Abstract
Drug delivery systems, particularly nanomaterial-based drug delivery systems, possess a tremendous amount of potential to improve diagnostic and therapeutic effects of drugs. Controlled drug delivery targeted to a specific disease is designed to significantly improve the pharmaceutical effects of drugs and reduce their side effects. Unfortunately, only a few targeted drug delivery systems can achieve high targeting efficiency after intravenous injection, even with the development of numerous surface markers and targeting modalities. Thus, alternative drug and nanomedicine targeting approaches are desired. Circulating cells, such as erythrocytes, leukocytes, and stem cells, present innate disease sensing and homing properties. Hence, using living cells as drug delivery carriers has gained increasing interest in recent years. This review highlights the recent advances in the design of cell-mediated drug delivery systems and targeting mechanisms. The approaches of drug encapsulation/conjugation to cell-carriers, cell-mediated targeting mechanisms, and the methods of controlled drug release are elaborated here. Cell-based “live” targeting and delivery could be used to facilitate a more specific, robust, and smart payload distribution for the next-generation drug delivery systems.
Keywords: drug delivery, nanoparticles, immune cells, circulating cells, stem cells, targeting
INTRODUCTION
Drug delivery systems (DDS) have been extensively studied in past several decades as a powerful tool to achieve improved therapeutic efficacy and diagnostic effects by remarkably improving the pharmacokinetics and pharmacodynamics. Recently, a great deal of effort has been made to design novel nano-scale DDS with controlled therapeutic outcomes, targeted modalities towards specific diseases, and minimized side effects. However, traditional DDS, including injection formulations, particles, liposomes, and hydrogels, cannot meet the ever-growing requirements of modern medicine, such as truly targeted therapies and personalized medicine. Thus, cell-mediated drug delivery has emerged as a new frontier in medicine. In this review, we will analyze the current design strategies of circulating cell-based drug delivery systems in terms of 1) selecting cells, 2) loading therapeutics into live cells or conjugating therapeutics to the surface of live cells, 3) allowing cell-mediated DDS to target specific diseases, and 4) achieving controlled drug release. The purpose of this review is to summarize the existing designs in constructing circulating cell-mediated DDS and to provide our perspectives on future directions of “live” drug delivery.
Conventional DDS and their challenges
Current DDS typically employ vehicles to carry therapeutics in order to improve the drug solubility, reduce toxicity, prolong circulating time, limit bio-distribution, achieve specific targeting, control drug release, and diminish immunogenicity. Ideal drug delivery vehicles should be biocompatible, biodegradable, easy to modify, and targeted towards specific diseases. A wide range of vehicles have been employed as controlled DDS, e.g. liposomes1, nanoparticles (NPs)2, micelles3, hydrogels4-5, fibers6-7, and films8. Particularly, nano- and micro-materials decorated with targeting ligands or molecules, such as peptides, antibodies, aptamers, and proteins, that are specific to the receptors expressed or overexpressed on aberrant cells and their surrounding microenvironments have been widely studied in targeted drug delivery9-10. Unfortunately, very few of these nanomedicines have shown clinical efficacy due to significant challenges associated with the trafficking and targeting in vivo.
Current targeting strategies can be categorized into passive and active targeting. Passive targeting is typically dependent on enhanced permeability and the retention (EPR) effect caused by leaky vasculature and poor lymphatic drainage. The EPR effect is a unique phenomenon, by which macromolecules and nanoparticles (NPs) escape from the blood flow and preferentially accumulate more in tumors rather than in normal tissues. It occurs frequently in solid tumors, in which blood vessels commonly have defects. Additionally, defective lymphatic drainage leads to the loss of the ability to clear infiltrating substances. These distinguishing anatomical and pathophysiological characteristics of solid tumors have been generally utilized in tumor targeting. However, passive targeting is largely chance-dependent. Therefore, active targeting, especially molecular targeting, has been the focus of drug delivery research in recent years. Until now, numerous surface markers have been found on abnormal cells or in their surrounding microenvironment. Integrins, folate, growth factors, and cytokines offer possibility for counter-ligand functionalized drug vehicles to recognize cells and tissue targets11-12. With the capability to bind to specific surface markers, circulating drug carriers may have a higher tendency to attach and accumulate at the sites of diseases such as cancers. In addition, a number of cell-penetrating molecules have also been discovered that can further improve the drug delivery efficiency13. Certainly, the combination of passive and active targeting for drug delivery may yield amplified results.
Despite tremendous efforts made towards discovering novel materials and biomolecule markers for targeted DDS, very few of them are truly specific after intravenous injection and the targeting still remains chance-dependent. Both active and passive targeting approaches require exogenous drug vehicles to disperse and voyage in circulation for a long time to pass through the leaky vasculature or detect the surface markers. However, the circulating environment, in which many drug vehicles cannot have a long enough circulating time to achieve targeted binding, is extremely complicated14-15. In addition, the human body has an innate defense mechanism for invasion. For example, the reticuloendothelial system (RES) rapidly recognizes foreign bodies and destroys them via a series of biological processes. The RES, also called the mononuclear phagocyte system, is comprised primarily of bone marrow progenitors, blood monocytes, and tissue macrophages16. Furthermore, the EPR effect is somehow heterogeneous in the tumor microenvironments and varies among patients17. For example, hypoxic regions of solid tumor generally do not even exhibit EPR effects due to poor angiogenesis17-18. Considering the complexity and sophistication of in vivo conditions, conventional passive and active targeting strategies still remain inadequate. Hence, developing novel DDS with truly specific targeting is a formidable challenge for modern medicine and nanotechnology.
Cell-mediated DDS
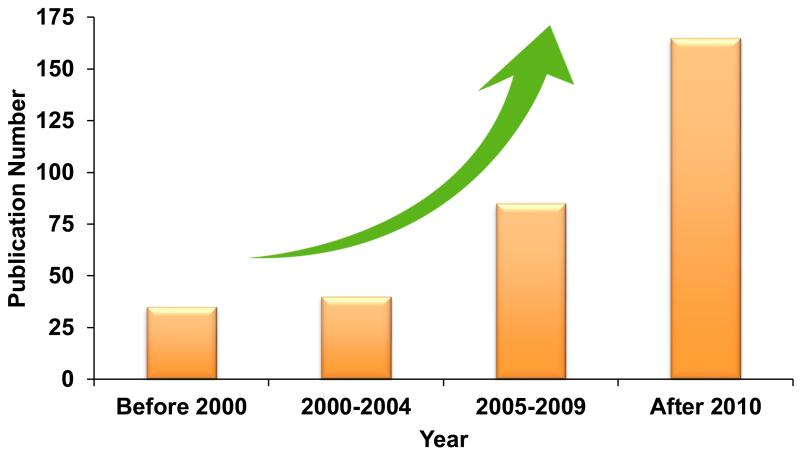
Recently, cell-mediated DDS have emerged as a promising strategy that is poised to address the above challenges. This novel strategy takes advantage of cell properties, such as long circulation time, abundant surface ligands, flexible morphology, cellular signaling, and metabolism, to offer a unique opportunity to maximize therapeutic outcomes as well as minimizing side effects. The increasing attention towards this field can be seen from the increasing numbers of publications according to Web of Science™ (Figure 1). Here, we review recent advances in the design of cell-mediated targeting and drug delivery systems. It is our hope that this review will synergize the current ongoing efforts and lead to future innovations in live cell-mediated DDS.
Figure 1.
The numbers of publications searched with the keywords of “Cell Mediated” and “Drug Delivery”. Source: Web of Science™.
CIRCULATING CELLS
Circulating cells can serve as ideal drug delivery carriers for a number of reasons. They are highly mobile and able to travel through blood flow without immunogenicity. Circulating cells are involved in various disease processes, including infection, inflammation, and cancers development, so they can offer multiple advantages for disease targeting. For instance, leukocytes have the innate ability to cross the blood brain barrier (BBB) to access tumor cells in the brain19. Inflammatory responses and wound healing of many diseases are involved in cell homing processes that spontaneously attract circulating cells to disease sites. Furthermore, using circulating cells as delivery vehicles is advantageous as it significantly reduces immune clearance and prolongs the biological half-time for drug delivery. Candidate cells to mediate drug delivery include erythrocytes, leukocytes, platelets, and stem cells, whose properties are summarized in Table 1.
Table 1.
Properties of erythrocytes, leukocytes, and stem cells.
| Erythrocytes | Leukocytes | Stem Cells | |
|---|---|---|---|
|
Amount
(/ml blood) Diameter (μm) |
4.2-6.0×106 | 4×103-11×103 | Unknown |
| ~7 | 7-15 | 30-40 | |
| Location | Blood circulation | Circulation, tissues and organs |
Circulation, tissues and organs |
| Life Span | ~120 days | A few days; years for memory lymphocytes |
Depending on cell types |
| Functions | Transport oxygen | Immune defense | Repair tissues and organs |
| Pros as DDS | Large encapsulating volume; large surface areas; longer life span; reversible deformation; RES targeting. |
Capable of cross biological membrane; tumor-homing; immune response. |
Potency; homing to injured cells, tissues and organs. |
| Cons as DDS | Rapid RES elimination; drug released off-site |
Short life-span; hard to handle. |
Hard to maintain potency in vitro. |
Red blood cells
Erythrocytes, or red blood cells (RBCs), make up the largest population of blood cells (>99%). Approximately 2 million new erythrocytes are continuously produced per second in the human body. RBCs are non-nuclear biconcave discs that average ~7 μm in diameter and ~2.5 μm in thickness, and have a large internal capacity volume of 185-191 μm3. Utilizing hemoglobin as an iron-containing protein, RBCs transport oxygen from the respiratory organs to the rest of body. In addition to oxygen, RBCs can carry a range of valuable payloads from therapeutics to imaging contrast agents.
RBC-based drug delivery has attracted increasing attention for many reasons. RBCs can be easily isolated, frozen, and stored for an extended period of at least ten years20. RBCs in the blood circulation have a life span of 120 days and thus may act as a reservoir for sustained drug release21. The biconcave shape and non-nuclear architecture allow RBCs to encapsulate a large amount of drugs. The membranes of RBCs have reversible deformability, making them capable for taking up payloads via physical methods. Additionally, RBCs are completely biodegradable without producing toxic byproducts, as the RES recognizes old and incompatible RBCs and rapidly removes them. The clearance pathways of RBCs have been widely employed in targeting the RES of the liver, spleen and bone marrow.
The use of RBCs as drug delivery vehicles, however, still encounters several challenges. RBC carriers can be rapidly eliminated by the RES after drug encapsulation or cellular modification due to potential morphological and functional alterations. The RES rapidly recognizes and eliminates modified RBCs before they reach disease sites, which makes non-RES targeting particularly challenging. Drugs may also be released from destructed cells and result in cytotoxicity. The abundance of RBCs could be a double-edged sword that prevents efficient delivery of therapeutics to disease sites. In addition, RBCs are generally not as selective as other circulating cells in terms of targeting disease sites and promoting the healing process.
Leukocytes
Leukocytes, or white blood cells (WBCs,) play significant roles in the immune system, by cleaning cellular debris and defending the body against infections and diseases. Normal human blood contains 4-11×109 leukocytes per mL22. Leukocytes are found in five major types, neutrophils (40% - 75%), eosinophils (1% - 6%), basophils (less than 1%), monocytes (2%-10%), and lymphocytes (20%-45%). Although the lifespan of leukocytes (up to 20 days) is typically shorter than that of RBCs, their specialized functions make them attractive as drug delivery carriers because leukocytes are involved in various immune responses, cellular interactions, cell adhesion, and are capable of penetrating through biological barriers into non-vascular areas. Neutrophils, monocytes/macrophages, and lymphocytes are all potential candidates for delivering therapeutics to treat various diseases.
Neutrophils, also known as polymorphonuclear granulocytes (PMNs) that contain distinctive cytoplasmic granules, are the most abundant leukocyte found in the human body. They are the first cells that arrive at the sites of infection or inflammation, produce cytokines to recruit other cells, and are cleared after a few days23. Neutrophils also engulf invading microorganisms or foreign substances and consequently eliminate the invaders using digestive enzymes (e.g. lysozyme, hydrolytic enzymes and myeloperoxidase) or respiratory burst24. Unfortunately, neutrophils have the average lifespan of 5.4 days in circulation and only a few hours after their isolation from blood25. The short life span of neutrophils limits their applications in DDS, but their ability to immediately migrate and transport make them attractive as drug carrier candidates.
Monocytes are mononuclear leukocytes with kidney-shaped nuclei and clear cytoplasm. They are produced from stem cell precursors in the bone marrow. Monocytes circulate in the bloodstream and migrate to tissues, particularly the liver, lymph nodes, and lungs. Meanwhile, monocytes continuously migrate to and accumulate at disease sites in association with infection or inflammation26-27. Once leaving the blood flow, monocytes differentiate into macrophages in response to various stimulations. Otherwise, they return to the bone marrow without activation. Macrophages play versatile roles in inflammation, cell recruitment, secretion of cytokines and growth factors, and bacteria/cellular debris clearance. Emerging studies also indicate that macrophages are the major players in disease microenvironments and disease progression, such as in cancer invasion28-29. Additionally, monocytes/macrophages present phagocytic capability that enables the spontaneous encapsulation of drugs/particles.
Lymphocytes are identified by their large nucleus surrounded by a thin layer of cytoplasm. The average diameter of lymphocytes is 7-15 μm. Lymphocytes are primarily found in the circulation and central lymphoid organs such as the spleen, tonsils, and lymph nodes30. T cells and B cells are the major types of lymphocytes and are responsible for the adaptive immune system. T cells mature in the thymus and play a critical role in cell-mediated immunity and can be broadly divided into helper T cells, cytotoxic T cells, and regulatory T cells31. When an antigen appears, antigen-presenting cells (APCs) recognize and present the antigen to T cells. Then, helper T cells secrete various cytokines, which stimulate toxic T cells to directly kill abnormal cells. Regulatory T cells are also activated to suppress immune response in order to maintain immunological tolerance. B-cells are produced in the bone marrow and involved in humoral immunity. B cells make antibodies against antigens and can be characterized by the presence of immunoglobulin on their surface32. Meanwhile, B cells can differentiate into memory B cells, which respond rapidly when exposed to the same antigen. Thus, both lymphocytes present multiple functions in human immunity and are involved in numerous diseases: detecting antigens, infiltrating disease sites, and attacking abnormal cells. Clearly, lymphocytes could serve as a potential platform to deliver drugs specifically.
Overall, leukocytes have a rapid response and intrinsic homing properties with respect to infections, inflammations, and tumors. Such sensitive detections and biological barriers infiltration abilities give rise to opportunities for leukocytes-mediated drug delivery. However, vulnerable leukocytes are difficult to harvest and handle with relative short life spans, which hinder the manipulation processes for loading drugs and NPs.
Stem cells
Stem cells are self-renewable with a potential to differentiate into various cell types and therefore essential in tissue repair and regeneration. They are broadly classified into two categories according to their source and plasticity: embryonic stem cells and adult stem cells. Embryonic stem cells originate from the inner cell mass of a blastocyst in an early-stage embryo, which are pluripotent that can generate all cell types in the body. Adult stem cells are tissue-specific stem cells that can differentiate into limited specialized cells33. Stem cell therapy has been significantly explored in tissue engineering, regenerative medicine, and even translated into clinical trials34-35. Utilizing stem cells as drug carriers can be particularly beneficial since it may add another dimension into existing stem cell therapy. Current stem cell therapies including those for neurological disease, heart disease, and cancer can all be improved by incorporating therapeutics into stem cells. Interestingly, stem cells are inherently tumor homing, which is mediated by inflammatory factors, tumor-derived factors, and tumor-specific receptors36-37. Generally, stem cells take 2-4 days to migrate to tumor sites38. Thus, stem cells also have natural living targeting capabilities that response to diseases and tissue regeneration. Additionally, stem cells are relatively easy to harvest, culture in vitro, and can be induced to differentiate into specialized cells under certain conditions. These properties make stem cells promising candidates for targeted drug delivery.
ENCAPSULATION/CONJUGATION OF DRUGS/PARTICLES TO CIRCULATING CELLS
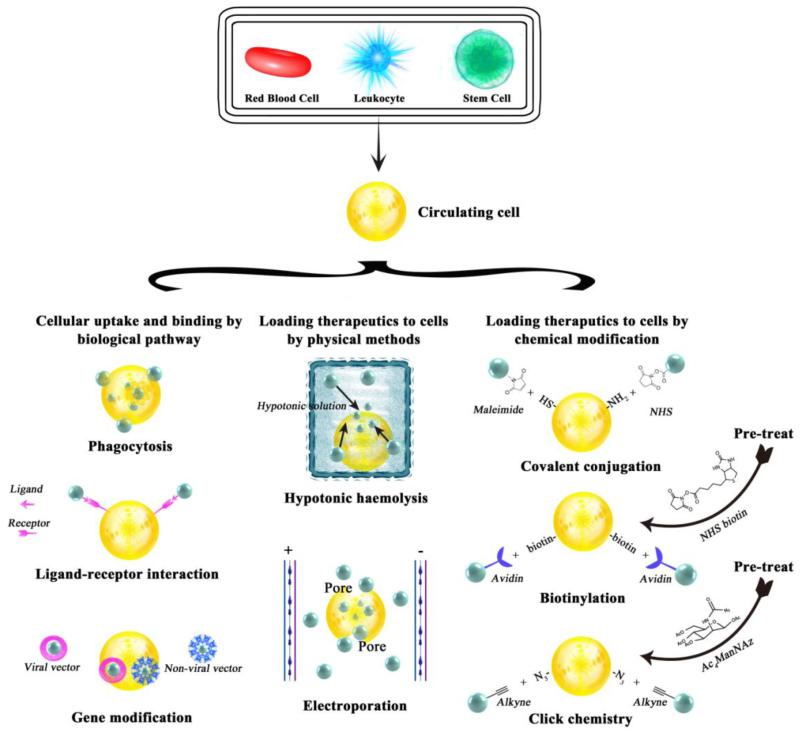
In order to obtain cell-mediated drug delivery, drugs or drug-loaded particles should be able to attach onto or internalized into cells to form drug-cell or particle-cell complexes. The basic requirements for drugs and drug-loaded particles in cell-mediated delivery are no or low toxicity to cell carriers, particle degradability, and controlled release of drugs. For drug/particle-cell complexes, strong binding between payloads and cell carriers, long circulating time, and low immunogenicity are desired. The use of cell carriers should not only transport drugs in high specificity to target tissues, but also regulate pharmacokinetics and pharmacodynamics via altering the properties of biomaterials that are used to encapsulate therapeutics. To date, drug vehicles can be added to cells by biological pathways, physical approaches or chemical modifications (Figure 2).
Figure 2.
Strategies of encapsulation and conjugation of drugs/particles onto/into circulating cells, including red blood cells (RBCs), leukocytes, and stem cells, by biological, physical and chemical means.
Cellular uptake and binding by biological pathways
The most straightforward way to load drugs with circulating cells is using unmodified cells, which possesses natural biological pathways that can be utilized. Biological pathways represent a series of actions that regulate cell behavior and fate at the molecular level. Various cells are able to load drugs via different pathways. In these cases, their morphology and functional integrity can be largely preserved. In this section, we will review the pathways that facilitate the encapsulation of drugs or genes within cells and/or conjugating nanoparticles onto the surface of cells. Loading therapeutics via physical and chemical modifications of cells will be reviewed in later two sections, respectively.
Endocytosis
Endocytosis is a fundamental cellular process, by which eukaryotic cells engulf fluid, molecules, and even other cells39. Most substances, especially large and polar molecules, cannot directly pass through the hydrophobic cell membranes. Endocytosis provides a pathway to internalize these substances via plasma membrane deformation. During this process, plasma membranes invaginate and form intracellular vesicles around the substances to be internalized, followed by the membrane fusion. Endocytosis is dominated by several parameters such as sizes, shapes, charges, and mechanical properties of the foreign substances40. It offers an opportunity to regulate the encapsulation of drug vehicles by an endocytosis-dependent pathway.
Pinocytosis is a mode of endocytosis that can non-specifically “drink” the surrounding liquid and molecules. Eukaryotic cells continuously conduct pinocytosis and are prone to engulf relatively small molecules. This plasma membrane homeostasis process has been utilized encapsulation bare drugs into erythrocytes in early attempts. Drugs, such as hydrocortisone, primaquine, vinblastine, and chlorpromazine, can directly induce erythrocyte membrane internalization by pinocytosis41-43. The formation of vacuoles is age-related44. Pinocytosis is adenosine triphosphate (ATP)-dependent and influenced by the drug concentration, temperature, pH, and the balance between the concentrations of magnesium and calcium in the cell membrane45-46. These drug-loading methods are based on simple absorption; however, they tend to induce potential cytotoxicity.
In contrast to pinocytosis, phagocytosis sporadically occurs in specialized cells (e.g. neutrophils, macrophages, lymphocytes, and dendritic cells). Stimulated by immune responses, these white blood cells can internalize particles in nano or micro sizes via an actin-dependent mechanism associated with receptor reorganizations such as Fc receptors, complements, and mannoses47. The sizes of drug vehicles, including NPs, micelles, and liposomes, commonly fall in this range. Then, the engulfed particles fuse with lysosomes that are later broken down by abundant digestive enzymes in lysosomes. Since phagocytosis is a type of immune defense procedures against foreign invaders, including drugs or drug-encapsulated particles, it gains tremendous interest in cell-mediated DDS design. Drug-laden particles are susceptible to rapid RES clearance in vivo, which means immune cells are the first cells to sense and catch particles during circulation. Instead of designing particles to overcome rapid RES elimination, phagocytic cells can be rationally utilized as delivery carriers. Further, phagocytosis can be significantly enhanced by opsonization, by which particles are coated with proteins and/or antibodies to enable the rapid reorganization of particles by the mononuclear phagocytic system48. For example, immunoglobulin and albumin were coated on superparamagnetic iron oxide nanoparticles (SPIO NPs) to facilitate the monocyte-macrophage uptake49. Compared with pristine SPIO NPs, a larger amount of opsonized-SPIO NPs accumulated in monocytes nuclear periphery49.
Numerous nanomaterials, including inorganic NPs, polymeric NPs, and liposomes, have been successfully loaded into phagocytic cells and delivered to target various diseases (Figure 3A)50-54. One important understanding in the application of phagocytic cell carriers is that the material geometry plays a significant role in regulating cell internalization. Specifically, particle size is crucial in this process, since phagocytosis takes place at the nano/microscale level55-56. IgG-opsonized and non-opsonized polystyrene (PS) microspheres with an average diameter of 2-3 μm exhibited the highest internalization in a rat alveolar macrophages phagocytosis model55-56. Material geometry also affects phagocytosis. Champion et al. studied phagocytosis of alveolar macrophages and cultured the cells with PS particles with various sizes and shapes57. Results suggested that macrophage internalization largely depends on the local particle shape at point of initial contact. The local particle shape is defined as the angel (Ω) between the normal of initial cell/particle contact point and a vector from the initial contact point to the center point of the particle contour. Thus, Ω represents the local curvature. There is a critical point, Ω = 45°, where the internalization cannot be observed. Phagocytosis does not occur any more where is Ω > 45°57. The relationship between local particle shape and phagocytosis leads to so-called shape engineering, which is important in cell-mediated drug delivery. Additionally, macrophage phagocytosis is regulated by the mechanical properties of the substrates, as macrophages show a strong preference to engulf rigid objects58. For example, macrophages internalized about 6 times IgG opsonized polyacrylamide micro-beads with a higher crosslinking rate (stiff beads) than those soft beads with 4 times lower crosslinking rate.58. The effect of surface charge on phagocytosis, however, raises a controversy. Ari et al. coated drugs with poloxamer and/or phospholipid surfactants to form NPs with various surface charges. They found that positively charged NPs accumulate more in macrophages than negatively charged NPs51. But liposomes with negative charges on surfaces were uptaken more by macrophages through the scavenger receptor recognition59-60. These paradoxical observations might be due to various phagocytic pathways adopted in the cellular uptake of different materials.
Figure 3.
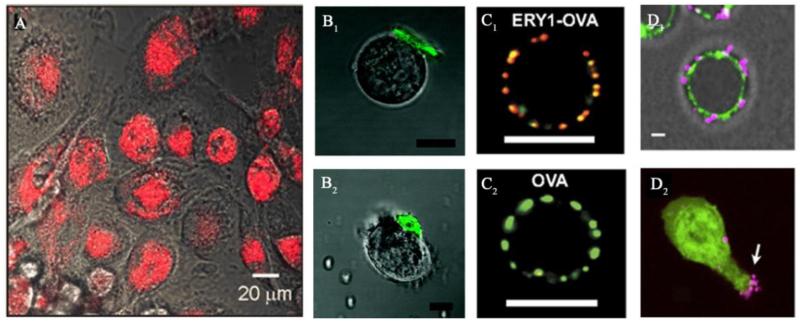
Examples of drugs/NPs internalized into or conjugated onto circulating cells via innate cellular uptake/binding. (A): Endocytosis pathway. Fluorescence microscopy images of phagocytosis of gold silica nanoshells (red) by macrophages54. Reprinted with permission from Ref 54. Copyright © 2012, PLOS ONE. (B): Ligand-receptor interaction. Confocal microscopy images of HA functionalized PEM-based cellular patches (green) attached on the surface of a T cell (B1) and a B cell (B2) via CD44 reorganization. Scale bar, 10 μm62. Reprinted with permission from Ref 62. Copyright © 2008, American Chemical Society. (C): Specific ligand-receptor interaction that exclusively occurs in certain cells. Confocal microscopy images of OVA (red) conjugated ERY1 peptide, binding to a mouse RBC (labeled with anti-mouse glycophorin-A, green) through glycophorin-A, C1; instead, no free-OVA observed on RBC, C2. Scale bar, 5 μm70. Reprinted with permission from Ref 70. Copyright © National Academy of Sciences USA. (D): Covalent conjugation. Confocal microscopy images of D1: Maleimide-functionalized liposome-coated NPs (purple) conjugated on T cells (green) via thiol groups; D2: NPs relocated during migration. Scale bar, 2 μm71. Reprinted with permission from Ref 71. Copyright © 2012 Elsevier Ltd.
Certainly, it is also important to prevent drug release within phagocytic cells before reaching the target disease site, since it may compromise the therapeutic effects and also damage cell carriers. In some cases, it is necessary to inhibit phagocytosis since cells may rapidly degrade the internalized particles. Thus, the design of drug-loaded particles is critical to achieve ideal particle location. Generally, it is considered that surface anchored particles would be ideal. For example, worm-like particles with very high aspect ratios (>20) were designed to partially inhibit macrophage phagocytosis in order to protect the drug during the circulating and targeting processes61.
Therefore, endocytosis is a powerful and tricky pathway to load drugs and/or NPs. However, due to its complexity involving diverse receptors and a series of fusion and fission events, precisely controlling cell internalization is still a significant challenge. New techniques to fine-tune particle properties and endocytosis processes are still needed to improve endocytic cell-mediated nanoparticle delivery.
Ligand-receptor interactions
Ligand-receptor interactions are one of the most basic ways for cells to communicate with each other and the environment. Cellular receptors are generally embedded within plasma membrane surfaces, which contain specific sites for ligand binding and vice versa. Ligand-receptor interactions have been utilized by currently active nanoparticle targeting strategies due to its selectivity and specificity. Thus, this approach also can be used to load particles onto/into circulating cells. Considering that circulating cells are relatively rich in receptors on the surface, a variety of drug carriers have been linked to them via ligand-receptor interactions.
As an example, CD44 markers are involved in cell-adhesion and extensively expressed on a large number of circulating cells, e.g. leukocytes and stem cells. CD44 is also a receptor for hyaluronic acid (HA), which is a glycosaminoglycan that can be found in the extracellular matrix. HA is a promising drug delivery material that is known for its biocompatibility and intrinsic binding capability to CD44. HA coated polyelectrolyte multilayer (PEM) cell patches were developed to attach on the surfaces of B and T lymphocyte by binding to the CD44 on surfaces (Figure 3B)62-63. The cell-PEM-HA complexes retained strong retention that can be controlled by varying the size of the patches and the ratio of cells to patches64. The binding efficiency between PEM-HA and B cells increased at lower pH and higher NaCl concentrations 63. This phenomenon may result from the change of HA conformation that exposed more binding sites to CD44 receptor63. Compared to conventional spherical particles of equal volume, the low curvature particles significantly decreased cell uptake. Interestingly, flat PEM-based disks with ~6 μm average diameters that exhibited low phagocytosis were able to tightly attach on cell surfaces65. This so called cellular backpack is an alternative approach for cell-mediated drug delivery by attaching drug-loaded particles on cell surfaces, maximizing protection for therapeutic payloads and cell integrity. The cellular backpack achieved controlled release of drugs since the drugs were encapsulated in biodegradable poly(lactic-co-glycolic acid)65. These approaches are promising in preventing drug release off-site, avoiding RES elimination, and enhancing the retention time of NPs in blood flow. Most recently, self-assembled thermo-sensitive poly(ethylene glycol) methacrylate-HA nanogels were found rapidly phagocytized by macrophages and accumulated in the liver and spleen 13 minutes after intravenous injection in mice. This study also suggested that HA-CD44 binding was effective for attaching nanocarriers on macrophages66. These macrophage/nanogel complexes have shown potential in drug delivery for macrophage-associated diseases.
The cell-particle encapsulation/conjugation also can be achieved by antibody–antigen interactions. Silica nanorattles, namely hollow silica spheres with moving cores, were bioconjugated with monoclonal antibody to recognize membrane proteins of CD73 and CD90 on mesenchymal stem cells (MSCs)67. After antibody–antigen recognition, antibody-modified nanorattles were internalized within MSCs, suggesting that multiple pathways were involved in the process67. Each MSC can retain up to 1,500 nanorattles for at least 48 hours, which is sufficient for MSCs to migrate to tumor sites67.
Additionally, some receptors only exist in particular cell types, granting opportunities for cells taking up particles in the circulation instead of assembling the cell-drug complex in vitro. For instance, complement receptor type 1 (CR1) is primarily expressed on RBCs. Monoclonal antibody (mAb) against CR1 (Anti-CR1) was conjugated on a tissue-type plasminogen (tPA) activator in order to couple with RBC carriers68. Anti-CR1/tPA can rapidly bind to RBCs to prolong circulation time68. Glycophorin A is another erythrocyte specific surface marker. ERY1 is a 12 amino acid peptide that is able to specifically bind to the RBC surface via glycophorin A69. Antigen ovalbumin conjugated with ERY1 spontaneously recognized RBCs within 30 minutes and remained tightly bind for at least 72 hours after intravenous injection in mice (Figure 3C)70. However, to the best of our knowledge, no drug-loaded particle has been conjugated to circulating cells through these specific interactions.
Thus, ligand-receptor binding is a powerful method to attach particles to circulating cells. Advances in cellular engineering with discoveries of new surface markers and binding ligands are beneficial for ligand-based drug carrier design. Improving the binding efficiency on particular cell types is still remaining as a challenge. It is also desired to accomplish in vivo specific binding within the blood circulation.
Gene modification and delivery
Great efforts have been made to insert therapeutic plasmid DNA, mRNA, and siRNA into living cells to build novel gene or drug delivery systems. Transduction and transfection, both of which are powerful ways to obtain a large number of functional cells in vitro and in vivo, can introduce foreign genes, including therapeutic genes, into host cells72-73.
Cell-mediated viral delivery to tumors has been well studied. Oncolytic virus (OV) is an anti-cancer agent that can selectively induce cell death in cancer cells, while leaving normal cells healthy74. Transduction can effectively introduce OVs into circulating cells based on a virus-mediated DNA transfer mechanism. Furthermore, OVs can be transported with cell carriers to tumors, followed by local replication and amplification to suppress tumor growth. A variety of OVs, such as adenovirus, herpes simplex virus, poxvirus, vesicular stomatitis virus, measles virus, Newcastle disease virus, influenza virus, and reovirus, has been combined with different cell-based delivery systems. For example, mesenchymal progenitor cells (MPCs) have served as vehicles to deliver oncolytic adenoviruses75. To enhance transduction efficiency, oncolytic adenoviruses were first genetically modified with cellular integrin-binding motifs76. Transfused MPCs maintained their intrinsic capacity of sensing tumors and further improved the therapeutic effects in an ovarian carcinoma model75. Additionally, human activated T lymphocytes infected with oncolytic measles viruses were able to transfer viruses to tumor cells via the heterologous cell fusion, and subsequently deliver viruses to myeloma77.
Virus therapy is widely studied, but its clinical safety is still a concern due to rapid proliferation of viruses, off-target cytotoxicity, and body antiviral immunity. Nonviral transfection is an alternative strategy to introduce therapeutic nucleic acids into cellular carriers. Hu et al. developed nonviral vehicles, based on low molecular weight polyethylenimine-co-β-cyclodextrins (β-CD), to transfect tumor necrosis factor (TNF)-related apoptosis-inducing ligands (TRAILs) into circulating MSCs78. MSC-TRAILs efficiently killed tumor cells in a lung metastasis model. In order to achieve non-invasive drug delivery and MRI contrast capabilities, light sensitive, gold-coated SPIO core-shell magnetic NPs (SPIO@AuNPs) were transfected into cell adipose-derived MSCs79. Adipose-derived -MSCs retained high cell viability, osteogenic differentiation, and disease homing ability after transfection. Interestingly, SPIO@AuNPs stimulated cell growth at a certain concentration (<10 μg/mL) and did not induce pro-inflammation, which is typically promoted by other SPIO NPs80.
With the help of genetic modifications, cell carriers are capable of constantly expressing desired therapeutic or imaging molecules. Since transduction/transfection permanently alters cell properties, it inevitably gives rise to safety concerns and even ethical issues. Researchers should take them into account when applying genetic modifications.
Loading therapeutics to cells by physical methods
RBCs possess various reversible transformation capabilities that allow for drug loading simply with external physical treatments such as hypotonic haemolysis and electroporation (Figure 2). These approaches are relatively easier to handle in vitro and eliminates the use of chemical reagents to reduce possible alterations of RBCs. RBCs can recover to their normal morphology and maintain the role of transporting therapeutics and particles to targeted sites.
Hypotonic haemolysis
Hypotonic haemolysis has been widely applied in RBC encapsulation. RBCs behave like osmometers that shrink in hypertonic conditions and swell in hypotonic conditions. Pores open on cell membranes, when the critical haemolytic volume is reached. The increased cell membrane permeability in hypotonic solution enables the entrapment of drugs, biomacromolecules, and NPs in RBCs by a passive mechanism81-82. The principle of reversible swelling is based on the fact that the absence of a superfluous membrane allows cells to accommodate additional volume by changing the shape from biconcave to spherical. Several hypotonic haemolysis techniques have been generated, such as hypotonic dialysis, hypotonic dilution, and hypotonic pre-swelling83-84. Hypotonic dialysis is predominantly applied in encapsulating enzymes, proteins, and contrast agents due to its relative ease of use, ability to preserve cell characteristics and high encapsulation rate. In the process, erythrocytes with a hematocrit value of 70-80% are prepared in a dialysis tube and immersed in a hypotonic buffer for a few hours under gentle stirring. The techniques have been applied in encapsulating drugs, enzymes, and proteins since 1970s85-90. Peptide nucleic acids (PNAs), as therapeutic agents, were loaded into RBCs via hypotonic dialysis and achieved 82% encapsulation after 18 hours incubation88. PNAs-laden RBCs underwent opsonization with ZnCl2 and bis-sulfosuccynimidil-suberate treatment, and then specifically targeted macrophages. PNAs were effectively delivered and resulted in reduced production of nitric oxide and inhibited protein expression of the enzyme nitric oxide synthase88.
Electroporation
Electroporation is a physical method that uses an electrical pulse to create temporary pores on cell surfaces91. Cells are typically suspended in a conductive solution and exposed to a high-intensity external electrical field. The disturbance of the phospholipid bilayer induces temporary dysfunctions of the cell membranes, resulting in the encapsulation of exogenous molecules in cells. During the process, cells keep their morphology when engulfed molecules are larger than the size of electropores. However, cells may swell and the cell membranes may rupture if the substances are smaller than the size of electropores92. Various compounds such as drugs, enzymes, and genes have been encapsulated within RBCs accomplished with sustained release93-95. The drawbacks of electroporation are obvious. The electrical force causes irreversible deterioration and the recovery rate is commonly low95. Thus, it is necessary to optimize the applied voltage to keep cell integrity in electroporation.
Loading/conjugating therapeutics into/onto cells by chemical modifications
Compared to biological and physical methods to load drugs into cells, chemical modification may fix drugs and drug vehicles onto/into circulating cells and avoid the metabolic degradation of materials and drugs within the carrier cells. The surface of circulating cells is a highly heterogeneous structure composed of different proteins, lipids, and carbohydrates with various functional groups for chemical modification opportunities as mentioned previously96-97. Furthermore, chemical modification is not solely dependent on the naturally available cell surface molecules. The complexity of the cell membrane offers alternatives to introduce additional functional groups for a number of conjugating reactions. Compared to pristine cells, modified cells enable convenient conjugations with therapeutics and particles via versatile chemistry tools (Figure 2).
Covalent conjugation onto surface markers
A number of functional groups have been discovered on different cell surfaces that allow bioconjugation with molecules or particles. Among the available functional groups, primary amine and thiol groups are mostly used for covalent reactions due to the ease of labeling and low toxicity of the reactions. N-hydroxysuccinimide (NHS) ester-based crosslinkers are most popular for primary amines modification via carbodiimide reaction. Rossi et al. grafted hyperbranched polyglycerol (HPG) onto the surface of RBCs to generate immunocamouflaged cells98. HPG is a hemocompatible polymer with abundant hydroxyl groups that are ideal for surface modification. HPG was functionalized with succinimidyl succinate (SS) groups by initially reacting with succinic anhydride and then with NHS98. SS-HPG was eventually grafted with primary amine groups presented on the RBC surfaces. Also, the thiol groups in cysteine containing membrane proteins are available for coupling via maleimide-thiol conjugation. Maleimide-functionalized liposome-coated NPs were conjugated on the surface of lymphocytes and MSCs99. The attachment of NPs did not activate lymphocytes or alter stem cell tumor-homing ability99. Stephan et al. also found maleimide-functionalized NPs rapidly relocated to T-cell/tumor cell contact zone following antigen recognition (Figure 3D)71.
Covalent coupling typically exerts stronger binding than ligand-receptor interactions to avoid drug vehicle detachment during cell migration. Versatile conjugation chemistry enables exclusively covalent reactions with amino or thiol groups that are naturally expressed on cells. These covalent bindings help anchoring drug carriers on the surfaces of cells, which have advantages over engulfing inside cells in terms of cellular safety and drug protection.
Biotinylation
Biotin is a water-soluble vitamin that has a high affinity for avidin and streptavidin proteins100. Biotinylated cells can be conjugated with various proteins, enzymes, and NPs without compromising their viability and biological activities. NHS ester-activated biotins are widely-accepted biotinylation reagents that can introduce biotins on living cell membranes that contain primary amino groups101-102. NHS esters form stable amide bonds with primary amino groups in buffers within a few hours at room temperature, which can be tolerated by most cells. NeutrAvidin-coated NPs were anchored on the biotinylated membranes of human mesenchymal stem cells (hMSCs) for up to 2 days, while a certain amount of NPs were observed within the cytoplasm of hMSCs possibly resulting from the membrane recognition of NPs103. hMSCs maintained the tumor-homing capability after nanoparticle attachment103. RBCs were also coupled with a thrombolytics agent on their surface via avidin-biotin bridges, serving as Trojan horses for prophylactic fibrinolysis104-105. With increasing attention to cell surface biotinylation, additional biotinylation reagents have been developed to target different functional groups such as sulfhydryls, carboxyls and carbohydrates. This diversity provides multiple choices in terms of surface modification of circulating cells to bind and load drugs or NPs.
Click chemistry
“Click” chemistry represents a rapid, selective, and high-yielding bio-orthogonal reaction that is also capable of immobilizing materials on cell surfaces106. Numerous click chemistry strategies have been recently developed for living cells, including copper(I)-catalyzed azide-alkyne (CuAAC), strain-promoted azide–alkyne cycloaddition (SPAAC), thiol-ene, Diels–Alder, and Pseudo-Click reactions. Unlike many traditional synthetic routes, click reactions take place in water solution at physiological pH (6-8) and temperature (37°C) without toxic by-products, which are ideal platforms for loading drug vehicles onto circulating cells107-108. To improve the binding efficiency, abiotic functional groups are initially introduced onto cells allowing counter groups on drugs and particles to undergo click chemistry. To date, CuAAC, SPAAC, and thiol-ene reactions have been applied to click living cells109
CuAAC and SPAAC are [3+2] azide-alkyne cycloaddition in the context of Staudinger Ligation110. Azide groups, which are naturally absent on mammalian cell surfaces, need to be introduced to enable click reactions between cells and therapeutics via biosynthesis. Cell glycosylation, which is also called carbohydrate reaction, provides accessibility to incorporate azido sugars onto membranes through sialic acid. Generally, azido sugars can be metabolically inserted into the cell membranes after 24-48 hours of incubation. The azide-functionalized cells subsequently undergo reaction with drugs or NPs containing activated alkyne groups. The reaction, however, is thermodynamically favorable at high temperature or pressure, which is not applicable in living systems. Thus, CuAAC utilizes Cu+ catalyst to accelerate the reaction within 30 minutes at physiological conditions111. Unfortunately, the cytotoxic Cu+ catalyst limits the in vitro and in vivo applications. Importantly, studies have indicated that alkyne activation can be achieved by introducing ring strain, namely SPAAC, which is a Cu-free click chemistry for selective biomolecule labeling in living organisms based on different glycosylation degrees between abnormal and normal cells107, 112. Xu et al. immobilized highly branched polyamidoamine dendrimers on azido-modified macrophage surfaces by SPAAC copper-free click chemistry113. Cell motility and its common stress-activated signaling pathways, including AKT, p65, and p38, have no significant alteration during the cell-nanoparticle hybridization process. An alternative approach to employ click chemistry is the thiol-ene reaction. Briefly, methacryloyl groups can be incorporated onto cell surfaces through a glycosylation pathway and then clicked with a thiol-terminal polymer. The thiol-ene reaction was explored on clicking cancer cells114. Despite few developments on clicking circulating cells, click chemistry has shown potential in delivering therapeutics and imaging agents. A shortcoming of linking cell and therapeutics by click chemistry is that relatively complicated surface chemistry is required.
TARGETING STRATEGIES OF CELL-MEDIATED DRUG DELIVERY
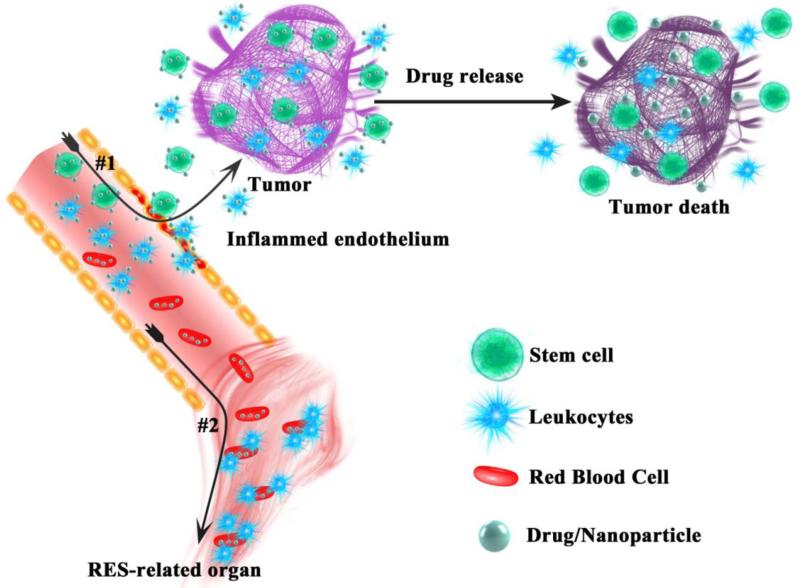
Once drug vehicles are assembled with circulating cells either in vitro or in vivo, the complexes can travel and diffuse within the blood circulation and further navigate to the desired targets (Table 2). By taking advantages of carrier cells’ intrinsic capabilities, controlled drug/particle delivery to specific disease sites can be achieved (Figure 4.). In this section, we will review the existing circulating cell-based targeting strategies for various objectives.
Table 2.
Summary of current drug vehicle encapsulation/conjugation methods for different cell-mediated drug delivery and their targeted applications.
| Pathway | Mechanism | Cell Type | Drug/ Particles | Target | Ref . |
|
|---|---|---|---|---|---|---|
| Unmodified Cells |
Endocytosis | Endocytosis | Monocyte, macrophage |
(IgG/ albumin coated) SPIO NPs, |
Lymphoid tissues | 49 |
| Monocyte, macrophage |
Au nanoshells | BBB and hypoxia |
53, 54 |
|||
| Macrophage | Liposomal doxorubicin |
Lung cancer | 50 | |||
| Macrophage | Catalyse- polyethyleneimi ne- poly(ethylene glycol) |
Parkinson’s disease |
52 | |||
| Macrophage | Indinavir NPs | HIV-1 | 115 | |||
|
|
||||||
| Anti- endocytosis |
Macrophage | Polyelectrolyte multilayer- based disks |
Macrophage associated diseases |
65 | ||
|
| ||||||
| Ligand- receptor binding |
Hyaluronic acid (HA)- CD44 |
B and T lymphocyte |
Polyelectrolyte multilayer |
ICAM-coated surface |
62- 64 |
|
| Macrophage | poly(ethylene glycol) methacrylate- HA nanogel |
Liver and spleen macrophage- associated diseases |
66 | |||
|
| ||||||
| Antibody- CD73 and CD90 |
MSC | Doxorubicin- silica nanorattles- antibody |
Glioma tumor | 67 | ||
|
| ||||||
| Antibody- complement receptor type 1 |
RBC | Tissue-type plasminogen activator- antibody |
Thromboprophyla xis |
68 | ||
| Peptide (ERY1)- glycophorin A |
RBC | Antigen ovalbumin- ERY1 |
T cells | 70 | ||
|
| ||||||
| Covalent conjugation |
NHS-NH3 | RBC | Hyperbranched polyglycerols |
RES-related organs |
98 | |
|
|
||||||
| Maleimide- thiol |
T cell, MSC | Liposome- coated NPs |
Prostate cancer, lymphoma |
71, 99 |
||
|
| ||||||
| Physical Modificatio n |
Hypotonic haemolysis |
Membrane flexibility |
RBC | Peptide nucleic acids |
Macrophages | 88 |
|
| ||||||
| Electroporatio n |
Temporary dysfunction s of membrane semi- permeability |
RBC | Alcohol dehydrogenase and aldehyde dehydrogenase |
RES-related organs |
116 | |
|
| ||||||
| Chemical Modificatio n |
Biotinylation | Avidin- biotin bridges |
hMSC | NeutrAvidin- coated NPs |
Liver tumor | 103 |
| RBC | Thrombolytics agent |
Thrombosis |
104, 105 |
|||
|
| ||||||
| Click Chemistry |
Strain- promoted azide– alkyne cycloadditio n |
Macrophage | Polyamidoamin e dendrimers |
98 | ||
|
| ||||||
| Transduction | Genetic transfer via viral vectors |
T cell, MSC | Oncolytic virus | Myeloma ovarian, carcinoma |
76, 77 |
|
|
| ||||||
| Transfection | Genetic transfer via non-viral vectors |
MSC | TRAILs | Lung cancer metastases |
78 | |
| MSC | SPIO@AuNPs | Head and neck cancer |
79 | |||
Figure 4.
Illustration of circulating cell-mediated targeting and drug delivery pathways. #1: living targeting to disease sites via leukocytes and stem cells. #2: RES targeting via RBCs.
Passive targeting: RES/non-RES targeting
The first rational living cell-based targeted drug delivery strategy is passive targeting, including RES and non-RES effects. For example, as RBCs age, they are recognized by the RES and subsequently undergo cell membrane lysis by lysosomal enzymes, hemoglobin degradation, and eventually lose their flexibility, integrity, and functionality117-118. Taking advantage of the metabolic pathways in physiologic conditions, RBCs-based drug/particle complexes can easily and efficiently target RES-related regions such as liver, spleen and bone marrow. RES clearance can be accelerated by altering the properties of RBCs during drug-loading processes in order to enhance the targeting efficiency. This strategy has been demonstrated in drug delivery for over 30 years117, 119. Its applications range from RES organ targeting to targeting specific cell types, including hepatic Kupffer cells, alveolar macrophages, peritoneal macrophages and peripheral blood monocytes, for delivering drugs to treat diverse diseases such as infections, inflammations, leukemia and cancers120-125.
However, rapid clearance by the RES significantly limits the employment of RBC carriers in non-RES targeting. To address this problem, several approaches have been developed to permit the survival of heterologous or even xenogeneic RBCs in the circulation. A straightforward strategy to modify RBCs is by conjugating with nonimmunogenic materials onto the surfaces of RBCs in order to generate immunocamouflaged cells. Polyethylene glycol (PEG) is a bioinert and hydrophilic polymer that is widely used for cell stealth purposes. Using the same technique, methoxy(polyethylene glycol) (mPEG) was attached on the surface of RBCs and the immunogenity of modified RBCs was adjusted by varying the surface density and chain size of the coating polymer126. The mPEG-modified RBCs still retained normal morphology, osmotic fragility, oxygen transportation, and high cell viability, but became resistant to RES clearance with prolonged circulation time127-128. An alternative polymer, HPG, has similar antigen “mask” effects98. HPG is a hyperbranched polymer with the capability of further modification to achieve controlled release. Furthermore, stimuli-responsive RBCs, such as ultrasound-responsive RBCs129 and magnetic-responsive RBCs130, can be directed to disease sites, providing a platform for non-RES targeting. However, cell-based passive targeting strategies are still similar to the passive targeting of bare NPs, which typically cannot reach the desired targeting and drug delivery efficiency. Additionally, RBCs are mostly used in passive targeting pathways, which are less favorable for many diseases.
Live cell-mediated targeting strategies
Many circulating cells such as neutrophils, monocytes/macrophages, and stem cells present targeting capabilities to many diseases, including cancer, wounds, and ischemic tissues. For example, tumor-homing, one of the most studied targeting mechanisms of living cells, suggests that various cells can not only sense solid tumors, but can also track circulating cancer cells in the blood flow and reach the primary and metastatic tumor sites. Targeting solid tumors, tumor microenvironments, and cancer metastases will be reviewed, respectively here.
Leukocytes are the cells involved in immune response so they are essential for inflammation, disease development, regulation, and healing processes. The general paradigm of neutrophil and monocyte extravasation includes tethering, rolling, adhesion, crawling, and eventually transmigration131-132. For many diseases, an injured endothelium is activated directly by pathogens or indirectly by pro-inflammation133-134. Neutrophils are well recognized as the first cells to be recruited by chemoattractants (e.g. platelet-activating factor, interleukin 8, interleukin 17, formyl-methionyl-leucyl-phenylalanine, and complement component 5a), cytokines (e.g. TNF-α), growth factors (e.g. granulocyte-macrophage colony-stimulating factor), and bacterial products (e.g. formylated peptides and lipopolysaccharide)135-136. With the increasing expression of adhesion molecules, P-selectin and E-selectin in particular, neutrophils tether and slowly roll onto the surface of the endothelium137-139. Mediated by chemokines and adhesion molecules, neutrophils firmly attach on the endothelium and subsequently crawl towards endothelial adjunctions140-143. Finally, neutrophils cross the endothelium, basement membrane, pericytes, and reach disease sites144. Monocytes are also recruited by similar chemokines, cytokines and growth factors132, 145. Afterwards, monocytes differentiate into highly specialized macrophages to participate in the following immune responses146. Similarly, lymphocytes can infiltrate tumor sites and display a series of immune responses147-148. Therefore, the tumor-homing mechanisms of leukocytes provide active and “living” approaches for cell-mediated drug delivery. Doxorubicin (Dox) encapsulated liposome-carrying macrophages accumulated in lung tumors and released Dox after 24 hours of administration, but a smaller amount of Dox was observed without using macrophage carries (Figure 5A)50. Adhesion molecule-associated extravasation also provides targeting options. PEM functionalized T cells can migrate to ICAM-coated surface62. A recent study has demonstrated that RGD modified single-walled carbon nanotubes can target monocytes and enhance the delivery efficiency of monocyte carriers via integrin mediation (Figure 5B)149.
Figure 5.
Cell-mediated targeting approaches to different disease sites via leukocytes and stem cells that retain their intrinsic capacities of crossing biological barriers and homing to tumor after nanoparticle assembly. (A): Bioluminescence images of macrophages carrying liposome-doxorubicin, which accumulated at lung cancer metastasis sites50. Reprinted with permission from Ref 50. Copyright © 2012, Elsevier Ltd. (B): Single-walled carbon nanotube-laden monocytes (grey) bypassed blood vessels (red) and infiltrated tumor (green) interstitium. Scale bar, 50 μm149. Reprinted with permission from Ref 149. Copyright © 2014, Nature Publishing Group. (C): Ex vivo images of monocytes/macrophages that loaded with fluorescence labeled microspheres located within brain metastatic tumor (green) at 24 h post-injection in a mouse model54. Reprinted with permission from Ref 54. Copyright © 2012, Springer. (D): Fluorescence images of hMSCs (green) anchored with NeutrAvidin-coated NPs (red) sensed and responded to tumor spheroid (overlaid phase-contrast image) in vitro, indicated by cell polarization103. Reprinted with permission from Ref 103. Copyright © 2010, American Chemical Society.
A particularly important application of leukocyte-mediated drug/nanoparticle delivery is the brain drug delivery across the BBB. The BBB, which is a tight assembly of endothelium cells and astrocytes, separates the brain from the circulatory system118. The barrier is therefore highly selective and only allows the passage of essential nutrition such as water, soluble lipid molecules, amino acids, and glucose while blocking other substances. The BBB protects the brain from harmful substances; however, it also establishes a huge obstacle for delivering therapeutic agents to the brain. Interestingly, monocytes/macrophages are able to transmigrate across the BBB. Therefore, they can potentially serve as carriers for sheathed brain drug delivery. Bone-marrow-derived macrophages were found to be able to infiltrate into the brain and deliver nanozyme in a Parkinson’s disease model150. MRI contrast agent-labeled murine monocytes/macrophages also targeted and accumulated in rat brain tumors151. A similar concept was applied in FluoSpheres® NeutrAvidin® labeled microspheres delivered to brain metastasis of breast cancer via monocytes/macrophages (Figure 5C)54.
The tumor microenvironment, which involves a vast amount of cells, vessels, and stroma, is another potential target for circulating cell-mediated therapeutic delivery. Solid tumors generally contain hypoxic regions with low oxygen levels due to poor angiogenesis. Hypoxia is associated with necrosis and facilitates tumor mutation152. Due to the lack of blood vessels, hypoxic cells are resistant to traditional drug delivery approaches. Promisingly, monocytes, tumor-associated macrophages, and tumor infiltrating lymphocytes are frequently found within tumor microenvironments including hypoxic and necrotic regions, suggesting that leucocytes could access in the depth of tumors. Choi et al. utilized monocytes/macrophages as “Trojan Horses” to deliver gold nanoshells to the hypoxic regions of cancer and their results suggested that malignant cells were completely killed within these areas, where free nanoshells could not access53.
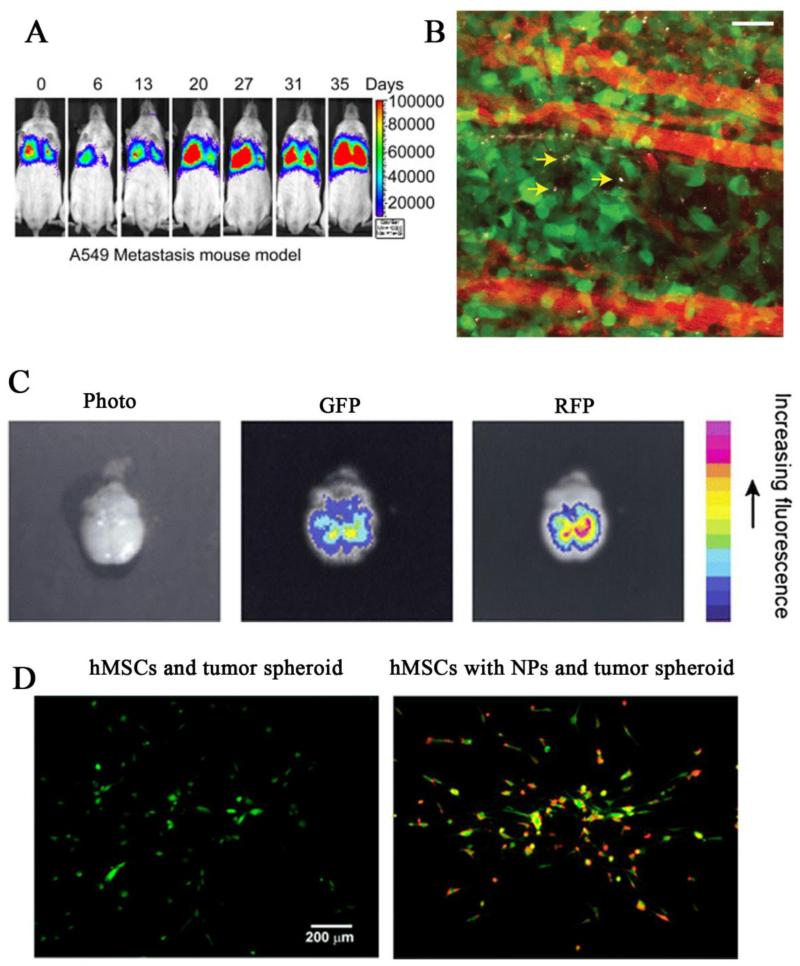
Stem cells can also naturally migrate towards injured tissues as part of the healing process. In addition, stem cells display tumor-homing and BBB-infiltrating properties 153. Emerging evidences suggest that various types of stem cells have exclusive tropism for tumors and can thus mediate drug delivery. Previous attempts have emphasized on producing a large number of therapeutic agents in situ via genetically-modified stem cells. For example, interferon-β gene transduced/transfected hMSCs preferentially accumulated in gliomas, melanomas, and pulmonary metastases, then locally released interferon-β, and induced tumor regression154-156. In another study, neural stem cells migrated toward intracranial gliomas and delivered the oncolytic drug, 5-fluorouracil, to reduce about 80% of the tumor mass within 2 weeks38. Although stem cell-mediated gene therapy showed great outcomes, recent studies have put more attention on developing drug/biomaterial-laden cell carriers to reduce the potential risk of gene modification. NeutrAvidin nanoparticle-coated hMSCs were found be able to sense liver tumor spheroids in vitro (Figure 5D)103. MSCs surface-modified with Dox-loaded silica nanorattles also successfully tracked glioma cells and released Dox to kill cancer cells67. Recently, multi-functional theranostic NPs, such as SPIO-loaded gold NPs and photosensitizer silica NPs, were successfully delivered to different tumors via MSC-mediated approaches157-158.
Cancer cells are particularly lethal when they reach the metastatic state. Unfortunately, delivering therapeutics to metastatic circulating tumor cells (CTCs) has been a significant challenge for decades due to their scarcity in the blood circulation. CTCs are capable of leaving a primary tumor, seeding metastases in distant sites. Effective therapies that directly remove CTCs from the blood circulation have not been found yet since CTCs circulate in the bloodstream with a low concentration (average 8 cells/ml) and are difficult to detect159. Interestingly, studies have indicated that tumor cells exhibit interactions with leukocytes and endothelial cells via adhesion molecules, which provide a platform for targeting CTCs through leukocytes. Inspired by selectin-dependent rolling along the vessel, human E-selectin, and cancer-specific TRAIL functionalized leukocytes were used to target CTCs, which displayed sialylated carbohydrate ligands for selectin under blood flow resulting in effective tumor killing outcome160. Studies have also found that tumor cells express adhesion molecules such as intercellular adhesion molecule 1 (ICAM-1, also known as CD54) that can bind to lymphocyte function-associated antigen (LFA-1, also known as CD11a) and macrophage-1 antigen (MAC-1, also known as CD11b) in the circulation11, 161.
Therefore, circulating cells have shown promise as a powerful drug delivery system with an innate ability to detect diseases, cross biological barriers, and release drugs to disease sites. Compared to conventional drug delivery and nanomedicine, utilizing the natural ability of different cells is a completely different targeting mechanism, in which living cells act as guiders and drivers for delivering therapeutics and NPs. This strategy has also opened up new avenues for treatments of diseases that cannot be easily reached by traditional DDS.
Engineered targeting/binding
To maximize the targeting efficiency, engineered unnatural targeting/binding strategies have been developed for living cell-mediated DDS, allowing flexibility and diversity in binding pathways. Antibody decoration is one of the most popular techniques, by which cell carriers spontaneously search for specific antigens and even trigger the clearance of certain cells. Lipophilic anti-CD45 and anti-CD20 (Rituximab) surface painted RBCs effectively depleted CD45 positive leukocytes and CD19 +/CD20 +/CD45 + human lymphoma cells162.
An alternative approach is to use an external magnetic field to enhance the accumulation of cell carriers in targets. Magnetic nanomaterials can enable remote control of the location of drug vehicles including circulating cells by an external magnetic field. SPION NPs have been widely explored due to their biocompatibility, ease of handling, and good MRI contrast. SPIO NPs loaded RBCs successfully targeted to tumor cells under magnetic stimulation, further reducing the RES aggregation163. The combination of molecular binding and cell-mediated drug delivery is a new and emerging field. The techniques in cellular engineering and biomaterial modifications offer significant opportunities for targeted drug and nanoparticle delivery.
CONTROLLED DRUG RELEASE
The controlled release of therapeutics is desired in medicine164. It is even more critical for circulating cell-mediated drug delivery, since it requires not only maximum treatment effects, but also limited drug release in carrier cells. Generally, the encapsulation or conjugation of free drugs in cell carriers is not ideal due to the cytotoxicity of drugs and drug-cell interactions. Drugs are thus protected in vehicles made with various materials forming new complexes to ultimately control the payload release. In this section, strategies for controlled drug release from circulating cells will be reviewed.
Degradation controlled drug release
Biodegradable polymers are the most widely used biomaterials for controlled drug release, since the release can be controlled by degradation mechanisms. Hydrolytic and enzymatic degradations are typical approaches to control drug release rates after being uptaken by cells. Abundant enzymes are located in the cytoplasm of phagocytic cells and able to facilitate the breakdown of engulfed substances. The degradation can be fine-tuned to obtain minimal release in circulating cells and release at desired disease sites. Liposomes are popular drug vehicles that have been used in many commercialized pharmaceutical formulations. Through elegant engineering designs, the desired release of drugs from liposomes can also be achieved after being delivered by living cells. For example, when liposome-Dox-loaded macrophages arrived at the tumor sites, drugs were released via macrophage death cause by leaky Dox from liposome degradation50. Biodegradable polymeric particles, for instance, polylactic acid (PLA) NPs, have manageable degradation profiles that are ideal for controlled drug release165. The slow degradation may ensure safety to circulating carrier cells and programmed release upon reaching disease sites. However, degradation controlled release may not achieve the ultimate safety and therapeutic effects due to the complicity of the in vivo environment and the fact that drug diffusion is also somewhat independent from material degradation. Thus, stimuli-triggered release from a smart drug delivery system can be advantageous.
Stimulated drug release by cells and local microenvironment
Environmental responsive/smart drug release has gained much attention in recent decades in the field of drug delivery, as payloads can be discharged by responding the local cellular/physiological changes, which may have resulted from disease development or inflammation166-167. It is also promising to combine the cell-mediated “live” targeting mechanism with a “smart” releasing mechanism. For instance, glutathione can reduce disulfide bonds and is available for responsive drug release within cytoplasm. An acute lymphoblastic leukemia drug, L-asparaginase, was covalently conjugated with cell-penetrating peptides (CPP) via disulfide linkages, followed by the encapsulation by RBCs without any membrane alteration168. The disulfide linkage disassociation occurred within the cytoplasm of RBCs, which contains glutathione, and resulted in the release of L-asparaginase release. RBCs spontaneously triggered drug release in the absence of external interventions, but underwent apoptosis to release the drug out of the cells168.
Another pathway to facilitate drug release from cell carriers is discharging the drug-loaded nanoparticle as a whole. Generally, cells retain their equilibrium via constantly engulfing foreign substances and liberating engulfed particles. In contrast to endocytosis, exocytosis allows secreting drug vehicles out of cells, as a potential approach for nanoparticle delivery. In a recent study, bone-marrow-derived macrophages internalized catalase/polyethyleneimine-PEG nanozymes within 40–60 min and sustained released the cargos for 4-5 days150. And the release rate can be enhanced by phorbol myristate acetate treatment52. While the integrity of cell carriers can be maintained after the drug is released by taking advantages of exocytosis, the hemostasis may occur and the chances of off-site targeting may rise.
External stimulation triggered drug release
External stimulation is an attractive alternative to trigger the localized release of therapeutic agents after reaching the disease sites, if the local microenvironmental change is not available or not strong enough. Particularly, stimuli-responsive polymers have unique characteristics that respond to small changes in the microenvironment and are therefore being widely used in smart drug delivery166, 169. Drugs are securely protected by polymeric vehicles until external stimulation, which ensures rapid, transient and precise drug release is applied. Again, combining external stimuli-responsive release with living cell-mediated targeting is a potential area to be explored.
Until now, numerous smart drug vehicles have been developed that respond to external stimuli, such as temperature, light, pressure, light, or ultrasound. In some cases, drugs can be released by multiple stimulations to ensure an on-demand administration. For example, Swiston et al. functionalized lymphocytes with PEM coatings by combining both pH-sensitive and temperature-sensitive polymers. And these lymphocytes were able to regulate drug release by dual factors62. Overall, stimuli-responsive drug release can be crucial for a cell based DDS, even more significant than for the conventional DDS.
CONCLUSIONS AND FUTURE PROSPECTS
Circulating cell-mediated drug delivery has emerged as a new concept that focuses on the targeted delivery of therapeutics by living cells instead of passive factors or surface markers. A summary of comparison between conventional DDS and cell-mediated DDS is shown in Table 3. As we have discussed, various cell types in the circulation, including RBCs, leukocytes, and stem cells, are available for carrying drugs and particles to targeted diseases. Generally, three steps are considered in terms of design strategies: drugs/particles loading by cell carriers, cell carriers targeted to desired locations, and controlled drug release/treatment. Compared to traditional targeted drug delivery and nanomedicine strategies, additional factors must be considered for cell-mediated drug delivery. The drugs/particles must not be toxic to the cell carrier and should prevent cell alteration after drug loading. Thus, more attention has been paid to anchoring therapeutics on the surface of living cells. Certainly, high drug/particle loading or binding efficiency is favorable. We have elaborated on the feasibility and variety of cell targeting pathways to target different diseases. Rational selection of appropriate cells is necessary to achieve high targeting efficiency for new cell-mediated drug delivery system. Finally, it is imperative to employ a controlled drug releasing mechanism to ensure safety and maximize therapeutic effects. New treatment methods, such as photothermal and magnetic hyperthermia therapies, can also benefit from living cell-based delivery systems. The development of suitable biomaterials and biomedical engineering techniques is particularly important in creating new and effective cell-mediated drug delivery systems.
Table 3.
A comparison of conventional DDS and cell-mediated DDS.
| Conventional DDS | Cell-mediated DDS | |
|---|---|---|
| Drug Encapsulation | Physical or chemical methods | Biological, physical or chemical methods |
| Circulating Time | Short, easy to be eliminated via RES system; Stealth coating generally used |
Longer, cells are capable of circulating in blood |
| Targeting | Decorated with targeting ligands and molecules; Mostly depend on EPR effects; Difficult in transmembrane penetration; Access to vascular areas only |
Natural homing ability; Possess cell signaling; Penetrate through biological barriers; Reach to non-vascular areas |
| Drug Release | Controlled drug release via diffusion, degradation or external stimulation |
Controlled drug release via exocytosis, degradation or external stimulation |
| Safety | May have off-site drug release | |
| Biocompatibility | May cause unwanted immune response; |
Natural biocompatibility; May cause unwanted immune response after modification. |
Although novel cell-mediated drug/nanomedicine delivery holds promises in improving diagnosis and therapeutic effects, it is debatable whether this strategy is safe enough for clinical practices. Many challenges still remain for this state-of-art approach. Generally, candidate cells are isolated and functionalized in vitro, followed by re-injection into the circulation or disease sites. Although current techniques allow effective isolation and expansion of certain cells in vitro, cell-carriers are susceptible to contamination and other risks. Alternatively, drugs and drug-engulfed vehicles can be directly injected into the circulation to bind with cell carriers in vivo. However, the drug loading efficiency may be compromised due to immune surveillance and drug vehicles’ limited binding ability. Potential therapeutics leaking from the carrying cells is another concern. Additionally, a wide spectrum of cells playing multiple roles in disease development and the healing process may participate in the drug delivery procedure. Introducing cell carriers may interrupt the balance of natural physiological conditions. For example, leukocytes are attracted to inflammation sites to deliver medicine in the short term, but they can also cause chronic inflammation issues later. Since the mechanism and development of many diseases are not well understood, foreign cells with drug complexes may also exacerbate pathological conditions. In the meantime, more evidence is needed to verify the applicability of cell-mediated DDS in various diseases, including, but not limited to, cancers, wound healing, brain diseases, and cardiovascular diseases.
Regardless of these challenges, cell-mediated drug delivery holds great potential to revolutionize current diagnostic and medical techniques. Advances in biomaterials may open up the door to a new paradigm of DDS designs. Engineering tools for manipulating living cells and fabricating drug vehicles, such as NPs, will create efficient and intelligent targeting, releasing, and imaging strategies. Combining living circulating cells and nanotechnology will be the new direction for controlled drug delivery, while offering a powerful technique to improve overall diagnostic and therapeutic outcomes.
ACKNOWLEDGMENT
The authors are grateful for the funding support from a National Institutes of Health (NIH) Awards NIBIB EB012575, NCI CA182670, and NHLBI HL118498, and National Science Foundation (NSF) Awards DMR1313553, CMMI 1266116 and CBET-BME 1330663.
ABBREVIATIONS
- DDS
drug delivery systems
- NPs
nanoparticles
- EPR
enhanced permeability and retention
- RES
reticuloendothelial system
- BBB
blood brain barrier
- RBC
red blood cell
- WBC
white blood cell
- PMN
polymorphonuclear granulocyte
- APC
antigen-presenting cell
- SPIO
superparamagnetic iron oxide
- PS
polystyrene
- HA
hyaluronic acid
- PEM
polyelectrolyte multilayer
- MSC
mesenchymal stem cells
- hMSC
human mesenchymal stem cells
- CR1
complement receptor type 1
- tPA
tissue-type plasminogen
- mAb
monoclonal antibody
- NHS
N-hydroxysuccinimide
- HPG
hyperbranched polyglycerol
- SS
succinimidyl succinate
- PNAs
peptide nucleic acids
- CuAAC
copper(I)-catalyzed azide-alkyne
- SPAAC
strain-promoted azide–alkyne cycloaddition
- OV
oncolytic virus
- MPC
mesenchymal progenitor cells
- TNF
transfect tumor necrosis factor
- TRAIL
TNF-related apoptosis-inducing ligands
- PEG
polyethylene glycol
- Dox
doxorubicin
- CTCs
circulating tumor cells
- CPP
cell-penetrating peptide
- NRI
near-infrared
- mTHPP
5,10,15,20-tetrakis(meso-hydroxyphenyl)porphyrin
- PSS
poly(styrene sulfonate)
Footnotes
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
REFERENCES
- 1.Allen TM, Cullis PR. Liposomal drug delivery systems: from concept to clinical applications. Adv. Drug Del. Rev. 2013;65:36–48. doi: 10.1016/j.addr.2012.09.037. [DOI] [PubMed] [Google Scholar]
- 2.Parveen S, Misra R, Sahoo SK. Nanoparticles: a boon to drug delivery, therapeutics, diagnostics and imaging. Nanomed. Nanotechnol. Biol. Med. 2012;8:147–166. doi: 10.1016/j.nano.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 3.Kedar U, Phutane P, Shidhaye S, Kadam V. Advances in polymeric micelles for drug delivery and tumor targeting. Nanomed. Nanotechnol. Biol. Med. 2010;6:714–729. doi: 10.1016/j.nano.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 4.Ashley GW, Henise J, Reid R, Santi DV. Hydrogel drug delivery system with predictable and tunable drug release and degradation rates. Proceedings of the National Academy of Sciences. 2013;110:2318–2323. doi: 10.1073/pnas.1215498110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamidi M, Azadi A, Rafiei P. Hydrogel nanoparticles in drug delivery. Adv. Drug Del. Rev. 2008;60:1638–1649. doi: 10.1016/j.addr.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 6.Cui W, Zhou Y, Chang J. Electrospun nanofibrous materials for tissue engineering and drug delivery. Science and Technology of Advanced Materials. 2010;11:014108. doi: 10.1088/1468-6996/11/1/014108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoo HS, Kim TG, Park TG. Surface-functionalized electrospun nanofibers for tissue engineering and drug delivery. Adv. Drug Del. Rev. 2009;61:1033–1042. doi: 10.1016/j.addr.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 8.Prausnitz MR, Langer R. Transdermal drug delivery. Nat. Biotechnol. 2008;26:1261–1268. doi: 10.1038/nbt.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keefe AD, Pai S, Ellington A. Aptamers as therapeutics. Nature Reviews Drug Discovery. 2010;9:537–550. doi: 10.1038/nrd3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Torchilin VP. Multifunctional nanocarriers. Adv. Drug Del. Rev. 2012;64:302–315. doi: 10.1016/j.addr.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 11.Brannon-Peppas L, Blanchette JO. Nanoparticle and targeted systems for cancer therapy. Adv. Drug Del. Rev. 2012;64(Supplement):206–212. doi: 10.1016/j.addr.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 12.Muro S. Challenges in design and characterization of ligand-targeted drug delivery systems. J. Control. Release. 2012;164:125–137. doi: 10.1016/j.jconrel.2012.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Snyder EL, Dowdy SF. Cell penetrating peptides in drug delivery. Pharm. Res. 2004;21:389–393. doi: 10.1023/B:PHAM.0000019289.61978.f5. [DOI] [PubMed] [Google Scholar]
- 14.Torchilin VP, Trubetskoy VS. Which polymers can make nanoparticulate drug carriers long-circulating? Adv. Drug Del. Rev. 1995;16:141–155. [Google Scholar]
- 15.Greenwald RB, Choe YH, McGuire J, Conover CD. Effective drug delivery by PEGylated drug conjugates. Adv. Drug Del. Rev. 2003;55:217–250. doi: 10.1016/s0169-409x(02)00180-1. [DOI] [PubMed] [Google Scholar]
- 16.Alexis F, Pridgen E, Molnar LK, Farokhzad OC. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol. Pharm. 2008;5:505–515. doi: 10.1021/mp800051m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fang J, Nakamura H, Maeda H. The EPR effect: unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv. Drug Del. Rev. 2011;63:136–151. doi: 10.1016/j.addr.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 18.Tatum JL. Hypoxia: importance in tumor biology, noninvasive measurement by imaging, and value of its measurement in the management of cancer therapy. Int. J. Radiat. Biol. 2006;82:699–757. doi: 10.1080/09553000601002324. [DOI] [PubMed] [Google Scholar]
- 19.Qin J, Yang X, Zhang RX, Luo YX, Li JL, Hou J, Zhang C, Li YJ, Shi J, Lu L, Wang JX, Zhu WL. Monocyte mediated brain targeting delivery of macromolecular drug for the therapy of depression. Nanomedicine. 2014 doi: 10.1016/j.nano.2014.09.012. [DOI] [PubMed] [Google Scholar]
- 20.Berezina TL, Zaets SB, Morgan C, Spillert CR, Kamiyama M, Spolarics Z, Deitch EA, Machiedo GW. Influence of storage on red blood cell rheological properties. J. Surg. Res. 2002;102:6–12. doi: 10.1006/jsre.2001.6306. [DOI] [PubMed] [Google Scholar]
- 21.Muzykantov VR. Drug delivery by red blood cells: vascular carriers designed by mother nature. Expert opinion on drug delivery. 2010;7:403–427. doi: 10.1517/17425241003610633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hollowell JG, van Assendelft OW, Gunter EW, Lewis BG, Najjar M, Pfeiffer C. Vital Health Stat. Vol. 11. United States Bureau of the Census; 2005. Hematological and Iron-Related Analytes-Reference Data for Persons Aged 1 Year and Over: United States, 1988-94; pp. 1–156. [PubMed] [Google Scholar]
- 23.Amulic B, Cazalet C, Hayes GL, Metzler KD, Zychlinsky A. Neutrophil function: from mechanisms to disease. Annu. Rev. Immunol. 2012;30:459–489. doi: 10.1146/annurev-immunol-020711-074942. [DOI] [PubMed] [Google Scholar]
- 24.Smith JA. Neutrophils, host defense, and inflammation: a double-edged sword. J. Leukoc. Biol. 1994;56:672–686. doi: 10.1002/jlb.56.6.672. [DOI] [PubMed] [Google Scholar]
- 25.Pillay J, den Braber I, Vrisekoop N, Kwast LM, de Boer RJ, Borghans JAM, Tesselaar K, Koenderman L. In vivo labeling with 2H2O reveals a human neutrophil lifespan of 5.4 days. Blood. 2010;116:625–627. doi: 10.1182/blood-2010-01-259028. [DOI] [PubMed] [Google Scholar]
- 26.Adams DH, Rlloyd A. Chemokines: leucocyte recruitment and activation cytokines. The Lancet. 1997;349:490–495. doi: 10.1016/s0140-6736(96)07524-1. [DOI] [PubMed] [Google Scholar]
- 27.McGuinness C, Humphries J, Waltham M, Burnand K, Collins M, Smith A. Recruitment of labelled monocytes by experimental venous thrombi. THROMBOSIS AND HAEMOSTASIS-STUTTGART. 2001;85:1018–1024. [PubMed] [Google Scholar]
- 28.Bingle L, Brown NJ, Lewis CE. The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. J. Pathol. 2002;196:254–265. doi: 10.1002/path.1027. [DOI] [PubMed] [Google Scholar]
- 29.Sica A, Allavena P, Mantovani A. Cancer related inflammation: the macrophage connection. Cancer Lett. 2008;267:204–215. doi: 10.1016/j.canlet.2008.03.028. [DOI] [PubMed] [Google Scholar]
- 30.Kunkel EJ, Butcher EC. Chemokines and the tissue-specific migration of lymphocytes. Immunity. 2002;16:1–4. doi: 10.1016/s1074-7613(01)00261-8. [DOI] [PubMed] [Google Scholar]
- 31.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 32.Mauri C, Bosma A. Immune regulatory function of B cells. Annu. Rev. Immunol. 2012;30:221–241. doi: 10.1146/annurev-immunol-020711-074934. [DOI] [PubMed] [Google Scholar]
- 33.Wagers AJ, Weissman IL. Plasticity of adult stem cells. Cell. 2004;116:639–648. doi: 10.1016/s0092-8674(04)00208-9. [DOI] [PubMed] [Google Scholar]
- 34.Bianco P, Robey PG. Stem cells in tissue engineering. Nature. 2001;414:118–121. doi: 10.1038/35102181. [DOI] [PubMed] [Google Scholar]
- 35.Kim SU, de Vellis J. Stem Cell-Based Cell Therapy in Neurological Diseases: A Review. J. Neurosci. Res. 2009;87:2183–2200. doi: 10.1002/jnr.22054. [DOI] [PubMed] [Google Scholar]
- 36.Reagan MR, Kaplan DL. Concise Review: Mesenchymal Stem Cell Tumor Homing: Detection Methods in Disease Model Systems. Stem Cells. 2011;29:920–927. doi: 10.1002/stem.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tu S-M, Lin S-H, Logothetis CJ. Stem-cell origin of metastasis and heterogeneity in solid tumours. The lancet oncology. 2002;3:508–513. doi: 10.1016/s1470-2045(02)00820-3. [DOI] [PubMed] [Google Scholar]
- 38.Aboody KS, Brown A, Rainov NG, Bower KA, Liu S, Yang W, Small JE, Herrlinger U, Ourednik V, Black PM. Neural stem cells display extensive tropism for pathology in adult brain: Evidence from intracranial gliomas. Proceedings of the National Academy of Sciences. 2000;97:12846–12851. doi: 10.1073/pnas.97.23.12846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pastan I, Willingham MC. Endocytosis. Springer; 1985. The pathway of endocytosis; pp. 1–44. [Google Scholar]
- 40.Canton I, Battaglia G. Endocytosis at the nanoscale. Chemical Society Reviews. 2012;41:2718–2739. doi: 10.1039/c2cs15309b. [DOI] [PubMed] [Google Scholar]
- 41.Ben-Bassat I, Bensch KG, Schrier SL. Drug-induced erythrocyte membrane internalization. J. Clin. Invest. 1972;51:1833. doi: 10.1172/JCI106985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ginn FL, Hochstein P, Trump BF. Membrane Alterations in Hemolysis: Internalization of Plasmalemma Induced by Primaquine. Science. 1969;164:843–845. doi: 10.1126/science.164.3881.843. [DOI] [PubMed] [Google Scholar]
- 43.Gopal V, Kumar AR, Usha N, Karthik A, Udupa N. Effective drug targeting by erythrocytes as carrier systems. Curr. Trends Biotechnol. Pharm. 2007;1:18–33. [Google Scholar]
- 44.Matovcik LM, Junga IG, Schrier SL. Drug-induced endocytosis of neonatal erythrocytes. Blood. 1985;65:1056–1063. [PubMed] [Google Scholar]
- 45.Ben-Bassat I, Bensch KG, Schrier SL. Drug-induced erythrocyte membrane internalization. The Journal of clinical investigation. 1972;51:1833–44. doi: 10.1172/JCI106985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schrier SL, Zachowski A, Devaux PF. Mechanisms of amphipath-induced stomatocytosis in human erythrocytes. Blood. 1992;79:782–6. [PubMed] [Google Scholar]
- 47.Aderem A, Underhill DM. Mechanisms of phagocytosis in macrophages. Annu. Rev. Immunol. 1999;17:593–623. doi: 10.1146/annurev.immunol.17.1.593. [DOI] [PubMed] [Google Scholar]
- 48.Owens DE, III, Peppas NA. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int. J. Pharm. 2006;307:93–102. doi: 10.1016/j.ijpharm.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 49.Beduneau A, Ma Z, Grotepas CB, Kabanov A, Rabinow BE, Gong N, Mosley RL, Dou H, Boska MD, Gendelman HE. Facilitated Monocyte-Macrophage Uptake and Tissue Distribution of Superparmagnetic Iron-Oxide Nanoparticles. PLoS One. 2009;4:e4343. doi: 10.1371/journal.pone.0004343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Choi J, Kim H-Y, Ju EJ, Jung J, Park J, Chung H-K, Lee JS, Lee JS, Park HJ, Song SY, Jeong S-Y, Choi EK. Use of macrophages to deliver therapeutic and imaging contrast agents to tumors. Biomaterials. 2012;33:4195–4203. doi: 10.1016/j.biomaterials.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 51.Nowacek AS, Miller RL, McMillan J, Kanmogne G, Kanmogne M, Mosley RL, Ma Z, Graham S, Chaubal M, Werling J. NanoART synthesis, characterization, uptake, release and toxicology for human monocyte-macrophage drug delivery. Nanomedicine. 2009;4:903–917. doi: 10.2217/nnm.09.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Batrakova EV, Li S, Reynolds AD, Mosley RL, Bronich TK, Kabanov AV, Gendelman HE. A macrophage-nanozyme delivery system for Parkinson’s disease. Bioconjug. Chem. 2007;18:1498–1506. doi: 10.1021/bc700184b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Choi M-R, Stanton-Maxey KJ, Stanley JK, Levin CS, Bardhan R, Akin D, Badve S, Sturgis J, Robinson JP, Bashir R. A cellular Trojan Horse for delivery of therapeutic nanoparticles into tumors. Nano Lett. 2007;7:3759–3765. doi: 10.1021/nl072209h. [DOI] [PubMed] [Google Scholar]
- 54.Choi M-R, Bardhan R, Stanton-Maxey KJ, Badve S, Nakshatri H, Stantz KM, Cao N, Halas NJ, Clare SE. Delivery of nanoparticles to brain metastases of breast cancer using a cellular Trojan horse. Cancer Nanotechnol. 2012;3:47–54. doi: 10.1007/s12645-012-0029-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Doshi N, Mitragotri S. Macrophages Recognize Size and Shape of Their Targets. PLoS One. 2010;5:e10051. doi: 10.1371/journal.pone.0010051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Champion J, Walker A, Mitragotri S. Role of Particle Size in Phagocytosis of Polymeric Microspheres. Pharm. Res. 2008;25:1815–1821. doi: 10.1007/s11095-008-9562-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Champion JA, Mitragotri S. Role of target geometry in phagocytosis. Proc. Natl. Acad. Sci. U. S. A. 2006;103:4930–4934. doi: 10.1073/pnas.0600997103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Beningo KA, Wang Y.-l. Fc-receptor-mediated phagocytosis is regulated by mechanical properties of the target. J. Cell Sci. 2002;115:849–856. doi: 10.1242/jcs.115.4.849. [DOI] [PubMed] [Google Scholar]
- 59.Fujiwara M, Baldeschwieler JD, Grubbs RH. Receptor-mediated endocytosis of poly(acrylic acid)-conjugated liposomes by macrophages. Biochim. Biophys. Acta. 1996;1278:59–67. doi: 10.1016/0005-2736(95)00183-2. [DOI] [PubMed] [Google Scholar]
- 60.Juliano RL, Stamp D. The effect of particle size and charge on the clearance rates of liposomes and liposome encapsulated drugs. Biochem. Biophys. Res. Commun. 1975;63:651–658. doi: 10.1016/s0006-291x(75)80433-5. [DOI] [PubMed] [Google Scholar]
- 61.Champion J, Mitragotri S. Shape Induced Inhibition of Phagocytosis of Polymer Particles. Pharm. Res. 2009;26:244–249. doi: 10.1007/s11095-008-9626-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Swiston AJ, Cheng C, Um SH, Irvine DJ, Cohen RE, Rubner MF. Surface functionalization of living cells with multilayer patches. Nano Lett. 2008;8:4446–4453. doi: 10.1021/nl802404h. [DOI] [PubMed] [Google Scholar]
- 63.Vasconcellos FC, Swiston AJ, Beppu MM, Cohen RE, Rubner MF. Bioactive polyelectrolyte multilayers: Hyaluronic acid mediated B lymphocyte adhesion. Biomacromolecules. 2010;11:2407–2414. doi: 10.1021/bm100570r. [DOI] [PubMed] [Google Scholar]
- 64.Swiston AJ, Gilbert JB, Irvine DJ, Cohen RE, Rubner MF. Freely Suspended Cellular “Backpacks” Lead to Cell Aggregate Self-Assembly. Biomacromolecules. 2010;11:1826–1832. doi: 10.1021/bm100305h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Doshi N, Swiston AJ, Gilbert JB, Alcaraz ML, Cohen RE, Rubner MF, Mitragotri S. Cell-Based Drug Delivery Devices Using Phagocytosis-Resistant Backpacks. Advanced Materials. 2011;23:H105–H109. doi: 10.1002/adma.201004074. [DOI] [PubMed] [Google Scholar]
- 66.Fernandes Stefanello T, Szarpak-Jankowska A, Appaix F, Louage B, Hamard L, De Geest BG, van der Sanden B, Nakamura CV, Auzély-Velty R. Thermoresponsive hyaluronic acid nanogels as hydrophobic drug carrier to macrophages. Acta Biomater. 2014;10:4750–4758. doi: 10.1016/j.actbio.2014.07.033. [DOI] [PubMed] [Google Scholar]
- 67.Li L, Guan Y, Liu H, Hao N, Liu T, Meng X, Fu C, Li Y, Qu Q, Zhang Y, Ji S, Chen L, Chen D, Tang F. Silica Nanorattle–Doxorubicin-Anchored Mesenchymal Stem Cells for Tumor-Tropic Therapy. ACS nano. 2011;5:7462–7470. doi: 10.1021/nn202399w. [DOI] [PubMed] [Google Scholar]
- 68.Zaitsev S, Danielyan K, Murciano J-C, Ganguly K, Krasik T, Taylor RP, Pincus S, Jones S, Cines DB, Muzykantov VR. Human complement receptor type 1-directed loading of tissue plasminogen activator on circulating erythrocytes for prophylactic fibrinolysis. Blood. 2006;108:1895–1902. doi: 10.1182/blood-2005-11-012336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kontos S, Hubbell JA. Improving protein pharmacokinetics by engineering erythrocyte affinity. Mol. Pharm. 2010;7:2141–7. doi: 10.1021/mp1001697. [DOI] [PubMed] [Google Scholar]
- 70.Kontos S, Kourtis IC, Dane KY, Hubbell JA. Engineering antigens for in situ erythrocyte binding induces T-cell deletion. Proceedings of the National Academy of Sciences. 2013;110:E60–E68. doi: 10.1073/pnas.1216353110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stephan MT, Stephan SB, Bak P, Chen J, Irvine DJ. Synapse-directed delivery of immunomodulators using T-cell-conjugated nanoparticles. Biomaterials. 2012;33:5776–5787. doi: 10.1016/j.biomaterials.2012.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Naldini L, Blömer U, Gallay P, Ory D, Mulligan R, Gage FH, Verma IM, Trono D. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272:263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- 73.Tjuvajev JG, Stockhammer G, Desai R, Uehara H, Watanabe K, Gansbacher B, Blasberg RG. Imaging the expression of transfected genes in vivo. Cancer Res. 1995;55:6126–6132. [PubMed] [Google Scholar]
- 74.Chiocca EA. Oncolytic viruses. Nature Reviews Cancer. 2002;2:938–950. doi: 10.1038/nrc948. [DOI] [PubMed] [Google Scholar]
- 75.Komarova S, Kawakami Y, Stoff-Khalili MA, Curiel DT, Pereboeva L. Mesenchymal progenitor cells as cellular vehicles for delivery of oncolytic adenoviruses. Mol. Cancer Ther. 2006;5:755–766. doi: 10.1158/1535-7163.MCT-05-0334. [DOI] [PubMed] [Google Scholar]
- 76.Pereboeva L, Komarova S, Mikheeva G, Krasnykh V, Curiel DT. Approaches to utilize mesenchymal progenitor cells as cellular vehicles. Stem Cells. 2003;21:389–404. doi: 10.1634/stemcells.21-4-389. [DOI] [PubMed] [Google Scholar]
- 77.Ong HT, Hasegawa K, Dietz AB, Russell SJ, Peng KW. Evaluation of T cells as carriers for systemic measles virotherapy in the presence of antiviral antibodies. Gene Ther. 2006;14:324–333. doi: 10.1038/sj.gt.3302880. [DOI] [PubMed] [Google Scholar]
- 78.Hu YL, Huang B, Zhang TY, Miao PH, Tang GP, Tabata Y, Gao JQ. Mesenchymal stem cells as a novel carrier for targeted delivery of gene in cancer therapy based on nonviral transfection. Mol. Pharm. 2012;9:2698–2709. doi: 10.1021/mp300254s. [DOI] [PubMed] [Google Scholar]
- 79.Melancon MP, Lu W, Zhong M, Zhou M, Liang G, Elliott AM, Hazle JD, Myers JN, Li C, Jason Stafford R. Targeted multifunctional gold-based nanoshells for magnetic resonance-guided laser ablation of head and neck cancer. Biomaterials. 2011;32:7600–7608. doi: 10.1016/j.biomaterials.2011.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Huang D-M, Hsiao J-K, Chen Y-C, Chien L-Y, Yao M, Chen Y-K, Ko B-S, Hsu S-C, Tai L-A, Cheng H-Y, Wang S-W, Yang C-S, Chen Y-C. The promotion of human mesenchymal stem cell proliferation by superparamagnetic iron oxide nanoparticles. Biomaterials. 2009;30:3645–3651. doi: 10.1016/j.biomaterials.2009.03.032. [DOI] [PubMed] [Google Scholar]
- 81.Sanz S, Lizano C, Luque J, Pinilla M. In vitro and in vivo study of glutamate dehydrogenase encapsulated into mouse erythrocytes by a hypotonic dialysis procedure. Life Sci. 1999;65:2781–9. doi: 10.1016/s0024-3205(99)00546-9. [DOI] [PubMed] [Google Scholar]
- 82.Kwant W, Seeman P. The erythrocyte ghost is a perfect osmometer. The Journal of general physiology. 1970;55:208–219. doi: 10.1085/jgp.55.2.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.DeLoach J, Droleskey R, Andrews K. Encapsulation by hypotonic dialysis in human erythrocytes: a diffusion or endocytosis process. Biotechnol. Appl. Biochem. 1991;13:72–82. [PubMed] [Google Scholar]
- 84.Deloach JR. Carrier erythrocytes. Med. Res. Rev. 1986;6:487–504. doi: 10.1002/med.2610060406. [DOI] [PubMed] [Google Scholar]
- 85.De Flora A, Zocchi E, Guida L, Polvani C, Benatti U. Conversion of encapsulated 5-fluoro-2′-deoxyuridine 5′-monophosphate to the antineoplastic drug 5-fluoro-2′-deoxyuridine in human erythrocytes. Proc. Natl. Acad. Sci. U. S. A. 1988;85:3145–9. doi: 10.1073/pnas.85.9.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Alvarez FJ, Jordan JA, Herraez A, Diez JC, Tejedor MC. Hypotonically loaded rat erythrocytes deliver encapsulated substances into peritoneal macrophages. J. Biochem. 1998;123:233–9. doi: 10.1093/oxfordjournals.jbchem.a021927. [DOI] [PubMed] [Google Scholar]
- 87.Pérez MT, Alvarez FJ, García-Pérez A-I, Lucas L, Tejedor MC, Sancho P. Heterogeneity of hypotonically loaded rat erythrocyte populations as detected by counter-current distribution in aqueous polymer two-phase systems. Journal of Chromatography B: Biomedical Sciences and Applications. 1996;677:45–51. doi: 10.1016/0378-4347(95)00433-5. [DOI] [PubMed] [Google Scholar]
- 88.Chiarantini L, Cerasi A, Fraternale A, Andreoni F, Scarí S, Giovine M, Clavarino E, Magnani M. Inhibition of Macrophage iNOS by Selective Targeting of Antisense PNA†. Biochemistry. 2002;41:8471–8477. doi: 10.1021/bi020079f. [DOI] [PubMed] [Google Scholar]
- 89.Deloach J, Ihler G. A dialysis procedure for loading erythrocytes with enzymes and lipids. Biochim. Biophys. Acta. 1977;496:136–45. doi: 10.1016/0304-4165(77)90121-0. [DOI] [PubMed] [Google Scholar]
- 90.Foroozesh M, Hamidi M, Zarrin A, Mohammadi-Samani S, Montaseri H. Preparation and in-vitro characterization of tramadol-loaded carrier erythrocytes for long-term intravenous delivery. J. Pharm. Pharmacol. 2011;63:322–332. doi: 10.1111/j.2042-7158.2010.01207.x. [DOI] [PubMed] [Google Scholar]
- 91.Weaver JC. Electroporation of biological membranes from multicellular to nano scales. Dielectrics and Electrical Insulation, IEEE Transactions on. 2003;10:754–768. [Google Scholar]
- 92.Tsong TY. Electroporation of cell membranes. Biophys. J. 1991;60:297–306. doi: 10.1016/S0006-3495(91)82054-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Flynn G, Hackett TJ, McHale L, McHale AP. Encapsulation of the thrombolytic enzyme, brinase, in photosensitized erythrocytes: a novel thrombolytic system based on photodynamic activation. Journal of Photochemistry and Photobiology B-Biology. 1994;26:193–196. doi: 10.1016/1011-1344(94)07037-7. [DOI] [PubMed] [Google Scholar]
- 94.Mangal PC, Kaur A. Electroporation of red blood cell membrane and its use as a drug carrier system. Indian J. Biochem. Biophys. 1991;28:219–21. [PubMed] [Google Scholar]
- 95.Lizano C, Perez MT, Pinilla M. Mouse erythrocytes as carriers for coencapsulated alcohol and aldehyde dehydrogenase obtained by electroporation in vivo survival rate in circulation, organ distribution and ethanol degradation. Life Sci. 2001;68:2001–16. doi: 10.1016/s0024-3205(01)00991-2. [DOI] [PubMed] [Google Scholar]
- 96.Chen I, Howarth M, Lin W, Ting AY. Site-specific labeling of cell surface proteins with biophysical probes using biotin ligase. Nat. Methods. 2005;2:99–104. doi: 10.1038/nmeth735. [DOI] [PubMed] [Google Scholar]
- 97.Ferguson MA, Williams AF. Cell-surface anchoring of proteins via glycosyl-phosphatidylinositol structures. Annu. Rev. Biochem. 1988;57:285–320. doi: 10.1146/annurev.bi.57.070188.001441. [DOI] [PubMed] [Google Scholar]
- 98.Rossi NAA, Constantinescu I, Kainthan RK, Brooks DE, Scott MD, Kizhakkedathu JN. Red blood cell membrane grafting of multi-functional hyperbranched polyglycerols. Biomaterials. 2010;31:4167–4178. doi: 10.1016/j.biomaterials.2010.01.137. [DOI] [PubMed] [Google Scholar]
- 99.Stephan MT, Moon JJ, Um SH, Bershteyn A, Irvine DJ. Therapeutic cell engineering with surface-conjugated synthetic nanoparticles. Nat. Med. 2010;16:1035–1041. doi: 10.1038/nm.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Diamandis EP, Christopoulos TK. The biotin-(strept) avidin system: principles and applications in biotechnology. Clin. Chem. 1991;37:625–636. [PubMed] [Google Scholar]
- 101.Parrott MB, Adams KE, Mercier GT, Mok H, Campos SK, Barry MA. Metabolically biotinylated adenovirus for cell targeting, ligand screening, and vector purification. Mol. Ther. 2003;8:688–700. doi: 10.1016/s1525-0016(03)00213-2. [DOI] [PubMed] [Google Scholar]
- 102.Suzuki T, Dale GL. Biotinylated erythrocytes: in vivo survival and in vitro recovery. Blood. 1987;70:791–795. [PubMed] [Google Scholar]
- 103.Cheng H, Kastrup CJ, Ramanathan R, Siegwart DJ, Ma M, Bogatyrev SR, Xu Q, Whitehead KA, Langer R, Anderson DG. Nanoparticulate cellular patches for cell-mediated tumoritropic delivery. ACS nano. 2010;4:625–631. doi: 10.1021/nn901319y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Murciano J-C, Medinilla S, Eslin D, Atochina E, Cines DB, Muzykantov VR. Prophylactic fibrinolysis through selective dissolution of nascent clots by tPA-carrying erythrocytes. Nat Biotech. 2003;21:891–896. doi: 10.1038/nbt846. [DOI] [PubMed] [Google Scholar]
- 105.Murciano J-C, Higazi AA-R, Cines DB, Muzykantov VR. Soluble urokinase receptor conjugated to carrier red blood cells binds latent pro-urokinase and alters its functional profile. J. Control. Release. 2009;139:190–196. doi: 10.1016/j.jconrel.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Baskin JM, Bertozzi CR. Bioorthogonal click chemistry: Covalent labeling in living systems. QSAR & Combinatorial Science. 2007;26:1211–1219. [Google Scholar]
- 107.Chang PV, Prescher JA, Sletten EM, Baskin JM, Miller IA, Agard NJ, Lo A, Bertozzi CR. Copper-free click chemistry in living animals. Proceedings of the National Academy of Sciences. 2010;107:1821–1826. doi: 10.1073/pnas.0911116107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kolb HC, Sharpless KB. The growing impact of click chemistry on drug discovery. Drug Discov. Today. 2003;8:1128–1137. doi: 10.1016/s1359-6446(03)02933-7. [DOI] [PubMed] [Google Scholar]
- 109.Hong V, Steinmetz NF, Manchester M, Finn MG. Labeling Live Cells by Copper-Catalyzed Alkyne-Azide Click Chemistry. Bioconjug. Chem. 2010;21:1912–1916. doi: 10.1021/bc100272z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Prescher JA, Bertozzi CR. Chemistry in living systems. Nat. Chem. Biol. 2005;1:13–21. doi: 10.1038/nchembio0605-13. [DOI] [PubMed] [Google Scholar]
- 111.Hein JE, Fokin VV. Copper-catalyzed azide-alkyne cycloaddition (CuAAC) and beyond: new reactivity of copper (I) acetylides. Chemical Society Reviews. 2010;39:1302–1315. doi: 10.1039/b904091a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Baskin JM, Prescher JA, Laughlin ST, Agard NJ, Chang PV, Miller IA, Lo A, Codelli JA, Bertozzi CR. Copper-free click chemistry for dynamic in vivo imaging. Proceedings of the National Academy of Sciences. 2007;104:16793–16797. doi: 10.1073/pnas.0707090104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Xu L, Zolotarskaya OY, Yeudall WA, Yang H. Click Hybridization of Immune Cells and Polyamidoamine Dendrimers. Advanced Healthcare Materials. 2014;3:1430–1438. doi: 10.1002/adhm.201300515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Iwasaki Y, Matsuno H. Metabolic Delivery of Methacryloyl Groups on Living Cells and Cell Surface Modification via Thiol Ene “Click” Reaction. Macromol. Biosci. 2011;11:1478–1483. doi: 10.1002/mabi.201100242. [DOI] [PubMed] [Google Scholar]
- 115.Dou H, Destache CJ, Morehead JR, Mosley RL, Boska MD, Kingsley J, Gorantla S, Poluektova L, Nelson JA, Chaubal M. Development of a macrophage-based nanoparticle platform for antiretroviral drug delivery. Blood. 2006;108:2827–2835. doi: 10.1182/blood-2006-03-012534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lizano C, Pérez MT, Pinilla M. Mouse erythrocytes as carriers for coencapsulated alcohol and aldehyde dehydrogenase obtained by electroporation: In vivo survival rate in circulation, organ distribution and ethanol degradation. Life Sci. 2001;68:2001–2016. doi: 10.1016/s0024-3205(01)00991-2. [DOI] [PubMed] [Google Scholar]
- 117.Lynch WE, Sartiano GP, Ghaffar A. Erythrocytes as carriers of chemotherapeutic agents for targeting the reticuloendothelial system. Am. J. Hematol. 1980;9:249–259. doi: 10.1002/ajh.2830090303. [DOI] [PubMed] [Google Scholar]
- 118.Knutson M, Wessling-Resnick M. Iron metabolism in the reticuloendothelial system. Crit. Rev. Biochem. Mol. Biol. 2003;38:61–88. doi: 10.1080/713609210. [DOI] [PubMed] [Google Scholar]
- 119.Zocchi E, Tonetti M, Polvani C, Guida L, Benatti U, Flora A. In vivo liver and lung targeting of adriamycin encapsulated in glutaraldehyde treated murine erythrocytes. Biotechnol. Appl. Biochem. 1988;10:555–562. [PubMed] [Google Scholar]
- 120.Mishra P, Jain N. Biotinylated methotrexate loaded erythrocytes for enhanced liver uptake.‘A study on the rat’. Int. J. Pharm. 2002;231:145–153. doi: 10.1016/s0378-5173(01)00847-x. [DOI] [PubMed] [Google Scholar]
- 121.Crinelli R, Antonelli A, Bianchi M, Gentilini L, Scaramucci S, Magnani M. Selective Inhibition of NF-kB Activation and TNF-α Production in Macrophages by Red Blood Cell-Mediated Delivery of Dexamethasone. Blood Cells Mol. Dis. 2000;26:211–222. doi: 10.1006/bcmd.2000.0298. [DOI] [PubMed] [Google Scholar]
- 122.Deloach J, Peters S, Pinkard O, Glew R, Hiler G. Effect of glutaraldehyde treatment on enzyme-loaded erythrocytes. Biochimica et Biophysica Acta (BBA)-General Subjects. 1977;496:507–515. doi: 10.1016/0304-4165(77)90332-4. [DOI] [PubMed] [Google Scholar]
- 123.Zocchi E, Tonetti M, Polvani C, Guida L, Benatti U, De Flora A. Encapsulation of doxorubicin in liver-targeted erythrocytes increases the therapeutic index of the drug in a murine metastatic model. Proceedings of the National Academy of Sciences. 1989;86:2040–2044. doi: 10.1073/pnas.86.6.2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kwon YM, Chung HS, Moon C, Yockman J, Park YJ, Gitlin SD, David AE, Yang VC. L-Asparaginase encapsulated intact erythrocytes for treatment of acute lymphoblastic leukemia (ALL) J. Control. Release. 2009;139:182–189. doi: 10.1016/j.jconrel.2009.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rossi L, Serafini S, Antonelli A, Pierigé F, Carnevali A, Battistelli V, Malatesta M, Balestra E, Caliò R, Perno C-F. Macrophage depletion induced by clodronate-loaded erythrocytes. J. Drug Target. 2005;13:99–111. doi: 10.1080/10611860500064123. [DOI] [PubMed] [Google Scholar]
- 126.Bradley AJ, Murad KL, Regan KL, Scott MD. Biophysical consequences of linker chemistry and polymer size on stealth erythrocytes: size does matter. Biochim. Biophys. Acta. 2002;1561:147–158. doi: 10.1016/s0005-2736(02)00339-5. [DOI] [PubMed] [Google Scholar]
- 127.Murad KL, Mahany KL, Brugnara C, Kuypers FA, Eaton JW, Scott MD. Structural and functional consequences of antigenic modulation of red blood cells with methoxypoly (ethylene glycol) Blood. 1999;93:2121–2127. [PubMed] [Google Scholar]
- 128.Scott MD, Murad KL, Koumpouras F, Talbot M, Eaton JW. Chemical camouflage of antigenic determinants: Stealth erythrocytes. Proceedings of the National Academy of Sciences. 1997;94:7566–7571. doi: 10.1073/pnas.94.14.7566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Price RJ, Skyba DM, Kaul S, Skalak TC. Delivery of colloidal particles and red blood cells to tissue through microvessel ruptures created by targeted microbubble destruction with ultrasound. Circulation. 1998;98:1264–7. doi: 10.1161/01.cir.98.13.1264. [DOI] [PubMed] [Google Scholar]
- 130.Sprandel U, Lanz DJ, von Horsten W. Magnetically responsive erythrocyte ghosts. Methods Enzymol. 1987;149:301–12. doi: 10.1016/0076-6879(87)49068-x. [DOI] [PubMed] [Google Scholar]
- 131.Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nature Reviews Immunology. 2013;13:159–175. doi: 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- 132.Schenkel AR, Mamdouh Z, Muller WA. Locomotion of monocytes on endothelium is a critical step during extravasation. Nat. Immunol. 2004;5:393–400. doi: 10.1038/ni1051. [DOI] [PubMed] [Google Scholar]
- 133.Albelda SM, Smith CW, Ward P. Adhesion molecules and inflammatory injury. The FASEB Journal. 1994;8:504–512. [PubMed] [Google Scholar]
- 134.Pober JS, Cotran RS. The role of endothelial cells in inflammation. Transplantation. 1990;50:537–544. doi: 10.1097/00007890-199010000-00001. [DOI] [PubMed] [Google Scholar]
- 135.Luster AD, Alon R, von Andrian UH. Immune cell migration in inflammation: present and future therapeutic targets. Nat. Immunol. 2005;6:1182–1190. doi: 10.1038/ni1275. [DOI] [PubMed] [Google Scholar]
- 136.Sampson A. The role of eosinophils and neutrophils in inflammation. Clin. Exp. Allergy. 2000;30:22–27. doi: 10.1046/j.1365-2222.2000.00092.x. [DOI] [PubMed] [Google Scholar]
- 137.Carlos TM, Harlan JM. Leukocyte-endothelial adhesion molecules. Blood. 1994;84:2068–2101. [PubMed] [Google Scholar]
- 138.Bevilacqua MP. Endothelial-leukocyte adhesion molecules. Annu. Rev. Immunol. 1993;11:767–804. doi: 10.1146/annurev.iy.11.040193.004003. [DOI] [PubMed] [Google Scholar]
- 139.Diacovo T, Roth S, Buccola J, Bainton D, Springer T. Neutrophil rolling, arrest, and transmigration across activated, surface-adherent platelets via sequential action of P-selectin and the beta 2-integrin CD11b/CD18. Blood. 1996;88:146–157. [PubMed] [Google Scholar]
- 140.Lo SK, Lee S, Ramos RA, Lobb R, Rosa M, Chi-Rosso G, Wright SD. Endothelial-leukocyte adhesion molecule 1 stimulates the adhesive activity of leukocyte integrin CR3 (CD11b/CD18, Mac-1, alpha m beta 2) on human neutrophils. The Journal of experimental medicine. 1991;173:1493–1500. doi: 10.1084/jem.173.6.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.von Andrian UH, Chambers JD, McEvoy LM, Bargatze RF, Arfors K-E, Butcher EC. Two-step model of leukocyte-endothelial cell interaction in inflammation: distinct roles for LECAM-1 and the leukocyte beta 2 integrins in vivo. Proceedings of the National Academy of Sciences. 1991;88:7538–7542. doi: 10.1073/pnas.88.17.7538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Phillipson M, Heit B, Colarusso P, Liu L, Ballantyne CM, Kubes P. Intraluminal crawling of neutrophils to emigration sites: a molecularly distinct process from adhesion in the recruitment cascade. The Journal of experimental medicine. 2006;203:2569–2575. doi: 10.1084/jem.20060925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Williams MR, Azcutia V, Newton G, Alcaide P, Luscinskas FW. Emerging mechanisms of neutrophil recruitment across endothelium. Trends Immunol. 2011;32:461–469. doi: 10.1016/j.it.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nature Reviews Immunology. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 145.Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nature Reviews Immunology. 2011;11:762–774. doi: 10.1038/nri3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of Monocytes, Macrophages, and Dendritic Cells. Science. 2010;327:656–661. doi: 10.1126/science.1178331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Pages F, Galon J, Dieu-Nosjean M, Tartour E, Sautes-Fridman C, Fridman W. Immune infiltration in human tumors: a prognostic factor that should not be ignored. Oncogene. 2009;29:1093–1102. doi: 10.1038/onc.2009.416. [DOI] [PubMed] [Google Scholar]
- 148.Kawakami Y, Eliyahu S, Delgado CH, Robbins PF, Sakaguchi K, Appella E, Yannelli JR, Adema GJ, Miki T, Rosenberg SA. Identification of a human melanoma antigen recognized by tumor-infiltrating lymphocytes associated with in vivo tumor rejection. Proceedings of the National Academy of Sciences. 1994;91:6458–6462. doi: 10.1073/pnas.91.14.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Smith BR, Ghosn EEB, Rallapalli H, Prescher JA, Larson T, Herzenberg LA, Gambhir SS. Selective uptake of single-walled carbon nanotubes by circulating monocytes for enhanced tumour delivery. Nature nanotechnology. 2014 doi: 10.1038/nnano.2014.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Batrakova EV, Li S, Reynolds AD, Mosley RL, Bronich TK, Kabanov AV, Gendelman HE. A Macrophage–Nanozyme Delivery System for Parkinson’s Disease. Bioconjug. Chem. 2007;18:1498–1506. doi: 10.1021/bc700184b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Valable S, Barbier EL, Bernaudin M, Roussel S, Segebarth C, Petit E, Rémy C. In vivo MRI tracking of exogenous monocytes/macrophages targeting brain tumors in a rat model of glioma. Neuroimage. 2008;40:973–983. doi: 10.1016/j.neuroimage.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 152.Harris AL. Hypoxia—a key regulatory factor in tumour growth. Nature Reviews Cancer. 2002;2:38–47. doi: 10.1038/nrc704. [DOI] [PubMed] [Google Scholar]
- 153.Roger M, Clavreul A, Venier-Julienne M-C, Passirani C, Sindji L, Schiller P, Montero-Menei C, Menei P. Mesenchymal stem cells as cellular vehicles for delivery of nanoparticles to brain tumors. Biomaterials. 2010;31:8393–8401. doi: 10.1016/j.biomaterials.2010.07.048. [DOI] [PubMed] [Google Scholar]
- 154.Studeny M, Marini FC, Champlin RE, Zompetta C, Fidler IJ, Andreeff M. Bone marrow-derived mesenchymal stem cells as vehicles for interferon-β delivery into tumors. Cancer Res. 2002;62:3603–3608. [PubMed] [Google Scholar]
- 155.Studeny M, Marini FC, Dembinski JL, Zompetta C, Cabreira-Hansen M, Bekele BN, Champlin RE, Andreeff M. Mesenchymal stem cells: potential precursors for tumor stroma and targeted-delivery vehicles for anticancer agents. J. Natl. Cancer Inst. 2004;96:1593–1603. doi: 10.1093/jnci/djh299. [DOI] [PubMed] [Google Scholar]
- 156.Nakamizo A, Marini F, Amano T, Khan A, Studeny M, Gumin J, Chen J, Hentschel S, Vecil G, Dembinski J. Human bone marrow-derived mesenchymal stem cells in the treatment of gliomas. Cancer Res. 2005;65:3307–3318. doi: 10.1158/0008-5472.CAN-04-1874. [DOI] [PubMed] [Google Scholar]
- 157.Zhao J, Vykoukal J, Abdelsalam M, Recio-Boiles A, Huang Q, Qiao Y, Singhana B, Wallace M, Avritscher R, Melancon MP. Stem cell-mediated delivery of SPIO-loaded gold nanoparticles for the theranosis of liver injury and hepatocellular carcinoma. Nanotechnology. 2014;25:405101. doi: 10.1088/0957-4484/25/40/405101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Cao B, Yang M, Zhu Y, Qu X, Mao C. Stem Cells Loaded with Nanoparticles as a Drug Carrier for In Vivo Breast Cancer Therapy. Advanced Materials. 2014 doi: 10.1002/adma.201401550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Allard WJ, Matera J, Miller MC, Repollet M, Connelly MC, Rao C, Tibbe AGJ, Uhr JW, Terstappen LWMM. Tumor Cells Circulate in the Peripheral Blood of All Major Carcinomas but not in Healthy Subjects or Patients With Nonmalignant Diseases. Clin. Cancer Res. 2004;10:6897–6904. doi: 10.1158/1078-0432.CCR-04-0378. [DOI] [PubMed] [Google Scholar]
- 160.Mitchell MJ, Wayne E, Rana K, Schaffer CB, King MR. TRAIL-coated leukocytes that kill cancer cells in the circulation. Proceedings of the National Academy of Sciences. 2014;111:930–935. doi: 10.1073/pnas.1316312111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Liang S, Slattery MJ, Wagner D, Simon SI, Dong C. Hydrodynamic shear rate regulates melanoma-leukocyte aggregation, melanoma adhesion to the endothelium, and subsequent extravasation. Ann. Biomed. Eng. 2008;36:661–671. doi: 10.1007/s10439-008-9445-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Mukthavaram R, Shi G, Kesari S, Simberg D. Targeting and depletion of circulating leukocytes and cancer cells by lipophilic antibody-modified erythrocytes. J. Control. Release. 2014;183:146–153. doi: 10.1016/j.jconrel.2014.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wang C, Sun X, Cheng L, Yin S, Yang G, Li Y, Liu Z. Multifunctional Theranostic Red Blood Cells For Magnetic-Field-Enhanced in vivo Combination Therapy of Cancer. Advanced Materials. 2014 doi: 10.1002/adma.201400158. [DOI] [PubMed] [Google Scholar]
- 164.Soppimath KS, Aminabhavi TM, Kulkarni AR, Rudzinski WE. Biodegradable polymeric nanoparticles as drug delivery devices. J. Control. Release. 2001;70:1–20. doi: 10.1016/s0168-3659(00)00339-4. [DOI] [PubMed] [Google Scholar]
- 165.Xie Z, Zhang Y, Liu L, Weng H, Mason RP, Tang L, Nguyen KT, Hsieh JT, Yang J. Development of Intrinsically Photoluminescent and Photostable Polylactones. Advanced Materials. 2014;26:4491–4496. doi: 10.1002/adma.201306070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Schmaljohann D. Thermo-and pH-responsive polymers in drug delivery. Adv. Drug Del. Rev. 2006;58:1655–1670. doi: 10.1016/j.addr.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 167.Bajpai A, Shukla SK, Bhanu S, Kankane S. Responsive polymers in controlled drug delivery. Progress in Polymer Science. 2008;33:1088–1118. [Google Scholar]
- 168.He H, Ye J, Wang Y, Liu Q, Chung HS, Kwon YM, Shin MC, Lee K, Yang VC. Cell-penetrating peptides meditated encapsulation of protein therapeutics into intact red blood cells and its application. J. Control. Release. 2014;176:123–132. doi: 10.1016/j.jconrel.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Bawa P, Pillay V, Choonara YE, Du Toit LC. Stimuli-responsive polymers and their applications in drug delivery. Biomed Mater. 2009;4:022001. doi: 10.1088/1748-6041/4/2/022001. [DOI] [PubMed] [Google Scholar]