Abstract
An imbalance of excitatory and inhibitory functions has been shown to contribute to numerous pathological disorders. Accumulating evidence supports the idea that a change in hypothalamic γ-aminobutyric acid (GABA)-ergic inhibitory and glutamatergic excitatory synaptic functions contributes to exacerbated neurohumoral drive in prevalent cardiovascular disorders, including hypertension. However, the precise underlying mechanisms and neuronal substrates are still not fully elucidated. In the present study, we combined quantitative immunohistochemistry with neuronal tract tracing to determine whether plastic remodeling of afferent GABAergic and glutamatergic inputs into identified RVLM-projecting neurons of the hypothalamic paraventricular nucleus (PVN-RVLM) contributes to an imbalanced excitatory/inhibitory function in reno-vascular hypertensive rats (RVH). Our results indicate that both GABAergic and glutamatergic innervation densities increased in oxytocin-positive, PVN-RVLM (OT-PVN-RVLM) neurons in RVH rats. Despite this concomitant increase, time-dependent and compartment-specific differences in the reorganization of these inputs resulted in an altered balance of excitatory/inhibitory inputs in somatic and dendritic compartments. A net predominance of excitatory over inhibitory inputs was found in OT-PVN-RVLM proximal dendrites. Our results indicate that, along with previously described changes in neurotransmitter release probability and postsynaptic receptor function, remodeling of GABAergic and glutamatergic afferent inputs contributes as an underlying mechanism to the altered excitatory/ inhibitory balance in the PVN of hypertensive rats.
Keywords: synaptic, sympathetic, plasticity, brainstem, immunohistochemistry, hypertension
A proper balance of excitatory and inhibitory functions is required for nearly all brain functions, including cognitive, sensory, motor, and autonomic functions (Cline, 2005; Hensch and Fagiolini, 2005). An imbalance of these opposing neurotransmitter systems, on the other hand, contributes to numerous neurological disorders, including epilepsy, autism, and schizophrenia (Cline, 2005; Dudek and Staley, 2007; Rubenstein and Merzenich, 2003; Wassef et al., 2003). A change in central nervous system (CNS) γ-aminobutyric acid (GABA)ergic inhibitory and glutamatergic excitatory synaptic functions has been shown to be involved in the exacerbated neurohumoral drive in prevalent cardiovascular disorders, including hypertension and heart failure (Bergamaschi et al., 1995; Haywood et al., 2001; Hermes et al., 2008; Ito et al., 2001; Kleiber et al., 2008; Li and Pan, 2005, 2007b; Li and Patel, 2003; Mei et al., 2003). Despite this evidence, the precise underlying mechanisms and neuronal substrates involved are not fully elucidated.
The hypothalamic paraventricular nucleus (PVN) plays an important role in the modulation of sympathetic vaso-motor tone and blood pressure (Allen, 2002; Coote et al., 1998; Dampney et al., 2005; Martin and Haywood, 1992; Swanson and Sawchenko, 1983). In addition to the well-established projection to sympathetic preganglionic neurons in the intermediolateral cell column of the spinal cord, the rostral ventrolateral medulla (RVLM) is another major PVN target that mediates its influence on cardiovascular control (Allen, 2002; Ciriello et al., 1985; Coote et al., 1998; Dampney et al., 1987; Hardy, 2001; Kubo et al., 2000; Tagawa and Dampney, 1999; Yang and Coote, 1998). Activation of the PVN-RVLM pathway results in increased vasomotor RVLM neuronal activity (Yang et al., 2001; Yang and Coote, 1998) as well as pressor and sympathoexcitatory responses (Allen, 2002; Tagawa and Dampney, 1999; Yang and Coote, 1998).
Among the different peptides expressed in long-descending PVN neurons, oxytocin (OT) and vasopressin (VP) are the most prevalent (Swanson and Sawchenko 1982). A predominance of OT over VP expression in PVN-brainstem projections, including the RVLM, has been reported (Aguado et al., 2003; Gomez et al., 1993; Mack et al., 2002; Sawchenko and Swanson, 1982; Stocker et al., 2006). This is in agreement with other studies showing a predominance of OT over VP in the brainstem, with respect to both their levels and their immunoreactivities (Hancock and Nicholas, 1987; Lang et al., 1983; Nilaver et al., 1980). Microinjections of OT within the RVLM has been shown to induce an increase in mean arterial pressure and heart rate (Mack et al., 2002) as well as playing an important role in mediating cardiovascular responses to environmental stressors. Overall, these studies support the PVNRVLM-OT pathway as playing an important role in mediating PVN influences on sympathetic and cardiovascular control.
The PVN-RVLM pathway has been implicated in enhanced sympathetic vasomotor tone in hypertensive rats. Lesions of the PVN attenuated the development of hyper-tension and lowered blood pressure in spontaneously hypertensive rats (SHRs) and RVH rats (Allen, 2002; Ciriello et al., 1984; Earle and Pittman, 1995; Goto et al., 1981; Herzig et al., 1991; Takeda et al., 1991). In addition, increased expression of FOS immunoreactivity was reported in the PVN of RVH rats (Jung et al., 2004), supporting an increased activation of PVN neurons. Importantly, an enhanced PVN excitatory drive to the RVLM was reported to mediate enhanced sympathetic vasomotor tone in hyper-tensive rats (Allen, 2002). Despite the evidence supporting a role for the PVN in enhanced sympathoexcitatory outflow during hypertension, the underlying cellular and synaptic mechanisms remain to be elucidated.
The excitability of PVN neurons, and consequently sympathetic outflow from this region, is highly dependent on a balanced activity between excitatory and inhibitory synaptic inputs. GABA, acting most predominantly on GABAA receptors, is the major inhibitory neurotransmitter in the hypothalamus, restraining PVN neuronal activity and autonomic outflow (Chen et al., 2003; Decavel and Van den Pol, 1990; Tasker and Dudek, 1993; Zhang et al., 2002). Conversely, glutamate, acting both on AMPA and NMDA iono-tropic receptors, is the major excitatory neurotransmitter (Boudaba et al., 1997; Decavel and van den Pol, 1992; Herman et al., 2000), stimulating PVN activity and autonomic outflow (Chen et al., 2003; Herman et al., 2002; Latchford and Ferguson, 2004; Li and Pan, 2006; Li et al., 2003).
An altered glutamatergic and GABAergic synaptic function was shown to contribute to exacerbated PVN neuronal activity and sympathetic outflow during hypertension. For example, an enhanced glutamatergic input into the PVN is required to maintain elevated sympathetic activity in hypertensive rats (Allen, 2002; Li and Pan, 2007b), a phenomenon that involves, at least in part, enhanced presynaptic glutamate release, as well as up-regulation of postsynaptic NMDA receptors (Li et al., 2008). The contribution of altered PVN GABAergic function to sympathoex-citation in hypertension is less clear. For example, a blunted PVN GABAergic function has been shown to play a role in exacerbated sympathoexcitation in chronically SHRs (Li and Pan, 2006, 2007). In RVH rats, however, a diminished and an enhanced PVN GABAergic inhibition was observed at early and late hypertensive stages, respectively (Haywood et al., 2001; Martin and Haywood, 1998). Thus, dynamic and time-dependent changes in GABAergic inhibitory function in renovascular hypertension could contribute to the onset of sympathoexcitation at early stages, while acting as a compensatory mechanism at late stages of hypertension.
Although these data support a role for altered glutamatergic/GABAergic balance as an important mechanism underlying altered PVN sympathetic control in hypertension, the precise underlying mechanisms are not fully delineated. Previous studies support both changes in neurotransmitter release probability and changes in numbers and types of postsynaptic receptors (Li et al., 2008a,b). However, whether remodeling of afferent synaptic inputs also occurs in hypertensive conditions is at present unknown. Given the growing evidence supporting an important role for the PVN-RVLM pathway in cardiovascular control, as well as its involvement in the pathophysiology of hypertension, we used in this study a combination of quantitative immunohistochemistry with neuronal tract tracing to investigate afferent synaptic remodeling in identified OT-PVN-RVLM neurons in RVH rats.
MATERIALS AND METHODS
Animals
Male Wistar rats (150–180 g) were purchased from Harlan Laboratories (Indianapolis, IN). Rats were maintained under a 12-hour:12-hour light-dark cycle and given free access to food and water. All procedures were carried out in agreement with the Medical College of Georgia Institutional Animal Care and Use Committee guidelines.
Induction of two-kidney one-clip RVH rats
RVH rats were generated as previously described (Bergamaschi et al., 1995; Sonner et al., 2008). Briefly, rats were anesthetized with ketamine and xylazine (40 mg and 10 mg/kg, respectively, ip); the left renal artery was exposed through an abdominal incision, and the renal artery and renal vein were dissected free from the adherent tissues. The left renal artery was partially obstructed with a silver clip of 0.2-mm width. No clip obstruction was applied to the sham-operated group (n = 15). Animals were submitted to the final experimental procedures 3 or 6 weeks after the surgical procedure. These two groups were designated as early (3 weeks, n = 3) and late (6 weeks, n = 12) RVH rats (Carretero and Gulati, 1978; Martinez-Maldonado, 1991). Systolic blood pressure was measured in conscious rats using a pneumatic tail-cuff method (RTBP 2000; Kent Scientific). An average of three measurements was obtained per animal.
Retrograde labeling of RVLM-projecting PVN neurons (PVN-RVLM)
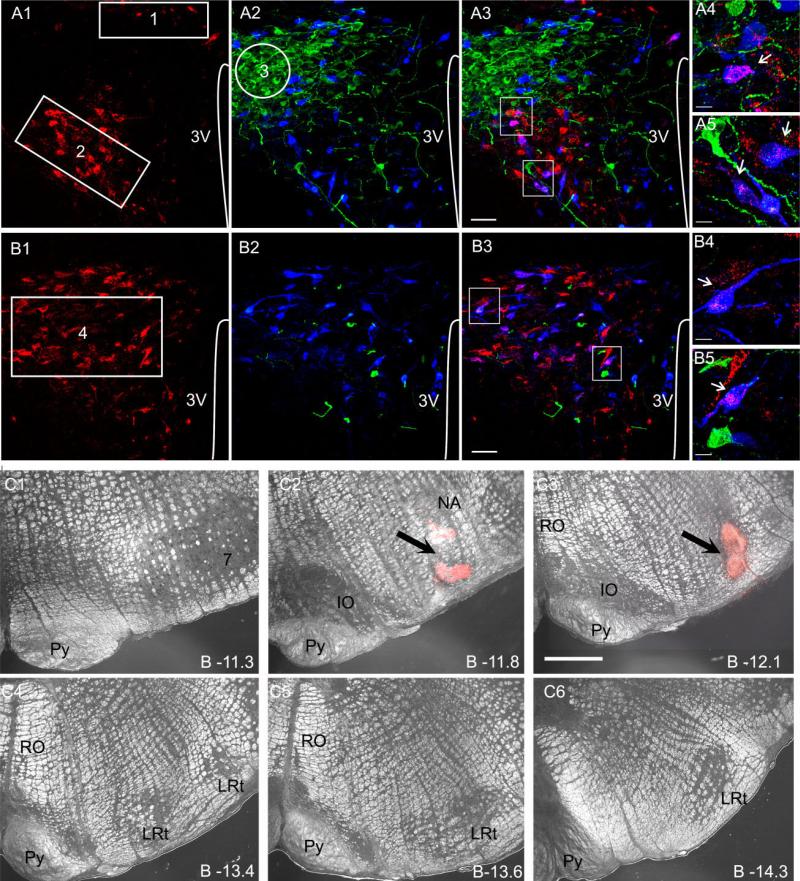
Labeling of PVN-RVLM neurons was done as previously described (Li et al., 2003; Sonner et al., 2008; Sonner and Stern, 2007). Briefly, rats were anesthetized (ketamine/ xylazine mixture, 90 and 5 mg/kg, respectively, i.p.), and the head of the rat was placed in a stereotaxic frame. For injections in the RVLM, an ~4-mm burr hole was made in the skull, and rhodamine-labeled microspheres (Lumaflor, Naples FL) were pressure injected unilaterally (400 nl) using the coordinates bregma –11.96, lateral –2.0, dorsal –8.0. Animals were allowed to recover and were killed 5–7 days after surgery, and the brain was dissected for immunohistochemical experiments. The location of the injections sites were confirmed histologically. Injections in the RVLM usually extended from the caudal pole of the facial nucleus to ~1 mm caudally and were ventrally located with respect to the nucleus ambiguous (see Fig. 1; Li et al., 2003; Sonner et al., 2008; Sonner and Stern, 2007; Stern and Zhang, 2003). Cases showing a misplaced injection site were not included in the analysis. Hypothalamic sections were then processed for double immunohistochemistry.
Figure 1.
Identification of various PVN subnuclei, RVLM-projecting neurons, and OT-and VP-ir neurons in the PVN. A: Representative confocal photomicrographs obtained at the medial level of PVN (~1.8–2.1 mm caudal to bregma). Retrogradely labeled PVN-RVLM neurons (A1, red) and OT-and VP-ir neurons (A2, blue and green, respectively) are shown. In A3, both images were superimposed, and the areas contained within the white squares were reimaged at higher magnification using different thresholds and are displayed in A4 and A5. The numbered regions in A1 and A2 depict the dorsal cap (1, DC), ventromedial parvocellular (2, VM), and lateral magnocellular (3, LM) subnuclei. B: Representative confocal photomicrographs obtained at the caudal level of PVN (~2.1–2.3 mm caudal to bregma). Retrogradely labeled PVN-RVLM neurons (B1, red) and OT-and VP-ir neurons (B2, blue and green, respectively) are shown in B1 and B2. In B3, both images were superimposed, and the areas contained within the white squares were reimaged at higher magnification using different thresholds and are displayed in B4 and B5. Arrows point to examples of double-labeled OT-PVN-RVLM neurons. C: Representative example of a rhodamine bead injection site in the RVLM, displayed at various rostrocaudal levels in the ventral medulla (C1–C6). The arrows in C2,3 point to the center of the injection site. 3V, third ventricle; Py, pyramidal tract; IO, inferior olive; RO, nucleus raphe obscurus; LRt, lateral reticular nucleus. A magenta-green copy of this figure is available as Supporting Information Figure 1. Scale bars = 50 μm in A,B; 12 μm in insets; 100 μm in C.
Immunohistochemistry
Rats were deeply anesthetized with sodium pentobarbital (100 mg/kg i.p.) and perfused transcardiacally with 0.01 M phosphate-buffered saline (PBS; pH 7.3–7.4; 150 ml) followed by 4% paraformaldehyde (350 ml). Brains were postfixed in 4% paraformaldehyde for 3 hours at 4°C. Fixed brains were cryoprotected at 4°C with 0.01 M PBS containing 30% sucrose for a minimum of 72 hours. Sequential 25-μm sections through the hypothalamus, between bregma –1.80 and –2.12 (Paxinos et al., 1999), were cut with a cryostat, transferred into 0.01 M PBS, and incubated in a solution of 0.01 M PBS with 0.01% Triton X-100, 0.04% NaN3, and 5% normal horse serum for 1 hour. Hypothalamic sections were incubated for 24 hours in a cocktail of primary antibodies containing either a guinea pig antivesicular glutamate transporter 2 (VGLUT2) polyclonal antibody (catalogue No. AB5907, lot No. 25020802; Chemicon, Temecula, CA; 1:10,000) or a mouse antiglutamic acid decarboxylase 67 (GAD67) monoclonal antibody (catalogue No. MAB5406; Chemicon; 1:800), along with a rabbit antioxytocin (OT) polyclonal antibody (catalogue No. T-4084, Bachem, Torrance, CA; 1:15,000). Reactions with primary antibodies were followed by a 4-hour incubation in the presence of fluorescently labeled secondary antibody (anti-guinea pig or mouse Cy5-labeled antibody, 1:50; and donkey anti-rabbit FITC-labeled, 1:400). Rhodamine-labeled microsphere labeling was readily detected by fluorescence illumination. For the initial characterization of OT and VP immunoreactivity in retrogradely labeled PVNRVLM neurons, a cocktail of primary antibodies containing the rabbit anti-OT antibody along with a guinea pig anti-VP polyclonal antibody (catalogue No. T-5048, lot No. 061305; Bachem; 1:15,000) was used, followed by an incubation in secondary antibodies (anti-guinea pig FITC-labeled, 1:400; and anti-rabbit Cy5-labeled, 1:50), as described above. All antibodies were diluted with PBS containing 0.01% Triton X-100 and 0.04% NaN3. All secondary antibodies were obtained from Jackson Immunore-search Laboratories (West Grove, PA).
Antibody characterization
In all cases, primary antibodies were titrated to determine the dilutions that produced the maximal number of immunoreactive elements with a minimal level of background staining. General control experiments included omission of primary or secondary antibodies and extensive tests of cross-reactivity of secondary antibodies. In all these cases, staining was completely absent. The guinea pig anti-VGLUT2 antibody (Chemicon) was raised against a synthetic peptide corresponding to a region of the VGLUT2 protein sequence (VQESAQDAYSYKDRDDYS). This sequence does not overlap with that of VGLUT1 or VGLUT3. According to Chemicon, preabsorption of the VGLUT2 an tiserum with immunogen peptide eliminated all immuno-staining. The specificity of this antibody has been previously determined by others using immunofluorescence protocols similar to those described here (Hrabovszky et al., 2004; Stocker et al., 2006). In both studies, preabsortion of the antibody with the synthetic peptide used as immunogen resulted in complete lack of immunolabeling. In our hands, the staining pattern obtained with the VGLUT2 antibody corresponds to the patterns previously described by others using the same antibody in the hypothalamus (Castelli et al., 2007; Hrabovszky et al., 2004, 2005; Kiss et al., 2007; Stocker et al., 2006; Wittmann et al., 2005) as well as the pattern obtained using a different antibody (Fremeau et al., 2001; Fujiyama et al., 2001; Kaneko et al., 2002; Sakata-Haga et al., 2001; Tong et al., 2001; Varoqui et al., 2002).
The mouse monoclonal anti-GAD67 antibody (Chemicon) was raised against a recombinant fusion protein containing the unique N-terminal regions of GAD67 not shared by GAD65 protein. According to Chemicon, this antibody reacts with the 67-kDa isoform of glutamate decarboxylase (GAD67) of rat, mouse, and human origin and possesses no detectable cross-reactivity with GAD65 by Western blot on rat brain lysate compared with a blot probed with AB1511, which reacts with both GAD65 and GAD67. The specificity of this antibody has been previously determined by others using immunofluorescence protocols similar to those described here (Benagiano et al., 2005; Sweatt et al., 2004). In both of those studies, preabsortion of the antibody with the synthetic peptide used as immunogen resulted in complete lack of immunolabeling. In our hands, and as we previously described (Park et al., 2006; Richards et al., 2005), the staining pattern obtained with the GAD67 antibody corresponds to the patterns previously described by others using the same antibody in the hypothalamus (Al-Noori et al., 2008; Castelli et al., 2007) and other brain regions (Singec et al., 2004; Sweatt et al., 2004).
The rabbit anti-OT antibody (Bachem) was raised against the antigen sequence H-Cys-Tyr-Ile-Gln-Asn-Cys-Pro-Leu-Gly-NH2. According to Bachem, this antibody has no cross-reactivity with other peptides typically found in the hypothalamus, including arg-vasopressin, α-ANP, CRF, or GFR. The specificity of this antibody was tested in our laboratory using OT-knockout mice generously provided by Dr. Mariana Morris (Wright State University, Dayton, Ohio), in which labeling was completely absent. In rats, the appropriate cellular distribution pattern was observed, with strong somatic immunofluorescence staining found in the supraoptic and paraventricular nucleus as well as fiber staining in the median eminence and posterior pituitary. This staining pattern overlapped with that obtained using a rabbit OT antiserum raised against the C terminus of OT (also kindly provided by Dr. Mariana Morris) as well as with a mouse anti-OT-neurophysin (PS38) antibody (generously provided by Dr. Hal Gainer, NIH; Richards et al., 2005) raised against rat OT-neurophysin. The specificity of PS38 has been extensively characterized previously (Ben-Barak et al., 1985; Whitnall et al., 1985).
The guinea pig anti-VP antibody (Bachem) was raised against the antigen sequence H-Cys-Tyr-Phe-Gln-Asn-Cys-Pro-Arg-Gly-NH2. According to Bachem, this antibody has no cross-reactivity with other peptides typically found in the hypothalamus, including OT, Met-enkephalin, PACAP38, or desamino-(D-Arg8)-VP). In our hands, this antibody gave the appropriate cellular distribution pattern expected for VP, with strong somatic immunofluorescence staining found in the supraoptic nucleus, PVN, and suprachiasmatic nucleus, along with fiber staining in the median eminence and posterior pituitary. This staining pattern overlapped with that obtained using a mouse anti-VP-neurophysin (PS46) antibody (also generously provided by Dr. Hal Gainer; Richards et al., 2005) raised against rat VP-neurophysin. The specificity of PS46 has been extensively characterized previously (Ben-Barak et al., 1985; Whitnall et al., 1985).
Confocal imaging acquisition and analysis
Acquisition and quantification of immunoreactive signals were performed as previously described (Higa-Taniguchi et al., 2007). Stained sections were examined with a Leica TCS SL (Leica Microsystems, Mannheim, Germany) confocal microscope system. A helium-neon laser was used to excite fluorescein isothiocyanate (FITC) and rhodamine fluorochromes at 488 and 543 nm, respectively, and an argon-krypton laser was used to excite Cy5 fluorochrome at 633 nm. Fluorescent signal cross-talk among the channels was avoided by setting image-acquisition parameters with individually labeled sections. All images from the different experimental groups were digitized with identical acquisition settings.
Quantification of GAD67 and VGLUT2 immunoreactivities within specific PVN subnuclei
Stacks of six consecutive optical focal planes (z-step 1 μm, image size 1,024 × 1,024 pixels, ×20 objective) were obtained from three to five sections/rat at two rostrocaudal levels of the PVN, according to Swanson and Kuypers (1980). These included 1) the middle one-third of the PVN, containing the lateral magnocellular (LM), dorsal cap (DC), and ventromedial (VM) subnuclei and 2) the most caudal one-third of the PVN, containing the posterior parvocellular (PaPo) subnucleus (Armstrong et al., 1980; Swanson and Kuypers, 1980). As previously reported (Li et al., 2003; Pyner and Coote, 2000; Stocker et al., 2004, 2006), these rostrocaudal regions contain the highest density of PVNRVLM projecting neurons.
Imaging analysis was performed in Metamorph software (Universal Imaging Corporation, Downingtown, PA). An automated tracing procedure incorporating a threshold paradigm (Alvarez et al., 2004) was applied to the channel containing the VGLUT2 or the GAD67 immunoreactivity information. Background intensity was calculated from random adjacent areas in the neuropil, and the threshold was set to pass intensities 1.5 times above background immunofluorescence. Regions of interest (ROIs; n = 3) of predetermined sizes were drawn within the LM, VM, DC, and PaPo PVN subnuclei. The distributions of retrogradely labeled and OT-ir neurons were used as landmarks to delineate the various subnuclei (see Fig. 1). The density of VGLUT2 and GAD67 thresholded signal within each ROI (expressed as percentage threshold area = area occupied by thresholded signal/total ROI area · 100) was calculated, and a mean value for each sampled section was obtained. Then, a mean value of all the sampled sections/animal was calculated, resulting in mean VGLUT2 and GAD67 density values per PVN subnuclei per animal (Higa-Taniguchi et al., 2007; Jansen et al., 2003). Mean VGLUT2 and GAD67 density values were then obtained for each experimental condition/group. For control purposes, similar measurements and analyses were obtained from a region ventrally located to the main PVN as well as from the suprachiasmatic nucleus (SCN).
Quantification of VGLUT2 and GAD67 immunoreactivities in identified OTergic, PVN-RVLM-projecting neurons
Double-labeled OT-PVN-RVLM neurons were identified by merging confocal images obtained from the FITC and rhoda-mine fluorophores. A positive colocalization was considered by the appearance of yellow (red + green) profiles in the merged image (see Fig. 1). Stacks of confocal images containing the three channels (FITC, rhodamine, and Cy5) and spanning the whole extension of double-labeled neurons (~50 sections, z-step 0.5 μm, image size 1,024 × 1,024 pixels, ×63 oil immersion lense, ×3 digital zoom) were then obtained. Only double-labeled neurons whose somata were fully contained within the z-stack were selected for quantification. In most cases, segments of proximal dendrites were also included, and comparisons between somatic and these proximal dendrites were performed. All neurons contained within the sampled sections fulfilling these criteria were used for quantification purposes. These image stacks were imported into Volocity (Improvision, Lexington, MA), and neurons, along with associated VGLUT2ir or GAD67ir boutons, were reconstructed in three dimensions. Because the retrograde tracer used to identify PVN-RVLM neurons results in a punctate staining, the OT signal, which provides a diffuse, homogenous staining of the neuron, and the VGLUT2 and/or GAD67 channels were classified to establish an intensity threshold for neuronal and bouton signals. Threshold was set to pass intensities five times above background immunofluorescence, as determined from the frequency distribution of voxel intensities calculated for each channel. The surface area of positive elements in the neuronal and bouton channels was measured independently. Volocity software algorithms allow colocalization analysis, discriminating between boutons that overlap with the neuron from those that do not. The density of overlapping boutons was calculated by dividing the number of overlapping boutons by the respective neuronal surface area, also provided by Volocity. To calculate and compare dendrites/somata immunoreactive ratios among groups, ratios were determined on the same neuronal profile and then averaged. These approaches have been recently validated in our laboratory (Higa-Taniguchi et al., 2007) to study changes in (nor)adrenergic synaptic innervation density under physiological and pathological conditions, as well as by others (see, e.g., Sykes and Condron, 2005; Wimmer et al., 2006). All quantitative analyses were carried out blindly. To enhance contrasts and reduce noise, all photographs shown were adjusted for contrast and brightness and frequently filtered with a Gaussian filter (Adobe Photoshop version 10.0.1). Final composition was performed in CorelDraw 12.
Statistical analysis
Results are expressed as mean ± SEM. Results were analyzed using a one-way or two-way ANOVA as indicated, followed by Bonferroni's post hoc tests. Differences were considered significant at P < 0.05. To assess differences in the incidence of OT and VP immunoreactivity in PVNRVLM neurons, χ2 tests were used.
RESULTS
Blood pressure measurements
Most of our studies involved sham and late-stage (6 weeks) RVH rats. However, as indicated below, a subset of experiments was also performed on early-stage (3 weeks) RVH rats. Systolic blood pressure values from sham, early-stage RVH, and late-stage RVH rats were 121 ± 6 mmHg, 152 ± 5 mmHg (P < 0.0004 vs. sham), and 208 ± 14 mmHg (P < 0.0001 vs. sham and P < 0.0020 vs. early-stage RVH), respectively. No differences in body weight were observed among experimental groups (results not shown).
Distribution of GABAergic and glutamatergic terminals in identified PVN subnuclei
To identify autonomically related and magnocellular neuroendocrine PVN subnuclei, we retrogradely labeled PVN neurons with rhodamine beads injected in the RVLM (as described in Materials and Methods) and immunohistochemically identified OT-and VP-containing neurons. As previously described (Armstrong et al., 1980; Stern, 2001; Swanson and Kuypers, 1980; Swanson and Sawchenko, 1983), PVN-RVLM neurons were found at two major rostrocaudal levels of the PVN: medial (~1.8 –2.1 mm caudal to bregma) and caudal (~2.1– 2.3 mm caudal to bregma). The PVN-RVLM neurons were concentrated within three major subnuclei: the dorsal cap (DC), the parvocellular ventromedial (VM) subnuclei (both located within the medial PVN), and the parvocellular posterior (PaPo) subnucleus (located in the caudal PVN). Magnocellular OT and VP neurons, on the other hand, were concentrated in the lateral magno-cellular subnucleus (LM; Fig. 1). In several instances, double-labeled OT-PVN-RVLM and VP-PVN-RVLM neurons were observed throughout the PVN regions sampled (Fig. 1). An initial colocalization analysis in control rats revealed a significantly higher predominance of OT over VP staining in PVN-RVLM neurons. Among a total of 686 PVN-RVLM sampled neurons, 139 (~20%) and 53 (~7%) were found to be OT-and VP-ir, respectively (P < 0.0001, χ2 test). Given the low incidence of VP colocalization with PVN-RVLM neurons, the remainder of the study was focused on OT-ir PVN-RVLM neurons. A more detailed analysis in this population indicated that the incidence of colocalization was similar between sham normotensive and late-stage RVH rats and did not vary between medial (DC and VM subnuclei) and caudal (PaPo subnucleus) aspects of the PVN: sham-caudal = ~22% colocalization (100/466), RVH-caudal =~21% colocalization (85/408), sham-medial =~18% colocalization (39/220), RVH-medial =~26% colocalization (96/372; P > 0.1, χ2).
GABAergic and glutamatergic terminals in the PVN were identified by their selective immunoreactivity to GAD67 and VGLUT2, respectively (Fremeau et al., 2001). In both cases and as shown in the representative examples in Figure 2, dense immunoreactivity for both markers was found in the PVN, which displayed a characteristic neuropile punctate staining. No somatic staining was observed in either case.
Figure 2.
Triple immunofluorescent staining for oxytocin (A; green), GAD67 (B; red), and VGLUT2 (C; white) in the caudal PVN. In D, all images are superimposed. In E, the region contained within the rectangular region in D is shown at higher magnification. Note the characteristic neuropile punctuate staining of GAD67 and VGLUT2. 3V, third ventricle. A blue-green copy of this figure is available as Supporting Information Figure 2. Scale bars == 50 μm in A; 50 μm in D (applies to B–D); 25 μm in E.
Changes in GAD67 and VGLUT2 immunoreactivities within identified PVN subnuclei in late-stage RVH rats
To determine whether the density of PVN GAD67 immunoreactivity changed in late-stage RVH rats and to assess whether this change was dependent on its topographical distribution within the PVN, we measured GAD67 immuno-reactivity within identified PVN subnuclei in sham and late-stage RVH rats (n = 6 in each group). Results are summarized in Figure 3. As shown, PVN GAD67 immunoreactivity was significantly increased in RVH rats (F = 34.7, P < 0.0001, two-way ANOVA), an effect that was observed in all the subnuclei sampled (P < 0.001 for DC, VM and PaPo subnuclei; P < 0.05 for LM; Bonferroni post hoc test). On the other hand, no differences in GAD67 immunoreactivity between sham and late-stage RVH rats were observed in a region ventrally located to the main PVN (sham 19.7% ± 4.0% density, RVH 23.8% ± 4.6% density; P > 0.5) or in the suprachiasmatic nucleus (sham 10.3% ± 3.2% density, RVH 14.3% ± 3.8% density; P > 0.5).
Figure 3.
GAD67 immunoreactivity in the PVN of normotensive sham and RVH rats. A: Representative confocal photomicro-graphs obtained at the medial level of PVN (1.8 –2.1 mm caudal to bregma) in sham (A1) and late-stage RVH (A2) rats, showing retrogradely labeled PVN-RVLM neurons (red) and GAD67 immunore-activity (white). For better comparisons, representative regions of A1 and A2 are displayed at higher magnification in A3 and A4, respectively. B: Representative confocal photomicrographs obtained at the caudal level of PVN (2.1–2.3 mm caudal to bregma) in sham (B1) and RVH (B2) rats, showing retrogradely labeled PVN-RVLM neurons (red) and GAD67 immunoreactivity (white). For better comparisons, representative regions of B1 and B2 are displayed at higher magnification in B3 and B4, respectively. C: Summary showing a significant increase in the relative GAD67 immunoreactivity density in RVH rats in all PVN subnuclei analyzed. 3V, third ventricle. *P < 0.05 vs. sham, n = 6 in each group. Scale bars = 50 μm in A (applies to A,B); 10 μm in insets.
A similar approach was used to measure changes in VGLUT2 immunoreactivity in sham and late-stage RVH rats (n = 6 in each group). Results are summarized in Figure 4. Similarly to the case for GAD67, we found PVN VGLUT2 immunoreactivity to be significantly increased in RVH rats (F = 15.6, P < 0.0001, two-way ANOVA). Although a trend toward an increased VGLUT2 immuno-reactivity was observed in the DC, VM, and PaPo subnucleus, differences reached statistical significance only in the latter (P < 0.01, Bonferroni post hoc test). Unlike the case for the PVN, a small though significantly diminished VGLUT2 immunoreactivity was found in a region located ventrally to the main PVN (sham 23.8% ± 1.6% density, RVH 18.8% ± 1.4% density; P > 0.05). No significant differences were observed, however, in the suprachiasmatic nucleus (sham 8.1% ± 2.3% density, RVH 7.2% ± 3.8% density; P > 0.5).
Figure 4.
VGLUT2 immunoreactivity in the PVN of normotensive sham and RVH rats. A: *P < 0.05 vs. sham, n = 6 in each group. Representative confocal photomicrographs obtained at the medial level of PVN (1.8–2.1 mm caudal to bregma) in sham (A1) and RVH (A2) rats, showing retrogradely labeled PVN-RVLM neurons (red) and VGLUT2 immunoreactivity (white). For better comparisons, representative regions of A1 and A2 are displayed at higher magnification in A3 and A4, respectively. B: Representative confocal photomicro-graphs obtained at the caudal level of PVN (2.1–2.3 mm caudal to bregma) in sham (B1) and RVH (B2) rats, showing retrogradely labeled PVN-RVLM neurons (red) and VGLUT2 immunoreactivity (white). For better comparisons, representative regions of B1 and B2 are displayed at higher magnification in B3 and B4, respectively. C: Summary showing a significant increase in the relative VGLUT2 immunoreactivity density in the PaPo subnucleus. 3V, third ventricle. *P < 0.05 vs. sham, n = 6 in each group. Scale bars = 50 μm in A (applies to A,B); 10 μm in insets.
Measurements of GABAergic and glutamatergic boutons in close apposition to identified OT-positive PVN-RVLM neurons
Given the high neurochemical and functional cellular heterogeneity characteristic of the PVN, specific neuronal populations could be differentially affected despite an apparent global change in the density of immunore-active terminals in RVH rats. Thus, to determine whether changes in GABAergic and glutamatergic PVN immunoreactive terminals were associated with OT-positive PVN-RVLM projecting neurons, the densities of GAD67-and VGLUT2-ir boutons in close apposition to these neurons were calculated. Representative examples of 3D-reconstructed, retrogradely labeled PVNRVLM neurons and associated GAD67 and VGLUT2 immunoreactive boutons are shown in Figure 5. Despite the changes reported above in general GAD67 immuno-reactivity in middle regions of the PVN, containing the VM and DC subnuclei, the density of GAD67- and VGLUT2-ir boutons in close apposition with OT-PVNRVLM neurons in the VM and DC subnuclei did not change in late-stage RVH rats (GAD67 density: sham 0.012 ± 0.003 counts/μm2, RVH 0.018 ± 0.004 counts/μm2; P > 0.1, n = 7; VGLUT2: sham 0.021 ± 0.003 counts/μm2, RVH 0.017 ± 0.005 counts/μm2; P > 0.3, n = 14). On the other hand, and as summarized below, significant changes in the densities and distributions of these inputs were observed in neurons located in the most caudal aspects of the PVN. Thus, the reminder of our work is focused on OT-PVN-RVLM neurons located in the PaPo subnucleus. A detailed analysis of the distribution of GAD67-and VGLUT2-ir boutons apposing somatic and proximal dendritic compartments of these neurons in sham and two developmental stages of RVH [i.e., early (3 weeks) and late (6 weeks)] is summarized below. Because no differences in any of the parameters evaluated were observed between the sham groups at the two time periods (not shown), data from these control groups were pooled together for further analysis.
Figure 5.
Three-dimensional rendered images of OT-positive, retrogradely labeled PVN-RVLM neurons and associated GAD67-and VGLUT2-ir boutons. A: Representative example of an OT-ir (A1, green), PVN-RVLM retrogradely labeled (rhodamine beads, white, A2) neuron located in the PaPo subnucleus. In A3, the two images were superimposed. B: Example of a PVN-RVLM retrogradely labeled, OT-positive neuron located in the PaPo subnucleus and associated GAD67-ir boutons, shown in two different spatial orientations. For better display, only the OT staining (green) is shown. Boutons in red and white colors represent those overlapping and those that are close to, but not overlapping, the OT-positive PVN-RVLM neuron, respectively. C: Example of a different PVN-RVLM retrogradely labeled, OT-positive neuron located in the PaPo subnucleus and associated VGLUT2-ir boutons, shown in two different spatial orientations. For better display, only the OT staining is shown. Boutons in red and white colors represent those overlapping and those that are close to, but not overlapping, the OT-positive PVN-RVLM neuron, respectively. Insets show representative overlapping GAD67 and VGLUT2 boutons, respectively (white boxes), at a higher magnification. A magenta-green copy of this figure is available as Supporting Information Figure 3. Scale bars = 10 μm in A; 10 μm in B (applies to B,C); 1.5 μm in insets.
Changes in the density of GAD67-ir boutons overlapping OT-PVN-RVLM neurons in the PaPo subnucleus
The surface area of reconstructed neurons was calculated, and results are summarized in Table 1. No differences in overall neuronal surface area were observed among groups (F = 0.4, P > 0.1, one-way ANOVA). Similarly, no differences in somatic or proximal dendritic surface areas were observed among groups (F = 1.7 and 0.6, respectively, P > 0.2, one-way ANOVA). Finally, no correlations between neuronal surface area and density of GAD67-or VGLUT2-ir boutons were observed (R2 = 0.13 and 0.33, respectively).
TABLE 1.
Whole-Cell and Somatic and Proximal Dendritic Surface Area of PVN-RVLM Neurons in Sham and Early-Stage and Late-Stage RVH Rats1
| Sham (n = 37; μm2) | Early-stage RV (n = 13; μm2) | Late-stage RV (n = 26; μm2) | |
|---|---|---|---|
| Whole cell | 7,733 ± 546.9 | 7,624 ± 528.8 | 6,983 ± 724.4 |
| Soma | 5,819 ± 497.5 | 6,964 ± 626.3 | 4,797 ± 630.8 |
| Dendrites | 1,652 ± 182.8 | 1,270 ± 286.3 | 2,024 ± 496.5 |
Values are means ± SE for n neurons. No significant differences were observed among groups (F = 0.4, P > 0.1, one-way ANOVA), and no differences in somatic or proximal dendritic surface were observed (F = 1.7 and 0.6, respectively, P > 0.2, one-way ANOVA).
The overall density of GAD67-ir boutons overlapping OT-PVN-RVLM neurons increased significantly in neurons from RVH compared with those of sham rats (F = 19.1, respectively, P < 0.0001, one-way ANOVA) and were observed both at early and late stages of hypertension (P < 0.0001 in both cases, Bonferroni post hoc test; Fig. 6A).
Figure 6.
Summary data showing differences in GAD67-ir bouton densities in OT-positive, retrogradely labeled PVN-RVLM neurons in the PaPo subnucleus from sham rats (n = 18 neurons) and early-stage (e-RVH, n = 6 neurons) and late-stage (l-RVH, n = 13 neurons) hypertensive rats. Differences were quantified at the whole-cell level (A1) and somatic (A2) and proximal dendritic (A3) compartments. *P < 0.05, **P < 0.0001, Bonferroni post hoc test.
Differential changes in somatic and proximal dendritic compartments were observed. In OT-PVN-RVLM cell bodies, the density of GAD67-ir boutons increased significantly in RVH rats (F = 18.5, P < 0.0001, one-way ANOVA), both at early and at late stages (see Fig. 6B). On the other hand, no significant differences were observed in the density of GAD67 immunoreactivity in proximal dendrites (F = 3.1, P > 0.05, one-way ANOVA).
To examine further the differences and changes in GAD67 innervation between cell bodies and proximal dendrites, the relative density of GAD67 immunoreactivity between these two neuronal compartments was calculated in each group. In all groups, GAD67-ir bouton density was more prominent in proximal dendrites vs. cell bodies (i.e., dendritic/somatic ratio > 1; see Fig. 8A). Even though no significant differences were observed among groups (F = 2.3, P > 0.1, one-way ANOVA), a tendency for a diminished dendritic/somatic ratio was observed in RVH rats.
Figure 8.
Summary data showing the proximal dendritic/somatic relative GAD67 (A) and VGLUT2 (B) immunoreactivity densities in OT-positive, retrogradely labeled PVN-RVLM neurons in the PaPo subnucleus from sham (n = 18 and 19 for GAD67 and VGLUT2, respectively) and early-stage (e-RVH, n = 6 and 7 for GAD67 and VGLUT2, respectively) and late-stage (l-RVH, n = 13 for both GAD67 and VGLUT2) hypertensive rats. Note that, in all groups, GAD67-and VGLUT2-ir bouton densities were more prominent in proximal dendrites vs. cell bodies (i.e., proximal dendritic/somatic ratio > 1), although a tendency for a diminished dendritic/somatic GAD67 ratio was observed in RVH rats.
Changes in the density of VGLUT2-ir boutons overlapping OT-PVN-RVLM neurons in the PaPo subnucleus
The overall density of VGLUT2-ir boutons overlapping OT-PVN-RVLM neurons increased significantly in neurons from RVH rats compared with those from sham rats (F = 9.7, P < 0.0001, one-way ANOVA), and changes were observed both at early and at late RVH stages (P < 0.1, Bonferroni post hoc test; see Fig. 7A).
Figure 7.
Summary data showing differences in VGLUT2-ir bouton densities in OT-positive, retrogradely labeled PVN-RVLM neurons in the PaPo subnucleus from sham rats (n = 19 neurons) and early-stage (e-RVH, n = 7 neurons) and late-stage (l-RVH, n = 13 neurons) hypertensive rats. Differences were quantified at the whole-cell level (A1) and somatic (A2) and proximal dendritic (A3) compartments. *P < 0.05, **P < 0.0001, Bonferroni post hoc test.
Similarly to GAD67-ir boutons, somatic and dendritic compartments were differentially affected. The density of VGLUT2-immunoreactive boutons in OT-PVN-RVLM cell bodies increased significantly in RVH rats (F = 16.7, P < 0.0001, one-way ANOVA), both at early and at late stages (see Fig. 7B). In contrast to GAD67 immunoreactivity, VGLUT2-ir bouton dendritic density also increased significantly in RVH rats (F = 4.5, P < 0.05, one-way ANOVA), both at early and at late stages (see Fig. 7C).
As in the case of GAD67 immunoreactivity, VGLUT2-ir bouton density in PVN-RVLM neurons was more prominent in proximal dendrites over cell bodies (i.e., dendritic/ somatic ratio > 1; Fig. 8B), and no significant differences were observed among groups (F = 0.3, P > 0.5, one-way ANOVA).
Shift in the balance of GAD67/VGLUT2 innervation of proximal dendrites in OT-PVN-RVLM neurons in the PaPo subnucleus in hypertensive rats
An imbalance in glutamatergic/GABAergic functions has been shown to contribute to numerous neurological disorders (Cline, 2005). Therefore, we also sought to determine whether the balance of VGLUT2/GAD67 inputs at somatic and proximal dendritic compartments was changed in RVH rats. For these comparisons, we replotted the data presented in Figures 6 and 7 into Figure 9, to compare better the relative predominance of these two inputs in each experimental group. The density of VGLUT2 immunoreactivity in OT-PVN-RVLM somata from sham rats was more prominent than that of GAD67 immunoreactivity (P < 0.01, mean VGLUT2/mean GAD67 immunoreactivity ratio = 1.91). On the other hand, similar densities of these two types of inputs were observed in proximal dendrites (P > 0.5). A similar pattern was observed in OT-PVN-RVLM neurons from early-stage RVH rats: the density of VGLUT2 immunoreactivity in cell bodies was significantly more prominent than the density of GAD67 immunoreactivity (P < 0.05, mean VGLUT2/mean GAD67 immunoreactivity ratio = 1.85), whereas no differences were observed in proximal dendrites (P > 0.5). Finally, in OT-PVN-RVLM neurons of late-stage RVH rats, the somatic predominance of VGLUT2 over GAD67 was no longer present (P > 0.5), whereas the density of VGLUT2 immunoreactivity became more prominent than GAD67 immunoreactivity in proximal dendritic compartments (P < 0.05, mean VGLUT2/mean GAD67 ratio = 1.90).
Figure 9.
Summary data comparing VGLUT2-and GAD67-ir boutons in somatic (A) and proximal dendritic (B) compartments in OT-positive, retrogradely labeled PVN-RVLM neurons in the PaPo subnucleus from sham (n = 18 and 19 for GAD67 and VGLUT2, respectively) and early-stage (e-RVH, n = 6 and 7 for GAD67 and VGLUT2, respectively) and late-stage (l-RVH, n = 13 for both GAD67 and VGLUT2) hypertensive rats. *P < 0.05, **P < 0.01 compared with GAD67 within the same experimental group, Bonferroni post hoc test.
DISCUSSION
The PVN-RVLM pathway is a major neuronal substrate underlying the hypothalamic control of autonomic outflow to the cardiovascular system (Allen, 2002; Ciriello et al., 1985; Coote et al., 1998; Dampney et al., 1987; Hardy, 2001; Kubo et al., 2000; Tagawa and Dampney, 1999; Yang et al., 2001; Yang and Coote, 1998). Although PVN neurons giving rise to this pathway (as well as other medullary targets) are neurochemically heterogeneous, both anatomical and functional studies support OT as the predominant neuropeptide expressed in PVN neurons inner-vating the ventrolateral medulla (Gomez et al., 1993; Hancock and Nicholas, 1987; Lang et al., 1983; Mack et al., 2002; Nilaver et al., 1980; Sawchenko and Swanson, 1982; Stocker et al., 2006). In this study, we found that ~25% of the retrogradely labeled PVN-RVLM neurons expressed OT. Given recent studies supporting the involvement of PVN-RVLM neurons in elevated sympathoexcitation in hypertensive conditions (Allen, 2002), our goal was to determine whether a reorganization of afferent GABAergic and glutamatergic synaptic inputs in OT-PVN-RVLM neurons constitutes a potential mechanism contributing to the imbalance of inhibitory/excitatory functions known to occur in hypertensive conditions, including renovascular hypertension (Allen, 2002; Bergamaschi et al., 1995; Haywood et al., 2001; Horn et al., 1998; Ito et al., 2000; Li and Pan, 2006, 2007a,b; Li et al., 2008; Martin and Haywood, 1998; Sonner et al., 2008). The main results of this study can be summarized as follows: 1) GABAergic and glutamatergic innervation densities increased in RVH rats in various PVN subnuclei, most prominently in caudal aspects of the PVN; 2) changes in excitatory and inhibitory afferent input densities differentially affected neuronal somatic and proximal dendritic compartments of identified OT-positive PVN-RVLM neurons located in caudal aspects of the PVN, whereas an increase in glutamatergic innervation density was observed both in somata and in proximal dendrites, and GABAergic afferent inputs were selectively increased in somatic compartments; and 3) remodeling of GABAergic and glutamatergic afferent inputs was observed both at early and at late hypertensive stages.
Remodeling of GABAergic and glutamatergic afferent inputs during hypertension
In this study, we used two complementary approaches to determine whether changes in the distribution and density of GABAergic and glutamatergic inputs occurred in the PVN of hypertensive rats. First, GAD67 and VGLUT2 immunoreactivities were quantified within specific PVN subnuclei at two rostrocaudal levels of the PVN. Our results support an overall increase of both GABAergic and glutamatergic immunoreactivities in the PVN of hypertensive rats. A more detailed analysis, however, indicated that, whereas VGLUT2 immunoreactivity was significantly increased only in the most caudal aspects of the PVN (PaPo subnucleus), GAD67 immunoreactivity increased both at the medial and at the caudal PVN levels. Taken together, these results could then suggest an increased predominance of inhibitory inputs in the medial aspects of the PVN, whereas a balanced excitatory/inhibitory inner-vation state is maintained in the caudal regions of the PVN of hypertensive rats. Care should be taken, however, when drawing such conclusions, given the high degree of cellular heterogeneity within the PVN. Thus, to circumvent this potential problem, we performed a quantitative analysis of GAD67-and VGLUT2-ir boutons in close apposition with somata and proximal dendrites of identified OT-PVN-RVLM projecting neurons at two different stages during the development of the hypertensive condition. Our results show that, despite concomitant increases in both GABAergic and glutamatergic inputs in OT-PVN-RVLM neurons located in the PaPo, their differential and compartment-specific reorganization resulted in a time-dependent, altered balance of excitatory/inhibitory inputs. Thus, in sham and early-stage RVH rats, a predominance of glutamatergic over GABAergic inputs was found in OT-PVNRVLM somata, whereas equal densities were observed in proximal dendrites. Conversely, at late hypertensive stages, the glutamatergic innervation predominance shifted from the somata to proximal dendrites. Similarly, concomitant changes in both excitatory and inhibitory synapses, as well as compartment-specific changes in the balance between excitatory and inhibitory inputs, were previously described in cortical and hippocampal neurons (Cossart et al., 2001; Knott et al., 2002). Interestingly, despite the overall increase in GAD67 immunoreactivity found in PVN subnuclei located in medial regions of the PVN, we found no changes in the density of GAD67-ir (and VGLUT2-ir) boutons in close apposition with OT-PVN-RVLM neurons in the VM and DC subnuclei in RVH rats. Whether other PVN neuronal populations located in these subnuclei reflected changes in GAD67 apposing boutons remains to be determined. The significance of the subnucleus-dependent reorganization of afferent inputs to OT-PVNRVLM neurons during hypertension is at present unknown. Nonetheless, our results underscore the importance of performing such studies within specifically identified neuronal populations, particularly in a highly heterogeneous nucleus such as the PVN.
Multiple mechanisms could account for changes in synaptic function and/or efficacy, including altered postsynaptic (e.g., changes in numbers and/or types of postsynaptic receptors) and presynaptic (e.g, changes in neurotransmitter release probability) mechanisms, as well as changes in the number and/or distribution of synaptic contacts (Conti et al., 1994; Mody et al., 1994). Results from the present study, along with those from previous studies, support the view that multiple mechanisms acting in concert, including changes in neurotransmitter release probability, postsynaptic receptor densities (Li and Pan, 2006, 2007b), and structural remodeling of afferent inputs (this study), contribute to altered PVN inhibitory GABAergic and excitatory glutamatergic synaptic functions in hypertensive disorders (Allen, 2002; Haywood et al., 2001; Horn et al., 1998; Li and Pan, 2006, 2007a,b; Li et al., 2006, 2008a,b). Thus, in addition to contributing to network adaptation and optimization in response to physiological challenges such as lactation (Hatton, 1997; Theodosis and Poulain, 2001) and changes in circulating levels of estrogen, leptin, and ghrelin (Neal-Perry et al., 2008; Pinto et al., 2004) synaptic remodeling in hypothalamic circuits could also constitute an underlying pathophysio-logical mechanism, as previously described for other regions of the CNS (Dudek and Staley, 2007; Rubenstein and Merzenich, 2003; Wassef et al., 2003).
Potential mechanisms underlying afferent input remodeling during hypertension
A dynamic reorganization of afferent inputs in the PVN during hypertension could be mediated by a variety of potential underlying mechanisms. A common mechanism that operates within hypothalamic networks in response to physiological challenges involves the active removal of neuronal-glial wrapping processes, allowing new synaptic contacts to be formed on neuronal surfaces free of astrocytic coverage (Tweedle and Hatton, 1984). A well-established example is the activity-dependent removal of astrocytic coverage of OT magnocellular neurosecretory neurons in the supraoptic nucleus of lactating rats, resulting in increased GABAergic, glutamatergic, and noradrenergic innervation densities (Hatton, 2004; Theodosis et al., 2006). A similar relationship between concomitant changes in the degree of astrocytic coverage and density of synaptic inputs has been described in the arcuate nucleus during fluctuations in sex steroid levels (Garcia-Segura et al., 1994a,b). Although astrocytic structural and functional changes have been reported in different animal models of hypertension (Ishida et al., 2006; Pietranera et al., 2006; Tomassoni et al., 2004; Yamagata et al., 2006, 2008), a mechanistic relationship between synaptic and glial remodeling in the PVN of hypertensive rats remains to be established.
Activation of angiotensin II (AngII) receptors in brain astrocytes was shown to induce changes in intracellular Ca2+ levels (Gebke et al., 1998; Wang et al., 1996) and to regulate astrocyte growth and proliferation (Clark and Gonzalez, 2007; Clark et al., 2008). Thus, given the well-established role of AngII in the pathophysiology of reno-vascular hypertension and sympathoexcitation (Fink, 1997), future studies are warranted to evaluate thoroughly the role of AngII-mediated mechanisms on the altered excitatory/inhibitory afferent balance described in these studies.
Functional implications of excitatory/ inhibitory afferent remodeling during hypertension
Neuronal firing activity is strongly influenced by an interplay between excitatory and inhibitory inputs, and their balanced organization and activity are necessary for proper neuronal network functionality. The functional consequences of the excitatory/inhibitory afferent remodeling reported here in OT-PVN-RVLM neurons during hyper-tension are at present unknown. Nonetheless, there are some important aspects that should be discussed.
First, an increased density of both excitatory and inhibitory terminals in PVN-RVLM neurons during hypertension could be a mechanism to compensate for an increased neuronal size, as previously observed in hypothalamic magnocellular neurosecretory neurons during lactation (El Majdoubi et al., 1997; Theodosis and Poulain, 2001). The lack of significant changes in PVN-RVLM neuronal surface area during hypertension and the lack of correlation between densities of overlapping boutons and neuronal surface area, however, argue against this possibility.
Second, somatic and dendritic synaptic inputs serve different functional roles. Whereas somatic-projecting inputs influence the generation of Na+ spikes and thus neuronal output, dendritic-projecting inputs, in particular the balance of excitatory and inhibitory postsynaptic potentials (EPSPs and IPSPs, respectively), influence the availability and activation of dendritic low-threshold voltage-gated Ca2+ currents and somatodendritic propagation of Ca2+ and Na+ spikes (Egger et al., 2005; Llinas and Nicholson, 1971; Magee and Johnston, 1995; Miles et al., 1996; Spruston et al., 1995). We have recently shown that low-threshold Ca2+ channels, located predominantly in dendritic compartments, play a major role in controlling PVNRVLM membrane excitability (Li et al., 2008; Sonner and Stern, 2007; Stern, 2001), in part by influencing somatodendritic propagation of Ca2+ signals, which we found to be exacerbated in RVH rats (Sonner et al., 2007, 2008). Thus, it is possible that a shift in the innervation pattern observed in hypertensive rats, leading to a net predominance of excitatory over inhibitory inputs in proximal dendrites of PVN-RVLM neurons, could facilitate the generation and propagation of larger dendritic EPSPs and consequently a more efficient activation of dendritic low-threshold Ca2+ channels.
Finally, it is also important to consider that even a balanced, concomitant increase in excitatory and inhibitory synaptic inputs could result in altered postsynaptic neuronal function. This has been shown in magnocellular neurosecretory neurons, in which a balanced increase in excitatory and inhibitory inputs resulted in an elevated firing rate and linearization of their input/output function (Leng et al., 2001). This could be due in part to an overall increase in background synaptic noise, recently shown to strongly influence the firing discharge probability and firing pattern in these neurons (Li et al., 2007).
We acknowledge that, despite the fact that several of the hypertensive-related changes in PVN GAD67 and VGLUT2 immunoreactivities reported here were observed at early stages of this condition, our experimental approach precluded us from determining whether the structural remodeling of GABAergic and glutamatergic inputs is causative or reactive to the elevated blood pressure in this animal model. Future studies addressing the time course relationship between the development of hypertension and the immunoreactive changes reported herein are warranted to address this issue precisely, as well as the functional consequences of the afferent synaptic remodeling in PVN neurons of hypertensive rats.
Methodological considerations and limitations
To identify PVN-RVLM neurones, rhodamine-labeled fluorescent latex microspheres were injected into the RVLM. This retrograde tracer results in highly restricted and well-defined injection sites (Katz et al., 1984) and is commonly used to trace CNS pathways, including PVN projections to brainstem nuclei (Cato and Toney, 2005; Cham et al., 2006; Li and Pan, 2005, 2006; Zahner et al., 2007). Although this tracer is not taken up by fibers in passage (Katz et al., 1984; Katz and Iarovici, 1990; Krug et al., 1998; Sasek and Helke, 1989), we cannot rule out potential labeling of severed axons in the area of the injection. Similarly, whereas for the most part the tracer was localized within the RVLM, we cannot rule potential leakage of tracer into the caudal ventrolateral medulla (CVLM) or the nucleus ambiguous. This is, however, unlikely, in part because injections that were misplaced rostrally, caudally, or laterally to the RVLM [areas containing PVN descending axons running toward the dorsal brainstem or the spinal cord (Luiten et al., 1985)] failed to label neurons in the PVN retrogradely.
Confocal microscopy and 3D reconstructions were used to quantify immunoreactive boutons overlapping target-specific, sympathetic-related PVN neurons. We have recently used a similar approach to assess exercise-induced reorganization of (nor)adrenergic afferent inputs in the PVN (Higa-Taniguchi et al., 2007). Moreover, a similar approach was recently validated as an efficient tool to assess the plasticity of neurotransmitter innervation in identified neurons after prolonged stimulation (Mueller et al., 2005). Thus, although our approach likely reflects reorganization of putative synaptic contacts in PVN-RVLM neurons, electron microscopy studies will be needed to verify more conclusively the synaptic nature of the overlapping boutons quantified in our studies.
Another caveat of our studies is that labeled dendrites could be followed only within the thickness of a given section and, in many instances, were obscured by labeled neighboring neurons or dendrites. As a result, quantification was limited to somata and proximal dendrites (see Materials and Methods). Thus, whether changes similar to those reported in proximal dendrites also occur in more distal dendritic segments is at present unknown.
Finally, based on the evidence supporting a major contribution of the PVN-RVLM pathway to enhanced sympathoexcitation during hypertension (Allen, 2002) and OT, one of the most predominant neurochemical phenotypes of this neuronal population, our studies were focused on OT-PVN-RVLM neurons. It is well-established, however, that other neurochemically and target diverse descending pathways are involved in mediating PVN influences on autonomic and cardiovascular control. This includes a direct PVN projection to preganglionic sympathetic neurons in the spinal cord. In fact, a small proportion of presympathetic PVN neurons has been reported to project to both the RVLM and the spinal cord (Pyner and Coote, 2000; Shafton et al., 1998), a subpopulation that could have been included within the sampled OT-PVN-RVLM group in our study. Whether the changes reported in our studies are cell specific or, conversely, whether they affect other PVN neuronal populations (e.g., PVN-dorsal vagal complex projecting, magnocellular neuroendocrine) remains to be determined.
Supplementary Material
Acknowledgments
Grant sponsor: National Institutes of Health; Grant number: R01 HL68725 (to J.E.S); Grant sponsor: CAPES; Grant number: BEX0325/ 03-1.
Footnotes
Additional Supporting Information may be found in the online version of this article.
LITERATURE CITED
- Aguado F, Carmona MA, Pozas E, Aguilo A, Martinez-Guijarro FJ, Alcantara S, Borrell V, Yuste R, Ibanez CF, Soriano E. BDNF regulates spontaneous correlated activity at early developmental stages by increasing synaptogenesis and expression of the K+/Cl− co-transporter KCC2. Development. 2003;130:1267–1280. doi: 10.1242/dev.00351. [DOI] [PubMed] [Google Scholar]
- Al-Noori S, Sanders NM, Taborsky GJ, Jr, Wilkinson CW, Zavosh A, West C, Sanders CM, Figlewicz DP. Recurrent hypoglycemia alters hypothalamic expression of the regulatory proteins FosB and synaptophysin. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1446–1454. doi: 10.1152/ajpregu.90511.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen AM. Inhibition of the hypothalamic paraventricular nucleus in spontaneously hypertensive rats dramatically reduces sympathetic vasomotor tone. Hypertension. 2002;39:275–280. doi: 10.1161/hy0202.104272. [DOI] [PubMed] [Google Scholar]
- Alvarez FJ, Villalba RM, Zerda R, Schneider SP. Vesicular glutamate transporters in the spinal cord, with special reference to sensory primary afferent synapses. J Comp Neurol. 2004;472:257–280. doi: 10.1002/cne.20012. [DOI] [PubMed] [Google Scholar]
- Armstrong WE, Warach S, Hatton GI, McNeill TH. Subnuclei in the rat hypothalamic paraventricular nucleus: a cytoarchitectural, horseradish peroxidase and immunocytochemical analysis. Neuroscience. 1980;5:1931–1958. doi: 10.1016/0306-4522(80)90040-8. [DOI] [PubMed] [Google Scholar]
- Ben-Barak Y, Russell JT, Whitnall MH, Ozato K, Gainer H. Neurophysin in the hypothalamo-neurohypophysial system. I. Production and characterization of monoclonal antibodies. J Neurosci. 1985;5:81–97. doi: 10.1523/JNEUROSCI.05-01-00081.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benagiano V, Lorusso L, Coluccia A, Tarullo A, Flace P, Girolamo F, Bosco L, Cagiano R, Ambrosi G. Glutamic acid decarboxylase and GABA immunoreactivities in the cerebellar cortex of adult rat after prenatal exposure to a low concentration of carbon monoxide. Neuroscience. 2005;135:897–905. doi: 10.1016/j.neuroscience.2005.06.058. [DOI] [PubMed] [Google Scholar]
- Bergamaschi C, Campos RR, Schor N, Lopes OU. Role of the rostral ventrolateral medulla in maintenance of blood pressure in rats with Goldblatt hypertension. Hypertension. 1995;26:1117–1120. doi: 10.1161/01.hyp.26.6.1117. [DOI] [PubMed] [Google Scholar]
- Boudaba C, Schrader LA, Tasker JG. Physiological evidence for local excitatory synaptic circuits in the rat hypothalamus. J Neurophysiol. 1997;77:3396–3400. doi: 10.1152/jn.1997.77.6.3396. [DOI] [PubMed] [Google Scholar]
- Carretero OA, Gulati OP. Effects of angiotensen antagonist in rats with acute, subacute, and chronic two-kidney renal hypertension. J Lab Clin Med. 1978;91:264–271. [PubMed] [Google Scholar]
- Castelli MP, Piras AP, Melis T, Succu S, Sanna F, Melis MR, Collu S, Grazia Ennas M, Diaz G, Mackie K, Argiolas A. Cannabinoid CB1 receptors in the paraventricular nucleus and central control of penile erection: immunocytochemistry, autoradiography and behavioral studies. Neuroscience. 2007;147:197–206. doi: 10.1016/j.neuroscience.2007.02.062. [DOI] [PubMed] [Google Scholar]
- Cato MJ, Toney GM. Angiotensin II excites paraventricular nucleus neurons that innervate the rostral ventrolateral medulla: an in vitro patch-clamp study in brain slices. J Neurophysiol. 2005;93:403–413. doi: 10.1152/jn.01055.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cham JL, Owens NC, Barden JA, Lawrence AJ, Badoer E. P2X purinoceptor subtypes on paraventricular nucleus neurones projecting to the rostral ventrolateral medulla in the rat. Exp Physiol. 2006;91:403–411. doi: 10.1113/expphysiol.2005.032409. [DOI] [PubMed] [Google Scholar]
- Chen QH, Haywood JR, Toney GM. Sympathoexcitation by PVN-injected bicuculline requires activation of excitatory amino acid receptors. Hypertension. 2003;42:725–731. doi: 10.1161/01.HYP.0000085197.20043.44. [DOI] [PubMed] [Google Scholar]
- Ciriello J, Kline RL, Zhang TX, Caverson MM. Lesions of the paraventricular nucleus alter the development of spontaneous hypertension in the rat. Brain Res. 1984;310:355–359. doi: 10.1016/0006-8993(84)90159-8. [DOI] [PubMed] [Google Scholar]
- Ciriello J, Caverson MM, Calaresu FR. Lateral hypothalamic and peripheral cardiovascular afferent inputs to ventrolateral medullary neurons. Brain Res. 1985;347:173–176. doi: 10.1016/0006-8993(85)90908-4. [DOI] [PubMed] [Google Scholar]
- Clark MA, Gonzalez N. Angiotensin II stimulates rat astrocyte mitogen-activated protein kinase activity and growth through EGF and PDGF receptor transactivation. Regul Pept. 2007;144:115–122. doi: 10.1016/j.regpep.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Clark MA, Guillaume G, Pierre-Louis HC. Angiotensin II induces proliferation of cultured rat astrocytes through c-Jun N-terminal kinase. Brain Res Bull. 2008;75:101–106. doi: 10.1016/j.brainresbull.2007.07.028. [DOI] [PubMed] [Google Scholar]
- Cline H. Synaptogenesis: a balancing act between excitation and inhibition. Curr Biol. 2005;15:R203–R205. doi: 10.1016/j.cub.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Conti F, Minelli A, Brecha NC. Cellular localization and laminar distribution of AMPA glutamate receptor subunits mRNAs and proteins in the rat cerebral cortex. J Comp Neurol. 1994;350:241–259. doi: 10.1002/cne.903500208. [DOI] [PubMed] [Google Scholar]
- Coote JH, Yang Z, Pyner S, Deering J. Control of sympathetic outflows by the hypothalamic paraventricular nucleus. Clin Exp Pharmacol Physiol. 1998;25:461–463. doi: 10.1111/j.1440-1681.1998.tb02235.x. [DOI] [PubMed] [Google Scholar]
- Cossart R, Dinocourt C, Hirsch JC, Merchan-Perez A, De Felipe J, Ben-Ari Y, Esclapez M, Bernard C. Dendritic but not somatic GABAergic inhibition is decreased in experimental epilepsy. Nat Neurosci. 2001;4:52–62. doi: 10.1038/82900. [DOI] [PubMed] [Google Scholar]
- Dampney RA, Czachurski J, Dembowsky K, Goodchild AK, Seller H. Afferent connections and spinal projections of the pressor region in the rostral ventrolateral medulla of the cat. J Auton Nerv Syst. 1987;20:73–86. doi: 10.1016/0165-1838(87)90083-x. [DOI] [PubMed] [Google Scholar]
- Dampney RA, Horiuchi J, Killinger S, Sheriff MJ, Tan PS, McDowall LM. Long-term regulation of arterial blood pressure by hypothalamic nuclei: some critical questions. Clin Exp Pharmacol Physiol. 2005;32:419–425. doi: 10.1111/j.1440-1681.2005.04205.x. [DOI] [PubMed] [Google Scholar]
- Decavel C, Van den Pol AN. GABA: a dominant neurotransmitter in the hypothalamus. J Comp Neurol. 1990;302:1019–1037. doi: 10.1002/cne.903020423. [DOI] [PubMed] [Google Scholar]
- Decavel C, van den Pol AN. Converging GABA-and glutamate-immunoreactive axons make synaptic contact with identified hypothalamic neurosecretory neurons. J Comp Neurol. 1992;316:104–116. doi: 10.1002/cne.903160109. [DOI] [PubMed] [Google Scholar]
- Dudek FE, Staley KJ. How does the balance of excitation and inhibition shift during epileptogenesis? Epilepsy Curr. 2007;7:86–88. doi: 10.1111/j.1535-7511.2007.00181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earle ML, Pittman QJ. Involvement of the PVN and BST in 1K1C hypertension in the rat. Brain Res. 1995;669:41–47. doi: 10.1016/0006-8993(94)01222-4. [DOI] [PubMed] [Google Scholar]
- Egger V, Svoboda K, Mainen ZF. Dendrodendritic synaptic signals in olfactory bulb granule cells: local spine boost and global low-threshold spike. J Neurosci. 2005;25:3521–3530. doi: 10.1523/JNEUROSCI.4746-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Majdoubi M, Poulain DA, Theodosis DT. Lactation-induced plasticity in the supraoptic nucleus augments axodendritic and axosomatic GABAergic and glutamatergic synapses: an ultrastructural analysis using the disector method. Neuroscience. 1997;80:1137–1147. doi: 10.1016/s0306-4522(97)00193-0. [DOI] [PubMed] [Google Scholar]
- Fink GD. Long-term sympatho-excitatory effect of angiotensin II: a mechanism of spontaneous and renovascular hypertension. Clin Exp Pharmacol Physiol. 1997;24:91–95. doi: 10.1111/j.1440-1681.1997.tb01789.x. [DOI] [PubMed] [Google Scholar]
- Fremeau RT, Jr, Troyer MD, Pahner I, Nygaard GO, Tran CH, Reimer RJ, Bellocchio EE, Fortin D, Storm-Mathisen J, Edwards RH. The expression of vesicular glutamate transporters defines two classes of excitatory synapse. Neuron. 2001;31:247–260. doi: 10.1016/s0896-6273(01)00344-0. [DOI] [PubMed] [Google Scholar]
- Fujiyama F, Furuta T, Kaneko T. Immunocytochemical localization of candidates for vesicular glutamate transporters in the rat cerebral cortex. J Comp Neurol. 2001;435:379–387. doi: 10.1002/cne.1037. [DOI] [PubMed] [Google Scholar]
- Garcia-Segura LM, Chowen JA, Parducz A, Naftolin F. Gonadal hormones as promoters of structural synaptic plasticity: cellular mechanisms. Prog Neurobiol. 1994a;44:279–307. doi: 10.1016/0301-0082(94)90042-6. [DOI] [PubMed] [Google Scholar]
- Garcia-Segura LM, Luquin S, Parducz A, Naftolin F. Gonadal hormone regulation of glial fibrillary acidic protein immunoreactivity and glial ultrastructure in the rat neuroendocrine hypothalamus. Glia. 1994b;10:59–69. doi: 10.1002/glia.440100108. [DOI] [PubMed] [Google Scholar]
- Gebke E, Muller AR, Jurzak M, Gerstberger R. Angiotensin II-induced calcium signalling in neurons and astrocytes of rat circumventricular organs. Neuroscience. 1998;85:509–520. doi: 10.1016/s0306-4522(97)00601-5. [DOI] [PubMed] [Google Scholar]
- Gomez RE, Cannata MA, Milner TA, Anwar M, Reis DJ, Ruggiero DA. Vasopressinergic mechanisms in the nucleus reticularis lateralis in blood pressure control. Brain Res. 1993;604:90–105. doi: 10.1016/0006-8993(93)90356-r. [DOI] [PubMed] [Google Scholar]
- Goto A, Ikeda T, Tobian L, Iwai J, Johnson MA. Brain lesions in the paraventricular nuclei and catecholaminergic neurons minimize salt hypertension in Dahl salt-sensitive rats. Clin Sci. 1981;61(Suppl 7):53s–55s. doi: 10.1042/cs061053s. [DOI] [PubMed] [Google Scholar]
- Hancock MB, Nicholas AP. Oxytocin-immunoreactive projections onto medullary adrenaline neurons. Brain Res Bull. 1987;18:213–219. doi: 10.1016/0361-9230(87)90192-4. [DOI] [PubMed] [Google Scholar]
- Hardy SG. Hypothalamic projections to cardiovascular centers of the medulla. Brain Res. 2001;894:233–240. doi: 10.1016/s0006-8993(01)02053-4. [DOI] [PubMed] [Google Scholar]
- Hatton GI. Function-related plasticity in hypothalamus. Annu Rev Neurosci. 1997;20:375–397. doi: 10.1146/annurev.neuro.20.1.375. [DOI] [PubMed] [Google Scholar]
- Hatton GI. Dynamic neuronal–glial interactions: an overview 20 years later. Peptides. 2004;25:403–411. doi: 10.1016/j.peptides.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Haywood JR, Mifflin SW, Craig T, Calderon A, Hensler JG, Hinojosa-Laborde C. Gamma-aminobutyric acid (GABA)—a function and binding in the paraventricular nucleus of the hypothalamus in chronic renal-wrap hypertension. Hypertension. 2001;37:614–618. doi: 10.1161/01.hyp.37.2.614. [DOI] [PubMed] [Google Scholar]
- Hensch TK, Fagiolini M. Excitatory-inhibitory balance and critical period plasticity in developing visual cortex. Prog Brain Res. 2005;147:115–124. doi: 10.1016/S0079-6123(04)47009-5. [DOI] [PubMed] [Google Scholar]
- Herman JP, Eyigor O, Ziegler DR, Jennes L. Expression of ionotropic glutamate receptor subunit mRNAs in the hypothalamic paraventricular nucleus of the rat. J Comp Neurol. 2000;422:352–362. [PubMed] [Google Scholar]
- Herman JP, Tasker JG, Ziegler DR, Cullinan WE. Local circuit regulation of paraventricular nucleus stress integration: glutamate–GABA connections. Pharmacol Biochem Behav. 2002;71:457–468. doi: 10.1016/s0091-3057(01)00681-5. [DOI] [PubMed] [Google Scholar]
- Hermes SM, Mitchell JL, Silverman MB, Lynch PJ, McKee BL, Bailey TW, Andresen MC, Aicher SA. Sustained hyper-tension increases the density of AMPA receptor subunit, GluR1, in baroreceptive regions of the nucleus tractus solitarii of the rat. Brain Res. 2008;1187:125–136. doi: 10.1016/j.brainres.2007.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzig TC, Buchholz RA, Haywood JR. Effects of paraventricular nucleus lesions on chronic renal hypertension. Am J Physiol. 1991;261:H860–H867. doi: 10.1152/ajpheart.1991.261.3.H860. [DOI] [PubMed] [Google Scholar]
- Higa-Taniguchi KT, Silva FC, Silva HM, Michelini LC, Stern JE. Exercise training-induced remodeling of paraventricular nucleus (nor)adrenergic innervation in normotensive and hypertensive rats. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1717–R1727. doi: 10.1152/ajpregu.00613.2006. [DOI] [PubMed] [Google Scholar]
- Horn EM, Shonis CA, Holzwarth MA, Waldrop TG. Decrease in glutamic acid decarboxylase level in the hypothalamus of spontaneously hypertensive rats. J Hypertens. 1998;16:625–633. doi: 10.1097/00004872-199816050-00010. [DOI] [PubMed] [Google Scholar]
- Hrabovszky E, Turi GF, Kallo I, Liposits Z. Expression of vesicular glutamate transporter-2 in gonadotropin-releasing hormone neurons of the adult male rat. Endocrinology. 2004;145:4018–4021. doi: 10.1210/en.2004-0589. [DOI] [PubMed] [Google Scholar]
- Hrabovszky E, Wittmann G, Turi GF, Liposits Z, Fekete C. Hypophysiotropic thyrotropin-releasing hormone and corticotropin-releasing hormone neurons of the rat contain vesicular glutamate transporter-2. Endocrinology. 2005;146:341–347. doi: 10.1210/en.2004-0856. [DOI] [PubMed] [Google Scholar]
- Ishida H, Takemori K, Dote K, Ito H. Expression of glucose transporter-1 and aquaporin-4 in the cerebral cortex of stroke-prone spontaneously hypertensive rats in relation to the blood–brain barrier function. Am J Hypertens. 2006;19:33–39. doi: 10.1016/j.amjhyper.2005.06.023. [DOI] [PubMed] [Google Scholar]
- Ito S, Komatsu K, Tsukamoto K, Sved AF. Excitatory amino acids in the rostral ventrolateral medulla support blood pressure in spontaneously hypertensive rats. Hypertension. 2000;35:413–417. doi: 10.1161/01.hyp.35.1.413. [DOI] [PubMed] [Google Scholar]
- Ito S, Komatsu K, Tsukamoto K, Sved AF. Tonic excitatory input to the rostral ventrolateral medulla in Dahl salt-sensitive rats. Hypertension. 2001;37:687–691. [PubMed] [Google Scholar]
- Jansen AS, Schmidt ED, Voorn P, Tilders FJ. Substance induced plasticity in noradrenergic innervation of the paraventricular hypothalamic nucleus. Eur J Neurosci. 2003;17:298–306. doi: 10.1046/j.1460-9568.2003.02453.x. [DOI] [PubMed] [Google Scholar]
- Jung JY, Lee JU, Kim WJ. Enhanced activity of central adrenergic neurons in two-kidney, one-clip hypertension in Sprague-Dawley rats. Neurosci Lett. 2004;369:14–18. doi: 10.1016/j.neulet.2004.07.029. [DOI] [PubMed] [Google Scholar]
- Kaneko T, Fujiyama F, Hioki H. Immunohistochemical localization of candidates for vesicular glutamate transporters in the rat brain. J Comp Neurol. 2002;444:39–62. doi: 10.1002/cne.10129. [DOI] [PubMed] [Google Scholar]
- Katz LC, Iarovici DM. Green fluorescent latex micro-spheres: a new retrograde tracer. Neuroscience. 1990;34:511–520. doi: 10.1016/0306-4522(90)90159-2. [DOI] [PubMed] [Google Scholar]
- Katz LC, Burkhalter A, Dreyer WJ. Fluorescent latex micro-spheres as a retrograde neuronal marker for in vivo and in vitro studies of visual cortex. Nature. 1984;310:498–500. doi: 10.1038/310498a0. [DOI] [PubMed] [Google Scholar]
- Kiss J, Halasz B, Csaki A, Liposits Z, Hrabovszky E. Vesicular glutamate transporter 2 protein and mRNA containing neurons in the hypothalamic suprachiasmatic nucleus of the rat. Brain Res Bull. 2007;74:397–405. doi: 10.1016/j.brainresbull.2007.07.023. [DOI] [PubMed] [Google Scholar]
- Kleiber AC, Zheng H, Schultz HD, Peuler JD, Patel KP. Exercise training normalizes enhanced glutamate-mediated sympathetic activation from the PVN in heart failure. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1863–R1872. doi: 10.1152/ajpregu.00757.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knott GW, Quairiaux C, Genoud C, Welker E. Formation of dendritic spines with GABAergic synapses induced by whisker stimulation in adult mice. Neuron. 2002;34:265–273. doi: 10.1016/s0896-6273(02)00663-3. [DOI] [PubMed] [Google Scholar]
- Krug K, Smith AL, Thompson ID. The development of topography in the hamster geniculo-cortical projection. J Neurosci. 1998;18:5766–5776. doi: 10.1523/JNEUROSCI.18-15-05766.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo T, Hagiwara Y, Sekiya D, Chiba S, Fukumori R. Cholinergic inputs to rostral ventrolateral medulla pressor neurons from hypothalamus. Brain Res Bull. 2000;53:275–282. doi: 10.1016/s0361-9230(00)00343-9. [DOI] [PubMed] [Google Scholar]
- Lang RE, Heil J, Ganten D, Hermann K, Rascher W, Unger T. Effects of lesions in the paraventricular nucleus of the hypothalamus on vasopressin and oxytocin contents in brainstem and spinal cord of rat. Brain Res. 1983;260:326–329. doi: 10.1016/0006-8993(83)90690-x. [DOI] [PubMed] [Google Scholar]
- Latchford KJ, Ferguson AV. ANG II-induced excitation of paraventricular nucleus magnocellular neurons: a role for glutamate interneurons. Am J Physiol Regul Integr Comp Physiol. 2004;286:R894–R902. doi: 10.1152/ajpregu.00603.2003. [DOI] [PubMed] [Google Scholar]
- Leng G, Brown CH, Bull PM, Brown D, Scullion S, Currie J, Blackburn-Munro RE, Feng J, Onaka T, Verbalis JG, Russell JA, Ludwig M. Responses of magnocellular neurons to osmotic stimulation involves coactivation of excitatory and inhibitory input: an experimental and theoretical analysis. J Neurosci. 2001;21:6967–6977. doi: 10.1523/JNEUROSCI.21-17-06967.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Tripathi PK, Armstrong WE. Differences in spike train variability in rat vasopressin and oxytocin neurons and their relationship to synaptic activity. J Physiol. 2007;581:221–240. doi: 10.1113/jphysiol.2006.123810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li DP, Pan HL. Angiotensin II attenuates synaptic GABA release and excites paraventricular-rostral ventrolateral medulla output neurons. J Pharmacol Exp Ther. 2005;313:1035–1045. doi: 10.1124/jpet.104.082495. [DOI] [PubMed] [Google Scholar]
- Li DP, Pan HL. Plasticity of GABAergic control of hypothalamic presympathetic neurons in hypertension. Am J Physiol Heart Circ Physiol. 2006;290:H1110–1119. doi: 10.1152/ajpheart.00788.2005. [DOI] [PubMed] [Google Scholar]
- Li DP, Pan HL. Glutamatergic inputs in the hypothalamic paraventricular nucleus maintain sympathetic vasomotor tone in hypertension. Hypertension. 2007a;49:916–925. doi: 10.1161/01.HYP.0000259666.99449.74. [DOI] [PubMed] [Google Scholar]
- Li DP, Pan HL. Role of gamma-aminobutyric acid (GABA)A and GABAB receptors in paraventricular nucleus in control of sympathetic vasomotor tone in hypertension. J Pharmacol Exp Ther. 2007b;320:615–626. doi: 10.1124/jpet.106.109538. [DOI] [PubMed] [Google Scholar]
- Li DP, Yang Q, Pan HM, Pan HL. Plasticity of pre-and postsynaptic GABAB receptor function in the paraventricular nucleus in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2008a;295:H807–H815. doi: 10.1152/ajpheart.00259.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li DP, Yang Q, Pan HM, Pan HL. Pre-and postsynaptic plasticity underlying augmented glutamatergic inputs to hypothalamic presympathetic neurons in spontaneously hyper-tensive rats. J Physiol. 2008b;586:1637–1647. doi: 10.1113/jphysiol.2007.149732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YF, Patel KP. Paraventricular nucleus of the hypothalamus and elevated sympathetic activity in heart failure: the altered inhibitory mechanisms. Acta Physiol Scand. 2003;177:17–26. doi: 10.1046/j.1365-201X.2003.01043.x. [DOI] [PubMed] [Google Scholar]
- Li Y, Zhang W, Stern JE. Nitric oxide inhibits the firing activity of hypothalamic paraventricular neurons that inner-vate the medulla oblongata: role of GABA. Neuroscience. 2003a;118:585–601. doi: 10.1016/s0306-4522(03)00042-3. [DOI] [PubMed] [Google Scholar]
- Li YF, Cornish KG, Patel KP. Alteration of NMDA NR1 receptors within the paraventricular nucleus of hypothalamus in rats with heart failure. Circ Res. 2003b;93:990–997. doi: 10.1161/01.RES.0000102865.60437.55. [DOI] [PubMed] [Google Scholar]
- Li YF, Jackson KL, Stern JE, Rabeler B, Patel KP. Interaction between glutamate and GABA systems in the integration of sympathetic outflow by the paraventricular nucleus of the hypothalamus. Am J Physiol Heart Circ Physiol. 2006;291:H2847–H2856. doi: 10.1152/ajpheart.00625.2005. [DOI] [PubMed] [Google Scholar]
- Llinas R, Nicholson C. Electrophysiological properties of dendrites and somata in alligator Purkinje cells. J Neurophysiol. 1971;34:532–551. doi: 10.1152/jn.1971.34.4.532. [DOI] [PubMed] [Google Scholar]
- Luiten PG, ter Horst GJ, Karst H, Steffens AB. The course of paraventricular hypothalamic efferents to autonomic structures in medulla and spinal cord. Brain Res. 1985;329:374–378. doi: 10.1016/0006-8993(85)90554-2. [DOI] [PubMed] [Google Scholar]
- Mack SO, Kc P, Wu M, Coleman BR, Tolentino-Silva FP, Haxhiu MA. Paraventricular oxytocin neurons are involved in neural modulation of breathing. J Appl Physiol. 2002;92:826–834. doi: 10.1152/japplphysiol.00839.2001. [DOI] [PubMed] [Google Scholar]
- Magee JC, Johnston D. Synaptic activation of voltage-gated channels in the dendrites of hippocampal pyramidal neurons. Science. 1995;268:301–304. doi: 10.1126/science.7716525. [DOI] [PubMed] [Google Scholar]
- Martin DS, Haywood JR. Sympathetic nervous system activation by glutamate injections into the paraventricular nucleus. Brain Res. 1992;577:261–267. doi: 10.1016/0006-8993(92)90282-e. [DOI] [PubMed] [Google Scholar]
- Martin DS, Haywood JR. Reduced GABA inhibition of sympathetic function in renal-wrapped hypertensive rats. Am J Physiol. 1998;275:R1523–R1529. doi: 10.1152/ajpregu.1998.275.5.R1523. [DOI] [PubMed] [Google Scholar]
- Martinez-Maldonado M. Pathophysiology of renovascular hypertension. Hypertension. 1991;17:707–719. doi: 10.1161/01.hyp.17.5.707. [DOI] [PubMed] [Google Scholar]
- Mei L, Zhang J, Mifflin S. Hypertension alters GABA receptor-mediated inhibition of neurons in the nucleus of the solitary tract. Am J Physiol Regul Integr Comp Physiol. 2003;285:R1276–R1286. doi: 10.1152/ajpregu.00255.2003. [DOI] [PubMed] [Google Scholar]
- Miles R, Toth K, Gulyas AI, Hajos N, Freund TF. Differences between somatic and dendritic inhibition in the hippocampus. Neuron. 1996;16:815–823. doi: 10.1016/s0896-6273(00)80101-4. [DOI] [PubMed] [Google Scholar]
- Mody I, De Koninck Y, Otis TS, Soltesz I. Bridging the cleft at GABA synapses in the brain. Trends Neurosci. 1994;17:517–525. doi: 10.1016/0166-2236(94)90155-4. [DOI] [PubMed] [Google Scholar]
- Mueller NK, Di S, Paden CM, Herman JP. Activity-dependent modulation of neurotransmitter innervation to vasopressin neurons of the supraoptic nucleus. Endocrinology. 2005;146:348–354. doi: 10.1210/en.2004-0539. [DOI] [PubMed] [Google Scholar]
- Neal-Perry GS, Zeevalk GD, Shu J, Etgen AM. Restoration of the luteinizing hormone surge in middle-aged female rats by altering the balance of GABA and glutamate transmission in the medial preoptic area. Biol Reprod. 2008 doi: 10.1095/biolreprod.108.069831. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilaver G, Zimmerman EA, Wilkins J, Michaels J, Hoffman D, Silverman AJ. Magnocellular hypothalamic projections to the lower brain stem and spinal cord of the rat. Immunocyto-chemical evidence for predominance of the oxytocinneurophysin system compared with the vasopressinneurophysin system. Neuroendocrinology. 1980;30:150–158. doi: 10.1159/000122991. [DOI] [PubMed] [Google Scholar]
- Park JB, Skalska S, Stern JE. Characterization of a novel tonic gamma-aminobutyric acidA receptor-mediated inhibition in magnocellular neurosecretory neurons and its modulation by glia. Endocrinology. 2006;147:3746–3760. doi: 10.1210/en.2006-0218. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Carrive P, Wang H, Wang PU. Chemoarchitec-tonic atlas of the rat brainstem. Academic Press; San Diego: 1999. [Google Scholar]
- Pietranera L, Saravia F, Gonzalez Deniselle MC, Roig P, Lima A, De Nicola AF. Abnormalities of the hippocampus are similar in deoxycorticosterone acetate-salt hypertensive rats and spontaneously hypertensive rats. J Neuroendocrinol. 2006;18:466–474. doi: 10.1111/j.1365-2826.2006.01436.x. [DOI] [PubMed] [Google Scholar]
- Pinto S, Roseberry AG, Liu H, Diano S, Shanabrough M, Cai X, Friedman JM, Horvath TL. Rapid rewiring of arcuate nucleus feeding circuits by leptin. Science. 2004;304:110–115. doi: 10.1126/science.1089459. [DOI] [PubMed] [Google Scholar]
- Pyner S, Coote JH. Identification of branching paraventricular neurons of the hypothalamus that project to the rostroventrolateral medulla and spinal cord. Neuroscience. 2000;100:549–556. doi: 10.1016/s0306-4522(00)00283-9. [DOI] [PubMed] [Google Scholar]
- Richards DS, Villalba RM, Alvarez FJ, Stern JE. Expression of GABAB receptors in magnocellular neurosecretory cells of male, virgin female and lactating rats. J Neuroendocrinol. 2005;17:413–423. doi: 10.1111/j.1365-2826.2005.01324.x. [DOI] [PubMed] [Google Scholar]
- Rubenstein JL, Merzenich MM. Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2003;2:255–267. doi: 10.1034/j.1601-183x.2003.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakata-Haga H, Kanemoto M, Maruyama D, Hoshi K, Mogi K, Narita M, Okado N, Ikeda Y, Nogami H, Fukui Y, Kojima I, Takeda J, Hisano S. Differential localization and colocalization of two neuron-types of sodium-dependent inorganic phosphate cotransporters in rat forebrain. Brain Res. 2001;902:143–155. doi: 10.1016/s0006-8993(01)02290-9. [DOI] [PubMed] [Google Scholar]
- Sasek CA, Helke CJ. Enkephalin-immunoreactive neuronal projections from the medulla oblongata to the intermediolateral cell column: relationship to substance P-immunoreactive neurons. J Comp Neurol. 1989;287:484–494. doi: 10.1002/cne.902870407. [DOI] [PubMed] [Google Scholar]
- Sawchenko PE, Swanson LW. Immunohistochemical identification of neurons in the paraventricular nucleus of the hypothalamus that project to the medulla or to the spinal cord in the rat. J Comp Neurol. 1982;205:260–272. doi: 10.1002/cne.902050306. [DOI] [PubMed] [Google Scholar]
- Shafton AD, Ryan A, Badoer E. Neurons in the hypothalamic paraventricular nucleus send collaterals to the spinal cord and to the rostral ventrolateral medulla in the rat. Brain Res. 1998;801:239–243. doi: 10.1016/s0006-8993(98)00587-3. [DOI] [PubMed] [Google Scholar]
- Singec I, Knoth R, Ditter M, Volk B, Frotscher M. Neurogranin is expressed by principal cells but not interneurons in the rodent and monkey neocortex and hippocampus. J Comp Neurol. 2004;479:30–42. doi: 10.1002/cne.20302. [DOI] [PubMed] [Google Scholar]
- Sonner PM, Stern JE. Functional role of A-type potassium currents in rat presympathetic PVN neurones. J Physiol. 2007;582:1219–1238. doi: 10.1113/jphysiol.2007.134379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonner PM, Filosa JA, Stern JE. Intracellular Ca2+ dynamics in presympathetic PVN neurons during repetitive firing. FASEB J. 2007;21:A471. [Google Scholar]
- Sonner PM, Filosa JA, Stern JE. Diminished A-type potassium current and altered firing properties in presympathetic PVN neurones in renovascular hypertensive rats. J Physiol. 2008;586:1605–1622. doi: 10.1113/jphysiol.2007.147413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spruston N, Jonas P, Sakmann B. Dendritic glutamate receptor channels in rat hippocampal CA3 and CA1 pyramidal neurons. J Physiol. 1995;482:325–352. doi: 10.1113/jphysiol.1995.sp020521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern JE. Electrophysiological and morphological properties of pre-autonomic neurones in the rat hypothalamic paraventricular nucleus. J Physiol. 2001;537:161–177. doi: 10.1111/j.1469-7793.2001.0161k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern JE, Zhang W. Preautonomic neurons in the paraventricular nucleus of the hypothalamus contain estrogen receptor beta. Brain Res. 2003;975:99–109. doi: 10.1016/s0006-8993(03)02594-0. [DOI] [PubMed] [Google Scholar]
- Stocker SD, Cunningham JT, Toney GM. Water deprivation increases Fos immunoreactivity in PVN autonomic neurons with projections to the spinal cord and rostral ventrolateral medulla. Am J Physiol Regul Integr Comp Physiol. 2004;287:R1172–R1183. doi: 10.1152/ajpregu.00394.2004. [DOI] [PubMed] [Google Scholar]
- Stocker SD, Simmons JR, Stornetta RL, Toney GM, Guyenet PG. Water deprivation activates a glutamatergic projection from the hypothalamic paraventricular nucleus to the rostral ventrolateral medulla. J Comp Neurol. 2006;494:673–685. doi: 10.1002/cne.20835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW, Kuypers HG. The paraventricular nucleus of the hypothalamus: cytoarchitectonic subdivisions and organization of projections to the pituitary, dorsal vagal complex, and spinal cord as demonstrated by retrograde fluorescence double-labeling methods. J Comp Neurol. 1980;194:555–570. doi: 10.1002/cne.901940306. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE. Hypothalamic integration: organization of the paraventricular and supraoptic nuclei. Annu Rev Neurosci. 1983;6:269–324. doi: 10.1146/annurev.ne.06.030183.001413. [DOI] [PubMed] [Google Scholar]
- Sweatt AJ, Garcia-Espinosa MA, Wallin R, Hutson SM. Branched-chain amino acids and neurotransmitter metabolism: expression of cytosolic branched-chain aminotransferase (BCATc) in the cerebellum and hippocampus. J Comp Neurol. 2004;477:360–370. doi: 10.1002/cne.20200. [DOI] [PubMed] [Google Scholar]
- Sykes PA, Condron BG. Development and sensitivity to serotonin of Drosophila serotonergic varicosities in the central nervous system. Dev Biol. 2005;286:207–216. doi: 10.1016/j.ydbio.2005.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagawa T, Dampney RA. AT(1) receptors mediate excita-tory inputs to rostral ventrolateral medulla pressor neurons from hypothalamus. Hypertension. 1999;34:1301–1307. doi: 10.1161/01.hyp.34.6.1301. [DOI] [PubMed] [Google Scholar]
- Takeda K, Nakata T, Takesako T, Itoh H, Hirata M, Kawasaki S, Hayashi J, Oguro M, Sasaki S, Nakagawa M. Sympathetic inhibition and attenuation of spontaneous hypertension by PVN lesions in rats. Brain Res. 1991;543:296–300. doi: 10.1016/0006-8993(91)90040-3. [DOI] [PubMed] [Google Scholar]
- Tasker JG, Dudek FE. Local inhibitory synaptic inputs to neurones of the paraventricular nucleus in slices of rat hypothalamus. J Physiol. 1993;469:179–192. doi: 10.1113/jphysiol.1993.sp019810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodosis DT, Poulain DA. Maternity leads to morphological synaptic plasticity in the oxytocin system. Prog Brain Res. 2001;133:49–58. doi: 10.1016/s0079-6123(01)33004-2. [DOI] [PubMed] [Google Scholar]
- Theodosis DT, Trailin A, Poulain DA. Remodeling of astrocytes, a prerequisite for synapse turnover in the adult brain? Insights from the oxytocin system of the hypothalamus. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1175–R1182. doi: 10.1152/ajpregu.00755.2005. [DOI] [PubMed] [Google Scholar]
- Tomassoni D, Avola R, Di Tullio MA, Sabbatini M, Vitaioli L, Amenta F. Increased expression of glial fibrillary acidic protein in the brain of spontaneously hypertensive rats. Clin Exp Hypertens. 2004;26:335–350. doi: 10.1081/ceh-120034138. [DOI] [PubMed] [Google Scholar]
- Tong Q, Ma J, Kirchgessner AL. Vesicular glutamate transporter 2 in the brain–gut axis. Neuroreport. 2001;12:3929–3934. doi: 10.1097/00001756-200112210-00015. [DOI] [PubMed] [Google Scholar]
- Tweedle CD, Hatton GI. Synapse formation and disappearance in adult rat supraoptic nucleus during different hydration states. Brain Res. 1984;309:373–376. doi: 10.1016/0006-8993(84)90607-3. [DOI] [PubMed] [Google Scholar]
- Varoqui H, Schafer MK, Zhu H, Weihe E, Erickson JD. Identification of the differentiation-associated Na+/PI transporter as a novel vesicular glutamate transporter expressed in a distinct set of glutamatergic synapses. J Neurosci. 2002;22:142–155. doi: 10.1523/JNEUROSCI.22-01-00142.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Martens JR, Posner P, Sumners C, Gelband CH. Angiotensin II regulation of intracellular calcium in astroglia cultured from rat hypothalamus and brainstem. J Neurochem. 1996;67:996–1004. doi: 10.1046/j.1471-4159.1996.67030996.x. [DOI] [PubMed] [Google Scholar]
- Wassef A, Baker J, Kochan LD. GABA and schizophrenia: a review of basic science and clinical studies. J Clin Psychopharmacol. 2003;23:601–640. doi: 10.1097/01.jcp.0000095349.32154.a5. [DOI] [PubMed] [Google Scholar]
- Whitnall MH, Key S, Ben-Barak Y, Ozato K, Gainer H. Neurophysin in the hypothalamo-neurohypophysial system. II. Im munocytochemical studies of the ontogeny of oxytocinergic and vasopressinergic neurons. J Neurosci. 1985;5:98–109. doi: 10.1523/JNEUROSCI.05-01-00098.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimmer VC, Horstmann H, Groh A, Kuner T. Donut-like topology of synaptic vesicles with a central cluster of mitochondria wrapped into membrane protrusions: a novel structure–function module of the adult calyx of Held. J Neurosci. 2006;26:109–116. doi: 10.1523/JNEUROSCI.3268-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann G, Lechan RM, Liposits Z, Fekete C. Glutamatergic innervation of corticotropin-releasing hormone-and thyrotropin-releasing hormone-synthesizing neurons in the hypothalamic paraventricular nucleus of the rat. Brain Res. 2005;1039:53–62. doi: 10.1016/j.brainres.2005.01.090. [DOI] [PubMed] [Google Scholar]
- Yamagata K, Shoji Y, Terashima T, Yokogoshi H. Glutamate reduces secretion of l-serine in astrocytes isolated from stroke-prone spontaneously hypertensive rats. Neuroscience. 2006;143:729–737. doi: 10.1016/j.neuroscience.2006.08.050. [DOI] [PubMed] [Google Scholar]
- Yamagata K, Tagami M, Yamori Y. Nitric oxide reduces astrocytic lactate production and induces neuronal vulnerability in stroke-prone spontaneously hypertensive rats. Glia. 2008;56:387–393. doi: 10.1002/glia.20621. [DOI] [PubMed] [Google Scholar]
- Yang Z, Coote JH. Influence of the hypothalamic paraventricular nucleus on cardiovascular neurones in the rostral ventrolateral medulla of the rat. J Physiol. 1998;513:521–530. doi: 10.1111/j.1469-7793.1998.521bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Bertram D, Coote JH. The role of glutamate and vasopressin in the excitation of RVL neurones by paraventricular neurones. Brain Res. 2001;908:99–103. doi: 10.1016/s0006-8993(01)02593-8. [DOI] [PubMed] [Google Scholar]
- Zahner MR, Li DP, Pan HL. Benzodiazepine inhibits hypothalamic presympathetic neurons by potentiation of GABAergic synaptic input. Neuropharmacology. 2007;52:467–475. doi: 10.1016/j.neuropharm.2006.08.024. [DOI] [PubMed] [Google Scholar]
- Zhang K, Li YF, Patel KP. Reduced endogenous GABA-mediated inhibition in the PVN on renal nerve discharge in rats with heart failure. Am J Physiol Regul Integr Comp Physiol. 2002;282:R1006–R1015. doi: 10.1152/ajpregu.00241.2001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.