of miR-132 or BDNF were poorly reflected by the density of T. gondii nucleic acids in brain areas. Few brain regions showed a weak correlation. For females, correlations between levels of miR-132 and BAG1 occurred only in the hippocampus (r = 0.56; P = 0.021; n = 9) and hypothalamus (r = −0.51; P = 0.03; n = 9). For males, levels of BAG1 were correlated with miR-132 in the hippocampus (r = −0.69; P = 0.021; n = 7) and with BDNF in the cerebellum (r = −0.48; P = 0.039; n = 9).
Because downregulation of miR-132 and BDNF occurred in different brain regions in males and females, a correlation analysis was not conducted between these measures.
DISCUSSION
We have previously shown that acute in vivo and in vitro T. gondii infection induces miR-132 expression (Xiao et al. 2014). The brain represents an important target organ for T. gondii in terms of establishing persistence and altering host behavior. We thus examined the effect of persistent T. gondii infection on miR-132 in multiple brain areas of experimentally infected mice. We found that persistent infection resulted in a statistically significant decrease of miR-132. This effect varied amon regions of the brain and presented in a sex-dependent manner, with females displaying more susceptibility than males. Although BDNF can regulate neuronal expression of miR-132, a coordinated expression between these molecules was not noted. Moreover, T. gondii was distributed throughout the tested brain regions and a selective tropism of the parasite toward hippocampus based on bradyzoite density was observed in both sexes. However, the changes in gene expression were poorly related to the quantity of parasite present in brain regions. Our findings highlight the importance of investigating the miR-132-mediated neuronal function in mice infected with T. gondii.
We found that, while acute infection of T. gondii increases the expression of miR-132 (Xiao et al. 2014), chronic infection of T. gondii has the opposite effect. The molecular basis for the alterations in miR-132 expression awaits further investigation. Multiple lines of evidence in vitro and in vivo support the fact that miR-132 is linked to multiple functions, including neuronal cell development, synaptic plasticity, inflammation, and angiogenesis (Wanet et al. 2012). MiR-132 overexpression has been confirmed to stimulate neurite outgrowth and synaptic plasticity (Lambert et al. 2010; Wibrand et al. 2010), while miR-132 reduction impairs acquisition of trace fear memory (Wang et al. 2013). However, it is worth noting that transgenic mice overexpressing miR-132 exhibited increased neuronal spine density but impaired novel object recognition and spatial memory (Hansen et al. 2010; Hansen et al. 2013), indicating that appropriate miR-132 expression is required for normal memory formation. Thus, either excess or shortage of expression of miR-132, which is the case in T. gondii infection, could lead to memory impairment.
Sex seems an independent determinant for the expression of miR-132 in murine models of chronic T. gondii infection because females had more affected brain areas than males (hippocampus, hypothalamus, striatum and cerebellum vs. hippocampus). This is consistent with previous findings indicating a general increased susceptibility of female mice over male mice to T. gondii infection (Roberts et al. 1995). Recent studies document the role of miR-132 dysregulation in several neurological disorders such as schizophrenia, Alzheimer, Parkinson’s disease and tauopathies (Miller et al. 2012; Wanet et al. 2012). It is intriguing to speculate that behavioral changes triggered by T. gondii may be partly due to changes in levels of miR-132. It is also tempting to speculate that gender differences in the behavioral effects of T. gondii infection (Lindová et al. 2010; Xiao et al. 2012) may be due to sex-dependent dysregulation of miR-132. Although our recent work suggested that elevated miR-132 in acute infection is associated with altered dopamine pathway in infected mice by repressing the expression of relevant proteins (Xiao et al. 2014), little is known about the effects of miR-132 in chronic infection. Further elucidation of miR-132-mediated pathways may have important implications for the neuropathology and therapy of neurological disorders.
The hippocampus is particularly interesting for the current study in that 1) this brain region is the only one that displayed significant miR-132 downregulation in both males and females following infection; 2) this region has a higher parasite density among the tested brain regions with regard to both sexes. The miR-132-mediated functions in the hippocampal region have been examined before. Magill et al. (2010) deleted the miR-212/132 locus and found that miR-132 is required for normal dendritic maturation in newborn neurons in the adult hippocampus. Wang et al. (2013) reported that significantly reduced hippocampal expression of miR-132 impairs acquisition of trace fear memory. Hermes et al. (2008) noted pronounced pathological changes in the hippocampus of mice with latent toxoplasmosis using immunestaining. There is an association of hippocampal abnormalities with a number of neurological diseases of humans including Alzheimer’s disease, depression, and schizophrenia (Monje et al. 2003; Lazarov et al. 2005; Airan et al. 2007; McHugh et al. 2007). Our previous study suggested that olfactory memory, a proposed function of hippocampus, is potentially modulated in mice with chronic T. gondii infection (Xiao et al. 2012). Given the importance of the hippocampus, further experiments are warranted to ascertain its role in chronic T. gondii infection.
We explored T. gondii tropism on brain regions in reference to bradyzoite number (parasite density). The tropism of T. gondii to specific brain regions based on cyst density has not been clearly established. Several areas of the brain are implicated, such as amygdala (Vyas et al. 2007), cortex and diencephalon (Hermes et al. 2008), and amygdala, hippocampus, olfactory bulbs and a number of cortical regions (Berenreiterova et al. 2011). We observed a selective tropism of T. gondii to the hippocampus based on bradyzoite density in both males and females. However, our results do not necessarily indicate that there is a tropism for the number of cysts toward the hippocampus. In fact, there may be no tropism in the cyst number, but there could be larger cysts in the hippocampus and therefore more bradyzoite gene expression was detected. On the other hand, the difference between PCR-based and microscope-based methods might have also contributed to the apparent tropism in our study. The microscope-based technique computes the density of parasite per volume of brain tissue, while PCR-based technique computes density of parasites per number of reference molecule, here β-actin. Therefore specific differences in the density of parasites can be obtained with PCR-based technique when the distribution of parasites in brain is random but the number of host cells or phenotype of host cells differs between brain regions. Clearly, further investigations that combine microscopic examination and PCR-based method with multiple reference molecules will be required to distinguish between these possibilities.
In line with prior studies indicating T. gondii cysts were distributed throughout the brain (Berenreiterová et al. 2011), we found parasite DNA/RNA in all analyzed brain regions in both sexes. However, the number of parasites was weakly associated with levels of miR-132 or BDNF in tested brain regions in infected mice, suggesting the parasites may indirectly modulate neuronal function. Several previous studies have also reported that parasite load is not the decisive factor driving behavioral changes or brain inflammation (Haroon et al. 2012; Ingram et al. 2013). Instead, the chronicity of infection has been suggested to account for the altered neuronal function (Haroon et al. 2012).
Although BDNF can regulate neuronal expression of miR-132, the two factors were not correlated in the brain of T. gondii infected mice. This finding suggests that other transcriptional regulators such as CREB and REST (RE1-Silencing Transcription factor) must be involved in miR-132 expression (Wanet et al. 2012) in the mouse model of T. gondii infection. It has been reported that the mature olfactory bulb harbors high levels of BDNF, which is essential to the differentiation of regenerating cells within the olfactory bulb (Benraiss et al. 2001). Spinocerebellar ataxias (SCAs) are a group of progressive degenerative disorders characterized by incoordination of gait and often associated with poor coordination of hands, speech, and eye movements. In the cerebellum of SCA6 (Takahashi et al. 2012) and SCA1 (Hourez et al. 2011), BDNF mRNA is significantly suppressed. The significance of BDNF downregulation in the olfactory bulb and cerebellum for females and males, respectively, awaits future investigation. Interestingly, there is evidence that chronically infected mice exhibit motor coordination and sensory deficits (Hermes et al. 2008; Gulinello et al. 2010).
Collectively, our results demonstrated that chronic toxoplasma infection specifically influences expression of miR-132 and BDNF. The effects vary among brain regions and were displayed in a sex-dependent manner. Future efforts should be directed toward the elucidation of miR- 132/BDNF regulated genes and associated pathways. These studies might lead to a better understanding of the mechanisms of T. gondii-induced alterations in host behavior.
KEY FINDINGS.
While acute T. gondii infection induces miR-132, chronic infection has the opposite effect.
The downregulation of miR-132 varied among brain regions in a sex-dependent manner.
BDNF and miR-132 were not co-varies in the brain areas of T. gondii infected mice.
T. gondii displayed a selective tropism for the hippocampus based on bradyzoite density.
The density of T. gondii in brain areas poorly reflected changes in gene expression.
Acknowledgments
FINANCIAL SUPPORT
Mental Health (NIMH) P50 Silvio O. Conte Center at Johns Hopkins (grant# MH-94268).
References
- Airan RD, Meltzer LA, Roy M, Gong Y, Chen H, Deisseroth K. High-speed imaging reveals neurophysiological links to behavior in an animal model of depression. Science. 2007;317:819–823. doi: 10.1126/science.1144400. [DOI] [PubMed] [Google Scholar]
- Benraiss A, Chmielnicki E, Lerner K, Roh D, Goldman SA. Adenoviral brain-derived neurotrophic factor induces both neostriatal and olfactory neuronal recruitment from endogenous progenitor cells in the adult forebrain. The Journal of Neuroscience. 2001;21:6718–6731. doi: 10.1523/JNEUROSCI.21-17-06718.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berenreiterová M, Flegr J, Kuběna AA, Němec P. The distribution of Toxoplasma gondii cysts in the brain of a mouse with latent toxoplasmosis: implications for the behavioral manipulation hypothesis. PLoS One. 2011;6:e28925. doi: 10.1371/journal.pone.0028925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulinello M, Acquarone M, Kim JH, Spray DC, Barbosa HS, Sellers R, Tanowitz HB, Weiss LM. Acquired infection with Toxoplasma gondii in adult mice results in sensorimotor deficits but normal cognitive behavior despite widespread brain pathology. Microbes and Infection. 2010;12:528–537. doi: 10.1016/j.micinf.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen KF, Karelina K, Sakamoto K, Wayman GA, Impey S, Obrietan K. miRNA-132: a dynamic regulator of cognitive capacity. Brain Structure and Function. 2013;218:817–831. doi: 10.1007/s00429-012-0431-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen KF, Sakamoto K, Wayman GA, Impey S, Obrietan K. Transgenic miR132 alters neuronal spine density and impairs novel object recognition memory. PLoS One. 2010;5:e15497. doi: 10.1371/journal.pone.0015497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermes G, Ajioka JW, Kelly KA, Mui E, Roberts F, Kasza K, Mayr T, Kirisits MJ, Wollmann R, Ferguson DJ, Roberts CW, Hwang JH, Trendler T, Kennan RP, Suzuki Y, Reardon C, Hickey WF, Chen L, McLeod R. Neurological and behavioral abnormalities, ventricular dilatation, altered cellular functions, inflammation, and neuronal injury in brains of mice due to common, persistent, parasitic infection. Journal of Neuroinflammation. 2008;5:48. doi: 10.1186/1742-2094-5-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hourez R, Servais L, Orduz D, Gall D, Millard I, de Kerchove d'Exaerde A, Cheron G, Orr HT, Pandolfo M, Schiffmann SN. Aminopyridines correct early dysfunction and delay neurodegeneration in a mouse model of spinocerebellar ataxia type 1. The Journal of Neuroscience. 2011;31:11795–11807. doi: 10.1523/JNEUROSCI.0905-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert TJ, Storm DR, Sullivan JM. MicroRNA132 modulates short-term synaptic plasticity but not basal release probability in hippocampal neurons. PLoS One. 2010;5:e15182. doi: 10.1371/journal.pone.0015182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarov O, Robinson J, Tang YP, Hairston IS, Korade-Mirnics Z, Lee VM, Hersh LB, Sapolsky RM, Mirnics K, Sisodia SS. Environmental enrichment reduces Abeta levels and amyloid deposition in transgenic mice. Cell. 2005;120:701–713. doi: 10.1016/j.cell.2005.01.015. [DOI] [PubMed] [Google Scholar]
- Lindová J, Kubena AA, Sturcová H, Krivohlavá R, Novotná M, Rubesová A, Havlícek J, Kodym P, Flegr J. Pattern of money allocation in experimental games supports the stress hypothesis of gender differences in Toxoplasma gondii-induced behavioural changes. Folia Parasitologica (Praha) 2010;57:136–142. [PubMed] [Google Scholar]
- Magill ST, Cambronne XA, Luikart BW, Lioy DT, Leighton BH, Westbrook GL, Mandel G, Goodman RH. microRNA-132 regulates dendritic growth and arborization of newborn neurons in the adult hippocampus. Proceedings of the National Academy of Sciences of the USA. 2010;107:20382–20387. doi: 10.1073/pnas.1015691107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marler KJ, Suetterlin P, Dopplapudi A, Rubikaite A, Adnan J, Maiorano NA, Lowe AS, Thompson ID, Pathania M, Bordey A, Fulga T, Van Vactor DL, Hindges R, Drescher U. BDNF promotes axon branching of retinal ganglion cells via miRNA-132 and p250GAP. The Journal of Neuroscience. 2014;34:969–979. doi: 10.1523/JNEUROSCI.1910-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh TJ, Jones MW, Quinn JJ, Balthasar N, Coppari R, Elmquist JK, Lowell BB, Fanselow MS, Wilson MA, Tonegawa S. Dentate gyrus NMDA receptors mediate rapid pattern separation in the hippocampal network. Science. 2007;317:94–99. doi: 10.1126/science.1140263. [DOI] [PubMed] [Google Scholar]
- Miller BH, Zeier Z, Xi L, Lanz TA, Deng S, Strathmann J, Willoughby D, Kenny PJ, Elsworth JD, Lawrence MS, Roth RH, Edbauer D, Kleiman RJ, Wahlestedt C. MicroRNA-132 dysregulation in schizophrenia has implications for both neurodevelopment and adult brain function. Proceedings of the National Academy of Sciences of the USA. 2012;109:3125–3130. doi: 10.1073/pnas.1113793109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- Remenyi J, Hunter CJ, Cole C, Ando H, Impey S, Monk CE, Martin KJ, Barton GJ, Hutvagner G, Arthur JS. Regulation of the miR-212/132 locus by MSK1 and CREB in response to neurotrophins. The Biochemical Journal. 2010;428:281–291. doi: 10.1042/BJ20100024. [DOI] [PubMed] [Google Scholar]
- Roberts CW, Cruickshank SM, Alexander J. Sex-determined resistance to Toxoplasma gondii is associated with temporal differences in cytokine production. Infection and Immunity. 1995;63:2549–2555. doi: 10.1128/iai.63.7.2549-2555.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M, Ishikawa K, Sato N, Obayashi M, Niimi Y, Ishiguro T, Yamada M, Toyoshima Y, Takahashi H, Kato T, Takao M, Murayama S, Mori O, Eishi Y, Mizusawa H. Reduced brain-derived neurotrophic factor (BDNF) mRNA expression and presence of BDNF-immunoreactive granules in the spinocerebellar ataxia type 6 (SCA6) cerebellum. Neuropathology. 2012;32:595–603. doi: 10.1111/j.1440-1789.2012.01302.x. [DOI] [PubMed] [Google Scholar]
- Tomita T, Bzik DJ, Ma YF, Fox BA, Markillie LM, Taylor RC, Kim K, Weiss LM. The Toxoplasma gondii cyst wall protein CST1 is critical for cyst wall integrity and promotes bradyzoite persistence. PLoS Pathogens. 2013;9:e1003823. doi: 10.1371/journal.ppat.1003823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno A, Dautu G, Munyaka B, Carmen G, Kobayashi Y, Igarashi M. Toxoplasma gondii: Identification and characterization of bradyzoite-specific deoxyribose phosphate aldolase-like gene (TgDPA) Experimental Parasitology. 2009;121:55–63. doi: 10.1016/j.exppara.2008.09.018. [DOI] [PubMed] [Google Scholar]
- Vyas A, Kim SK, Giacomini N, Boothroyd JC, Sapolsky RM. Behavioral changes induced by Toxoplasma infection of rodents are highly specific to aversion of cat odors. Proceedings of the National Academy of Sciences of the USA. 2007;104:6442–6447. doi: 10.1073/pnas.0608310104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanet A, Tacheny A, Arnould T, Renard P. miR-212/132 expression and functions: within and beyond the neuronal compartment. Nucleic Acids Research. 2012;40:4742–4753. doi: 10.1093/nar/gks151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang RY, Phang RZ, Hsu PH, Wang WH, Huang HT, Liu IY. In vivo knockdown of hippocampal miR-132 expression impairs memory acquisition of trace fear conditioning. Hippocampus. 2013;23:625–633. doi: 10.1002/hipo.22123. [DOI] [PubMed] [Google Scholar]
- Wibrand K, Panja D, Tiron A, Ofte ML, Skaftnesmo KO, Lee CS, Pena JT, Tuschl T, Bramham CR. Differential regulation of mature and precursor microRNA expression by NMDA and metabotropic glutamate receptor activation during LTP in the adult dentate gyrus in vivo. European Journal of Neuroscience. 2010;31:636–645. doi: 10.1111/j.1460-9568.2010.07112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao J, Jones-Brando L, Talbot CC, Jr, Yolken RH. Differential effects of three canonical Toxoplasma strains on gene expression in human neuroepithelial cells. Infection and Immunity. 2011;79:1363–1373. doi: 10.1128/IAI.00947-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao J, Kannan G, Jones-Brando L, Brannock C, Krasnova IN, Cadet JL, Pletnikov M, Yolken RH. Sex-specific changes in gene expression and behavior induced by chronic Toxoplasma infection in mice. Neuroscience. 2012;206:39–48. doi: 10.1016/j.neuroscience.2011.12.051. [DOI] [PubMed] [Google Scholar]
- Xiao J, Li Y, Prandovszky E, Karuppagounder SS, Talbot CC, Jr, Dawson VL, Dawson TM, Yolken RH. MicroRNA-132 dysregulation in Toxoplasma gondii infection has implications for dopamine signaling pathway. Neuroscience. 2014;268:128–138. doi: 10.1016/j.neuroscience.2014.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haroon F, Händel U, Angenstein F, Goldschmidt J, Kreutzmann P, Lison H, Fischer KD, Scheich H, Wetzel W, Schlüter D, Budinger E. Toxoplasma gondii actively inhibits neuronal function in chronically infected mice. PLoS One. 2012;7:e35516. doi: 10.1371/journal.pone.0035516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram WM, Goodrich LM, Robey EA, Eisen MB. Mice infected with low-virulence strains of Toxoplasma gondii lose their innate aversion to cat urine, even after extensive parasite clearance. PLoS One. 2013;8:e75246. doi: 10.1371/journal.pone.0075246. [DOI] [PMC free article] [PubMed] [Google Scholar]