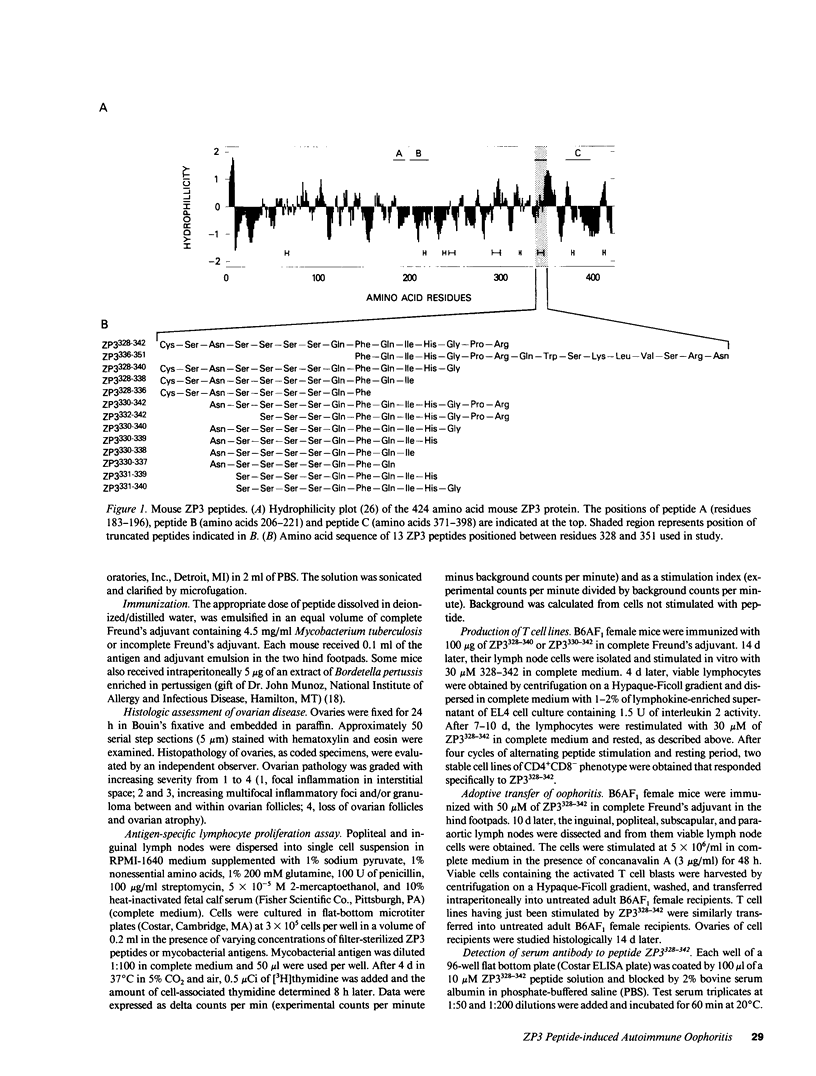
Abstract
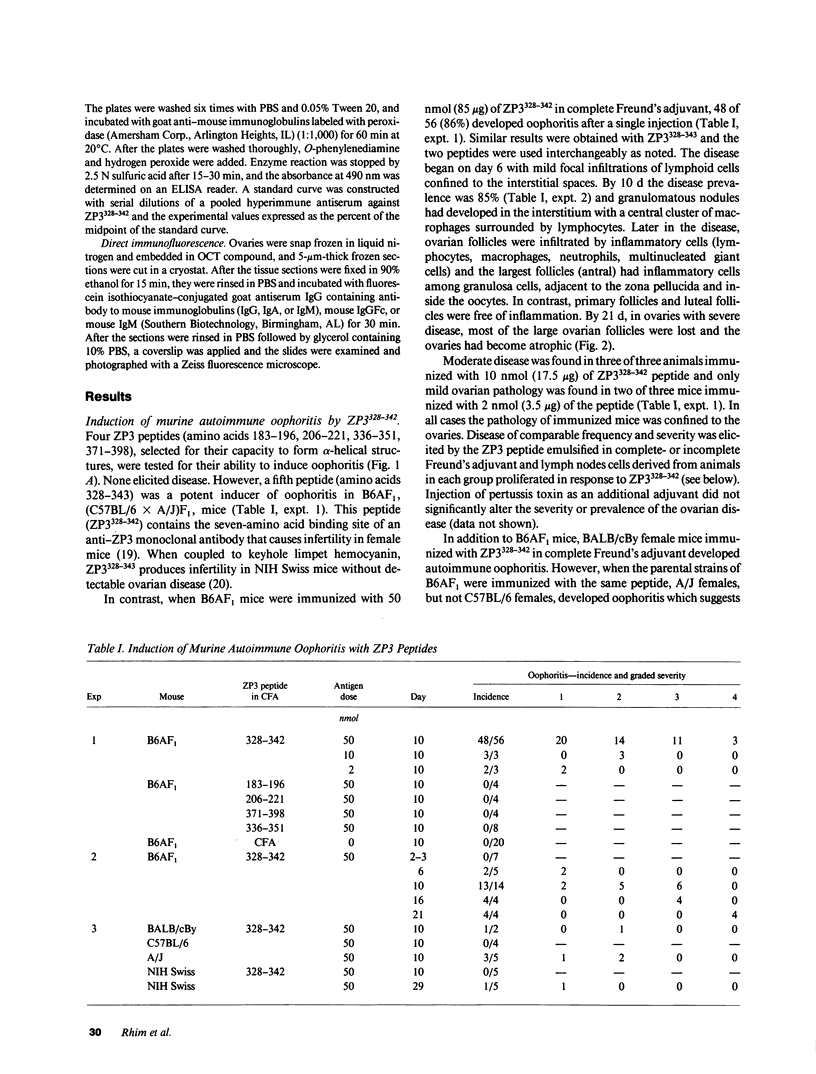
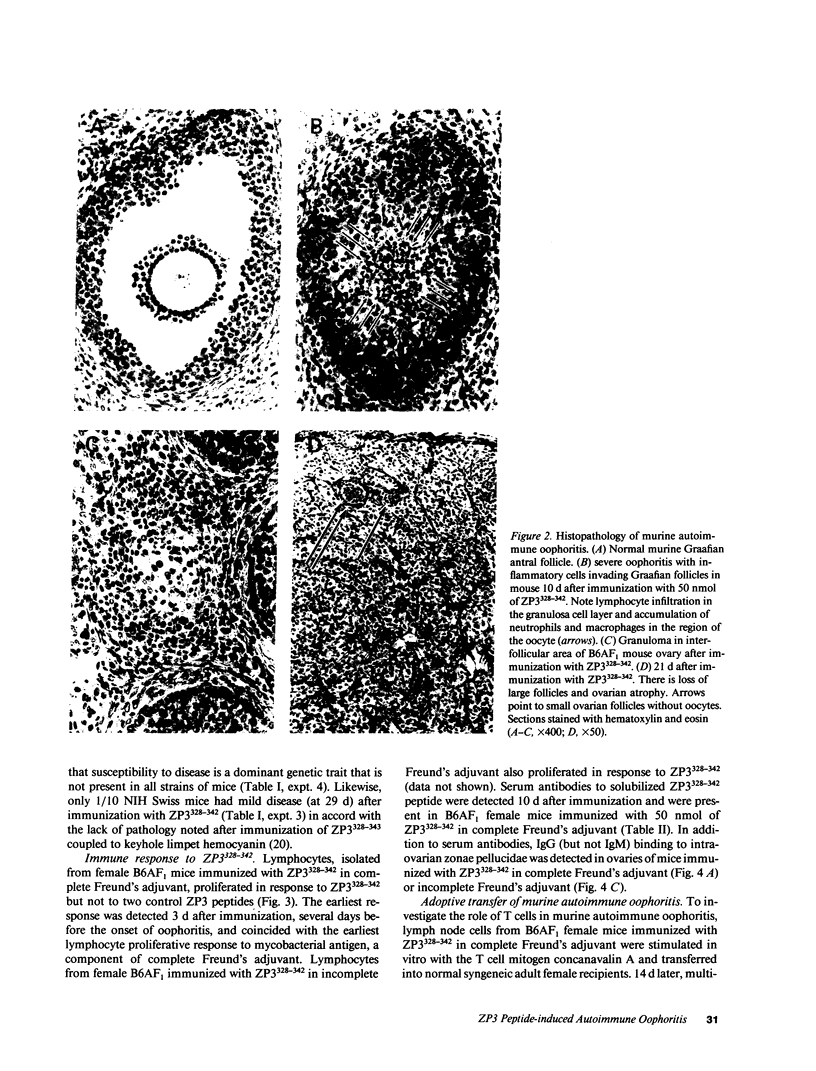
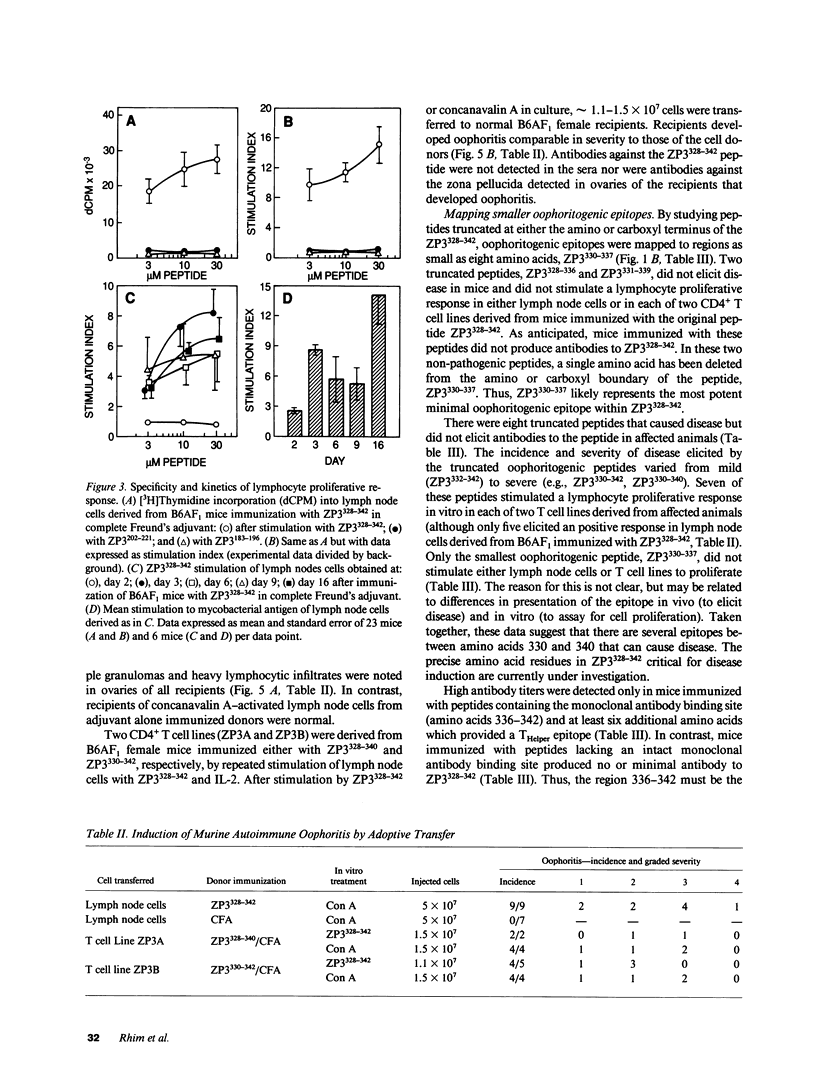
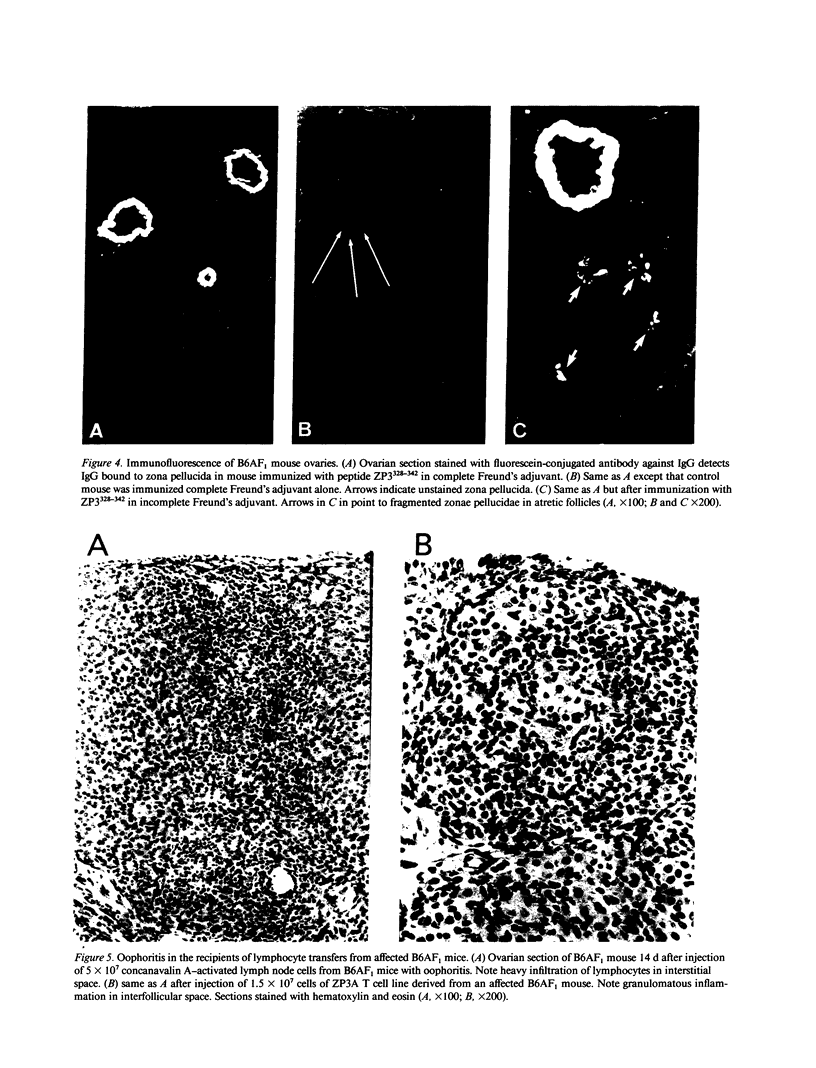
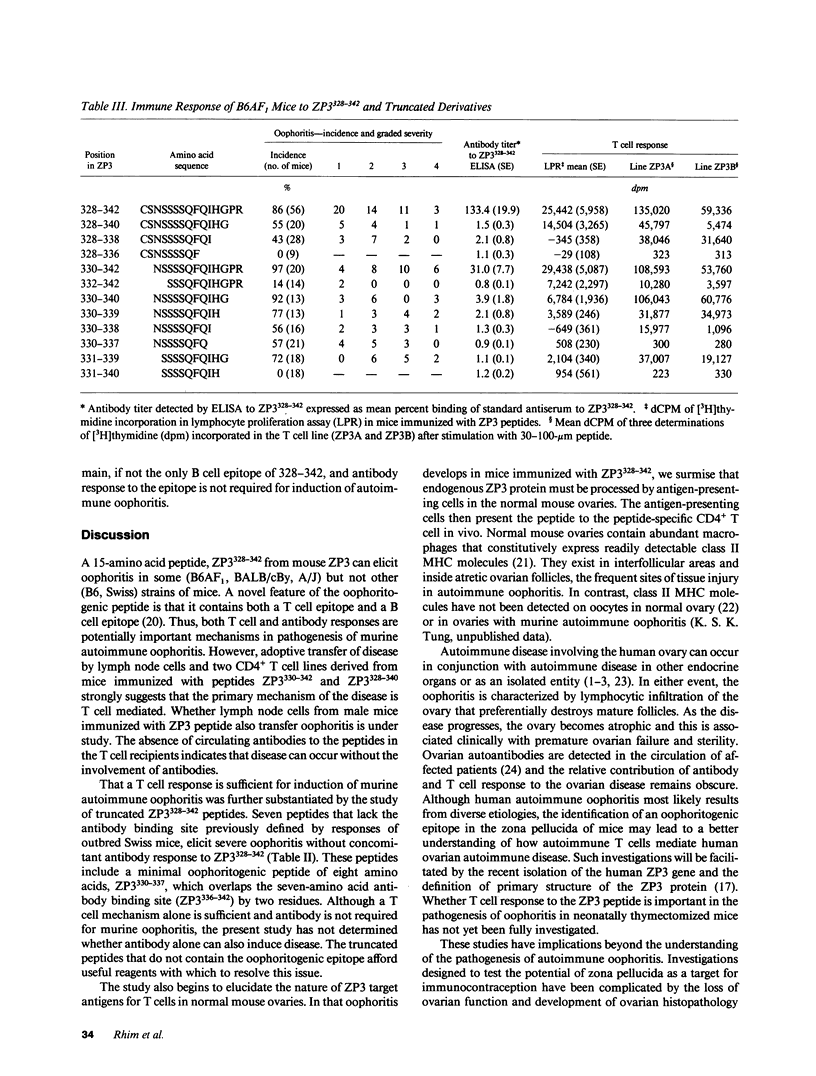
We describe a novel experimental system in mice for the study of ovarian autoimmune disease, a condition encountered in women with premature ovarian failure. The ovarian autoimmune disease is induced in B6AF1 mice by a 15-amino acid peptide (Cys-Ser-Asn-Ser-Ser-Ser-Ser-Gln-Phe-Gln-Ile-His-Gly-Pro-Arg) from mouse ZP3, the sperm-binding component of the zona pellucida that surrounds growing and mature oocytes. Whereas the peptide induces both T cell and antibody responses, adoptive transfer of CD4+ T cell lines derived from affected animals causes oophoritis without observable antibodies to the zona pellucida peptide. The primacy of the T cell response in the pathogenesis of disease is further substantiated by defining oophoritogenic peptides as small as eight amino acids (Asn-Ser-Ser-Ser-Ser-Gln-Phe-Gln) that do not elicit an antibody response to the full-length ZP3 peptide. The identification of a well characterized peptide as a causative agent of autoimmune oophoritis should facilitate understanding of the pathogenesis of this T cell-mediated autoimmune disease. Because the proteins of the zona pellucida are conserved among mammals (the mouse and human ZP3 proteins are 67% identical), this murine model may lead to better understanding of the pathogenesis of human autoimmune oophoritis.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biscotti C. V., Hart W. R., Lucas J. G. Cystic ovarian enlargement resulting from autoimmune oophoritis. Obstet Gynecol. 1989 Sep;74(3 Pt 2):492–495. [PubMed] [Google Scholar]
- Bleil J. D., Wassarman P. M. Structure and function of the zona pellucida: identification and characterization of the proteins of the mouse oocyte's zona pellucida. Dev Biol. 1980 Apr;76(1):185–202. doi: 10.1016/0012-1606(80)90371-1. [DOI] [PubMed] [Google Scholar]
- Bukovský A., Presl J., Zidovský J., Mancal P. The localization of Thy-1.1, MRC OX 2 and Ia antigens in the rat ovary and fallopian tube. Immunology. 1983 Mar;48(3):587–596. [PMC free article] [PubMed] [Google Scholar]
- Chamberlin M. E., Dean J. Genomic organization of a sex specific gene: the primary sperm receptor of the mouse zona pellucida. Dev Biol. 1989 Jan;131(1):207–214. doi: 10.1016/s0012-1606(89)80052-1. [DOI] [PubMed] [Google Scholar]
- Chamberlin M. E., Dean J. Human homolog of the mouse sperm receptor. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6014–6018. doi: 10.1073/pnas.87.16.6014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohr G. A., Motter W., Leitinger S., Desoye G., Urdl W., Winter R., Wilders-Truschnig M. M., Uchanska-Ziegler B., Ziegler A. Lack of expression of HLA [corrected] class I and class II molecules on the human oocyte. J Immunol. 1987 Jun 1;138(11):3766–3770. [PubMed] [Google Scholar]
- East I. J., Gulyas B. J., Dean J. Monoclonal antibodies to the murine zona pellucida protein with sperm receptor activity: effects on fertilization and early development. Dev Biol. 1985 Jun;109(2):268–273. doi: 10.1016/0012-1606(85)90454-3. [DOI] [PubMed] [Google Scholar]
- Gloor E., Hurlimann J. Autoimmune oophoritis. Am J Clin Pathol. 1984 Jan;81(1):105–109. doi: 10.1093/ajcp/81.1.105. [DOI] [PubMed] [Google Scholar]
- Henderson C. J., Hulme M. J., Aitken R. J. Contraceptive potential of antibodies to the zona pellucida. J Reprod Fertil. 1988 May;83(1):325–343. doi: 10.1530/jrf.0.0830325. [DOI] [PubMed] [Google Scholar]
- Hopp T. P., Woods K. R. Prediction of protein antigenic determinants from amino acid sequences. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3824–3828. doi: 10.1073/pnas.78.6.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinloch R. A., Roller R. J., Fimiani C. M., Wassarman D. A., Wassarman P. M. Primary structure of the mouse sperm receptor polypeptide determined by genomic cloning. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6409–6413. doi: 10.1073/pnas.85.17.6409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohno S., Munoz J. A., Williams T. M., Teuscher C., Bernard C. C., Tung K. S. Immunopathology of murine experimental allergic orchitis. J Immunol. 1983 Jun;130(6):2675–2682. [PubMed] [Google Scholar]
- LaBarbera A. R., Miller M. M., Ober C., Rebar R. W. Autoimmune etiology in premature ovarian failure. Am J Reprod Immunol Microbiol. 1988 Mar;16(3):115–122. doi: 10.1111/j.1600-0897.1988.tb00180.x. [DOI] [PubMed] [Google Scholar]
- Liang L. F., Chamow S. M., Dean J. Oocyte-specific expression of mouse Zp-2: developmental regulation of the zona pellucida genes. Mol Cell Biol. 1990 Apr;10(4):1507–1515. doi: 10.1128/mcb.10.4.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luborsky J. L., Visintin I., Boyers S., Asari T., Caldwell B., DeCherney A. Ovarian antibodies detected by immobilized antigen immunoassay in patients with premature ovarian failure. J Clin Endocrinol Metab. 1990 Jan;70(1):69–75. doi: 10.1210/jcem-70-1-69. [DOI] [PubMed] [Google Scholar]
- Millar S. E., Chamow S. M., Baur A. W., Oliver C., Robey F., Dean J. Vaccination with a synthetic zona pellucida peptide produces long-term contraception in female mice. Science. 1989 Nov 17;246(4932):935–938. doi: 10.1126/science.2479101. [DOI] [PubMed] [Google Scholar]
- Philpott C. C., Ringuette M. J., Dean J. Oocyte-specific expression and developmental regulation of ZP3, the sperm receptor of the mouse zona pellucida. Dev Biol. 1987 Jun;121(2):568–575. doi: 10.1016/0012-1606(87)90192-8. [DOI] [PubMed] [Google Scholar]
- Ringuette M. J., Chamberlin M. E., Baur A. W., Sobieski D. A., Dean J. Molecular analysis of cDNA coding for ZP3, a sperm binding protein of the mouse zona pellucida. Dev Biol. 1988 Jun;127(2):287–295. doi: 10.1016/0012-1606(88)90315-6. [DOI] [PubMed] [Google Scholar]
- Shimizu S., Tsuji M., Dean J. In vitro biosynthesis of three sulfated glycoproteins of murine zonae pellucidae by oocytes grown in follicle culture. J Biol Chem. 1983 May 10;258(9):5858–5863. [PubMed] [Google Scholar]
- Smith H., Chen I. M., Kubo R., Tung K. S. Neonatal thymectomy results in a repertoire enriched in T cells deleted in adult thymus. Science. 1989 Aug 18;245(4919):749–752. doi: 10.1126/science.2788921. [DOI] [PubMed] [Google Scholar]
- Taguchi O., Nishizuka Y. Autoimmune oophoritis in thymectomized mice: T cell requirement in adoptive cell transfer. Clin Exp Immunol. 1980 Nov;42(2):324–331. [PMC free article] [PubMed] [Google Scholar]
- Taguchi O., Nishizuka Y., Sakakura T., Kojima A. Autoimmune oophoritis in thymectomized mice: detection of circulating antibodies against oocytes. Clin Exp Immunol. 1980 Jun;40(3):540–553. [PMC free article] [PubMed] [Google Scholar]
- Tung K. S., Smith S., Teuscher C., Cook C., Anderson R. E. Murine autoimmune oophoritis, epididymoorchitis, and gastritis induced by day 3 thymectomy. Immunopathology. Am J Pathol. 1987 Feb;126(2):293–302. [PMC free article] [PubMed] [Google Scholar]
- Wassarman P. M. Zona pellucida glycoproteins. Annu Rev Biochem. 1988;57:415–442. doi: 10.1146/annurev.bi.57.070188.002215. [DOI] [PubMed] [Google Scholar]