Abstract
Treatment for chronic myeloid leukemia (CML) has improved substantially in the last 20 years, especially since the introduction of oral BCR-ABL inhibitors a decade ago. However, for patients to reap the benefits of BCR-ABL inhibitors, they must likely be on therapy for the remainder of their lives. In this situation, adherence to medication becomes a concern. Adherence to therapy for chronic health conditions, including CML, has been demonstrated to be poor. Studies have shown nonadherence in CML to be common in one-third or more of patients, and 100% adherence is rare. Furthermore, evidence suggests that reduced adherence to BCR-ABL inhibitors is associated with reduced efficacy and increased healthcare costs. Factors that can cause nonadherence, including dose, toxicity, time from diagnosis to prescription, and the number of concomitant medications, should be addressed and monitored by the physician. To maximize adherence, CML treatment should be individualized to the patient and simplified as appropriate.
Keywords: imatinib, dasatinib, nilotinib, chronic myelogenous leukemia, medical possession ratio
Introduction
Chronic myeloid leukemia (CML) is a hematopoietic stem cell disease that accounts for 15% of adult leukemias.1 Much progress has been made in the field of CML therapy over the last 20 years. Initially stem cell transplantation, which was intended to be curative, was the preferred therapy; however, its use is limited by donor availability and toxicity in older patients.1 The dismal prognosis faced by patients with CML when chemotherapy was the only nontransplant treatment option was modestly improved by the introduction of interferon.2 Substantial improvement in prognosis followed the approval of oral tyrosine kinase inhibitors targeting BCR-ABL (the oncoprotein associated with CML) beginning with imatinib in 2001.3 Before the introduction of BCR-ABL inhibitors, 5-year CML survival rates with interferon or chemotherapy were 57% and 42%, respectively.2 In contrast, first-line therapy with imatinib is associated with 5-year overall survival rates of 83% to 89%.4,5
Because most patients with CML are in the chronic phase (CP) at diagnosis,6 preventing disease progression typically will require a prolonged period of treatment. Although BCR-ABL inhibitor therapy has shown good efficacy, to maintain response patients may need to remain on therapy for the rest of their lives. As the median age of patients with CML at diagnosis is 66 years and the average life expectancy in the United States is 78 years of age, this can translate into a decade or more of continuous treatment.7,8
Until 2010, imatinib was the only BCR-ABL inhibitor indicated for the first-line treatment of CML.3 However, the second-generation BCR-ABL inhibitors (dasatinib and nilotinib) now have been approved for first-line as well as second-line therapy.9,10 Current guidelines and recommendations state that patients demonstrating adequate response to BCR-ABL inhibitors should be continued indefinitely on the established treatment.1,11 Data regarding long-term use of BCR-ABL inhibitors are available primarily for imatinib. In the most recent follow-up of the International Randomized Study of Interferon and STI571 (IRIS), at the 8-year data cutoff, 55% of patients remained on imatinib therapy and 45% had discontinued treatment: 16% due to unsatisfactory therapeutic outcome, 6% due to adverse events and safety, 3% due to stem cell transplantation, 3% due to death, and 17% for other reasons including withdrawal and lack of renewal of consent.12
Maintaining adherence to BCR-ABL inhibitor dose schedules is important to ensure patients receive prescribed treatment at the optimum dose intensity. Herein we discuss issues of oral medication adherence in chronic conditions in general and in CML specifically, including treatment- and non-treatment–related factors that may affect adherence and thus optimization of therapy.
Adherence to Oral Therapies of Chronic Conditions
Adherence may be defined as the degree to which a patient takes medication as prescribed by a physician. Methods to monitor adherence to patient-/caregiver-administered treatments include direct assessments (eg, observation and blood or urine collection to determine drug, metabolite, or biomarker levels [clinically applicable to only select agents]) and indirect assessments (eg, clinical response, patient interviews or surveys, medication diaries, pill counts at follow-up visits, medication event monitoring systems [MEMS] to record the date and time of bottle openings, and review of pharmacy or insurance records).13,14 Prescription refill history also is used to calculate medication possession ratio (MPR), defined as the total days’ supply of drug acquired in a year divided by 365, or as drug supply dispensed (days) divided by actual duration of dosing (days). A limitation of the MPR method is that it does not distinguish delays in medication renewals that arise from physician-prescribed treatment delays and/or dose reductions as opposed to patient nonadherence.
In addition to the lack of a gold standard for measuring adherence, there is no consensus as to what constitutes adequate adherence. Clinical trial investigators have used values varying from 80% to 95% of prescribed doses taken, although such cutoffs generally have little supporting evidence, and the level of adherence required to achieve the desired therapeutic outcome will vary depending on the treatment and regimen used.13,14 This issue is particularly relevant to oral therapies, for which the elimination half-life of a given agent can substantially influence the degree of nonadherence tolerable without affecting therapeutic outcomes.14 However, the effect of elimination half-life on adherence requirements has not been examined.
Adherence generally is high for physician-administered therapies, including infusion-based treatments. However, oral therapies often are preferred by patients because they offer greater convenience and autonomy; however, patient-/caregiver-administered oral therapies often are associated with poor adherence.15,16 Even in clinical trials, where adherence could be expected to be higher than in the general patient population (due to the rigorous monitoring requirements), treatment adherence can be less than optimal.17,18
Low adherence is of particular concern in the long-term treatment of chronic diseases, especially those that present asymptomatically, as patients may experience treatment-related adverse events without obvious benefits.14 In one retrospective cohort study of over 30,000 patients, it was estimated that only 43% of patients taking statin drugs were adherent after 6 months.15 Even in the field of oncology, where patient motivation may be expected to be high, nonadherence is an issue.16 This can take the form of overadherence (taking more medication than is prescribed), which can result in increased toxicity.14 However, a literature review of adherence studies that evaluated oncology patients taking oral therapy (conditions included hematologic malignancies, breast cancer, ovarian cancer, small cell lung cancer, and Hodgkin’s disease or non-Hodgkin lymphoma) found adherence to range from 100% to as low as 16% in clinical trials.14,19
Most studies of adherence to cancer therapy have been conducted in patients with breast cancer. A literature review found that, in the clinical practice setting, adherence to endocrine therapy for breast cancer (as determined by prescription refills recorded in an insurance database or telephone surveys) ranged from 50% to 70% over periods of 4 to 5 years.20 Similarly, patient surveys regarding adjuvant hormonal therapies (tamoxifen and/or aromatase inhibitor, N = 8769; and tamoxifen only, N = 2378) found that 49% and 77% of patients, respectively, were adherent to treatment.21,22 Furthermore, in a subset of the tamoxifen-only study (n = 309), filled prescriptions covered only 50% of treatment days during the fourth year of treatment.22
Poor treatment adherence has been estimated to account for 33% to 69% of medication-related hospital admissions and may be associated with higher rates of disease progression and mortality.13 An adherence outcomes study of breast cancer patients (N = 2080) that utilized a retrospective medical record analysis showed that lower adherence to tamoxifen treatment (ie, < 80% Adherence Index: days of coverage/duration of therapy) was associated with a small but significantly decreased rate of survival (hazard ratio, 1.10, 95% confidence interval [CI], 1.001–1.21; P = .046).23 In addition to the health effects potentially associated with poor adherence, worsening disease may be mistaken by the physician for drug resistance, leading to unnecessary diagnostic tests, hospitalization, and/or changes in treatment.14
Dosage and dosing schedule can offer insights into possible issues that may arise around adherence. For example, dosing often is an important factor in the risk-benefit assessment of anticancer agents. Dose-optimization studies of dasatinib in patients with CML have shown that different dosing schedules can significantly affect the safety profile of the drug.24,25
Factors Affecting Adherence to Therapy of Chronic Disease
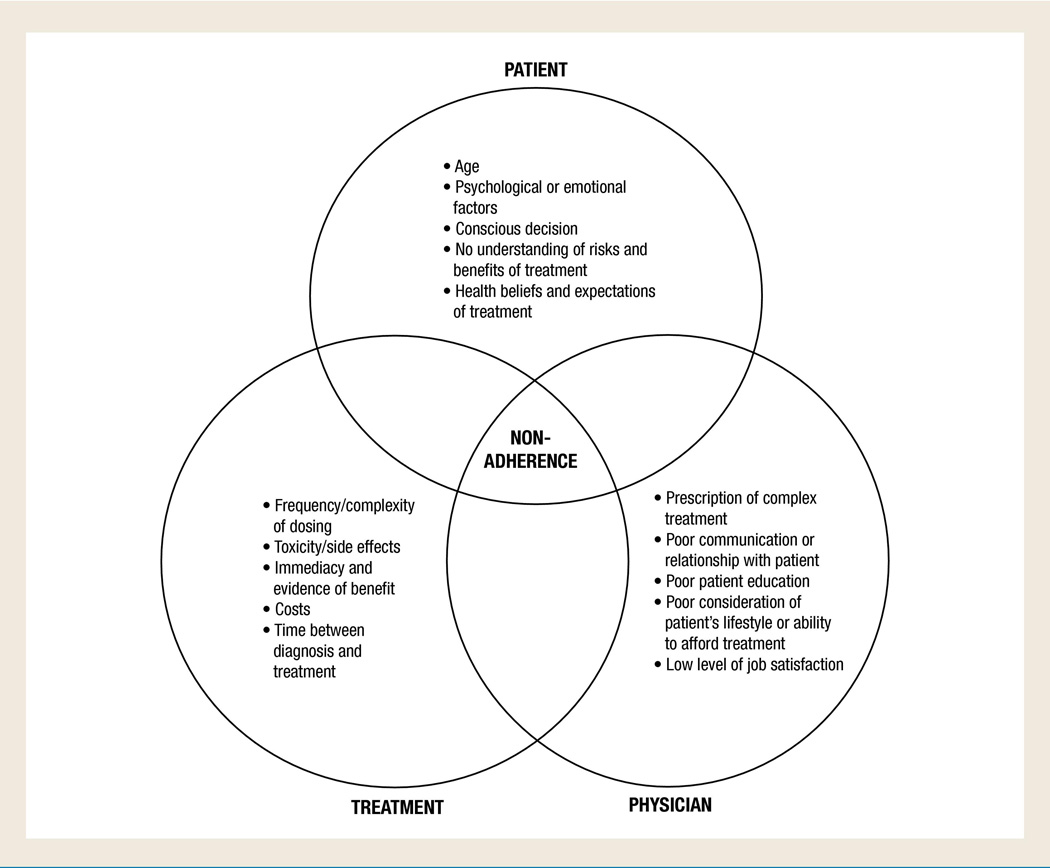
Barriers to adherence fall into 3 general areas: patient-, treatment-, and physician-related (including the healthcare system; Fig). Patient-related barriers can include age, psychological factors, and patient knowledge and beliefs. Studies have shown that adolescents are particularly prone to nonadherence with oral anticancer agents,14,16,19 while other research has found that patients with breast cancer aged <45 years or >75 years appear more likely to be nonadherent.21,22 Psychological factors, including depression or other mental illness as well as cognitive impairment also may lower adherence.13,14 Conscious reasons cited by patients for nonadherence include forgetfulness, other priorities, decision to omit doses, lack of information, and emotional factors.13 Adherence also may suffer if taking the medication as prescribed requires a substantial change in patient behavior.14 In addition, if a patient believes that the medication is doing them more harm than good, he or she may not adhere to the therapy as prescribed.13,14
Figure 1.
Factors Likely to Promote Treatment Nonadherence
Treatment-related barriers can include dose frequency or complexity, side effects (including toxicity), immediacy/evidence of benefit, cost of treatment, and time from diagnosis.13,14,19 A recent systematic review of 20 prospective studies used MEMS to examine the impact of dosing frequency (1–4 times daily) on adherence to oral medications for chronic conditions associated with asymptomatic periods (all noncancer conditions).18 Once-daily oral regimens were associated with an increase in days of adherence versus more frequent dosing (2% to 44% more days than twice-daily regimens and 22% to 41% more than three-times-daily regimens across the individual studies), which reached statistical significance (P < .05) in 15 of the 20 studies.18
The relationship between patient and practitioner can also play a role in adherence. Physicians and/or medical staff may exacerbate nonadherence by: prescribing complex regimens, not ensuring that the patient understands the proper use of the medication, not adequately explaining how the disease develops or progresses or the risks and benefits of treatment, and/or by not taking patient lifestyle or medication/insurance costs into consideration. Any of these events can translate into impaired physician-patient communication or a poor physician-patient relationship.13,14
Healthcare system–related barriers to adherence include limited/inconvenient access to healthcare (which in turn would affect the frequency and timing of obtaining and filling prescriptions) and high cost of treatment.13,14 A formulary change (requiring patients to switch to a new medication that may have new dosing requirements or safety concerns) or negative patient experience with their overall care also have the potential to affect patient adherence to a prescribed treatment regimen.
Adherence to BCR-ABL Inhibitor Therapy in Patients With CML
Because patients with CML-CP may present without symptoms and because lifelong medical treatment currently is required to prevent disease progression, achieving 100% adherence may be challenging. Adherence to BCR-ABL inhibitor treatment has been predominantly examined in studies of patients prescribed imatinib for CML which have found that a substantial proportion of patients with CML are nonadherent.26,27
In a retrospective study of pharmacy prescription data (N = 4043) for imatinib, which included patients with CML or gastrointestinal stromal tumors (GIST), Tsang et al found that patients with CML had a mean MPR of 78% (slightly higher than the mean MPR of 73% observed in the subset of patients with GIST).28 A retrospective cohort study by St Charles et al using employer-based prescription data from patients with CML who received ≥ 2 prescriptions of imatinib (n = 430) defined nonadherence as an MPR of ≤ 85%. In this study, 40% of patients were classified as nonadherent over a 12-month follow-up period.29 In a retrospective survey of healthcare claims data from 267 patients with CML on imatinib, Darkow et al found a mean MPR of 77% during the first year of treatment. In this study, 31% of patients interrupted therapy (ie, failed to refill imatinib within 30 days of the previous prescription’s runout date), although all of these patients resumed treatment within the 12-month follow-up period.26 A second retrospective survey of healthcare claims data in patients with CML (N = 592) on imatinib by Wu et al found a mean MPR of 79%, with 41% of patients having an MPR of < 85%.27
The Adherence Assessment with Glivec: Indicators and Outcomes (ADAGIO) prospective study investigated the prevalence of imatinib adherence in 169 Belgian patients with CML.30 According to the Basel Assessment of Adherence Scale (BAAS) with Immunosuppressive Medication (a 4-question interview, adapted for imatinib), 36% of patients at baseline and 33% at follow-up were considered nonadherent. Among patients in this study, only 14% took imatinib exactly as prescribed and achieved 100% adherence according to pill counts and patient self-reports.30
Poor adherence may be associated with poor response to treatment. In CML, direct and indirect measures of dose intensity can be used to identify an association between continuous, adequate BCR-ABL inhibitor exposure and a higher likelihood of response and positive outcomes. For example, in a Hammersmith Hospitals retrospective analysis of prospectively obtained data from patients with CML-CP (N = 204) receiving imatinib, dosing was started at 400 mg/day and adjusted as needed to address patient tolerance and response. In this analysis, there was a positive association between imatinib dose and the probability of achieving complete cytogenetic response (CCyR). Specifically, among patients remaining in CP at 12 months, those who had received a mean dose of imatinib > 350 mg/day over the first 6 months of therapy had a higher cumulative incidence of CCyR over 5 years than those who had received lower doses (89% vs 62%; P = .003).5 While dose reductions in this study were based on patient tolerance or response and not poor patient adherence, these results highlight the impact of inadequate dose levels on patient outcomes as is observed with reduced adherence to therapy.
A study of 280 patients with any-stage CML (n = 276) or Philadelphia chromosome–positive acute lymphoblastic leukemia (Ph+ ALL, n = 4) treated with dasatinib or nilotinib in first- and second-line settings (mean follow-up, 20 months) demonstrated that dose reductions and/or interruptions were associated with reduced failure-free survival in the overall population (P < .001). When patient subsets were analyzed by disease stage, this association also was found in individuals with CML-CP receiving dasatinib or nilotinib as first-line treatment (n = 98; P < .03); however, no similar association was observed with other patient subsets, possibly due to their small size (n ≤ 75). BCR-ABL inhibitor dose reduction and/or interruption had no effect on rates of major cytogenetic response or CCyR in any of the patient subsets.31
Subsequent research has shown that nonadherence, which may lead to decreased dose intensity, may be associated with lower response rates. In addition, dose interruptions, reductions, or discontinuations for the management myelosuppression9 may contribute to adverse outcomes.32
In the ADAGIO study, suboptimal response to treatment was defined as an incomplete hematologic response at 3 months, less than a partial cytogenetic response at 6 months, less than a major molecular response (MMR) at 18 months, or loss of MMR with other limitations or chromosomal abnormalities.30 Patients in this study with a suboptimal response to imatinib had a higher mean percentage of imatinib doses not taken than those achieving an optimal response (23% vs 7% based on pill counts [percentages calculated as proportions multiplied by 100]; P = .005).30
Another study from the Hammersmith Hospitals assessed adherence to imatinib using MEMS in patients with CML-CP (N = 87) who had achieved CCyR with imatinib 400 mg/day after a median treatment duration of 60 months. This analysis found that the degree of adherence (calculated as being > 90% or ≤ 90%), measured over a median of 91 days, was an independent predictor significantly associated with an inability to achieve molecular response.33 In addition, the 6-year probability of MMR and complete molecular response (CMR) strongly correlated with adherence rates: MMR rates were 14% versus 94% with an adherence of ≤ 90% and > 90%, respectively (P < .001), and CMR rates were 0% versus 44% (P = .002; Table 1). No patients with an adherence of ≤80% achieved a molecular response. In addition, patients whose imatinib dose was increased appeared to have a lower adherence rate (86%).33
Table 1.
Probable Rates of 6-Year MMR and CMR by Level of Adherence to Imatinib33
| Response | Rate of Adherence | ||
|---|---|---|---|
| ≤ 90% | ➢90% | ||
| MMR | 14% | 94% | P < .001 |
| CMR | 0% | 44% | P = .002 |
CMR = complete molecular response; MMR = major molecular response.
Current management guidelines state that if resistance to BCR-ABL inhibitors is encountered in the clinic, nonadherence should be investigated.1 Furthermore, poor outcomes may increase costs. In the surveys of CML imatinib healthcare claims data from American insurance providers conducted by Darkow et al and Wu et al, poor adherence to imatinib treatment was associated with increased healthcare costs. Darkow et al found that MPR inversely correlated with both medical costs (defined as all costs except prescription costs; P < .001) and healthcare costs (defined as medical and prescription costs, excluding imatinib; P < .001).26 Wu et al showed that patients with low MPR (< 85%) had more all-cause inpatient visits (4.1 vs 0.4; P < .001) and inpatient days (14.8 vs 1.8; P < .001) compared with those with a high MPR (≥ 85%; Table 2). Non-imatinib costs also were 283% higher (US$56,324) in the low-MPR group compared with the high-MPR group (P < .001).27 Although MPR was inversely correlated with medical costs and healthcare expenditures in both studies,26,27 it is unclear whether nonadherence caused increased medical complications and thereby healthcare costs, or whether increased medical complications and hospital admissions (and consequently, healthcare costs) caused nonadherence to therapy.
Table 2.
Mean Number of All-Cause Inpatient Visits or Days by Level of Adherence to Imatinib27
| Inpatient Requirements | Rate of Adherence | ||
|---|---|---|---|
| < 85% | ≥ 85% | ||
| Inpatient visits | 4.1 | 0.4 | P < .001 |
| Inpatient days | 14.8 | 1.8 | P < .001 |
Factors Affecting Adherence to BCR-ABL Inhibitor Therapy in Patients With CML
Predictors of BCR-ABL inhibitor nonadherence also have been predominantly studied in patients receiving imatinib. In general, data regarding the effect of age, gender, or length of imatinib exposure on adherence are inconsistent. However, several significant predictors of adherence have been identified across studies, including imatinib dose, time between CML diagnosis and imatinib prescription, and number of concomitant medications.
In their retrospective survey of employer-based prescription data for imatinib, St Charles et al showed that an MPR of < 85% was significantly associated with younger age (P < .05), shorter duration of exposure to imatinib (P < .001), an imatinib starting dose of ≤ 400 mg/day (P < .005), longer time between CML diagnosis and imatinib prescription fill (P < .0005), a higher number of concomitant prescriptions (P < .05), and a higher copayment percentage (P < .01).29 In their retrospective survey using pharmacy prescription data for patients with either CML or GIST, Tsang et al measured adherence by MPR and medication persistency (defined as time on therapy without significant gaps in refills). Patients initially treated with imatinib 300 to 400 mg/day showed the greatest persistency (13.0 and 12.9 months, respectively, vs 8.5 months average for all patients) and the greatest adherence (38.5% with 100% adherence vs 11.5% for other regimens).28
In their retrospective survey of CML imatinib healthcare claims data, Darkow et al examined both MPR and cancer complexity. Complexity was measured according to diagnosis codes for disease activity (eg, polyneuropathy in malignant disease, ICD-9-CM 357.3) or adverse events (eg, malnutrition, ICD-9-CM 263.9) that were indicative of increased difficulties in the clinical management of the disease.26 This study found that MPR was lower among women (P = .003), patients with high cancer complexity (P = .003), and patients with a higher imatinib starting dose (≥ 600 mg vs ≤ 400 mg, P = .04); further, MPR was inversely correlated with the number of concomitant medications (P = .002). Women were more likely to interrupt treatment than men (P = .009), as were those with high cancer complexity (P = .03).26
In the ADAGIO study, there was a weak correlation between patient-reported “bothersomeness” of symptoms and adherence behavior (correlation: rbs= −0.240, P = .007).30 This multivariate analysis was performed to evaluate adherence parameters (capturing BAAS scores, 10-cm visual analog scale ratings converted to a 0–100 score, and pill counts) using data from ≥ 160 patients with CML.30 In this analysis, other parameters correlated with nonadherence were (in descending order of canonical loading) higher age (0.649), longer time since CML diagnosis (0.272), living alone (0.246), median duration of treatment follow-up visits (0.237), male sex (0.194), longer time on imatinib (0.193), and imatinib dose ≥ 600 mg/day (0.193). The parameters correlated with the most substantial improvement in adherence (measured as decreased nonadherence) were the number of active patients with CML seen by the physician in the past year (−0.363) and patient knowledge of disease and treatment (−0.314).30
Toxicity, Complexity of Treatment, and Adherence to CML BCR-ABL Inhibitor Therapy
The adverse events predominantly associated with BCR-ABL inhibitor therapy are hematologic.3,9,10 QTc prolongation and liver function abnormalities (eg, elevated bilirubin) are class effects in BCR-ABL inhibitors3,9,10; however, they are asymptomatic and unlikely to affect adherence. Clinically symptomatic adverse events may include fluid retention, peripheral edema, rash, and musculoskeletal events for imatinib3; pleural effusion, bleeding events, rash, and musculoskeletal events for dasatinib9; and rash and musculoskeletal events for nilotinib.10
Polypharmacy that includes BCR-ABL inhibitors may negatively affect adherence, either by increasing treatment complexity (noted earlier as a known barrier to adherence) or by increasing toxicity through drug-drug interactions. Imatinib, dasatinib, and nilotinib are hepatic CYP3A4 substrates; therefore, the concomitant use of drugs that inhibit CYP3A4 may increase patient BCR-ABL inhibitor exposure, with subsequent increased risk of toxicity. Strong CYP3A4 inhibitors include azole antifungals, erythromycin, clarithromycin, indinavir, nelfinavir, ritonavir, and nefazodone. Grapefruit juice also is a CYP3A4 inhibitor and should be avoided while taking any of the above BCR-ABL inhibitors. Similarly, BCR-ABL inhibitors may reduce the metabolic clearance of other substrates of CYP3A4, raising the risk of toxicity from these agents. Additionally, inducers of CYP3A4 (eg, rifampin, rifabutin, phenytoin, dexamethasone, carbamazepine, phenobarbital) may decrease BCR-ABL inhibitor exposure, necessitating dose escalation.3,9,10
Other specific drug interactions with BCR-ABL inhibitors also are important to note. Because warfarin is metabolized by CYP2C9 and CYP3A4, patients receiving imatinib who require anticoagulation should receive low-molecular-weight or standard heparin instead of warfarin. Also, coadministration with imatinib may increase systemic exposure to acetaminophen,3 whereas use of acid-suppressing drugs may alter the pH-dependent solubility and subsequent exposure of dasatinib and nilotinib.9,10
Food restrictions also may increase the complexity of treatment. Fasting requirements in particular are one factor known to negatively affect treatment adherence.34 Patient surveys suggest that 12% to18% of patients are nonadherent with at least one of the bisphosphonate administration requirements, which include fasting rules.35 In the treatment of patients with CML, eating is known to increase nilotinib bioavailability and strict fasting is required 2 hours before and 1 hour after administration. Proactive patient discussions can help determine the likelihood that a patient’s schedule can readily support adherence to optimal timing required for medication in relation to meals.
Treatment Optimization to Ensure Adherence
Nontreatment factors such as healthcare access (eg, more convenient appointments and reduced patient cost burden) may improve adherence. Supervision of the patient’s treatment and patient-directed education also may improve adherence by helping the patient better understand the disease, their treatment, and the importance of self-monitoring for adverse events.13,19 However, a systematic review of over 6500 citations, with full review of 549 articles published between 1967 and 2001, did not find a predictably effective approach to ensuring optimal adherence; it was concluded that most methods for improving adherence for chronic health problems usually are complicated, costly, and not consistently successful.36 However, based on what we do know, prompt prescription of BCR-ABL inhibitors after CML diagnosis and effective patient supervision, which includes early and ongoing education regarding CML and its treatment, are likely to increase adherence.
Dealing proactively with toxicity due to BCR-ABL inhibitors also is important and may improve medication adherence. The patient should be educated to recognize and immediately report symptoms of key adverse events (eg, fever, unusual bruising or bleeding, swelling or weight gain, shortness of breath, significant fatigue). The physician should be diligent in monitoring the patient because discussions with patients at follow-up clinic visits may reveal signs of adverse events that the patient does not recognize for him- or herself. Toxicity resulting from BCR-ABL inhibitors generally is manageable through dose reduction or interruption and appropriate supportive care.37 Physicians should promptly provide appropriate management of adverse events when detected.
Patients who are already experiencing or are at risk for adverse events with a specific BCR-ABL inhibitor should be offered alternative treatment in accordance with current guidelines.1 Specifically, treatment options other than dasatinib should be considered in patients at risk for or already suffering from severe bleeding or pleural effusion. Nilotinib should not be preferentially chosen for patients with a history of or risk factors for diabetes mellitus, pancreatitis, hepatic disease, or rash. Imatinib should not be preferentially selected for patients with hepatic disease or fluid retention. However, in the first-line study setting, nilotinib-associated hyperglycemia has been reported as typically mild, transient, and manageable, and without causing treatment discontinuation in patients with type II diabetes.38 In addition to specific risks, it is useful to consider comedication issues. In general with BCR-ABL inhibitors, concomitant strong CYP3A4 inhibitors and inducers should be avoided whenever possible. If the use of strong CYP3A4 inhibitors or inducers cannot be avoided, BCR-ABL inhibitors should be delivered with appropriate dose adjustment, as recommended in the prescribing information. As drugs that alter stomach pH are known to reduce the solubility and exposure of dasatinib and nilotinib, simultaneous administration of these BCR-ABL inhibitors with proton pump inhibitors, H2 antagonists, and antacids should be avoided.9,10 In the event acid-suppressing drugs are necessary, these medications should only be administered several hours before or after a dasatinib or nilotinib dose. In general, reviewing concomitant treatments and simplifying or reducing them if possible may lead to improved adherence overall, as simpler, less complex regimens are associated with greater adherence.
Conclusions
Nonadherence to medication is a widespread problem. Although oral therapies generally are preferred by patients over injectable or intravenous ones, they also are known to have reduced adherence rates. Studies have indicated that adherence to oral therapies for chronic health problems, including cancer, can be quite poor; this is particularly true for conditions that may have asymptomatic presentations such as CML, which is most often diagnosed in the chronic phase. Nonadherence rates for first-line imatinib use have been reported to range from 20% to 40%, and the ADAGIO study (N = 169) found that just over one-tenth of patients were 100% adherent to imatinib therapy.
Evidence suggests that poor adherence to BCR-ABL inhibitor therapy is associated with reduced efficacy and increased healthcare costs. Studies that have measured adherence to imatinib have demonstrated that good adherence is significantly associated with higher molecular and cytogenetic response rates in patients with CML. Other retrospective claims database studies indicate that poor adherence leads to greater resource utilization (eg, inpatient visits and days) and increased non-imatinib costs.
Based on current evidence, there are several recommendations for BCR-ABL inhibitor use that can help optimize adherence. When indicated, a prescription for BCR-ABL inhibitor therapy should be provided promptly following CML diagnosis, as early prescribing has been a consistent predictor of adherence across studies. Another predictor of adherence is the number of concomitant medications being taken by the patient. Therefore, any steps that can be taken to simplify the overall medical management of the patient, ideally involving cooperation between the patient’s oncologist and primary care physician, should be a priority. Furthermore, clear and effective education and communication with the patient and proactive management of toxicity if identified are likely to improve the prospects for good adherence. Overall, BCR-ABL inhibitor treatment selection should be individualized for patients, taking into account comorbidities, concomitant medications, and their level of independence/responsibility.
Acknowledgments
This manuscript was supported by Bristol-Myers Squibb. The authors acknowledge StemScientific, funded by Bristol-Myers Squibb, for providing writing and editorial support. Bristol-Myers Squibb did not influence the content of the manuscript, nor did the authors receive financial compensation for authoring the manuscript.
Footnotes
Disclosures
Elias Jabbour has received honoraria from Bristol-Myers Squibb, Novartis, and Pfizer. Giuseppe Saglio is a consultant for and has received honoraria from Novartis and Bristol-Myers Squibb. Jerald Radich is a consultant for and has received research grants from Novartis and is a consultant for Bristol-Myers Squibb and Pfizer. Hagop Kantarjian has received research grants from Bristol-Myers Squibb, Pfizer, and Ariad and is a consultant for and has received research grants from Novartis.
References
- 1.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology™: Chronic Myelogenous Leukemia, v.2.2011. [Accessed December 8, 2011]; Available at: http://www.nccn.org/professionals/physician_gls/PDF/cml.pdf. [Google Scholar]
- 2.Chronic Myeloid Leukemia Trialists’ Collaborative Group. Interferon alfa versus chemotherapy for chronic myeloid leukemia: a meta-analysis of seven randomized trials. J Natl Cancer Inst. 1997;89:1616–1620. [PubMed] [Google Scholar]
- 3.Gleevec (imatinib) [prescribing information] East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2010. [Google Scholar]
- 4.Druker BJ, Guilhot F, O’Brien SG, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355:2408–2417. doi: 10.1056/NEJMoa062867. [DOI] [PubMed] [Google Scholar]
- 5.de Lavallade H, Apperley JF, Khorashad JS, et al. Imatinib for newly diagnosed patients with chronic myeloid leukemia: incidence of sustained responses in an intention-to-treat analysis. J Clin Oncol. 2008;26:3358–3363. doi: 10.1200/JCO.2007.15.8154. [DOI] [PubMed] [Google Scholar]
- 6.Faderl S, Talpaz M, Estrov Z, et al. Chronic myelogenous leukemia: biology and therapy. Ann Intern Med. 1999;131:207–219. doi: 10.7326/0003-4819-131-3-199908030-00008. [DOI] [PubMed] [Google Scholar]
- 7.Horner MJ, Ries LAG, Krapcho M, et al., editors. SEER Cancer Statistics Review, 1975–2006 (based on November 2008 SEER data submission) [Accessed December 8, 2011]; Updated January 28, 2010. Available at: http://seer.cancer.gov/csr/1975_2006/.
- 8.United Nations. World Population Prospects: The 2006 Revision. [Accessed December 8, 2011]; Available at: http://www.un.org/esa/population/publications/wpp2006/WPP2006_Highlights_rev.pdf. [Google Scholar]
- 9.Sprycel (dasatinib) [package insert] Princeton, NJ: Bristol-Myers Squibb Company; 2011. [Google Scholar]
- 10.Tasigna (nilotinib) [prescribing information] East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2011. [Google Scholar]
- 11.Baccarani M, Cortes J, Pane F, et al. Chronic myeloid leukemia: an update of concepts and management recommendations of European LeukemiaNet. J Clin Oncol. 2009;27:6041–6051. doi: 10.1200/JCO.2009.25.0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deininger M, O’Brien SG, Guilhot F, et al. International Randomized Study of Interferon vs STI571 (IRIS) 8-year follow up: sustained survival and low risk for progression or events in patients with newly diagnosed chronic myeloid leukemia in chronic phase (CML-CP) treated with imatinib [abstract] Blood. 2009;114:1126. [Google Scholar]
- 13.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487–497. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 14.Partridge AH, Avorn J, Wang PS, et al. Adherence to therapy with oral antineoplastic agents. J Natl Cancer Inst. 2002;94:652–661. doi: 10.1093/jnci/94.9.652. [DOI] [PubMed] [Google Scholar]
- 15.Benner JS, Glynn RJ, Mogun H, et al. Long-term persistence in use of statin therapy in elderly patients. JAMA. 2002;288:455–461. doi: 10.1001/jama.288.4.455. [DOI] [PubMed] [Google Scholar]
- 16.Weingart SN, Brown E, Bach PB, et al. NCCN Task Force report: oral chemotherapy. J Natl Compr Canc Netw. 2008;6(suppl 3):S1–S14. [PubMed] [Google Scholar]
- 17.Claxton AJ, Cramer J, Pierce C. A systematic review of the associations between dose regimens and medication compliance. Clin Ther. 2001;23:1296–1310. doi: 10.1016/s0149-2918(01)80109-0. [DOI] [PubMed] [Google Scholar]
- 18.Saini SD, Schoenfeld P, Kaulback K, et al. Effect of medication dosing frequency on adherence in chronic diseases. Am J Manag Care. 2009;15:e22–e33. [PubMed] [Google Scholar]
- 19.Ruddy K, Mayer E, Partridge A. Patient adherence and persistence with oral anticancer treatment. CA Cancer J Clin. 2009;59:56–66. doi: 10.3322/caac.20004. [DOI] [PubMed] [Google Scholar]
- 20.Chlebowski RT, Geller ML. Adherence to endocrine therapy for breast cancer. Oncology. 2006;71:1–9. doi: 10.1159/000100444. [DOI] [PubMed] [Google Scholar]
- 21.Hershman DL, Kushi LH, Shao T, et al. Early discontinuation and nonadherence to adjuvant hormonal therapy in a cohort of 8,769 early-stage breast cancer patients. J Clin Oncol. 2010;28:4120–4128. doi: 10.1200/JCO.2009.25.9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Partridge AH, Wang PS, Winer EP, et al. Nonadherence to adjuvant tamoxifen therapy in women with primary breast cancer. J Clin Oncol. 2003;21:602–606. doi: 10.1200/JCO.2003.07.071. [DOI] [PubMed] [Google Scholar]
- 23.McCowan C, Shearer J, Donnan PT, et al. Cohort study examining tamoxifen adherence and its relationship to mortality in women with breast cancer. Br J Cancer. 2008;99:1763–1768. doi: 10.1038/sj.bjc.6604758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kantarjian H, Cortes J, Kim D-W, et al. Phase 3 study of dasatinib 140 mg once daily versus 70 mg twice daily in patients with chronic myeloid leukemia in accelerated phase resistant or intolerant to imatinib: 15-month median follow-up. Blood. 2009;113:6322–6329. doi: 10.1182/blood-2008-11-186817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shah NP, Kim D-W, Kantarjian H, et al. Potent, transient inhibition of BCR-ABL with dasatinib 100 mg daily achieves rapid and durable cytogenetic responses and high transformation-free survival rates in chronic phase chronic myeloid leukemia patients with resistance, suboptimal response or intolerance to imatinib. Haematologica. 2010;95:232–240. doi: 10.3324/haematol.2009.011452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Darkow T, Henk HJ, Thomas SK, et al. Treatment interruptions and non-adherence with imatinib and associated healthcare costs: a retrospective analysis among managed care patients with chronic myelogenous leukaemia. Pharmacoeconomics. 2007;25:481–496. doi: 10.2165/00019053-200725060-00004. [DOI] [PubMed] [Google Scholar]
- 27.Wu EQ, Johnson S, Beaulieu N, et al. Healthcare resource utilization and costs associated with non-adherence to imatinib treatment in chronic myeloid leukemia patients. Curr Med Res Opin. 2010;26:61–69. doi: 10.1185/03007990903396469. [DOI] [PubMed] [Google Scholar]
- 28.Tsang J, Rudychev I, Pescatore SL. Prescription compliance and persistence in chronic myelogenous leukemia (CML) and gastrointestinal stromal tumor (GIST) patients (pts) on imatinib (IM) [abstract] J Clin Oncol. 2006;24(suppl):6119. [Google Scholar]
- 29.St Charles M, Bollu VK, Hornyak E, et al. Predictors of treatment non-adherence in patients treated with imatinib mesylate for chronic myeloid leukemia [abstract] Blood. 2009;114:2209. [Google Scholar]
- 30.Noens L, van Lierde M-A, De Bock R, et al. Prevalence, determinants, and outcomes of nonadherence to imatinib therapy in patients with chronic myeloid leukemia: the ADAGIO study. Blood. 2009;113:5401–5411. doi: 10.1182/blood-2008-12-196543. [DOI] [PubMed] [Google Scholar]
- 31.Santos FPS, Kantarjian H, Fava C, et al. Clinical impact of dose reductions and interruptions of second-generation tyrosine kinase inhibitors in patients with chronic myeloid leukemia. Br J Haematol. 2010;150:303–312. doi: 10.1111/j.1365-2141.2010.08245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Galinsky I, Buchanan S. Practical management for dasatinib for maximum patient benefit. Clin J Oncol Nurs. 2008;13:329–335. doi: 10.1188/09.CJON.329-335. [DOI] [PubMed] [Google Scholar]
- 33.Marin D, Bazeos A, Mahon FX, et al. Adherence is the critical factor for achieving molecular responses in patients with chronic myeloid leukemia who achieve complete cytogenetic responses on imatinib. J Clin Oncol. 2010;28:2381–2388. doi: 10.1200/JCO.2009.26.3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boonen S, Vanderschueren D, Venken K, et al. Recent developments in the management of postmenopausal osteoporosis with bisphosphonates: enhanced efficacy by enhanced compliance. J Intern Med. 2008;264:315–332. doi: 10.1111/j.1365-2796.2008.02010.x. [DOI] [PubMed] [Google Scholar]
- 35.Papaioannou A, Kennedy CC, Dolovich L, et al. Patient adherence to osteoporosis medications: problems, consequences and management strategies. Drugs Aging. 2007;24:37–55. doi: 10.2165/00002512-200724010-00003. [DOI] [PubMed] [Google Scholar]
- 36.McDonald HP, Garg AX, Haynes RB. Interventions to enhance patient adherence to medication prescriptions. JAMA. 2002;288:2868–2879. doi: 10.1001/jama.288.22.2868. [DOI] [PubMed] [Google Scholar]
- 37.Jabbour E, Deininger M, Hochhaus A. Management of adverse events associated with tyrosine kinase inhibitors in the treatment of chronic myeloid leukemia. Leukemia. 2011;25:201–210. doi: 10.1038/leu.2010.215. [DOI] [PubMed] [Google Scholar]
- 38.Saglio G, Larson RA, Hughes TP, et al. Efficacy and safety of nilotinib in chronic phase (CP) chronic myeloid leukemia (CML) patients (pts) with type 2 diabetes in the ENESTnd trial [abstract] Blood. 2010;116:3430. [Google Scholar]